Effect of Silver Nanoparticles on Growth of Wheat: Is It Stage-Specific or Not?
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Dispersions of AgNPs
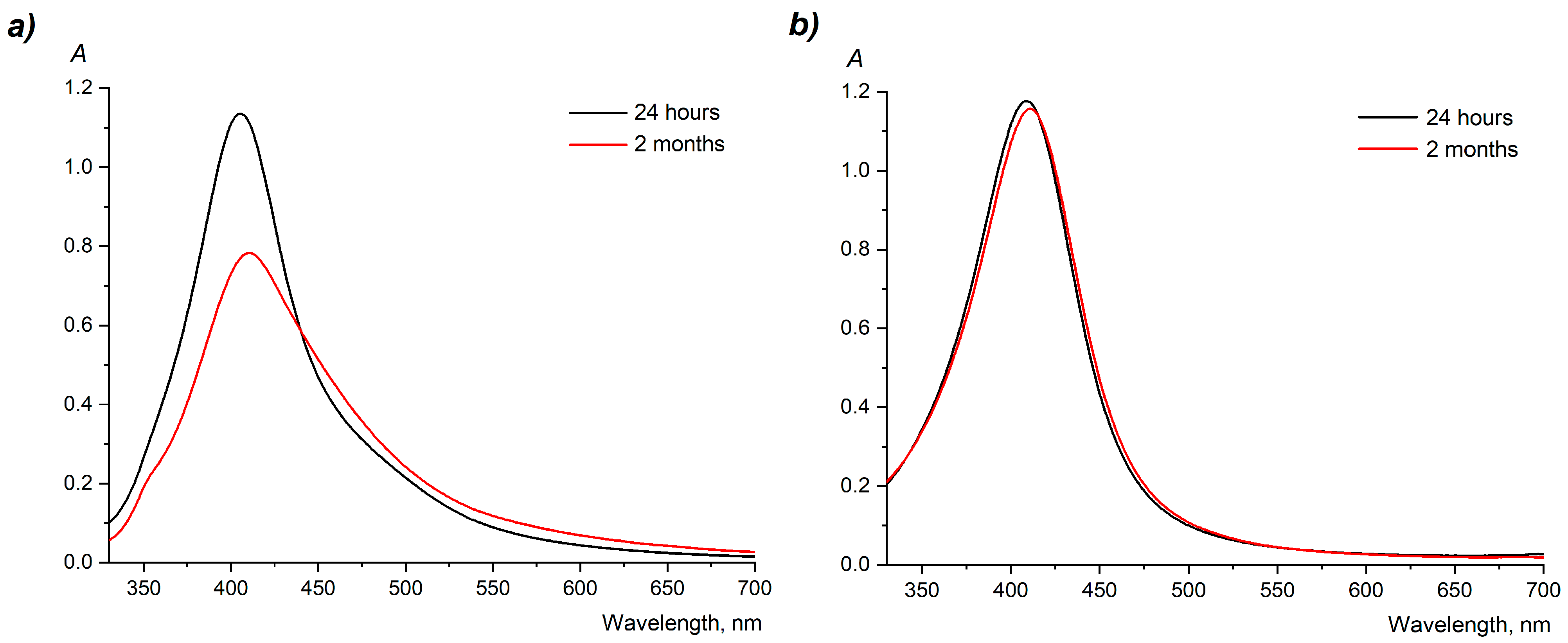
2.3. UV–Vis Spectrophotometry
2.4. TEM and SAED
2.5. DLS
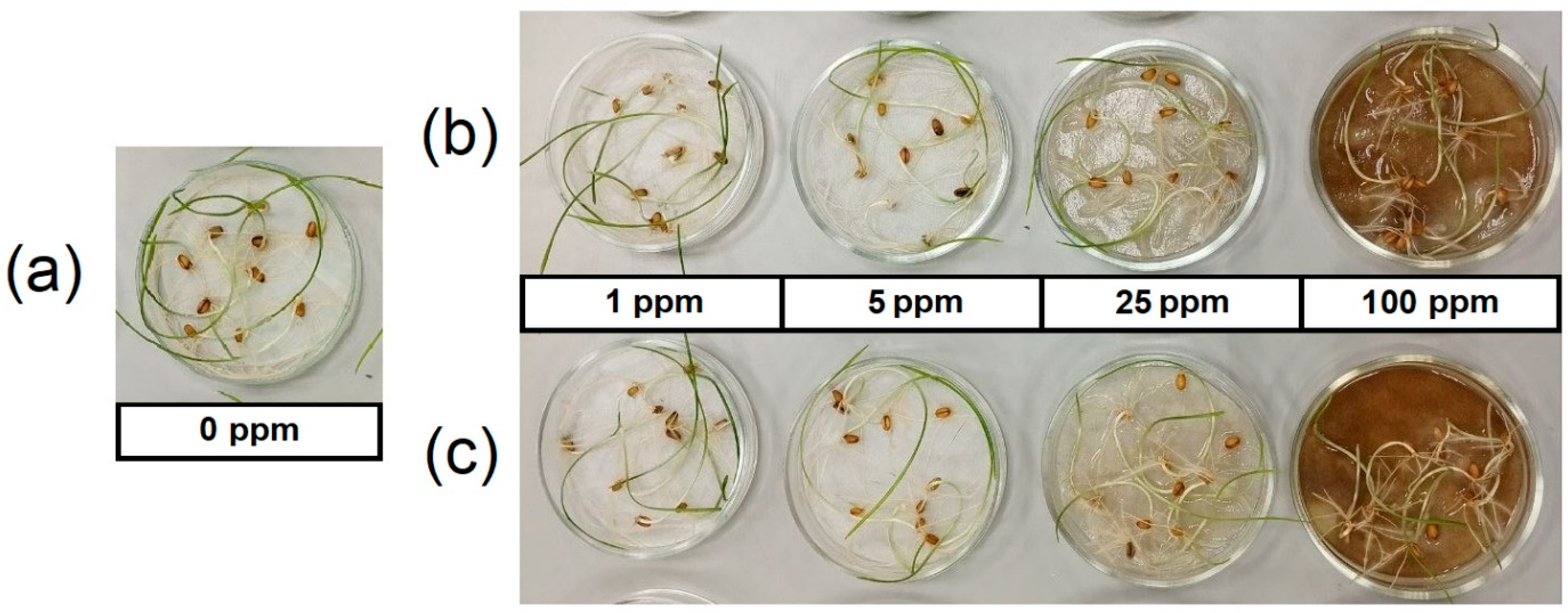
2.6. Effect of Priming with Dispersions of AgNPs on Seed Germination
2.7. Field Trials
- Control—background fertilization NPK 40:60:40 kg ha−1 (as N, P2O5, and K2O);
- NPK 40:60:40 + two foliar applications at tillering and stem elongation of an APCG solution at 200 mg∙L−1;
- NPK 40:60:40 + two foliar applications at tillering and stem elongation of a dispersion of AgNPs at 1 mg∙L−1;
- NPK 40:60:40 + two foliar applications at tillering and stem elongation of a dispersion of AgNPs at 5 mg∙L−1;
- NPK 40:60:40 + three foliar applications at tillering, stem elongation, and heading of a dispersion of AgNPs at 5 mg∙L−1;
- NPK 40:60:40 + two foliar applications at tillering and stem elongation of a dispersion of AgNPs at 25 mg∙L−1.
2.8. Antioxidant Enzyme Activity
2.8.1. Peroxidase (POD) Activity
2.8.2. Catalase (CAT) Activity
2.8.3. Polyphenol Oxidase (PPO) Activity
2.9. Statistical Analysis
3. Results
3.1. Preparation and Colloidal Characteristics of Dispersions of AgNPs
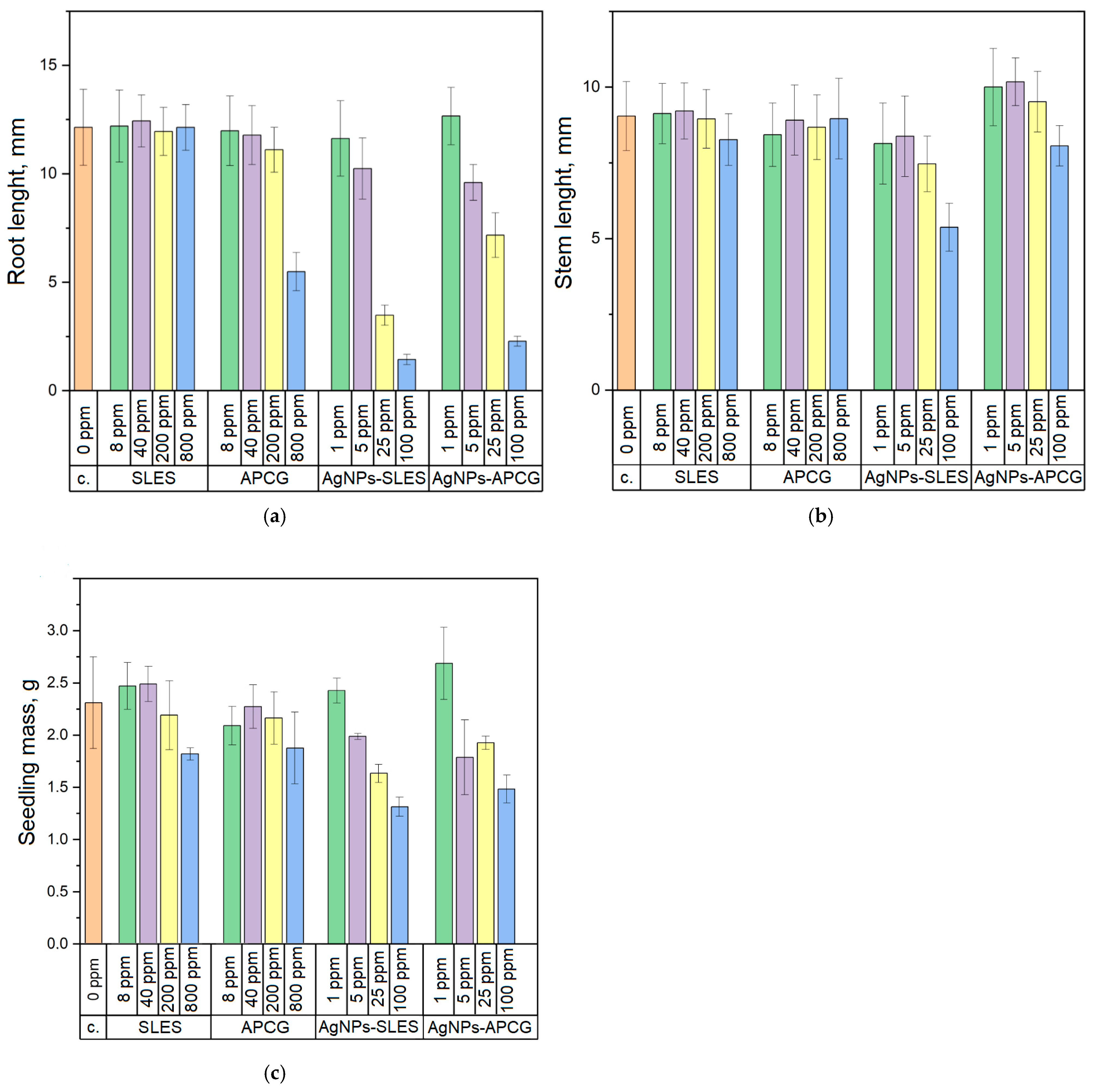
3.2. Effect of Seed Priming with Dispersions of AgNPs on Wheat Seedlings Growth
3.3. Field Trials
3.4. Antioxidant Enzyme Activity
4. Discussion
4.1. Preparation and Colloidal Characteristics of Dispersions of AgNPs
4.2. Effect of Seed Priming with Dispersions of AgNPs on Wheat Seedlings Growth
4.3. Field Trials
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABA | abscisic acid |
| AgNPs | silver nanoparticles |
| APCG | sodium amphopolycarboxyglycinate |
| CAT | catalase |
| DLS | dynamic light scattering |
| HSD | Honestly Significant Difference |
| LSD | least significant difference |
| MAPK | mitogen-activated protein kinases |
| POD | peroxidase |
| PPO | polyphenol oxidase |
| PVP | polyvinylpyrrolidone |
| ROS | reactive oxygen species |
| SAED | selected area electron diffraction |
| SD | standard deviation |
| SLES | sodium laureth sulfate |
| TEM | transmission electron microscopy |
| TKW | thousand-kernel weight |
| ζ-potential | electrokinetic potential (“zeta” potential) |
References
- Dawadi, S.; Katuwal, S.; Gupta, A.; Lamichhane, U.; Thapa, R.; Jaisi, S.; Lamichhane, G.; Bhattarai, D.P.; Parajuli, N. Current Research on Silver Nanoparticles: Synthesis, Characterization, and Applications. J. Nanomater. 2021, 2021, 6687290. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Green Silver Nanoparticles: An Antibacterial Mechanism. Antibiotics 2025, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Duman, H.; Eker, F.; Akdaşçi, E.; Witkowska, A.M.; Bechelany, M.; Karav, S. Silver Nanoparticles: A Comprehensive Review of Synthesis Methods and Chemical and Physical Properties. Nanomaterials 2024, 14, 1527. [Google Scholar] [CrossRef]
