Genotype-Specific Synergy Between Arbuscular Mycorrhizal Fungi and Olive Cultivars Enhances Drought Resilience in China’s Olive Belt
Abstract
1. Introduction
2. Materials and Methods
2.1. Soil Collection and Fungal and Plant Materials
2.2. Experimental Design and Treatments
2.2.1. Experimental Design
2.2.2. Water Treatments and Fungal Inoculation
2.3. Determination of Plant Biomass and Mycorrhizal Colonization
2.4. Determination of Photosynthetic and, Fluorescence Parameters and Plant Water Status
2.5. Determination of Chlorophylls and Carbon and Nitrogen Detection
2.6. Determination of Physiological Characteristics
2.7. Statistical Analyses
3. Results
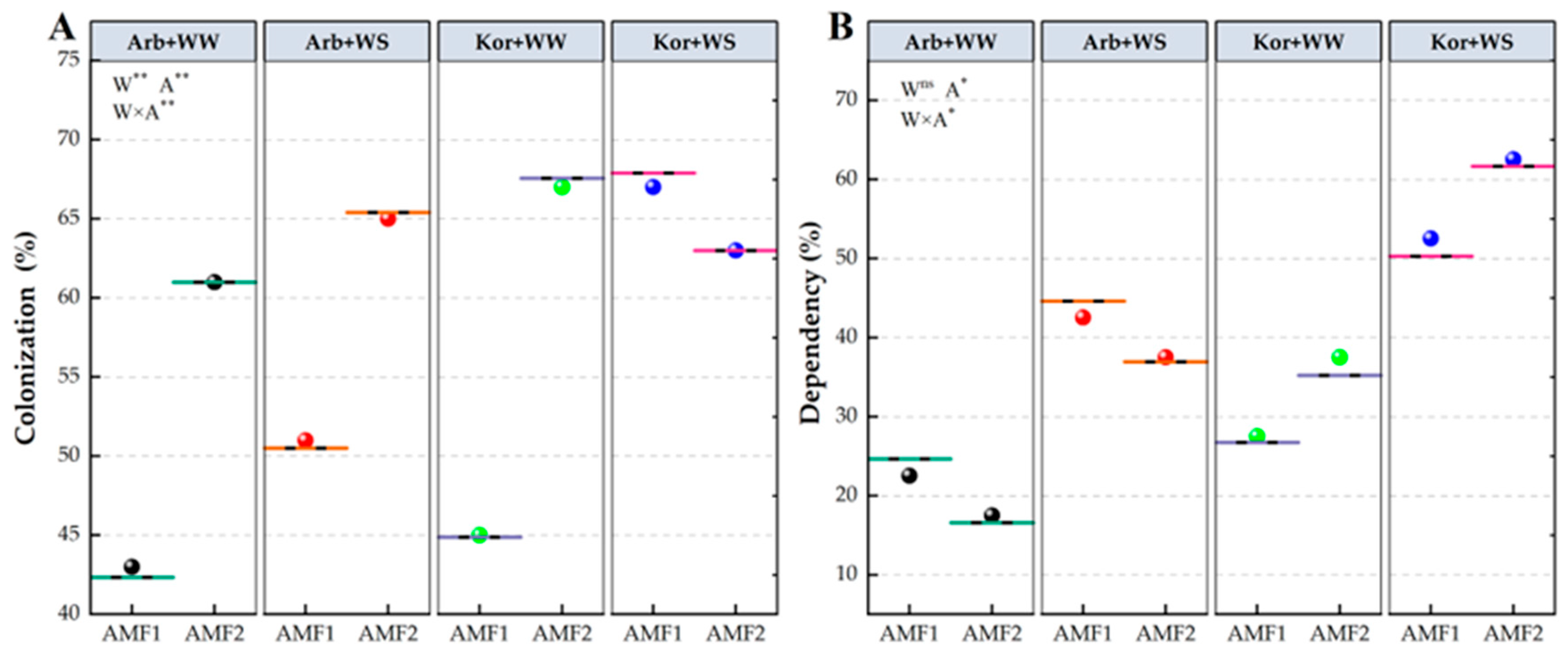
3.1. AMF Colonization and Mycorrhizal Dependency in Roots
3.2. Biomass Accumulation and Root Growth
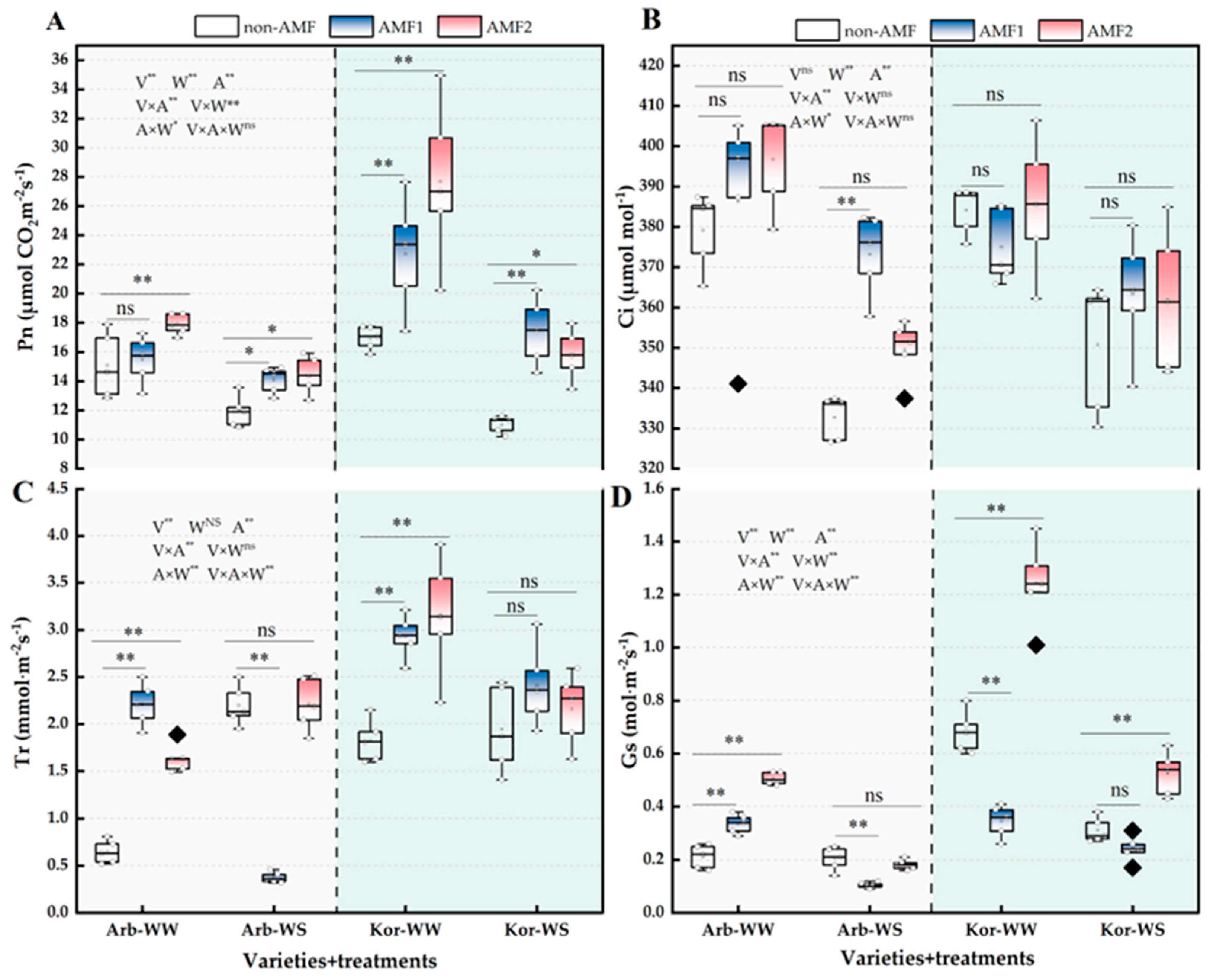
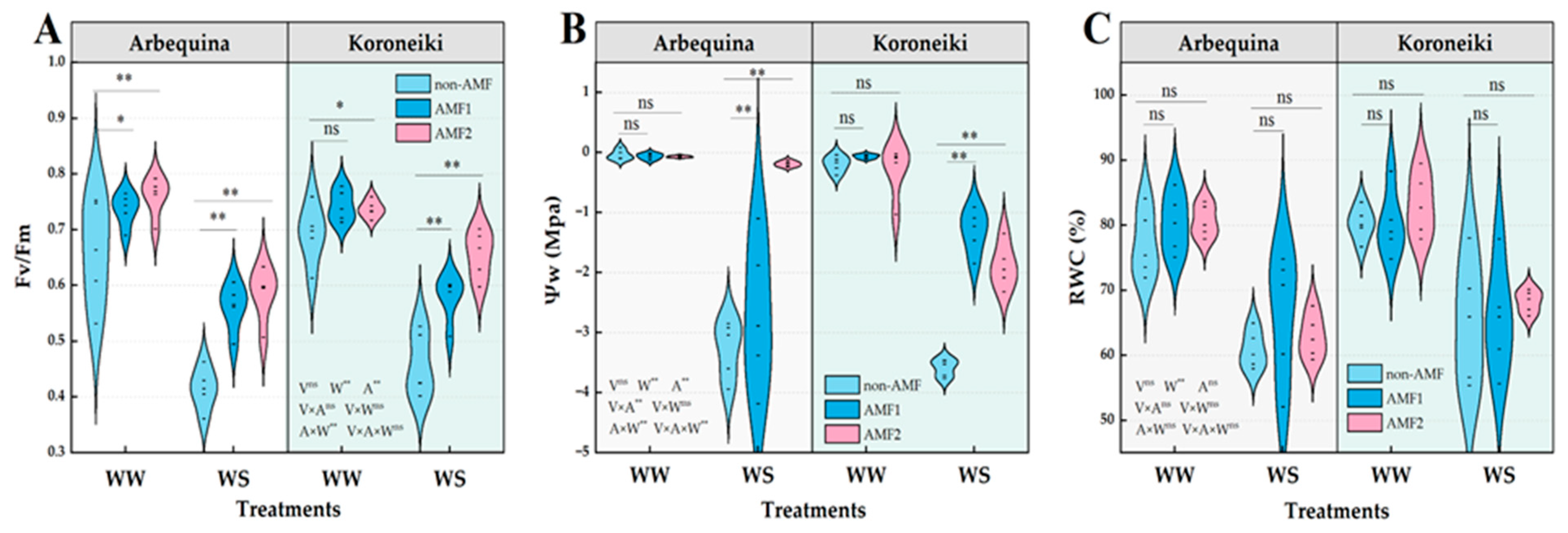
3.3. Plant Photosynthetic and Fluorescence Parameters and Water Status
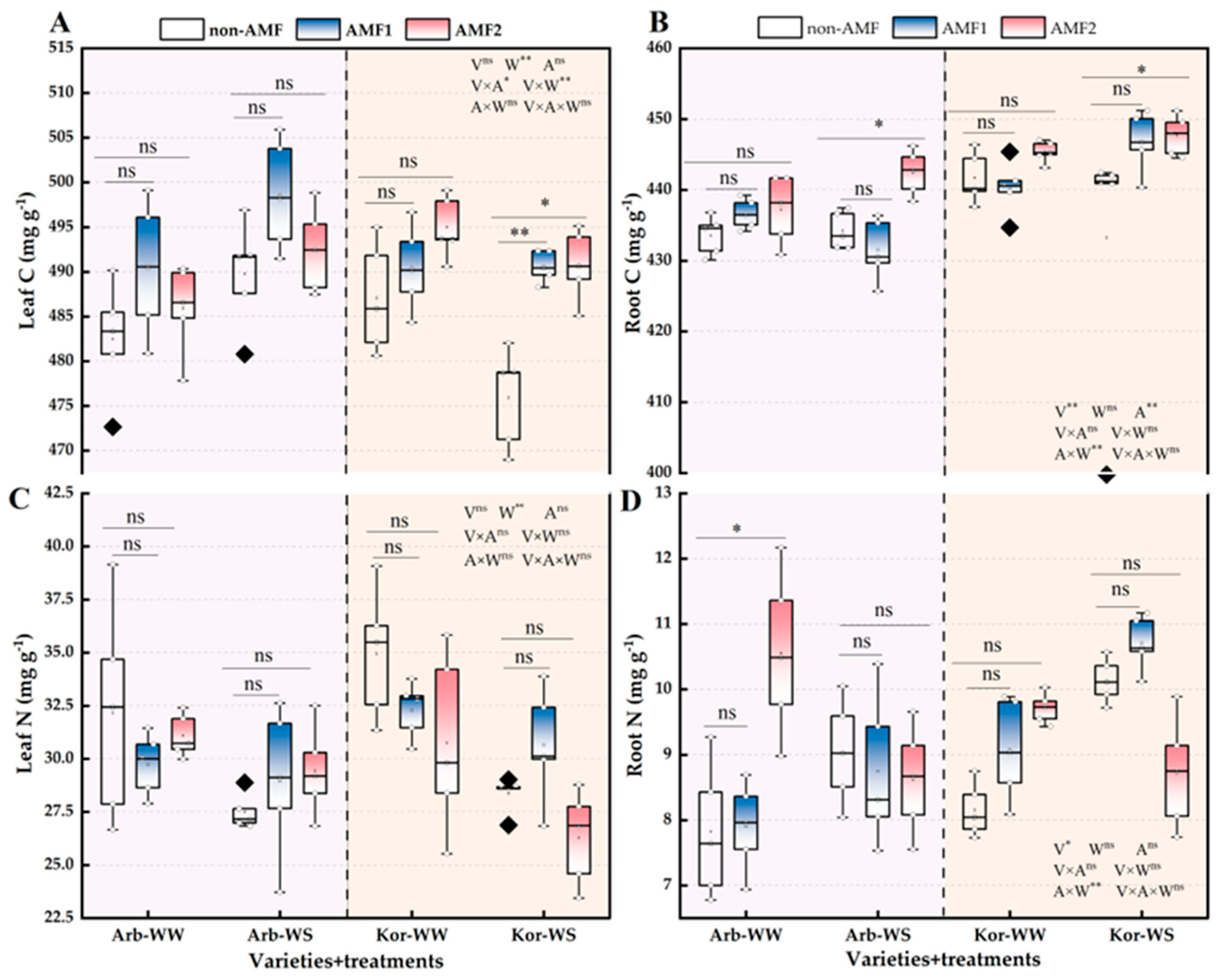
3.4. Photosynthetic Pigments and Nutrient Elements
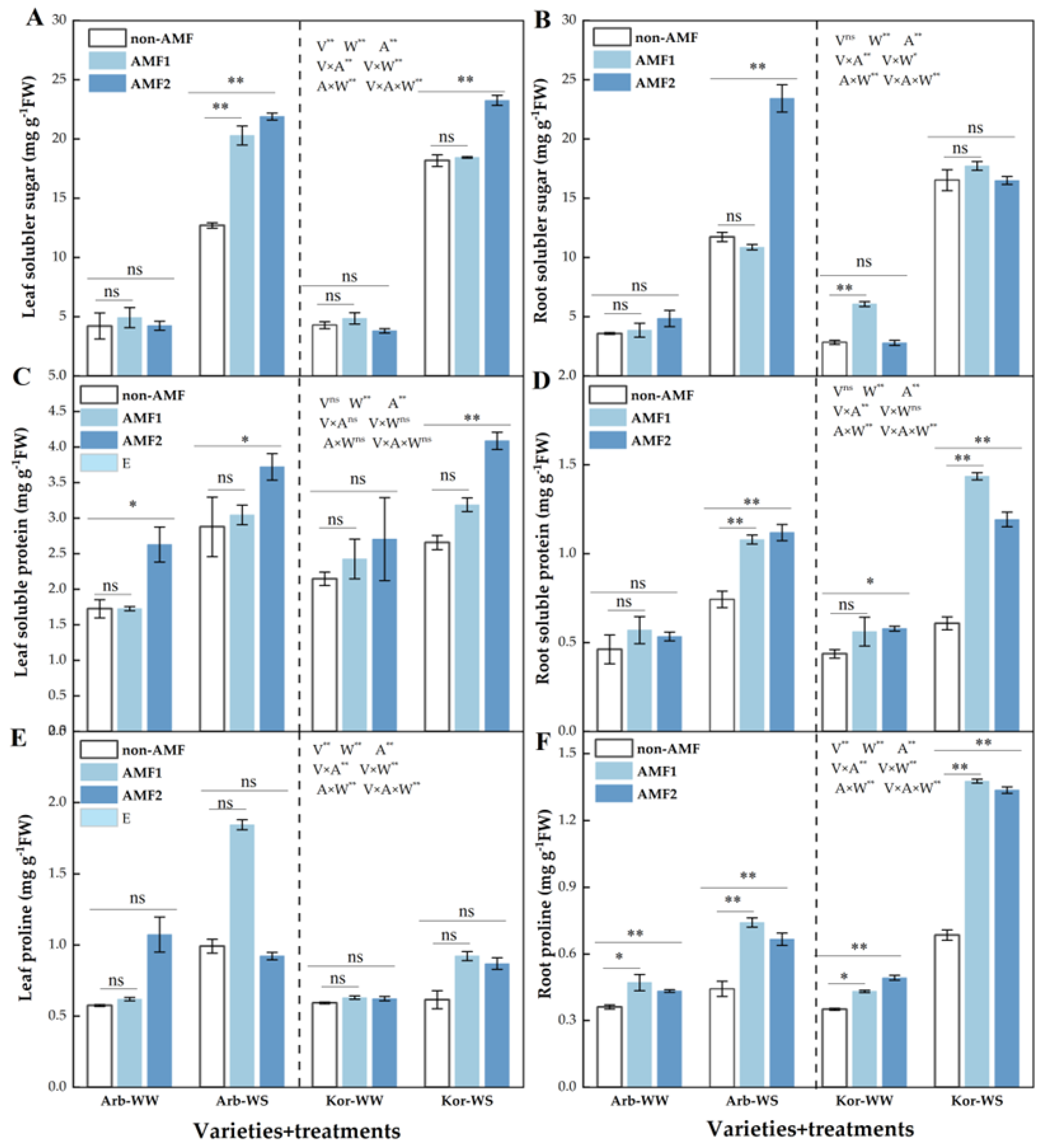
3.5. Antioxidant Enzymes and Osmoregulatory Substances
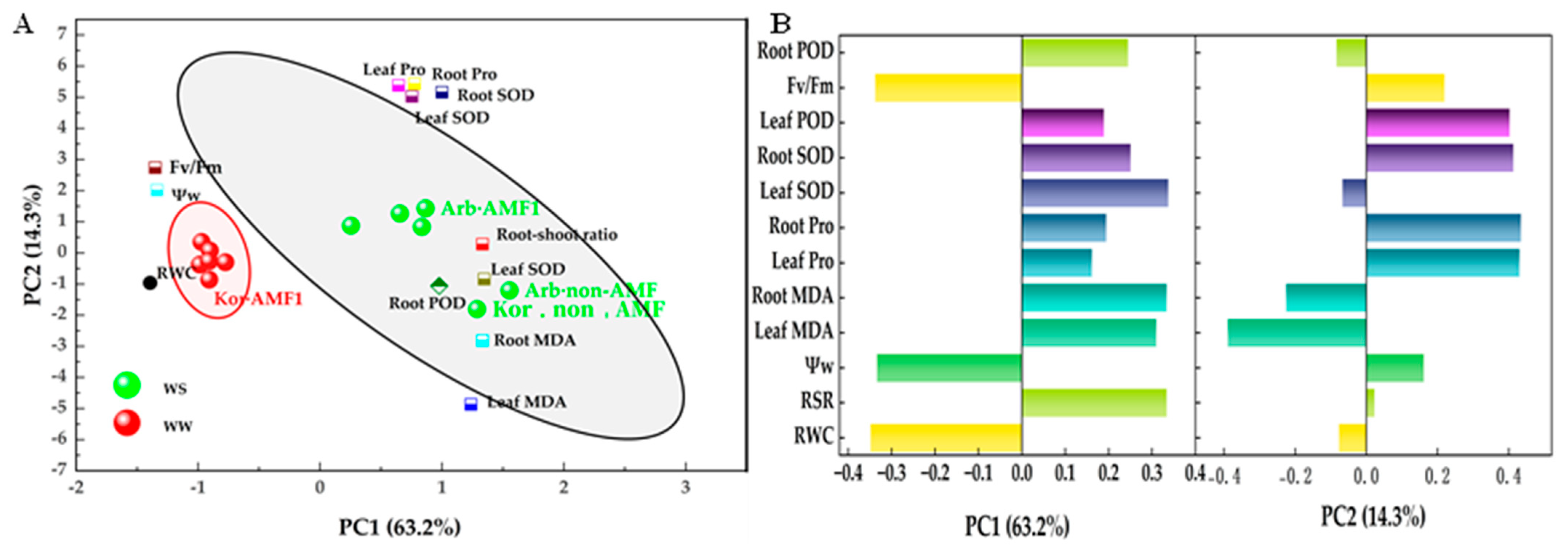
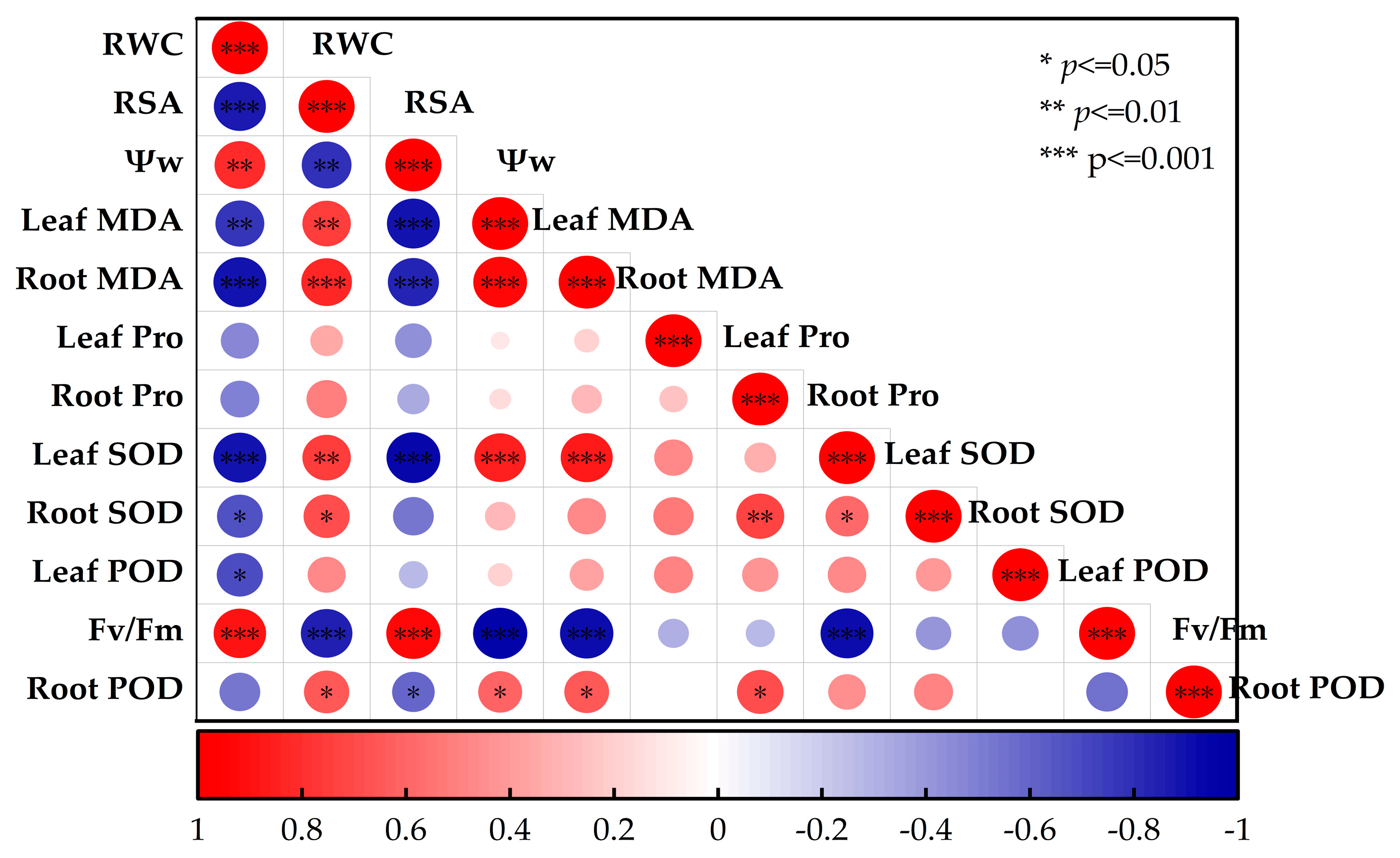
3.6. Principal Component and Correlation Analyses
4. Discussion
4.1. AMF Establishes a Symbiotic Relationship with Two Olive Varieties Under Different Water Conditions
4.2. Effects of AMF on Biomass Allocation and Root Growth in Two Olive Varieties Under Different Water Conditions
4.3. Effects of AMF on Photosynthesis, Fluorescence, and Water Status in Two Olive Varieties Under Different Water Conditions
4.4. Effects of AMF on Photosynthetic Pigments and Nutrient Elements in Two Olive Varieties Under Different Water Conditions
4.5. Effects of AMF on Antioxidant Enzymes and Osmoregulatory Substances in Two Olive Varieties Under Different Water Conditions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMF | Arbuscular mycorrhizal fungi |
| AMF1 | Rhizophagus intraradices |
| AMF2 | Funneliformis mosseae |
| Non-AMF | Non-mycorrhizal |
| WW | Well-watered |
| WS | Water-stressed |
| FC | Field capacity |
| Arb | Arbequina |
| Kor | Koroneiki |
| Arb-WW | Arbequina varieties with well-watered treatment |
| Kor-WW | Koroneiki varieties with well-watered treatment |
| Arb-WS | Arbequina varieties with water-stressed treatment |
| Kor-WS | Koroneiki varieties with water-stressed treatment |
| AFW | Aboveground fresh weight |
| UFW | Underground fresh weight |
| ADW | Aboveground dry weights |
| UDW | Underground dry weights |
| Pn | Photosynthetic rate |
| Ci | Intercellular CO2 concentration |
| Gs | Stomatal conductance |
| Tr | Transpiration rate |
| RWC | Relative water content |
| Ψw | Leaf water potential |
| Fv/Fm | Maximum photochemical efficiency of photosystem II |
| Chla | Chlorophyll a |
| Chlb | Chlorophyll b |
| C | Carbon |
| N | Nitrogen |
| MDA | Malondialdehyde |
| SOD | Superoxide dismutase |
| POD | Peroxidase |
| PCA | Principal component analysis |
References
- Gholami, R.; Fahadi Hoveizeh, N.; Zahedi, S.M.; Gholami, H.; Carillo, P. Effect of three water-regimes on morpho-physiological, biochemical and yield responses of local and foreign olive cultivars under field conditions. BMC Plant Biol. 2022, 22, 477. [Google Scholar] [CrossRef]
