UAV-Based Spectral and Thermal Indices in Precision Viticulture: A Review of NDVI, NDRE, SAVI, GNDVI, and CWSI
Abstract
1. Introduction
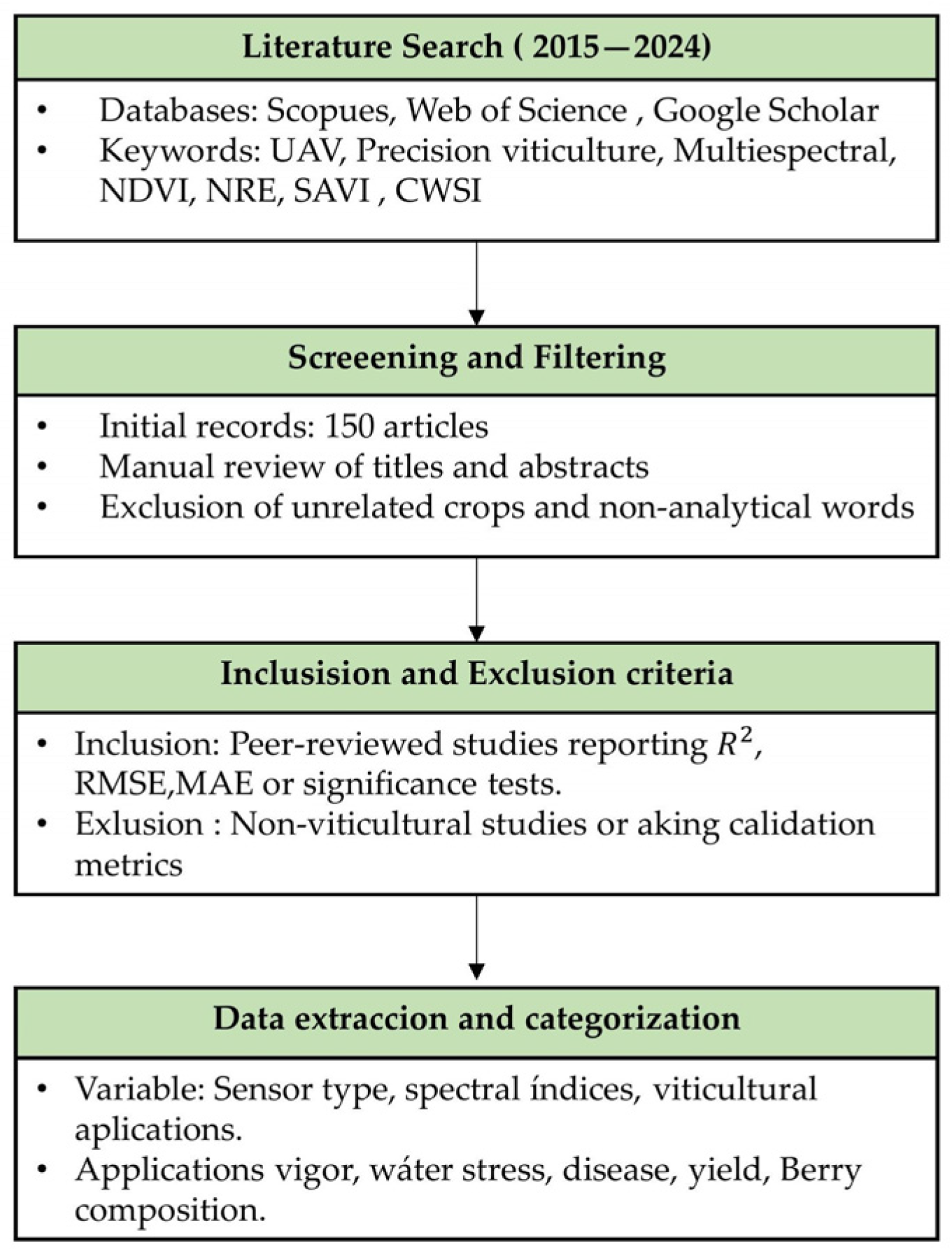
2. Methodology of Literature Selection
3. UAV Data Acquisition and Processing
3.1. General Concepts
3.2. Data Processing

4. Vegetation and Thermal Indices in Precision Viticulture
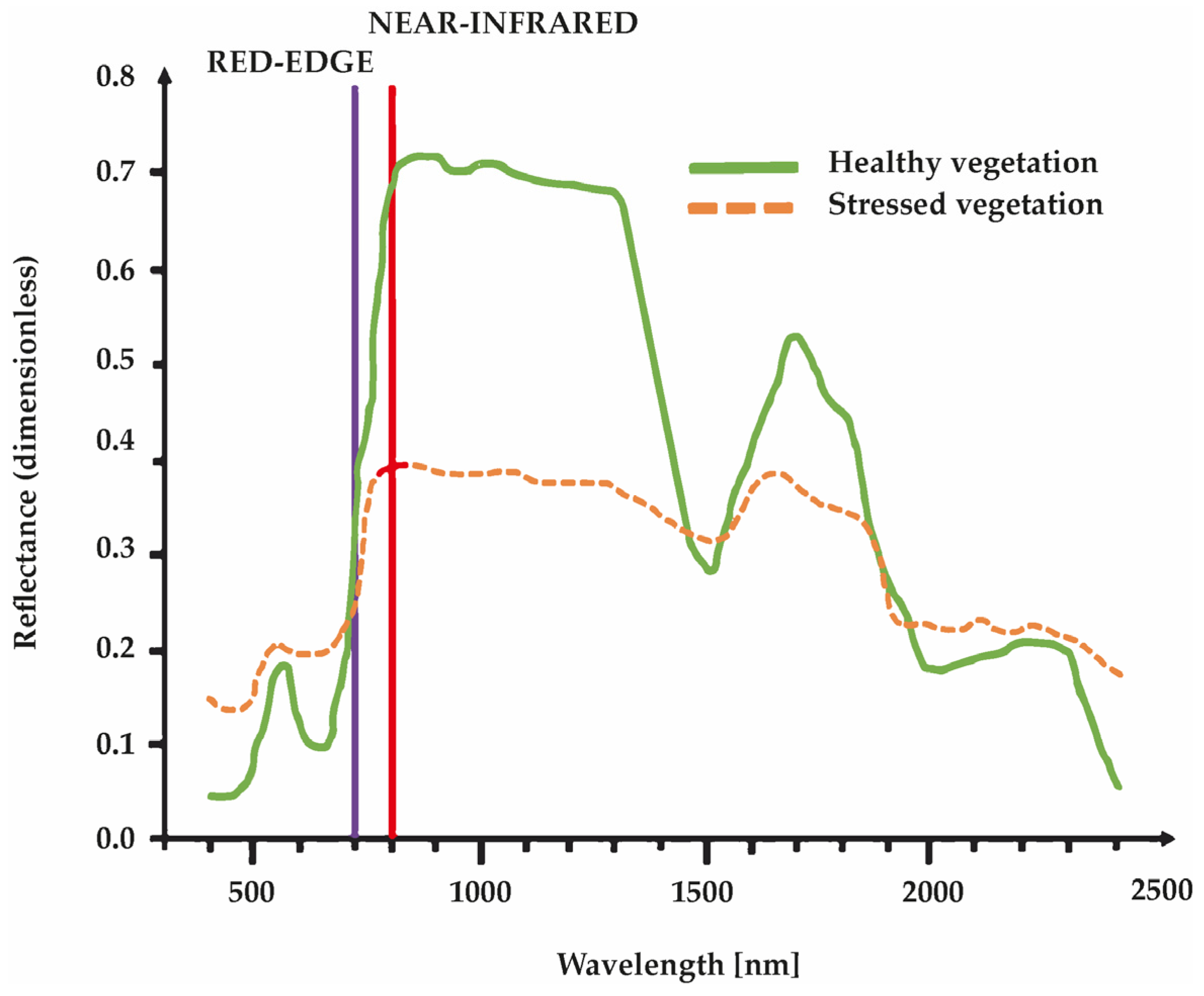
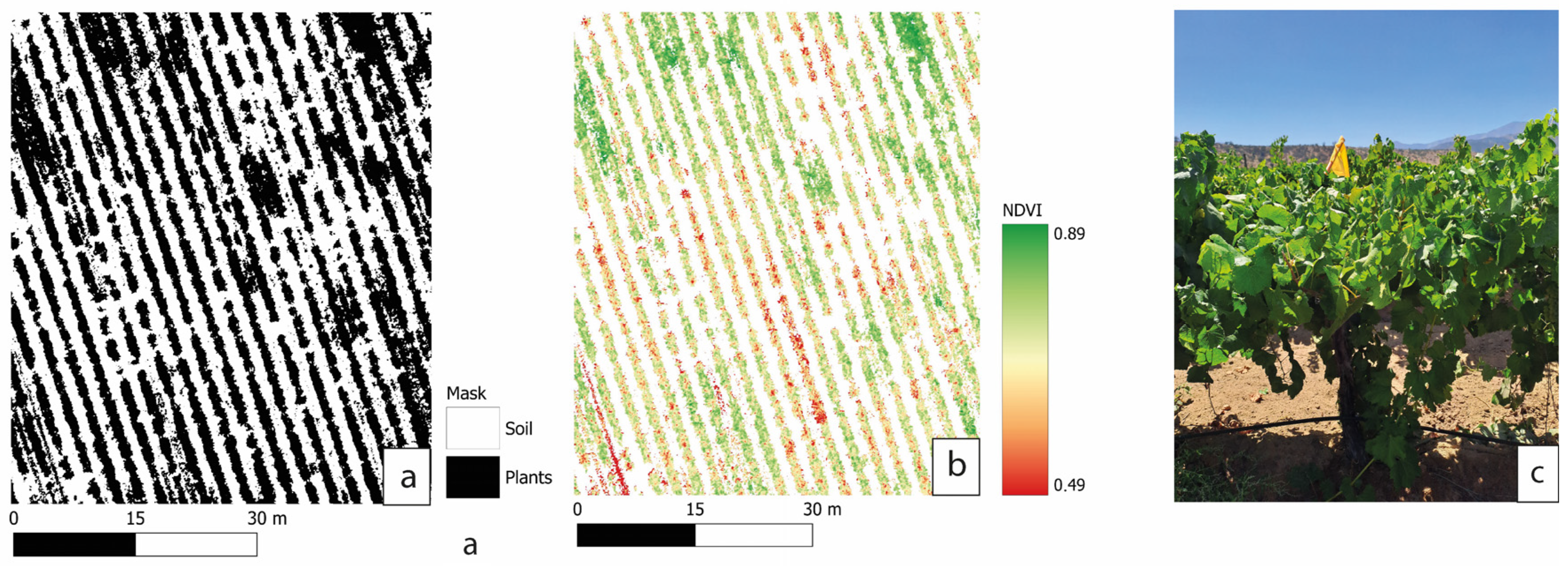
4.1. NDVI
4.2. GNDVI and SAVI
4.3. NDRE
4.4. CWSI
4.5. LAI (Leaf Area Index)
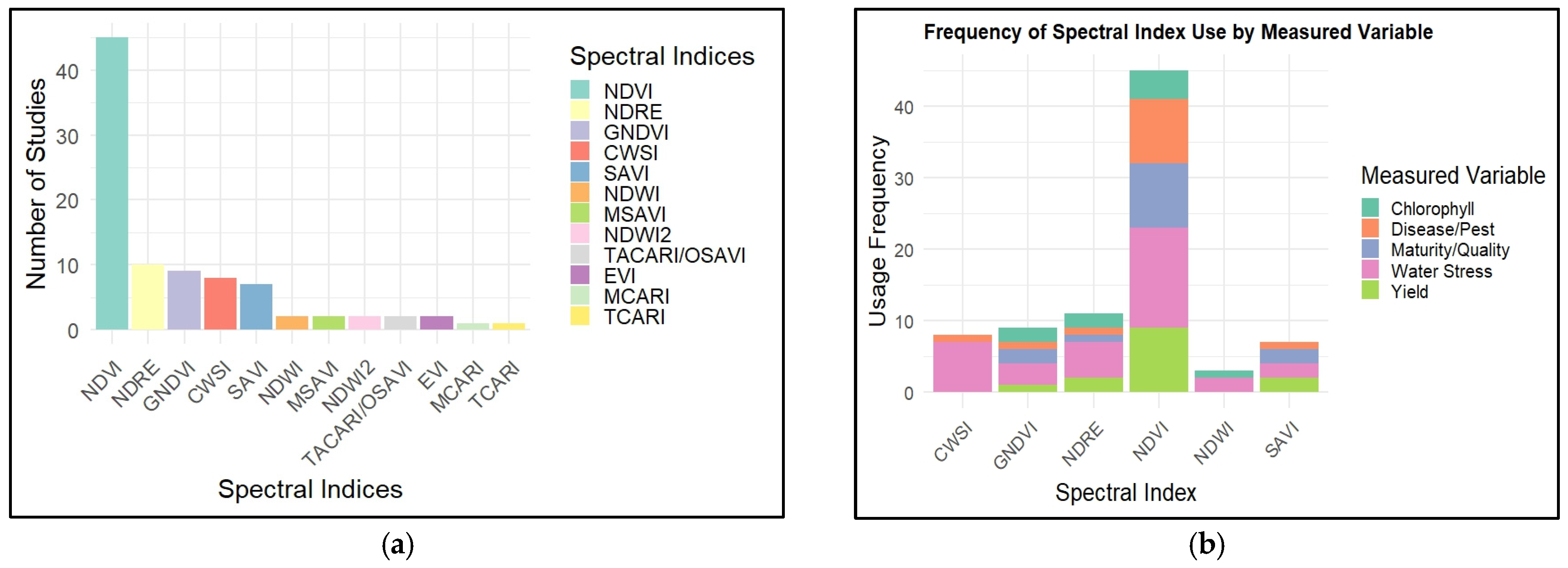
5. Applications of UAV-Based Indices
5.1. Water Stress and Irrigation
5.2. UAV for Pests and Diseases
5.3. UAV Applications in Grape Ripening and Plant Physiology
6. Limitations
7. Future Projections for UAV Implementation
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- International Organisation of Vine and Wine Intergovernmental Organisation. Annual Assessment of the World Vine and Wine Sector in 2022. 2023. Available online: https://www.oiv.int/sites/default/files/documents/OIV_Annual_Assessment-2023.pdf (accessed on 13 February 2024).
- Ugaglia, A.A.; Cardebat, J.-M.; Jiao, L. The French Wine Industry. In The Palgrave Handbook of Wine Industry Economics; Springer International Publishing: Cham, Switherland, 2019. [Google Scholar]