- Singh, K.R.B.; Natarajan, A.; Pandey, S.S. Bioinspired Multifunctional Silver Nanoparticles for Optical Sensing Applications: A Sustainable Approach. ACS Appl. Bio Mater. 2023, 6, 4549–4571. [Google Scholar] [CrossRef]
- Abbas, R.; Luo, J.; Qi, X.; Naz, A.; Khan, I.A.; Liu, H.; Yu, S.; Wei, J. Silver Nanoparticles: Synthesis, Structure, Properties and Applications. Nanomaterials 2024, 14, 1425. [Google Scholar] [CrossRef] [PubMed]
- Khina, A.G.; Krutyakov, Y.A. Similarities and Differences in the Mechanism of Antibacterial Action of Silver Ions and Nanoparticles. Appl. Biochem. Microbiol. 2021, 57, 683–693. [Google Scholar] [CrossRef]
- Krutyakov, Y.A.; Khina, A.G. Bacterial Resistance to Nanosilver: Molecular Mechanisms and Possible Ways to Overcome them. Appl. Biochem. Microbiol. 2022, 58, 493–506. [Google Scholar] [CrossRef]
- Mussin, C.; Giusiano, A. Biogenic Silver Nanoparticles as Antifungal Agents. Front. Chem. 2022, 10, 1023542. [Google Scholar] [CrossRef]
- Krutyakov, Y.A.; Kudrinskiy, A.A.; Zherebin, P.M.; Yapryntsev, A.D.; Pobedinskaya, M.A.; Elansky, S.N.; Denisov, A.N.; Mikhaylov, D.M.; Lisichkin, G.V. Tallow amphopolycarboxyglycinate-stabilized silver nanoparticles: New frontiers in development of plant protection products with a broad spectrum of action against phytopathogens. Mater. Res. Express 2016, 3, 075403. [Google Scholar] [CrossRef]
- Luceri, A.; Francese, R.; Lembo, D.; Ferraris, M.; Balagna, C. Silver Nanoparticles: Review of Antiviral Properties, Mechanism of Action and Applications. Microorganisms 2023, 11, 629. [Google Scholar] [CrossRef]
- Naganthran, A.; Verasoundarapandian, G.; Khalid, F.E.; Masarudin, M.J.; Zulkharnain, A.; Nawawi, N.M.; Karim, M.; Che Abdullah, C.A.; Ahmad, S.A. Synthesis, Characterization and Biomedical Application of Silver Nanoparticles. Materials 2022, 15, 427. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, Y.K.; Mishra, A.K.; Panda, J.; Chakrabartty, I.; Sarma, B.; Panda, S.K.; Chopra, H.; Zengin, G.; Moloney, M.G.; Sharifi-Rad, M. Promising Applications of Phyto-Fabricated Silver Nanoparticles: Recent Trends in Biomedicine. Biochem. Biophys. Res. Commun. 2023, 688, 149126. [Google Scholar] [CrossRef] [PubMed]
- Piluk, T.; Faccio, G.; Letsiou, S.; Liang, R.; Freire-Gormaly, M. A Critical Review Investigating the Use of Nanoparticles in Cosmetic Skin Products. Environ. Sci. Nano 2024, 11, 3674–3692. [Google Scholar] [CrossRef]
- Blinov, A.; Blinova, A.; Nagdalian, A.; Siddiqui, S.A.; Gvozdenko, A.; Golik, A.; Rekhman, Z.; Filippov, D.; Shariati, M.A.; Al-Farga, A.; et al. Sustainable Detergent-Disinfectant Agent Based on Whey Mineralizate and Silver Nanoparticles for Cleaner Production in Dairy Industry. Sci. Rep. 2024, 14, 23943. [Google Scholar] [CrossRef]