- Wang, B.; Shen, S.; Qu, J.; Xu, Z.; Feng, S.; Chen, T.; Ding, C.J.A. Optimizing total phenolic and oleuropein of Chinese olive (Olea europaea) leaves for enhancement of the phenols content and antioxidant activity. Agronomy 2021, 11, 686. [Google Scholar] [CrossRef]
- Mustapha, M.; Zineddine, M. An evaluative technique for drought impact on variation in agricultural LULC using remote sensing and machine learning. Environ. Monit. Assess. 2024, 196, 515. [Google Scholar] [CrossRef] [PubMed]
- Tangu, N.A. Effects on plant morphology of drought in olive. Turk. J. Agric. Nat. Sci. 2014, 1, 900–904. [Google Scholar]
- Kumar, A.; Sharma, N. Characterization of olive cultivars for drought tolerance potential under rainfed conditions of Himachal Pradesh. Indian J. Agric. Res. 2016, 50, 440–445. [Google Scholar]
- Hartmann, H. Olive flower-bud formation: Nutrients essential to tree during March and April when flower-buds are forming tests show. Calif. Agric. 1950, 4, 4–16. [Google Scholar]
- Gucci, R.; Lodolini, E.M.; Rapoport, H.F. Water deficit-induced changes in mesocarp cellular processes and the relationship between mesocarp and endocarp during olive fruit development. Tree Physiol. 2009, 29, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Lan, Y.; Tan, F.; Tu, Y.; Sun, Y.; Yougu, G.; Yang, Z.; Ding, C.; Li, T. Drip irrigation elevated olive productivity in Southwest China. HortTechnology 2019, 29, 122–127. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C.M. Drought stress effects and olive tree acclimation under a changing climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef]
- Majikumna, K.U.; Zineddine, M.; El Hilali Alaoui, A. Olive tree drought stress: A systematic review. J. Water Clim. Change 2024, 15, 5741–5762. [Google Scholar] [CrossRef]
- Bacelar, E.; Pinto, T.; Anjos, R.; Morais, M.C.; Oliveira, I.; Vilela, A.; Cosme, F. Impacts of climate change and mitigation strategies for some abiotic and biotic constraints influencing fruit growth and quality. Plants 2024, 13, 1942. [Google Scholar] [CrossRef]
- Boughalleb, F.; Hajlaoui, H. Physiological and anatomical changes induced by drought in two olive cultivars (cv Zalmati and Chemlali). Acta Physiol. Plant. 2011, 33, 53–65. [Google Scholar] [CrossRef]
- Parri, S. Drought and the Olive Tree in a Changing Climate: A Multi-Level Response Characterisation to Explore and Valorise Italian Cultivars. Ph.D. Thesis, Università degli Studi di Siena, Siena, Italy, 2024. [Google Scholar]
- Karimi, S.; Rahemi, M.; Rostami, A.A.; Sedaghat, S. Drought effects on growth, water content and osmoprotectants in four olive cultivars with different drought tolerance. Int. J. Fruit Sci. 2018, 18, 254–267. [Google Scholar] [CrossRef]
- Azimi, M.; Taheri, M.; Khoshzaman, T. Effect of drought stress on growth characteristics, osmolyte accumulation, and nutrient uptake of some olive (Olea europaea L.) cultivars. Int. J. Hortic. Sci. Technol. 2026, 13, 19–32. [Google Scholar]
- Denaxa, N.-K.; Damvakaris, T.; Roussos, P.A. Antioxidant defense system in young olive plants against drought stress and mitigation of adverse effects through external application of alleviating products. Sci. Hortic. 2020, 259, 108812. [Google Scholar] [CrossRef]
- Abdallah, M.B.; Methenni, K.; Nouairi, I.; Zarrouk, M.; Youssef, N.B. Drought priming improves subsequent more severe drought in a drought-sensitive cultivar of olive cv. Chétoui. Sci. Hortic. 2017, 221, 43–52. [Google Scholar] [CrossRef]
