Abstract
Biochar and arbuscular mycorrhizal fungi (AMF) make significant contributions to improving soil and plant mineral nutrition, primarily phosphorus (P). However, the response of soil and leaf P fractions dynamics to biochar and AMF amendment in paddy ecosystems remains unclear. A pot experiment in greenhouse was conducted to study the effects of three biochars produced from rice husk (HBC), maize straw (MBC), and wood chips (WBC) and Rhizophagus irregularis on soil and leaf P fractions, soil chemical properties, and rice growth. The combination of biochar and AMF increased soil content of labile inorganic P (38.25%, 50.87% and 23.65%, respectively) and decreased that of labile organic P (52.31%, 61.12% and 44.60%, respectively) compared to the control. Similarly, HBC and MBC with AMF combination increased leaf contents of inorganic (7.29% and 8.81%, respectively) and nucleic acid (18.75% and 14.73%, respectively) P, which were strongly correlated with soil labile P fractions. Biochar and AMF amendment governed the transformation of soil P by altering total P, organic matter, and pH. Meanwhile, the distribution of leaf P was influenced by leaf total P content, soil organic matter, and electrical conductivity (EC). In addition, MBC and HBC increased the rice mycorrhizal colonization rate by 6.78% and 18.19%, respectively. The application of HBC and AMF increased leaves’ and stems’ biomass (28.57% and 26.67%, respectively), and three biochars and AMF also facilitated P accumulation in rice. Therefore, these results provide the first evidence for the interaction between biochar and AMF to alter P distribution among leaf fractions in paddy fields.
1. Introduction
Phosphorus (P) is an essential nutrient for plant growth and development, as well as the overall productivity of agricultural ecosystems, and it is involved in major processes of biological metabolism [1,2]. In the paddy ecosystem, P limitation due to high weathering and leaching is common, which requires a large amount of P fertilizer input to improve agricultural productivity [3]. However, excessive application of P fertilizer can lead to the accumulation of a large amount of P in the soil, which precipitates with Fe (II) and Al under acidic conditions, or adsorbs on Fe (III) (hydroxyl) oxides [4]. This not only reduces the utilization efficiency of P fertilizer, but also increases the environmental risk of soil P release into the aquatic systems [5,6].
The chemical fraction and abundance of P in soil directly affect the absorption of P by plants, and soil P transformation is influenced by soil physics, chemistry, and microbial activity [7]. The labile P in soil, including P extracted by resin and NaHCO3, is a component that can be directly utilized by plants, whereas the insoluble P in soil needs to be transformed by long-term action of soil microorganisms and plant root exudates before it can be utilized by plants [8,9]. Similarly, there is also a large amount of moderately labile P extracted by NaOH in soil, which plays an important role in the transformation of insoluble P into labile P [4]. Given that the transformation of soil P directly regulates plant growth and productivity [10], it is necessary to find an effective and sustainable alternative to P fertilizer. In particular, the combined application of biochar and AMF is receiving increasing attention, as they may have great potential as biochar-based microbial fertilizers in restoring soil environments and sustainable agricultural development [11].
Biochar, a carbon-rich material produced from the pyrolysis of biomass under oxygen-limited conditions [12], possesses a large specific surface area, high porosity, and abundant P content. It is worth noting that although the addition of biochar can directly increase the availability of P in soil, this depends entirely on its initial material and pyrolysis conditions. Biochar could also enhance soil physicochemical properties such as aeration and cation exchange capacity [13]. These improvements create a favorable environment for microbial activity and root development, thereby facilitating the transformation of soil phosphorus and driving a positive plant-soil P cycle [14]. Consequently, biochar application represents a promising strategy for improving soil fertility and structure in agricultural systems.
Arbuscular mycorrhizal fungi (AMF), an indigenous microorganism, can form a symbiotic relationship with more than 80% of plants on land, helping host plants absorb nutrients, especially P from the surrounding soil [15,16]. Numerous studies have shown that AMF is particularly effective in improving plant P uptake [16,17,18]. For example, AMF hyphae can extend to soil areas beyond the reach of roots, significantly expanding the P absorption area of rice. The absorption efficiency of P by hyphae is 14 times that of roots, and even when flooding causes root hypoxia and a decrease in absorption capacity, difficult to dissolve P can still be efficiently absorbed by hyphae [16,17]. The AM fungal hyphae can also secrete organic acids and phosphatase to activate insoluble P in soil, thereby increasing soil P availability [19]. Under flooded conditions, AMF activates aquaporins such as CsPIP1:6 and accumulates osmoregulatory substances such as proline, helping rice roots maintain aerobic metabolism and cell osmotic balance, indirectly ensuring the energy required for P absorption [20]. Therefore, the colonization intensity of AMF is suppressed under flooded conditions, but residual AMF can still significantly promote rice P uptake.
In plants, leaves are considered to be the most sensitive organs to soil nutrient sufficiency or deficiency, and the distribution of P in leaves is a key strategy for coping with changes in soil P [21,22]. Studies have shown that changes in the concentration and abundance of different chemical forms of P in leaves are related to the distribution of soil P fraction and physicochemical properties of soil [10,23]. The chemical forms of P in leaves are divided into inorganic P, sugar P, nucleic acid P, and insoluble P [24]. The content of inorganic P in leaves reflects the supply level of inorganic P in soil [25]; sugar P is considered the most important fraction for studying P limitation, and it mainly participates in active metabolism [26]; nucleic acid P is an important component of RNA; therefore, its distribution is crucial for protein synthesis, and insoluble P mainly includes insoluble P compounds bound to phosphorylated proteins [27]. However, it is unclear how leaf P fractions respond to the addition of biochar and AMF in rice ecosystems.
Biochar and AMF combined amendment plays an important role in promoting P cycling between plants and soil [28,29]. While the synergistic benefits of biochar and AMF on plant P uptake in controlled or upland systems are increasingly recognized, critical knowledge gaps remain regarding their interactive effects on the internal phosphorus cycling within the soil-plant continuum of paddy ecosystems. Specifically, it is unclear how biochar and AMF affect the chemical speciation of P in both soil and plant leaves, and more importantly, how the dynamics of soil P fractions are functionally linked to plant P fractions. Therefore, this study aimed to investigate the responses of a comprehensive suite of soil and leaf P fractions to different biochar types and AMF in a rice paddy system. We hypothesized that (1) biochar and AMF together would enhance soil P turnover and availability; (2) biochar and AMF amendment would alleviate soil P limitation, thereby changing the distribution strategy of leaf P and promoting plant P accumulation.
2. Materials and Methods
2.1. Material Preparation
The soil used in this experiment was collected from a depth from the 0–20 cm layer of a paddy field (Alfisol) that continuously planting rice at Shenyang Agriculture University, Liaoning Province, China (41°48′ N, 123°33′ E). The air-dried soil was passed through a 2 mm sieve and then sterilized by autoclaving at 120 °C for 2 h. The biochar was pyrolyzed for 1 h under oxygen-free conditions from rice husk, maize straw, and wood chip at 500 °C. In this study, the addition of biochar was 1% (w/w), because it had a significant effect on the colonization of AMF in our previous study [30]. The basic properties of the biochar and soil are shown in Table S1.