- Ediyilyam, S.; George, B.; Shankar, S.S.; Dennis, T.T.; Wacławek, S.; Černík, M.; Padil, V.V.T. Chitosan/Gelatin/Silver Nanoparticles Composites Films for Biodegradable Food Packaging Applications. Polymers 2021, 13, 1680. [Google Scholar] [CrossRef]
- Mecha, A.C.; Chollom, M.N.; Babatunde, B.F.; Tetteh, E.K.; Rathilal, S. Versatile Silver-Nanoparticle-Impregnated Membranes for Water Treatment: A Review. Membranes 2023, 13, 432. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Plant Response to Silver Nanoparticles: A Critical Review. Crit. Rev. Biotechnol. 2022, 42, 973–990. [Google Scholar] [CrossRef]
- Khan, S.; Zahoor, M.; Khan, R.S.; Ikram, M.; Islam, N.U. The Impact of Silver Nanoparticles on the Growth of Plants: The Agriculture Applications. Heliyon 2023, 9, e16928. [Google Scholar] [CrossRef]
- Huang, D.; Dang, F.; Huang, Y.; Chen, N.; Zhou, D. Uptake, Translocation, and Transformation of Silver Nanoparticles in Plants. Environ. Sci. Nano 2022, 9, 12–39. [Google Scholar] [CrossRef]
- Yang, Q.; Shan, W.; Hu, L.; Zhao, Y.; Hou, Y.; Yin, Y.; Liang, Y.; Wang, F.; Cai, Y.; Liu, J.; et al. Uptake and Transformation of Silver Nanoparticles and Ions by Rice Plants Revealed by Dual Stable Isotope Tracing. Environ. Sci. Technol. 2019, 53, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Vannini, C.; Domingo, G.; Onelli, E.; Prinsi, B.; Marsoni, M.; Espen, L.; Bracale, M. Morphological and Proteomic Responses of Eruca sativa Exposed to Silver Nanoparticles or Silver Nitrate. PLoS ONE 2013, 8, e68752. [Google Scholar] [CrossRef]
- Vishwakarma, K.; Shweta; Upadhyay, N.; Singh, J.; Liu, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Tripathi, D.K.; Sharma, S. Differential Phytotoxic Impact of Plant Mediated Silver Nanoparticles (AgNPs) and Silver Nitrate (AgNO3) on Brassica sp. Front. Plant Sci. 2017, 8, 1501. [Google Scholar] [CrossRef]
- Jiang, H.-S.; Qiu, X.-N.; Li, G.-B.; Li, W.; Yin, L.-Y. Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ. Toxicol. Chem. 2014, 33, 1398–1405. [Google Scholar] [CrossRef]
- Flores-López, L.Z.; Espinoza-Gómez, H.; Somanathan, R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019, 39, 16–26. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Onelli, E.; De Mattia, F.; Bruni, I.; Marsoni, M.; Bracale, M. Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J. Plant Physiol. 2014, 171, 1142–1148. [Google Scholar] [CrossRef]
- Štefanić, P.P.; Cvjetko, P.; Biba, R.; Domijan, A.-M.; Letofsky-Papst, I.; Tkalec, M.; Šikić, S.; Cindrić, M.; Balen, B. Physiological, ultrastructural and proteomic responses of tobacco seedlings exposed to silver nanoparticles and silver nitrate. Chemosphere 2018, 209, 640–653. [Google Scholar] [CrossRef] [PubMed]