- Gilinsky, P.; Martínez-Falcó, J. The economic, social, and environmental value of the Spanish wine industry. In Handbook of Research on Sustainability Challenges in the Wine Industry; IGI Global Scientific Publishing: Palmdale, PA, USA, 2023; pp. 121–142. [Google Scholar]
- Butiuc-Keul, A.; Coste, A. Biotechnologies and Strategies for Grapevine Improvement. Horticulturae 2023, 9, 62. [Google Scholar] [CrossRef]
- Oliveira, A.F.; Serra, S.; Ligios, V.; Satta, D.; Nieddu, G. Assessing the Effects of Vineyard Soil Management on Downy and and Powdery Mildew Development. Horticulturae 2021, 7, 209. [Google Scholar] [CrossRef]
- Alchanatis, V.; Cohen, Y.; Cohen, S.; Moller, M.; Meron, M.; Tsipris, J.; Orlov, V.; Naor, A.; Charit, Z. Fusion of IR and Multispectral Images in the Visible Range for Empirical and Model Based Mapping of Crop Water Status. In Proceedings of the 2006 ASAE Annual Meeting, Portland, OR, USA, 9–12 July 2006; p. 061171. [Google Scholar]
- Singh, A.P.; Yerudkar, A.; Mariani, V.; Iannelli, L.; Glielmo, L. A Bibliometric Review of the Use of Unmanned Aerial Vehicles in Precision Agriculture and Precision Viticulture for Sensing Applications. Remote Sens. 2022, 14, 1604. [Google Scholar] [CrossRef]
- Buendía, M.R.P.; Martínez, J.M.M. The history of wine and the growing of grapes; as expressed in the Bible. Ensayos 2012, 27, 217–246. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=4202876 (accessed on 15 October 2024).
