1. Introduction
Soil quality is a crucial factor in determining crop yields, yet its parameters exhibit significant spatial variability even within a single field. One major contributor to this variability is topography, which directly influences the physicochemical characteristics of the soil, including water retention, nutrient distribution, and biochemical activity.
Since edaphic factors influence the activity of soil microorganisms, it is reasonable to assume that there is a close relationship between spatial variability and the physicochemical and biological properties of soil ecosystems. This has been supported by numerous studies. For instance, Broughton and Gross [
1] found that more productive and active microbial communities are found in the lower parts of slopes, which have higher levels of soil moisture, plant biomass, nitrogen (N) and carbon (C) content compared to the upper and middle parts of the slopes. Additionally, the activity of several soil enzymes, such as dehydrogenase, urease, phosphatase, arylsulfatase, β-glucosidase and chitinases, also depends on the position of the slope [
2,
3]. In a large-scale study, key edaphic factors of the terrain that determine the distribution of microbial communities were identified [
4]. Under conditions of high humidity, topographic parameters are positively correlated with the activity of denitrifying enzymes, microbial biomass, and denitrification rate. Local characteristics such as elevation, gradient, and curvature show a negative relationship with microbial activity [
4]. The highest microbial activity is observed in relief depressions, where moisture and organic matter accumulate. On slopes, aerated soils with increased bulk density and reduced enzymatic activity predominate. These patterns are significantly weakened under dry conditions when the topographic determinacy of spatial distribution parameters disappears.
Thus, soil microbial communities are shaped under the complex influence of abiotic and human factors. However, most research has focused on the impact of topography or land use in isolation. The combined and potentially hierarchical effects of a pronounced altitudinal gradient, hydrologically driven regimes (distance from rivers), and specific crop types within a single agricultural system remain poorly understood and represent a critical knowledge gap.
In this study, we go beyond investigating these factors in isolation and decode their unique interaction. We conducted a comprehensive analysis to understand how a pronounced altitudinal gradient, hydrological conditions, and specific agricultural land uses interact hierarchically to shape the structure and function of soil biota.
We hypothesized that the diversity and taxonomic composition of microbial communities would vary significantly across the topographic gradient, with the greatest differences observed between the upper slopes and floodplain areas. We also hypothesized that land use would play a key role in modulating community function, especially for fungi.
Understanding this hierarchy is essential for the advancement of precision agriculture. This study aims to provide a framework that allows for the development of spatially explicit management strategies. These strategies could include tailored erosion control measures and nutrient amendments based on specific topographic conditions.
Furthermore, by mapping the distribution of microbial functional groups, our work provides a basis for developing predictive models for assessing phytopathogenic risks. This allows for targeted interventions to prevent crop disease outbreaks in diverse landscapes.
2. Materials and Methods
The methodological approach was designed to thoroughly characterize the soil ecosystem at each sampling site. This was done through a multi-level analysis that included soil physicochemical properties, microbial community activity, and taxonomic and functional potential through DNA sequencing and enzymatic activity.
2.1. Study Area
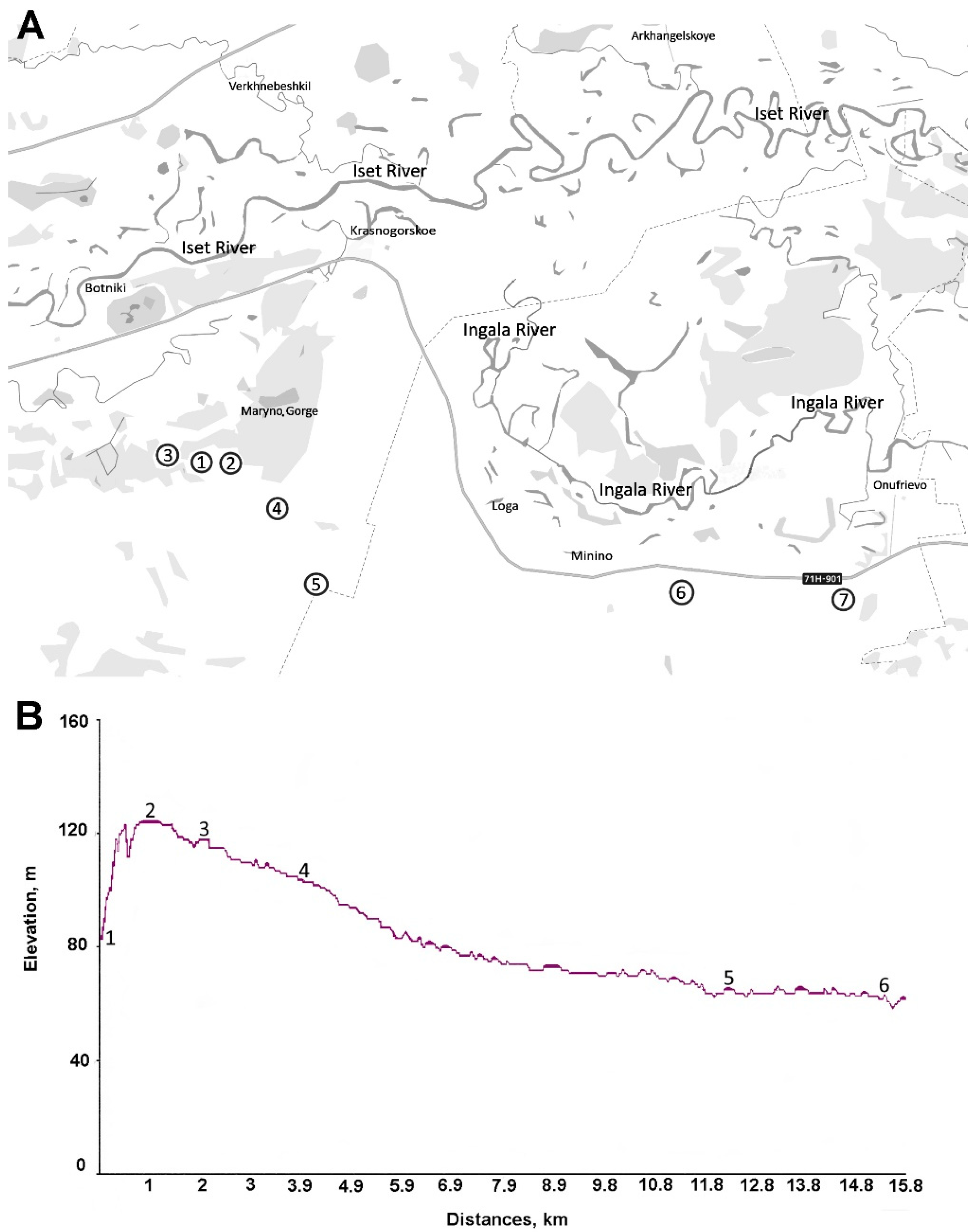
The study area is located in the southwestern part of the Tyumen Region, Russia, in the northern forest–steppe subzone of the West Siberian Lowland. Some of the sites are located in the floodplains of the Ingala and Iset Rivers, and are characterized by specific geomorphological conditions, dominated by sandy deposits. The terrain is gently rolling, with gently sloping hillsides that lead to the valleys of large rivers (
Figure 1). There are alternating drained ridges and depressions, partially occupied by marshes and lakes.
The climate in the region is sharply continental, with pronounced seasonal contrasts. Winters are long and cold, with stable snow cover down to −40 degrees Celsius and an average precipitation of 350–400 mm per year. Summers are warm, with temperatures reaching up to +35 degrees Celsius, and a growing season lasting 110–120 days. A list of sampling sites and their specific characteristics can be found in
Table 1 below.
