Abstract
Biochar amendment has been widely recognized for its potential to promote soil carbon sequestration and improve crop productivity; however, the microbial mechanisms underlying carbon sequestration at varying biochar application rates remain insufficiently understood. In this study, a field experiment was conducted in a typical fluvo-aquic soil region of the North China Plain under a maize–wheat rotation, with one-time biochar application at four levels: CK (0 t ha−1), B5 (5 t ha−1), B10 (10 t ha−1), and B20 (20 t ha−1). The effects of these treatments on soil physicochemical properties, organic carbon fractions, microbial community structure, and enzyme activities were systematically examined. The results showed that soil total nitrogen (TN) and pH increased consistently with higher biochar application rates, reaching maximum values under B20 treatment, where TN and pH rose by 35.56% and 7.00% relative to CK, respectively. In contrast, the contents of NH4+-N, available phosphorus (AP), and available potassium were mostly enhanced under B5 during the maize season, while in the wheat season, NH4+-N peaked under B10 and AP peaked under B5. Biochar addition significantly increased soil organic carbon fractions and the carbon pool management index (CMI). In the maize season, soil organic carbon (SOC), microbial biomass carbon (MBC), particulate organic carbon (POC), and CMI under B20 rose by 55.99%, 39.67%, 79.69% and 180.54% over CK, respectively, whereas dissolved organic carbon (DOC) peaked under B5. Throughout the wheat season, SOC, MBC, and POC contents under B20 were 53.70%, 64.31% and 147.81% higher than CK, while DOC peaked under B5 (+56.98%). Soil enzyme activities, including catalase, urease, invertase and alkaline phosphatase, were strongly stimulated by biochar, with B20 increasing their activities by 4.49–18.18%, 3.19–19.77%, 6.14–26.14% and 12.25–33.19%, respectively. Biochar also reshaped microbial community structure: the during maize season, it reduced the relative abundance of Glomeromycetes (65.31%) and Oligohymenophorea (51.64%) while enhancing Deltaproteobacteria (46.15%) and Gammaproteobacteria (29.03%); during wheat season; it enhanced Eurotiomycetes (85.77%) and Dothideomycetes (16.28%) but suppressed Deinococci (74.08%) and Alphaproteobacteria (4.39%). Pathway analysis further indicated that biochar amendments indirectly increased SOC fractions and CMI by simultaneously altering nutrient availability, regulating microbial community structure, and stimulating soil enzyme activities. Collectively, these findings highlight that the effects of biochar are dosage-specific: moderate rates (e.g., 5 t ha −1) are more suitable for the short-term improvement of soil fertility, while higher rates (e.g., 20 t ha−1) are more effective for long-term carbon sequestration; depending on the objective, biochar application can thus substantially modify soil physicochemical and biological processes to promote agroecosystem sustainability in the North China Plain.
1. Introduction
Soil organic carbon (SOC), formed through the microbial-mediated decomposition of humus and plant and animal residues, constitutes a fundamental component for soil biota and is the core of soil quality and function [1]. Soil organic carbon SOC is commonly categorized into labile and recalcitrant (or stable) fractions [2], which exhibit distinct turnover rates due to their differing chemical compositions, ultimately affecting their mechanisms and efficiency of sequestration in the soil carbon pool. Despite its relatively small proportion within the total SOC pool, the labile fraction undergoes rapid turnover and serves as a sensitive indicator of changes in soil carbon dynamics. In contrast, the recalcitrant SOC fraction, which includes heavy fraction organic carbon (HFOC) and mineral-associated organic carbon (MAOC), consists of compounds resistant to decomposition over extended periods, making it a key indicator of the long-term carbon sequestration potential in soil [3,4]. The dynamics of these SOC fractions are strongly influenced by a range of biotic and abiotic factors, including soil microbial community structure, extracellular enzyme activities, soil texture, mineralogy, and climatic conditions. In particular, microbial life in soil, governed by nutrient availability, pH, moisture, and the presence of inhibitory compounds, plays a decisive role in SOC stabilization and turnover [5,6]. Numerous studies have demonstrated that agricultural management practices significantly influence the soil carbon pool, with the labile SOC fractions being particularly responsive to practices like fertilization, tillage, and irrigation due to their direct involvement in microbial metabolism [7,8]. Therefore, a thorough understanding of the dynamics of labile SOC fractions is crucial for elucidating the underlying mechanisms of soil carbon sequestration when assessing the efficacy of agricultural management practices.
Biochar is produced through the pyrolysis of agricultural wastes like crop straw, endowing it with a stable aromatic structure, a developed pore system, and a high specific surface area. Functioning as an effective soil amendment [9], it plays a significant role in promoting agricultural sustainability and achieving carbon sequestration and emission reduction. Extensive research indicates that biochar application improves soil structure [10], enhances nutrient retention [11], increases soil organic carbon content [12], and consequently boosts crop yields [13]. In the context of soil carbon sequestration, recent research has increasingly focused on how biochar modifies the composition and stability of SOC fractions, rather than merely increasing the total SOC content. Previous studies have revealed that biochar application influences soil organic carbon fractions through multiple mechanisms [14]. On the one hand, the abundant functional groups on the biochar surface can effectively adsorb and stabilize soluble organic matter in the soil, reducing leaching losses [15]. On the other hand, biochar can form organo-mineral complexes with mineral particles, promoting the formation of macro-aggregates, thereby physically protecting particulate organic carbon (POC) and significantly increasing its content [16]. It has also been widely reported that the porous structure of biochar provides a favorable habitat for microbes, significantly shifts microbial community composition, and enriches beneficial plant-growth-promoting bacteria such as phosphate-solubilizing and nitrogen-fixing bacteria, thus enhancing organic matter accumulation [17].
Despite the considerable potential of biochar for soil carbon sequestration, its impact on SOC is highly variable, influenced by heterogeneity under natural conditions [18], inherent soil properties [19], biochar characteristics [20], and agronomic management practices [21]. The application rate of biochar is a key factor contributing to this variability. A critical knowledge gap persists in understanding the non-linear responses of SOC fractions to biochar application rates, particularly the underlying microbial mechanisms. A meta-analysis reported a positive correlation between biochar application rate and the magnitude of SOC enhancement [22]. In contrast, no significant difference was observed in SOC improvement when application rates varied within the range of 40–60 t ha−1 [23]. The pronounced discrepancies observed across studies are likely linked to microbial effects. High application rates may induce excessive immobilization of water and essential nutrients [24] or directly adsorb extracellular enzymes [25], consequently causing nutrient imbalances and aberrant microbial responses. Furthermore, biochar may contain inherent inhibitory compounds like polycyclic aromatic hydrocarbons (PAHs), which can exert toxic effects at localized high concentrations [26]. This can suppress the activity and functionality of specific microbial groups, thereby reducing their capacity to decompose and transform organic matter, leading to decreased soil carbon sequestration and enzyme activity [27]. Thus, clarifying the shifts in soil microbial community structure in response to varying application rates is crucial for revealing the mechanisms underlying biochar-induced carbon sequestration. Moreover, a meta-analysis on the integrated impact of biochar amendment on greenhouse gas emissions revealed that inappropriate application could potentially increase the global warming potential [28]. Based on DNDC model simulations, a recommended application rate of 40 t ha−1 was determined as optimal, following a comprehensive assessment that balanced greenhouse gas mitigation, SOC sequestration, and crop yield enhancement [12]. A profound understanding of the carbon sequestration mechanisms associated with different biochar application rates is essential for advancing green and low-carbon agricultural practices in the North China Plain.