- Pádua, L.; Adão, T.; Sousa, A.; Peres, E.; Sousa, J.J. Individual Grapevine Analysis in a Multi-Temporal Context Using UAV-Based Multi-Sensor Imagery. Remote Sens. 2020, 12, 139. [Google Scholar] [CrossRef]
- Weiss, M.; Jacob, F.; Duveiller, G. Remote sensing for agricultural applications: A meta-review. Remote Sens. Environ. 2020, 236, 111402. [Google Scholar] [CrossRef]
- Naidu, R.A.; Maree, H.J.; Burger, J.T. Grapevine Leafroll Disease and Associated Viruses: A Unique Pathosystem. Annu. Rev. Phytopathol. 2015, 53, 613–634. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An update on the impact of climate change in viticulture and potential adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Hall, A.; Lamb, D.W.; Holzapfel, B.P.; Louis, J.P. Within-season temporal variation in correlations between vineyard canopy and winegrape composition and yield. Precis. Agric. 2011, 12, 103–117. [Google Scholar] [CrossRef]
- Rejeb, A.; Abdollahi, A.; Rejeb, K.; Treiblmaier, H. Drones in agriculture: A review and bibliometric analysis. Comput. Electron. Agric. 2022, 198, 107017. [Google Scholar] [CrossRef]
- Sassu, A.; Gambella, F.; Ghiani, L.; Mercenaro, L.; Caria, M.; Pazzona, A.L. Advances in unmanned aerial system remote sensing for precision viticulture. Sensors 2021, 21, 956. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.F.; Nemani, R.; Hornbuckle, J.; Bastiaanssen, W.; Thoreson, B.; Tisseyre, B.; Pierce, L. Remote sensing for viticultural research and production. In The Geography of Wine: Regions, Terroir and Techniques; Dougherty, P.H., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 209–226. [Google Scholar] [CrossRef]
- Carroll, J.E.; Wilcox, W.F. Effects of humidity on the development of grapevine powdery mildew. Phytopathology 2003, 93, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Del-Campo-Sanchez, A.; Ballesteros, R.; Hernandez-Lopez, D.; Ortega, J.F.; Moreno, M.A. Quantifying the effect of Jacobiasca lybica pest on vineyards with UAVs by combining geometric and computer vision techniques. PLoS ONE 2019, 14, e0215521. [Google Scholar] [CrossRef] [PubMed]
- Kandylakis, Z.; Falagas, A.; Karakizi, C.; Karantzalos, K. Water Stress Estimation in Vineyards from Aerial SWIR and multispectral UAV data. Remote Sens. 2020, 12, 2499. [Google Scholar] [CrossRef]
- Cogato, A.; Jewan, S.Y.Y.; Wu, L.; Marinello, F.; Meggio, F.; Sivilotti, P.; Sozzi, M.; Pagay, V. Water Stress Impacts on Grapevines (Vitis vinifera L.) in Hot Environments: Physiological and Spectral Responses. Agronomy 2022, 12, 1819. [Google Scholar] [CrossRef]
- Yang, H.; Gao, T.; Li, Q.; Tan, W.; Sun, X.; Wang, D.; Cao, H. Effects of Grape Downy Mildew on Photosynthesis of ‘Red Globe’ Grape Leaves under High Temperature Stress. Int. J. Fruit Sci. 2022, 22, 581–594. [Google Scholar] [CrossRef]
- Shanmugapriya, P.; Rathika, S.; Ramesh, T.; Janaki, P. Applications of Remote Sensing in Agriculture—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2270–2283. [Google Scholar] [CrossRef]
- Reddy, M.A. Remote Sensing and Geographical Information Systems, 3rd ed.; B.S Publications: Hyderabad, India, 2010; pp. 40–50. ISBN 978-81-7800-135-7. [Google Scholar]
- Tanda, G.; Chiarabini, V. Use of multispectral and thermal imagery in precision viticulture. J. Phys. Conf. Ser. 2019, 1224, 012034. [Google Scholar] [CrossRef]
- Castañeda, C.S.; Almanza-Merchán, P.J.; Pinzón, E.H.; Cely, G.E.; Serrano, P.A. Chlorophyll concentration estimation using non-destructive methods in grapes (Vitis vinifera L.) cv. Riesling Becker. Rev. Colomb. Cienc. Hortícolas 2018, 12, 329–337. [Google Scholar] [CrossRef]
- Iatrou, G.; Mourelatos, S.; Gewehr, S.; Kalaitzopoulou, S.; Iatrou, M.; Zartaloudis, Z. Using multispectral imaging to improve berry harvest for wine making grapes. Cienc. E Tec. Vitivinic. 2017, 32, 33–41. [Google Scholar] [CrossRef]
- Fonfach-Badinella, B. Evolution of Water and Photosynthetic Status in Vitis vinifera L. Cultivating Country in the Maule Dryland and Its Correlation with Spectral Parameters; Universidad de Chile: Santiago, Chile, 2021. [Google Scholar]
- Putra, B.T.W.; Soni, P. Evaluating NIR-Red and NIR-Red edge external filters with digital cameras for assessing vegetation indices under different illumination. Infrared Phys. Technol. 2017, 81, 148–156. [Google Scholar] [CrossRef]
- Ferro, M.V.; Catania, P. Technologies and Innovative Methods for Precision Viticulture: A Comprehensive Review. Horticulturae 2023, 9, 399. [Google Scholar] [CrossRef]
- Palacios, F.; Melo-Pinto, P.; Diago, M.P.; Tardaguila, J. Deep learning and computer vision for assessing the number of actual berries in commercial vineyards. Biosyst. Eng. 2022, 218, 175–188. [Google Scholar] [CrossRef]
- Nonni, F.; Malacarne, D.; Pappalardo, S.E.; Codato, D.; Meggio, F.; De Marchi, M. Sentinel-2 data analysis and comparison with uav multispectral images for precision viticulture. GI_Forum 2018, 1, 105–116. [Google Scholar] [CrossRef]
- Gong, C.; Buddenbaum, H.; Retzlaff, R.; Udelhoven, T. An Empirical Assessment of Angular Dependency for RedEdge-M in Sloped Terrain Viticulture. Remote Sens. 2019, 11, 2561. [Google Scholar] [CrossRef]
- Olsson, P.-O.; Vivekar, A.; Adler, K.; Millan, V.E.G.; Koc, A.; Alamrani, M.; Eklundh, L. Radiometric correction of multispectral uas images: Evaluating the accuracy of the parrot sequoia camera and sunshine sensor. Remote Sens. 2021, 13, 577. [Google Scholar] [CrossRef]
- Mazzetto, F.; Calcante, A.; Mena, A. Comparing Commercial Optical Sensors for Crop Monitoring Tasks in Precision Viticulture. J. Agric. Eng. 2009, 40, 11–18. [Google Scholar] [CrossRef]
- Yang, C. A high-resolution airborne four-camera imaging system for agricultural remote sensing. Comput. Electron. Agric. 2012, 88, 13–24. [Google Scholar] [CrossRef]
- Deng, L.; Mao, Z.; Li, X.; Hu, Z.; Duan, F.; Yan, Y. UAV-based multispectral remote sensing for precision agriculture: A comparison between different cameras. ISPRS J. Photogramm. Remote Sens. 2018, 146, 124–136. [Google Scholar] [CrossRef]
- Thomas, M.L.; Kiefer, R.W.; Chipam, J. Remote Sensing and Image Interpretation, 7th ed.; Library of Congress Cataloging-in-Publication Data: Washington, DC, USA, 2015; Volume 7, pp. 1–500. [Google Scholar]
- Marinello, F. Last generation instrument for agriculture multispectral data collection. Agric. Eng. Int. CIGR J. 2017, 19, 87–93. [Google Scholar]
- Pádua, L.; Marques, P.; Hruška, J.; Adão, T.; Peres, E.; Morais, R.; Sousa, J.J. Multi-temporal vineyard monitoring through UAV-based RGB imagery. Remote Sens. 2018, 10, 1907. [Google Scholar] [CrossRef]
- Sun, L.; Gao, F.; Anderson, M.C.; Kustas, W.P.; Alsina, M.M.; Sanchez, L.; Sams, B.; McKee, L.; Dulaney, W.; White, W.A.; et al. Daily Mapping of 30 m LAI and NDVI for Grape Yield Prediction in California Vineyards. Remote Sens. 2017, 9, 317. [Google Scholar] [CrossRef]
- Žibrat, U. Remote Sensing And Advanced Plant Phenotyping Handbook; Agricultural Institute of Slovenia (Kmetijski Inštitut Slovenije): Ljubljana, Slovenia, 2021; 41p. [Google Scholar]
- López-García, P.; Ortega, J.F.; Pérez-Álvarez, E.P.; Moreno, M.A.; Ramírez, J.M.; Intrigliolo, D.S.; Ballesteros, R. Yield estimations in a vineyard based on high-resolution spatial imagery acquired by a UAV. Biosyst. Eng. 2022, 224, 227–245. [Google Scholar] [CrossRef]
- Urretavizcaya, I.; Royo, J.B.; Miranda, C.; Tisseyre, B.; Guillaume, S.; Santesteban, L.G. Relevance of sink-size estimation for within-field zone delineation in vineyards. Precis. Agric. 2017, 18, 133–144. [Google Scholar] [CrossRef]