At each study site, three spatially distant locations were selected. At each selected site, the top layer of soil (0–10 cm deep) was collected using the ‘checkboard’ sampling method (with four points in the corners and one in the center). The soil samples were transported to the laboratory and stored in sterile plastic bags at 4 °C until analysis.
2.2. Chemical Analysis of Soils
Soil pH was measured according to the international standard ISO 10390 (accessed on 7 November 2025,
https://www.iso.org/standard/75243.html). The pHKCl value was determined potentiometrically in a 1 M KCl solution, and the pHH
2O value was determined in an aqueous solution at a soil:solution ratio of 1:5 using an Orion Star A 111 pH-meter (Thermo Scientific, Waltham, MA, USA) [
5]. Total carbon (TC) and total nitrogen (TN) content were determined in triplicate using a Vario EL III elemental analyzer (Elementar, Langenselbold, Germany).
2.3. Determination of Soil Microbiological Properties
Before the start of microbiological measurements, sieved soil with a sieve diameter of 2 mm was freed from roots and large plant residues. It was moistened to 60% of its full moisture capacity and incubated in plastic bags at 22 °C for 3 days. The physiological activity of microorganisms in samples was assessed based on basal (microbial) soil respiration (BR) (CO
2 production) as described in the study [
6]. Samples were incubated during 24 h of incubation at 22 °C and 60% of the full moisture capacity.
Microbial biomass (MB
SIR) was determined by the substrate-induced respiration (SIR) method [
7]. CO
2 production (BR, SIR) was measured using a LI-830 gas analyzer (LI-COR Biosciences, Lincoln, NE, USA). The BR and SIR results were expressed in μg CO
2 g
−1 soil h
−1, a MB
SIR—in μg C g
−1 soil. Basal soil respiration and microbial biomass were determined for each biological replicate (3 analytical replicates each).
To assess the metabolic efficiency and physiological stress of the microbial community in response to environmental conditions, we calculated the following eco-physiological indices: the microbial respiration coefficient (QR = BR/SIR), which reflects energy allocation strategy, the metabolic coefficient or specific respiration of microbial biomass (
qCO
2 = BR/MB
SIR, mg CO
2 (g C h)
−1), the share of microbial biomass carbon in organic carbon (MB
SIR/TC, %), the relationship between C-use efficiency and the quality of available soil organic matter (
qCO
2/TC, mg CO
2 kg soil
−1 (gMB
SIR gC h)
−1). The description of eco-physiological indicators is detailed in our previous study [
8].
2.4. DNA Sequencing and Bioinformatics Analysis
To understand the taxonomic composition of bacterial and fungal communities, and to test the hypothesis that topography and land use influence community assembly, we performed high-throughput sequencing of the 16S rRNA gene and ITS region. Additionally, to gain a deeper understanding of the functional potential of these communities beyond taxonomic classifications, we conducted shotgun metagenomics on a subset of samples. DNA was extracted from 0.5 g soil samples using the Quick-DNA™ Fecal/Soil Microbe Miniprep Kit (ZymoResearch, Irvine, CA, USA). DNA concentration and purity were determined using a Qubit 4.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA) and a NanoPhotometer N120 spectrophotometer (Implen, Munich, Germany), respectively. The 27F (AGRGTTYGATYMTGGCTCAG) and 1492R (RGYTACCTTGTTACGACTT) universal primer set was used to amplify the full-length 16S rRNA gene from the genomic DNA. Both the forward and reverse 16S primers were tailed with sample specific PacBio barcode sequences to allow for multiplexed sequencing. To amplify the target region 18S-ITS-28S rRNA, we used a primer set containing the forward primer 3ndf complementary to the V4 region of the 18S rRNA gene GGCAAGTCTGGTGGTGCCAG and the reverse primer 21R complementary to the 28S rRNA gene GACGAGGCATTTGGCTATTTGGCTACCTT [
9].
The DNA amplified from each sample was then pooled in equimolar concentration prior to library preparation. The amplified DNA pool was processed using the SMRTbell prep kit 3.0 (PacBio, Menlo Park, CA, USA) according to the Procedure and Checklist—Amplification of Full-Length 16S Gene with Barcoded Primers for Multiplexed SMRTbell Library Preparation and Sequencing protocol. Fluorescence concentration and average size of the resulting library pool were checked using the Qubit HS DNA Kit (Qubit Fluorometer, Invitrogen, Waltham, MA, USA) and Fragment Analyzer (Agilent Technologies, Santa Clara, CA, USA). Sequencing was performed using the Sequel II Sequencing Kit 3.0 (PacBio, PacBio, Menlo Park, CA, USA) on the Sequel II PacBio system.
Shotgun metagenomic sequencing analysis. DNA libraries for shotgun metagenomic sequencing were prepared using the Illumina DNA Prep (M) Tagmentation Kit (Illumina, San Diego, CA, USA). Sequencing was performed on the Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA) following standard protocols for shotgun metagenomics, generating paired-end 2 × 100 bp reads.Pacific Biosciences data were demultiplexed using the PacBio ‘lima’ program (version 2.5.1). HiFi reads (CCS reads with predicted accuracy ≥ Q20) were extracted using SAMtools (version 1.13) [
10] and converted to FASTQ format using PacBio ‘bam2fasta’ (version 1.3.1). ITS sequences were isolated from sequencing data of the 18S-ITS-28S rRNA region using ITSx. Sequences containing both ITS1 and ITS2 were selected using a Perl script. The quality of the FASTQ files was analyzed using FastQC (version 0.11.5) software. Further processing was performed using the DADA2 algorithm [
11]. This algorithm, in brief, reorients sequences, removes forward and reverse primers, filters and trims sequences by length and average quality, dereplicates sequences, estimates sequencing errors using the PacBio error model, and determines sample composition. The ASVs were assigned taxonomically using the GTDB database [
12] for the bacterial communities and the UNITE database [
13] for the fungal communities.
The quality of the paired-end metagenome reads was assessed using FastQC (0.11.5) software [
14] and subsequently trimmed using Trimmomatic with the following parameters: SLIDINGWINDOW:4:20 MINLEN:50. Metagenome assembly was performed using metaSPAdes. Assembled contigs were annotated using reCOGnizer v. 1.6.4. This tool was employed to identify clusters of orthologous groups (COGs) in the predicted protein sequences.
2.5. Soil Enzyme Activities
To link the composition of microbial communities to ecosystem-level functioning and quantify the rates of key nutrient cycling, we measured the activity of hydrolytic enzymes specific to carbon (βG, βX, CBH), nitrogen (NAG, LAP), phosphorus (Phos), and sulfur (ARS) cycling using a fluorometric assay with methylumbelliferyl (MUB)-labeled substrates, following the method described by [
15]. The principle of the method is based on the enzymatic hydrolysis of a substrate labeled with a fluorescent dye (4-MUB). The hydrolysis reaction releases the fluorescent product, the concentration of which is quantified fluorometrically. Specifically, the activities of
β-1,4-glucosidase (
βG, EC 3.2.1.21),
β-1,4-xylosidase (
βX, EC 3.2.1.37), cellobiohydrolase (CBH, EC 3.2.1.91), phosphatase (Phos, EC 3.1.3.2), arylsulfatase (ARS, EC 3.1.6.1), and
β-1,4-N-acetylglucosaminidase (NAG, EC 3.2.1.30) were measured using their respective 4-MUB-labeled substrates: 4-MUB-β-D-glucopyranoside, 4-MUB-β-D-xylopyranoside, 4-MUB-β-D-cellobioside, 4-MUB-phosphate, 4-MUB-sulfate, and 4-MUB-N-acetyl-β-D-glucosaminide. For L-leucine aminopeptidase (LAP, EC3.4.11.1), the substrate L-leucine-7-amino-4-methylcoumarin labeled with 7-Amino-4-Methylcoumarin (AMC) was used. Fluorescent emission was measured using a Fluoroskan Ascent FL microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). The procedure for determining enzyme activity is described in detail in our previous study [
8]. In each biological replicate, enzymatic activity was determined in 8 analytical replicates.