The AM fungal Rhizophagus irregularis BGC JX04B was obtained from the Bank of Glomeromycota in China, the Institute of Plant Nutrition and Resources, Beijing Academy of Agriculture and Forestry Sciences, Beijing, China. The inoculum was propagated using Zea mays L. as the host plant and mixture of soil and river sand (1:1, w/w) as the substrate for four months in a greenhouse. The inoculum consisted of a substrate containing spores, root segments, and hyphae and 1 g inoculum contained 30 spores. This rice (Oryza sativa L.) cultivar plant was conventional japonica rice Shennong 265 (Rice Research Institute, Shenyang Agricultural University, China). The rice seeds were sterilized using 75% ethanol for 10 s and 1% sodium hypochlorite (NaClO) for 15 min, with shaking every five minutes. After washing three times with deionized water, they were placed in a Petri dish and cultured in a sterile environment for 10 days before being transplanted.
2.2. Experimental Design
The experiment was a 4 (biochar: Control, HBC, MBC, WBC) × 2 (AMF: AMF−, AMF+) factorial arrangement in a completely randomized design with three replications. The treatment information is shown in Table S2. Firstly, we filled 700 g of sterilized soil into each of 24 bottom-sealed plastic pots (diameter 14 cm, height 12 cm). This biochar was fully mixed with randomly selected 18 pots of soil at a rate of 1% (w/w) and each type of biochar accounted for 6 pots, while the remaining pots were not mixed with biochar. Then, for both each type of biochar and without biochar treatment, half of the pots were randomly selected and 10 g of air-dried AMF inoculants (300 spores) were placed 5 cm below the soil surface. Subsequently, for non-inoculated AMF treatments, the same amount of sterilized AMF inoculant was added to the same location in each pot. To maintain the bacterial community of the original soil, we added 10 mL of unsterilized soil filtrate (soil: ddH2O = 1:5, m/v) without AMF spores to each pot [31]. Finally, we transplanted a rice seedling with consistent growth in each pot.
This experiment was conducted in a greenhouse at Shenyang Agricultural University from May to August 2023. Rice plants were grown under a 16/8 h day/night cycle with temperatures of 30/25 °C and relative humidity of 50/65%. A combination of natural sunlight and supplemental LED lighting was used to maintain a light intensity of approximately 300 µmol/m2/s at the plant canopy level. The pots were re-randomized on a weekly basis throughout the cultivation period to minimize positional effects. All pots were watered daily with deionized water to maintain a waterlogged state (with a 1–2 cm standing water layer) and received a weekly supplementation of 10 mL of quarter-concentration Kimura B nutrient solution (nitrogen: 5.07 mg/kg soil, phosphorus: 1.11 mg/kg soil, potassium: 5.57 mg/kg soil) to support normal rice growth [32].
2.3. Plant and Soil Sampling
After 60 days of transplanting, we harvested the rice plants from each pot, then carefully cleaned the plant roots with deionized water. The plants were divided into leaves, stems, and roots and analyzed for P content, leaf P fractions, and dry matter weight following dry at 60 °C to constant weight. Subsequently, the soil was collected and mixed evenly from each pot. The sampled soil was air-dried and sieved through a 2 mm particle size sieve for measuring soil properties.
2.4. Plant Chemical Analysis
The plant samples (leaves, stems, roots) were ground, mixed, and sieved (<0.15 mm) and digested using H2SO4-H2O2. The P content in the leaves, stems, and roots of the plant was measured using the molybdenum blue colorimetric method. The fresh plant root sample was cut into 1 cm pieces and washed with 10% KOH and stained with 0.05% Trypan Blue. The mycorrhizal colonization rate was quantified using a cross over method under a 200× light microscope (Olympus Corporation, Tokyo, Japan) [33].
2.5. Leaf P Chemical Forms Analysis
Four P chemical forms (Inorganic P concentration, sugar P content, nucleic acid P content, insoluble P content) in rice leaves were analyzed using trichloroacetic acid (TCA) continuous extraction method [27]. Specifically, the dried leaf material after grinding was extracted by 25 mL 0.3 M cold TCA and centrifuged. Part of the supernatant was used to determine inorganic P content, while the remaining part was digested with H2SO4-H2O2 to determine sugar P content. The leaf residue was extracted and centrifuged with 25 mL 0.15 M hot TCA, and the supernatant was digested with H2SO4-H2O2 to determine nuclear acid P content. Thus, the insoluble P content was calculated as the difference between the total P content in the leaves and the other forms. The above P forms were determined using the molybdenum blue colorimetric method.
2.6. Soil P Fractions Analysis
Soil P fractions were analyzed according to the methods of Hedley et al. [7] and Sui et al. [34]. Specifically, we placed 1 g of soil in a 50 mL centrifuge tube and extracted it continuously with 30 mL deionized water, 30 mL 0.5 M NaHCO3, 30 mL 0.1 M NaOH, and 30 mL 1 M HCl following 16 h of shaking and centrifugation for 10 min. The total P contents of NaHCO3 and NaOH extracts were determined by digestion with potassium persulfate (K2S2O8) at 120 k Pa and 121 °C for 60 and 90 min, respectively. The content of organic P in the extract was calculated as the difference between total P and inorganic P. The residual P content in the soil residue was determined following digestion with 5 mL of concentrated H2SO4 and H2O2 at 360 °C. All P contents were measured using the molybdenum blue colorimetry method. The amount of P in the soil is divided into labile Pi (H2O-P, NaHCO3-Pi), labile Po (NaHCO3-Po), moderately labile Po (NaOH-Po), moderately labile Pi (NaOH-Pi), and sparingly labile P (HCl-Pi, residual P).
2.7. Soil Chemical Properties Analysis
The soil pH was measured using a ratio of 1:2.5 between soil and deionized water. The soil EC (electrical conductivity, μS cm−1) was measured using a conductivity meter with a soil water ratio of 1:5. The Element Analyzer (Elemental Macro Cube, Frankfurt, Germany) was used to determine the total C and N content of soil, and soil organic matter (SOM) content was calculated as 1.724 times the soil C content [10]. Soil dissolved organic carbon (DOC) and nitrogen (DON) content were measured using a TOC/TN analyzer (multiN/C 3100; Analytik, Jena, Germany) following 2 M KCl extraction.
2.8. Statistical Analysis
Two-way analysis of variance (ANOVA) was performed to determine the statistical significance (p < 0.05) of the influence of different biochar types and AMF and their interactive effects on variations using Duncan’s multiple range test; analyses were conducted in SPSS 26.0 (SPSS Inc, Chicago, IL, USA). All data were presented as means ± standard deviation (SD, n = 3). All data met assumptions of normality and variance homogeneity (p > 0.05). Pearson’s correlation between soil P fractions, leaf P fractions, and soil chemical properties was analyzed using Origin 2023b. Redundancy analysis (RDA) was performed using CANOCO (v5.0) to explore the main factors affecting soil P fractions and leaf P chemical forms. The linear regression relationship between soil P fractions and leaf P fractions was analyzed using Origin 2023b after logarithmic transformation of natural data.
