Referenced Transcriptomics Identifies a Core Set of Cytochrome P450 Genes Driving Broad-Spectrum Insecticide Detoxification in Phthonandria atrilineata
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Experimental Design
2.2. Genome Re-Annotation and P450 Gene Identification
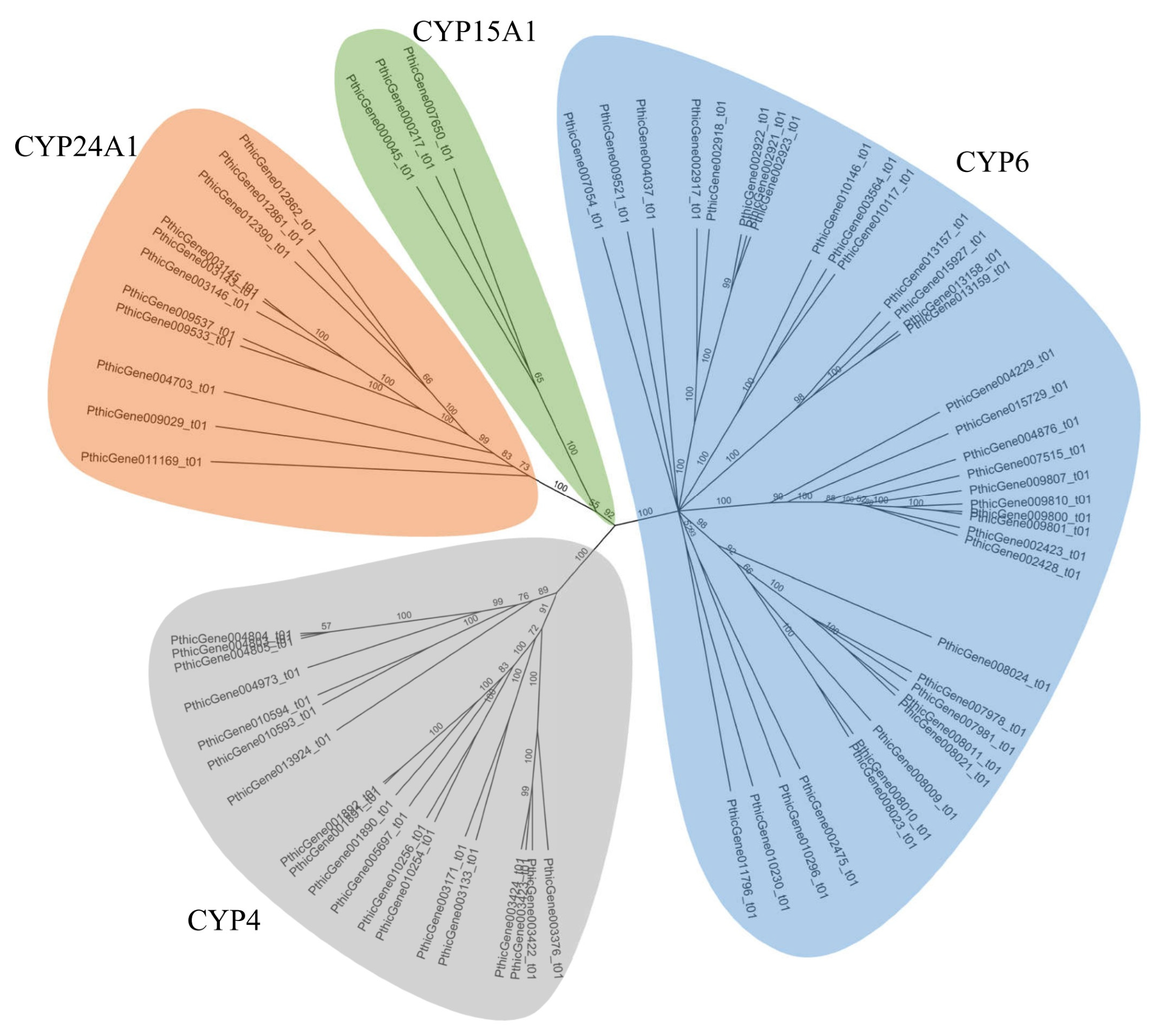
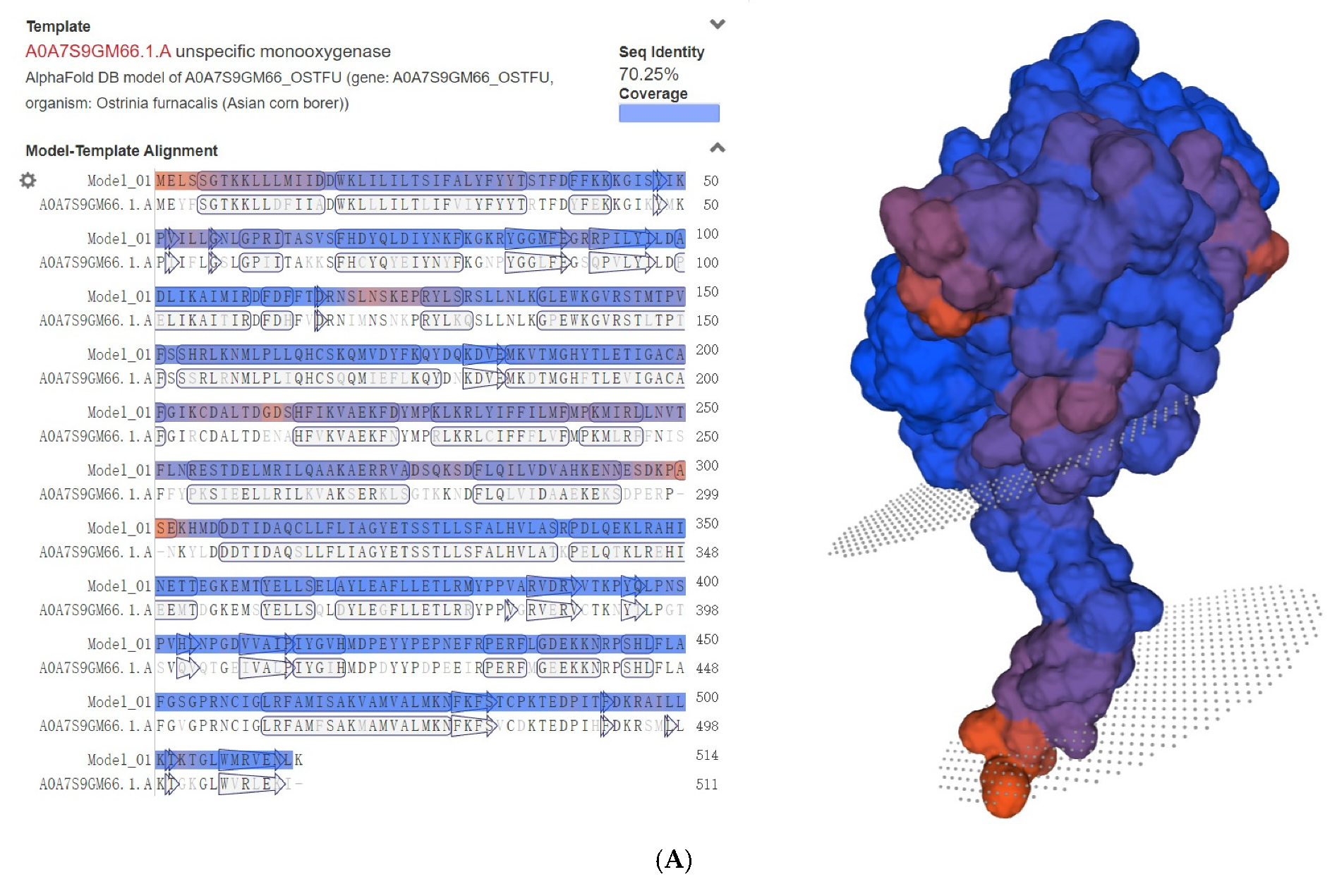
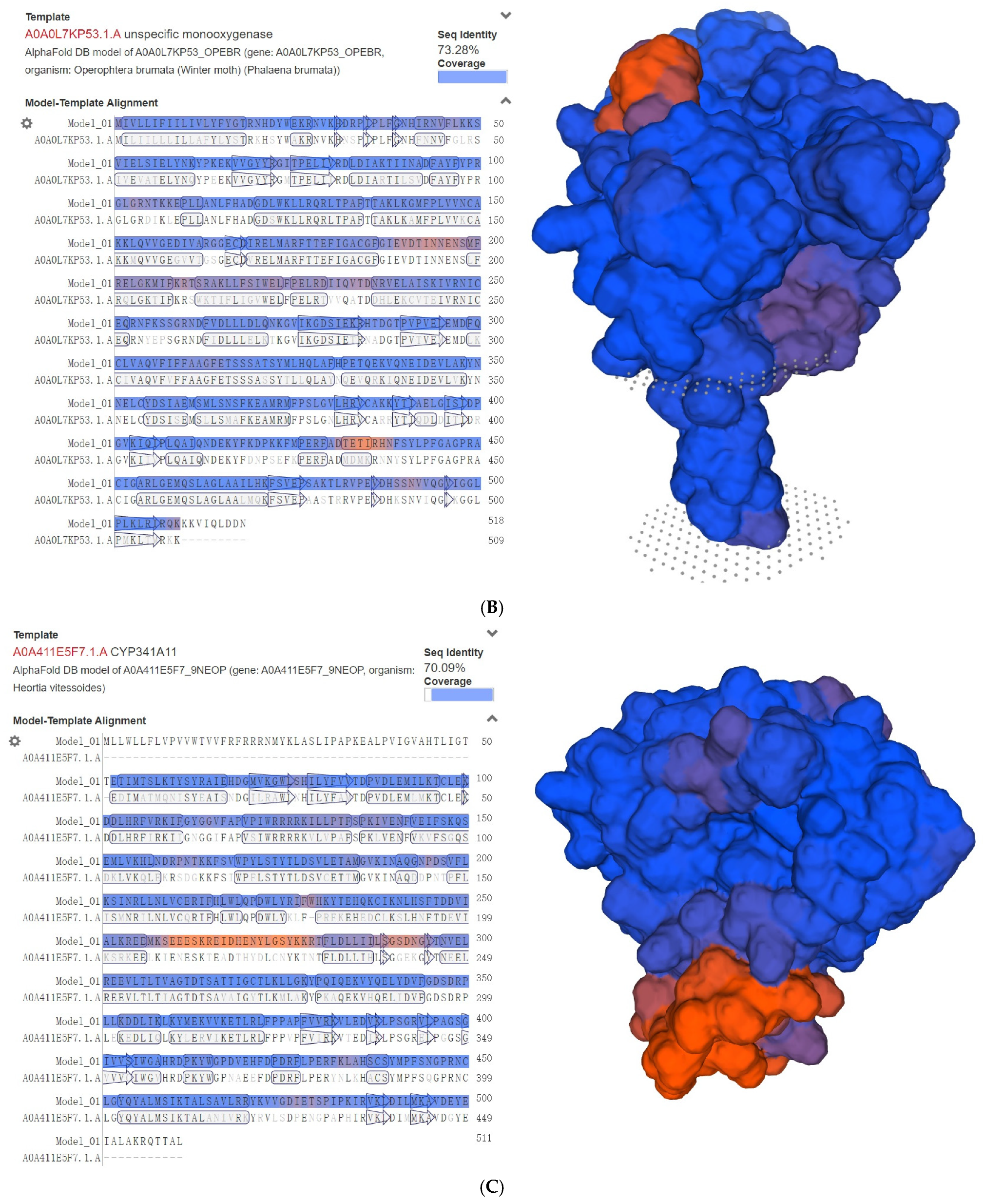
2.3. Phylogenetic, Genomic, and Structural Analysis
2.4. Transcriptomic Analysis and Differential Gene Expression
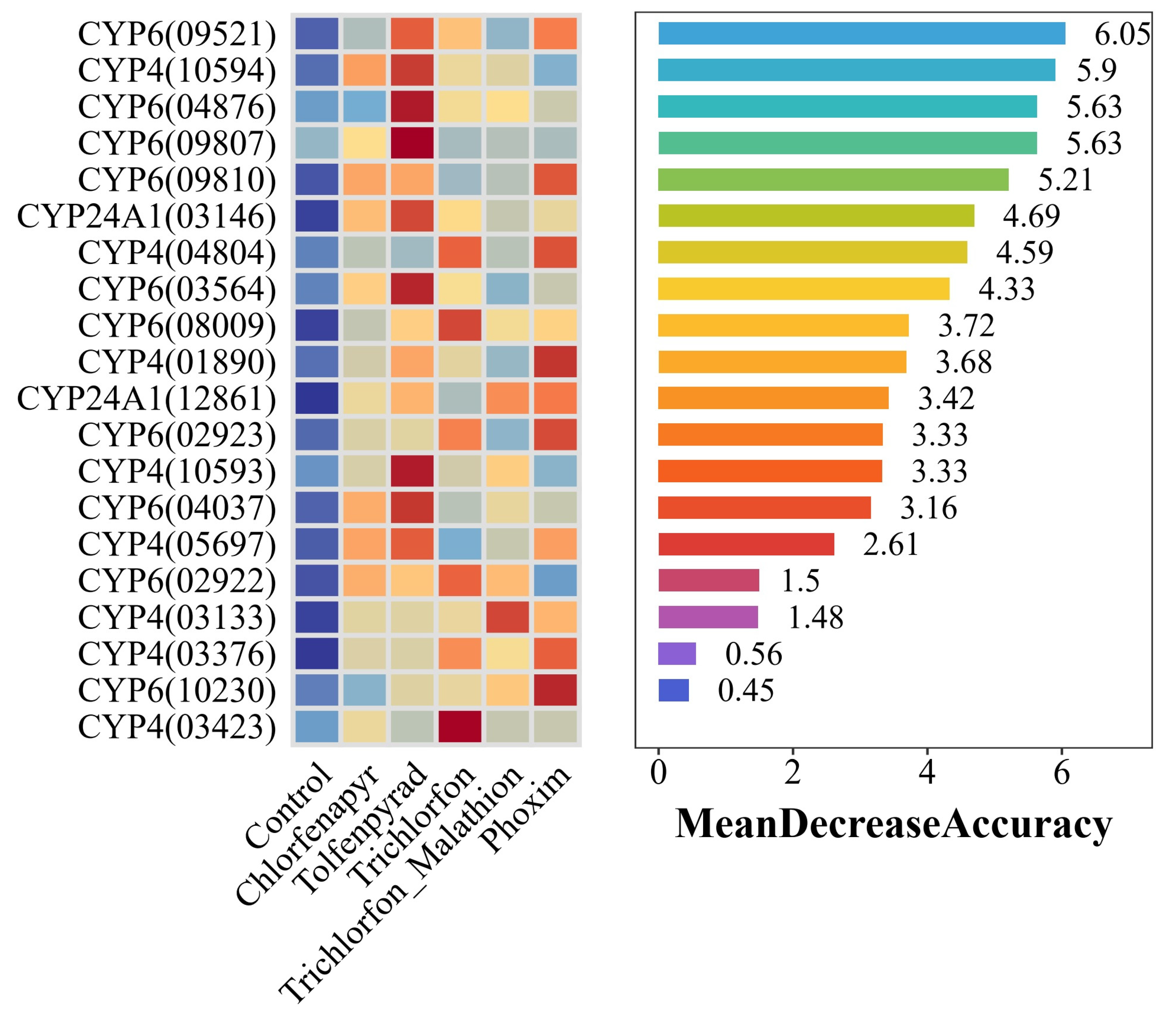
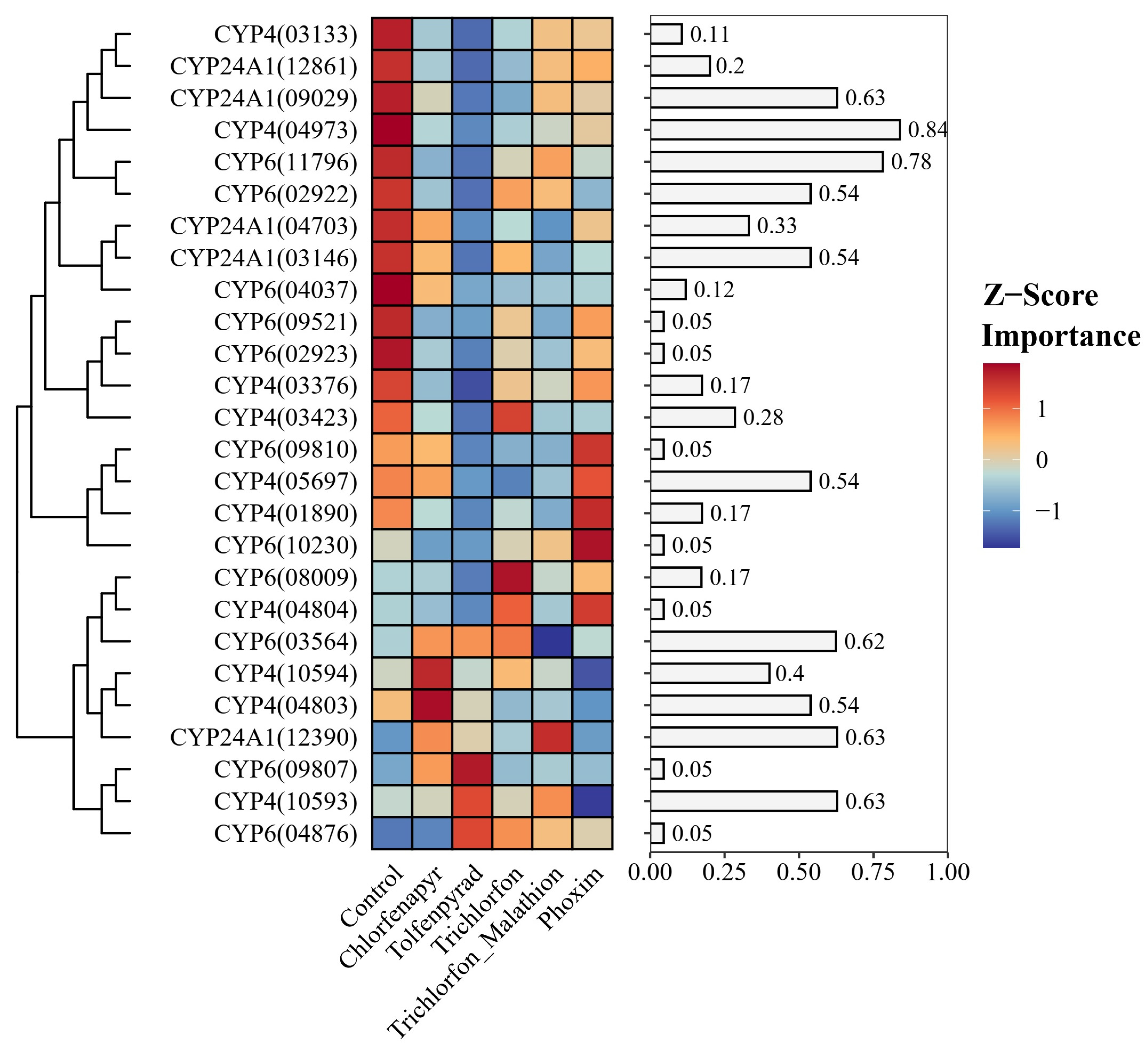
2.5. Machine Learning-Based Biomarker Identification
2.6. Statistical Analyses and Data Visualization
3. Results
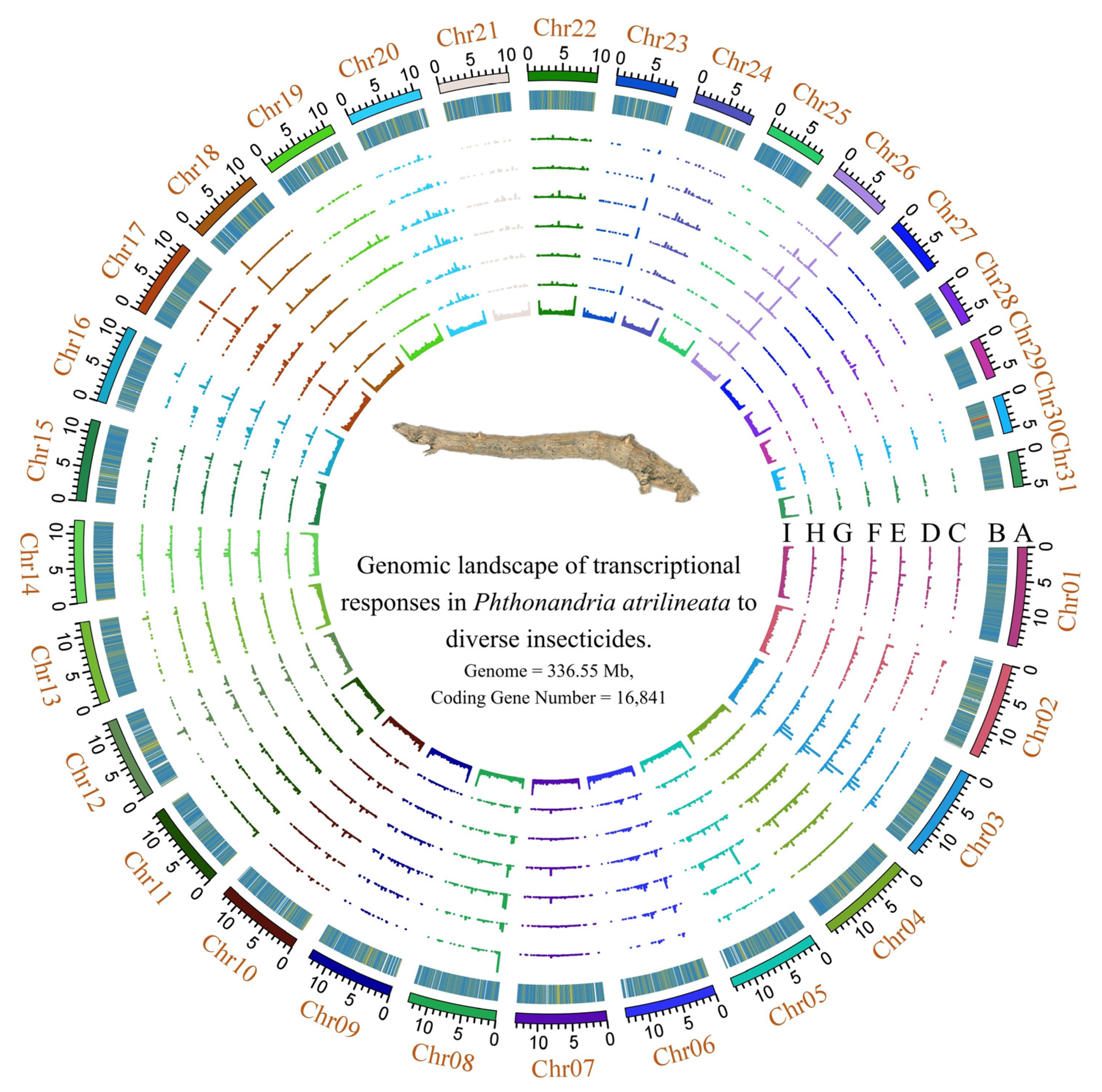
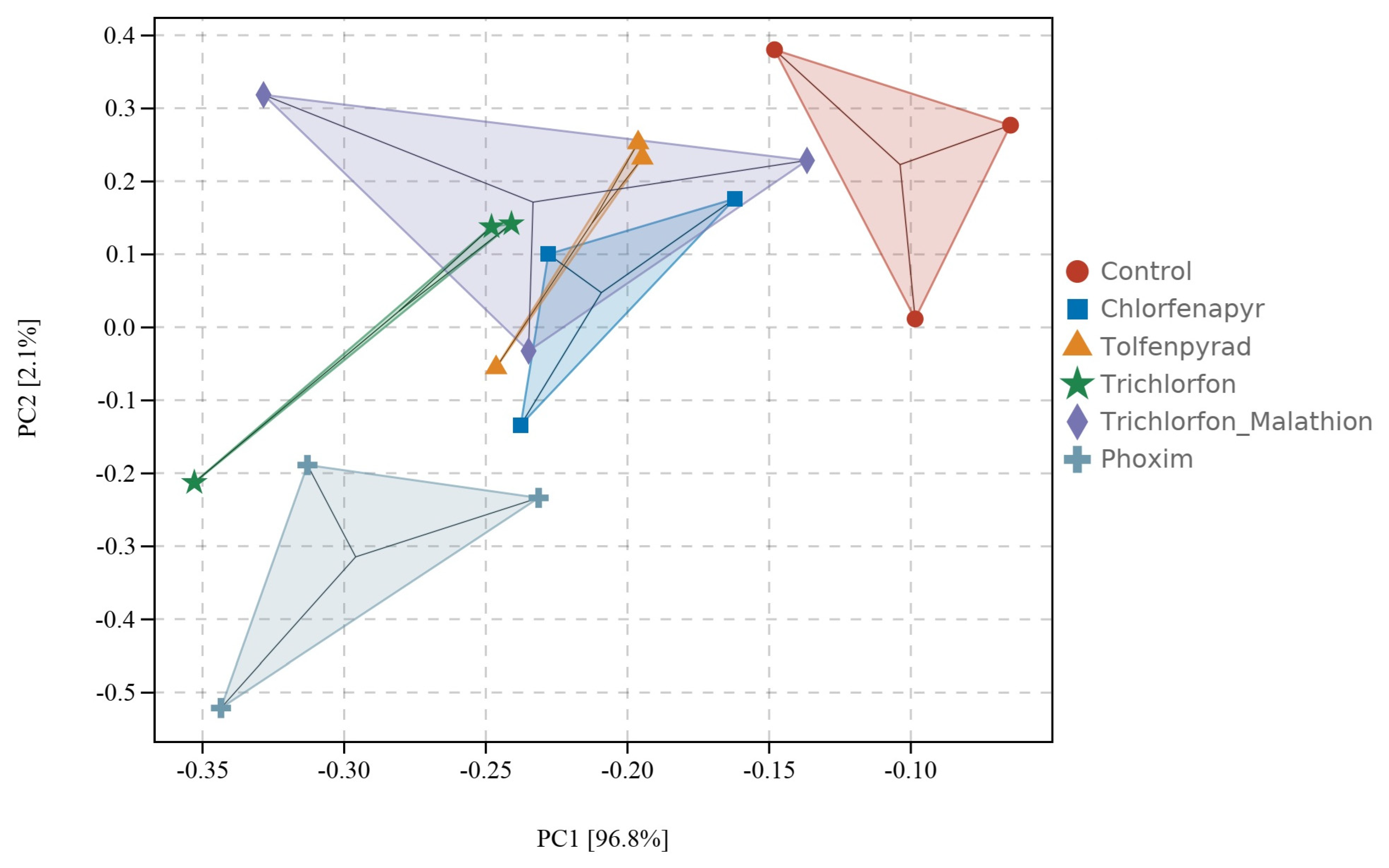
3.1. Genome Re-Annotation and Transcriptome-Wide Expression Landscape
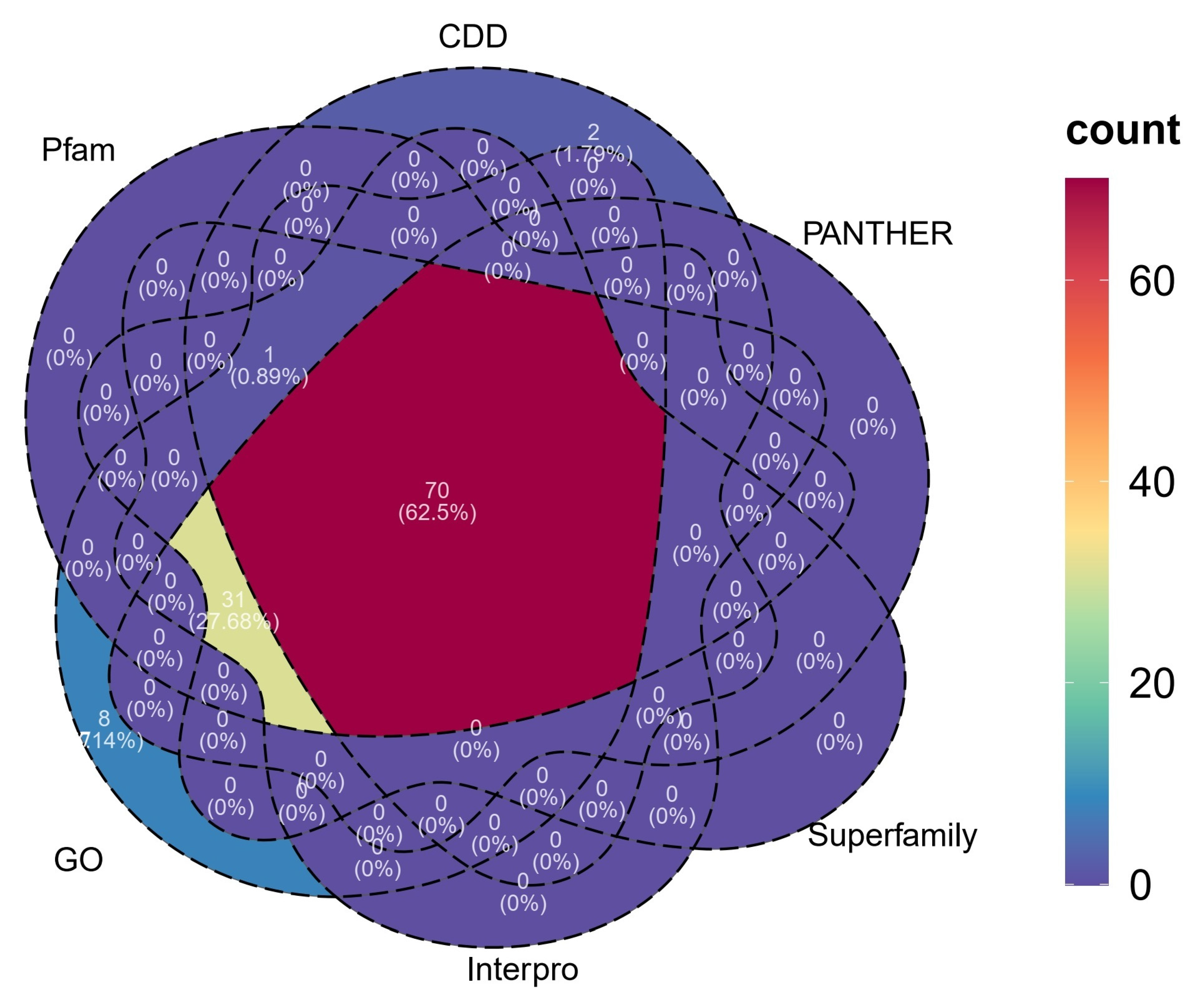
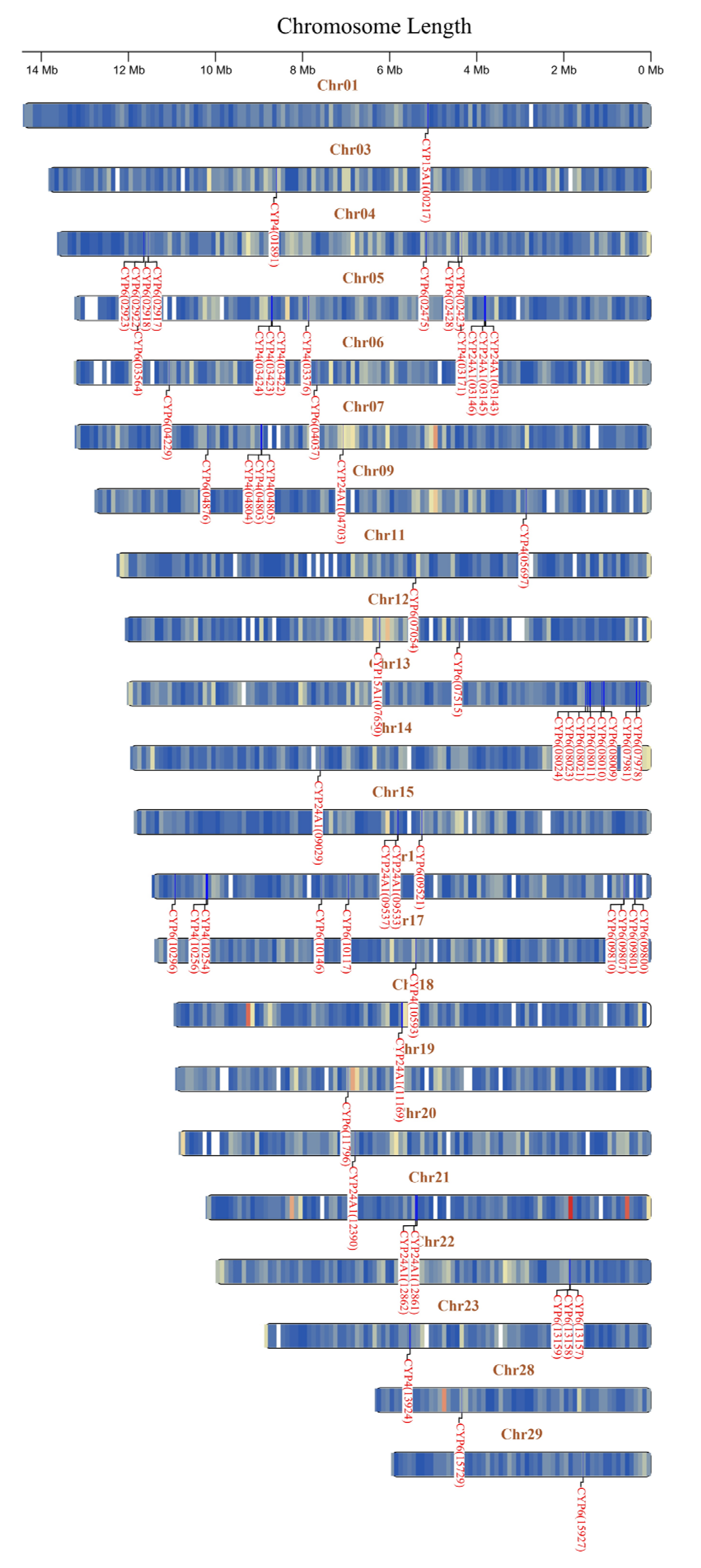
3.2. Identification and Annotation of the P450 Gene Complement
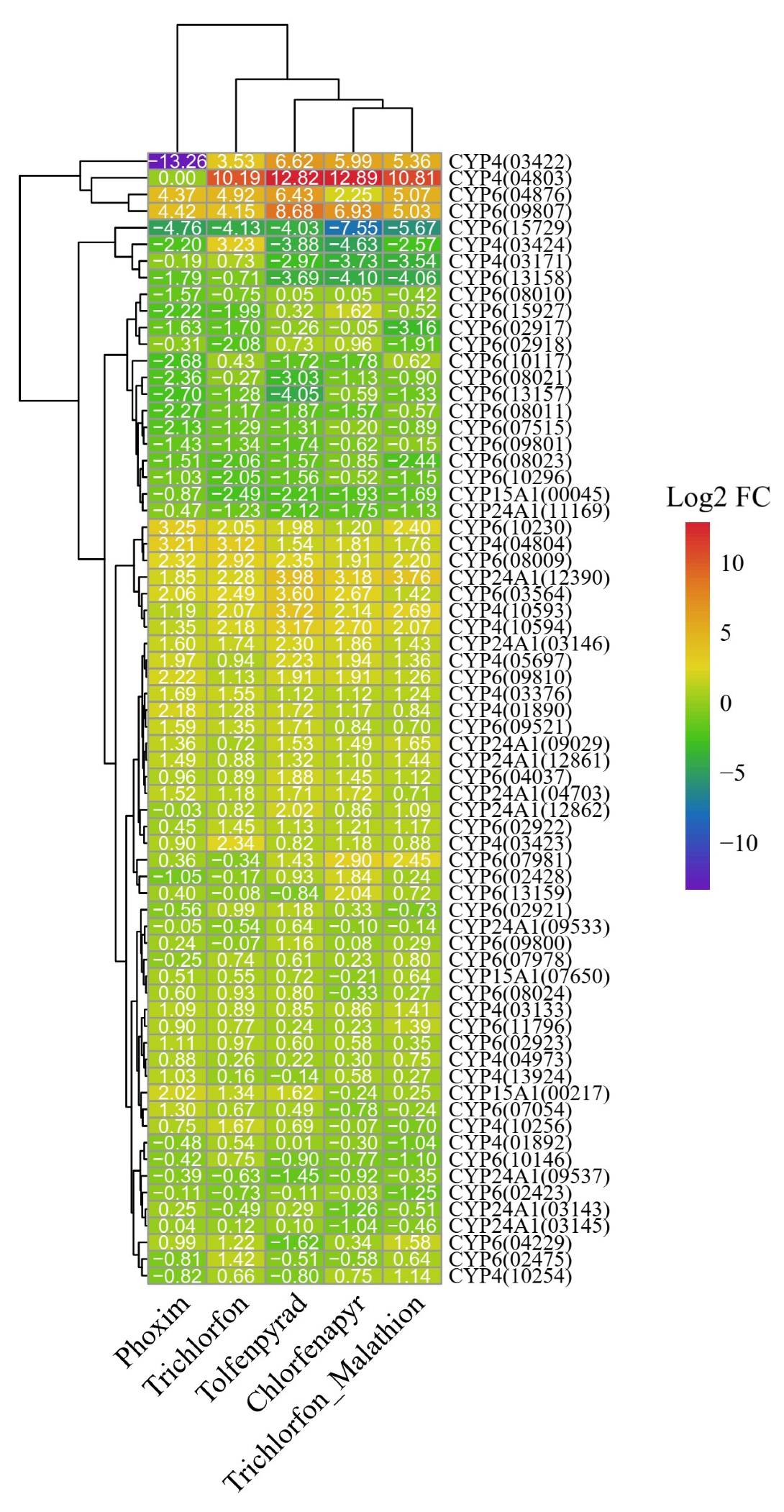
3.3. Transcriptional Response of P450 Genes to Insecticide Exposure
3.4. Convergent Evidence Pinpoints a Trio of P450s as Key Effectors of Broad-Spectrum Resistance
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zainab, R.; Hasnain, M.; Ali, F.; Abideen, Z.; Siddiqui, Z.S.; Jamil, F.; Hussain, M.; Park, Y.K. Prospects and challenges of nanopesticides in advancing pest management for sustainable agricultural and environmental service. Environ. Res. 2024, 261, 119722. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, W.B. Role of research and regulation in 50 years of pest management in agriculture Prepared for the 50th anniversary of the Journal of Agricultural and Food Chemistry. J. Agric. Food Chem. 2002, 50, 4151–4155. [Google Scholar] [CrossRef] [PubMed]