2.6. Statistical Processing
The Shapiro–Wilk test was used to assess the normal distribution of values. In the presence of a normal distribution, the two-sample t-test was used, whereas if normality was rejected, the Mann–Whitney test was used. A correction for multiple comparisons was applied using the Sequential Bonferroni (Holm-Bonferroni) method. A p-value of <0.05 was considered statistically significant for all tests. Principal coordinate analysis (PCA) was performed using Origin v. 2021 software (OriginLab Corporation, Northampton, MA, USA). One-way PERMANOVA (Bray–Curtis similarity index), the α-diversity was assessed using the Shannon and Chao1 indices were calculated using Past v.5.2.2.
3. Results
3.1. Physicochemical Soil Properties
Analysis of the TC and TN content revealed a pronounced spatial differentiation along the slope catena (
Figure S1). The most significant trend was the accumulation of both TC and TN in the floodplain soils (FA-W1, FA-W2). In contrast, the watershed slopes demonstrated high variability, with the lowest values recorded at the FAa1 site and the highest among the upland sites at FA-P1. Soils were consistently alkaline (pH
2O 7.05–8.15), with a texture gradient from sandy soils on the plateau (FAa2) to loams in the accumulation zones. All detailed numerical data, including TC, TN, C:N ratios, pH values, and particle size distribution, are consolidated in
Figure S1 and Table S1.
3.2. Analysis of Soil Microbiological Activity
Analysis of microbial physiological profiles revealed a clear contrast in metabolic strategies along the topographic gradient. Ecophysiological profiles revealed contrasting microbial metabolic strategies along the topographic gradient (
Table 2,
Figure S2). The sandy plateau (FAa2) showed indicators of energy stress, while the floodplain (FA-W1) supported highly efficient microbial communities. The northern slope fallow (FAa1) demonstrated a unique profile, with an exceptionally high proportion of soil organic carbon present as living microbial biomass (MB
SIR/TC), suggesting a system with rapid turnover of labile organic matter. Detailed data on basal respiration (BR) and microbial biomass (MB
SIR) can be found in
Figure S2.
3.3. Enzymatic Activity of Soils
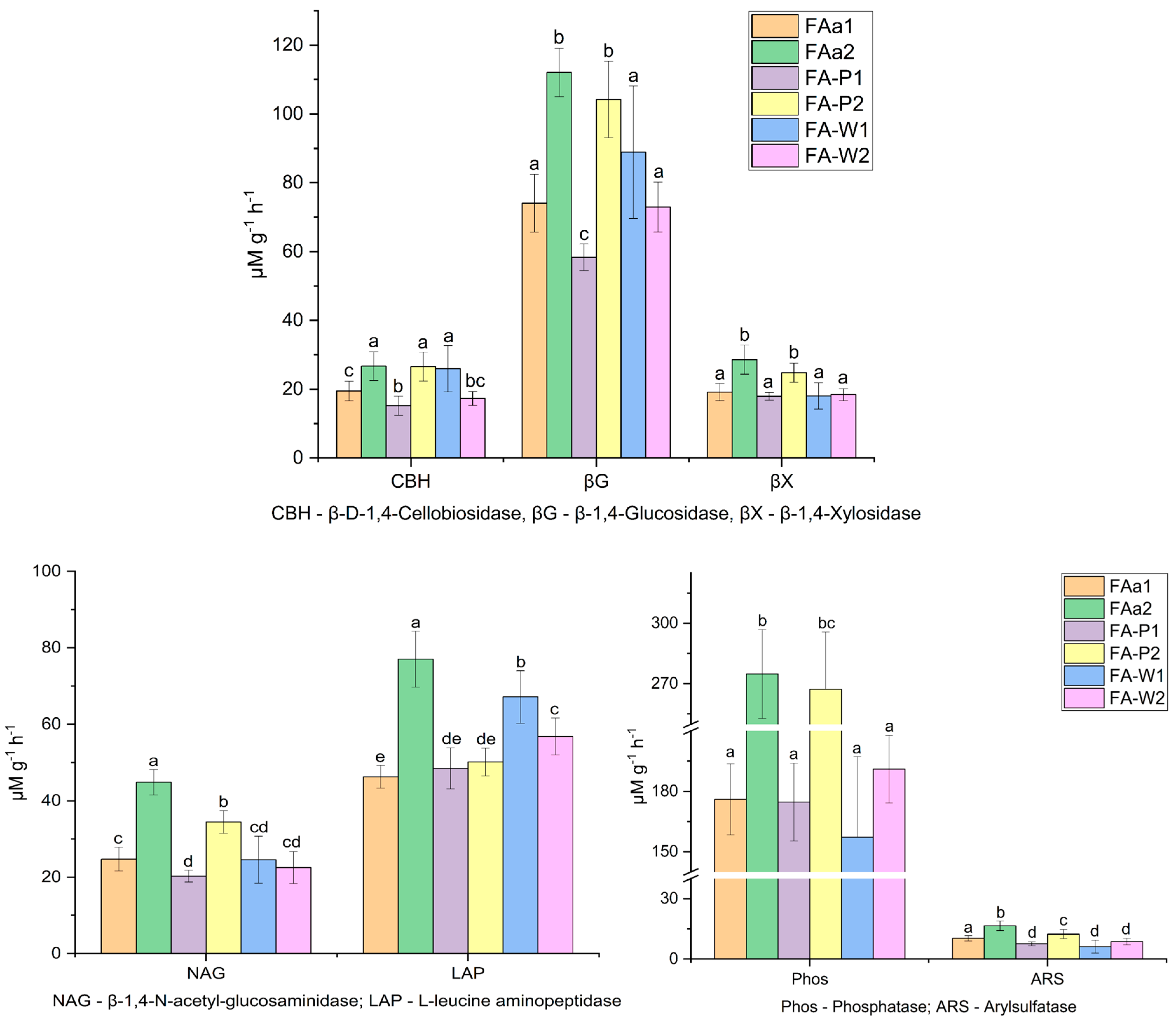
Analysis of enzymatic activities revealed distinct spatial patterns along the topographic gradient (
Figure 2). Fallow soils on the plateau consistently exhibited the highest overall enzymatic activity across multiple nutrient cycles (FAa2). For C-cycle enzymes (
Figure 2), significant differences were observed among land use types and topographic positions. Plateau fallow (FAa) showed substantially higher activity of CBH, βG, and βX compared to other sites. Pea cultivation sites generally demonstrated higher C-acquiring enzyme potential than wheat fields, particularly in the middle slope position (FA-P2).
The N-cycle enzymes displayed contrasting patterns between proteolytic and chitinolytic activities (
Figure 2). LAP activity was predominant in wheat fields and the northern slope fallow, while NAG activity dominated in the middle slope pea plot (FA-P2). The plateau fallow (FAa2) maintained elevated activity of both N-cycling enzymes compared to arable lands.
For P- and S-cycle enzymes, the most pronounced activity was recorded in the plateau fallow FAa2, with Phos and ARS values significantly exceeding those in other locations. Among arable sites, the middle slope pea plot FA-P2 showed notably higher phosphorus and sulfur acquisition potential compared to wheat fields.
The enzymatic profiles reflected a clear hierarchy of microbial functional strategies, with the sandy plateau fallow exhibiting signs of nutrient limitation stress, while accumulation zones demonstrated more balanced nutrient acquisition patterns.