Therefore, elucidating the carbon sequestration effects and underlying microbial mechanisms under different biochar application rates is essential for developing optimized application strategies. We hypothesized that biochar enhances soil carbon sequestration in a dose-dependent manner, driven by distinct microbial pathways that vary between cropping seasons. To test this, a field experiment was conducted in a typical maize–wheat rotation system on aquic soil in the North China Plain, employing a one-time application of biochar at 0, 5, 10, and 20 t ha−1. The specific objectives were as follows: (1) to quantify the responses of soil organic carbon fractions (including SOC, POC, DOC, EOC, and MBC) and the carbon pool management index to different biochar application rates in the maize–wheat rotation system, and (2) to elucidate the microbial mechanisms driving soil carbon pool dynamics, thereby providing a scientific basis for the rational application of biochar in sustainable agroecosystems in the North China Plain.
2. Materials and Methods
2.1. Experimental Site
The field experiment was conducted at the Original Seed Farm in Ningjin County, Xingtai City, Hebei Province, China (37°37′ N, 114°59′ E). The experimental site lies on the alluvial plain at the eastern foothills of the Taihang Mountains in the North China Plain, characterized by a warm-temperate semi-arid climate. The mean annual temperature is 12.8 °C, the mean annual precipitation is 449.1 mm, and the average frost-free period is 198 days. The soil at the site is classified as a medium loam aquic cinnamon soil. The basic physicochemical properties of the 0–20 cm layer are presented in Table 1.

Table 1.
Basic physicochemical properties of the experimental soil prior to biochar application.
2.2. Experimental Design
Four treatments were established: the experiment included a control treatment (CK) and three biochar-amended treatments (B5, B10, B20). For CK, a bulk blend fertilizer (156 kg N ha−1, 60 kg P2O5 ha−1, 72 kg K2O ha−1) was applied before maize sowing, while in the wheat season, a bulk blend fertilizer (120 kg N ha−1, 135 kg P2O5 ha−1, 105 kg K2O ha−1) was applied pre-sowing, followed by a topdressing of 120 kg N·ha−1 using urea at the jointing stage, with no biochar addition. Other treatments (B5, B10, B20) maintained identical chemical fertilizer rates as CK but were supplemented with 5, 10, or 20 t ha−1 biochar, respectively. A randomized block design was applied with three replicates per treatment. Each plot measures 9.5 m in length and 3.6 m in width, with an area of 34.2 m2. The experiment involves a rotation system of summer maize and winter wheat. The first-year summer maize was sown on 18 June 2017 and harvested on 3 October 2017. Winter wheat was sown on 26 October 2017 and harvested on 7 June 2018. The summer maize of the following year was sown on 13 June 2018 and harvested on 1 October 2018. Winter wheat was sown on 13 October 2018 and harvested on 6 June 2019. The summer maize of the third year was sown on 11 June 2019 and harvested on 28 September 2019. Winter wheat was sown on 11 October 2019 and harvested on 6 June 2020. A one-time application of biochar was conducted in June 2017. The biochar was spread on the soil surface before summer maize sowing and subsequently incorporated into the 0–20 cm topsoil layer via plowing. Biochar was produced from pruned fruit tree residues through pyrolysis at 600 °C under oxygen-limited conditions. The biochar contained 0.50 g kg−1 total N, 0.90 g kg−1 total P, 89.30 mg kg−1 available P, and 251.00 mg kg−1 available potassium. The maize cultivar Zhengdan 958 was sown at a density of 67,500 plants ha−1, the row spacing is 60 cm, and the plant spacing is 22 cm. The wheat cultivar Gaoyou 2018 was sown at a density of 262.5 kg ha−1. Irrigation, pest control and weeding all followed local conventional practices. Maize was irrigated twice (at sowing and the V12 stage), while wheat was irrigated three times (before overwintering, at green-up, and at jointing stages). The total irrigation amount during the growth period of maize was 90 mm, and during the growth period of wheat, it was 315 mm.
2.3. Sample Collection
Composite soil samples (0–20 cm) were collected using the five-point method. Baseline soils were sampled before fertilizer and biochar applications of the summer maize season in June 2017. Soil samples used to determine the experimental effects were collected at the crop harvest stage from each plot on 28 September 2019 in the maize season and 6 June 2020 in the wheat season. A portion of each soil sample was stored at 4 °C for the determination of soil moisture, NH4+-N, NO3−-N, DOC, and MBC, while the remaining portion was air-dried, cleaned, ground, and sieved for analysis of available P, available potassium, organic matter, enzyme activities (invertase, urease, alkaline phosphatase, catalase), and pH. Rhizosphere soils were collected by gently shaking roots of randomly selected plants, brushing off fine soils adhering to the root surface, and immediately storing the samples at −80 °C for microbial community analysis.
2.4. Parameters and Measurements
2.4.1. Soil Physicochemical Properties
Soil organic matter (SOM) was determined by potassium dichromate oxidation with external heating [29]. Total nitrogen (TN) was measured by the semi-micro Kjeldahl method (Beijing Ruibang Xingye (Beijing, China) KDY-9820 Kjeldahl Nitrogen Analyzer) [30]. NH4+-N and NO3−-N were extracted with 1 mol/L KCl and quantified using a flow injection analyzer (Skala Analytical Instruments (Shanghai, China) Co., LTD SKALAR SAN++ Continuous Flow Analyzer) [31]. Available phosphorus (AP) was measured by 0.5 mol/L NaHCO3 extraction followed by molybdenum–antimony colorimetry (Shanghai Youke Scanning (Shanghai, China) Ultraviolet-Visible Spectrophotometer UV759CRT), and available potassium (AK) was determined by 1 mol/L NaOAc extraction and flame photometry (Zhejiang Lichen Technology (Zhejiang, China) WGH-Flame Photometer) [32]. Soil pH was measured in a 1:2.5 soil-to-water suspension using a pH meter (FE28 pH Meter) [33]. Soil organic carbon was determined by K2Cr2O7-H2SO4 oxidation with external heating [34]. Dissolved organic carbon (DOC) was quantified following Ghani’s method using German Element-Elimonta Trading (Shanghai, China) Co., LTD liquiTOC after shaking and centrifugation [35]. Easily oxidizable organic carbon (EOC) was measured using the KMnO4 oxidation method [36], and microbial biomass carbon (MBC) was determined by the chloroform fumigation-extraction method using liquiTOC [37]. Particulate organic carbon was determined through sodium hexametaphosphate dispersion and dichromate oxidation [38,39].