- DI Gennaro, S.; Battiston, E.; DI Marco, S.; Facini, O.; Matese, A.; Nocentini, M.; Palliotti, A.; Mugnai, L. Unmanned Aerial Vehicle (UAV)-based remote sensing to monitor grapevine leaf stripe disease within a vineyard affected by esca complex. Phytopathol. Mediterr. 2015, 54, 241–252. [Google Scholar]
- Mamani, I.Y.E. Imagenes Multiespectrales en la Detección del Oídio (Erysiphe necator) en Vid (Vitis vinífera) Variedad Thompson Seedless, Titor- Arequipa. Tesis; Universidad Nacional de San Agustin Arequipa: Arequipa, Peru, 2022. [Google Scholar]
- Nowack, J.C.; Atencia-Payares, L.K.; Tarquis, A.M.; Gomez-Del-Campo, M. Application of Unmanned Aerial Vehicle (UAV) Sensing for Water Status Estimation in Vineyards under Different Pruning Strategies. Plants 2024, 13, 1350. [Google Scholar] [CrossRef]
- Baluja, J.; Diago, M.P.; Balda, P.; Zorer, R.; Meggio, F.; Morales, F.; Tardaguila, J. Assessment of vineyard water status variability by thermal and multispectral imagery using an unmanned aerial vehicle (UAV). Irrig. Sci. 2012, 30, 511–522. [Google Scholar] [CrossRef]
- Catania, C.; Avagnina, S. Grape ripening. In Advanced Wine Tasting Course; INTA, Ed.; INTA: Mendoza, Argentina, 2007; pp. 1–14. [Google Scholar]
- Gil Cortiella, M. Influencia de la Madurez de la Uva y de Ciertas Prácticas Vitivinícolas Sobre el Color, los Compuestos Fenólicos y los Polisacáridos del Vino Tinto; Universitat Rovira i Virgili: Tarragona, Spain, 2014. [Google Scholar]
- Gago, J.; Douthe, C.; Coopman, R.; Gallego, P.; Ribas-Carbo, M.; Flexas, J.; Escalona, J.; Medrano, H. UAVs challenge to assess water stress for sustainable agriculture. Agric. Water Manag. 2015, 153, 9–19. [Google Scholar] [CrossRef]
- Matese, A.; Baraldi, R.; Berton, A.; Cesaraccio, C.; Di Gennaro, S.F.; Duce, P.; Facini, O.; Mameli, M.G.; Piga, A.; Zaldei, A. Estimation of Water Stress in grapevines using proximal and remote sensing methods. Remote Sens. 2018, 10, 114. [Google Scholar] [CrossRef]
- Khanal, S.; Kc, K.; Fulton, J.P.; Shearer, S.; Ozkan, E. Remote sensing in agriculture—Accomplishments, limitations, and opportunities. Remote Sens. 2020, 12, 3783. [Google Scholar] [CrossRef]
- Giovos, R.; Tassopoulos, D.; Kalivas, D.; Lougkos, N.; Priovolou, A. Remote sensing vegetation indices in viticulture: A critical review. Agriculture 2021, 11, 457. [Google Scholar] [CrossRef]
- Ammoniaci, M.; Kartsiotis, S.-P.; Perria, R.; Storchi, P. State of the art of monitoring technologies and data processing for precision viticulture. Agriculture 2021, 11, 201. [Google Scholar] [CrossRef]
- Tardaguila, J.; Stoll, M.; Gutiérrez, S.; Proffitt, T.; Diago, M.P. Smart applications and digital technologies in viticulture: A review. Smart Agric. Technol. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Santesteban, L.G. Precision viticulture and advanced analytics. A short review. Food Chem. 2019, 279, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Comba, L.; Gay, P.; Primicerio, J.; Aimonino, D.R. Vineyard detection from unmanned aerial systems images. Comput. Electron. Agric. 2015, 114, 78–87. [Google Scholar] [CrossRef]
- Pichon, L.; Leroux, C.; Macombe, C.; Taylor, J.; Tisseyre, B. What relevant information can be identified by experts on unmanned aerial vehicles’ visible images for precision viticulture? Precis. Agric. 2019, 20, 278–294. [Google Scholar] [CrossRef]
- Zovko, M.; Žibrat, U.; Knapič, M.; Kovačić, M.B.; Romić, D. Hyperspectral remote sensing of grapevine drought stress. Precis. Agric. 2019, 20, 335–347. [Google Scholar] [CrossRef]
- Bramley, R.G.V. Precision Viticulture: Managing vineyard variability for improved quality outcomes. In Managing Wine Quality: Viticulture and Wine Quality; Woodhead Publishing: Cambridge, UK, 1999; pp. 445–480. [Google Scholar]
- Torre, G.; García, S.; Álvarez, E.; Fernández, J.; López, C.A.; Campos, A.M.; Arango, R.B. New experiences derived from the use of remote sensors for the development of decision-making support tools. In Proceedings of the V Jornadas de Ingeniería del Agua (JIA 2017), A Coruña, Spain, 24–26 October 2017; pp. 1–9. Available online: http://www.ingenieriadelagua.com/JIA/posters (accessed on 25 October 2024).
- Zhu, W.; Sun, Z.; Huang, Y.; Yang, T.; Li, J.; Zhu, K.; Zhang, J.; Yang, B.; Shao, C.; Peng, J.; et al. Optimization of multi-source UAV RS agro-monitoring schemes designed for field-scale crop phenotyping. Precis. Agric. 2021, 22, 1768–1802. [Google Scholar] [CrossRef]
- Seifert, E.; Seifert, S.; Vogt, H.; Drew, D.; van Aardt, J.; Kunneke, A.; Seifert, T. Influence of drone altitude, image overlap, and optical sensor resolution on multi-view reconstruction of forest images. Remote Sens. 2019, 11, 1252. [Google Scholar] [CrossRef]
- Pix4D, S.A.; Lausanne, S. Pix4D Mapper. Available online: https://www.pix4d.com (accessed on 14 December 2022).
- Agisoft Metashape, Version 1.6.3; AgiSoft LLC: St. Petersburg, Russia. Available online: https://www.agisoft.com (accessed on 6 May 2024).
- Di Gennaro, S.F.; Toscano, P.; Gatti, M.; Poni, S.; Berton, A.; Matese, A. Spectral Comparison of UAV-Based Hyper and Multispectral Cameras for Precision Viticulture. Remote Sens. 2022, 14, 449. [Google Scholar] [CrossRef]
- Modica, G.; Messina, G.; De Luca, G.; Fiozzo, V.; Praticò, S. Monitoring the vegetation vigor in heterogeneous citrus and olive orchards. A multiscale object-based approach to extract trees’ crowns from UAV multispectral imagery. Comput. Electron. Agric. 2020, 175, 105500. [Google Scholar] [CrossRef]
- García-Fernández, M.; Sanz-Ablanedo, E.; Rodríguez-Pérez, J.R. High-resolution drone-acquired RGB imagery to estimate spatial grape quality variability. Agronomy 2021, 11, 655. [Google Scholar] [CrossRef]
- Pádua, L.; Marques, P.; Adão, T.; Guimarães, N.; Sousa, A.; Peres, E.; Sousa, J.J. Vineyard variability analysis through UAV-based vigour maps to assess climate change impacts. Agronomy 2019, 9, 581. [Google Scholar] [CrossRef]
- Burgos, S.; Mota, M.; Noll, D.; Cannelle, B. Use of very high-resolution airborne images to analyse 3D canopy architecture of a vineyard. ISPRS—Int. Arch. Photogramm. Remote Sens. Spat. Inf. Sci. 2015, 40, 399–403. [Google Scholar] [CrossRef]
- Cantürk, M.; Zabawa, L.; Pavlic, D.; Dreier, A.; Klingbeil, L.; Kuhlmann, H. UAV-based individual plant detection and geometric parameter extraction in vineyards. Front. Plant Sci. 2023, 14, 1244384. [Google Scholar] [CrossRef]
- Barros, T.; Conde, P.; Gonçalves, G.; Premebida, C.; Monteiro, M.; Ferreira, C.S.S.; Nunes, U. Multispectral vineyard segmentation: A deep learning comparison study. Comput. Electron. Agric. 2022, 195, 106782. [Google Scholar] [CrossRef]
- Xue, J.; Su, B. Significant remote sensing vegetation indices: A review of developments and applications. J. Sens. 2017, 2017, 1353691. [Google Scholar] [CrossRef]
- Rouse, J.W.; Hass, R.H.; Schell, J. Monitoring vegetation systems in the great plains with earts. Remote Sensingcenter 1976, 24, 309–317. [Google Scholar]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a green channel in remote sensing of global vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Huete, A.R. A soil-Adjusted Vegetation Index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Matese, A.; Di Gennaro, S.F.; Berton, A. Assessment of a canopy height model (CHM) in a vineyard using UAV-based multispectral imaging. Int. J. Remote Sens. 2017, 38, 2150–2160. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Spectral Reflectance Changes Associated with Autumn Senescence of Aesculus hippocastanum L. and Acer platanoides L. Leaves. Spectral Features and Relation to Chlorophyll Estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Ferro, M.V.; Catania, P.; Miccichè, D.; Pisciotta, A.; Vallone, M.; Orlando, S. Assessment of vineyard vigour and yield spatio-temporal variability based on UAV high resolution multispectral images. Biosyst. Eng. 2023, 231, 36–56. [Google Scholar] [CrossRef]