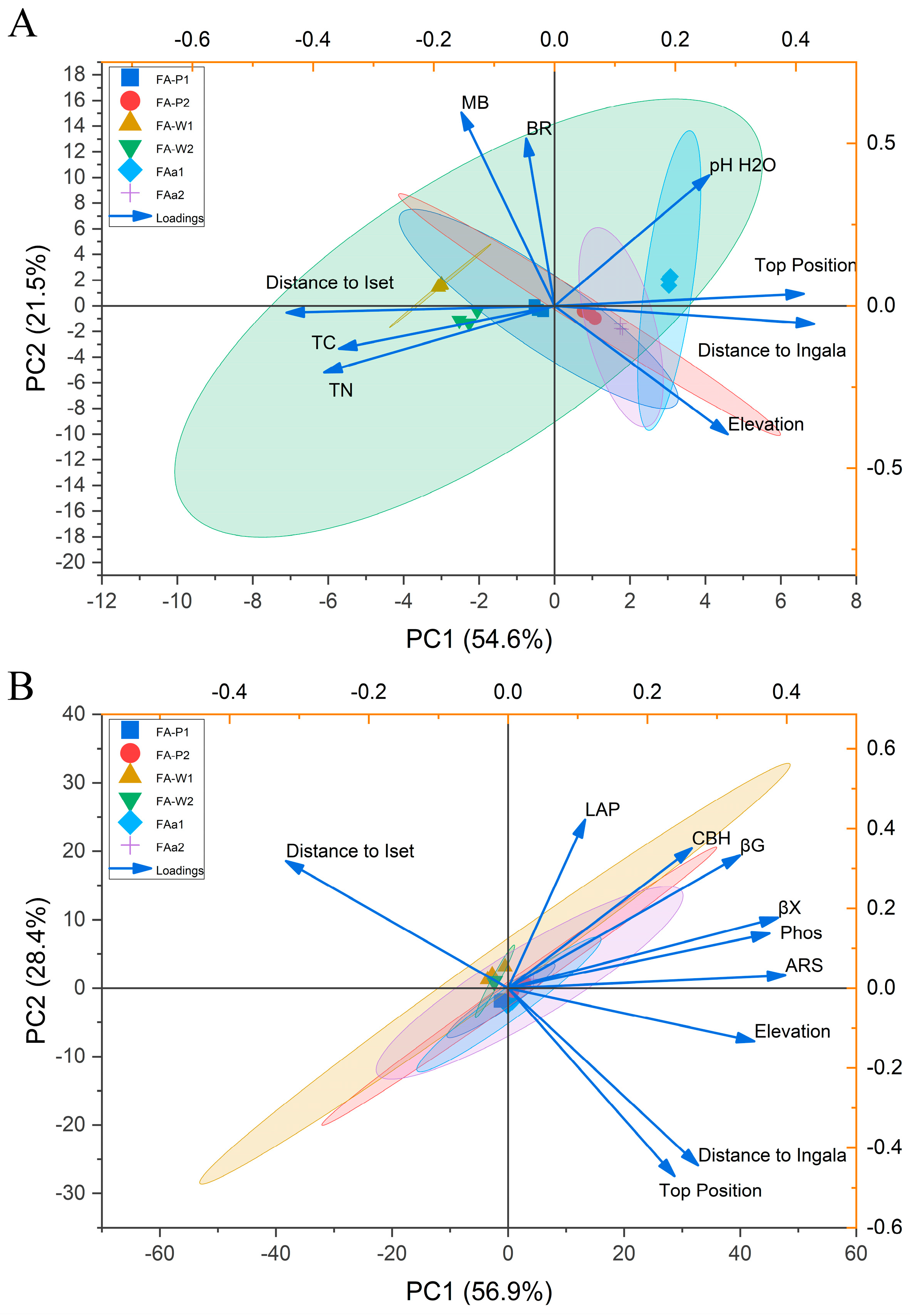
3.4. Spatial Distribution of Soil Properties and Microbial Activity: Principal Component Analysis
Principal component analysis (PCA) identified a two-factor structure that explains 76.1% of the overall variability in soil physicochemical properties and microbial physiological activity (
Figure 3A).
The first factor (PC1), accounting for 54.6% of variability, represents a large-scale hydrological and topographical gradient. The positive end of PC1 is strongly associated with distance from the Ingala River (loading = 0.430), topographic position (loading = 0.413) and altitude (loading = 0.287), while the negative end is determined by distance from Iset River (loadings = −0.444). Soil carbon and nitrogen distribution is specifically linked to this gradient as indicated by negative loading of total carbon (TC) (loading = −0.357) and total nitrogen (TN) (−0.341) with the negative pole of PC1 confirming the accumulation of organic matter in areas influenced by Iset river watershed.
The second component (PC2, accounting for 21.5% of the total variance) reflects an independent gradient of microbial community physiological activity. This gradient is strongly positively correlated with microbial biomass (MBSIR, loading = 0.594) and basal respiration (BR, loading = 0.515), but negatively correlated with altitude (loading = −0.395). Hydrological factors exhibit negligible loadings on this component (distance from Ingala: −0.055; distance from Iset: −0.021), demonstrating that the gradient of microbial activity and the influence of the river are orthogonal in this system.
A separate PCA of enzymatic activity revealed two orthogonal gradients that explained 85.3% of the total variation (
Figure 3B). The first gradient (PC1, 56.9%) represents an erosion-hydrological gradient. The activity of enzymes involved in carbon (β-xylosidase, βX, loading = 0.387; β-glucosidase, βG, loading = 0.333), phosphorus (phosphatase, Phos, loading = 0.375) and sulfur (arylsulfatase, ARS, loading = 0. 398) cycling was high in elevated areas of the watershed (elevation, loading = 0.353). This indicates increased enzymatic activity under conditions of elemental limitation in well-drained erosion-prone zones. The second gradient (PC2) contrasts zones with intense nitrogen metabolism. It showed a strong positive correlation with leucine aminopeptidase (LAP) (loading = 0.521) and a strong negative correlation with distance from the Iset River (loading = −0.458). This suggests that the local hydrological regime of the Iset floodplain plays a more significant role in controlling proteolytic activity than the general topographic position. Although a global PERMANOVA analysis of all measured parameters showed significant differences between sampling points (F = 36.6,
p < 0.001), pairwise analysis did not find any statistically significant differences (all
p > 0.05), suggesting that spatial heterogeneity is characterized by gradual change along PCA gradients rather than distinct groups.