3. Results
3.1. Soil P Fractions
The soil P fractions were dominated by sparingly labile P, followed by moderately labile Po and Pi, with the proportion of labile Po and Pi being low in all treatments (Figure 1a). In the absence of mycorrhizal fungi, the three biochars significantly increased soil labile Pi content, with increases of 32.90%, 37.77% and 8.28% compared to the control, respectively. Similarly, in the presence of mycorrhizal fungi, biochar also significantly increased labile Pi content by 38.25%, 50.87% and 23.65% and decreased labile Po content by 52.31%, 61.12% and 44.60%, respectively, compared to the control. Whether under non mycorrhizal or mycorrhizal inoculation conditions, biochar addition significantly reduced labile Po content compared to the control. In the treatment of non-mycorrhizal fungi, HBC and MBC significantly increased moderately labile Pi content by 6.21% and 10.06%, respectively, compared to the control, while WBC had no significant effect. Interestingly, under the conditions of mycorrhizal inoculation, the effects of three biochars on moderately labile Pi were the same as those of non-mycorrhizal treatment. Under non-mycorrhizal treatment, only WBC significantly increased moderately labile Po content compared to the control. When inoculated with mycorrhizal fungi, the effect of WBC on moderately labile Po was no longer significant compared to the mycorrhizal control. The content of sparingly labile P in soil amended with MBC was the highest under non-mycorrhizal and mycorrhizal conditions.
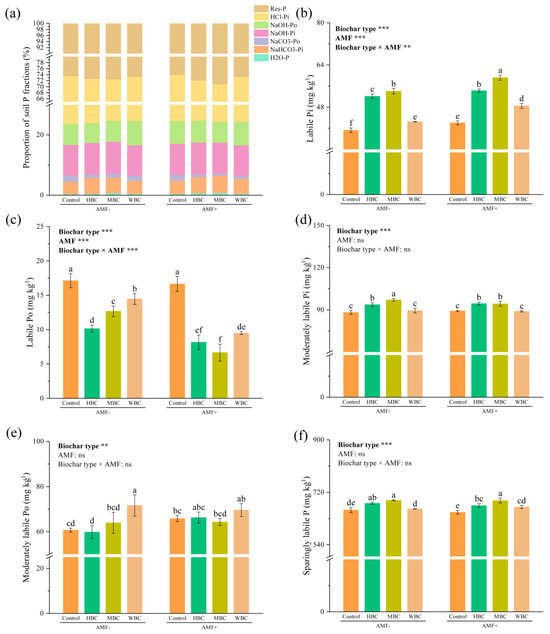
Figure 1.
The proportion of different soil P fractions (a). The variation in labile Pi content (b), labile Po content (c), moderately labile Pi content (d), moderately labile Po content (e), sparingly labile P content (f) in soil. Different lowercase letters indicate significant differences among different treatments (p < 0.05). Values are means ± standard deviation (n = 3). The bar represents the average standard deviation of the mean. ** p < 0.01, *** p < 0.001; ns, not significant.
3.2. Soil Chemical Properties
Regardless of the absence or presence of mycorrhizal fungi, biochar addition significantly increased soil pH and EC compared to the control (Figure 2a). However, mycorrhizal fungal inoculation significantly reduced soil pH compared to the control non-mycorrhizal soil. In the absence of mycorrhizal fungi, HBC and WBC significantly increased soil total nitrogen, with increases of 6.45% and 10.75% compared to the control, respectively. Interestingly, in the case of mycorrhizal inoculation, soil total nitrogen added with biochar was no longer better than that of the control mycorrhizal soil. The three biochars significantly increased soil organic matter compared to the control under non-mycorrhizal and mycorrhizal conditions. For non-mycorrhizal treatments, only MBC significantly increased soil dissolved organic carbon by 8.07%, compared to the control. In the case of mycorrhizal inoculation, both MBC and WBC significantly increased dissolved organic carbon, with increases of 12.36% and 17.44% compared to the control mycorrhizal soil, respectively. In the absence of mycorrhizal fungi, biochar addition had no significant effect on the dissolved organic nitrogen in soil. Interestingly, in the presence of mycorrhizal fungi, HBC significantly increased dissolved organic nitrogen compared to the control mycorrhizal soil.
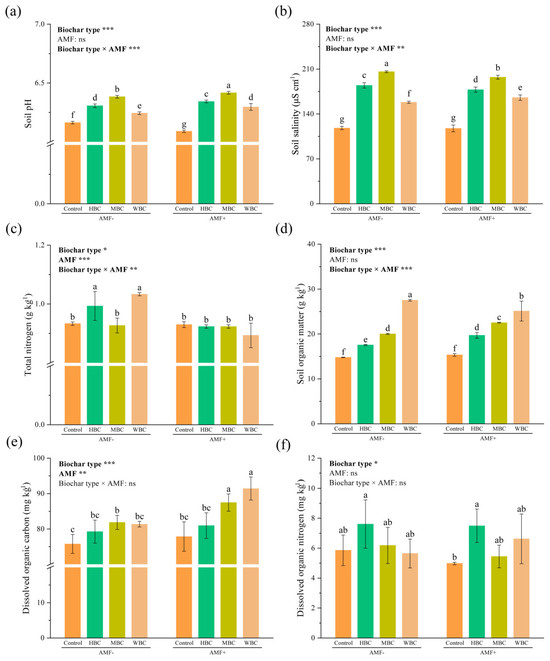
Figure 2.
Effects of biochar and AMF amendment on pH (a), EC (b), total nitrogen (c), organic matter (d), dissolved organic carbon (e) and dissolved organic nitrogen (f) in soil. Different lowercase letters indicate significant differences among different treatments (p < 0.05). Values are means ± standard deviation (n = 3). The bar represents the average standard deviation of the mean. * p < 0.05, ** p < 0.01, *** p < 0.001; ns, not significant.
3.3. Rice Leaf P Chemical Forms
The chemical form of P in rice leaves was dominated by inorganic P, followed by sugar P and nucleic acid P, while the proportion of insoluble P content was the lowest in each treatment (Figure 3a). In the absence of mycorrhizal inoculation, the three biochars significantly increased leaf inorganic P content, with increases of 11.65%, 18.38% and 4.77% compared to the control plant, respectively. In the presence of mycorrhizal fungi, HBC and MBC also significantly increased leaf inorganic P content by 7.29% and 8.81%, respectively, compared to the control. Interestingly, when inoculated with mycorrhizal fungi, the effect of WBC on inorganic P content was no longer significant compared to the control plant. Under the non-mycorrhizal condition, only HBC significantly reduced leaf sugar P content compared to the control. Interestingly, in the presence of mycorrhizal fungi, the effect of HBC on sugar P was no longer significant, while WBC significantly reduced sugar P content compared to the control plant. In non-mycorrhizal plants, compared to the control plant, three biochars significantly increased leaf nucleic acid P content by 18.75%, 14.73% and 8.04%, respectively. Under the presence of mycorrhizal fungi, the rice leaf nucleic acid P content amended with MBC was the highest among all treatments. Whether mycorrhizal fungi were present or not, WBC significantly increased leaf insoluble P content compared to the control.
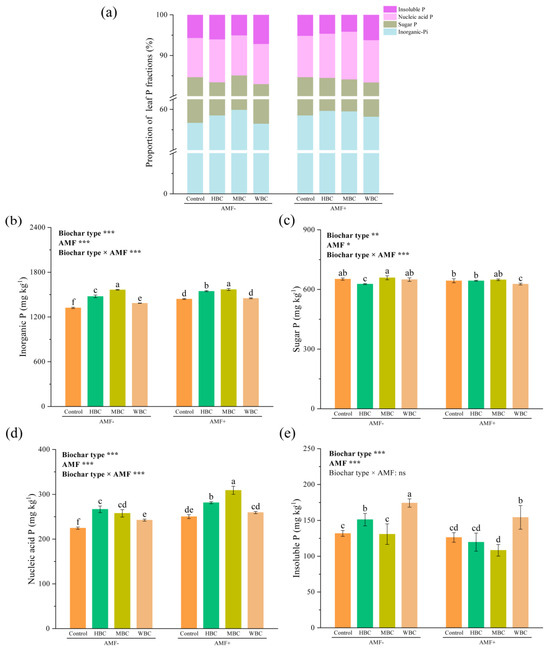
Figure 3.