- Sbbar, N.; Lahbouki, S.; Er-Raki, S.; Douira, A.; El Bakkali, A.; Boutasknit, A.; El Modafar, C.; Meddich, A. Beneficial microorganisms: A sustainable strategy to enhance morpho-physiological traits in drought-tolerant olive cultivars. Plant Biosyst. 2025, 159, 191–203. [Google Scholar] [CrossRef]
- Qu, J.; Chen, Z.; Wang, B.; Feng, S.; Tong, Z.; Chen, T.; Zhou, L.; Peng, Z.; Ding, C. Molecular mechanisms regulating the oil biosynthesis in olive (Olea europaea L.) fruits revealed by transcriptomic analysis. Agronomy 2022, 12, 2718. [Google Scholar] [CrossRef]
- Ruano-Rosa, D.; Valverde-Corredor, A.; Gómez-Lama Cabanás, C.; Sesmero, R.; Mercado-Blanco, J. What lies beneath: Root-associated bacteria to improve the growth and health of olive trees. In Soil Biological Communities and Ecosystem Resilience; Springer: Cham, Switzerland, 2017; pp. 107–122. [Google Scholar]
- Maksoud, M.; El-Shamma, M.; Saleh, M.A.; Zaied, N.S.; Hafez, O.M. Effect of different compost sorts and biofertilizers on chemlali olive trees grown in calcareous soil. Middle-East J. Sci. Res. 2012, 12, 1046–1049. [Google Scholar]
- Golubkina, N.; Krivenkov, L.; Sekara, A.; Vasileva, V.; Tallarita, A.; Caruso, G. Prospects of arbuscular mycorrhizal fungi utilization in production of Allium plants. Plants 2020, 9, 279. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic press: Cambridge, MA, USA, 2010. [Google Scholar]
- Guo, X.; Wang, P.; Wang, X.; Li, Y.; Ji, B. Specific plant mycorrhizal responses are linked to mycorrhizal fungal species interactions. Front. Plant Sci. 2022, 13, 930069. [Google Scholar] [CrossRef]
- Sadhana, B. Arbuscular mycorrhizal fungi (AMF) as a biofertilizer-a review. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 384–400. [Google Scholar]
- Jamiołkowska, A.; Księżniak, A.; Gałązka, A.; Hetman, B.; Kopacki, M.; Skwaryło-Bednarz, B. Impact of abiotic factors on development of the community of arbuscular mycorrhizal fungi in the soil: A review. Int. Agrophys. 2018, 32, 133–140. [Google Scholar] [CrossRef]
- Wahid, F.; Fahad, S.; Danish, S.; Adnan, M.; Yue, Z.; Saud, S.; Siddiqui, M.H.; Brtnicky, M.; Hammerschmiedt, T.; Datta, R. Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture 2020, 10, 334. [Google Scholar] [CrossRef]
- Gai, J.; Christie, P.; Feng, G.; Li, X. Twenty years of research on community composition and species distribution of arbuscular mycorrhizal fungi in China: A review. Mycorrhiza 2006, 16, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Parihar, M.; Rakshit, A.; Meena, V.S.; Gupta, V.K.; Rana, K.; Choudhary, M.; Tiwari, G.; Mishra, P.K.; Pattanayak, A.; Bisht, J.K. The potential of arbuscular mycorrhizal fungi in C cycling: A review. Arch. Microbiol. 2020, 202, 1581–1596. [Google Scholar] [CrossRef] [PubMed]
- Lone, R.; Shuab, R.; Koul, K. AMF association and their effect on metabolite mobilization, mineral nutrition and nitrogen assimilating enzymes in saffron (Crocus sativus) plant. J. Plant Nutr. 2016, 39, 1852–1862. [Google Scholar] [CrossRef]
- Borde, M.; Dudhane, M.; Kulkarni, M. Role of arbuscular mycorrhizal fungi (AMF) in salinity tolerance and growth response in plants under salt stress conditions. In Mycorrhiza-Eco-Physiology, Secondary Metabolites, Nanomaterials; Springer: Cham, Switzerland, 2017; pp. 71–86. [Google Scholar]