3.5. Diversity and Taxonomic Composition of Bacterial and Fungal Communities in Soil
3.5.1. Fungal Diversity, Composition, and Functional Guilds
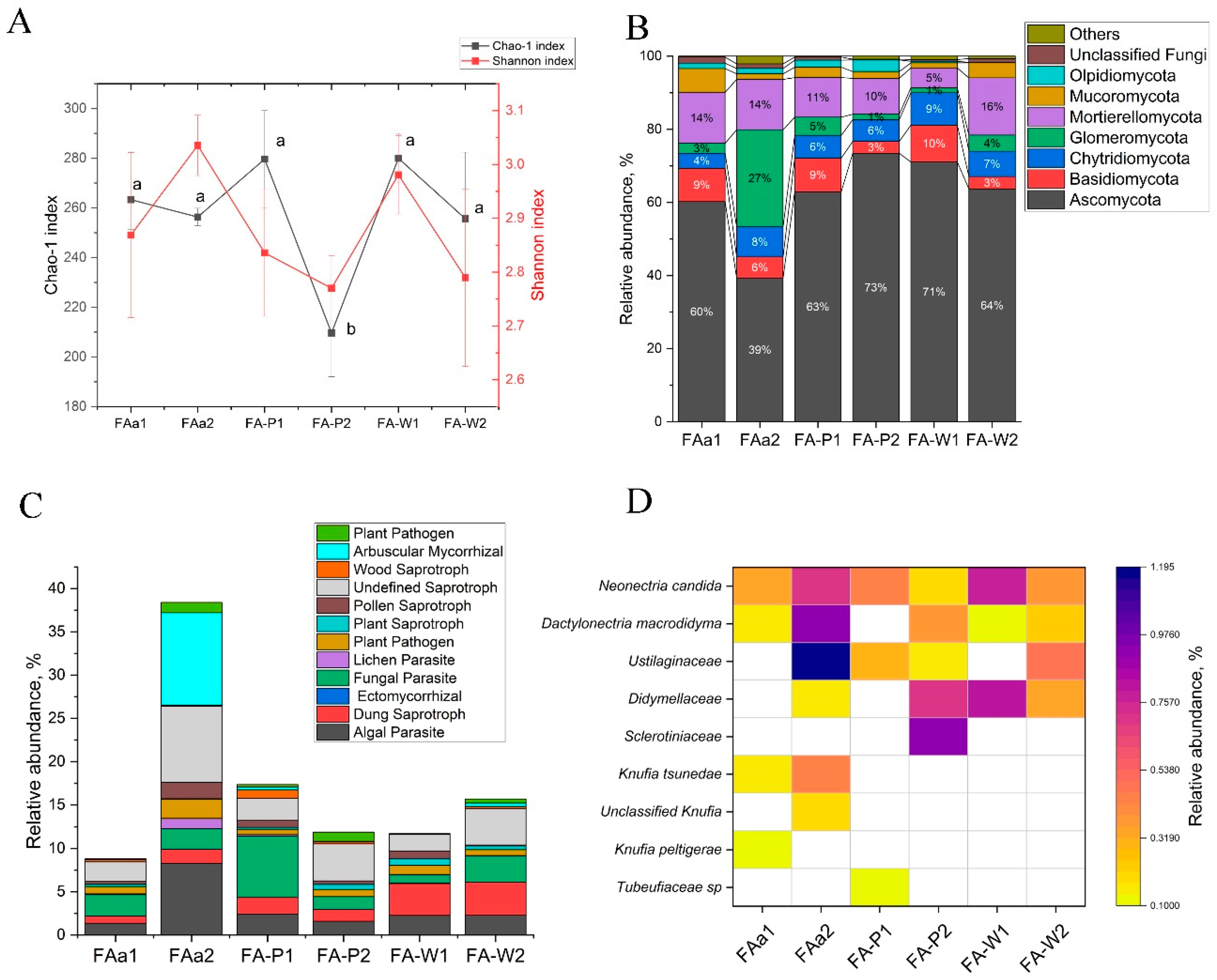
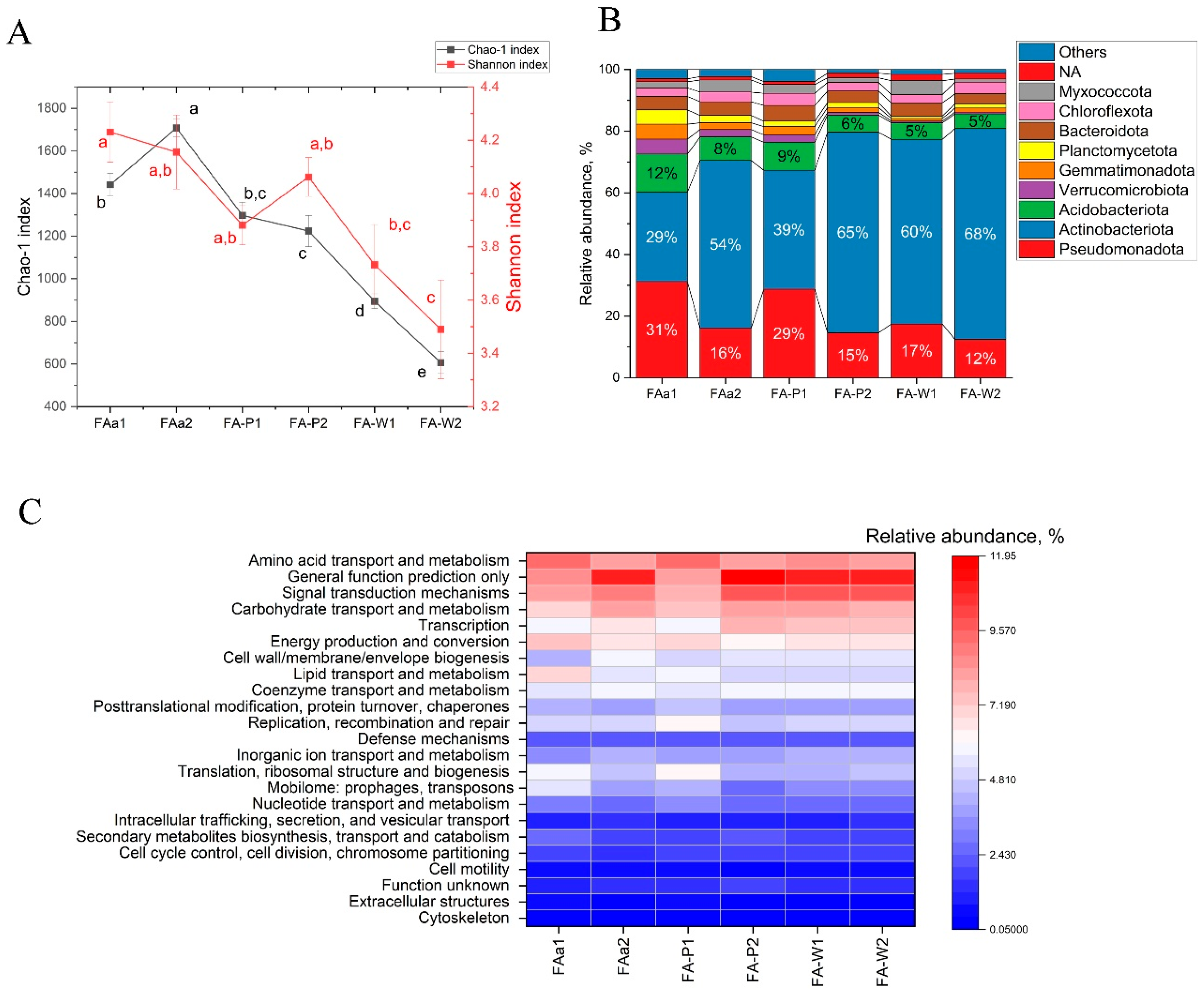
Fungal alpha-diversity, expressed by the Shannon index, showed consistency across topographic gradients and land use types, ranging from 2.81 to 3.04, without statistically significant variation. In contrast, the Chao1 index showed substantial variation ranging from 199 to 299 with significant differences between specific sites (
Figure 4A).
Significant differences were found in the proportion of Glomeromycota, a group of arbuscular mycorrhizal fungi. Their share increased substantially under FAa2 conditions (up to 26.5%), whereas in FA-P1 and FAa1, it remained moderate, not exceeding 1.8% (
Figure 4B). The dominant phylum Ascomycota also showed an increase in relative abundance in agrosystems (FA-P2: 73.4%, FA-W1: 71.0%) compared to most fallow and legume sites. Mortierellomycota abundance was highest on the low floodplain terrace (FA-W2: 15.6%) and at one fallow site (FAa1: 13.9%), but it decreased in other samples, exhibiting no clear topographic pattern.