The proportion of different leaf P fractions (a). The variation in inorganic P content (b), sugar P content (c), nucleic acid P content (d), insoluble P content (e) in rice leaves. Different lowercase letters indicate significant differences among different treatments (p < 0.05). Values are means ± standard deviation (n = 3). The bar represents the average standard deviation of the mean. * p < 0.05, ** p < 0.01, *** p < 0.001; ns, not significant.
3.4. Dry Weight, P Content, Total P Uptake, and Mycorrhizal Colonization Rate in Rice
In the absence of mycorrhizal fungi, only MBC significantly increased biomass of rice leaves and stems by 21.43% and 20.00%, respectively, compared with the control (Table 1). In the presence of mycorrhizal fungi, compared with the control, HBC significantly increased biomass of rice leaves and stems by 28.57% and 26.67%, respectively, while MBC no longer significantly increased. In non-mycorrhizal plants, only HBC significantly increased root biomass of rice by 28.57%, compared to the control plant. Under non-mycorrhizal conditions, three biochars significantly increased P content in rice leaves, stems, and roots compared to the control plant. When inoculated with mycorrhizal fungi, the three biochars only significantly increased P content in rice stems, while the amendment with MBC and WBC significantly reduced P content in rice roots. The total P uptake in the presence of mycorrhizal conditions was highest in rice plants fertilized with HBC. For non-mycorrhizal treatment, no mycorrhizal fungal colonization was detected. The HBC and MBC significantly increased mycorrhizal colonization rate of rice by 6.78% and 18.19%, respectively, compared to the control, while WBC had no significant effect.

Table 1.
The dry weight, P content, total P uptake and mycorrhizal colonization rate of rice.
3.5. Correlation Analysis Between Leaf P Chemical Forms, Soil P Fractions, and Soil Chemical Properties
As shown in Figure 4 and Table S3, there was a positive correlation between soil labile Pi and leaf inorganic P and nucleic acid P (p < 0.01). However, soil labile Po had adverse effects on leaf P. Interestingly, there was a significant positive correlation between soil total P and leaf inorganic P and nucleic acid P (p < 0.01). There was a significant positive correlation between leaf P and soil EC, for example, soil EC was related to leaf inorganic P, sugar P, and insoluble P (p < 0.01). Similarly, the response of inorganic P and nucleic acid P in leaves to soil pH was also positive, while there was no significant relationship between sugar P and insoluble P with soil pH.
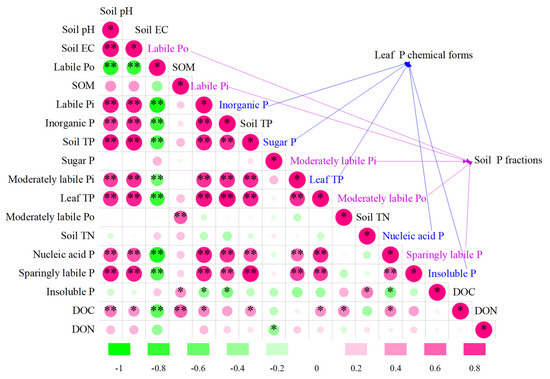
Figure 4.
Pearson’s correlation analysis between leaf P chemical forms, soil P fractions, and soil properties. The depth of color and the size of the circle represent the size of the Pearson’s correlation coefficient. Green represents a negative Pearson’s coefficient, and red represents a positive Pearson’s coefficient. The closer to ±1, the stronger the correlation. The closer to 0, the weaker the correlation. Significances are shown only if p values < 0.05. * p < 0.05, ** p < 0.01. SOM, soil organic matter; Soil TN, soil total nitrogen; DOC, dissolved organic carbon; DON, dissolved organic nitrogen.
The major factors affecting soil P fractions and leaf P chemical forms were identified by redundancy analysis (Figure 5). The direction of an environmental factor arrow indicates the direction of increase in that variable. The length of the arrow corresponds to its importance in explaining the variation in the data. The angle between the arrows indicates their correlation (acute angle represents positive correlation; obtuse angle represents negative correlation). The position of a treatment symbol relative to an arrow indicates the relative value of that variable in that treatment. The soil chemical properties can explain the variability of 88.4% and 92.8% in soil P fractions and leaf P chemical forms, respectively. There was a significant correlation between soil P fractions and total P content, SOM content, and pH in soil. Likewise, there was a significant correlation between leaf P fractions and leaf P content, SOM content, and soil salinity.
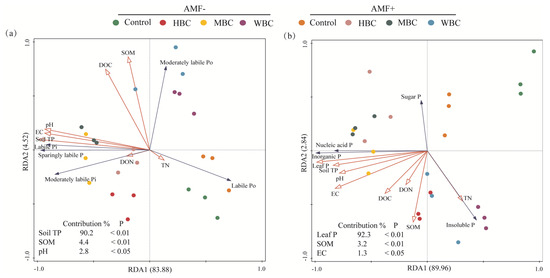
Figure 5.
Redundancy analysis (RDA) of the effects of biochar and AMF amendment on soil P fractions (a) and chemical forms of P in rice leaves (b). The red arrows represent explanatory variables and the blue arrows represent the response variables. SOM, soil organic matter; TN, soil total nitrogen; DOC, dissolved organic carbon; DON, dissolved organic nitrogen.
3.6. Linear Relationship Between Leaf P Chemical Forms and Soil P Fractions
The linear relationship between leaf P chemical forms and soil P fractions was explored (Figure 6). In general, this result indicated a significant positive correlation between leaf P and soil labile Pi, and a significant negative correlation with soil labile Po. Notably, there was a significant positive correlation between inorganic P and nucleic acid P in leaves and labile Pi in soil. Similarly, it had the same positive correlation with moderately labile Pi and sparingly labile P in soil. At the same time, a significant negative correlation was found between leaf inorganic P and nucleic acid P and labile Po in soil. Overall, leaf P was dominated by labile P in the soil.
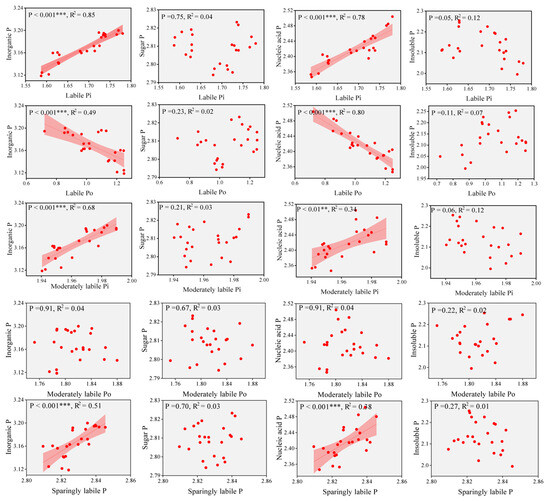
Figure 6.
Linear correlation between soil P fractions and leaf P chemical forms. Shaded areas the 95% confidence regions of the regression curves. The red dots represent the value of each sample. R2 and p values for linear trend lines are shown on each plot. ** p < 0.01, *** p < 0.001.