- Syu, Y.-Y.; Hung, J.-H.; Chen, J.-C.; Chuang, H.-W. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef]
- Bawskar, M.; Bansod, S.; Rathod, D.; Santos, C.A.D.; Ingle, P.; Rai, M.; Gade, A. Silver nanoparticles as nanofungicide and plant growth promoter: Evidences from morphological and chlorophyll ‘a’ fluorescence analysis. Adv. Mater. Lett. 2021, 12, 1–7. [Google Scholar] [CrossRef]
- Krutyakov, Y.A.; Khina, A.G.; Mukhina, M.T.; Shapoval, O.A.; Lisichkin, G.V. Effect of treatment with colloidal silver dispersions stabilized with polyhexamethylene biguanide on the yield and biochemical parameters of potato plants in a field trial. Nanobiotechnol. Rep. 2023, 18, 362–370. [Google Scholar] [CrossRef]
- Zakharova, O.V.; Gusev, A.A.; Zherebin, P.M.; Skripnikova, E.Y.; Shashkova, E.N.; Bugaeva, L.I.; Sotnikova, M.V.; Nizovtseva, I.N.; Kudrinsky, A.A.; Krutyakov, Y.A. Sodium Tallow Amphopolycarboxyglycinate–Stabilized Silver Nanoparticles Suppress Early and Late Blight of Solanum lycopersicum and Stimulate the Growth of Tomato Plants. BioNanoScience 2017, 7, 692–702. [Google Scholar] [CrossRef]
- Kim, E.; Kim, S.-G.; Kim, H.-C.; Lee, S.G.; Lee, S.J.; Jeong, S.W. Growth Inhibition of Aquatic Plant Caused by Silver and Titanium Oxide Nanoparticles. Toxicol. Environ. Health Sci. 2011, 3, 1–6. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, L.R.; Dubey, B. Evaluation of Developmental Responses of Two Crop Plants Exposed to Silver and Zinc Oxide Nanoparticles. Sci. Total Environ. 2013, 452–453, 321–332. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Hatata, M.M.; Al-Huqail, A.A.; Ibrahim, M.M. Preparation, Characterization of Silver Phyto Nanoparticles and Their Impact on Growth Potential of Lupinus termis L. Seedlings. Saudi J. Biol. Sci. 2018, 25, 313–319. [Google Scholar] [CrossRef]
- Prażak, R.; Święciło, A.; Krzepiłko, A.; Michałek, S.; Arczewska, M. Impact of Ag Nanoparticles on Seed Germination and Seedling Growth of Green Beans in Normal and Chill Temperatures. Agriculture 2020, 10, 312. [Google Scholar] [CrossRef]
- Almutairi, Z.; Alharbi, A. Effect of Silver Nanoparticles on Seed Germination of Crop Plants. J. Adv. Agric. 2015, 4, 280–285. [Google Scholar] [CrossRef]
- Sosan, A.; Svistunenko, D.; Straltsova, D.; Tsiurkina, K.; Smolich, I.; Lawson, T.; Subramaniam, S.; Golovko, V.; Anderson, D.; Sokolik, A.; et al. Engineered Silver Nanoparticles Are Sensed at the Plasma Membrane and Dramatically Modify the Physiology of Arabidopsis thaliana Plants. Plant J. 2015, 85, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Vinković, T.; Štolfa-Čamagajevac, I.; Tkalec, M.; Goessler, W.; Domazet-Jurašin, D.; Vinković Vrček, I. Does Plant Growing Condition Affects Biodistribution and Biological Effects of Silver Nanoparticles? Span. J. Agric. Res. 2018, 16, e0803. [Google Scholar] [CrossRef]