- Joußen, N.; Heckel, D.G. Saltational evolution of a pesticide-metabolizing cytochrome P450 in a global crop pest. Pest Manag. Sci. 2021, 77, 3325–3332. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Xing, Z.; Dong, H.; Chen, X.; Ren, M.; Liu, K.; Rao, C.; Tan, A.; Su, J. Cytochrome P450 CYP6AE70 Confers Resistance to Multiple Insecticides in a Lepidopteran Pest, Spodoptera exigua. J. Agric. Food Chem. 2024, 72, 23141–23150. [Google Scholar] [CrossRef]
- Casida, J.E. Why Prodrugs and Propesticides Succeed. Chem. Res. Toxicol. 2017, 30, 1117–1126. [Google Scholar] [CrossRef]
- Guan, D.L.; Qin, Y.C.; Chen, Y.Z.; Zhang, S.H.; Liu, J.P.; Yi, H.Y.; Li, X.D. A high-quality chromosome-level genome assembly of the mulberry looper, Phthonandria atrilineata. Sci. Data 2025, 12, 186. [Google Scholar] [CrossRef]
- Guan, D.; Qin, Y.; Song, J.; Qin, Y.; Zhang, S.; Xin, L.; Li, X. Comparative transcriptomics reveal universal and compound-specific mechanisms of insecticide response in the mulberry looper (Phthonandria atrilineata). Genomics 2025, 117, 111121. [Google Scholar] [CrossRef]
- Vontas, J.; Katsavou, E.; Mavridis, K. Cytochrome P450-based metabolic insecticide resistance in Anopheles and Aedes mosquito vectors: Muddying the waters. Pestic. Biochem. Physiol. 2020, 170, 104666. [Google Scholar] [CrossRef]
- Liu, N.; Li, M.; Gong, Y.; Liu, F.; Li, T. Cytochrome P450s—Their expression, regulation, and role in insecticide resistance. Pestic. Biochem. Physiol. 2015, 120, 77–81. [Google Scholar] [CrossRef]
