Abstract
The soil of abandoned rural residential land is often deficient in organic matter and low in nutrient content, which limits agricultural productivity. Organic carbon input (OCI) is recognized as an effective strategy to enhance soil quality, yet it remains unclear which active carbon and nitrogen fractions drive yield enhancement and how their cycles are coupled. A three-year field experiment included five treatments: an unfertilized control (CK) and four OCI levels applied at an equal total N rate of 270 kg N ha−1: 0.51 t ha−1 (T1), 0.77 t ha−1 (T2), 1.02 t ha−1 (T3), and 2.56 t ha−1 (T4). Compared with CK, T1–T4 treatments significantly increased dissolved organic carbon (DOC) by 56.04–137.25%, readily oxidizable organic carbon (ROC) by 56.46–85.29%, particulate organic carbon (POC) by 35.26–50.17%, microbial biomass carbon (MBC) by 33.87–49.90%, acid-hydrolyzable ammonium nitrogen (AN) by 21.54–30.66%, acid-hydrolyzable amino sugar nitrogen (ASN) by 11.05–24.21%, acid-hydrolyzable amino acid nitrogen (AAN) by 23.56–31.92%, and rice yield by 44.50–69.56%. Overall, among T1–T4 treatments, T2 and T3 treatments performed best in improving soil fertility and rice yield in the current study. Structural equation modeling (SEM) analysis indicated that ROC significantly influenced total hydrolyzable nitrogen (THN), which in turn was the main direct determinant of rice yield. Collectively, these findings demonstrate that a medium OCI rate (0.77–1.02 t ha−1 in the current study) at 270 kg N ha−1 delivers the most balanced improvement in soil C-N cycling and yield formation, providing a sound theoretical and practical basis for optimizing organic fertilization strategies in abandoned rural residential land soil.
1. Introduction
Land serves as the foundation for human survival and development, supporting both human production activities and meeting living needs. However, global human–land conflict is intensifying due to the gap between a growing population and limited arable land. Projections indicate that the global population will reach 9.31 billion by 2050 [1], while the current total global cultivated land area remains stable at approximately 1.6 billion hectares [2], and the imbalance between the supply and demand of human–land resources will further intensify. Meanwhile, the rapid advancement of urbanization has aggravated the idleness and waste of rural land resources; a large number of rural residents have migrated to cities, leading to the widespread abandonment of rural residential land. At present, the global area of rural residential land has increased by 14 million hectares, and the United Nations further estimates that 68% of the global population will reside in urban areas by 2050 [3,4]. Reclaiming these abandoned rural residential lands has thus emerged as a critical strategy to supplement arable land and safeguard food security. The soil of these unreclaimed lands, hereafter referred to as “abandoned rural residential land soil”, originates from lands previously used for rural housing, yards, and auxiliary facilities. Characterized by high construction waste content and distinct anthropogenic modification, this soil is classified as Technosols under the World Reference Base for Soil Resources (WRB), a category encompassing soils strongly shaped by human activities. A key challenge with abandoned rural residential land soils is their inherent degradation: low organic matter content, poor nutrient status, and reduced microbial activity collectively result in significantly lower crop productivity compared to conventional farmland [5,6]. Addressing this degradation is essential to unlocking the agricultural potential of reclaimed residential lands.
Engineering remediation and chemical remediation are core technical measures for the reclamation of rural residential land soil; the former focuses on addressing the physical obstacles left by rural residential lands, such as residual construction waste, insufficient site leveling, and soil compaction, and rapidly restores the basic soil structure through physical intervention [7], while the latter centers on chemical improvement, specifically solving chemical problems such as soil acidification, salinization, nutrient imbalance, and mild heavy metal pollution by applying modifiers or adjusting soil chemical parameters (e.g., pH, redox potential), thereby optimizing the soil chemical environment [8,9]. However, these two remediation methods pay insufficient attention to the core limiting factors commonly existing in rural residential land soil, such as poor fertility and low productivity; soil organic matter is the material basis of soil fertility, and its content directly determines the soil’s ability to retain and supply nutrients as well as its productivity level [10,11], while organic carbon input (OCI), by supplementing soil organic matter and promoting carbon–nitrogen cycling, serves as a fundamental and sustainable strategy to improve soil fertility and enhance soil functions, which can effectively fill the gap of engineering and chemical remediation in terms of fertility restoration [12,13,14,15]. Previous studies have demonstrated that exogenous OCI can enhance soil organic carbon sequestration [16,17]. Labile organic carbon fractions such as dissolved organic carbon (DOC), readily oxidizable organic carbon (ROC), particulate organic carbon (POC), and microbial biomass carbon (MBC) play vital roles in short-term soil fertility improvement. These fractions facilitate microbial metabolism and aggregate formation, thereby regulating over 90% of nutrient turnover efficiency [18,19]. Soil organic nitrogen, which accounts for more than 90% of total nitrogen, constitutes the primary reservoir of soil nitrogen availability [20,21]. Total hydrolyzable nitrogen (THN) includes acid-hydrolyzable ammonium nitrogen (AN), acid-hydrolyzable amino sugar nitrogen (ASN), acid-hydrolyzable amino acid nitrogen (AAN), and acid-hydrolyzable unknown nitrogen (HUN), serving as a key indicator of nitrogen availability and cycling [22,23].
Studies have shown that the combined application of organic carbon input and chemical nitrogen fertilizer can affect soil nitrogen availability by altering soil organic nitrogen and its mineralization. This is primarily reflected in the increase in total soil organic nitrogen and its fractionations (AN, ASN, and AAN), with AAN contributing most strongly to the increase in total organic nitrogen [24]. The main reason is that the co-application of organic carbon input and chemical fertilizer increases the soil C/N ratio, stimulates microbial activity, and promotes the accumulation of amino acid nitrogen, thereby enhancing soil nitrogen retention capacity and expanding the soil nitrogen pool [25,26,27].
However, most existing studies about OCI focus on conventional farmland, with limited attention given to abandoned rural residential land soils that have undergone intense anthropogenic disturbance. Owing to structural degradation, organic matter deficiency, and disrupted microbial community, the patterns of carbon and nitrogen transformation in these soils may differ from those in conventional farmland [28]. As a powerful multivariate causal relationship analysis method, structural equation modeling (SEM) can effectively integrate observed variables and latent variables and quantify direct and indirect effects. To decipher these unique transformation pathways, this study employed SEM to specifically quantify the direct and indirect influence pathways through which organic carbon input influences soil organic carbon, available nitrogen, and crop yield in abandoned rural residential land soils.
Accordingly, we proposed the following hypotheses: (1) Increasing OCI enhances soil fertility and rice yield by enriching labile carbon and nitrogen fractions. (2) The improvement in rice yield is primarily mediated by the rearrangement of labile carbon pools that stimulate nitrogen availability. Against this background, a three-year field experiment was conducted with an unfertilized control (CK) and four OCI levels (0.51, 0.77, 1.02, 2.56 t ha−1) at equal total N input. The objectives are to (1) investigate the effects of OCI on soil organic carbon fractions, organic nitrogen fractions, and rice yield and (2) identify the key pathways and mechanisms through which OCI influences yield via carbon and nitrogen fractions.
2. Materials and Methods
2.1. Experimental Site
This study was carried out on a parcel of supplementary cultivated land located in Gongdao Town, Hanjiang District, Yangzhou City, Jiangsu Province, China (119°19′10″ E, 32°34′13″ N). The area is a typical landform of the alluvial plain, with an elevation of 3–4 m. The area is characterized by a subtropical monsoon climate, with a mean annual temperature of 15 °C and an average annual precipitation of 1063.2 mm, predominantly occurring between June and September. Following the demolition of the village in 2012, the abandoned rural homestead area was first cleared of construction debris, hardened surfaces, waste, and other miscellaneous materials. The reconstruction process consisted primarily of excavation, removal of the original topsoil, and backfilling with imported Haplic Stagnic Anthrosols as topsoil. The land surface was then leveled to restore an appropriate slope (<5°) and ensure proper irrigation and drainage. In 2013, the reconstructed land remained idle for a year, and from 2014 to 2021, it was used for rice–wheat rotation. Since 2022, the effects of organic carbon input on soil fertility and rice yield improvement have been studied. According to the IUSS Working Group WRB, the soil tested in this study is inherently classified as Technosols. Additionally, the basic soil physicochemical properties before the experiment were as follows: pH 6.60, total organic carbon (TOC) content of 10.35 g·kg−1, total nitrogen (TN) content of 0.96 g·kg−1, available phosphorus (AP) content of 10.70 mg·kg−1, and available potassium (AK) content of 130 to148 mg·kg−1.