- Krutyakov, Y.A.; Kudrinsky, A.A.; Gusev, A.A.; Zakharova, O.V.; Klimov, A.I.; Yapryntsev, A.D.; Zherebin, P.M.; Shapoval, O.A.; Lisichkin, G.V. Synthesis of positively charged hybrid PHMB-stabilized silver nanoparticles: The search for a new type of active substances used in plant protection products. Mater. Res. Express 2017, 4, 075018. [Google Scholar] [CrossRef]
- Wang, D.; Jaisi, D.P.; Yan, J.; Jin, Y.; Zhou, D. Transport and Retention of Polyvinylpyrrolidone-Coated Silver Nanoparticles in Natural Soils. Vadose Zone J. 2015, 14, vzj2015-01. [Google Scholar] [CrossRef]
- Santa-Cruz, J.; Vasenev, I.I.; Gaete, H.; Peñaloza, P.; Krutyakov, Y.A.; Neaman, A. Metal Ecotoxicity Studies with Artificially Contaminated versus Anthropogenically Contaminated Soils: Literature Review, Methodological Pitfalls and Research Priorities. Russ. J. Ecol. 2021, 52, 479–485. [Google Scholar] [CrossRef]
- Khina, A.G.; Lisichkin, G.V.; Krutyakov, Y.A. Effect of Silver Nanoparticles on the Physiology of Higher Plants. Russ. J. Plant Physiol. 2024, 71, 169. [Google Scholar] [CrossRef]
- Flors, V.; Kyndt, T.; Mauch-Mani, B.; Pozo, M.J.; Ryu, C.-M.; Ton, J. Enabling sustainable crop protection with induced resistance in plants. Front. Sci. 2024, 2, 1407410. [Google Scholar] [CrossRef]
- Kudrinskiy, A.; Zherebin, P.; Gusev, A.; Shapoval, O.; Pyee, J.; Lisichkin, G.; Krutyakov, Y. New Relevant Descriptor of Linear QNAR Models for Toxicity Assessment of Silver Nanoparticles. Nanomaterials 2020, 10, 1459. [Google Scholar] [CrossRef] [PubMed]
- Abramenko, N.; Demidova, T.B.; Krutyakov, Y.A.; Zherebin, P.M.; Krysanov, E.Y.; Kustov, L.M.; Peijnenburg, W. The Effect of Capping Agents on the Toxicity of Silver Nanoparticles to Danio rerio Embryos. Nanotoxicology 2019, 13, 1–13. [Google Scholar] [CrossRef]
- López-Miranda, A.; López-Valdivieso, A.; Viramontes-Gamboa, G. Silver Nanoparticles Synthesis in Aqueous Solutions Using Sulfite as Reducing Agent and Sodium Dodecyl Sulfate as Stabilizer. J. Nanoparticle Res. 2012, 14, 1101. [Google Scholar] [CrossRef]
- Parrey, S.H.; Maseet, M.; Ahmad, R.; Khan, A.B. Deciphering the Kinetic Study of Sodium Dodecyl Sulfate on Ag Nanoparticle Synthesis Using Cassia siamea Flower Extract as a Reducing Agent. ACS Omega 2021, 6, 12155–12167. [Google Scholar] [CrossRef] [PubMed]
- Federal State Budgetary Institution “State Commission of the Russian Federation for Selection Achievements Test and Protection” (Gossortkomissiya). In Methods of State Variety Testing of Agricultural Crops; Issue One: General Part; Gossortkomissiya: Moscow, Russia, 2019; 329p, Available online: https://gossortrf.ru/upload/2019/08/metodica_1.pdf (accessed on 28 October 2025).
