Functional Characterization of Nuclear Receptor MuFTZ-F1 in the Bean Flower Thrips, Megalurothrips usitatus
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Rearing
2.2. In Silico Analysis and Phylogenetic Analysis of MuFTZ-F1
2.3. Stage- and Tissue-Specific Expression Profiling of MuFTZ-F1
2.4. dsRNA Synthesis
2.5. Oral RNAi Bioassay
2.6. Gene Expression Analysis Following RNAi
2.7. Reverse Transcriptase-Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
2.8. 20E and Dopamine Titer Quantification
2.9. Statistical Analysis
3. Results
3.1. MuFTZ-F1 Cloning and Phylogenetic Analysis
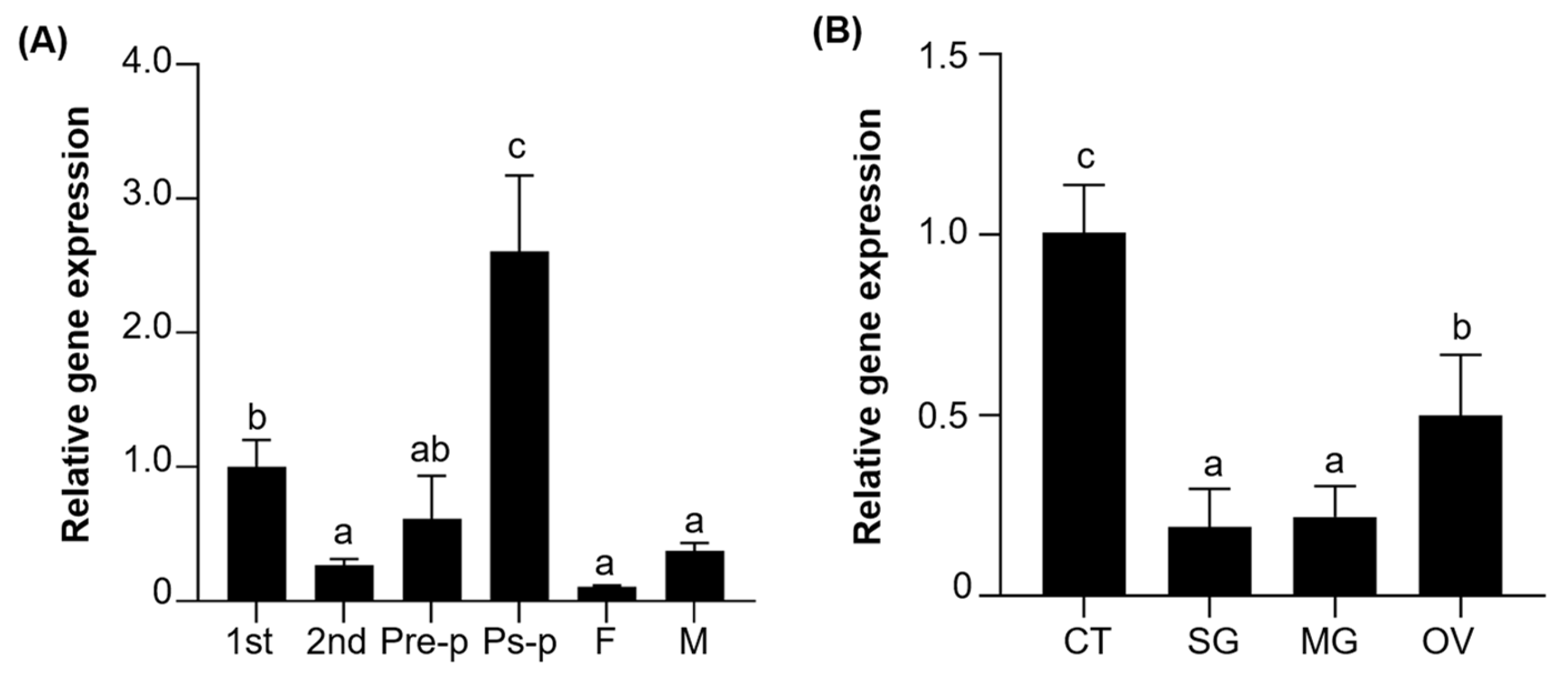
3.2. Spatiotemporal Expression Profiles of MuFTZ-F1
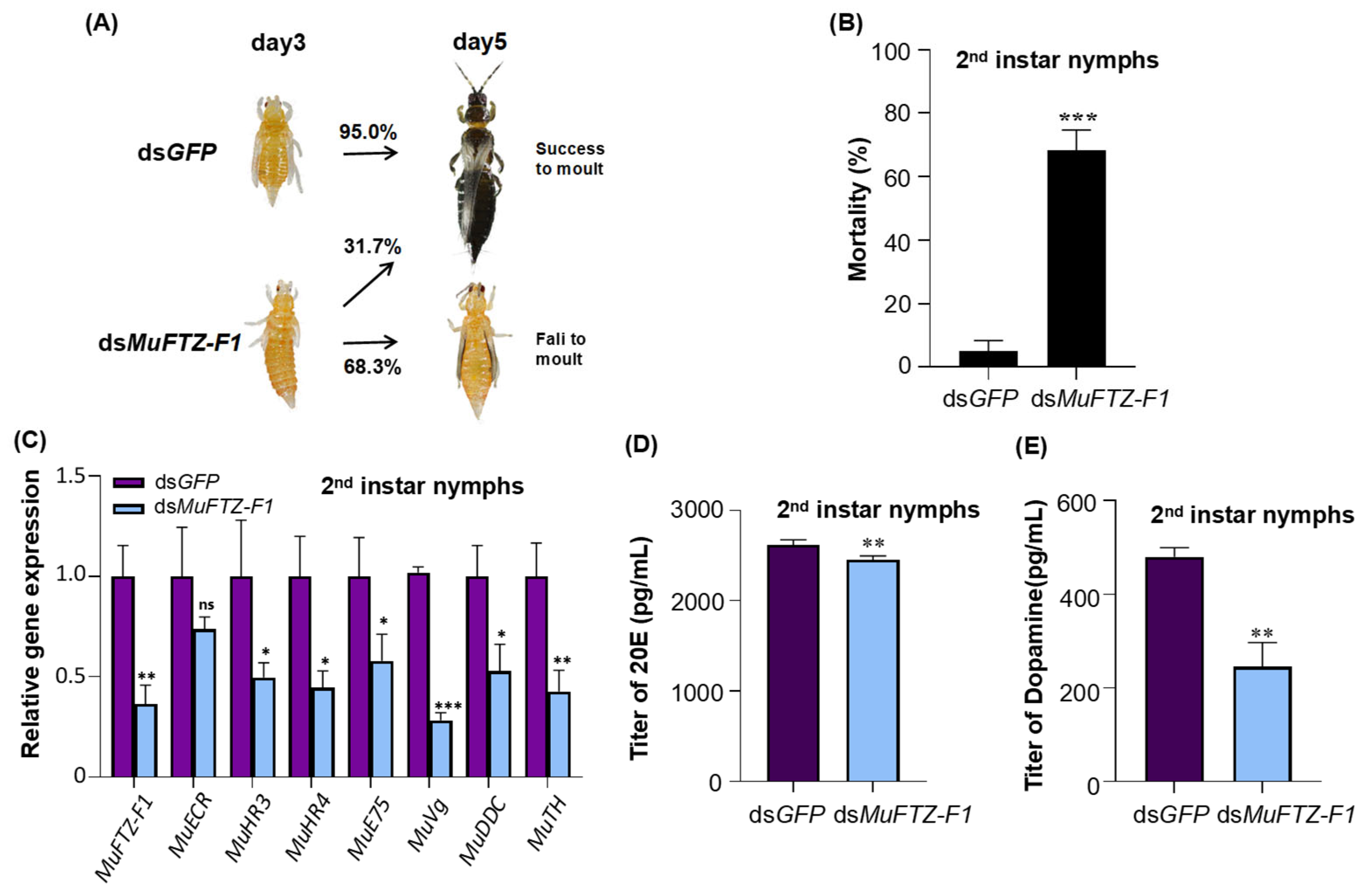
3.3. Effect of dsMuFTZ-F1 on M. usitatus Nymphs
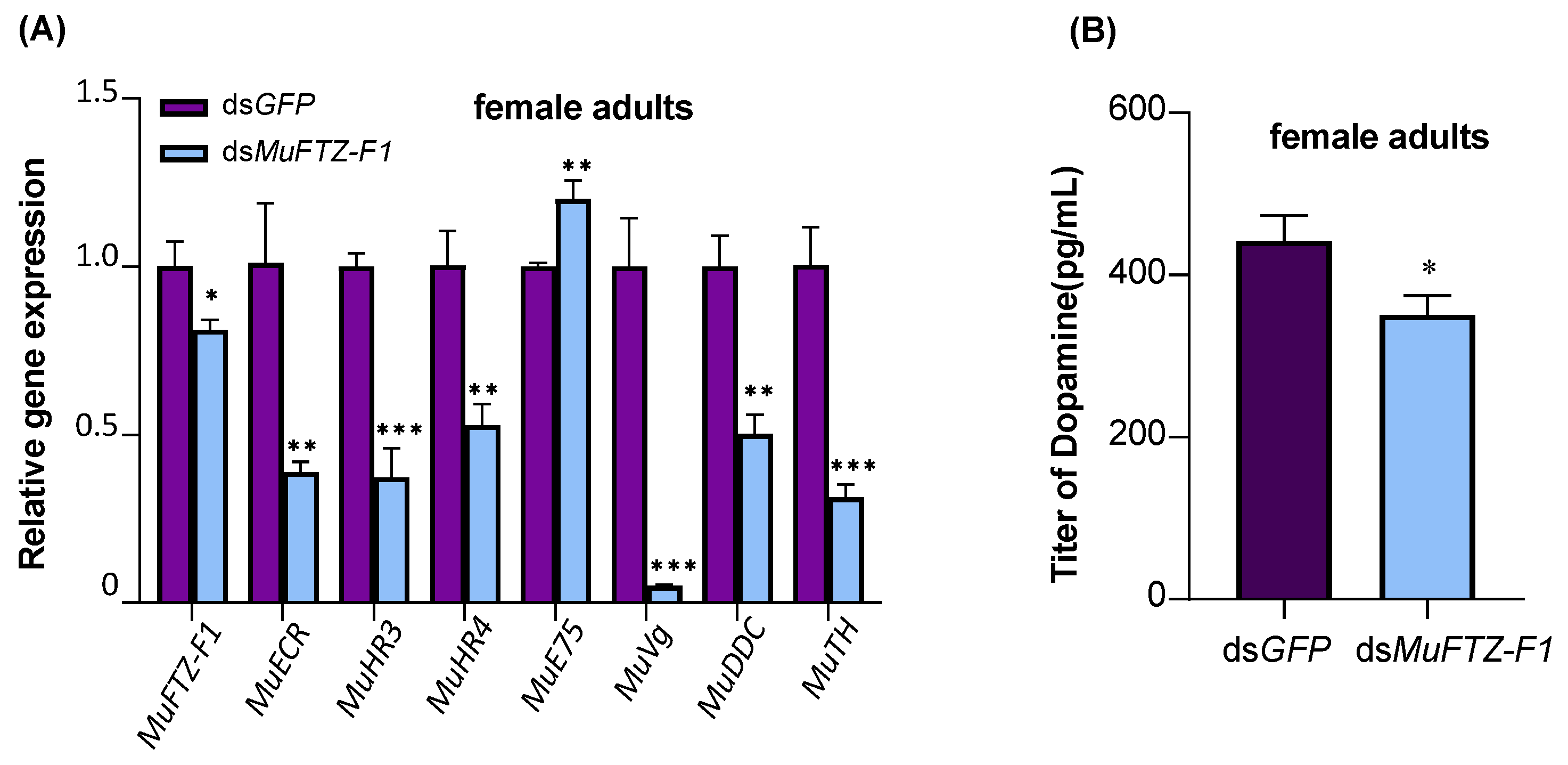
3.4. Effect of dsMuFTZ-F1 on M. usitatus Female Adults
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tang, L.D.; Yan, K.L.; Fu, B.L.; Wu, J.H.; Liu, K.; Lu, Y.Y. The life table parameters of Megalurothrips usitatus (Thysanoptera: Thripidae) on four leguminous crops. Fla. Entomol. 2015, 98, 620–625. [Google Scholar] [CrossRef]
- He, Y.C.; Gao, Y.; Hong, H.N.; Geng, J.M.; Chen, Q.L.; Zhou, Y.; Zhu, Z.R. Megalurothrips usitatus directly causes the black-heads and black-tail symptoms of cowpea along with the production of insect-resistance flavonoids. Plants 2023, 12, 3865. [Google Scholar] [CrossRef]
- Fu, B.L.; Tao, M.; Xue, H.; Jin, H.F.; Liu, K.; Qiu, H.Y.; Yang, S.Y.; Yang, X.; Gui, L.Y.; Zhang, Y.J.; et al. Spinetoram resistance drives interspecific competition between Megalurothrips usitatus and Frankliniella intonsa. Pest Manag. Sci. 2022, 78, 2129–2140. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Palli, S.R. Mechanisms, applications, and challenges of insect RNA interference. Annu. Rev. Entomol. 2020, 65, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.M.; Nanda, S.; Zhang, Y.J.; Zhou, X.G.; Yang, C.X.; Pan, H.P. Risk assessment of RNAi-based biopesticides. New Crops 2024, 1, 100019. [Google Scholar] [CrossRef]