2.4.2. Soil Enzyme Activities
Soil urease activity was determined by indophenol blue colorimetry. Procedure: 5 g air-dried soil was treated with toluene; urea solution and buffer were added and incubated at 37 °C for 24 h and then filtered. Filtrate reacted with chromogenic agent, and absorbance at 578 nm was measured using a UV759CRT spectrophotometer. Enzyme activity was calculated against a standard curve. Soil catalase activity was determined by potassium permanganate titration. Procedure: 2 g of soil was mixed with distilled water and H2O2 solution; after sealed shaking, saturated alum was added to terminate the reaction, and the filtrate was titrated with standardized KMnO4 using a Titrette burette until a stable purple endpoint was reached. Soil-free controls were established, with enzyme activity calculated from KMnO4 consumption. Soil invertase activity was determined by 3,5-dinitrosalicylic acid (DNS) colorimetry. Procedure: 5 g of soil was mixed with sucrose solution and buffer; after toluene treatment, samples were incubated at 37 °C for 24 h. The filtrate was reacted with the DNS reagent after filtration; color development was achieved by boiling in a water bath before dilution; absorbance at 508 nm was measured using a UV759CRT spectrophotometer; both substrate-free and soil-free controls were established; and enzyme activity was calculated against a standard curve. Soil alkaline phosphatase activity was determined using disodium phenyl phosphate colorimetry. Procedure: 5 g of sieved air-dried soil was mixed with toluene and disodium phenyl phosphate solution; after 37 °C incubation, samples were filtered. The filtrate was reacted with the buffer solution and 4-aminoantipyrine and potassium ferricyanide reagent to develop color; after dilution, absorbance at 510 nm was measured using a UV759CRT spectrophotometer. Soil-free controls and standard curves were established; enzyme activity was calculated based on phenol production.
2.4.3. Soil Microbial Community Compositions
High-throughput sequencing of the soil bacterial and fungal communities was conducted by Guangdong Magigene Technology Co., Ltd. (Guangzhou, China) on an Illumina MiSeq platform. Briefly, the bacterial 16S rRNA gene V3-V4 region and the fungal ITS2 region were amplified using primer sets with index sequences. The PCR amplicons were purified from 2% agarose gels, quantified, and then pooled in equimolar amounts for library construction. The constructed libraries passed quality control (including concentration and insert size assessment) before being subjected to paired-end sequencing (2 × 300 bp) using the Illumina (San Diego, CA, USA) MiSeq Reagent Kit v3 (600-cycle). The resulting sequence data were processed and analyzed using Magigene’s standard bioinformatics pipeline, enabling simultaneous analysis of both communities.
Bioinformatics analysis of soil microbial data was conducted by splicing the raw data obtained from sequencing using Flash (V1.2.11) software, the spliced fragments were clustered using Usearch (V8.0.1517) software, operational taxonomic units (OTU) and taxonomic community distributions were analyzed in R software 4.4.3. Alpha diversity indices were calculated from normalized OTU tables using QIIME’s (v1.9.1) alpha_diversity.py script, the alpha diversity index difference test was conducted using R software. Redundancy analysis (RDA) and chart modification of soil fungal and bacterial phylum horizontal community abundance and chemical factors were conducted using R software. All analyses were conducted on the Magigene Cloud Platform (Guangdong Magigene Biotechnology Co., Ltd., China).
2.5. Statistical Analysis
The impacts of biochar application on soil properties, organic carbon components, and enzymatic activities were analyzed by one-factor analysis of variance (ANOVA), and correlation analyses of physicochemical properties, carbon components, and enzyme activities were conducted using Pearson correlation analysis. Path analysis elucidated intrinsic relationships between soil enzyme activities and organic carbon fractions under biochar treatments. The application rate of biochar was set as an exogenous variable, soil enzyme activity as an endogenous variable, and the composition of soil organic carbon was an endogenous explanatory variable. Model reliability was verified by the χ2 test, degrees of freedom (Df), and p-value thresholds. Statistical analysis, heatmap, and path analysis result graphs were performed using R software, and other graphical outputs were generated using Origin 2021.
3. Results
3.1. Response of Soil Physicochemical Properties to Biochar Application
During maize season, soil pH and TN showed a clear upward trend with increasing biochar application rates, with the highest values observed under B20 treatment, where pH and TN rose by 7.0% and 35.6%, respectively, compared with CK (Table 2). Soil NH4+-N, AP and AK peaked under B5 treatment, increasing by 47.2%, 1.1%, and 5.0% relative to CK, respectively, while bulk density showed no significant differences among treatments. In the wheat season, TN continued to rise significantly with increasing biochar input, reaching 36.2% higher under B20 treatment than CK. Concentrations of NO3−-N and AP peaked under B5, whereas NH4+-N reached its maximum at B10, increasing by 7.5%, 28.6%, and 56.2% relative to CK. No significant differences in soil pH and bulk density were detected among treatments during the wheat season.

Table 2.
Soil physicochemical properties respond to biochar application in the North China Plain.
3.2. Response of Soil Carbon Fractions to Biochar Application
Biochar addition markedly influenced SOC and its fractions in the maize–wheat rotation system (Figure 1). The SOC, MBC, POC and CMI increased steadily with rising biochar application rates. In the maize season, the B20 treatment enhanced these parameters by 56.0%, 39.7%, 79.7% and 180.5%, respectively, compared with CK, while in the wheat season the corresponding increments were 53.7%, 64.3%, 147.8% and 75.5%. DOC, however, showed a different response pattern, peaking at B5, with increases of 56.98% in wheat and 60.82% in maize relative to CK. Easily oxidizable organic carbon was most enhanced under B20 in maize (+120.4%) and under B10 in wheat (+71.4%) compared with CK.
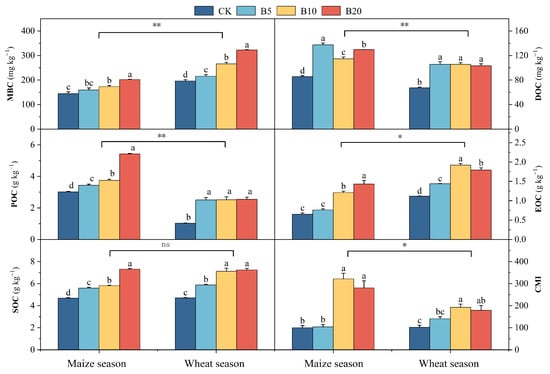
Figure 1.
Soil organic carbon fractions and carbon pool management index respond to biochar application in the North China Plain. MBC, microbial biomass carbon; DOC, dissolved organic carbon; POC, particulate organic carbon; EOC, easily oxidizable organic carbon; SOC, soil organic carbon; CMI, carbon pool management index. Significant differences in the measured parameters between cropping seasons are indicated by asterisks (* p ≤ 0.05, ** p ≤ 0.01; ns, p > 0.05). Different lowercase letters indicate significant differences between treatments within the same crop season (p < 0.05). Error bars represent standard error.