- Chance, B.; Maehly, A.C. Assay of Catalases and Peroxidases. Methods Enzymol. 1955, 2, 764–775. [Google Scholar] [CrossRef]
- Aebi, H. Catalase In Vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Mayer, A.M.; Harel, E.; Ben-Shaul, R. Assay of Catechol Oxidase—A Critical Comparison of Methods. Phytochemistry 1966, 5, 783–789. [Google Scholar] [CrossRef]
- Dospekhov, B.A. Methods of Field Experiment (with Fundamentals of Statistical Processing of Research Results), 5th ed.; Agropromizdat: Moscow, Russia, 1985; 351p. [Google Scholar]
- Huynh, K.A.; Chen, K.L. Aggregation Kinetics of Citrate and Polyvinylpyrrolidone Coated Silver Nanoparticles in Monovalent and Divalent Electrolyte Solutions. Environ. Sci. Tech. 2011, 45, 5564–5571. [Google Scholar] [CrossRef] [PubMed]
- Alabdallah, N.M.; Hasan, M.M. Plant-based green synthesis of silver nanoparticles and its effective role in abiotic stress tolerance in crop plants. Saudi J. Biol. Sci. 2021, 28, 5631–5639. [Google Scholar] [CrossRef]
- Iqbal, M.; Raya, N.I.; Mashwani, Z.-U.-M.; Hussain, M.; Ejaz, M.; Yasmeen, M. Effect of Silver Nanoparticles on Growth of Wheat Under Heat Stress. Iran J. Sci. Technol. Trans. A Sci. 2017, 43, 387–395. [Google Scholar] [CrossRef]
- Saeb, A.T.M.; Alshammari, A.S.; Al-Brahim, H.; Al-Rubeaan, K.A. Production of Silver Nanoparticles with Strong and Stable Antimicrobial Activity against Highly Pathogenic and Multidrug Resistant Bacteria. Sci. World J. 2014, 2014, 704708. [Google Scholar] [CrossRef]
- Qiao, Y.; Kang, J.; Song, C.; Zhou, N.; Zhang, P.; Song, G. Further Study on the Particle Size, Stability, and Complexation of Silver Nanoparticles under the Composite Effect of Bovine Serum Protein and Humic Acid. RSC Adv. 2024, 14, 2621–2632. [Google Scholar] [CrossRef]
- Karami Mehrian, S.; Heidari, R.; Rahmani, F.; Najafi, S. Effect of Chemical Synthesis Silver Nanoparticles on Germination Indices and Seedlings Growth in Seven Varieties of Lycopersicon esculentum Mill (Tomato) Plants. J. Clust. Sci. 2016, 27, 327–340. [Google Scholar] [CrossRef]