Analysis of fungal communities revealed distinct functional patterns driven primarily by land management practices. Long-term fallow on the plateau (FAa2) created a unique symbiotic system characterized by the highest observed proportion of arbuscular mycorrhizal fungi (10.7%) and a high abundance of saprotrophic fungi (e.g., Undefined Saprotroph, 8.8%) (
Figure 4C). In contrast, fallow on the slope (FAa1) had a pathogen-dominated community, with a combined share of pathogens exceeding 3.2%.
Agricultural sites shifted towards saprotrophic and pathogenic niches. A key finding was a sharp increase in dung saprotrophs in the floodplain wheat fields (FA-W1, FA-W2: 3.7–3.8%). In contrast, pea crops on the slopes (FA-P1, FA-P2) were associated with a higher abundance of fungal parasites (up to 7.1% in FA-P1), suggesting crop-specific pathogenic pressures (
Figure 4C).
The analysis of phytopathogenic fungal communities revealed significant differences in their taxonomic structure and relative abundance between the studied samples. The highest taxonomic diversity and total proportion of pathogens were characteristic of the samples FAa1. Representatives of
Ustilaginaceae,
Dactylonectria macrodidyma, and
Neonectria candida dominated, with a total of 1.19%, 0.93%, and 0.69%, respectively, while species of the genus
Knufia and members of the
Didymellaceae were also present. In contrast, sample FA-P2 stood out for its uniquely high content of unidentified members of the family
Didymellacea (0.71%), as well as the presence of species from
Sclerotiniacea (0.92%). These findings were not observed at other sampling sites. FA-W1 и FA-W2 samples are dominated by
Didymellaceae (0.35% и 0.80%), respectively, and
Neonectria candida (0.38% и 0.77%). While FA-P1 and FA-P2 are characterized by a more uniform distribution and a smaller overall pathogen pool. The exception is
Neonectria candida in FA-P1 (45%), and there are also residual amounts of other taxa present (
Figure 4D). Thus, the samples from FAa1 и FA-P2 can be considered the most problematic phytopathogenic zones.
3.5.2. Bacterial Diversity, Composition, and Functional Potential
The spatial structure of the bacterial community, assessed at the level of dominant phyla, demonstrates a clear dependence on edaphic and topographic gradients. A key finding is the significant imbalance in the community on low floodplain terraces of the Ingala River. This community exhibits significantly reduced alpha diversity, with a Shannon index ranging from 3.67 to 3.9 and a Chao1 index ranging from 649 to 905 (
Figure 5A). The community is dominated by Actinomycetota, accounting for 59.2–67.6% of the total community, with a corresponding reduction in Pseudomonadota, which accounts for 12.3–17.2%. Acidobacteria, Verrucomicrobia, and Planctomycetes are also present, but at much lower abundances (<2%), as shown in
Figure 5B. Bacterial communities on elevated interfluve and slope sites (FAa1, FAa2, FA-P1, and FA-P2, with altitudes greater than 80 cm) exhibited higher alpha diversity indices (Shannon index of 3.94 to 4.19 and Chao1 index of 1238 to 1703), compared to floodplain sites. The composition of these upland communities varied in the proportions of the dominant phyla, including Actinomycetota, Pseudomonadota, and Acidobacteriota. At site FAa2 with sandy Chernozem, Actinomycetota exhibited the highest dominance within this group, accounting for 64.4% of the total bacterial community (
Figure 5B).
3.5.3. Functional Potential of the Soil Metagenome
The analysis of the functional potential of metagenomes has revealed statistically significant differences in the relative representation of key categories of COG genes between the studied areas (
Figure 5C). These differences indicate the formation of different metabolic strategies in response to environmental conditions.
In the floodplain agrosystems (FA-W1 and FA-W2), a stress response strategy has been confirmed, as shown by a significant increase in the proportion of genes involved in signal transduction mechanisms (9.61–9.74% vs. 7.77–8.99%). At the same time, a decrease in the representation of genes related to mobilome (3.23–3.46% vs. 3.66–5.39%) and secondary metabolites biosynthesis (1.76–1.95% vs. 1.79–2.66%) has been observed.
In fallow areas, there was a contrast in metabolic status. On the plateau, a high abundance of general function prediction genes (11.01%) and inorganic ion transport and metabolism (4.16%) was recorded. In contrast, on the slope, the maximum representation of mobilome genes (5.39%) and secondary metabolites biosynthesis (2.66%) was found.
In agricultural ecosystems on flat lands, there is an intermediate nature of the functional profile with a tendency towards a decrease in mobilome genes and an increase in carbohydrate transport and metabolism.
4. Discussion
4.1. Influence of Topography and Land Use on Soil Physicochemical Properties and Microbial Physiology
The concept of the soil-topographic catena as a template for soil properties is well-established, and recent work has extended this framework to soil microbial communities [
16]. Our integrated study reveals that the spatial organization of soil microbial communities in an agricultural landscape is not random but follows a predictable pattern governed by a hierarchy of ecological filters. The spatial distribution of soil properties and microbial communities on slopes is complex and non-linear. Slope topography plays a crucial role in determining the distribution of moisture, heat, and the movement of elements, leading to spatial heterogeneity in organic matter content, nutrient availability, and pH levels [
17,
18,
19,
20]. This modulates the composition and functional activity of soil microbial communities [
4,
21]. One of the key factors that mediates the influence of topography is the particle size distribution of soils [
22]. As the results of the present study show, the interaction between relief and granulometry determines the final pattern of organic matter distribution and the functional activity of microbial communities.
4.1.1. The Influence of Particle Size Distribution and Moisture Deficit on the Microbial Community Is Limited
Despite the relatively high total carbon content, the most pronounced stress was observed at the plateau site (FAa2). The predominance of sand fractions (75.9%) results in low water-holding capacity and cation exchange capacity (CEC), which acts as a key limiting factor for microbial growth [
23,
24,
25,
26,
27] is evidenced by the low values of MB
SIR (microbial biomass specific respiration) and its share in the total carbon pool. Under these conditions, despite the abundance of available substrate (C:N ratio = 5.22), the community is experiencing physiological stress. The microorganisms are forced to redirect the energy they receive not towards growth (MB
SIR increase), but towards maintaining life under unfavorable conditions, as indicated by abnormally high values of the metabolic quotient (
qCO
2) and the coefficient of microbial respiration (QR) [
28].
A similar, albeit less pronounced, stress situation was observed in the arable land plot on the central southern slope (FA-P2). The sharp increase in the sand fraction compared to the arable land at the top of the slope is likely the result of water erosion, which led to the removal of silt and clay particles [
29]. This led to destabilization of conditions: moisture fluctuations, a drop in CEC, and nutrient deficiencies. The influx of fresh organic matter (low C:N = 4.48) stimulated respiration (high BR), but the energy was used by microorganisms primarily to cope with stress (high
qCO
2 and QR) rather than for efficient biomass growth [
30].
4.1.2. Balanced Functioning Under Optimal Conditions of Particle Size Distribution and Resource Availability Is Essential for the Health of Ecosystems
Sites with loamy soil, on the other hand, have highly efficient carbon utilization due to the unique characteristics of their microbial communities. For instance, the arable soil on the top of the southern slope (FA-P1) has a high content of stable organic matter, which is unusual for an arable horizon. It can be assumed that the medium-loam composition provides effective physicochemical protection for organic matter, as indicated by the low
qCO
2/TC ratio [
31,
32]. The presence of a high-quality and labile pool of organic carbon (C:N = 6.16) also supports high metabolic activity and a significant amount of microbial biomass [
33,
34]. The microbial community in this area functions in a balanced way, efficiently utilizing readily available carbon without excessive energy expenditure [
35].