- Bahadur, A.; Batool, A.; Nasir, F.; Jiang, S.; Mingsen, Q.; Zhang, Q.; Pan, J.; Liu, Y.; Feng, H. Mechanistic insights into arbuscular mycorrhizal fungi-mediated drought stress tolerance in plants. Int. J. Mol. Sci. 2019, 20, 4199. [Google Scholar] [CrossRef]
- Abdalla, M.; Bitterlich, M.; Jansa, J.; Püschel, D.; Ahmed, M.A. The role of arbuscular mycorrhizal symbiosis in improving plant water status under drought. J. Exp. Bot. 2023, 74, 4808–4824. [Google Scholar] [CrossRef]
- Yang, G.; Liu, N.; Lu, W.; Wang, S.; Kan, H.; Zhang, Y.; Xu, L.; Chen, Y. The interaction between arbuscular mycorrhizal fungi and soil phosphorus availability influences plant community productivity and ecosystem stability. J. Ecol. 2014, 102, 1072–1082. [Google Scholar] [CrossRef]
- Gao, X.; Liu, Y.; Liu, C.; Guo, C.; Zhang, Y.; Ma, C.; Duan, X. Individual and combined effects of arbuscular mycorrhizal fungi and phytohormones on the growth and physiobiochemical characteristics of tea cutting seedlings. Front. Plant Sci. 2023, 14, 1140267. [Google Scholar] [CrossRef]
- Powell, J.R.; Rillig, M.C. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef] [PubMed]
- Melloni, R.; Cardoso, E.J. Microbiome associated with olive cultivation: A review. Plants 2023, 12, 897. [Google Scholar] [CrossRef]
- Ouledali, S.; Ennajeh, M.; Zrig, A.; Gianinazzi, S.; Khemira, H. Estimating the contribution of arbuscular mycorrhizal fungi to drought tolerance of potted olive trees (Olea europaea). Acta Physiol. Plant. 2018, 40, 81. [Google Scholar] [CrossRef]
- Khabou, W.; Hajji, B.; Zouari, M.; Rigane, H.; Abdallah, F.B. Arbuscular mycorrhizal fungi improve growth and mineral uptake of olive tree under gypsum substrate. Ecol. Eng. 2014, 73, 290–296. [Google Scholar] [CrossRef]
- Chenchouni, H.; Mekahlia, M.N.; Beddiar, A. Effect of inoculation with native and commercial arbuscular mycorrhizal fungi on growth and mycorrhizal colonization of olive (Olea europaea L.). Sci. Hortic. 2020, 261, 108969. [Google Scholar] [CrossRef]
- Phillips, J.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158-IN118. [Google Scholar] [CrossRef]
- Barrs, H.; Weatherley, P. A re-examination of the relative turgidity technique for estimating water deficits in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1. [Google Scholar] [CrossRef]
- Gao, W.T.; Zhang, C.Y.; Dong, T.F.; Xu, X. Effects of arbuscular mycorrhizal fungi on the root growth of male and female Populus cathayana individuals grown under different sexual combination patterns. Chin. J. Plant Ecol. 2019, 43, 37–45. [Google Scholar]
- Mocquot, B.; Vangronsveld, J.; Clijsters, H.; Mench, M. Copper toxicity in young maize (Zea mays L.) plants: Effects on growth, mineral and chlorophyll contents, and enzyme activities. Plant Soil 1996, 182, 287–300. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Du, Z.; Bramlage, W.J. Modified thiobarbituric acid assay for measuring lipid oxidation in sugar-rich plant tissue extracts. J. Agric. Environ. Ethics 1992, 40, 1566–1570. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Wang, W.; Chen, J.; Zhu, L. Polybrominated diphenyl ethers and polychlorinated biphenyls induced rice” diabetes” by disturbing the transport and decomposition of soluble sugars. Environ. Pollut. 2024, 358, 124523. [Google Scholar] [CrossRef]
- Eris, A.; Gulen, H.; Barut, E.; Asuman, C. Annual patterns of total soluble sugars and proteins related to coldhardiness in olive (Olea europaea L.‘Gemlik’). Hortic. Sci. Biotechnol. 2007, 82, 597–604. [Google Scholar] [CrossRef]