2.2. Experimental Materials and Design
This experiment was initiated on 20 June 2022 and conducted continuously for three years during the annual rice growing season (2022–2024). A randomized block design was adopted. The rice cultivar used in the study was Nanjing 3908; each year, seedlings at the 3.5–4.0 leaf stage were mechanically transplanted in mid-June, with a transplanting density of approximately 26.7 × 104 holes·ha−1 to ensure consistent population establishment across treatments. The experiment included 15 experimental plots in total, corresponding to 5 treatments with 3 biological replicates each, and each plot had a uniform area of 36 m2 (6 m × 6 m). To prevent lateral leakage of fertilizers between adjacent plots and avoid mutual interference of nutrients, each plot was surrounded by 40 cm high plastic partitions, which were inserted at least 10 cm into the soil to form a closed isolation zone.
Two types of fertilizers were used in the experiment: an exogenous organic carbon source and chemical fertilizers. Commercial sheep manure was selected as the exogenous organic carbon source, with the following key basic properties: a total organic carbon (TOC) content of 153.99 g·kg−1, total nitrogen (TN) content of 16.23 g·kg−1, carbon-to-nitrogen ratio (C/N) of 9.49, total phosphorus (calculated as P2O5) content of 1.14 g·kg−1, and total potassium (calculated as K2O) content of 2.25 g·kg−1. For chemical fertilizers, urea (containing 46% N), single superphosphate (containing 12% P2O5), and potassium chloride (containing 60% K2O) were used to supply inorganic nitrogen, phosphorus, and potassium, respectively. Five treatments were established. The unfertilized treatment was used as the control (CK), the other treatments received an equal total 270 kg N ha−1 input but differed in the ratio of organic to chemical N (2:8, 3:7, 4:6, and 10:0), corresponding to four levels of OCI (0.51 t ha−1, 0.77 t ha−1,1.02 t ha−1, 2.56 t ha−1 OCI). Detailed information on fertilizer application is provided in Table 1. Fertilizer application was carried out uniformly before rice transplanting, following the same operational standards across the three experimental years. For organic fertilizer, commercial sheep manure was first evenly spread on the soil surface of each plot and then incorporated into the 0–20 cm plow layer via a single mechanical plowing operation to ensure full mixing with the soil. For chemical fertilizers, application was carried out according to local conventional rice cultivation practices: single superphosphate and potassium chloride were applied as base fertilizers, incorporated into the soil together with organic fertilizer; urea was split into base fertilizer and topdressing.

Table 1.
Experimental design under the condition of abandoned rural residential land soils.
2.3. Plant and Soil Sampling and Analysis
Given the study’s focus on the cumulative, stable effect of organic carbon input, all data presented in this manuscript (including soil carbon/nitrogen fractions and rice yield) are derived from the 2024 samples (final year of the experiment). Details of plant and soil sampling and analysis are as follows:
Plant and soil samples were collected from each treatment plot, with three adjacent sampling points selected per plot. Within a delineated 1 m × 1 m area at each point, rice plants were harvested, threshed, and cleaned to remove impurities. Grains were air-dried, and their dry weight was measured repeatedly to obtain an average value. The actual rice yield per hectare was subsequently calculated.
Soil samples were collected from the 0–20 cm depth at five points using the diagonal sampling method. The five independent soil samples were thoroughly mixed, passed through a 2 mm sieve, and homogenized into one composite sample. The composite sample was divided into two portions: one was air-dried for the analysis of basic soil chemical properties, and the other part was stored at 4 °C for the determination of soil organic carbon and nitrogen fractions.
Dissolved organic carbon (DOC) was extracted with 0.5 mol·L−1 K2SO4 (soil-to-solution ratio of 1:5) followed by analysis with a TOC analyzer (Shimadzu Corporation, Kyoto, Japan) [29]. Readily oxidizable carbon (ROC) was determined by the 333 mmol·L−1 KMnO4 oxidation colorimetric method with colorimetric detection performed at 565 nm [30]. Particulate organic carbon (POC) was measured using the sodium hexametaphosphate dispersion method (5 g·L−1) as described by Cambardella and Elliott [31]. Microbial biomass carbon (MBC) was quantified using the chloroform fumigation–K2SO4 extraction method (24 h fumigation) and analyzed by Suzhou Chishan Biotechnology Co., Ltd. (Suzhou, China).
Soil organic nitrogen fractionation was performed using the classic acid hydrolysis method [23], which separates nitrogen into total hydrolyzable nitrogen (THN) and non-hydrolyzable nitrogen. The THN fraction was further subdivided into acid-hydrolyzable ammonium nitrogen (AN), amino sugar nitrogen (ASN), amino acid nitrogen (AAN), and hydrolyzable unknown nitrogen (HUN). The acid hydrolysate was prepared as follows: 10 g of air-dried soil (sieved through 0.15 mm) was placed in a 150 mL flat-bottomed flask. After adding two drops of n-octanol and 20 mL of 6 mol·l−1 HCl, the mixture was shaken well, fitted with a condenser, and hydrolyzed at 120 °C for 12 h. After cooling, the hydrolysate was filtered through quantitative filter paper. The filtrate was adjusted to pH 6.5 through the dropwise addition of NaOH solution (typically 10 mol·L−1) with continuous stirring and then transferred to a 100 mL volumetric flask and made to volume with ultra-pure water. This stock solution was used for the subsequent determination of organic nitrogen fractions. Detailed procedures for the quantification of each nitrogen fraction are provided in Appendix A.1.
2.4. Statistical Analysis
SPSS 26.0 (SPSS Inc., Chicago, IL, USA) was used to process the data. Prior to conducting a one-way analysis of variance (ANOVA), the data were first checked for normality and homogeneity of variances, and these statistical assumptions were confirmed to be met. Subsequently, a one-way ANOVA was performed, and Duncan’s method was adopted for multiple comparisons. Data visualization was carried out with Origin 2024 (Origin Lab Corporation, Northampton, MA, USA). Correlation analysis between different organic carbon input rates and organic carbon and nitrogen fractions was performed using the Correlation Plot plugin of Origin software (Version 2024). Random forest analysis was implemented in R version 4.4 (R Foundation for Statistical Computing, Vienna, Austria). Random forest is a machine learning algorithm based on the principle of “ensemble learning”, whose core lies in constructing multiple independent decision trees and integrating their results to achieve accurate analysis and prediction of agriculture-related data—making it particularly suitable for complex scenarios involving multivariable and nonlinear relationships. Additionally, structural equation modeling (SEM) was performed using IBM SPSS Amos 27 (SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Response Characteristics of Soil Organic Carbon, Nitrogen Fractions, and Rice Yield to Different Organic Carbon Input Levels
3.1.1. Soil Organic Carbon Fractions
Under the specific soil, climate, and general conditions of this experiment, organic carbon input (OCI) significantly affected soil organic carbon fractions on abandoned rural residential land (Figure 1). Compared with the control (CK), T1–T4 treatments significantly increased dissolved organic carbon (DOC) by 56.04–137.25%, readily oxidizable organic carbon (ROC) by 56.46–85.29%, particulate organic carbon (POC) by 35.26–50.17%, and microbial biomass carbon (MBC) by 33.87–49.90% (p < 0.05). With increasing OCI, DOC increased correspondingly, with T4 exhibiting the maximum DOC contents. Both T3 and T4 showed the highest ROC contents, which were greater than those in T1 and T2. POC content in T2 and T3 was higher than in T1 and T4, although the differences were not significant (p > 0.05). Similarly, MBC content in T2 and T3 tended to be higher than in T1 and T4, but no significant difference was observed among T1, T2, and T3 (p > 0.05, Figure 1).
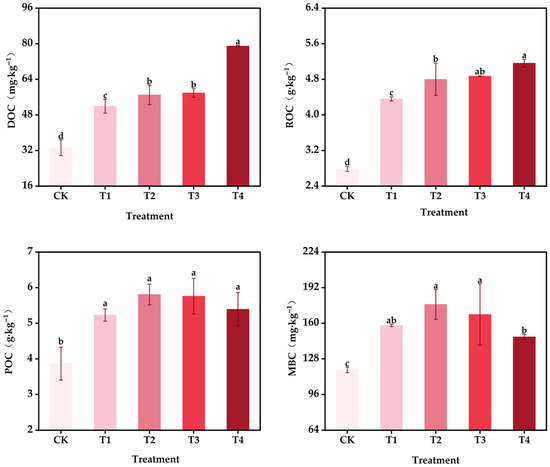
Figure 1.