4. Discussions
4.1. Effects of Biochar Combined with AMF on Soil P Fractions
The labile P fraction in soil, most easily accessible available P pool for plants, is composed of organic and inorganic fractions. The labile Pi fraction (H2O-P and NaHCO3-Pi) is a weakly adsorbed component in soil, while the labile Po fraction (NaHCO3-Po) is a relatively mineralized component in soil solution [8]. In this study, biochar amendment increased soil labile Pi content and decreased labile Po content. This may be due to the direct input of a large amount of available P by biochar addition [35], and we found that the changes in labile Pi content in the three biochar treatments were consistent with the effective P content of the biochar itself. Among the biochars, MBC had the highest EC and total P (Table S1), which may explain its stronger enhancement in labile P availability. Another possible explanation is that biochar increased soil pH, causing hydroxyl groups (OH−) to compete with phosphate groups (PO43−) for coordination sites on the surface of soil iron aluminum (hydrogen) oxides, resulting in the desorption of P that was originally fixed by covalent bonds with ligands [36]. In addition, the surface of biochar contains abundant carboxyl and phenolic hydroxyl groups, which have strong affinity for metal oxide surfaces and can “displace” adsorbed phosphate ions into soil solutions [4]. We found that the presence of AMF increased labile Pi content, but had no significant effect on labile Po. The extraradical hyphae of AMF can secrete organic acids and recruit beneficial bacteria to dissolve and mineralize organic P in soil, thereby increasing soil P availability [37]. We also found that biochar and AMF together significantly increased soil labile Pi content and decreased labile Po content, and this effect was more significant than the addition of biochar and AMF alone (especially maize straw biochar). One possible explanation is that biochar had a porous structure and abundant oxygen-containing functional groups, which improved soil aeration and nutrient retention capacity, and provided a comfortable environment for AMF spore germination and hyphae growth [38]. Another possible reason is that biochar and AMF together promoted much more organic P mineralization in soil than when either AMF or biochar was present, leading to the transformation of labile Po to Pi in soil [39,40].
The moderately labile P fraction, relatively insoluble fraction in soil, composed of NaOH-Pi and NaOH-Po, and coexisting with iron and aluminum oxides and humus, played an important role in the transformation of soil P fractions [8]. Our results indicated that the combination of HBC and MBC with AMF increased soil moderately labile Pi content. The increase in moderately labile Pi may be due to the introduction of iron and aluminum oxides related to P, or from the transformation of insoluble P [4]. The correlation analysis showed that there was a significant positive correlation between soil labile Pi and moderately labile Pi with total P content (Figure 4), which also verified the transformation of closed and non-available P fractions into bioavailable P. In addition, we found that the combination of HBC and AMF increased dissolved organic nitrogen in soil, and this mineralization of organic P related to soil nitrogen availability was widely observed in natural vegetation communities.
Sparingly labile P (HCl-Pi and Res-P), the dominant P fraction in the soil, represents a relatively stable pool that is not directly available to plants [41]. In this study, HBC and MBC treatments increased the content of this pool in the absence of AMF, while WBC did so when combined with AMF. We speculate that this increase likely represents a temporary immobilization rather than permanent sequestration. It is primarily attributed to the introduction of inherent, sparingly labile P from the biochar itself (Table S1). Crucially, this pool can act as a slow-release reservoir; processes such as microbial activity (particularly with AMF) and the gradual decomposition of recalcitrant organic compounds (e.g., lignin-encapsulated cellulose) that initially adsorb or precipitate P [42], may facilitate its subsequent release into more labile forms over time. Therefore, while this initial increase might temporarily reduce immediate availability, it potentially enhances long-term P sustainability by buffering the soil P pool against fixation and leaching.
4.2. Effects of Biochar Combined with AMF on Leaf P Fractions and Rice Physiology
In this study, we found that inorganic P dominated the chemical forms of P in the leaves, followed by sugar P and nucleic acid P, while insoluble P had the lowest content among all treatments (Figure 3a). Both biochar and AMF alone or together increased leaf inorganic P content, which may be related to changes in soil P availability. Furthermore, the inorganic P in leaves was limited by the content of organic P and available P in the soil, and high metabolic activity can also lead to an increase in the content of available P and inorganic P in leaves [25]. The correlation analysis also indicated a significant positive correlation between leaf inorganic P content and available P (labile Pi content) in soil (Figure 4).
Nucleic acid P is the main organic form of P in plants, and it is an important component of RNA (especially rRNA), while sugar P mainly participates in active metabolism in plants [25]. We found that three biochars significantly increased leaf nucleic acid P content, and this effect was more significant in the presence of mycorrhizal fungi (except for wood biochar treatment). Our results also indicated a positive correlation between leaf nucleic acid P content and the content of labile Pi and moderately labile Pi in soil. The positive correlation can be interpreted as evidence that this soil P pool acts as a reserve supporting the labile P pool under sustained AMF activity. This process is critical for long-term P supply, as the AMF-mediated mobilization of moderately labile Pi ensures a steady P flux to the plant. This P is then strategically allocated to nucleic acid P, promoting the synthesis of RNA and the proteins necessary for photosynthesis and development [10], thereby directly linking the utilization of a stable soil P reserve to enhanced plant growth.
Interestingly, the HBC treatment and the WBC+AMF combination significantly reduced the content of sugar P in rice leaves, a phenomenon previously associated with alleviated P limitation [22]. The decrease in leaf sugar P content carries important physiological implications for rice metabolism. Specifically, the redistribution from sugar P to inorganic and nucleic acid P directly influences the plant’s energy balance and growth capacity. The replenished inorganic P pool is crucial for sustaining ATP synthesis, thereby ensuring ample energy for photosynthetic metabolism. Meanwhile, the increased allocation to nucleic acid P supports the RNA and protein synthesis that underlies the observed biomass increase. This shift, triggered by the improved P availability from the amendments, indicates a transition from a P-limited state, where P is tied up in basic metabolites, to a growth-driven state where P is invested in energy transfer and genetic expression [22,43].
In this study, we found that the combination of HBC and MBC with AMF significantly increased rice leaf biomass, but WBC had no significant effect. At the same time, we also observed that HBC and MBC significantly increased the mycorrhizal colonization rate, while WBC had no significant effect. We attribute this disparity to the intrinsic properties of WBC derived from its woody feedstock. Firstly, WBC typically possesses a lower inherent nutrient content (including P) and a smaller specific surface area compared to HBC and MBC (Table S1), limiting its direct nutrient contribution and capacity to retain water and nutrients in the rhizosphere. Secondly, and perhaps more critically, the high lignin content of woody biomass leads to the formation of a more recalcitrant carbon structure upon pyrolysis. This recalcitrance likely resulted in a slower release of labile organic compounds, providing less available carbon to stimulate the soil microbial community and, specifically, to effectively fuel the carbon-demanding symbiotic association with AMF [44]. Consequently, the weaker microbial and mycorrhizal stimulation by WBC translated into diminished effects on soil P fraction mobilization and ultimately, plant biomass growth.