The most favorable conditions are found in the arable land at the foot of the slope (FA-W1), which acts as a zone for the accumulation of matter and energy. The influx of fine-dispersed material and labile organic matter from the slopes above, combined with an optimal particle size distribution, has created the conditions necessary for the formation of a highly efficient microbial community [
36,
37]. This is reflected in the maximum MB
SIR values and an optimal MB
SIR/TC ratio, with minimal energy losses (low
qCO
2 and QR). Microorganisms actively utilize the labile carbon pool, while the primary pool remains stable and protected.
4.1.3. The Influence of Specific Hydrological Regimes
A special case is the northern slope fallow (FAa1). The balanced loamy composition is expected to promote organic matter accumulation, but an anomalously low content was recorded here. This discrepancy can be explained by the development of a hydromorphic regime on the northern slope, which leads to increased moisture conditions. Under these conditions, the labile pools of carbon and nitrogen are actively involved in microbial turnover, and, without the protection of vegetation (in a fallow), are partially leached from the soil profile. The microbial community functions very efficiently under these conditions (low
qCO
2 and QR), directing energy toward growth and reproduction [
30,
38,
39]. his results in the formation of a large biomass with high respiratory activity. Almost all soil carbon is present as living microbial biomass (high MB
SIR/TC), indicating a very high rate of organic matter turnover [
40].
4.1.4. Spatial Heterogeneity of Accumulation Sites
The sharp contrast between two adjacent sites at the foot of the slope (FA-W1 and FA-W2) emphasizes the high spatial variability in conditions even within a single geomorphic site. Although located in the accumulation zone, site FA-W2, with its lighter texture, has lower fertility and biological activity compared to site FA-W1. This lighter texture results in rapid mineralization of labile organic matter (C:N = 5.00), likely creating less stable moisture conditions. As a result, although the community processes carbon efficiently (low QR), it operates in a state of mild stress, expending additional energy to overcome suboptimal conditions (
qCO
2 is higher than in FA-W1) [
30]. This suggests a non-linear nature of the accumulation processes, which may be related to the microtopography, such as the presence of riverbed ridges from an ancient terrace, which determine local conditions for the deposition and preservation of fine-grained material.
The functioning of microbial communities in the catena studied is an indicator of the stability of the soil ecosystem. The particle size distribution, which is modified by topography and erosion processes, plays a key role in determining the availability of moisture and nutrients. This, in turn, directly affects the physiological state of microorganisms and their ability to use organic matter efficiently.
Our observations of increased microbial metabolic stress (higher
qCO
2) on the plateau (FAa2) and higher efficiency in the accumulation zone (FA-W1) align with the understanding that topography plays a significant role in determining the distribution of resources and water in the ecosystem, thereby shaping the physiological status of microorganisms [
41].
However, the specific factors that drive these processes in our agricultural system seem to be more complex than previously thought. While Mohammadi et al. (2017) linked microbial biomass and activity in a forest ecosystem primarily to the accumulation of soil organic carbon and water along a catena, our data suggest that soil texture plays a critical role in mediating these topographic effects [
41].
The extreme stress at the sandy site FAa2, despite its relatively high carbon content, demonstrates how texture-induced limitations on water and nutrient holding capacity can override the influence of total organic carbon pools in determining microbial physiological stress in agricultural ecosystems.
4.2. Spatial Differentiation of Enzymatic Activity as an Indicator of the Functional State of Microbial Communities in a Slope Catena
The study found clear spatial variation in soil enzymatic activity along a slope, reflecting the complex impact of topography, particle size, and land use on microbial metabolism. Analysis of enzyme profiles allows us to not only assess resource availability, but also to diagnose the physiological status of soil biota, identifying signs of both healthy functioning and chronic stress.
4.2.1. Enzymatic Indicators of Stress Caused by Texture and Erosion
Plots with light textures, such as plateau, FAa2, and eroded cropland, FA-P2, exhibit similar patterns of physiological stress in microbial communities due to resource scarcity. These plots are characterized by extremely high phosphatase activity in response to severe phosphorus deficiency caused by nutrient leaching from light soils [
42].
However, on the eroded cropland (FA-P2), phosphatase activity is 1.5 times higher than on stable cropland (FA-P1). This indicates catastrophic depletion of the silt-clay fraction due to erosion [
43]. Against this backdrop, the key difference is in the carbon metabolism strategy. While moderate cellobiohydrolase and
β-glucosidase activity on the plateau indicates a preference for labile substrates, on eroded cropland, a sharp increase in their activity reflects the community’s desperate attempt to compensate for the depletion of its resource base by breaking down more difficult-to-access compounds, such as cellulose.
This shift is supported by a significant change in the nitrogen cycle, which is an increase in the NAG:LAP ratio, indicating a shift towards nitrogen utilization from chitin [
44]. The need to produce a wide variety of enzymes to address combined elemental deficiencies directly contributes to the highest energy expenditure (
qCO
2, QR) in these areas of the catena [
45,
46,
47].
4.2.2. The Specificity of Enzymatic Profiles in Hydromorphic Conditions with Balanced Nutrition Is Contrasted with Those of Stressed Communities
Areas with optimal conditions exhibit energy-efficient enzymatic strategies, although the mechanisms behind these strategies differ from those in stressed communities. The northern slope fallow soil profile (FAa1) reflects adaptation to a hydromorphic regime, which is characterized by dominance of phosphatase activity, confirming phosphorus limitation in waterlogged soils with leaching processes [
43]. The low CBH and
βX activities combined with high
βG activity indicate that degradation processes focus on the final stages of decomposition, consistent with the hypothesis of predominance of labile microbial organic matter. This is evidenced by the low NAG:LAP ratio, which indicates the dominance of proteins and peptides as the main nitrogen source. The most balanced and efficient profile is found in the arable land at the top of the southern slope (FA-P1). This is due to the stabilizing role of the medium loamy texture and the enrichment of the soil with bioavailable nitrogen through pea cultivation, which alleviates the severe limitation of key elements [
48,
49]. This allows the community to avoid the need for excessive synthesis of exoenzymes, as reflected in moderate phosphatase activity [
50]. The low NAG:LAP ratio and minimal metabolic quotients (
qCO2, QR) indicate an efficient use of readily available resources, with energy being allocated to biomass growth rather than compensating for deficits [
51,
52].
4.2.3. The Role of Accumulation Sites in the Formation of Enzymatic Patterns
The accumulation sites at the foot of the slope, FA-W1 and FA-W2, exhibit contrasting enzymatic patterns that are not solely explained by their texture, which is heavy loam and medium loam, respectively. The soil at site FA-W1 provides conditions for a highly productive ecosystem, with minimal AP activity indicating a sufficient phosphorus supply due to effective accumulation and stabilization of this nutrient. At the same time, high
βG and LAP activity levels indicate an intense flow of labile organic matter, maintaining maximum values for MB
SIR and respiratory rate [
33]. An extremely low NAG:LAP ratio confirms the abundance of readily available nitrogen-containing compounds. This enzyme profile corresponds to a state of physiological well-being and high metabolic efficiency.
In contrast, the plot profile of FA-W2 (medium loam) shows a development of moderate resource limitation. The elevated AP activity indicates a lack of available phosphorus, which, despite its potential for accumulation, is not being compensated for. This creates an additional metabolic burden on the ecosystem, as evidenced by higher CO2 levels compared to baseline (FA-W1). The stark contrast between these two sites highlights the significant spatial variability in soil properties and nutrient flows at the base of the slope. This variability may be attributed to microtopographic features (such as channel ridges from an ancient terrace), differences in the hydrologic regime, or historical land use, which require further investigation.