- Filippetti, I.; Allegro, G.; Valentini, G.; Pastore, C.; Colucci, E.; Intrieri, C. Influence of vigour on vine performance and berry composition of cv. Sangiovese (vitis vinifera L.). J. Int. Sci. Vigne Vin 2013, 47, 21–33. [Google Scholar] [CrossRef]
- Paz Pellat, F.; Enrique Romero Sanchez, M.; Palacios Vélez, E.; Bolaños González, M.; René Valdez Lazalde, J.; Aldrete, A. Scopes and Limitations of Spectral Vegetation Indices: Analysis of Broad Band Indices. Terra Latinoam 2015, 33, 23. [Google Scholar]
- Xie, Q.; Dash, J.; Huang, W.; Peng, D.; Qin, Q.; Mortimer, H.; Casa, R.; Pignatti, S.; Laneve, G.; Pascucci, S.; et al. Vegetation Indices Combining the Red and Red-Edge Spectral Information for Leaf Area Index Retrieval. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2018, 11, 1482–1493. [Google Scholar] [CrossRef]
- Jorge, J.; Vallbé, M.; Soler, J.A. Detection of irrigation inhomogeneities in an olive grove using the NDRE vegetation index obtained from UAV images. Eur. J. Remote Sens. 2019, 52, 169–177. [Google Scholar] [CrossRef]
- Romero, M.; Luo, Y.; Su, B.; Fuentes, S. Vineyard water status estimation using multispectral imagery from an UAV platform and machine learning algorithms for irrigation scheduling management. Comput. Electron. Agric. 2018, 147, 109–117. [Google Scholar] [CrossRef]
- Drissi, R.; Goutouly, J.; Forget, D.; Gaudillere, J. Nondestructive measurement of grapevine leaf area by ground normalized difference vegetation index. Agron. J. 2009, 101, 226–231. [Google Scholar] [CrossRef]
- Vélez, S.; Poblete-Echeverría, C.; Rubio, J.A.; Vacas, R.; Barajas, E. Estimation of leaf area index in vineyards by analysing projected shadows using uav imagery. OENO One 2021, 55, 159–180. [Google Scholar] [CrossRef]
- Ilniyaz, O.; Du, Q.; Shen, H.; He, W.; Feng, L.; Azadi, H.; Kurban, A.; Chen, X. Leaf area index estimation of pergola-trained vineyards in arid regions using classical and deep learning methods based on UAV-based RGB images. Comput. Electron. Agric. 2023, 207, 107723. [Google Scholar] [CrossRef]
- Perroy, R.L.; Hughes, M.; Keith, L.M.; Collier, E.; Sullivan, T.; Low, G. Examining the utility of visible near-infrared and optical remote sensing for the early detection of rapid’ohi’a death. Remote Sens. 2020, 12, 1846. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote sensing of chlorophyll concentration in higher plant leaves. Adv. Space Res. 1998, 22, 689–692. [Google Scholar] [CrossRef]
- Mahajan, U.; Bundel, B.R. Drones for Normalized Difference Vegetation Index (NDVI), to Estimate Crop Health for Precision Agriculture: A Cheaper Alternative for Spatial Satellite Sensors. In Proceedings of the International Conference on Innovative Research in Agriculture, Food Science, Forestry, Horticulture, Aquaculture, Animal Sciences, Biodiversity, Ecological Sciences and Climate Change (AFHABEC-2016), Delhi, India, 22 October 2016; pp. 38–41. [Google Scholar]
- Matese, A.; Di Gennaro, S.F. Practical applications of a multisensor UAV platform based on multispectral, thermal and RGB high resolution images in precision viticulture. Agriculture 2018, 8, 116. [Google Scholar] [CrossRef]
- Carloni, G. Effects of Variable Rate Mechanical Pruning Under Distinct NDVI (Normalised Differential Vegetation Index) Levels on the Wine Chemical Composition Using the Grapevine Variety Trincadeira (Vitis vinifera L.); Universodadde de Lisboa: Lisbon, Portugal, 2022. [Google Scholar]
- Dorin, B.; Reynolds, A.; Jollineau, M.; Lee, H.-S.; Shemrock, A. Utilization of unmanned aerial vehicles for zonal winemaking in cool-climate Riesling vineyards. OENO One 2022, 56, 327–341. [Google Scholar] [CrossRef]
- Zhou, J.; Khot, L.R.; Bahlol, H.Y.; Boydston, R.; Miklas, P.N. Evaluation of ground, proximal and aerial remote sensing technologies for crop stress monitoring. IFAC-PapersOnLine 2016, 49, 22–26. [Google Scholar] [CrossRef]
- Wei, H.-E.; Grafton, M.; Bretherton, M.; Irwin, M.; Sandoval, E. Evaluation of the Use of UAV-Derived Vegetation Indices and Environmental Variables for Grapevine Water Status Monitoring Based on Machine Learning Algorithms and SHAP Analysis. Remote Sens. 2022, 14, 5918. [Google Scholar] [CrossRef]
- Huete, A.; Hua, G.; Qi, J.; Chehbouni, A.; van Leeuwen, W.J.D. Normalization of multidirectional red and NIR reflectances with the SAVI. Remote Sens. Environ. 1992, 41, 143–154. [Google Scholar] [CrossRef]
- Aboutalebi, M.; Torres-Rua, A.F.; McKee, M.; Kustas, W.; Nieto, H.; Coopmans, C. Behavior of vegetation/soil indices in shaded and sunlit pixels and evaluation of different shadow compensation methods using UAV high-resolution imagery over vineyards. In Proceedings of the SPIE—The International Society for Optical Engineering 2018, San Francisco, CA, USA, 27 January–1 February 2018; p. 6. [Google Scholar]
- Jełowicki, Ł.; Sosnowicz, K.; Ostrowski, W.; Osińska-Skotak, K.; Bakuła, K. Evaluation of rapeseed winter crop damage using UAV-Based multispectral imagery. Remote Sens. 2020, 12, 2618. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H.; Sauer, T.J.; Dold, C.; O’brien, P.; Wacha, K. Applications of Vegetative Indices from Remote Sensing to Agriculture: Past and Future. Inventions 2019, 4, 71. [Google Scholar] [CrossRef]
- Devia, C.A.; Rojas, J.P.; Carol, E.P.; Ivan, M.; Patino, F.M.D.; Colorado, J. High-Throughput Biomass Estimation in Rice Crops Using UAV Multispectral Imagery. J. Intell. Robot. Syst. 2019, 96, 573–589. [Google Scholar] [CrossRef]
- Venancio, L.P.; Mantovani, E.C.; Amaral, C.H.D.; Neale, C.M.U.; Gonçalves, I.Z.; Filgueiras, R.; Campos, I. Forecasting corn yield at the farm level in Brazil based on the FAO-66 approach and soil-adjusted vegetation index (SAVI). Agric. Water Manag. 2019, 225, 105779. [Google Scholar] [CrossRef]
- Messina, G.; Praticò, S.; Badagliacca, G.; Di Fazio, S.; Monti, M.; Modica, G. Monitoring Onion Crop “Cipolla Rossa di Tropea Calabria IGP” Growth and Yield Response to Varying Nitrogen Fertilizer Application Rates Using UAV Imagery. Drones 2021, 5, 61. [Google Scholar] [CrossRef]
- Campos, I.; Neale, C.M.; Calera, A.; Balbontín, C.; González-Piqueras, J. Assessing satellite-based basal crop coefficients for irrigated grapes (Vitis vinifera L.). Agric. Water Manag. 2010, 98, 45–54. [Google Scholar] [CrossRef]
- Qiao, L.; Tang, W.; Gao, D.; Zhao, R.; An, L.; Li, M.; Sun, H.; Song, D. UAV-based chlorophyll content estimation by evaluating vegetation index responses under different crop coverages. Comput. Electron. Agric. 2022, 196, 106775. [Google Scholar] [CrossRef]
- Darra, N.; Psomiadis, E.; Kasimati, A.; Anastasiou, A.; Anastasiou, E.; Fountas, S. Remote and proximal sensing-derived spectral indices and biophysical variables for spatial variation determination in vineyards. Agronomy 2021, 11, 741. [Google Scholar] [CrossRef]
- Boiarskii, B. Comparison of NDVI and NDRE Indices to Detect Differences in Vegetation and Chlorophyll Content. J. Mech. Contin. Math. Sci. 2019, 4, 20–29. [Google Scholar] [CrossRef]
- Marty, C.; Khare, S.; Rossi, S.; Lafond, J.; Boivin, M.; Paré, M.C. Detection of Management Practices and Cropping Phases in Wild Lowbush Blueberry Fields Using Multispectral UAV Data. Can. J. Remote Sens. 2022, 48, 469–480. [Google Scholar] [CrossRef]