- Yu, Y.B.; Zhong, Z.X.; Li, J.X.; Liu, X.X.; Zhou, X.G.; Xie, W.; Zhang, Y.J.; Pan, H.P. Double-stranded RNA degrading nuclease 3 (dsRNase3) influencing the RNAi efficiency in the bean flower thrips, Megalurothrips usitatus. Entomol. Gen. 2025, accepted. [Google Scholar]
- Chen, J.; Li, J.X.; Zhong, Z.X.; Yu, Y.B.; Yang, C.X.; Zhang, C.Q.; Tang, R.Y.; Xie, W.; Zhang, Y.J.; Pan, H.P. Synergistic control of Megalurothrips usitatus through sequential RNAi targeting novel lethal genes and Serratia marcescens HvSm-1. J. Agric. Food Chem. 2025, 73, 26892–26899. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.S.; Chen, L.; Sun, G.Q.; Raikhel, A.S. The competence factor βFTZ-F1 potentiates ecdysone receptor activity via recruiting a p160/SRC coactivator. Mol. Cell. Biol. 2006, 26, 9402–9412. [Google Scholar] [CrossRef]
- Yamada, M.a.; Murata, T.; Hirose, S.; Lavorgna, G.; Suzuki, E.; Ueda, H. Temporally restricted expression of transcription factor βFTZ-F1: Significance for embryogenesis, molting and metamorphosis in Drosophila melanogaster. Development 2000, 127, 5083–5092. [Google Scholar] [CrossRef]
- Akagi, K.; Sarhan, M.; Sultan, A.R.; Nishida, H.; Koie, A.; Nakayama, T.; Ueda, H. A biological timer in the fat body comprising Blimp-1, βFTZ-F1 and Shade regulates pupation timing in Drosophila melanogaster. Development 2016, 143, 2410–2416. [Google Scholar]
- Zhu, J.S.; Chen, L.; Raikhel, A.S. Posttranscriptional control of the competence factor βFTZ-F1 by juvenile hormone in the mosquito Aedes aegypti. Proc. Natl. Acad. Sci. USA 2003, 100, 13338–13343. [Google Scholar] [CrossRef]