- Poury, N.; Seifi, E.; Alizadeh, M. Effects of salinity and proline on growth and physiological characteristics of three olive cultivars. Gesunde Pflanz. 2023, 75, 1169–1180. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Zhang, L.J.E. AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Robã, B.; Robã; Abdelghafour, D.; Hafidh, Z.; Soufan, W.; Fathi, A.; Abdellatif, B. Arbuscular mycorirrhizal response of five olive (Olea Europaea L.) introduced to an arid zone in Algeria. Sustain. Dev. 2024, 14, 335–354. [Google Scholar]
- Chen, J.; Xie, J.; Tang, M. Effects of arbuscular mycorrhizal fungi on the growth and drought resistance of Amorpha fruticosa under water stress. J. Beijing For. Univ. 2014, 36, 142–148. [Google Scholar]
- Ji, L.; Tan, W.; Chen, X. Arbuscular mycorrhizal mycelial networks and glomalin-related soil protein increase soil aggregation in Calcaric Regosol under well-watered and drought stress conditions. Soil Tillage Res. 2019, 185, 1–8. [Google Scholar] [CrossRef]
- Huang, D.; Ma, M.; Wang, Q.; Zhang, M.; Jing, G.; Li, C.; Ma, F. Arbuscular mycorrhizal fungi enhanced drought resistance in apple by regulating genes in the MAPK pathway. Plant Physiol. Biochem. 2020, 149, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Golubkina, N.; Gomez, L.D.; Kekina, H.; Cozzolino, E.; Simister, R.; Tallarita, A.; Torino, V.; Koshevarov, A.; Cuciniello, A.; Maiello, R. Joint selenium–iodine supply and arbuscular mycorrhizal fungi inoculation affect yield and quality of chickpea seeds and residual biomass. Plants 2020, 9, 804. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Meng, P.; Feng, H.; Wang, C. Effects of arbuscular mycorrhizal fungi on growth and physiological performance of Catalpa bungei CAMey. under drought stress. Forests 2020, 11, 1117. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, L.; Chen, K.; Sun, H.; Wang, P. Effects of arbuscular mycorrhizal fungi on the growth and physiological performance of Sophora davidii seedling under low-phosphorus stress. J. Plant Growth Regul. 2024, 43, 2383–2395. [Google Scholar] [CrossRef]
- Zhang, F.; Zou, D.; Wang, J.; Xiong, B.; Gao, L.; Guo, P.; Du, H.; Ma, M.; Rennenberg, H. Co-inoculation of rhizobia and AMF improves growth, nutrient uptake, and cadmium resistance of black locust grown in sand culture. Physiol. Plant 2024, 176, e14205. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Huang, Y. Effects of arbuscular mycorrhizal fungi on the drought tolerance of Cyclobalanopsis glauca seedlings under greenhouse conditions. New For. 2014, 45, 545–556. [Google Scholar] [CrossRef]
- Aalipour, H.; Nikbakht, A.; Etemadi, N.; Rejali, F.; Soleimani, M. Biochemical response and interactions between arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria during establishment and stimulating growth of Arizona cypress (Cupressus arizonica G.) under drought stress. Sci. Hortic. 2020, 261, 108923. [Google Scholar] [CrossRef]
- Guerfel, M.; Baccouri, O.; Boujnah, D.; Zarrouk, M. Changes in lipid composition, water relations and gas exchange in leaves of two young ‘Chemlali’and ‘Chetoui’olive trees in response to water stress. Plant Soil 2008, 311, 121–129. [Google Scholar] [CrossRef]
- Boutaj, H.; Meddich, A.; Wahbi, S.; El Alaoui-Talibi, Z.; Douira, A.; Filali-Maltouf, A.; El Modafar, C. Mycorrhizal fungus Rhizophagus irregularis mitigates symptoms of Verticillium wilt and enhances growth of olive trees (Olea europaea L.). Plant Pathol. 2025, 172, 337–353. [Google Scholar] [CrossRef]