3.3. Response of Soil Enzyme Activities and Microbial Community Structure to Biochar Application
Across both cropping seasons, biochar application significantly stimulated soil enzyme activities (Figure 2). Under B20, CAT, URE, INV, and ALP activities increased by 4.5–18.2%, 3.2–19.8%, 6.1–26.1% and 12.3–33.2%, respectively, compared with CK. Meanwhile, biochar substantially altered microbial community composition (Figure 3). In the wheat season, the abundances of Eurotiomycetes and Dothideomycetes increased by 85.8% and 16.3%, respectively, whereas in the maize season, Glomeromycetes and Oligohymenophorea decreased by 65.3% and 51.6%, respectively. Among bacterial groups, Gammaproteobacteria and Deltaproteobacteria were enriched during the maize season (+29.0% and +46.2%, respectively), while Deinococci and Alphaproteobacteria were suppressed during the wheat season (−74.1% and −4.4%, respectively).
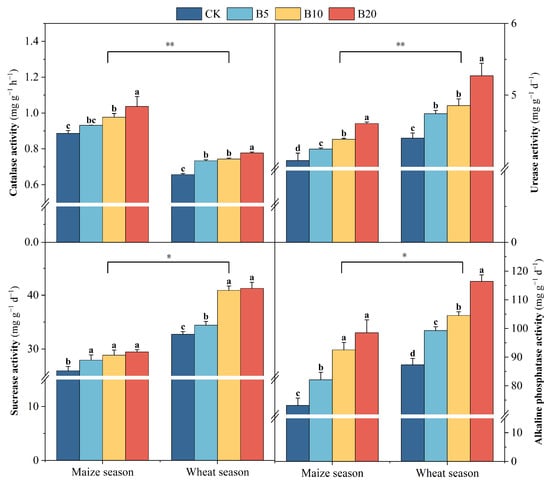
Figure 2.
Soil enzyme activities respond to biochar application in the North China Plain. Significant differences in the measured parameters between cropping seasons are indicated by asterisks (* p ≤ 0.05, ** p ≤ 0.01). Different lowercase letters indicate significant differences between treatments within the same crop season (p < 0.05). Error bars represent standard error.
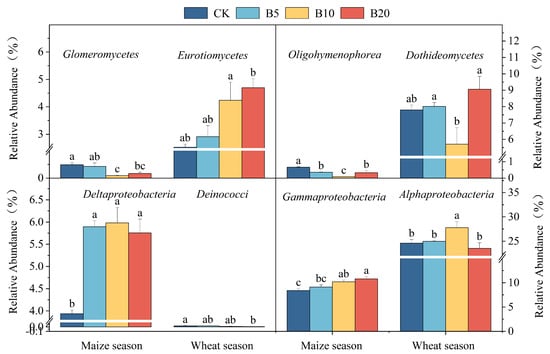
Figure 3.
Soil microbial community compositions respond to biochar application in the North China Plain. Different lowercase letters indicate significant differences between treatments within the same crop season (p < 0.05). Error bars represent standard error.
3.4. Interaction Mechanisms Among Soil Carbon Fractions, Microbial Communities, Enzyme Activities, and Soil Properties Under Biochar Application
Correlation analysis revealed distinct linkages between microbial taxa and soil enzyme activities (Figure 4). In the maize season, Oligohymenophorea and Glomeromycetes exhibited significant negative correlations with multiple enzyme activities, whereas Gammaproteobacteria and Deltaproteobacteria correlated positively with CAT, UE, and ALP (Figure 4a). In the wheat season, Eurotiomycetes and Dothideomycetes were positively associated with most enzyme activities, while Deinococci showed negative correlations (Figure 4b).
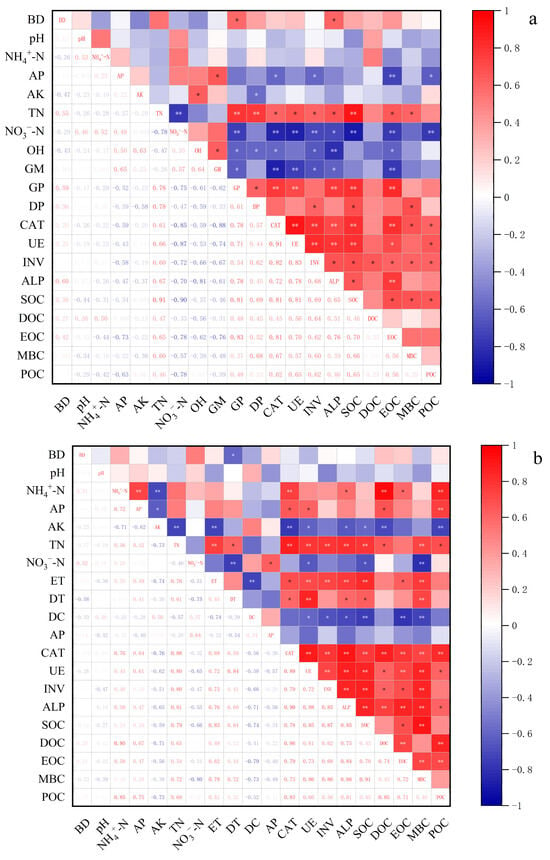
Figure 4.
Correlations among soil physicochemical properties, microbial abundance, enzyme activities, and organic carbon fractions in biochar-amended soils of the North China Plain. (a), Maize season; (b), wheat season. BD, bulk density; AP, available phosphorus; AK, available potassium; TN, total nitrogen. ET, Eurotiomycetes DT, Dothideomycetes; DC, Deinococci; AP, Alphaproteobacteria; CAT, catalase; UE, urease; INV, invertase; ALP, alkaline phosphatase; SOC, soil organic carbon; DOC, dissolved organic carbon; EOC, easily oxidizable organic carbon; MBC, microbial biomass carbon; POC, particulate organic carbon. Asterisks mark their significance: * p < 0.05, ** p < 0.01.
Path analysis further clarified the regulatory pathways under biochar amendment in the North China Plain (Figure 5). During the maize season, biochar significantly increased soil pH and TN, which promoted bacterial community proliferation (path coefficient = 0.773) but suppressed fungal communities (−0.794). Increased bacterial abundance enhanced soil enzyme activities (0.736), which in turn promoted SOC fractions (0.714) (Figure 5a). In the wheat season, biochar similarly improved soil pH and TN, but in this case, it stimulated fungal communities (0.790). Enhanced fungal abundance elevated enzyme activities (0.461), which subsequently promoted SOC fractions (0.437) (Figure 5b). These results suggest that biochar regulates SOC accumulation indirectly through a cascade of soil nutrient changes, microbial community restructuring, and enzyme activation, rather than solely by its direct effects.