- Lu, K.; Song, Y.; Zeng, R. The role of cytochrome P450-mediated detoxification in insect adaptation to xenobiotics. Curr. Opin. Insect Sci. 2021, 43, 103–107. [Google Scholar] [CrossRef]
- Lao, S.H.; Huang, X.H.; Huang, H.J.; Liu, C.W.; Zhang, C.X.; Bao, Y.Y. Genomic and transcriptomic insights into the cytochrome P450 monooxygenase gene repertoire in the rice pest brown planthopper, Nilaparvata lugens. Genomics 2015, 106, 301–309. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Sohpal, V.K.; Dey, A.; Singh, A. MEGA biocentric software for sequence and phylogenetic analysis: A review. Int. J. Bioinform. Res. Appl. 2010, 6, 230–240. [Google Scholar] [CrossRef]
- Xie, J.; Chen, Y.; Cai, G.; Cai, R.; Hu, Z.; Wang, H. Tree Visualization By One Table (tvBOT): A web application for visualizing, modifying and annotating phylogenetic trees. Nucleic Acids Res. 2023, 51, W587–W592. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhuang, H.; Jiang, X.H.; Zou, R.H.; Wang, H.Y.; Fan, Z.N. Unveiling the key genes, environmental toxins, and drug exposures in modulating the severity of ulcerative colitis: A comprehensive analysis. Front. Immunol. 2023, 14, 1162458. [Google Scholar] [CrossRef] [PubMed]
- Kebede, M.M.; Le Cornet, C.; Fortner, R.T. In-depth evaluation of machine learning methods for semi-automating article screening in a systematic review of mechanistic literature. Res. Synth. Methods 2023, 14, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Murphy, D. Application of ggplot2 to Pharmacometric Graphics. CPT Pharmacomet. Syst. Pharmacol. 2013, 2, e79. [Google Scholar] [CrossRef]