Effects of organic carbon input on carbon fractions of a soil of abandoned rural residential land at Jiangsu Province, China. CK, unfertilized input, T1, 0.51 t ha−1 OCI + 270 kg N ha−1 (organic N: chemical N = 2:8); T2, 0.77 t ha−1 OCI + 270 kg N ha−1 (organic N: chemical N = 3:7); T3,1.02 t ha−1 OCI + 270 kg N ha−1 (organic N: chemical N = 4:6); and T4, 2.56 t ha−1 OCI + 270 kg N ha−1 (organic N: chemical N = 10:0, fully organic fertilizer application). The same as below. DOC: dissolved organic carbon; ROC: readily oxidizable organic carbon; POC: particulate organic carbon; MBC: microbial biomass carbon. In the figure, the data in the bar charts are presented as mean ± standard error with three replicates (number of replicates = 3); different lowercase letters indicate statistically significant differences among different treatments.
3.1.2. Soil Organic Nitrogen Fractions
Under the specific soil, climate, and other conditions of this experiment, organic carbon input (OCI) significantly influenced some of the organic nitrogen fractions in the soil of the abandoned rural residential land (Figure 2). Compared with CK, T1–T4 treatments significantly increased soil acid-hydrolyzable ammonium nitrogen (AN) by 21.54–30.66%, acid-hydrolyzable amino sugar nitrogen (ASN) by 11.05–24.21%, and acid-hydrolyzable amino acid nitrogen (AAN) by 23.56–31.92% (p < 0.05). Among these treatments, the contents of AN, ASN, and AAN in the T2 and T3 treatments were significantly higher than those in the T1 and T4 treatments (p < 0.05), while the HUN content in the T4 treatment was significantly higher than that in the T1-T3 treatments (Figure 2).

Figure 2.
Effects of organic carbon input on nitrogen fractions of a soil of abandoned rural residential land at Jiangsu Province, China. AN: acid-hydrolyzable ammonium nitrogen, ASN: acid-hydrolyzable amino sugar nitrogen, AAN: acid-hydrolyzable amino acid nitrogen, HUN: acid-hydrolyzable unknown nitrogen. In the figure, the data in the bar charts are presented as mean ± standard error with three replicates (number of replicates = 3); different lowercase letters indicate statistically significant differences among different treatments.
3.1.3. Rice Yield
Under the specific soil, climate, and other conditions of this experiment, organic carbon input significantly affected the rice yield in the soil of abandoned rural residential lands (Figure 3). Compared with CK, T1–T4 treatments significantly increased rice yield by 44.50–69.56%. Among these treatments, T2 and T3 achieved the highest rice yields, reaching 10.17 t ha−1 and 10.02 t ha−1, respectively, while T4 had the lowest yield of 8.67 t ha−1.

Figure 3.
Effects of organic carbon input on rice yield (vertical axis) in a soil of an abandoned rural residential land at Jiangsu Province, China. In the figure, the data in the bar charts are presented as mean ± standard error with three replicates (number of replicates = 3); different lowercase letters indicate statistically significant differences among different treatments.
3.2. Correlation Among Organic Carbon Fractions, Nitrogen Fractions, and Rice Yield Under Different Organic Carbon Input Levels
Under the specific soil, climate and other conditions of this experiment, Pearson correlation analysis showed that OCI had an extremely significant positive correlation with DOC (p < 0.001, see Figure 4). DOC was positively correlated with ROC, POC, AN, ASN, and AAN (p < 0.05). There were relatively strong positive correlations among POC, ROC, and MBC (p < 0.01); additionally, AN, ASN, and AAN exhibited extremely significant positive correlations with one another (p < 0.001). POC, MBC, AN, and AAN were negatively correlated with HUN (p < 0.05), while both ROC and POC showed relatively strong positive correlations with AN, ASN, and AAN (p < 0.01). Rice yield had an extremely significant positive correlation with ROC, POC, MBC, AN, ASN, and AAN (p < 0.001) and an extremely significant negative correlation with HUN (p < 0.001).

Figure 4.
Correlation analysis between organic carbon input, organic carbon and nitrogen fractions, and rice yields in a soil of an abandoned rural residential land at Jiangsu Province, China. OCI: organic carbon input; DOC: organic carbon; ROC: readily oxidizable organic carbon; POC: particulate organic carbon; MBC: microbial biomass carbon. AN: acid-hydrolyzable ammonium nitrogen, ASN: acid-hydrolyzable amino sugar nitrogen, AAN: acid-hydrolyzable amino acid nitrogen, HUN: acid-hydrolyzable unknown nitrogen. *, **, and *** indicate statistical significance at p <0.05, p < 0.01, and p < 0.001, respectively.
3.3. Drivers and Pathways of Rice Yield Under Organic Carbon Input
The random forest model accounted for 91.5% of the variation in rice yield across different OCI levels. The results showed that under the specific soil, climate and other conditions of this experiment, soil THN in abandoned rural residential land was the most important predictor of rice yield (p < 0.05; Figure 5), followed by OCI, DOC, MBC, ROC, and POC. Variance explained represents the correlation between features and the target variable; the higher the value, the stronger the correlation. The increase in mean squared error serves as a measure of a feature’s importance to the prediction result.
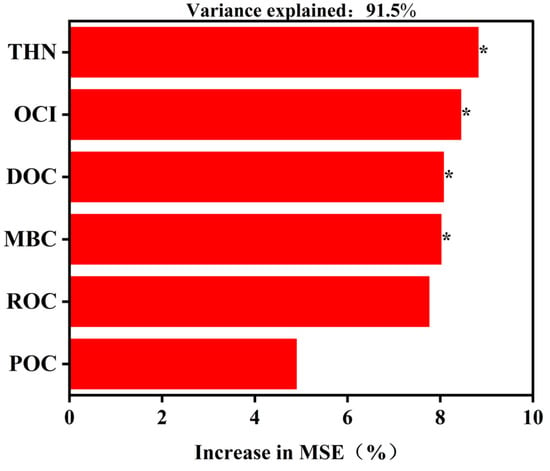
Figure 5.
Random forest analysis of rice yield and its influencing factors in an experiment that studied the effects of organic carbon input in the soil of an abandoned rural residential land in Jiangsu Province, China. THN: total hydrolyzable nitrogen; OCI: organic carbon input; DOC: organic carbon; ROC: readily oxidizable organic carbon; POC: particulate organic carbon; MBC: microbial biomass carbon. * indicates a statistical significance level of p < 0.05.
Structural equation modeling (SEM) revealed that OCI affects rice yield both directly and indirectly through its effects on labile organic carbon fractions and THN in abandoned rural residential lands (Figure 6). The model indicated that OCI explained 98%, 96%, and 94% of the variance in ROC, DOC, and THN, respectively, highlighting its dominant role in driving changes in these parameters. Path coefficients derived from the SEM demonstrated that the standardized total effects of OCI, ROC, and THN on rice yield were 0.43, 0.51, and 0.56, respectively (Figure 7), indicating that OCI enhances rice yield primarily through the mediation of ROC and subsequent promotion of THN accumulation.
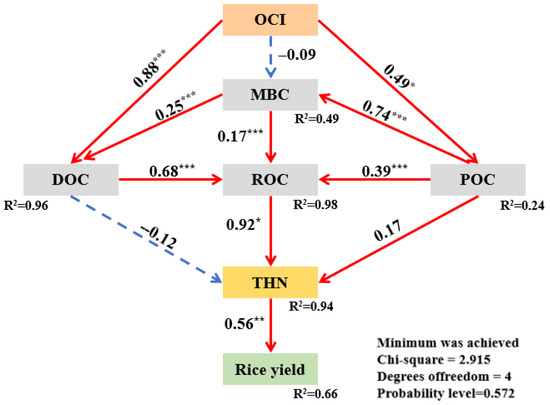
Figure 6.
Structural equation model (SEM) showing the direct and indirect effects of organic carbon input on rice yield in the soil of an abandoned rural residential land in Jiangsu Province, China. OCI: organic carbon input; DOC: organic carbon; ROC: readily oxidizable organic carbon; POC: particulate organic carbon; MBC: microbial biomass carbon; THN: total hydrolyzable nitrogen. * indicates a statistical significance level of p < 0.05. Solid red lines and dashed blue lines represent significant positive and negative paths, respectively. The thickness of the arrows is proportional to the strength of the relationships. *, **, and *** indicate statistical significance at p < 0.05, p < 0.01, and p < 0.001, respectively.