- Pan, R.; Zhang, Z.; Li, Y.; Zhu, S.; Anwar, S.; Huang, J.; Zhang, C.; Yin, L. Stage-Specific Effects of Silver Nanoparticles on Physiology During the Early Growth Stages of Rice. Plants 2024, 13, 3454. [Google Scholar] [CrossRef] [PubMed]
- Noori, A.; Ngo, A.; Gutierrez, P.; Theberge, S.; White, J.C. Silver Nanoparticle Detection and Accumulation in Tomato (Lycopersicon esculentum). J. Nanoparticle Res. 2020, 22, 131. [Google Scholar] [CrossRef]
- Sabir, S.; Arshad, M.; Ilyas, N.; Naz, F.; Amjad, M.S.; Malik, N.Z.; Chaudhari, S.K. Protective Role of Foliar Application of Green-Synthesized Silver Nanoparticles Against Wheat Stripe Rust Disease Caused by Puccinia striiformis. Green Process. Synth. 2022, 11, 29–43. [Google Scholar] [CrossRef]
- Mittler, R.; Zandalinas, S.I.; Fichman, Y.; Van Breusegem, F. Reactive oxygen species signalling in plant stress responses. Nat. Rev. Mol. Cell Biol. 2022, 23, 663–679. [Google Scholar] [CrossRef]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
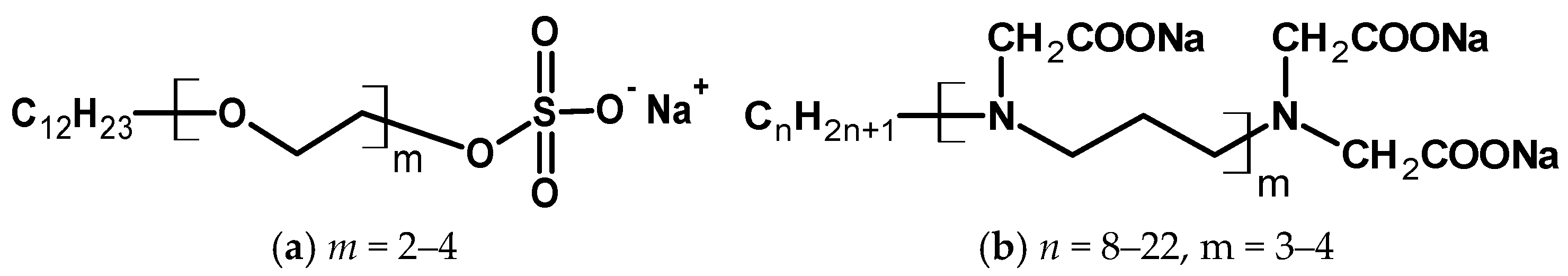
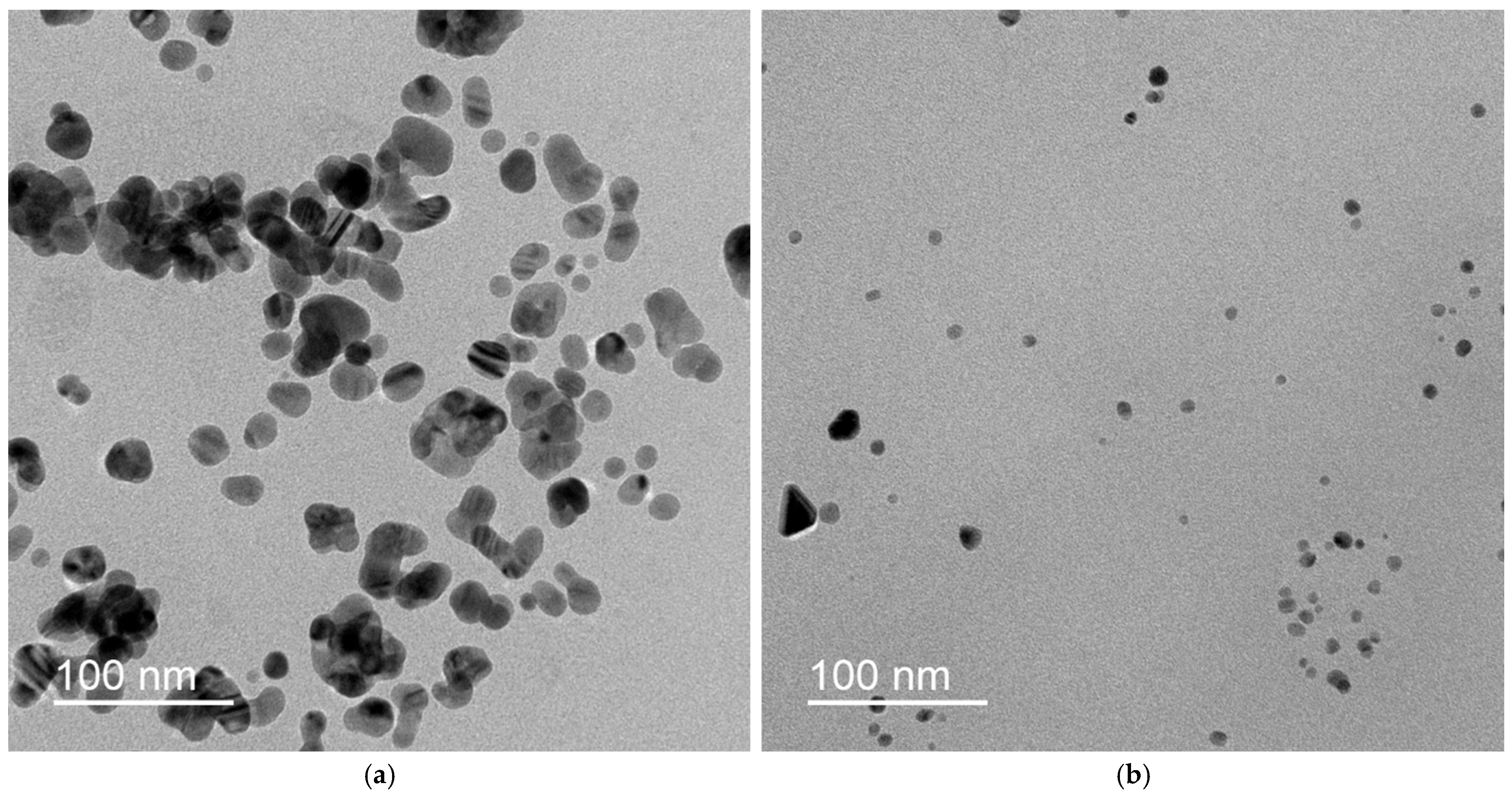

| Stabilizer | dTEM, nm | dDLS, nm | ζ, mV |
|---|---|---|---|
| SLES | 21.9 ± 9.1 | 60.2 ± 8.8 | −28.2 |
| APCG | 10.4 ± 4.9 | 27.2 ± 5.3 | −52.6 |
| Treatment No. | Growth Stage | |||
|---|---|---|---|---|
| Tillering | Stem Elongation | Heading | Milk Stage | |
| 1 | 407 | 378 | 364 | 352 |
| 2 | 404 | 372 | 368 | 349 |
| 3 | 410 | 384 | 375 | 358 |
| 4 | 425 | 395 | 382 | 367 |
| 5 | 440 | 406 | 395 | 380 |
| 6 | 418 | 392 | 386 | 372 |
| LSD0.05 | 12 | 10 | 10 | 9 |
| Treatment No. | Plant Height (mm) | Spikelets per Spike | Grains per Spike | Grain Weight per Spike (g∙Spike−1) | Thousand-Kernel Weight (g) | Grain Yield (t∙ha−1) |
|---|---|---|---|---|---|---|
| 1 | 560 | 19.0 | 29.0 | 1.24 | 42.8 | 5.89 |
| 2 | 555 | 19.3 | 28.5 | 1.23 | 43.2 | 5.82 |
| 3 | 588 | 19.8 | 31.0 | 1.31 | 42.3 | 5.99 |
| 4 | 605 | 20.6 | 30.6 | 1.42 | 46.4 | 6.32 |
| 5 | 625 | 21.0 | 31.2 | 1.46 | 46.8 | 6.60 |
| 6 | 615 | 20.8 | 30.9 | 1.44 | 46.6 | 6.50 |
| LSD0.05 | 25 | 1.0 | 1.5 | 0.06 | 1.0 | 0.18 |