- Lyu, F.; Han, F.; Ge, C.; Mao, W.; Chen, L.; Hu, H.; Chen, G.; Lang, Q.; Fang, C. OmicStudio: A composable bioinformatics cloud platform with real-time feedback that can generate high-quality graphs for publication. iMeta 2023, 2, e85. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, K.; Jang, S.; Itoh, H.; Kikuchi, Y. Insecticide resistance governed by gut symbiosis in a rice pest, Cletus punctiger, under laboratory conditions. Biol. Lett. 2021, 17, 20200780. [Google Scholar] [CrossRef]
- Booth, W. Population genetics as a tool to understand invasion dynamics and insecticide resistance in indoor urban pest insects. Curr. Opin. Insect Sci. 2024, 62, 101166. [Google Scholar] [CrossRef]
- Wang, B.; Shahzad, M.F.; Zhang, Z.; Sun, H.; Han, P.; Li, F.; Han, Z. Genome-wide analysis reveals the expansion of Cytochrome P450 genes associated with xenobiotic metabolism in rice striped stem borer, Chilo suppressalis. Biochem. Biophys. Res. Commun. 2014, 443, 756–760. [Google Scholar] [CrossRef]
- Xuan, N.; Guo, X.; Xie, H.Y.; Lou, Q.N.; Lu, X.B.; Liu, G.X.; Picimbon, J.F. Increased expression of CSP and CYP genes in adult silkworm females exposed to avermectins. Insect Sci. 2015, 22, 203–219. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Li, G.Y.; Li, L.; Song, Q.S.; Stanley, D.; Wei, S.J.; Zhu, J.Y. Genome-wide and expression-profiling analyses of the cytochrome P450 genes in Tenebrionidea. Arch. Insect Biochem. Physiol. 2022, 111, e21954. [Google Scholar] [CrossRef]