The spatial patterns of enzyme activities that we observed support the idea that topography creates a template for biogeochemical “hotspots” [
53]. However, when comparing our findings with other ecosystems, we found that the specific expression of these hotspots is highly dependent on the local context. For example, Keller et al. (2023) reported that C-cycle enzyme activity was highest in a forested catchment on drier slopes, while P-cycle (phosphatase) enzyme activity was more pronounced in the wet valley of a river [
54]. In contrast, our study in an agricultural area found that the highest overall activity of enzymes involved in the C, N, P, and S cycles occurred on a well-drained sandy plateau (FAa2), which is a site with severe nutrient limitation and physiological stress. This key difference highlights a fundamental distinction: in managed systems, historical erosion and land use can create localized areas of extreme resource depletion (like FAa2 and FA-P2), which can become powerful drivers for enzyme synthesis, potentially overpowering the more generalized topographic patterns seen in natural ecosystems. As a result, the “topographic template” in agriculture is often a modified one, where the legacy of soil degradation determines microbial function.
4.3. Response of Bacterial and Fungal Community Structure and Function to Environmental Gradients
Our metagenomic analysis of COG categories has revealed differences in the functional potential of microbial communities along the topographic gradient. However, it is crucial to contextualize these genetic predictions within the broader framework of microbial ecology.
The presence of genes identified by COG annotation indicates metabolic potential, but it does not guarantee their phenotypic expression at any given moment. This is supported by the recent study by Teslya et al. (2024), which showed a clear disconnect between the relative abundance of functional genes in metagenomes and the actual activity of corresponding enzymes in soil [
55]. The authors concluded that changes in enzyme activity are likely due to post-genomic regulatory mechanisms.
This principle is essential for interpreting our own integrated dataset and understanding the complex interactions between genes, enzymes, and the environment. While the functional potential inferred from COGs provides a valuable layer of explanation for stress response in floodplains, direct measurements of enzyme activity and ecophysiological coefficients (qCO2, QR) represent the realized functional output of microbial communities in situ. Combining these approaches, which predict potential and measure activity, provides a more robust and holistic understanding of community function than either method alone. This helps bridge the gap between genetic capabilities and ecosystem-level processes, providing a more complete picture of microbial community functioning.
A comprehensive study has identified a hierarchy of ecological factors that influence the structure and function of soil microbial communities in an agricultural landscape with slopes. By synthesizing all the available data, we can propose a comprehensive framework for understanding the distribution of ecological niches and metabolic strategies within these communities.
Our results convincingly demonstrate that the topographic position and the resulting hydrological regime are the primary factors that drive the spatial organization of the microbiome which is consistent with the results of other studies [
56]. This is particularly evident in the case of bacteria, where the absolute dominance of the Actinomycetota phylum (59.2–67.6%) and the suppression of other bacterial phyla in waterlogged floodplain soils (FA-W1 and FA-W2), in contrast to a background of reduced taxonomic richness (Chao1: 649–905), clearly indicates that these conditions are stressful, leading to the selection of a highly specialized microbial community.
Within a given topographic gradient, the history and type of land use determine the metabolic strategy of the microbial community. This is where the fundamental difference between bacterial and fungal communities emerges. Fungi act as direct indicators of land use. A sharp increase in the proportion of Glomeromycota (arbuscular mycorrhiza), to 26.5%, in long-term fallow land (FAa2), and a shift toward phytopathogens in agroecosystems reflect a direct restructuring of trophic interactions dependent on vegetation cover [
57]. The functional shifts we observed in fungal communities—from symbiotic (mycorrhizal) in fallow fields to pathogenic and saprotrophic in crop fields—align with broader ecological principles of community assembly. A study by Wang et al. (2023), on an eroding landscape, described a shift in fungal taxa from r-selected (e.g., Ascomycota) to K-selected (Basidiomycota), driven by changes in carbon availability [
16]. While our study did not detect a clear topographic pattern of taxa, we can draw a parallel at the functional level. The increase in saprotrophs and pathogens in agroecosystems may reflect a r-selected strategy, favoring species that can rapidly exploit readily available resources under disturbed conditions. In contrast, mycorrhizal communities in long-term fallow fields may represent a K-selected and stable assemblage. This suggests that, in addition to taxonomy, land use has a significant impact on the life cycle strategies of soil fungi.
Bacteria exert their influence through changes in their functional efficiency. This mechanism is revealed by data on enzymatic activity and ecophysiological coefficients. The FAa2 site exhibits a paradoxical situation: despite having low microbial biomass and organic carbon content, it shows maximum activity of enzymes involved in C-, N-, P- and S-cycling. This indicates a state of acute energy stress, confirmed by high CO
2 and QR, where the community has to intensify its enzymatic activity to degrade inaccessible organic matter. The presence of Actinomycetota, known for their hydrolytic abilities, supports this hypothesis [
58].
4.4. Topographic-Hydrological Gradient as the Primary Community Filter
The most profound divide in our system was between the floodplain and the watershed slope sites, overriding the influence of agricultural management. This was unequivocally demonstrated by the stark restructuring of the bacterial community in the periodically flooded soils (FA-W1, FA-W2), which exhibited significantly reduced alpha diversity and a strong dominance of actinobacteria. This pattern is a classic signature of an environmental filter selecting for stress-tolerant specialists. The hypoxic conditions and physical disturbances associated with flooding likely favor actinobacteria, known for their metabolic versatility and ability to produce spores to withstand adverse conditions [
59,
60]. This taxonomic shift is further corroborated by the metagenomic data, which showed an enrichment in genes related to signal transduction—a typical stress-response strategy—at the expense of genes for secondary metabolite biosynthesis and mobile genetic elements, indicating a reduction in competitive and evolutionary potential.
The influence of topography extended beyond taxonomy to ecosystem-level functions. The catena position directly governed the accumulation and quality of organic matter, leading to a gradient from carbon-depleted, erosion-prone upper slopes to carbon-enriched accumulation zones in the floodplain. This physical template preconditioned the metabolic landscape for microorganisms, setting the boundaries within which land use practices could modulate microbial activity.
4.5. Model Context and Forward Look
Our findings reveal a clear hierarchical structure of microbial communities across the topography. It is important to consider this model as a spatial framework that may vary over time. The functional hotspots and metabolic strategies we describe are linked to the landscape, but their intensity may be influenced by seasonal and inter-annual fluctuations that we have not addressed here. To validate the temporal stability of this pattern, especially for assessing phytopathogen risks, future longitudinal studies are needed.
The strength of the topographic and land use signals, as evidenced by the high effect sizes in our multivariate models, gives us confidence in the primary drivers. However, the incorporation of additional variables, such as precise management histories and explicit spatial modeling, could further enhance the predictive power of our framework for precision agriculture applications. This represents a promising avenue for translating our ecological model into actionable management strategies.
5. Conclusions
Our study shows that in agricultural landscapes with a pronounced topography, the composition of soil microbial communities is determined by a hierarchy of ecological factors. Topography acts as the main filter, controlling hydrology and soil development and thus shaping the structure and function of bacterial communities. Land use and its associated soil texture serve as secondary filters, refining the system by influencing the metabolic efficiency of microorganisms and the guild structure of fungal communities within this topographic context.
The crucial role of soil texture in influencing microbial responses emphasizes the need for context-specific soil management. A one-size-fits-all approach is not suitable for spatially diverse systems. Instead, we recommend adopting a topography-informed precision management strategy. This involves implementing targeted measures based on the landscape’s characteristics: prioritizing erosion prevention and organic matter addition on degraded slopes and carefully managing nutrient input in naturally fertile areas. Implementing this stratified approach will optimize resource use and maximize the potential of soil microorganisms to enhance agricultural productivity and sustainability.