- Nanda, M.K.; Giri, U.; Bera, N. Canopy Temperature-Based Water Stress Indices: Potential and Limitations. In Advances in Crop Environment Interaction; Springer: Singapore, 2018; pp. 365–385. [Google Scholar]
- López-García, P.; Intrigliolo, D.S.; Moreno, M.A.; Martínez-Moreno, A.; Ortega, J.F.; Pérez-Álvarez, E.P.; Ballesteros, R. Assessment of vineyard water status by multispectral and rgb imagery obtained from an unmanned aerial vehicle. Am. J. Enol. Vitic. 2021, 72, 285–297. [Google Scholar] [CrossRef]
- Möller, M.; Alchanatis, V.; Cohen, Y.; Meron, M.; Tsipris, J.; Naor, A.; Ostrovsky, V.; Sprintsin, M.; Cohen, S. Use of thermal and visible imagery for estimating crop water status of irrigated grapevine. J. Exp. Bot. 2007, 58, 827–838. [Google Scholar] [CrossRef]
- Cetin, M.; Alsenjar, O.; Aksu, H.; Golpinar, M.S.; Akgul, M.A. Estimation of crop water stress index and leaf area index based on remote sensing data. Water Supply 2023, 23, 1390–1404. [Google Scholar] [CrossRef]
- Ezenne, G.; Jupp, L.; Mantel, S.; Tanner, J. Current and potential capabilities of UAS for crop water productivity in precision agriculture. Agric. Water Manag. 2019, 218, 158–164. [Google Scholar] [CrossRef]
- Romero, P.; Botía, P.; Gil-Muñoz, R.; del Amor, F.M.; Navarro, J.M. Evaluation of the Effect of Water Stress on Clonal Variations of Cv. Monastrell (Vitis vinifera L.) in South-Eastern Spain: Physiology, Nutrition, Yield, Berry, and Wine-Quality Responses. Agronomy 2023, 13, 433. [Google Scholar] [CrossRef]
- Abbatantuono, F.; Lopriore, G.; Tallou, A.; Brillante, L.; Ali, S.A.; Camposeo, S.; Vivaldi, G.A. Recent progress on grapevine water status assessment through remote and proximal sensing: A review. Sci. Hortic. 2024, 338, 113658. [Google Scholar] [CrossRef]
- Stobbelaar, P.; Neinavaz, E.; Nyktas, P. Prediction of leaf area index using thermal infrared data acquired by UAS over a mixed temperate forest. Int. J. Appl. Earth Obs. Geoinf. 2022, 114, 103049. [Google Scholar] [CrossRef]
- Mesas-Carrascosa, F.J.; Ramírez, P.; Torres-Sánchez, J.; León-Gutiérrez, J.M.; Pérez-Porras, F.; López-Granados Fernando, F. Estimación de LAI en un viñedo de la variedad ‘Pedro Ximénez‘ mediante teledetección hiperespectral UAV. In IV Jornadas del Grupo de Viticultura: Acta de Horticultura: Comunicaciones Técnicas Sociedad Española de Ciencias Hortícolas: 26–28 de octubre 2022, Pamplona/Iruña; SECH (Sociedad Española de Ciencias Hortícolas): Córdoba, Spain, 2018; pp. 53–54. [Google Scholar]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.S.P. Response of plants to water stress. Front Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.D.; Idso, S.B.; Reginato, R.J.; Pinter, P.J., Jr. Canopy temperature as a crop water stress indicator. Water Resour. Res. 1981, 17, 1133–1138. [Google Scholar] [CrossRef]
- Araújo-Paredes, C.; Portela, F.; Mendes, S.; Valín, M.I. Using Aerial Thermal Imagery to Evaluate Water Status in Vitis vinifera cv. Loureiro. Sensors 2022, 22, 8056. [Google Scholar] [CrossRef]
- Lee, L.; Reynolds, A.; Dorin, B.; Shemrock, A. Potential of a Remotely Piloted Aircraft System with Multispectral and Thermal Sensors to Monitor Vineyard Characteristics for Precision Viticulture. Plants 2025, 14, 137. [Google Scholar] [CrossRef]
- Burchard-Levine, V.; Borra-Serrano, I.; Peña, J.M.; Kustas, W.P.; Guerra, J.G.; Dorado, J.; Mesías-Ruiz, G.; Herrezuelo, M.; Mary, B.; McKee, L.M.; et al. Evaluating the precise grapevine water stress detection using unmanned aerial vehicles and evapotranspiration-based metrics. Irrig. Sci. 2024, 43, 65–85. [Google Scholar] [CrossRef]
- Berry, A.; Vivier, M.A.; Poblete-Echeverría, C. Evaluation of canopy fraction-based vegetation indices, derived from multispectral UAV imagery, to map water status variability in a commercial vineyard. Irrig. Sci. 2024, 43, 135–153. [Google Scholar] [CrossRef]
- Campos, I.; Neale, C.M.; Suyker, A.E.; Arkebauer, T.J.; Gonçalves, I.Z. Reflectance-based crop coefficients REDUX: For operational evapotranspiration estimates in the age of high producing hybrid varieties. Agric. Water Manag. 2021, 187, 140–153. [Google Scholar] [CrossRef]
- Gautam, D.; Ostendorf, B.; Pagay, V. Estimation of grapevine crop coefficient using a multispectral camera on an unmanned aerial vehicle. Remote Sens. 2021, 13, 2639. [Google Scholar] [CrossRef]
- Pádua, L.; Marques, P.; Hruška, J.; Adão, T.; Bessa, J.; Sousa, A.; Peres, E.; Morais, R.; Sousa, J.J. Vineyard properties extraction combining UAS-based RGB imagery with elevation data. Int. J. Remote Sens. 2018, 39, 5377–5401. [Google Scholar] [CrossRef]
- Peeters, A.; Cohen, Y. Using Time Series of High-Resolution Planet Satellite Images to Monitor Grapevine Stem Water Potential in Commercial Vineyards. Remote Sens. 2018, 10, 1615. [Google Scholar] [CrossRef]
- Reynolds, A.G.; Lee, H.-S.; Dorin, B.; Brown, R.; Jollineau, M.; Shemrock, A.; Crombleholme, M.; Poirier, E.J.; Zheng, W.; Gasnier, M.; et al. Mapping Cabernet Franc vineyards by unmanned aerial vehicles (UAVs) for variability in vegetation indices, water status, and virus titer. E3S Web Conf. 2018, 50, 1–6. [Google Scholar] [CrossRef]
- Zuñiga, C.; Khot, L.R.; Sindhuja, S.; Pete, W.J. High Resolution Multispectral and Thermal Remote Sensing-Based Water Stress Assessment in Subsurface Irrigated Grapevines. Remote Sens. 2017, 9, 961. [Google Scholar]
- Nuske, S.; Achar, S.; Bates, T.; Narasimhan, S.; Singh, S. Yield estimation in vineyards by visual grape detection. In Proceedings of the 2011 IEEE/RSJ International Conference on Intelligent Robots and Systems, San Francisco, CA, USA, 25–30 September 2011; pp. 2352–2358. [Google Scholar]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, R.L.; et al. Advanced methods of plant disease detection: A review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Pu, R.; Gonzalez-Moreno, P.; Yuan, L.; Wu, K.; Huang, W. Monitoring plant diseases and pests through remote sensing technology: A review. Comput. Electron. Agric. 2019, 165, 104943. [Google Scholar] [CrossRef]
- Vélez, S.; Ariza-Sentís, M.; Valente, J. Mapping the spatial variability of Botrytis bunch rot risk in vineyards using UAV multispectral imagery. Eur. J. Agron. 2023, 142, 126691. [Google Scholar] [CrossRef]
- Daglio, G.; Gallo, R.; Rinaldi, M.F.; Massa, N.; Todeschini, V.; Mazzetto, F. Use of a Multirotor-UAV Equipped with a Multispectral Camera to Detect Vineyard Diseases: A Case Study on Barbera and Dolcetto Cultivars. Lect. Notes Civ. Eng. 2020, 67, 803–809. [Google Scholar]
- Albetis, J.; Duthoit, S.; Guttler, F.; Jacquin, A.; Goulard, M.; Poilvé, H.; Féret, J.-B.; Dedieu, G. Detection of Flavescence dorée grapevine disease using Unmanned Aerial Vehicle (UAV) multispectral imagery. Remote Sens. 2017, 9, 308. [Google Scholar] [CrossRef]
- Al-Saddik, H.; Simon, J.; Brousse, O.; Cointault, F. Multispectral band selection for imaging sensor design for vineyard disease detection: Case of Flavescence Dorée. Adv. Anim. Biosci. 2017, 8, 150–155. [Google Scholar] [CrossRef]
- Vanegas, F.; Bratanov, D.; Weiss, J.; Powell, K.; Gonzalez, F. Multi and hyperspectral UAV remote sensing: Grapevine phylloxera detection in vineyards. In Proceedings of the 2018 IEEE Aerospace Conference, Big Sky, MT, USA, 3–10 March 2018; pp. 1–9. [Google Scholar]
- Jiménez-Brenes, F.M.; López-Granados, F.; Torres-Sánchez, J.; Peña, J.M.; Ramírez, P.; Castillejo-González, I.L.; de Castro, A.I. Automatic UAV-based detection of Cynodon dactylon for site-specific vineyard management. PLoS ONE 2019, 14, e0218132. [Google Scholar] [CrossRef]