| Dispersion | AgNPs Concentration (mg∙L−1) | POD Activity (U∙g−1 FW∙s−1) | CAT Activity (μmol H2O2∙g−1 FW∙min−1) | PPO Activity (U∙g−1 FW∙min−1) |
|---|---|---|---|---|
| - | 0 | 189 ± 4 | 1119 ± 26 | 22.9 ± 1.2 |
| AgNPs-SLES | 1 | 251 ± 4 | 1432 ± 40 | 28.1 ± 1.4 |
| 5 | 270 ± 8 | 1600 ± 35 | 30.3 ± 1.3 | |
| 25 | 190 ± 7 | 1151 ± 29 | 23.1 ± 1.2 | |
| 100 | 45 ± 10 | 279 ± 19 | 6.6 ± 1.3 | |
| AgNPs-APCG | 1 | 253 ± 7 | 1510 ± 31 | 29.5 ± 1.0 |
| 5 | 285 ± 9 | 1685 ± 38 | 31.2 ± 1.4 | |
| 25 | 228 ± 8 | 1405 ± 33 | 26.3 ± 1.1 | |
| 100 | 81 ± 12 | 469 ± 30 | 9.4 ± 0.8 |
| Treatment No. | Peroxidase Activity (U∙g−1 FW∙s−1) | Catalase Activity (μmol H2O2∙g−1 FW∙min−1) | Polyphenol Oxidase Activity (U∙g−1 FW∙min−1) |
|---|---|---|---|
| 1 | 234 ± 16 | 1259 ± 43 | 21.2 ± 0.8 |
| 2 | 244 ± 19 | 1212 ± 43 | 21.3 ± 0.8 |
| 3 | 236 ± 18 | 1150 ± 26 | 20.3 ± 1.0 |
| 4 | 268 ± 14 | 1395 ± 35 | 23.8 ± 1.2 |
| 5 | 252 ± 12 | 1320 ± 31 | 22.6 ± 1.1 |
| 6 | 277 ± 10 | 1441 ± 41 | 24.6 ± 1.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khina, A.G.; Biktasheva, L.R.; Gordeev, A.S.; Mikhaylov, D.M.; Mukhina, M.T.; Lisichkin, G.V.; Krutyakov, Y.A. Effect of Silver Nanoparticles on Growth of Wheat: Is It Stage-Specific or Not? Agronomy 2025, 15, 2540. https://doi.org/10.3390/agronomy15112540
Khina AG, Biktasheva LR, Gordeev AS, Mikhaylov DM, Mukhina MT, Lisichkin GV, Krutyakov YA. Effect of Silver Nanoparticles on Growth of Wheat: Is It Stage-Specific or Not? Agronomy. 2025; 15(11):2540. https://doi.org/10.3390/agronomy15112540
Chicago/Turabian StyleKhina, Alexander G., Liliya R. Biktasheva, Alexander S. Gordeev, Dmitry M. Mikhaylov, Maria T. Mukhina, Georgii V. Lisichkin, and Yurii A. Krutyakov. 2025. "Effect of Silver Nanoparticles on Growth of Wheat: Is It Stage-Specific or Not?" Agronomy 15, no. 11: 2540. https://doi.org/10.3390/agronomy15112540
APA StyleKhina, A. G., Biktasheva, L. R., Gordeev, A. S., Mikhaylov, D. M., Mukhina, M. T., Lisichkin, G. V., & Krutyakov, Y. A. (2025). Effect of Silver Nanoparticles on Growth of Wheat: Is It Stage-Specific or Not? Agronomy, 15(11), 2540. https://doi.org/10.3390/agronomy15112540