4.3. Relationship Between Soil P Fractions, Leaf P Fractions, and Soil Chemical Properties
Labile P is an important soil P fraction for studying the potential correlation between plant P nutrient and soil P [22]. It is considered a fraction that plants can directly utilize in the short term and is related to the chemical form of P in plants [23]. We found that soil labile Pi (H2O-P or NaHCO3-Pi) had a strong and positive correlation with leaf P fractions and total P content, while labile Po (NaHCO3-Po) had a negative correlation (Figure 4), and the contribution of soil labile P fractions to leaf P chemical forms was greater than that of other soil P fractions. Similarly, Galván-Tejada et al. [45] also found a great correlation between P extracted by resin and P extracted by sodium bicarbonate and leaf P content. Gao et al. [22] also found that the contribution of soil labile P (Po+Pi) fractions to leaf P chemical forms was higher than other soil P fractions in P limited desert ecosystems. Previous studies have shown that plant P uptake came not only from labile P in the soil, but also from moderately labile P or even insoluble P pools [46]. Conversely, studies have also found that the process and time of activating moderately labile and stable P in soil to supplement the available P pool will be extremely long, especially in arid soil areas [47]. In this study, it was also found that the contents of moderately labile Pi and sparingly labile P had a significant positive correlation with leaf P (nucleic acid P and inorganic P) fractions (Figure 6), indicating that they were also the main source and potential utilization of P in plants. We speculate that this may be caused by the large amount of additional moderately labile P brought by biochar.
Environmental factors are key driving factors for soil P fractions and plant P chemical forms. Our analysis showed that soil contents of total P and organic matter and pH in soil were the main driving factors for changes in soil P fractions (Figure 5a), while leaf P content, soil organic matter content, and soil EC were the main driving factors for changes in plant P fractions (Figure 5b), indicating that soil organic matter content is closely related to the mineralization of P by soil microorganisms. Furthermore, soil microorganisms can decompose soil organic matter to produce more organic acids, thereby improving the soil’s ability to convert labile P, ultimately leading to an increase in plant uptake of soil P [22].
4.4. Effect of Biochar Combined with AMF on Sustainable Fertilization of Rice
Soil properties are closely related to plant-soil nutrient cycling [48]. The significant increase in soil dissolved organic C and N under the combined biochar and AMF treatments (Figure 2) served as a critical stimulant for microbial-mediated P mineralization. This input of readily available C and N likely enhanced microbial biomass and activity, which in turn promoted the synthesis of phosphatase enzymes. This process exemplifies C-P coupling, where the availability of labile carbon directly drives the microbial mining of organic P [49]. Biochar’s porous structure further supported this process by providing a niche for the proliferating microbial community. Thus, the co-application did not merely add nutrients but engineered a more active microbial environment that accelerated the conversion of stable P into labile forms. It is noteworthy that biochar amendment significantly increased soil EC due to the release of soluble salts inherent to the material. This salinity increase presents a legitimate concern, as high EC can not only directly impair rice growth but also inhibit the colonization and symbiotic efficiency of AMF, which are highly salt-sensitive, thereby potentially negating the P acquisition benefits of the partnership [50]. While our study did not observe severe negative effects, likely because EC remained below a critical threshold, the risk necessitates mitigation strategies for practical applications. Prospective solutions include employing pre-washed biochar, utilizing lower application rates, and inoculating with salt-tolerant AMF strains to safeguard both crop productivity and microbial functionality.
Our research provides valuable insights into the synergistic effects of biochar and AMF on P cycling in a simulated paddy environment; however, its limitations warrant consideration. Firstly, the relatively short duration of the greenhouse experiment may not fully represent long-term P dynamics or the perennial nature of AMF, potentially missing critical feedback mechanisms. A key constraint is the simplified growth environment, as the small pot volume and controlled conditions cannot fully replicate the complex biogeochemical interactions of an actual flooded field. Secondly, while we observed significant changes in P availability and plant uptake, the absence of data on related enzymatic activities (e.g., phosphatase) and broader microbial community shifts means the precise mechanistic pathways are not fully elucidated.
Therefore, future investigations should prioritize long-term field studies over multiple growing seasons to verify the persistence of these effects under real-world conditions. Mechanistic understanding could be advanced by quantifying phosphatase activity and profiling the entire microbial community’s response to biochar, with particular focus on the AMF community. The use of isotopic tracing (e.g., 32P or 33P) is recommended to directly quantify P flux from biochar and soil pools via the AMF pathway to the plant. Practically, our results suggest that co-applying biochar and AMF is a promising strategy for reducing dependency on synthetic P fertilizers in paddy systems. This approach enhances the availability of native soil P and improves plant acquisition efficiency, thereby potentially lowering production costs and environmental impacts while maintaining soil health. Future work should assess the economic feasibility and scalability of this integrated management practice to facilitate its widespread adoption in sustainable rice agriculture.
5. Conclusions
Our results suggest that the combined application of biochar and AMF, particularly HBC and WBC, plays a crucial role in promoting soil P turnover and affecting the distribution of P in plant leaves in paddy ecosystems (Figure 7). (i) The co-application of biochar and AMF exhibited a synergistic effect, significantly enhancing the pool of labile Pi in the paddy soil. (ii) This synergy facilitated a strategic reallocation of P within rice leaves, increasing the proportion of P in metabolically active forms. (iii) Our results highlight the potential of integrating biochar and AMF as a sustainable management strategy to improve P use efficiency and reduce dependence on mineral P fertilizers in paddy systems.
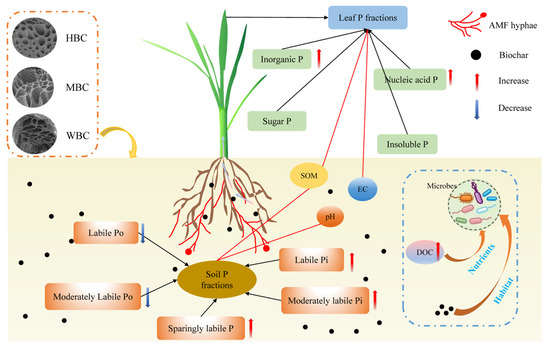
Figure 7.
Mechanism diagram of biochar combined with AMF promoting soil P transformation and leaf P distribution. The red line represents the correlation between two variables. The mechanism within the blue dashed box has not been validated in this study. SOM, soil organic matter. EC, electrical conductivity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15112562/s1, Table S1: Physicochemical properties of biochars derived from maize straw (MBC), rice husk (HBC), and wood chips (WBC), and of the experimental soil prior to treatment; Table S2: Experimental treatment codes and descriptions; Table S3: Pearson correlation analysis between leaf P chemical forms, soil P fractions, and soil properties.
Author Contributions
Conceptualization, Z.W., X.Y. and J.M.; Methodology, Z.W., X.Y., X.Z., Y.S. and H.Z.; Formal analysis, Z.W., X.Z., Y.S. and H.Z.; Investigation, Z.W., X.Z., Y.S. and H.Z.; Data curation, Z.W.; Visualization, X.Z., Y.S. and H.Z.; Writing—original draft, Z.W.; Writing—review and editing, Z.W., X.Y. and J.M.; Supervision, J.M.; Project administration, Z.W.; Funding acquisition, X.Y. and J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financially supported by the National Key R&D Program of China (2024YFD2301200), the National Natural Science Foundation of China (42207382), the Science and Technology Project of Liaoning Province (2024JH2/102400001), and the Earmarked Fund for China Agriculture Research System (CARS-01-51).