- Zhang, W.N.; Ma, L.; Liu, X.Y.; Peng, Y.C.; Liang, G.M.; Xiao, H.J. Dissecting the roles of FTZ-F1 in larval molting and pupation, and the sublethal effects of methoxyfenozide on Helicoverpa armigera. Pest Manag. Sci. 2021, 77, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Mello, T.R.P.; Aleixo, A.C.; Pinheiro, D.G.; Nunes, F.M.F.; Cristino, A.S.; Bitondi, M.M.G.; Barchuk, A.R.; Simões, Z.L.P. Hormonal control and target genes of FTZ-F1 expression in the honeybee Apis mellifera: A positive loop linking juvenile hormone, FTZ-F1, and vitellogenin. Insect Mol. Biol. 2019, 28, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Brunet, J.; Eichner, C.; Male, R. The FTZ-F1 gene encodes two functionally distinct nuclear receptor isoforms in the ectoparasitic copepod salmon louse (Lepeophtheirus salmonis). PLoS ONE 2021, 16, e0251575. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.Q.; Nanda, S.; Yang, C.X.; Chen, S.M.; Guo, M.J.; Khan, M.M.; Qiu, B.L.; Zhang, Y.J.; Zhou, X.G.; Pan, H.P. RNAi suppression of the nuclear receptor FTZ-F1 impaired ecdysis, pupation, and reproduction in the 28-spotted potato ladybeetle, Henosepilachna vigintioctopunctata. Pestic. Biochem. Physiol. 2022, 182, 105029. [Google Scholar] [CrossRef]
- Cruz, J.; Nieva, C.; Mané-Padrós, D.; Martín, D.; Bellés, X. Nuclear receptor BgFTZ-F1 regulates molting and the timing of ecdysteroid production during nymphal development in the hemimetabolous insect Blattella germanica. Dev. Dyn. 2008, 237, 3179–3191. [Google Scholar] [CrossRef]
- Mazina, M.Y.; Kocheryzhkina, E.V.; Nikolenko, J.V.; Krasnov, A.N. Nuclear receptors EcR, Usp, E75, DHR3, and ERR regulate transcription of ecdysone cascade genes. Dokl. Biochem. Biophys. 2017, 473, 145–147. [Google Scholar] [CrossRef]
- King-Jones, K.; Thummel, C.S. Nuclear receptors—A perspective from Drosophila. Nat. Rev. Genet. 2005, 6, 311–323. [Google Scholar] [CrossRef]
- Liu, S.H.; Yang, B.J.; Wang, A.Y.; Luo, J.; Tang, J. RNA interference of tyrosine hydroxylase caused rapid mortality by impairing cuticle formation in Nilaparvata lugens (Hemiptera: Delphacidae). Pest Manag. Sci. 2020, 76, 2225–2232. [Google Scholar] [CrossRef]