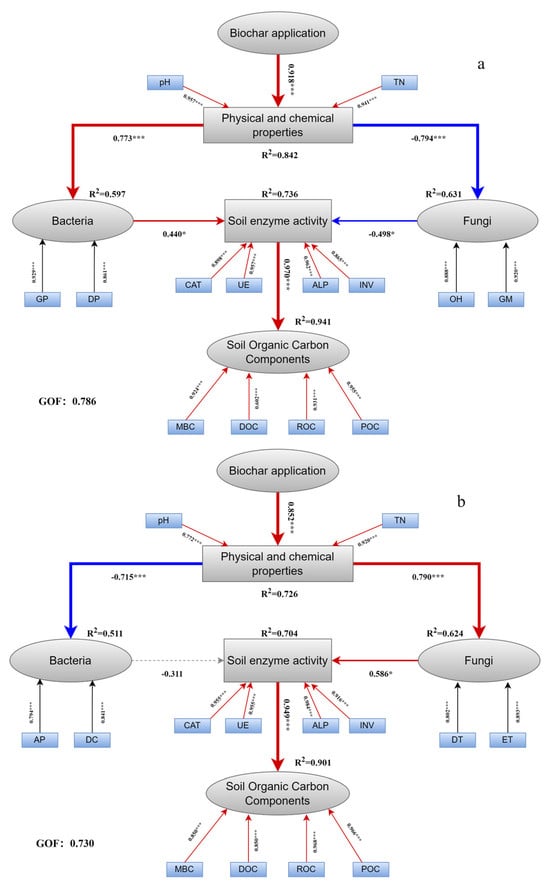
Figure 5.
Pathway analysis of soil physicochemical properties, microbial abundance, enzyme activities, and organic carbon fractions in biochar-amended soils of the North China Plain. (a), Maize season; (b), wheat season. TN, total nitrogen; GP, Gammaproteobacteria; DP, Deltaproteobacteria; CAT, Catalase; UE, Urease; ALP, Alkaline phosphatase; INV, Invertase; OH, Oligohymenophorea; GM, Glomeromycetes; AP, Alphaproteobacteria; DC, Deinococci; DT, Dothideomycetes; ET, Eurotiomycetes; MBC, microbial biomass carbon; DOC, dissolved organic carbon; EOC, easily oxidizable organic carbon; POC, particulate organic carbon. Asterisks mark their significance: * p ≤ 0.05,*** p ≤ 0.001. Red line, positive correlation; blue line, negative correlation.
4. Discussion
4.1. Responses of Soil Carbon Fractions and Physicochemical Properties to Biochar Application
Biochar amendment significantly altered both the composition and dynamics of the SOC pool, and these effects exhibited clear dose dependence as well as crop season specificity. Our results showed that SOC content increased progressively with rising biochar application levels during both maize (+56.0%) and wheat (+53.70%) seasons, reaching its maximum under the highest rate (B20). Consistent with previous studies [40], biochar application significantly enhanced native SOC content in the 0–30 cm layer, with increases of 39%, 49%, and 63% for the B30, B60, and B90 treatments, respectively, demonstrating a clear response to application rate. This increase can be primarily attributed to two mechanisms: the direct input of recalcitrant organic carbon inherent to biochar and the physical protection of native soil carbon facilitated by improved soil aggregation, particularly the formation of macroaggregates. Together, these processes promoted SOC stabilization and accumulation, consistent with the dynamic changes in SOC fractions observed in different growth stages of maize and wheat in previous rotation cycles [41].
The responses of labile carbon fractions were more complex. Both MBC and POC exhibited significant increases with higher biochar application, with the greatest enhancements under B20 treatment showing an average increase of 29.8% and 92.4%, respectively. Consistent with prior studies [16,42], biochar application enhanced both MBC and POC. This trend was observed in POC across all treatments (BC2.5-BC40), with increases ranging from 8.31% to 66.78% compared to CK [16,42]. It indicates that biochar at high rates not only provided a porous structure favorable for microbial colonization and proliferation but also enhanced soil aggregation through increased organic matter inputs and root growth [43], thereby physically protecting POC against rapid mineralization (citation needed) [39,44]. In contrast, DOC showed a non-monotonic trend: it peaked under the B5 treatment in both seasons but declined under the B20 treatment. Biochar’s impact on soil DOC is inconsistent: short-term increases are common, while long-term or specific soil/treatment conditions may show decreases or no significant effects [45]. These outcomes are strongly influenced by soil type, pH, application rate, and temporal scale [45]. This suggests that moderate rates (B5) primarily stimulated microbial activity and fresh organic matter decomposition, leading to DOC accumulation, whereas high rates (B20) likely reduced DOC bioavailability by adsorbing it onto biochar’s extensive surface area, binding it through functional groups, or forming insoluble complexes with polyvalent cations such as Ca2+ [46,47,48]. Easily oxidizable organic carbon also displayed season-dependent responses, with maize season peaking at B10 and wheat season at B20. These discrepancies may reflect seasonal differences in soil water-temperature regimes, root exudation, and biochar processes, coupled with microbial resource utilization strategies.
The CMI, which integrates SOC quality and dynamics, increased markedly with biochar dosage, reaching its maximum at B20 treatment. This finding demonstrates that despite possible reductions in labile fractions such as DOC, the dominant effect of high-rate biochar was to substantially elevate both SOC storage and stabilized fractions like POC, thereby improving overall soil carbon quality and sequestration potential. The larger CMI enhancement observed in the wheat season (+75.5%) compared with the maize season (+180.5%) under the B20 treatment may be explained by the prolonged aging effect of biochar across multiple crop cycles and its specific interactions with wheat root systems. Previous studies demonstrated that a biochar application rate of 2% significantly increased the CMI by 15.22% at 24 months [49]. These results highlight the long-lasting and crop-dependent effects of biochar on soil carbon stabilization.
4.2. Alteration of Biological Properties Reshapes Soil Carbon Fractions
A central finding of this study is that biochar regulates microbial communities in a directional rather than a universal manner. Instead of indiscriminately increasing microbial abundance, biochar selectively enriched functional groups critical to carbon cycling. During the maize season, biochar significantly enriched Gammaproteobacteria (+29.03%) and Deltaproteobacteria (+46.15%). Consistent with prior findings [50,51], maize-straw biochar increased the relative abundances of key bacterial classes, with Alphaproteobacteria, Gammaproteobacteria, Sphingobacteria, and Deltaproteobacteria rising by 7.87%, 9.81%, 1.24%, and 37%, respectively [50,51]. Many Gammaproteobacteria are r-strategists, such as Pseudomonas, which proliferate rapidly by exploiting biochar-derived DOC and pore habitats [52]. Their enrichment is directly associated with the accelerated turnover of labile carbon fractions. Meanwhile, the enrichment of Myxobacteria within Deltaproteobacteria indicated increased microbial food web complexity, as these taxa function as top-level predators. This top-down control enhanced microbial turnover rates and promoted the formation of microbial necromass, a major contributor to stable MAOC [53]. Furthermore, Deltaproteobacteria groups such as Anaeromyxobacter and Desulfovibrio maintained nitrogen fixation capacity under elevated nitrogen conditions, converting nitrate to ammonium for plant uptake. This not only increased soil nitrogen availability and supported root growth but also enhanced SOC accumulation. These findings suggest a bacterial succession strategy that shifts from rapid exploitation of labile resources to the establishment of a more structured microbial ecosystem.