Data Availability Statement
The original contributions presented in this study are included in this article/Supplementary Materials; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Neset, T.S.S.; Cordell, D. Global phosphorus scarcity: Identifying synergies for a sustainable future. J. Sci. Food Agric. 2012, 92, 2–6. [Google Scholar] [CrossRef]
- Mo, Q.F.; Li, Z.A.; Sayer, E.J.; Lambers, H.; Li, Y.W.; Zou, B.; Tang, J.W.; Heskel, M.; Ding, Y.Z.; Wang, F.M. Foliar phosphorus fractions reveal how tropical plants maintain photosynthetic rates despite low soil phosphorus availability. Funct. Ecol. 2019, 33, 503–513. [Google Scholar] [CrossRef]
- Hu, B.; Yang, B.; Pang, X.; Bao, W.; Tian, G. Responses of soil phosphorus fractions to gap size in a reforested spruce forest. Geoderma 2016, 279, 61–69. [Google Scholar] [CrossRef]
- Chen, G.H.; Weil, R.R.; Hill, R.L. Effects of compaction and cover crops on soil least limiting water range and air permeability. Soil Tillage Res. 2014, 136, 61–69. [Google Scholar] [CrossRef]
- Elser, J.J.; Bracken, M.E.S.; Cleland, E.E.; Gruner, D.S.; Harpole, W.S.; Hillebrand, H.; Ngai, J.T.; Seabloom, E.W.; Shurin, J.B.; Smith, J.E. Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol. Lett. 2007, 10, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Z.; Chen, C.R.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D.K. Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 2016, 276, 1–6. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.; Chauhan, B.S. Changes in Inorganic and Organic Soil Phosphorus Fractions Induced by Cultivation Practices and by Laboratory Incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Mao, X.L.; Xu, X.L.; Lu, K.P.; Gielen, G.; Luo, J.F.; He, L.Z.; Donnison, A.; Xu, Z.X.; Xu, J.; Yang, W.Y.; et al. Effect of 17 years of organic and inorganic fertilizer applications on soil phosphorus dynamics in a rice-wheat rotation cropping system in eastern China. J. Soils Sediments 2015, 15, 1889–1899. [Google Scholar] [CrossRef]
- Johnson, A.H.; Frizano, J.; Vann, D.R. Biogeochemical implications of labile phosphorus in forest soils determined by the Hedley fractionation procedure. Oecologia 2003, 135, 487–499. [Google Scholar] [CrossRef]
- Hei, J.; Xie, H.Y.; Yang, L.M.; Wang, W.Q.; Sardans, J.; Wang, C.; Tariq, A.; Zeng, F.J.; Peñuelas, J. Effects of contrasting N-enriched biochar applications on paddy soil and rice leaf phosphorus fractions in subtropical China. Sci. Total Environ. 2023, 877, 162949. [Google Scholar] [CrossRef]
- Gujre, N.; Soni, A.; Rangan, L.; Tsang, D.C.W.; Mitra, S. Sustainable improvement of soil health utilizing biochar and arbuscular mycorrhizal fungi: A review. Environ. Pollut. 2021, 268, 115549. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.F.; Meng, J.; Han, X.R.; Lan, Y.; Zhang, W.M. Past, present, and future of biochar. Biochar 2019, 1, 75–87. [Google Scholar] [CrossRef]
- Yang, X.; Yuan, J.; Bi, Y.H.; Wang, L.D.; Zhang, J.Q.; Wei, C.Q.; Cui, X.; Li, H.; Luo, P.Y.; Meng, J.; et al. Assessing the methane mitigation potential of biochar and stover incorporation: Insights from the emission dynamics and soil microbiome in maize agroecosystems. Soil. Till. Res. 2025, 251, 106554. [Google Scholar] [CrossRef]
- Li, F.Y.; Liang, X.Q.; Niyungeko, C.; Sun, T.; Liu, F.; Arai, Y. Effects of biochar amendments on soil phosphorus transformation in agricultural soils. Adv. Agron. 2019, 158, 131–172. [Google Scholar]
- Smith, S.E.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Nutrition and Growth: New Paradigms from Cellular to Ecosystem Scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, W.; Feng, Z.; Feng, G.; Zhu, H.; Yao, Q. Arbuscular mycorrhizal fungus differentially regulates P mobilizing bacterial community and abundance in rhizosphere and hyphosphere. Appl. Soil Ecol. 2022, 170, 104294. [Google Scholar] [CrossRef]
- Wang, G.W.; Jin, Z.X.; George, T.S.; Feng, G.; Zhang, L. Arbuscular mycorrhizal fungi enhance plant phosphorus uptake through stimulating hyphosphere soil microbiome functional profiles for phosphorus turnover. New Phytol. 2023, 238, 2578–2593. [Google Scholar] [CrossRef]
- Lin, Z.; Xu, M.; Yu, L.; Zhang, F.; Hodge, A.; Gu, F. Carbon and phosphorus exchange may enable cooperation between an arbuscular mycorrhizal fungus and a phosphate-solubilizing bacterium. New Phytol. 2016, 210, 1022–1032. [Google Scholar] [CrossRef]
- Sato, T.; Hachiya, S.; Inamura, N.; Ezawa, T.; Cheng, W.G.; Tawaraya, K. Secretion of acid phosphatase from extraradical hyphae of the arbuscular mycorrhizal fungus Rhizophagus clarus is regulated in response to phosphate availability. Mycorrhiza 2019, 29, 599–605. [Google Scholar] [CrossRef]
- Chowdhary, N.A.; Songachan, L.S. Boosting sustainable agriculture by arbuscular mycorrhizal fungi under abiotic stress condition. Plant Stress 2025, 17, 100945. [Google Scholar] [CrossRef]
- Sulpice, R.; Ishihara, H.; Schlereth, A.; Cawthray, G.R.; Encke, B.; Giavalisco, P.; Ivakov, A.; Arrivault, S.; Jost, R.; Krohn, N.; et al. Low levels of ribosomal RNA partly account for the very high photosynthetic phosphorus-use efficiency of Proteaceae species. Plant Cell Environ. 2013, 37, 1276–1298. [Google Scholar] [CrossRef]
- Gao, Y.; Tariq, A.; Zeng, F.; Graciano, C.; Zhang, Z.; Sardans, J.; Peñuelas, J. Allocation of foliar-P fractions of Alhagi sparsifolia and its relationship with soil-P fractions and soil properties in a hyperarid desert ecosystem. Geoderma 2022, 407, 115546. [Google Scholar] [CrossRef]
- Niederberger, J.; Kohler, M.; Bauhus, J. Distribution of phosphorus fractions with different plant availability in German forest soils and their relationship with common soil properties and foliar P contents. Soil 2019, 5, 189–204. [Google Scholar] [CrossRef]
- Close, D.C.; Beadle, C.L. Total, and chemical fractions, of nitrogen and phosphorus in Eucalyptus seedling leaves: Effects of species, nursery fertiliser management and transplanting. Plant Soil 2004, 259, 85–95. [Google Scholar] [CrossRef]
- Veneklaas, E.J.; Lambers, H.; Bragg, J.; Finnegan, P.M.; Lovelock, C.E.; Plaxton, W.C.; Price, C.A.; Scheible, W.R.; Shane, M.W.; White, P.J.; et al. Opportunities for improving phosphorus-use efficiency in crop plants. New Phytol. 2012, 195, 306–320. [Google Scholar] [CrossRef] [PubMed]