- Li, Z.Y.; Chen, W.Y.; Wang, X.S.; Sang, W.; Pan, H.P.; Ali, S.K.; Tang, L.D.; Wu, J.H. Transcriptome analysis of Megalurothrips usitatus (Bagnall) identifies olfactory genes with ligands binding characteristics of MusiOBP1 and MusiCSP1. Front. Physiol. 2022, 13, 978534. [Google Scholar] [CrossRef]
- Pan, H.P.; Yang, X.W.; Romeis, J.; Siegfried, B.D.; Zhou, X.G. Dietary RNAi toxicity assay exhibits differential responses to ingested dsRNAs among lady beetles. Pest Manag. Sci. 2020, 76, 3606–3614. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.Y.; Li, Z.Y.; Zhou, C.Y.; Ali, A.; Ali, S.K.; Wu, J.H. RNA interference in cytochrome P450 monooxygenase (CYP) gene results in reduced insecticide resistance in Megalurothrips usitatus Bagnall. Front. Physiol. 2023, 14, 1130389. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, K.L.; Liu, K.H.; Wang, X.; Ma, M.Y.; Luo, X.W.; Chen, W.Y.; Chen, A.; Peng, Z.P.; Zhang, D.Y. Role of nuclear receptors NlHR3 and NlFTZ-F1 in regulating molting and reproduction in Nilaparvata lugens (Stål). Front. Physiol. 2023, 14, 1123583. [Google Scholar] [CrossRef]
- Choa, K.H.; Daubnerová, I.; Park, Y.; Zitnanb, D.; Adams, M.E. Secretory competence in a gateway endocrine cell conferred by the nuclear receptor βFTZ-F1 enables stage-specific ecdysone responses throughout development in Drosophila. Dev. Biol. 2014, 385, 253–262. [Google Scholar] [CrossRef]
- Liu, X.P.; Fu, K.Y.; Lu, F.G.; Meng, Q.W.; Guo, W.C.; Li, G.Q. Involvement of FTZ-F1 in the regulation of pupation in Leptinotarsa decemlineata(Say). Insect Biochem. Mol. Biol. 2014, 55, 51–60. [Google Scholar] [CrossRef]
- Yan, S.Y.; Ma, L.X.; Xu, K.K.; Li, C.; Yang, W.J. Nuclear receptor FTZ-F1 is required for larval-pupal molting by regulating ecdysteroidogenesis and chitin metabolism in Lasioderma serricorne. J. Stored Prod. Res. 2023, 101, 102096. [Google Scholar] [CrossRef]