- Duarte, A.; Pym, A.; Garrood, W.T.; Troczka, B.J.; Zimmer, C.T.; Davies, T.G.E.; Nauen, R.; O’Reilly, A.O.; Bass, C. P450 gene duplication and divergence led to the evolution of dual novel functions and insecticide cross-resistance in the brown planthopper Nilaparvata lugens. PLoS Genet. 2022, 18, e1010279. [Google Scholar] [CrossRef]
- Yu, J.; Tehrim, S.; Wang, L.; Dossa, K.; Zhang, X.; Ke, T.; Liao, B. Evolutionary history and functional divergence of the cytochrome P450 gene superfamily between Arabidopsis thaliana and Brassica species uncover effects of whole genome and tandem duplications. BMC Genom. 2017, 18, 733. [Google Scholar] [CrossRef] [PubMed]
- Doddapaneni, H.; Chakraborty, R.; Yadav, J.S. Genome-wide structural and evolutionary analysis of the P450 monooxygenase genes (P450ome) in the white rot fungus Phanerochaete chrysosporium: Evidence for gene duplications and extensive gene clustering. BMC Genom. 2005, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Fernandez, D.; Gao, Y.; Pierre, S.; Gao, Y.; Dai, G. Enzymology, Histological and Ultrastructural Effects of Ar-Turmerone on Culex pipiens pallens Larvae. Insects 2020, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Peng, M.; Zhang, Y.; Wu, P.; Zheng, X.; Zhang, X.; Guo, S.; Ding, Y.; Yang, N.; Li, M.; et al. Transcriptome reanalysis and gene expression of 13 detoxification genes for avermectin and pyridaben resistance in Panonychus citri. Sci. Rep. 2024, 14, 25857. [Google Scholar] [CrossRef]
- Gu, Z.; Zhou, Y.; Xie, Y.; Li, F.; Ma, L.; Sun, S.; Wu, Y.; Wang, B.; Wang, J.; Hong, F.; et al. The adverse effects of phoxim exposure in the midgut of silkworm, Bombyx mori. Chemosphere 2014, 96, 33–38. [Google Scholar] [CrossRef]