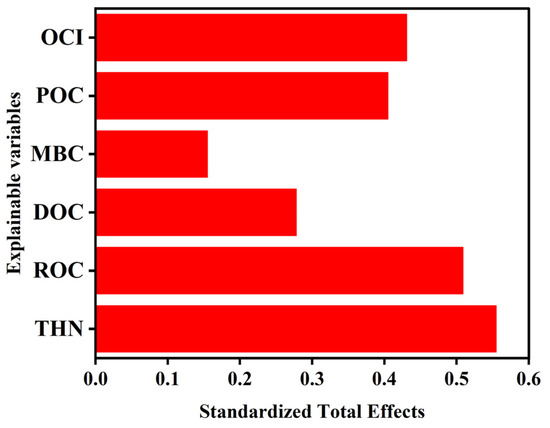
Figure 7.
An illustration of the standardized total effects of the variables on rice yield extracted from the SEM analysis with data from an experiment that studied the impact of organic carbon input in the soil of an abandoned rural residential land in Jiangsu Province, China. OCI: organic carbon input; DOC: organic carbon; ROC: readily oxidizable organic carbon; POC: particulate organic carbon; MBC: microbial biomass carbon; THN: total hydrolyzable nitrogen.
4. Discussion
4.1. Responses of Labile Carbon Fractions in Abandoned Rural Residential Land Soils to Organic Carbon Input and Their Carbon Pool Turnover Mechanisms
The significant increases in DOC, ROC, POC, and MBC under OCI treatments indicate that organic carbon input effectively enhanced the activity and availability of soil organic carbon pools in abandoned rural residential land soils. The marked rise in DOC with increasing OCI, particularly under treatment with the highest OCI rate (T4, OCI rate of 2.56 t ha−1, 270 kg N ha−1), further supports this. As the most labile soil carbon fraction in soil, DOC exhibits strong mobility and is easily decomposed and utilized by soil microorganisms. Exogenous organic carbon input can significantly increase the content of DOC [31], and this finding is supported by correlation analysis. This indicates that exogenous organic carbon input provides soluble and easily decomposable substrates for microbial utilization, thereby improving carbon turnover and nutrient cycling [32]. Notably, this increase implies that continued OCI may increase the risk of DOC leaching, particularly in abandoned rural residential land soils with poor physical structure. Although ROC also increased with OCI, its response plateaued at higher input levels (T3–T4), implying a saturation effect of labile carbon. The POC and MBC content in T2 (OCI rate of 0.77 t ha−1, 270 kg N ha−1) and T3 (OCI rate of 1.02 t ha−1, 270 kg N ha−1) treatments was higher than in the T4 treatment (OCI rate of 2.56 t ha−1, 270 kg N ha−1, organic–chemical N:10:0), suggesting that excess organic inputs do not proportionally increase microbial or particulate carbon once the system’s capacity for labile carbon stabilization is reached.
This pattern may be closely associated with the unique microbial metabolic activity and carbon pool turnover characteristics of abandoned rural residential land soils. When exogenous OCI exceeds the capacity of the indigenous microbial community, it triggers adaptive shifts in the microbial composition [33,34]. Under high OCI levels, the abundance of recalcitrant-carbon-degrading microbes (e.g., lignin decomposers) increases, whereas the activity of labile-carbon-degrading microorganisms is suppressed, resulting in reduced efficiency of the conversion of exogenous organic carbon into labile fractions [35,36,37].
Structural equation modeling revealed that DOC, as a short-term turnover carbon pool, was primarily directly driven by recent OCI, with a standardized total effect of 0.95 (see Appendix A; Figure A1). In contrast, ROC, POC, and MBC reflect intermediate processes within the soil carbon turnover continuum. Among these, ROC emerged as a key transformation product of OCI, accounting for up to 98% of the variance explained by OCI. Its dominant turnover pathway involves OCI ultimately driving the transformation of ROC by regulating DOC, POC, and MBC dynamics, with a standardized total effect of 0.88 for this pathway.
These findings clarify the functional differentiation among carbon fractions during the transformation and accumulation of exogenous organic carbon. Notably, this pattern markedly differs from the mature organic carbon transformation systems observed in conventional farmland [38]. Abandoned rural residential land soils typically exhibit a compromised native carbon pool due to construction-related disturbance, characterized by fragmented aggregate structure and poor stability; the microbial community is also in the early stage of reconstruction, showing limited diversity and functional integrity [39]. This immature soil system has a reduced capacity to channel and transform OCI: although DOC is highly responsive to recent input, it is susceptible to loss or rapid decomposition due to the weak water and nutrient retention capacity of abandoned rural residential land soils, preventing it from serving as a core vehicle for carbon transformation. POC depends on physical protection within aggregates, yet the underdeveloped aggregate architecture in these abandoned rural residential land soils limits its stabilization and contribution to carbon sequestration. Although MBC reflects microbial activity, the early-stage microbial community exhibits low efficiency in organic carbon utilization and cannot directly govern carbon turnover. Under these constraints, ROC functions as a pivotal hub for carbon turnover, converging dispersed carbon fluxes into a coordinated transformation pathway. It compensates for the limited independent turnover capacity of individual carbon fractions in abandoned rural residential land soils and serves as the principal medium for organic carbon transformation [40,41,42].
4.2. Response of Organic Nitrogen Fractions to Organic Carbon Input in Abandoned Rural Residential Land Soils
Organic carbon input (OCI) markedly improved the composition and transformation of organic nitrogen fractions in abandoned rural residential land soils, indicating a close coupling between carbon addition and nitrogen cycling [43,44]. The significant increases in acid-hydrolyzable ammonium nitrogen (AN), amino sugar nitrogen (ASN), and amino acid nitrogen (AAN) under OCI treatments reflect enhanced microbial activity and nitrogen mineralization stimulated by exogenous organic substrates [45]. The higher AN, ASN, and AAN contents in T2 (OCI rate of 0.77 t ha−1, 270 kg N ha−1) and T3 (OCI rate of 0.77 t ha−1, 270 kg N ha−1) treatments suggest that moderate OCI levels promoted the microbial synthesis and turnover of nitrogenous compounds. In contrast, as an unclassified fraction among soil acid-hydrolyzable nitrogen components, hydrolyzable unknown nitrogen (HUN) is mainly derived from complex nitrogen-containing polymers formed during the humification process. It is difficult for such components to be utilized by microorganisms and crops [46]. Moreover, the content of HUN is calculated as the difference between total hydrolyzable nitrogen (THN) and the sum of AN, ASN, and AAN; thus, HUN shows an opposite variation trend to other nitrogen fractions. In this study, the decrease in HUN under the T1–T3 treatments (with OCI rates of 0.51, 0.77, and 1.02 t ha−1, respectively, and a total N rate of 270 kg N ha−1) indicated that an appropriate amount of organic carbon input promoted the decomposition and transformation of part of the recalcitrant organic nitrogen into more labile forms via microbial activity [47]. This observation was consistent with the increases in AN, ASN, and AAN under the T1–T3 treatments. However, the relatively higher HUN in the T4 treatment (OCI rate of 2.56 t ha−1) implies that excessive OCI might cause an imbalance in the C/N ratio, shifting microbial metabolism from nitrogen acquisition to adaptation under nitrogen-limited conditions. Although carbon is abundant, nitrogen scarcity represses the synthesis of nitrogen-intensive enzymes responsible for decomposing recalcitrant organic N. This reduction in enzymatic capacity slows HUN mineralization, leading to its accumulation [48]. Furthermore, as the release of AN, ASN, and AAN from HUN decomposition declines, microorganisms increasingly assimilate these readily available nitrogen forms to meet their growth demands rather than releasing them into the soil. This leads to a stabilization or decline in the concentrations of AN, ASN, and AAN [49]. These nitrogen dynamics parallel the responses of ROC, POC, and MBC among soil carbon fractions, further corroborating the microbial-mediated coordination between carbon and nitrogen cycling in abandoned rural residential land soils [50,51].
4.3. Regulatory Mechanisms of Organic Carbon Fractions and Total Hydrolyzable Nitrogen on Rice Yields
Organic carbon input (OCI) significantly increased rice yield in abandoned rural residential land soils, demonstrating that organic amendments effectively enhanced soil fertility and crop productivity. This trend closely aligned with the behavior of soil organic carbon and nitrogen fractions, further supporting the regulatory role of carbon input in the productivity of degraded soils [52,53,54,55]. The highest yields observed in T2 (OCI rate of 0.77 t ha−1, 270 kg N ha−1) and T3 (OCI rate of 1.02 t ha−1, 270 kg N ha−1) treatments that balanced carbon and nitrogen inputs optimized soil biochemical processes and nutrient synchronization with plant demand [56,57]. The results of this experiment also showed that except for hydrolyzable unknown nitrogen (HUN), the contents of other organic carbon and nitrogen fractions as well as rice yield were significantly higher than those in the blank control group. This result further indicates that due to long-term anthropogenic disturbances, the soil chemical properties of rural residential land have been severely damaged, directly exhibiting the characteristics of soil impoverishment [55]. Another point worthy of attention is that the applicable conditions of the results in this study also need to be focused on. Existing studies have shown that under different soil types and climatic conditions, there are inherent differences in the initial soil carbon and nitrogen fractions [58]. Meanwhile, different types of organic carbon vary in their own nutrient contents and decomposition rates after being applied to the soil, leading to differences in their specific effects on soil carbon and nitrogen fractions. However, overall, all types of organic carbon show consistency in their effect of increasing the contents of soil carbon and nitrogen fractions [59,60].