- Campbell, P.; Bendek, C.; Latorre, B.A. Risk of powdery mildew (Erysiphe necator) outbreaks on grapevines in relation to cluster development. Cienc. E Investig. Agrar. 2007, 34, 5–11. [Google Scholar] [CrossRef]
- Noronha, H. The Effect of High-Temperature on Sugar Transport in Grape Cells. Master’s Thesis, Universidade do Minho Escola de Ciências Henrique Luis Silva de Noronha, Braga, Portugal, October 2010. [Google Scholar]
- van Leeuwen, C.; Darriet, P. The Impact of Climate Change on Viticulture and Wine Quality. J. Wine Econ. 2016, 11, 150–167. [Google Scholar] [CrossRef]
- Mpelasoka, B.S.; Schachtman, D.P.; Treeby, M.T.; Thomas, M.R. A review of potassium nutrition in grapevines with special emphasis on berry accumulation. Aust. J. Grape Wine Res. 2003, 9, 154–168. [Google Scholar] [CrossRef]
- Kuhn, N.; Guan, L.; Dai, Z.W.; Wu, B.-H.; Lauvergeat, V.; Gomès, E.; Li, S.-H.; Godoy, F.; Arce-Johnson, P.; Delrot, S. Berry ripening: Recently heard through the grapevine. J. Exp. Bot. 2014, 65, 4543–4559. [Google Scholar] [CrossRef]
- Cortell, J.M.; Halbleib, M.; Gallagher, A.V.; Righetti, T.L.; Kennedy, J.A. Influence of vine vigor on grape (Vitis vinifera L. Cv. Pinot Noir) anthocyanins. 1. Anthocyanin concentration and composition in fruit. J Agric Food Chem. 2007, 55, 6575–6584. [Google Scholar] [CrossRef]
- Albetis, J.; Jacquin, A.; Goulard, M.; Poilvé, H.; Rousseau, J.; Clenet, H.; Dedieu, G.; Duthoit, S. On the potentiality of UAV multispectral imagery to detect Flavescence dorée and Grapevine Trunk Diseases. Remote Sens. 2019, 11, 23. [Google Scholar] [CrossRef]
- Lamb, D.W.; Weedon, M.M.; Bramley, R.G.V. Using remote sensing to predict grape phenolics and colour at harvest in a Cabernet Sauvignon vineyard: Timing observations against vine phenology and optimising image resolution. Aust. J. Grape Wine Res. 2004, 10, 46–54. [Google Scholar] [CrossRef]
- Soubry, I.; Patias, P.; Tsioukas, V. Monitoring vineyards with UAV and multi-sensors for the assessment ofwater stress and grape maturity. J. Unmanned Veh. Syst. 2017, 5, 37–50. [Google Scholar] [CrossRef]
- Taskos, D.G.; Koundouras, S.; Stamatiadis, S.; Zioziou, E.; Nikolaou, N.; Karakioulakis, K.; Theodorou, N. Using active canopy sensors and chlorophyll meters to estimate grapevine nitrogen status and productivity. Precis. Agric. 2015, 16, 77–98. [Google Scholar] [CrossRef]
- Matese, A.; Capraro, F.; Primicerio, J.; Gualato, G.; Di Gennaro, S.F.; Agati, G. Mapping of vine vigor by UAV and anthocyanin content by a non destructive fluorescence technique. In Precision Agriculture’13; Stafford, J.V., Ed.; Wageningen Academic: Wageningen, The Netherlands, 2013; pp. 201–208. [Google Scholar]
- Romboli, Y.; Di Gennaro, S.; Mangani, S.; Buscioni, G.; Matese, A.; Genesio, L.; Vincenzini, M. Vine vigour modulates bunch microclimate and affects the composition of grape and wine flavonoids: An unmanned aerial vehicle approach in a Sangiovese vineyard in Tuscany. Aust. J. Grape Wine Res. 2017, 23, 368–377. [Google Scholar] [CrossRef]
- Ferrer, M.; Echeverría, G.; Pereyra, G.; Gonzalez-Neves, G.; Pan, D.; Mirás-Avalos, J.M. Mapping vineyard vigor using airborne remote sensing: Relations with yield, berry composition and sanitary status under humid climate conditions. Precis. Agric. 2020, 21, 178–197. [Google Scholar] [CrossRef]
- Peng, X.; Chen, D.; Zhou, Z.; Zhang, Z.; Xu, C.; Zha, Q.; Wang, F.; Hu, X. Prediction of the Nitrogen, Phosphorus and Potassium Contents in Grape Leaves at Different Growth Stages Based on UAV Multispectral Remote Sensing. Remote Sens. 2022, 14, 2659. [Google Scholar] [CrossRef]
- García-Fernández, M.; Sanz-Ablanedo, E.; Pereira-Obaya, D.; Rodríguez-Pérez, J.R. Vineyard pruning weight prediction using 3D point clouds generated from UAV imagery and structure from motion photogrammetry. Agronomy 2021, 11, 2489. [Google Scholar] [CrossRef]
- Lyu, H.; Grafton, M.; Ramilan, T.; Irwin, M.; Wei, H.-E.; Sandoval, E. Using Remote and Proximal Sensing Data and Vine Vigor Parameters for Non-Destructive and Rapid Prediction of Grape Quality. Remote Sens. 2023, 15, 5412. [Google Scholar] [CrossRef]
- Brunori, E.; Maesano, M.; Moresi, F.V.; Antolini, A.; Bellincontro, A.; Forniti, R.; Biasi, R.; Mencarelli, F. Using UAV-based remote sensing to assess grapevine canopy damage due to fire smoke. J. Sci. Food Agric. 2020, 100, 4531–4539. [Google Scholar] [CrossRef]
- Moresi, F.V.; Cirigliano, P.; Rengo, A.; Brunori, E.; Biasi, R.; Mugnozza, G.S.; Maesano, M. Monitoring Abiotic Stressors in Rainfed Vineyards Involves Combining UAV and Field Monitoring Techniques to Enhance Precision Management. Remote Sens. 2025, 17, 803. [Google Scholar] [CrossRef]
- Gačnik, M.B.; Škraba, A.; Pažek, K.; Rozman, Č. Predicting Wine Quality Under Changing Climate: An Integrated Approach Combining Machine Learning, Statistical Analysis, and Systems Thinking. Beverages 2025, 11, 116. [Google Scholar] [CrossRef]
- Kasimati, A.; Espejo-Garcia, B.; Vali, E.; Malounas, I.; Fountas, S. Investigating a selection of methods for the prediction of total soluble solids among wine grape quality characteristics using normalized difference vegetation index data from proximal and remote sensing. Front. Plant Sci. 2021, 12, 1–10. [Google Scholar] [CrossRef]
- Guo, Y.; Senthilnath, J.; Wu, W.; Zhang, X.; Zeng, Z.; Huang, H. Radiometric calibration for multispectral camera of different imaging conditions mounted on a UAV platform. Sustainability 2019, 11, 978. [Google Scholar] [CrossRef]
- Daniels, L.; Eeckhout, E.; Wieme, J.; Dejaegher, Y.; Audenaert, K.; Maes, W.H. Identifying the Optimal Radiometric Calibration Method for UAV-Based Multispectral Imaging. Remote Sens. 2023, 15, 2909. [Google Scholar] [CrossRef]
- Choi, H.S.; Lee, S.; Ryu, H.; Shim, H.; Ha, C. Dynamics and simulation of the effects of wind on UAVs and airborne wind measurement. Trans. Jpn. Soc. Aeronaut. Space Sci. 2015, 58, 187–192. [Google Scholar] [CrossRef]
- Feng, L.; Wu, W.; Wang, J.; Zhang, C.; Zhao, Y.; Zhu, S.; He, Y. Wind field distribution of multi-rotor uav and its influence on spectral information acquisition of rice canopies. Remote Sens. 2019, 11, 602. [Google Scholar] [CrossRef]
- Tajima, Y.; Hiraguri, T.; Matsuda, T.; Imai, T.; Hirokawa, J.; Shimizu, H.; Kimura, T.; Maruta, K. Analysis of Wind Effect on Drone Relay Communications. Drones 2023, 7, 182. [Google Scholar] [CrossRef]
- Aboutalebi, M.; Torres-Rua, A.F.; Kustas, W.P.; Nieto, H.; Coopmans, C.; McKee, M. Assessment of different methods for shadow detection in high-resolution optical imagery and evaluation of shadow impact on calculation of NDVI, and evapotranspiration. Irrig. Sci. 2019, 37, 407–429. [Google Scholar] [CrossRef]
- Fuentes-Penailillo, F.; Ortega-Farias, S.; Rivera, M.; Bardeen, M.; Moreno, M. Using clustering algorithms to segment UAV-based RGB images. In Proceedings of the 2018 IEEE International Conference on Automation/XXIII Congress of the Chilean Association of Automatic Control (ICA-ACCA), Concepcion, Chile, 17–19 October 2018; IEEE: Piscataway, NJ, USA; pp. 1–5. [Google Scholar] [CrossRef]

| Index | Formula | Main Application | Limitations in Vineyards |
|---|---|---|---|
| NDVI | Vigor mapping, biomass estimation, zoning for grape/wine quality | Saturates at high biomass; influenced by soil background, shadows, and atmospheric effects [79,80] | |
| GNDVI | Chlorophyll estimation, nutrient status, yield prediction | Sensitive to soil background, cloud cover, haze; lower performance before ripening [79,81] | |