- Ravanfar, R.; Sheng, Y.; Gray, H.B.; Winkler, J.R. Tryptophan-96 in cytochrome P450 BM3 plays a key role in enzyme survival. FEBS Lett. 2023, 597, 59–64. [Google Scholar] [CrossRef]
- Doehmer, J.; Goeptar, A.R.; Vermeulen, N.P. Cytochromes P450 and drug resistance. Cytotechnology 1993, 12, 357–366. [Google Scholar] [CrossRef]
- Wu, P.; Huang, Y.; Zheng, J.; Zhang, Y.; Qiu, L. Regulation of CncC in insecticide-induced expression of cytochrome P450 CYP9A14 and CYP6AE11 in Helicoverpa armigera. Pestic. Biochem. Physiol. 2023, 197, 105707. [Google Scholar] [CrossRef]
- Wang, J.; Ohno, H.; Ide, Y.; Ichinose, H.; Mori, T.; Kawagishi, H.; Hirai, H. Identification of the cytochrome P450 involved in the degradation of neonicotinoid insecticide acetamiprid in Phanerochaete chrysosporium. J. Hazard. Mater. 2019, 371, 494–498. [Google Scholar] [CrossRef]
- Katsavou, E.; Riga, M.; Ioannidis, P.; King, R.; Zimmer, C.T.; Vontas, J. Functionally characterized arthropod pest and pollinator cytochrome P450s associated with xenobiotic metabolism. Pestic. Biochem. Physiol. 2022, 181, 105005. [Google Scholar] [CrossRef]
- Burns, A.R.; Baker, R.J.; Kitner, M.; Knox, J.; Cooke, B.; Volpatti, J.R.; Vaidya, A.S.; Puumala, E.; Palmeira, B.M.; Redman, E.M.; et al. Selective control of parasitic nematodes using bioactivated nematicides. Nature 2023, 618, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Rosli, M.A.F.; Syed Jaafar, S.N.; Azizan, K.A.; Yaakop, S.; Aizat, W.M. Omics approaches to unravel insecticide resistance mechanism in Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). PeerJ 2024, 12, e17843. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Ramasamy, G.G.; Pathak, J.; Nayyar, N.; Muthugounder, M.; Maria, P.; Rai, A.; Thiruvengadam, V. Deciphering the Molecular Mechanisms of Insecticide Resistance From the Transcriptome Data of Field Evolved Spinosad Resistant and Susceptible Populations of Plutella xylostella (Lepidoptera: Plutellidae). J. Econ. Entomol. 2022, 115, 1268–1278. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Xia, M.; Li, C.; Liu, X.; Archdeacon, L.; O’Reilly, A.O.; Yuan, G.; Wang, J.; Dou, W. CYP4CL2 Confers Metabolic Resistance to Pyridaben in the Citrus Pest Mite Panonychus citri. J. Agric. Food Chem. 2023, 71, 19465–19474. [Google Scholar] [CrossRef]
- Nufer, M.I.; Coates, B.S.; Abel, C.A.; O’Neill, P.; McCracken, M.; Jain, D.; Pierce, C.A., 3rd; Glover, J.; Towles, T.; Reddy, G.V.P.; et al. Anatomy of a pest control failure: Introgression of cytochrome P450 337B3 alleles from invasive old-world bollworm into native corn earworm (Lepidoptera: Noctuidae). J. Insect Sci. (Online) 2024, 24. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Fan, R.; Naz, H.; Bamisile, B.S.; Hafeez, M.; Ghani, M.I.; Wei, Y.; Xu, Y.; Chen, X. Insights into insecticide-resistance mechanisms in invasive species: Challenges and control strategies. Front. Physiol. 2022, 13, 1112278. [Google Scholar] [CrossRef]
- Kim, Y.H.; Soumaila Issa, M.; Cooper, A.M.; Zhu, K.Y. RNA interference: Applications and advances in insect toxicology and insect pest management. Pestic. Biochem. Physiol. 2015, 120, 109–117. [Google Scholar] [CrossRef]