The enrichment of fungal groups such as Eurotiomycetes (+85.77%) and Dothideomycetes (+16.28%) during the wheat season confirms the previously reported increase in Eurotiomycetes [54], reflecting the extended and deepened effects of biochar. These fungi are specialists in decomposing recalcitrant compounds such as cellulose and lignin. After one cropping season, biochar surfaces likely adsorbed large amounts of organic molecules, creating a microenvironment conducive to fungal colonization. Their extensive hyphal networks not only secrete extracellular enzymes to slowly degrade complex carbon but also act as structural scaffolds that stabilize aggregates [55,56]. This dual role contributes significantly to MBC and the construction of long-term stable carbon pools. Fungal-driven carbon cycling, therefore, complements bacterial-driven rapid turnover, jointly promoting SOC accumulation. Seasonal differences between maize- and wheat-associated microbial communities indicate that biochar effects are dynamic and strongly shaped by interactions among root exudates, environmental factors, and biochar aging.
The restructuring of microbial communities induced by biochar was directly reflected in soil enzyme activities. Biochar promoted biochemical reactions, accelerated nutrient cycling, and increased MBC, thereby enhancing substrate availability for enzymatic processes [43,57]. The 6.1–26.1% increase in invertase (INV) activity observed in our study aligns with previous findings showing a 46.76% enhancement in soil invertase activity with increasing biochar application rates in sandy soils [58]. It provides direct evidence of intensified microbial carbon metabolism, as enriched Gammaproteobacteria and fungi are efficient decomposers of carbohydrates [59,60]. Their proliferation elevated community-level sucrose degradation capacity, creating a positive feedback loop where enhanced degradation generated more soluble sugars, which in turn stimulated invertase synthesis and secretion. This feedback represents the core mechanism of the “Microbial Carbon Pump”, transforming both exogenous and native organic matter into microbial biomass and necromass [53]. Catalase activity was also significantly enhanced, a phenomenon consistent with the 33.29% increase reported for maple leaf-derived biochar [61] and carries dual implications. On the one hand, it reflects vigorous aerobic metabolism, particularly fungal-driven lignin degradation that generates hydrogen peroxide H2O2 as a by-product [62]. On the other hand, it demonstrates the strong self-detoxification capacity of the microbial community, essential for sustaining high metabolic activity. Elevated catalase activity thus signifies ecosystem resilience and stability, ensuring the efficient functioning of carbon transformation processes under biochar amendment.
5. Conclusions
This study clarified the carbon sequestration effects and mechanisms of biochar amendment in a maize–wheat rotation system on the North China Plain. Biochar application markedly enhanced SOC fractions in a dose-dependent manner. Under the B20 treatment, SOC increased by 53.7% during the maize season and 56.0% during the wheat season, while the CMI rose by 75.5% and 180.5%, respectively, demonstrating the pronounced contribution of high application rates to soil carbon stability. In contrast, DOC peaked under the B5 treatment with an increase of approximately 53.0%, indicating that moderate rates are more favorable for the accumulation of labile carbon pools.
Microbial analyses revealed that biochar promoted carbon transformation through the selective enrichment of key microbial taxa. The B20 treatment enhanced the abundance of Deltaproteobacteria by 46.2% in the maize season and Eurotiomycetes by 85.8% in the wheat season. This targeted enrichment, together with significantly elevated activities of catalase and invertase, synergistically facilitated the conversion and stabilization of SOC. Path analysis confirmed that biochar promoted carbon sequestration via a sequential mechanism: (a) improving soil physicochemical properties; (b) restructuring microbial communities; (c) enhancing enzyme activities; and (d) stabilizing SOC fractions.
The present research provides systematic evidence that biochar drives soil carbon sequestration through a hierarchical mechanism spanning from soil properties to microbial community structure and enzymatic activity. We further demonstrate that the effects of biochar are not only dose-dependent but also vary seasonally within a cropping system, underscoring the context-specific nature of its impact. The identification of specific microbial taxa (e.g., Deltaproteobacteria and Eurotiomycetes) as key responders to biochar amendment advances our mechanistic understanding of belowground carbon processes.
In conclusion, biochar application strategies should be differentiated according to agricultural objectives. Moderate rates (e.g., 5 t ha−1) are more suitable for short-term improvement of soil fertility, while higher rates (e.g., 20 t ha−1) are more effective for long-term carbon sequestration. These insights extend beyond the local context and offer a conceptual framework for optimizing biochar application in similar intensive cropping systems globally. Future research should focus on the long-term persistence of biochar-induced microbial shifts and their functional consequences, as well as the economic and environmental trade-offs associated with different application rates under varying pedoclimatic conditions. These findings provide a robust scientific basis for the rational use of biochar to support sustainable soil management and green agricultural development in the North China Plain.
Author Contributions
Y.W.: Conceptualization, Investigation, Software, Methodology, Data Curation, Visualization, Writing—Original Draft. M.Z.: Methodology, Formal Analysis, Data Curation, Software, Visualization, Writing—Review and Editing. A.S.: Methodology, Data Curation, Software, Visualization, Writing—Review and Editing. X.F. and Z.P.: Formal Analysis, Data Curation, Software. H.X. and C.X.: Conceptualization, Supervision, Writing—Review and Editing, Funding Acquisition, Validation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (no. 2021YFD1901005) and the Central Guidance for Local Technology Development Fund (236Z6402G).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kopittke, P.M.; Berhe, A.A.; Carrillo, Y.; Cavagnaro, T.R.; Chen, D.; Chen, Q.L.; Dobarco, M.R.; Dijkstra, F.A.; Field, D.J.; Grundy, M.J.; et al. Ensuring planetary survival: The centrality of organic carbon in balancing the multifunctional nature of soils. Crit. Rev. Environ. Sci. Technol. 2022, 52, 4308–4324. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, D.; Wang, Z.; Harrison, M.T.; Liu, K.; Song, Z.; Chen, F.; Yin, X. Challenges and strategies in estimating soil organic carbon for multi-cropping systems: A review. Carbon Footpr. 2024, 3, 19. [Google Scholar] [CrossRef]