- Bizos, G.; Papatheodorou, E.M.; Chatzistathis, T.; Ntalli, N.; Aschonitis, V.G.; Monokrousos, N. The role of microbial inoculants on plant protection, growth stimulation, and crop productivity of the olive tree (Olea europea L.). Plants 2020, 9, 743. [Google Scholar] [CrossRef]
- Ye, Q.; Wang, H.; Li, H. Arbuscular mycorrhizal fungi improve growth, photosynthetic activity, and chlorophyll fluorescence of Vitis vinifera L. cv. Ecolly under drought stress. Agronomy 2022, 12, 1563. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, M.; Cofré, N.; Calvo, F.; Silvente, S. Effects of arbuscular mycorrhizal fungi in the rhizosphere of two olive (Olea europaea) varieties Arbequina and Barnea under water deficit conditions. Funct. Plant Biol. 2024, 51, FP24108. [Google Scholar] [CrossRef]
- Baslam, M.; Esteban, R.; García-Plazaola, J.I.; Goicoechea, N. Effectiveness of arbuscular mycorrhizal fungi (AMF) for inducing the accumulation of major carotenoids, chlorophylls and tocopherol in green and red leaf lettuces. Appl. Microbiol. Biotechnol. 2013, 97, 3119–3128. [Google Scholar] [CrossRef]
- Qin, W.; Yan, H.; Zou, B.; Guo, R.; Ci, D.; Tang, Z.; Zou, X.; Zhang, X.; Yu, X.; Wang, Y. Arbuscular mycorrhizal fungi alleviate salinity stress in peanut: Evidence from pot-grown and field experiments. Food Energy Secur. 2021, 10, e314. [Google Scholar] [CrossRef]
- Sun, T.; Li, L. Toward the ‘golden’ era: The status in uncovering the regulatory control of carotenoid accumulation in plants. Plant Sci. 2020, 290, 110331. [Google Scholar] [CrossRef]
- Tekaya, M.; Mechri, B.; Mbarki, N.; Cheheb, H.; Hammami, M.; Attia, F. Arbuscular mycorrhizal fungus Rhizophagus irregularis influences key physiological parameters of olive trees (Olea europaea L.) and mineral nutrient profile. Photosynthetica 2017, 55, 308–316. [Google Scholar] [CrossRef]
- Zaferanchi, S.; Salmasi, S.Z.; Salehi Lisar, S.Y.; Sarikhani, M.R. Influence of organics and bio fertilizers on biochemical properties of Calendula officinalis L. Int. J. Hortic. Sci. Technol. 2019, 6, 125–136. [Google Scholar]
- He, Z.; He, C.; Zhang, Z.; Zou, Z.; Wang, H. Changes of antioxidative enzymes and cell membrane osmosis in tomato colonized by arbuscular mycorrhizae under NaCl stress. Colloids Surf. B Biointerfaces 2007, 59, 128–133. [Google Scholar] [CrossRef]
- Amini, Z. Effects of water deficit on proline content and activity of antioxidant enzymes among three olive (Olea europaea L.) cultivars. J. Plant Res. 2014, 27, 156–167. [Google Scholar]
- Fouad, M.O.; Essahibi, A.; Benhiba, L.; Qaddoury, A. Effectiveness of arbuscular mycorrhizal fungi in the protection of olive plants against oxidative stress induced by drought. Span. J. Agric. Res. 2014, 12, 763–771. [Google Scholar] [CrossRef]
- Seifi, E.; Teymoor, Y.S.; Alizadeh, M.; Fereydooni, H. Olive mycorrhization: Influences of genotype, mycorrhiza, and growing periods. Sci. Hortic. 2014, 180, 214–219. [Google Scholar] [CrossRef]
- del Mar Alguacil, M.; Torrecillas, E.; Kohler, J.; Roldán, A. A molecular approach to ascertain the success of “in situ” AM fungi inoculation in the revegetation of a semiarid, degraded land. Sci. Total Environ. 2011, 409, 2874–2880. [Google Scholar] [CrossRef] [PubMed]