We also found that all acid-hydrolyzed organic nitrogen fractions had an extremely significant effect on rice yield (p < 0.001), confirming that the acid-hydrolyzable organic nitrogen pool serves as the core functional nitrogen pool regulating productivity in abandoned rural residential land soils [61]. Among them, total hydrolyzable nitrogen (THN), defined as the sum of acid-hydrolyzable ammonium nitrogen (AN), acid-hydrolyzable amino sugar nitrogen (ASN), acid-hydrolyzable amino acid nitrogen (AAN), and hydrolyzable unknown nitrogen (HUN), is a key indicator for characterizing soil nitrogen availability and nitrogen cycling processes [33]. In contrast, different labile organic carbon fractions exhibited varying regulatory effects on rice yield: the correlations of ROC, POC, and MBC with rice yield were markedly stronger than those of DOC. Based on these results, we selected THN as the representative indicator of nitrogen fractions for random forest and structural equation modeling analyses. The results of random forest analysis indicated that THN exhibited the greatest standardized total effect on rice yield, which is consistent with previous research. Cassman et al. pointed out that approximately 60% of the nitrogen absorbed by rice throughout its growth period may originate from the mineralization of organic nitrogen [62]. As a dynamic reservoir of soil organic nitrogen, THN can directly supply substrates for this mineralization process, thereby mechanistically explaining its pronounced impact on rice yield under different OCI regimes observed in this study. Durani et al. further confirmed through field experiments that organic input can significantly affect the accumulation of various nitrogen forms; soil nitrogen fractions such as THN substantially contribute to crop nitrogen uptake and concurrently enhance crop yield [63]. This aligns with the dominant role of THN identified here across OCI gradients, indicating that THN remains the central driver of rice yield variation regardless of organic carbon input levels.
Notably, structural equation modeling revealed an additional key insight: the standardized total effects of OCI and ROC on rice yield were second only to THN, suggesting that OCI is indirectly modulated through its regulation of the ROC fraction, ultimately enhancing rice yield. Further analysis indicated that ROC was significantly linearly correlated with ASN and AAN (all p < 0.01; see Appendix A and Figure A2), implying that ROC primarily influences THN via these two nitrogen subfractions. This mechanism may diverge from that in conventional farmlands, likely because ROC serves as an efficient energy source for microbial metabolism [64]. It markedly stimulates proteases and peptidase activities, accelerating the conversion of proteins into AAN [65,66]. Concurrently, sustained ROC input promotes fungal biomass accumulation; upon fungal death, chitinous cell wall residues are released and converted into ASN [67]. This interplay between soil organic carbon and nitrogen fractions corroborates the regulatory effects of OCI on carbon and nitrogen dynamics in abandoned rural residential land soils observed in this study. Specifically, the ROC content—modulated by OCI—governs the dynamics of THN and its key constituents, thereby influencing rice yield formation. These insights provide an important theoretical foundation for understanding soil fertility–crop productivity relationships under diverse organic carbon management strategies.
4.4. Limitations and Future Research
This study has certain limitations that need attention. First of all, it is worth noting that the optimal application rate of organic carbon derived from the results of this experiment should be considered carefully in the context of soil conditions, as well as the climatic and topographic conditions of the experimental site. Moreover, the organic carbon input calculated in this experiment is based on limiting factors such as the proportion of organic nitrogen replacing chemical fertilizer nitrogen under nitrogen-equivalent conditions and the specific carbon-to-nitrogen ratio (9.49) of the organic matter. Second, microbial community analysis was not conducted. Microorganisms play a crucial role in soil carbon and nitrogen cycling, and the lack of data on microbial dynamics may restrict the comprehensive interpretation of how soil organic carbon–nitrogen fractions and rice yield respond to organic carbon input (OCI), particularly regarding the underlying mechanisms. Finally, no intermediate organic carbon input levels were set between the organic carbon input rates of 1.02 t·ha−1 (with a 4:6 substitution ratio of organic nitrogen to chemical fertilizer nitrogen) and 2.65 t·ha−1 (with a 10:0 substitution ratio of organic nitrogen to chemical fertilizer nitrogen). This gap in input levels prevents an accurate description of the variation patterns of soil properties and rice yield within the range of 1.02–2.65 t·ha−1, thereby limiting the refinement of the conclusions.
These limitations will be addressed in a targeted manner in our future studies. First, intermediate organic carbon input (OCI) levels between 1.02 t·ha−1 and 2.65 t·ha−1 will be incorporated, with specific settings of 1.28, 1.54, 1.79, and 2.05 t·ha−1 (corresponding to organic nitrogen-to-chemical fertilizer nitrogen substitution ratios of 5:5, 6:4, 7:3, and 8:2). This will clarify the continuous response patterns of soil carbon and nitrogen fractions as well as rice yield to OCI. Second, integrated analyses of soil properties and microbial metagenomics research will be conducted to fully elaborate the interactive mechanism among organic carbon input, soil microorganisms, transformation of organic carbon and nitrogen fractions, and rice yield formation. Ultimately, this will provide more comprehensive and reliable theoretical support for the sustainable utilization of abandoned rural residential land soils.
5. Conclusions
Organic carbon input (OCI) significantly increased dissolved organic carbon (DOC), readily oxidizable organic carbon (ROC), particulate organic carbon (POC), microbial biomass carbon (MBC), acid-hydrolyzable ammonium nitrogen (AN), acid-hydrolyzable amino sugar nitrogen (ASN), and acid-hydrolyzable amino acid nitrogen (AAN), as well as rice yield. Overall, the T2 treatment (OCI rate: 0.77 t ha−1, 270 kg N ha−1) and T3 treatment (OCI rate: 1.02 t ha−1, 270 kg N ha−1) performed best in improving soil fertility and rice yield; however, excessive OCI in this type of soil did not yield additional benefits—instead, it led to reduced nitrogen availability and rice yield. Mechanistically, OCI enhanced rice yield by directly regulating ROC and indirectly promoting total hydrolyzable nitrogen (THN) accumulation. This study provides a general framework for future research on the application of OCI in agricultural soil improvement, enabling the clarification of the dynamics of organic carbon and nitrogen fractions and their impacts on soil productivity. Consequently, it can also serve as a reference for soil reuse projects in abandoned rural residential lands.
Author Contributions
Conceptualization, J.J. and H.Z.; methodology, X.Z.; software, X.Z. and T.L.; validation, J.J., H.Z., and W.M.; formal analysis, X.Z.; investigation, X.Z., S.C., and W.S.; resources, H.Z.; data curation, X.Z.; writing—original draft preparation, X.Z.; writing—review and editing, X.Z. and J.J.; visualization, X.Z.; supervision, J.J.; project administration, W.M.; funding acquisition, H.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (2024YFD2300302).
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to privacy requirement.
Acknowledgments
We would like to thank the personnel who provided assistance during sample collection and laboratory analysis for their valuable support.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A
Appendix A.1. Determination Methods of Total Hydrolyzable Nitrogen Fractions
Determination of total hydrolyzable nitrogen (THN): Pipette 5 mL of acid hydrolysate into a 50 mL Kjeldahl tube, add 2 mL of concentrated sulfuric acid (H2SO4) and 0.5 g of catalyst, and then proceed with digestion. Place the Kjeldahl tube on an electric furnace and heat carefully until water evaporates and foaming stops. Then, increase the temperature to continue digestion until the mixture becomes clear, followed by gentle boiling for 1 h to complete the digestion process. After digestion, allow the Kjeldahl tube to cool, and then slowly add 10 mL of distilled water while swirling gently. Add 5 mL of boric acid indicator solution into a 100 mL Erlenmeyer flask with a calibration mark at the 35 mL position and place the flask under the condenser of the distillation apparatus—ensure the end of the condenser dips into the boric acid solution. Connect the Kjeldahl tube to the distillation apparatus. Place 10 mL of sodium hydroxide (NaOH) solution [c(NaOH) = 10 mol·L−1] into the funnel on top of the apparatus, open the funnel stopcock, and let the alkali solution flow slowly into the Kjeldahl tube. When approximately 0.5 mL of alkali solution remains in the funnel, quickly rinse the funnel with about 5 mL of distilled water and close the stopcock. Add another 2 mL of distilled water to the funnel. Pass steam to start distillation; stop distillation when the distillate reaches the 35 mL calibration mark of the receiving Erlenmeyer flask (distillation time: approximately 4 min). Rinse the tip of the condenser and collect all rinsate, adding it to the distillate. Titrate the distillate with a standard sulfuric acid solution until the color changes from green to the endpoint (pale pink). Perform a blank test following the same procedure.