- Zhou, Z.; Ren, C.; Wang, C.; Delgado-Baquerizo, M.; Luo, Y.; Luo, Z.; Du, Z.; Zhu, B.; Yang, Y.; Jiao, S.; et al. Global turnover of soil mineral-associated and particulate organic carbon. Nat. Commun. 2024, 15, 5329. [Google Scholar] [CrossRef]
- He, J.; Liu, X.; Meng, W.; Chen, X. Recent advances in studies of soil organic carbon stability in Karst areas. Front. For. Glob. Change 2024, 7, 1453615. [Google Scholar] [CrossRef]
- Hu, Q.; Thomas, B.W.; Powlson, D.; Hu, Y.; Zhang, Y.; Jun, X.; Shi, X.; Zhang, Y. Soil organic carbon fractions in response to soil, environmental and agronomic factors under cover cropping systems: A global meta-analysis. Agric. Ecosyst. Environ. 2023, 355, 108591. [Google Scholar] [CrossRef]
- You, Y.; Wang, J.; Huang, X.; Tang, Z.; Liu, S.; Sun, O.J. Relating microbial community structure to functioning in forest soil organic carbon transformation and turnover. Ecol. Evol. 2014, 4, 633–647. [Google Scholar] [CrossRef]
- Plaza-Bonilla, D.; Álvaro-Fuentes, J.; Cantero-Martínez, C. Identifying soil organic carbon fractions sensitive to agricultural management practices. Soil Tillage Res. 2014, 139, 19–22. [Google Scholar] [CrossRef]
- Song, J.; Wang, J.; Hou, Q.; Xing, Z.; Zhang, Z.; Du, S.; Liu, M. Short-term effects of irrigation and nitrogen management on paddy soil carbon pools under deep placement of basal fertilizer nitrogen. Sci. Rep. 2024, 14, 11329. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yang, J.; Yu, Y.; Shakoor, A.; Virk, A.L.; Li, F.M.; Yang, H.; Kan, Z.R. Crop straw converted to biochar increases soil organic carbon but reduces available carbon. Eur. J. Agron. 2025, 164, 127499. [Google Scholar] [CrossRef]
- do Nascimento, Í.V.; Fregolente, L.G.; de Araújo Pereira, A.P.; do Nascimento, C.D.V.; Mota, J.C.A.; Ferreira, O.P.; Sousa, H.H.d.F.; da Silva, D.G.G.; Simões, L.R.; Filho, A.S.; et al. Biochar as a carbonaceous material to enhance soil quality in drylands ecosystems: A review. Environ. Res. 2023, 233, 116489. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Y.; Rong, X.; Zhou, X.; Fei, J.; Peng, J.; Luo, G. Biochar and organic fertilizer applications enhance soil functional microbial abundance and agroecosystem multifunctionality. Biochar 2024, 6, 3. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, S.; Pang, Q.; Abdalla, M.; Qi, S.; Hu, J.; Qiu, H.; Smith, P. Optimizing biochar application rate and predicting of climate change impacts on net greenhouse gas emissions in paddy systems using DNDC-BC model. Agric. For. Meteorol. 2025, 364, 110461. [Google Scholar] [CrossRef]
- Ruan, R.; Zhang, P.; Lambers, H.; Xie, W.; Zhang, Z.; Xie, S.; Wang, Y.; Wang, Y. Biochar application improves maize yield on the Loess Plateau of China by changing soil pore structure and enhancing root growth. Sci. Total Environ. 2024, 956, 177379. [Google Scholar] [CrossRef]
- Chagas, J.K.M.; de Figueiredo, C.C.; Ramos, M.L.G. Biochar increases soil carbon pools: Evidence from a global meta-analysis. J. Environ. Manag. 2022, 305, 114403. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, W.; Xiu, L.; Gu, W.; Wu, D.; Tang, L.; Chen, W. Long-term fertilization regimes modulate dissolved organic matter molecular chemodiversity and greenhouse gas emissions in paddy soil. Biochar 2025, 7, 43. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, J.; Ye, J.; Liu, Y.; Lin, Y.; Yi, Z.; Wang, Y. Biochar affects organic carbon composition and stability in highly acidic tea plantation soil. J. Environ. Manag. 2024, 370, 122803. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Wong, J.T.F.; Hashimoto, Y.; Huang, L.; Rinklebe, J.; Chang, S.X.; Bolan, N.; Wang, H.; Ok, Y.S. Response of microbial communities to biochar-amended soils: A critical review. Biochar 2019, 1, 3–22. [Google Scholar] [CrossRef]
- Beillouin, D.; Corbeels, M.; Demenois, J.; Berre, D.; Boyer, A.; Fallot, A.; Feder, F.; Cardinael, R. A global meta-analysis of soil organic carbon in the Anthropocene. Nat. Commun. 2023, 14, 3700. [Google Scholar] [CrossRef] [PubMed]
- Edeh, I.G.; Mašek, O.; Buss, W. A meta-analysis on biochar’s effects on soil water properties–New insights and future research challenges. Sci. Total Environ. 2020, 714, 136857. [Google Scholar] [CrossRef]
- Yuan, Y.; Liang, Y.; Cai, H.; Yuan, J.; Li, C.; Liu, H.; Zhang, C.; Wang, L.; Zhang, J. Soil organic carbon accumulation mechanisms in soil amended with straw and biochar: Entombing effect or biochemical protection? Biochar 2025, 7, 33. [Google Scholar] [CrossRef]
- Li, B.; Guo, Y.; Liang, F.; Liu, W.; Wang, Y.; Cao, W.; Song, H.; Chen, J.; Guo, J. Global integrative meta-analysis of the responses in soil organic carbon stock to biochar amendment. J. Environ. Manag. 2024, 351, 119745. [Google Scholar] [CrossRef]
- Zhang, N.; Ye, X.; Gao, Y.; Liu, G.; Liu, Z.; Zhang, Q.; Liu, E.; Sun, S.; Ren, X.; Jia, Z.; et al. Environment and agricultural practices regulate enhanced biochar-induced soil carbon pools and crop yield: A meta-analysis. Sci. Total Environ. 2023, 905, 167290. [Google Scholar] [CrossRef]
- Xu, H.; Cai, A.; Wu, D.; Liang, G.; Xiao, J.; Xu, M.; Colinet, G.; Zhang, W. Effects of biochar application on crop productivity, soil carbon sequestration, and global warming potential controlled by biochar C: N ratio and soil pH: A global meta-analysis. Soil Tillage Res. 2021, 213, 105125. [Google Scholar] [CrossRef]
- Frimpong, K.A.; Owusu, S.; Darko, R.O.; Hanyabui, E.; Abbey, A.N.A.; Tetteh, D.A. Effect of biochar application rates on soil properties and growth of Amaranthus caudatus. Discov. Agric. 2025, 3, 21. [Google Scholar] [CrossRef]
- Foster, E.J.; Fogle, E.J.; Cotrufo, M.F. Sorption to biochar impacts β-glucosidase and phosphatase enzyme activities. Agriculture 2018, 8, 158. [Google Scholar] [CrossRef]
- Buss, W.; Hilber, I.; Graham, M.C.; Mašek, O. Composition of PAHs in biochar and implications for biochar production. ACS Sustain. Chem. Eng. 2022, 10, 6755–6765. [Google Scholar] [CrossRef]