- Salimonti, A.; Lucchese, P.G.; Benincasa, C.; Desando, M.; Nicoletti, R.; Santilli, E.; Lodolini, E.M.; Mercati, F.; Sunseri, F.; Carbone, F. Uncovering alternative physiological and molecular strategies to cope with water stress in olive tree. Plant J. 2025, 123, 70362. [Google Scholar] [CrossRef] [PubMed]

| Treatment Group | AMF Status | Water Regime | FC (%) |
|---|---|---|---|
| AMF1 + WW | Gl. intraradices | Well-watered (WW) | 80% |
| AMF1 + WS | G. intraradices | Water-stressed (WS) | 30% |
| AMF2 + WW | F. mosseae | Well-watered (WW) | 80% |
| AMF2 + WS | F. mosseae | Water-stressed (WS) | 30% |
| Non-AMF + WW | None | Well-watered (WW) | 80% |
| Non-AMF + WS | None | Water-stressed (WS) | 30% |
| Treatments | Height (cm) | Diameter (mm) | AFW (g) | UFW (g) | ADW (g) | UDW (g) | Root–Shoot Ratio | |
|---|---|---|---|---|---|---|---|---|
| non-AMF | Arb-WW | 90.03 ± 3.19 abc | 9.00 ± 0.37 b | 90.11 ± 2.16 b | 16.59 ± 1.85 abc | 32.76 ± 0.45 ab | 9.62 ± 0.82 ab | 0.35 ± 0.03 abc |
| Kor-WW | 95.65 ± 5.03 ab | 7.92 ± 0.29 bcd | 69.40 ± 2.70 cd | 9.91 ± 1.08 cd | 26.70 ± 1.43 bc | 5.12 ± 0.32 b | 0.27 ± 0.02 bc | |
| Arb-WS | 51.35 ± 3.19 f | 6.92 ± 0.33 d | 40.56 ± 2.90 e | 9.97 ± 1.76 cd | 18.71 ± 2.65 cd | 7.20 ± 1.73 ab | 0.52 ± 0.13 a | |
| Kor-WS | 71.60 ± 3.85 cde | 7.18 ± 0.60 cd | 39.44 ± 8.85 e | 6.38 ± 0.11 d | 14.89 ± 3.41 d | 6.25 ± 1.47 b | 0.43 ± 0.09 ab | |
| AMF1 | Arb-WW | 77.4 ± 3.21 bcde | 8.81 ± 0.83 bc | 122.00 ± 9.56 a | 20.80 ± 4.01 a | 40.84 ± 3.63 a | 10.05 ± 1.07 ab | 0.21 ± 0.01 c |
| Kor-WW | 71.77 ± 5.45 cde | 8.53 ± 0.55 bcd | 72.54 ± 10.41 bc | 20.04 ± 6.31 ab | 32.23 ± 7.08 ab | 11.72 ± 4.69 a | 0.26 ± 0.05 bc | |
| Arb-WS | 64.48 ± 4.26 def | 7.88 ± 0.28 bcd | 52.62 ± 3.18 cde | 12.09 ± 1.84 bcd | 23.21 ± 2.70 bcd | 6.76 ± 0.65 b | 0.43 ± 0.04 ab | |
| Kor-WS | 66.83 ± 8.31 def | 7.01 ± 0.52 d | 42.05 ± 1.57 e | 9.34 ± 1.37 cd | 20.67 ± 0.96 cd | 5.94 ± 0.99 b | 0.43 ± 0.04 ab | |
| AMF2 | Arb-WW | 82.93 ± 3.95 abcd | 9.18 ± 0.89 ab | 70.01 ± 2.61 bc | 13.84 ± 0.36 abcd | 26.48 ± 2.05 bc | 6.97 ± 0.58 b | 0.21 ± 0.02 c |
| Kor-WW | 98.40 ± 11.04 a | 10.56 ± 0.61 a | 94.60 ± 9.48 b | 16.98 ± 1.79 abc | 32.86 ± 4.82 ab | 8.39 ± 1.67 ab | 0.33 ± 0.07 bc | |
| Arb-WS | 62.33 ± 11.17 ef | 7.27 ± 0.30 cd | 44.65 ± 6.15 e | 10.21 ± 0.85 cd | 20.81 ± 3.04 cd | 6.50 ± 0.61 b | 0.39 ± 0.06 abc | |
| Kor-WS | 82.45 ± 4.00 abcde | 7.93 ± 0.27 bcd | 50.62 ± 2.92 de | 12.21 ± 1.69 bcd | 24.83 ± 1.61 bcd | 8.71 ± 0.81 ab | 0.45 ± 0.01 ab | |
| V | ** | ns | ** | ns | ns | ns | ns | |
| A | ** | ** | ** | * | * | * | ns | |
| W | ** | ** | ** | ** | ** | ** | ** | |
| V × A | ** | ns | ** | * | ns | ** | ns | |
| V × W | ns | ns | ** | * | ns | ns | ns | |
| A × W | ** | ns | ** | * | * | ** | ns | |
| V × A × W | ns | ns | * | ns | ns | ns | ns | |
| Treatments | Surface Area of Root (dm2) | Total Root Length (dm) | Roots Volume (cm3) | Number of Root Tips (×1000) | Average Diameter (mm) | |
|---|---|---|---|---|---|---|
| non-AMF | Arb-WW | 2.52 ± 0.20 cd | 12.82 ± 1.02 b | 11.40 ± 1.39 b | 1.93 ± 0.09 cd | 0.50 ± 0.03 bc |
| Kor-WW | 3.30 ± 0.22 ab | 16.40 ± 0.20 a | 15.69 ± 1.02 a | 2.25 ± 0.17 c | 0.63 ± 0.03 ab | |
| Arb-WS | 1.69 ± 0.09 de | 7.83 ± 0.54 d | 4.35 ± 1.55 c | 1.72 ± 0.16 d | 0.63 ± 0.09 ab | |
| Kor-WS | 1.62 ± 0.23 e | 9.03 ± 1.08 cd | 4.45 ± 0.60 c | 1.61 ± 0.14 d | 0.48 ± 0.05 c | |
| AMF1 | Arb-WW | 2.89 ± 0.40 bc | 16.47 ± 0.80 a | 14.16 ± 0.96 a | 3.56 ± 0.14 a | 0.45 ± 0.03 c |