| SAVI | Vigor assessment under sparse canopy or early growth stages | Requires calibration of L; less effective in dense canopy conditions [76] | |
| NDRE | Chlorophyll content monitoring, stress detection, water status | Less robust under heterogeneous soils; indirect indicator; needs calibration with ground data [82,83] | |
| CWSI | Direct indicator of vine water stress, stomatal conductance, irrigation scheduling | Requires wet/dry reference calibration; affected by canopy architecture, time of acquisition [51,84] | |
| LAI | Canopy architecture, vigor, photosynthetic capacity, yield estimation | Sensitive to lighting conditions, soil interference, mixed vegetation; tends to saturate under dense canopy [85,86,87] |
| NDVI Values | Plant Status |
|---|---|
| −1–0 | Dead Plant or Inanimate Object |
| 0–0.33 | Unhealthy Plant |
| 0.33–0.66 | Moderately Healthy Plant |
| 0.66–1 | Very healthy Plant |
| Index/Approach | Sensor Type | Key Findings in Vineyards | Limitations | Reference(s) |
|---|---|---|---|---|
| CWSI | Thermal | Strong correlation with stomatal conductance (r = 0.91) and Ψstem; reliable proxy of transpiration and water status; effective for regulated deficit irrigation | Requires calibration of wet/dry references; sensitive to canopy architecture and acquisition timing | [6,51,118,119,120] |
| NDVI | Multispectral | Correlated with Ψleaf, gs, transpiration, and grape yield; useful in mapping intra-vineyard variability and deficit irrigation strategies | Saturates under high biomass; indirect proxy of water status | [39,84,122,127] |
| NDRE | Multispectral | High sensitivity to chlorophyll concentration and mid-season stress; complementary to NDVI in ripening stages | Limited performance in sparse canopies and heterogeneous soils | [107,126] |
| GNDVI | Multispectral | Moderate correlation with crop coefficient (Kc, R2 = 0.36); useful for assessing nutritional and hydric stress | Sensitive to soil background; less robust at early phenological stages | [124] |
| RGB indices (GLI, VARI) | RGB UAV | Detected moderate-to-severe accumulated water stress (SΨ); low-cost alternative for monitoring | Low sensitivity to early or mild stress; limited use for irrigation scheduling | [109] |
| Integrated ML models (ANN, GLM) | Multispectral + weather data | Improved estimation of water stress index (ISW) by combining spectral and meteorological variables | Require site-specific calibration and large training datasets | [20,84] |
| TSEB-derived indices (CTSI, CSSI) | Thermal + energy balance models | Capture stomatal and transpiration stress with improved physiological relevance; better than empirical CWSI | Computationally complex; require partitioning of canopy fluxes | [121] |
| Disease/Pest | Sensor Type | Index/Approach | Key Findings in Vineyards | Limitations | Reference(s) |
|---|---|---|---|---|---|
| Grapevine Leaf Stripe Disease (GLSD) | Multispectral | NDVI | Differentiated symptomatic from healthy vines at canopy level | Limited in detecting early/asymptomatic infections | [44] |
| Powdery mildew (Uncinula necator) | Multispectral | NDVI | Strong correlation (r > 0.9) with disease severity under field conditions | Symptom expression varies across cultivars; canopy shading reduces accuracy | [45,138] |
| Grapevine Leafroll Virus (GLRaV) | Multispectral | NDVI, NDRE, REIP | NDRE and REIP improved virus detection compared to NDVI alone; linked to pigment changes | Variable accuracy between vineyards; canopy structure affects detection | [127] |
| Botrytis bunch rot | Multispectral + RGB | NDVI | Early signs detected through reflectance differences | Overlaps with abiotic stress; low specificity | [128] |
| Esca (Trunk disease) | Multispectral | NDVI, GNDVI | Diseased vines had consistently lower NDVI (0.68–0.79) vs. healthy vines | Ineffective at detecting mild/early infections | [133] |
| Phylloxera | Multispectral + Hyperspectral + RGB | Vegetation indices (MCARI, Red-edge indices) | Detected spectral traits linked to reduced vigor and chlorophyll | Confounded with abiotic stress; requires hyperspectral data | [136] |
| Flavescence Dorée (FD) | RGB + Multispectral | GRVI, RGI, NDVI, CI | High discrimination in red cultivars (AUC ≈ 1.0); band selection at 520–800 nm improved classification (>94%) | Accuracy lower in white cultivars; requires optimized sensor settings | [134,135] |
| Jacobiaska lybica (mealybug vector) | RGB | RGB indices | Effective mapping of symptomatic patches | Poor generalization to early stages; manual validation required | [18] |
| Cynodon dactylon (weed competition) | RGB + RGB-NIR | ExGR, GNDVI | Differentiated weeds from soil with >97% accuracy; enabled 48% reduction in herbicide use | Not specific to disease; sensitive to soil background | [137] |
| Index | Sensor | Key Findings in Vineyards | Limitations | Reference |
|---|---|---|---|---|
| NDVI, LAI | Multispectral | NDVI explained phenolic variability across vineyard blocks | Requires calibration with destructive sampling | [82] |
| NDVI | Multispectral | Strong correlation between NDVI and berry ripening parameters | NDVI saturates at high vigor | [150] |
| NDVI, NDRE | Multispectral | Red-edge indices improved sensitivity to chlorophyll/nutrient status | Sensitive to soil/background effects | [147] |
| NDVI | Multispectral, multiple | NDVI captured anthocyanin accumulation trends | Limited resolution at late ripening stages | [148] |
| NDVI | Multispectral | UAV-NDVI predicted °Brix variability | Dependent on local calibration | [157] |
| NDVI | Multispectral | Seasonal correlation between NDVI and berry composition | Correlations varied across seasons | [13] |
| NDVI | Multispectral | Early evidence linking canopy vigor to wine color | Did not include UAV imagery | [145] |
| TCARI/OSAVI | Multispectral + RGB + NIR | Combined indices improved detection of chlorophyll/carotenoids | Sensitive to light conditions | [146] |
| NDVI-RGBVI | Multispectral-RGB | Low-cost RGB indices captured ripening trends | Overlap with vigor effects Effective only at moderate–high stress | [149] |
| NDVI, OSAVI, MSAVI, MCARI | Multispectral | Nutrient status strongly linked with ripening (N, P, K) | Requires simultaneous leaf sampling | [151] |
| VARI, PRI, RGBVI | RGB | Detected phenolic maturity and malic acid changes | RGB less reliable under canopy shadow | [68] |
| NDVI, GNDVI, NDRE, MSAVI | Multispectral | Strong correlations with berry composition and yield | Cultivar-specific responses not standardized | [79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vera-Esmeraldas, A.; Pizarro-Oteíza, S.; Labbé, M.; Rojo, F.; Salazar, F. UAV-Based Spectral and Thermal Indices in Precision Viticulture: A Review of NDVI, NDRE, SAVI, GNDVI, and CWSI. Agronomy 2025, 15, 2569. https://doi.org/10.3390/agronomy15112569
Vera-Esmeraldas A, Pizarro-Oteíza S, Labbé M, Rojo F, Salazar F. UAV-Based Spectral and Thermal Indices in Precision Viticulture: A Review of NDVI, NDRE, SAVI, GNDVI, and CWSI. Agronomy. 2025; 15(11):2569. https://doi.org/10.3390/agronomy15112569
Chicago/Turabian StyleVera-Esmeraldas, Adrián, Sebastián Pizarro-Oteíza, Mariela Labbé, Francisco Rojo, and Fernando Salazar. 2025. "UAV-Based Spectral and Thermal Indices in Precision Viticulture: A Review of NDVI, NDRE, SAVI, GNDVI, and CWSI" Agronomy 15, no. 11: 2569. https://doi.org/10.3390/agronomy15112569
APA StyleVera-Esmeraldas, A., Pizarro-Oteíza, S., Labbé, M., Rojo, F., & Salazar, F. (2025). UAV-Based Spectral and Thermal Indices in Precision Viticulture: A Review of NDVI, NDRE, SAVI, GNDVI, and CWSI. Agronomy, 15(11), 2569. https://doi.org/10.3390/agronomy15112569