Determination of acid-hydrolyzable amino acid nitrogen (AAN): Place 5 mL of acid hydrolysate into a 100 mL Kjeldahl flask, add 1 mL of sodium hydroxide (NaOH) solution [c(NaOH) = 0.5 mol·L−1], and heat the flask in boiling water until the sample evaporates to a residual volume of 2–3 mL (approximately 20 min). After cooling, add 0.50 g of citric acid (C6H8O7) and 0.1 g of ninhydrin (C9H6O4), and then place the flask in a vigorously boiling water bath—ensure the spherical part of the Kjeldahl flask is completely immersed in the boiling water. After approximately 1 min, shake the Kjeldahl flask in the boiling water for a few seconds, and then keep the flask in the water bath for another 9 min. Once cooled, connect the Kjeldahl flask to the distillation apparatus, add 10 mL of phosphate–borate buffer and 1 mL of sodium hydroxide (NaOH) solution [c(NaOH) = 5 mol·L−1], and perform distillation, absorption, and titration following the same procedure used for the determination of total hydrolyzable nitrogen (distillation time: approximately 4 min). Conduct a blank test separately.
Determination of acid-hydrolyzable ammonium nitrogen (AN): Place 10 mL of acid hydrolysate into a 100 mL Kjeldahl flask, connect the flask to the distillation apparatus, and add 0.07 ± 0.01 g of magnesium oxide (MgO). Add 5 mL of boric acid indicator solution into a 100 mL Erlenmeyer flask with a calibration mark at the 20 mL position. Determine the amount of released ammonia (NH3) by distillation following the procedure used for the determination of total hydrolyzable nitrogen, and stop distillation when the distillate reaches the 20 mL calibration mark of the receiving Erlenmeyer flask (distillation time: approximately 2 min). Conduct a blank test separately.
Determination of acid-hydrolyzable ammonium nitrogen + amino sugar nitrogen (AN + ASN): Place 10 mL of acid hydrolysate into a 100 mL Kjeldahl flask, connect the flask to the distillation apparatus, and add 10 mL of phosphate-borax buffer (pH = 11.2). Determine the amount of ammonia nitrogen (NH3-N) released by distillation following the procedure used for the determination of total hydrolyzable nitrogen (distillation time: approximately 4 min). Conduct a blank test separately.
Calculation method:
Amino sugar nitrogen (ASN) = (AN + ASN) − AN
Hydrolyzable unknown nitrogen (HUN) = THN − (AN + ASN + AAN)
Appendix B
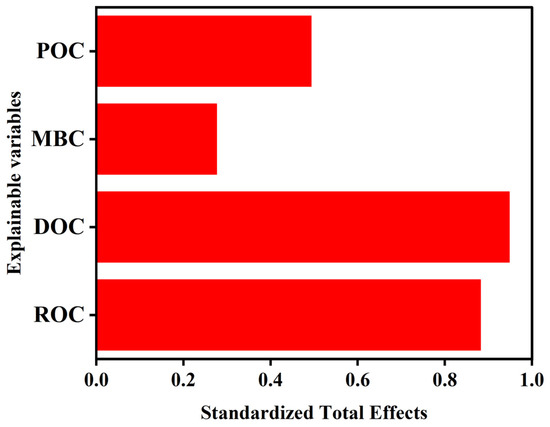
Figure A1.
Standardized total effects of each explanatory variable on OCI in structural equation modeling (SEM) (Figure 6 in the submitted manuscript). DOC: dissolved organic carbon; ROC: readily oxidizable organic carbon; POC: particulate organic carbon; MBC: microbial biomass carbon.
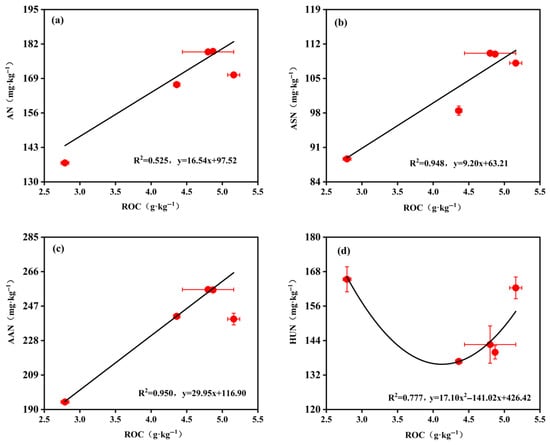
Figure A2.
(a) Linear fitting between ROC and AN under different OCI conditions. (b) Linear fitting between ROC and ASN under different OCI conditions. (c) Linear fitting between ROC and AAN under different OCI conditions. Nonlinear fitting between ROC and HUN under different OCI conditions (d). Each symbol represents the mean ± standard error (number of replicates = 3). R2 values were determined using quadratic regressions. OCI: organic carbon input; ROC: readily oxidizable carbon; AN: acid-hydrolyzable ammonium nitrogen; ASN: amino sugar nitrogen; AAN: amino acid nitrogen; HUN: hydrolyzable unknown nitrogen.
References
- Cleland, J. World Population Growth; Past, Present and Future. Environ. Resour. Econ. 2013, 55, 543–554. [Google Scholar] [CrossRef]
- FAO. FAOSTAT Analytical Briefs; FAO: Rome, Italy, 2025; Volume 107, p. 17. [Google Scholar]
- Hassanein, H.; Zorba, N.; Han, S.; Kanhere, S.S.; Shukair, M. Crowd Management. IEEE Commun. Mag. 2019, 57, 18–19. [Google Scholar] [CrossRef]
- Liu, R.; Yu, C.; Jiang, J.; Huang, Z.; Jiang, Y. Farmer differentiation, generational differences and farmers’ behaviors to withdraw from rural homesteads: Evidence from Chengdu, China. Habitat Int. 2020, 103, 102231. [Google Scholar] [CrossRef]
- Qi, L.; Zhou, P.; Yang, L.; Gao, M. Effects of land reclamation on the physical, chemical, and microbial quantity and enzyme activity properties of degraded agricultural soils. J. Soils Sediments 2020, 20, 973–981. [Google Scholar] [CrossRef]
- Lei, N.; Han, J.; Mu, X.; Sun, Z.; Wang, H. Effects of improved materials on reclamation of soil properties and crop yield in hollow villages in China. J. Soils Sediments 2019, 19, 2374–2380. [Google Scholar] [CrossRef]
- Meng, T.; Han, J.; Zhang, Y.; Sun, Y.; Liu, Z.; Zhang, R. Multifractal characteristics of soil particle size distribution of abandoned homestead reclamation under different forest management modes. Sci. Rep. 2024, 14, 8864. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Sun, Y.; Li, X.; Wang, N.; Wang, X.; Meng, T. Interaction force mechanism for the improvement of reclaimed soil aggregate stability in abandoned homestead by different organic-inorganic soil conditioners. Front. Environ. Sci. 2023, 11, 2023. [Google Scholar] [CrossRef]
- Wei, B. Effects of Different Amelioration Materials on Soil Fertility in Abandoned Homestead Reclaimed Soil. IOP Conf. Ser. Earth Environ. Sci. 2020, 514, 052038. [Google Scholar] [CrossRef]
- Sae-Tun, O.; Maftukhah, R.; Susanto, S.; Ngadisih, N.; Murtiningrum, M.; Hood-Nowotny, R.; Mentler, A.; Bodner, G.; Keiblinger, K.M. Organic carbon-based amendments effectively reclaim post-tin mining site via modified soil organic carbon characteristics. Plant Soil 2025, 508, 891–907. [Google Scholar] [CrossRef]
- Szili-Kovács, T.; Török, K.; Tilston, E.L.; Hopkins, D.W. Promoting microbial immobilization of soil nitrogen during restoration of abandoned agricultural fields by organic additions. Biol. Fertil. Soils 2007, 43, 823–828. [Google Scholar] [CrossRef]
- George, P.B.L.; Fidler, D.B.; Van Nostrand, J.D.; Atkinson, J.A.; Mooney, S.J.; Creer, S.; Griffiths, R.I.; McDonald, J.E.; Robinson, D.A.; Jones, D.L. Shifts in Soil Structure, Biological, and Functional Diversity Under Long-Term Carbon Deprivation. Front. Microbiol. 2021, 12, 2021. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Yoshida, T. Effect of organic acid transformations in submerged soils on growth of the rice plant. Soil Sci. Plant Nutr. 1973, 19, 39–45. [Google Scholar] [CrossRef][Green Version]
- Månsson, K.; Bengtson, P.; Falkengren-Grerup, U.; Bengtsson, G. Plant–microbial competition for nitrogen uncoupled from soil C:N ratios. Oikos 2009, 118, 1908–1916. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, G.; Ma, J.; Song, K.; Zhu, X.; Shen, W.; Xu, H. Dynamic interactions of nitrogen fertilizer and straw application on greenhouse gas emissions and sequestration of soil carbon and nitrogen: A 13-year field study. Agric. Ecosyst. Environ. 2022, 325, 107753. [Google Scholar] [CrossRef]