- Parvy, J.P.; Blais, C.; Bernard, F.; Warren, J.T.; Petryk, A.; Gilbert, L.I.; O’Connor, M.B.; Dauphin-Villemant, C. A role for βFTZ-F1 in regulating ecdysteroid titers during post-embryonic development in Drosophila melanogaster. Dev. Biol. 2005, 282, 84–94. [Google Scholar] [CrossRef]
- Knapp, E.M.; Li, W.; Singh, V.; Sun, J.J. Nuclear receptor FTZ-F1 promotes follicle maturation and ovulation partly via bHLH/PAS transcription factor sim. eLife 2020, 9, e54568. [Google Scholar] [CrossRef]
- Alborzi, Z.; Piulachs, M.D. Dual function of the transcription factor FTZ-F1 on oviposition in the cockroach Blattella germanica. Insect Mol. Biol. 2023, 32, 689–702. [Google Scholar] [CrossRef]
- Kang, X.L.; Zhang, J.Y.; Wang, D.; Zhao, Y.M.; Han, X.L.; Wang, J.X.; Zhao, X.F. The steroid hormone 20-hydroxyecdysone binds to dopamine receptor to repress lepidopteran insect feeding and promote pupation. PLoS Genet. 2019, 15, e1008331. [Google Scholar] [CrossRef]
- Gruntenko, N.Y.; Laukhina, O.V.; Rauschenbach, I.Y. Role of D1- and D2-like receptors in age-specific regulation of juvenile hormone and 20-hydroxyecdysone levels by dopamine in Drosophila. J. Insect Physiol. 2012, 58, 1534–1540. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.Z.; Cao, L.J.; Chen, J.C.; Chen, W.B.; Shen, X.J.; Song, W.; Yang, F.Y.; Wei, S.J. A nanocarrier-mediated dsRNA oral delivery enhances RNAi efficiency in thrips. Entomol. Gen. 2024, 44, 601–611. [Google Scholar] [CrossRef]
- Rakesh, V.; Singh, A.; Ghosh, A. Suppression of Thrips palmi population by spray-on application of dsRNA targeting V-ATPase-B. Int. J. Biol. Macromol. 2024, 280, 135576. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Kim, M.; Kim, Y. Greenhouse test of spraying dsRNA to control the western flower thrips, Frankliniella occidentalis, infesting hot peppers. BMC Biotechnol. 2023, 23, 10. [Google Scholar] [CrossRef]