- Ågren, G.I.; Wetterstedt, J.; Billberger, M.F.K. Nutrient limitation on terrestrial plant growth—Modeling the interaction between nitrogen and phosphorus. New Phytol. 2012, 194, 953–960. [Google Scholar] [CrossRef]
- Hidaka, A.; Kitayama, K. Allocation of foliar phosphorus fractions and leaf traits of tropical tree species in response to decreased soil phosphorus availability on Mount Kinabalu, Borneo. J. Ecol. 2011, 99, 849–857. [Google Scholar] [CrossRef]
- Figueira-Galan, D.; Heupel, S.; Duelli, G.; Tomasi Morgano, M.; Stapf, D.; Requena, N. Exploring the synergistic effects of biochar and arbuscular mycorrhizal fungi on phosphorus acquisition in tomato plants by using gene expression analyses. Sci. Total Environ. 2023, 884, 163506. [Google Scholar] [CrossRef]
- Li, S.; Chi, S.; Lin, C.; Cai, C.; Yang, L.; Peng, K.; Huang, X.; Liu, J. Combination of biochar and AMF promotes phosphorus utilization by stimulating rhizosphere microbial co-occurrence networks and lipid metabolites of Phragmites. Sci. Total Environ. 2022, 845, 157339. [Google Scholar] [CrossRef]
- Wen, Z.H.; Chen, Y.X.; Liu, Z.Q.; Meng, J. Biochar and arbuscular mycorrhizal fungi stimulate rice root growth strategy and soil nutrient availability. Eur. J. Soil Biol. 2022, 113, 103448. [Google Scholar] [CrossRef]
- Pacioni, G. 16 Wet-sieving and Decanting Techniques for the Extraction of Spores of Vesicular-arbuscular Fungi. Methods Microbiol. 1992, 24, 317–322. [Google Scholar] [CrossRef]
- Bao, X.Z.; Wang, Y.T.; Olsson, P.A. Arbuscular mycorrhiza under water—Carbon-phosphorus exchange between rice and arbuscular mycorrhizal fungi under different flooding regimes. Soil Biol. Biochem. 2019, 129, 169–177. [Google Scholar] [CrossRef]
- McGonigle, T.P.; Miller, M.H.; Evans, D.G.; Fairchild, G.L.; Swan, J.A. A new method which gives an objective measure of colonization of roots by vesicular—Arbuscular mycorrhizal fungi. New Phytol. 1990, 115, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Thompson, M.L.; Chao, S. Fractionation of Phosphorus in a Mollisol Amended with Biosolids. Soil Sci. Soc. Am. J. 1999, 63, 1174–1180. [Google Scholar] [CrossRef]
- Lehmann, J.; Gaunt, J.; Rondon, M. Biochar Sequestration in Terrestrial Ecosystems—A Review. Mitig. Adapt. Strat. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Yang, L.; Wu, Y.; Wang, Y.; An, W.; Jin, J.; Sun, K.; Wang, X. Effects of biochar addition on the abundance, speciation, availability, and leaching loss of soil phosphorus. Sci. Total Environ. 2021, 758, 143657. [Google Scholar] [CrossRef]
- Zhang, L.; Zhou, J.; George, T.S.; Limpens, E.; Feng, G. Arbuscular mycorrhizal fungi conducting the hyphosphere bacterial orchestra. Trends Plant Sci. 2022, 27, 402–411. [Google Scholar] [CrossRef]
- Hammer, E.C.; Forstreuter, M.; Rillig, M.C.; Kohler, J. Biochar increases arbuscular mycorrhizal plant growth enhancement and ameliorates salinity stress. Appl. Soil Ecol. 2015, 96, 114–121. [Google Scholar] [CrossRef]
- Yang, C.D.; Lu, S.G. Straw and straw biochar differently affect phosphorus availability, enzyme activity and microbial functional genes in an Ultisol. Sci. Total Environ. 2022, 805, 150325. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, N.; Fan, J.; Wang, F.; George, T.S.; Feng, G. Arbuscular mycorrhizal fungi stimulate organic phosphate mobilization associated with changing bacterial community structure under field conditions. Environ. Microbiol. 2018, 20, 2639–2651. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Z.; Zhong, X.; Xu, C.; Lin, Y.; Fan, Y.; Wang, M.; Chen, G.; Yang, Y. Decreases in soil P availability are associated with soil organic P declines following forest conversion in subtropical China. Catena 2021, 205, 105459. [Google Scholar] [CrossRef]
- Wang, W.J.; Baldock, J.A.; Dalal, R.C.; Moody, P.W. Decomposition dynamics of plant materials in relation to nitrogen availability and biochemistry determined by NMR and wet-chemical analysis. Soil Boil. Biochem. 2004, 36, 2045–2058. [Google Scholar] [CrossRef]
- Yin, X.L.; Peñuelas, J.; Sardans, J.; Xu, X.P.; Chen, Y.Y.; Fang, Y.Y.; Wu, L.Q.; Singh, B.P.; Tavakkoli, E.; Wang, W.Q. Effects of nitrogen-enriched biochar on rice growth and yield, iron dynamics, and soil carbon storage and emissions: A tool to improve sustainable rice cultivation. Environ. Pollut. 2021, 287, 117565. [Google Scholar] [CrossRef] [PubMed]
- Fellbaum, C.R.; Gachomo, E.W.; Beesetty, Y.; Choudhari, S.; Strahan, G.D.; Pfeffer, P.E.; Kiers, E.T.; Bücking, H. Carbon availability triggers fungal nitrogen uptake and transport in arbuscular mycorrhizal symbiosis. Proc. Natl. Acad. Sci. USA 2012, 109, 2666–2671. [Google Scholar] [CrossRef]
- Galván-Tejada, N.C.; Peña-Ramírez, V.; Mora-Palomino, L.; Siebe, C. Soil P fractions in a volcanic soil chronosequence of Central Mexico and their relationship to foliar P in pine trees. J. Plant Nutr. Soil Sci. 2014, 177, 792–802. [Google Scholar] [CrossRef]
- Niederberger, J.; Kohler, M.; Bauhus, J. The relevance of different soil phosphorus fractions for short-term tree nutrition: Results from a mesocosm bioassay. For. Int. J. For. Res. 2017, 90, 258–267. [Google Scholar] [CrossRef]
- Helfenstein, J.; Tamburini, F.; von Sperber, C.; Massey, M.S.; Pistocchi, C.; Chadwick, O.A.; Vitousek, P.M.; Kretzschmar, R.; Frossard, E. Combining spectroscopic and isotopic techniques gives a dynamic view of phosphorus cycling in soil. Nat. Commun. 2018, 9, 3226. [Google Scholar] [CrossRef]
- Tyler, G.; Olsson, T. Plant uptake of major and minor mineral elements as influenced by soil acidity and liming. Plant Soil 2001, 230, 307–321. [Google Scholar] [CrossRef]
- Wang, X.X.; Zhang, H.R.; Cao, D.; Wu, C.Y.; Wang, X.T.; Wei, L.; Guo, B.; Wang, S.; Ding, J.A.; Chen, H.; et al. Microbial carbon and phosphorus metabolism regulated by C: N: P stoichiometry stimulates organic carbon accumulation in agricultural soils. Soil Tillage Res. 2024, 242, 106152. [Google Scholar] [CrossRef]
- Zong, J.; Zhang, Z.; Huang, P.; Yang, Y. Arbuscular mycorrhizal fungi alleviates salt stress in Xanthoceras sorbifolium through improved osmotic tolerance, antioxidant activity, and photosynthesis. Front. Microbiol. 2023, 14, 2023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).