- Kim, Y.N.; Lee, J.H.; Seo, H.R.; Kim, J.W.; Cho, Y.S.; Lee, D.; Kim, B.H.; Yoon, J.H.; Choe, H.; Lee, Y.B.; et al. Co-Responses of Soil Organic Carbon Pool and Biogeochemistry to Different Long-Term Fertilization Practices in Paddy Fields. Plants 2022, 11, 3195. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.H.; Li, D.M.; Liu, K.L.; Yu, X.C.; Ye, H.C.; Hu, H.W.; Xu, X.L.; Wang, S.L.; Zhou, L.J.; Duan, Y.H.; et al. Effects of Long-Term Organic Amendments on Soil Organic Carbon in a Paddy Field: A Case Study on Red Soil. J. Integr. Agric. 2014, 13, 570–576. [Google Scholar] [CrossRef]
- Wei, W.; Huangfu, C.; Jia, Z.; Liu, S. Long-term organic amendments improved soil carbon sequestration to support crop production. J. Plant Nutr. Soil Sci. 2021, 184, 678–687. [Google Scholar] [CrossRef]
- Tian, J.; Lu, S.; Fan, M.; Li, X.; Kuzyakov, Y. Labile soil organic matter fractions as influenced by non-flooded mulching cultivation and cropping season in rice–wheat rotation. Eur. J. Soil Biol. 2013, 56, 19–25. [Google Scholar] [CrossRef]
- Schulten, H.R.; Schnitzer, M. The chemistry of soil organic nitrogen: A review. Biol. Fertil. Soils 1997, 26, 1–15. [Google Scholar] [CrossRef]
- Ichihashi, Y.; Date, Y.; Shino, A.; Shimizu, T.; Shibata, A.; Kumaishi, K.; Funahashi, F.; Wakayama, K.; Yamazaki, K.; Umezawa, A.; et al. Multi-omics analysis on an agroecosystem reveals the significant role of organic nitrogen to increase agricultural crop yield. Proc. Natl. Acad. Sci. USA 2020, 117, 14552–14560. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, Q.; Noll, L.; Zhang, S.; Wanek, W. Direct measurement of the in situ decomposition of microbial-derived soil organic matter. Soil Biol. Biochem. 2020, 141, 107660. [Google Scholar] [CrossRef]
- Bremner, J.M. Organic Nitrogen in Soils. In Soil Nitrogen; Wiley: Hoboken, NJ, USA, 1965; pp. 93–149. [Google Scholar]
- Wang, Z.; Liu, Z.; Hu, W.; Bai, H.; Ma, L.; Lv, X.; Zhou, Z.; Meng, Y. Crop residue return improved soil nitrogen availability by increasing amino acid and mineralization under appropriate N fertilization. Land Degrad. Dev. 2022, 33, 2197–2207. [Google Scholar] [CrossRef]
- Liu, X.; Hu, G.; He, H.; Liang, C.; Zhang, W.; Bai, Z.; Wu, Y.; Lin, G.; Zhang, X. Linking microbial immobilization of fertilizer nitrogen to in situ turnover of soil microbial residues in an agro-ecosystem. Agric. Ecosyst. Environ. 2016, 229, 40–47. [Google Scholar] [CrossRef]
- Lu, C.; Chen, H.; Teng, Z.; Yuan, L.; Ma, J.; He, H.; Chen, X.; Zhang, X.; Shi, Y. Effects of N fertilization and maize straw on the dynamics of soil organic N and amino acid N derived from fertilizer N as indicated by 15N labeling. Geoderma 2018, 321, 118–126. [Google Scholar] [CrossRef]
- Zhou, F.; Zhang, X.; Ma, S.; Li, Y.; Zhu, M.; Zhang, W.; Li, J.; Liu, X.; Hu, G.; Wang, X.; et al. Soil microbial necromass regulation of long-term fertilizer N retention influenced by maize stover mulching. Geoderma 2023, 433, 116453. [Google Scholar] [CrossRef]
- Yang, R.; Su, Y.Z.; Wang, T.; Yang, Q. Effect of chemical and organic fertilization on soil carbon and nitrogen accumulation in a newly cultivated farmland. J. Integr. Agric. 2016, 15, 658–666. [Google Scholar] [CrossRef]
- Jones, D.L.; Willett, V.B. Experimental evaluation of methods to quantify dissolved organic nitrogen (DON) and dissolved organic carbon (DOC) in soil. Soil Biol. Biochem. 2006, 38, 991–999. [Google Scholar] [CrossRef]
- Weil, R.; Stine, M.; Gruver, J.; Samson-Liebig, S. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar] [CrossRef]
- Cambardella, C.A.; Elliott, E.T. Particulate Soil Organic-Matter Changes across a Grassland Cultivation Sequence. Soil Sci. Soc. Am. J. 1992, 56, 777–783. [Google Scholar] [CrossRef]
- Qi, R.; Li, J.; Lin, Z.; Li, Z.; Li, Y.; Yang, X.; Zhang, J.; Zhao, B. Temperature effects on soil organic carbon, soil labile organic carbon fractions, and soil enzyme activities under long-term fertilization regimes. Appl. Soil Ecol. 2016, 102, 36–45. [Google Scholar] [CrossRef]
- Ren, T.; Ukalska-Jaruga, A.; Smreczak, B.; Cai, A. Dissolved organic carbon in cropland soils: A global meta-analysis of management effects. Agric. Ecosyst. Environ. 2024, 371, 109080. [Google Scholar] [CrossRef]
- Whitman, T.; DeCiucies, S.; Hanley, K.; Enders, A.; Woolet, J.; Lehmann, J. Microbial Community Shifts Reflect Losses of Native Soil Carbon with Pyrogenic and Fresh Organic Matter Additions and Are Greatest in Low-Carbon Soils. Appl. Environ. Microbiol. 2021, 87, e02555-20. [Google Scholar] [CrossRef]
- Mendoza, O.; De Neve, S.; Deroo, H.; Li, H.; Sleutel, S. Do interactions between application rate and native soil organic matter content determine the degradation of exogenous organic carbon? Soil Biol. Biochem. 2022, 164, 108473. [Google Scholar] [CrossRef]
- Kou, X.; Morriën, E.; Tian, Y.; Zhang, X.; Lu, C.; Xie, H.; Liang, W.; Li, Q.; Liang, C. Exogenous carbon turnover within the soil food web strengthens soil carbon sequestration through microbial necromass accumulation. Glob. Change Biol. 2023, 29, 4069–4080. [Google Scholar] [CrossRef]
- Huang, W.; Kuzyakov, Y.; Niu, S.; Luo, Y.; Sun, B.; Zhang, J.; Liang, Y. Drivers of microbially and plant-derived carbon in topsoil and subsoil. Glob. Change Biol. 2023, 29, 6188–6200. [Google Scholar] [CrossRef]
- Huang, Q.; Wang, B.; Shen, J.; Xu, F.; Li, N.; Jia, P.; Jia, Y.; An, S.; Amoah, I.D.; Huang, Y. Shifts in C-degradation genes and microbial metabolic activity with vegetation types affected the surface soil organic carbon pool. Soil Biol. Biochem. 2024, 192, 109371. [Google Scholar] [CrossRef]
- Li, T.T.; Zhang, J.Z.; Zhang, H.Y.; Chrisite, P.; Zhang, J.L. Fractionation of soil organic carbon in a calcareous soil after long-term tillage and straw residue management. J. Integr. Agric. 2022, 21, 3611–3625. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Cao, S.; Sun, Z.; Wang, N.; Zhang, Z.; Rong, Y. Variation Characteristics of Particle Surface Electrochemical Properties during the Improvement of Reclaimed Soil from Hollow Village in Loess Area. Sustainability 2022, 14, 11527. [Google Scholar] [CrossRef]
- Wang, M.; Feng, X.; Zhou, Z.; Ma, H.; Ge, T.; Tang, C.; Wang, D.; Chen, S. Labile organic carbon fractions in the rhizosphere contribute to nitrogen and phosphorus uptake in rice under long-term crop rotations and nitrogen application. Appl. Soil Ecol. 2024, 200, 105459. [Google Scholar] [CrossRef]