| Kor-WW | 2.76 ± 0.36 bcd | 12.21 ± 1.59 b | 11.42 ± 2.23 b | 1.76 ± 0.31 d | 0.58 ± 0.03 abc | |
| Arb-WS | 2.31 ± 0.26 cde | 11.36 ± 0.79 bc | 9.80 ± 0.93 b | 1.57 ± 0.04 d | 0.54 ± 0.03 bc | |
| Kor-WS | 2.12 ± 0.10 cde | 11.42 ± 1.22 bc | 9.48 ± 0.12 b | 1.92 ± 0.18 cd | 0.51 ± 0.02 bc | |
| AMF2 | Arb-WW | 1.91 ± 0.10 cde | 11.76 ± 0.74 bc | 10.50 ± 0.49 b | 2.22 ± 0.20 c | 0.45 ± 0.03 c |
| Kor-WW | 3.66 ± 0.07 a | 16.86 ± 0.34 a | 16.81 ± 0.17 a | 2.73 ± 0.11 b | 0.69 ± 0.01 a | |
| Arb-WS | 1.94 ± 0.08 cde | 11.63 ± 0.34 bc | 9.00 ± 0.45 b | 2.00 ± 0.14 cd | 0.53 ± 0.03 bc | |
| Kor-WS | 2.51 ± 0.09 cd | 12.69 ± 0.59 b | 10.56 ± 0.43 b | 2.38 ± 0.14 b | 0.54 ± 0.01 bc | |
| V | ns | ns | ns | ns | ns | |
| A | ** | ** | ** | ** | * | |
| W | ** | ** | ** | ** | ns | |
| V × A | ns | * | ns | ns | ** | |
| V × W | * | ns | ** | * | * | |
| A × W | ns | ns | * | * | ns | |
| V × A × W | ns | ns | ** | ** | ns | |
| Treatments | Chla (mg g−1) | Chlb (mg g−1) | Total Chl (mg g−1) | Carotenoids (mg g−1) | |
|---|---|---|---|---|---|
| non-AMF | Arb-WW | 1.31 ± 0.09 abc | 0.55 ± 0.04 bc | 1.86 ± 0.11 abc | 0.38 ± 0.03 bcd |
| Kor-WW | 1.31 ± 0.03 abc | 0.44 ± 0.02 cd | 1.75 ± 0.01 bcd | 0.43 ± 0.02 ab | |
| Arb-WS | 1.06 ± 0.09 cd | 0.34 ± 0.02 d | 1.43 ± 0.11 de | 0.25 ± 0.01 e | |
| Kor-WS | 0.96 ± 0.02 d | 0.35 ± 0.07 d | 1.31 ± 0.08 e | 0.29 ± 0.04 de | |
| AMF1 | Arb-WW | 1.44 ± 0.12 a | 0.73 ± 0.04 a | 2.17 ± 0.14 a | 0.47 ± 0.05 ab |
| Kor-WW | 1.34 ± 0.07 ab | 0.53 ± 0.02 c | 1.87 ± 0.08 abc | 0.49 ± 0.02 a | |
| Arb-WS | 1.05 ± 0.15 cd | 0.34 ± 0.04 d | 1.40 ± 0.19 de | 0.29 ± 0.02 de | |
| Kor-WS | 1.17 ± 0.08 abcd | 0.40 ± 0.03 cd | 1.50 ± 0.11 cde | 0.31 ± 0.03 d | |
| AMF2 | Arb-WW | 1.42 ± 0.14 a | 0.67 ± 0.07 ab | 2.09 ± 0.19 ab | 0.40 ± 0.03 bc |
| Kor-WW | 1.33 ± 0.05 ab | 0.53 ± 0.04 c | 1.87 ± 0.03 abc | 0.43 ± 0.02 ab | |
| Arb-WS | 1.08 ± 0.07 bcd | 0.39 ± 0.05 d | 1.47 ± 0.12 de | 0.32 ± 0.01 cde | |
| Kor-WS | 1.06 ± 0.13 cd | 0.30 ± 0.02 d | 1.44 ± 0.14 de | 0.29 ± 0.03 de | |
| V | ns | ** | ns | ns | |
| A | ns | * | ns | ns | |
| W | ** | ** | ** | ** | |
| V × A | ns | ns | ns | ns | |
| V × W | ns | ** | ns | ns | |
| A × W | ns | ns | ns | ns | |
| V × A × W | ns | ns | ns | ns | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Deng, Y.; Li, J.; Xu, Z.; Wang, B.; Xu, X.; Zhao, C. Genotype-Specific Synergy Between Arbuscular Mycorrhizal Fungi and Olive Cultivars Enhances Drought Resilience in China’s Olive Belt. Agronomy 2025, 15, 2568. https://doi.org/10.3390/agronomy15112568
Zhou J, Deng Y, Li J, Xu Z, Wang B, Xu X, Zhao C. Genotype-Specific Synergy Between Arbuscular Mycorrhizal Fungi and Olive Cultivars Enhances Drought Resilience in China’s Olive Belt. Agronomy. 2025; 15(11):2568. https://doi.org/10.3390/agronomy15112568
Chicago/Turabian StyleZhou, Junlin, Yan Deng, Junfei Li, Zhou Xu, Bixia Wang, Xiao Xu, and Chunyan Zhao. 2025. "Genotype-Specific Synergy Between Arbuscular Mycorrhizal Fungi and Olive Cultivars Enhances Drought Resilience in China’s Olive Belt" Agronomy 15, no. 11: 2568. https://doi.org/10.3390/agronomy15112568
APA StyleZhou, J., Deng, Y., Li, J., Xu, Z., Wang, B., Xu, X., & Zhao, C. (2025). Genotype-Specific Synergy Between Arbuscular Mycorrhizal Fungi and Olive Cultivars Enhances Drought Resilience in China’s Olive Belt. Agronomy, 15(11), 2568. https://doi.org/10.3390/agronomy15112568