- Ichinose, H.; Hatakeyama, M.; Yamauchi, Y. Sequence modifications and heterologous expression of eukaryotic cytochromes P450 in Escherichia coli. J. Biosci. Bioeng. 2015, 120, 268–274. [Google Scholar] [CrossRef]
- Hu, J.; Zhang, Y.; Wu, Y.; Zheng, J.; Yu, Z.; Qian, H.; Yu, J.; Cheng, Z.; Chen, J. Heterologous expression of bacterial cytochrome P450 from Microbacterium keratanolyticum ZY and its application in dichloromethane dechlorination. Environ. Pollut. 2021, 287, 117597. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, D.; Song, J.; Qin, Y.; Xin, L.; Li, X.; Zhang, S. Referenced Transcriptomics Identifies a Core Set of Cytochrome P450 Genes Driving Broad-Spectrum Insecticide Detoxification in Phthonandria atrilineata. Agronomy 2025, 15, 2561. https://doi.org/10.3390/agronomy15112561
Guan D, Song J, Qin Y, Xin L, Li X, Zhang S. Referenced Transcriptomics Identifies a Core Set of Cytochrome P450 Genes Driving Broad-Spectrum Insecticide Detoxification in Phthonandria atrilineata. Agronomy. 2025; 15(11):2561. https://doi.org/10.3390/agronomy15112561
Chicago/Turabian StyleGuan, Delong, Jing Song, Yue Qin, Lei Xin, Xiaodong Li, and Shihao Zhang. 2025. "Referenced Transcriptomics Identifies a Core Set of Cytochrome P450 Genes Driving Broad-Spectrum Insecticide Detoxification in Phthonandria atrilineata" Agronomy 15, no. 11: 2561. https://doi.org/10.3390/agronomy15112561
APA StyleGuan, D., Song, J., Qin, Y., Xin, L., Li, X., & Zhang, S. (2025). Referenced Transcriptomics Identifies a Core Set of Cytochrome P450 Genes Driving Broad-Spectrum Insecticide Detoxification in Phthonandria atrilineata. Agronomy, 15(11), 2561. https://doi.org/10.3390/agronomy15112561