- Zhang, Y.; Dou, S.; Ndzelu, B.S.; Ma, R.; Zhang, D.; Zhang, X.; Ye, S.; Wang, H. Effects of returning corn straw and fermented corn straw to fields on the soil organic carbon pools and humus composition. SOIL 2022, 8, 605–619. [Google Scholar] [CrossRef]
- Yan, S.; Zhang, S.; Yan, P.; Wei, Z.; Niu, X.; Zhang, H. Biochar application increased soil carbon sequestration by altering organic carbon components in aggregates. Soil Tillage Res. 2026, 255, 106795. [Google Scholar] [CrossRef]
- Almaraz, M.; Wong, M.Y.; Geoghegan, E.K.; Houlton, B.Z. A review of carbon farming impacts on nitrogen cycling, retention, and loss. Ann. N. Y. Acad. Sci. 2021, 1505, 102–117. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, D.; Teng, W.; Gu, F.; Zhang, R.; Cheng, K.; Liu, Z.; Zhao, Y.; Yang, F. A state of art review on carbon, nitrogen, and phosphorus cycling and efficient utilization in paddy fields. Plant Soil 2025, 513, 1689–1709. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, Q.; Noll, L.; Hu, Y.; Wanek, W. Environmental effects on soil microbial nitrogen use efficiency are controlled by allocation of organic nitrogen to microbial growth and regulate gross N mineralization. Soil Biol. Biochem. 2019, 135, 304–315. [Google Scholar] [CrossRef]
- Jones, D.L.; Kielland, K.; Sinclair, F.L.; Dahlgren, R.A.; Newsham, K.K.; Farrar, J.F.; Murphy, D.V. Soil organic nitrogen mineralization across a global latitudinal gradient. Glob. Biogeochem. Cycles 2009, 23, GB1016. [Google Scholar] [CrossRef]
- Hu, Y.; Zheng, Q.; Zhang, S.; Noll, L.; Wanek, W. Significant release and microbial utilization of amino sugars and d-amino acid enantiomers from microbial cell wall decomposition in soils. Soil Biol. Biochem. 2018, 123, 115–125. [Google Scholar] [CrossRef]
- Chen, M.; Xu, J.; Li, Z.; Li, D.; Wang, Q.; Zhou, Y.; Guo, W.; Ma, D.; Zhang, J.; Zhao, B. Long-term nitrogen fertilization-induced enhancements of acid hydrolyzable nitrogen are mainly regulated by the most vital microbial taxa of keystone species and enzyme activities. Sci. Total Environ. 2023, 874, 162463. [Google Scholar] [CrossRef]
- Ding, S.; Xin, X.; Yang, W.; Zhang, X.; Zhu, A.; Huang, S.; Yang, J.; Ren, G.; Li, M. Transformation of fertilizer nitrogen in fluvo-aquic soils with different textures and its influencing factors. Plant Soil 2022, 471, 541–558. [Google Scholar] [CrossRef]
- Zhuang, W.; Yan, X.; Zhang, J.; Zhang, J.; Zhao, C. Nitrogen deposition and water addition affect microbial carbon and nitrogen cycling: Insights from a 10-year experiment in a semi-arid steppe. CATENA 2025, 259, 109322. [Google Scholar] [CrossRef]
- Xue, S.; Yi, X.; Peng, J.; Bak, F.; Zhang, L.; Duan, G.; Liesack, W.; Zhu, Y. Fulvic Acid Enhances Nitrogen Fixation and Retention in Paddy Soils through Microbial-Coupled Carbon and Nitrogen Cycling. Environ. Sci. Technol. 2024, 58, 18777–18787. [Google Scholar] [CrossRef]
- Ma, Y.; Woolf, D.; Fan, M.; Qiao, L.; Li, R.; Lehmann, J. Global crop production increase by soil organic carbon. Nat. Geosci. 2023, 16, 1159–1165. [Google Scholar] [CrossRef]
- Gupta Choudhury, S.; Yaduvanshi, N.P.S.; Chaudhari, S.K.; Sharma, D.R.; Sharma, D.K.; Nayak, D.C.; Singh, S.K. Effect of nutrient management on soil organic carbon sequestration, fertility, and productivity under rice-wheat cropping system in semi-reclaimed sodic soils of North India. Env. Monit Assess 2018, 190, 117. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, T.; Wang, G.; Ju, J.; Mao, W.; Zhao, H. Response of Rice Grain Yield and Soil Fertility to Fertilization Management under Three Rice-Based Cropping Systems in Reclaimed Soil. Agronomy 2023, 13, 1840. [Google Scholar] [CrossRef]
- Hao, X.; Ma, X.; Sun, L.; Liu, S.; Ji, J.; Zhou, B.; Zhao, Y.; Zheng, Y.; Kuang, E.; Liu, Y.; et al. High Ratio of Manure Substitution Enhanced Soil Organic Carbon Storage via Increasing Particulate Organic Carbon and Nutrient Availability. Plants 2025, 14, 2045. [Google Scholar] [CrossRef]
- Xu, H.; Cai, A.; Yang, X.; Zhang, S.; Huang, S.; Wang, B.; Zhu, P.; Colinet, G.; Sun, N.; Xu, M.; et al. Long-Term Organic Substitution Promotes Carbon and Nitrogen Sequestration and Benefit Crop Production in Upland Field. Agronomy 2023, 13, 2381. [Google Scholar] [CrossRef]
- Huang, Y.; Wei, F. Climate controls the global distribution of soil organic and inorganic carbon. Ecol. Indic. 2025, 175, 113514. [Google Scholar] [CrossRef]
- Thomas, C.L.; Acquah, G.E.; Whitmore, A.P.; McGrath, S.P.; Haefele, S.M. The Effect of Different Organic Fertilizers on Yield and Soil and Crop Nutrient Concentrations. Agronomy 2019, 9, 776. [Google Scholar] [CrossRef]
- Li, X.; Li, D.; Lu, Q.; Wang, D.; Ren, X.; Lv, L.; Ahmed, T.; Yan, J.; Li, B. Effects of Different Organic Fertilizers on Sweet Potato Growth and Rhizosphere Soil Properties in Newly Reclaimed Land. Agronomy 2022, 12, 1649. [Google Scholar] [CrossRef]
- Breza, L.C.; Mooshammer, M.; Bowles, T.M.; Jin, V.L.; Schmer, M.R.; Thompson, B.; Grandy, A.S. Complex crop rotations improve organic nitrogen cycling. Soil Biol. Biochem. 2023, 177, 108911. [Google Scholar] [CrossRef]
- Cassman, K.G.; Peng, S.; Olk, D.C.; Ladha, J.K.; Reichardt, W.; Dobermann, A.; Singh, U. Opportunities for increased nitrogen-use efficiency from improved resource management in irrigated rice systems. Field Crops Res. 1998, 56, 7–39. [Google Scholar] [CrossRef]
- Durani, A.; Brar, B.S.; Dheri, G.S. Soil Nitrogen Fractions in Relation to Rice-Wheat Productivity: Effects of Long-Term Application of Mineral Fertilizers and Organic Manures. J. Crop Improv. 2016, 30, 399–420. [Google Scholar] [CrossRef]
- Xu, C.Y.; Du, C.; Jian, J.S.; Hou, L.; Wang, Z.K.; Wang, Q.; Geng, Z.C. The interplay of labile organic carbon, enzyme activities and microbial communities of two forest soils across seasons. Sci. Rep. 2021, 11, 5002. [Google Scholar] [CrossRef]
- Stevenson, F.J. Organic Forms of Soil Nitrogen. In Nitrogen in Agricultural Soils; Agronomy Monographs; Wiley: Hoboken, NJ, USA, 1982; pp. 67–122. [Google Scholar]
- Xu, L.; Chen, H.; Zhou, Y.; Zhang, J.; Nadeem, M.Y.; Miao, C.; You, J.; Li, W.; Jiang, Y.; Ding, Y.; et al. Long-term straw returning improved soil nitrogen sequestration by accelerating the accumulation of amino acid nitrogen. Agric. Ecosyst. Environ. 2024, 362, 108846. [Google Scholar] [CrossRef]
- Bai, Z.; Bodé, S.; Huygens, D.; Zhang, X.; Boeckx, P. Kinetics of amino sugar formation from organic residues of different quality. Soil Biol. Biochem. 2013, 57, 814–821. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).