- Howard, J.D.; Beghyn, M.; Dewulf, N.; De Vos, Y.; Philips, A.; Portwood, D.; Kilby, P.M.; Oliver, D.; Maddelein, W.; Brown, S.; et al. Chemically modified dsRNA induces RNAi effects in insects in vitro and in vivo: A potential new tool for improving RNA-based plant protection. J. Biol. Chem. 2022, 298, 102311. [Google Scholar] [CrossRef]
- Chen, S.M.; Luo, X.M.; Nanda, S.; Yang, C.X.; Li, Z.Y.; Zhang, Y.; Zhou, X.; Pan, H.P. RNAi-based biopesticides against 28-spotted lady-beetle, Henosepilachna vigintioctopunctata does not harm the insect predator Propylea japonica. J. Agric. Food Chem. 2023, 71, 3373–3384. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, Y.; Wang, Z.; An, X.; Wei, S.; Andronis, C.; Vontas, J.; Wang, J.J.; Niu, J. dsRNAEngineer: A web-based tool of comprehensive dsRNA design for pest control. Trends Biotechnol. 2025, 43, 969–983. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, Z.; Li, J.; Chen, J.; Yang, C.; Zhang, C.; Tang, R.; Xie, W.; Zhang, Y.; Pan, H. Functional Characterization of Nuclear Receptor MuFTZ-F1 in the Bean Flower Thrips, Megalurothrips usitatus. Agronomy 2025, 15, 2593. https://doi.org/10.3390/agronomy15112593
Zhong Z, Li J, Chen J, Yang C, Zhang C, Tang R, Xie W, Zhang Y, Pan H. Functional Characterization of Nuclear Receptor MuFTZ-F1 in the Bean Flower Thrips, Megalurothrips usitatus. Agronomy. 2025; 15(11):2593. https://doi.org/10.3390/agronomy15112593
Chicago/Turabian StyleZhong, Zexin, Jianxing Li, Jian Chen, Chunxiao Yang, Chaoqun Zhang, Riyuan Tang, Wen Xie, Youjun Zhang, and Huipeng Pan. 2025. "Functional Characterization of Nuclear Receptor MuFTZ-F1 in the Bean Flower Thrips, Megalurothrips usitatus" Agronomy 15, no. 11: 2593. https://doi.org/10.3390/agronomy15112593
APA StyleZhong, Z., Li, J., Chen, J., Yang, C., Zhang, C., Tang, R., Xie, W., Zhang, Y., & Pan, H. (2025). Functional Characterization of Nuclear Receptor MuFTZ-F1 in the Bean Flower Thrips, Megalurothrips usitatus. Agronomy, 15(11), 2593. https://doi.org/10.3390/agronomy15112593