- Holatko, J.; Kucerik, J.; Mustafa, A.; Lonova, K.; Siddiqui, M.H.; Naveed, M.; Hammerschmiedt, T.; Kintl, A.; Malicek, O.; Chorazy, T.; et al. Influence of biochar feedstock blends on soil enzyme activity, nutrient cycling, lettuce biomass accumulation and photosynthesis. BMC Plant Biol. 2025, 25, 323. [Google Scholar] [CrossRef]
- He, Y.; Zhou, X.; Jiang, L.; Li, M.; Du, Z.; Zhou, G.; Shao, J.; Wang, X.; Xu, Z.; Bai, S.; et al. Effects of biochar application on soil greenhouse gas fluxes: A meta-analysis. Gcb Bioenergy 2017, 9, 743–755. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Zhao, X.; Liu, Z.; Xu, H.; Cao, K.; Ye, L. Impact of Reduced Chemical Fertilizer and Organic Amendments on Yield, Nitrogen Use Efficiency, and Soil Microbial Dynamics in Chinese Flowering Cabbage. Horticulturae 2025, 11, 859. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Tang, Z.; Yang, Q. Impact of Reducing Nitrogen Fertilizer with Biochar on Flavor Substance and Nitrogen Balance in Different Swollen-Stem-Mustard Varieties. Agronomy 2024, 14, 1254. [Google Scholar] [CrossRef]
- Nong, T.; Yang, X.; Pan, R.; Zhao, Y.; Liu, X.; Wang, J.; Yin, Z.; Yan, B.; Xia, L.; An, S.; et al. Spartina alterniflora invasion exacerbates soil microbial carbon and phosphorus co-limitations and alters microbial carbon and nitrogen use efficiency in the coastal wetlands of eastern China. Ecol. Process. 2025, 14, 72. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, C.; Kong, Y.; Cao, X.; Zhu, L.; Zhang, Y.; Ning, Y.; Tian, W.; Zhang, H.; Yu, Y.; et al. Biochar application alleviated rice salt stress via modifying soil properties and regulating soil bacterial abundance and community structure. Agronomy 2022, 12, 409. [Google Scholar] [CrossRef]
- Chen, Y.; Tian, X.; Wang, J.H.; Zhang, Y.; Wang, J.; Li, Z.T.; Zhao, K.L.; Wu, J.Z. Silicon-iron modified biochar remediates cadmium and arsenic co-contaminated paddy soil by regulating cadmium and arsenic speciation. Front. Microbiol. 2025, 16, 1579213. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Peng, Y.; Li, M.; Li, X.; Li, H.; Dabu, X.; Yang, Y. Different active exogenous carbons improve the yield and quality of roses by shaping different bacterial communities. Front. Microbiol. 2025, 16, 1558322. [Google Scholar] [CrossRef]
- Li, W.; Guo, Z.; Li, J.; Han, J. Response of the characteristics of organic carbon mineralization of soft rock and soil composed of sand to soil depth. PeerJ 2021, 9, e11572. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Li, Q.; Wang, C.; Li, B.; Stomph, T.J.; Yang, J.; Tao, Q.; Yuan, S.; Tang, X.; Ge, J.; et al. Negative effects of urbanization on agricultural soil easily oxidizable organic carbon down the profile of the Chengdu Plain, China. Land Degrad. Dev. 2020, 31, 404–416. [Google Scholar] [CrossRef]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Xiang, Q.; Ma, T.; Wang, X.; Yang, Q.; Lv, L.; Wang, R.; Li, J.; Ma, J. Effects of Different Living Grass Mulching on Soil Carbon and Nitrogen in an Apple Orchard on Loess Plateau. Agronomy 2024, 14, 1917. [Google Scholar] [CrossRef]
- Mao, X.; Sun, T.; Zhu, L.; Wanek, W.; Cheng, Q.; Wang, X.; Zhou, J.; Liu, X.; Ma, Q.; Wu, L.; et al. Microbial adaption to stoichi-ometric imbalances regulated the size of soil mineral-associated organic carbon pool under continuous organic amendments. Geoderma 2024, 445, 116883. [Google Scholar] [CrossRef]
- Lu, H.; Xu, C.; Zhang, J.; Du, C.; Wu, G.; Luo, L. The characteristics of alkaline phosphatase activity and phoD gene community in heavy-metal contaminated soil remediated by biochar and compost. Bull. Environ. Contam. Toxicol. 2022, 109, 298–303. [Google Scholar] [CrossRef]
- Yao, Q. Effects of Biochar Application on Soil Organic Carbon Components and Nutrients in Farmland of the North China Plain; Hebei Agricultural University: Baoding, China, 2021. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Dijkstra, F.A.; Liu, X.R.; Wang, Y.D.; Huang, J.; Lu, N. Effects of biochar on soil microbial biomass after four years of consecutive application in the north China plain. PLoS ONE 2014, 9, e102062. [Google Scholar] [CrossRef]
- Ali, A.; Jabeen, N.; Chachar, Z.; Chachar, S.; Ahmed, S.; Ahmed, N.; Laghari, A.A.; Sahito, Z.A.; Farruhbek, R.; Yang, Z. The role of biochar in enhancing soil health & interactions with rhizosphere properties and enzyme activities in organic fertilizer substit-ution. Front. Plant Sci. 2025, 16, 1595208. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Liang, Y.; Xue, L.; Zamanian, K.; Sun, S.; Li, W.; Zhang, S.; Zhao, X. Five years of biochar amendment combined with reduced fertilization and irrigation improved the soil organic carbon composition and structure in a solonchak. Sci. Rep. 2025, 15, 21823. [Google Scholar] [CrossRef]
- Pietikäinen, J.; Kiikkilä, O.; Fritze, H. Charcoal as a habitat for microbes and its effect on the microbial community of the underlying humus. Oikos 2000, 89, 231–242. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Römkens, P.F.; Bril, J.; Salomons, W. Interaction between Ca2+ and dissolved organic carbon: Implications for metal mobilization. Appl. Geochem. 1996, 11, 109–115. [Google Scholar] [CrossRef]
- Qiu, H.; Hu, Z.; Liu, J.; Zhang, H.; Shen, W. Effect of biochar on labile organic carbon fractions and soil carbon pool management index. Agronomy 2023, 13, 1385. [Google Scholar] [CrossRef]
- Qi, Y.; Liu, H.; Wang, J.; Wang, Y. Effects of different straw biochar combined with microbial inoculants on soil environment in pot experiment. Sci. Rep. 2021, 11, 14685. [Google Scholar] [CrossRef]
- Meng, J.; Li, W.; Qiu, Y.; Li, Z.; Li, L.; Luo, Y.; Guo, H.; Yu, Y.; Shan, S.; Chen, H. Responses of soil microbial communities to manure and biochar in wheat cultivation of a rice-wheat rotation agroecosystem in East China. Pedosphere 2023, 33, 893–904. [Google Scholar] [CrossRef]
- Kolton, M.; Graber, E.R.; Tsehansky, L.; Elad, Y.; Cytryn, E. Biochar-stimulated plant performance is strongly linked to microbial diversity and metabolic potential in the rhizosphere. New Phytol. 2017, 213, 1393–1404. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Shan, M.; Chen, J.; Penttinen, P.; Qin, H. Contrasting dynamics of polychlorinated biphenyl dissipation and fungal community composition in low and high organic carbon soils with biochar amendment. Environ. Sci. Pollut. Res. 2018, 25, 33432–33442. [Google Scholar] [CrossRef]
- Wang, G.; Ma, Y.; Chenia, H.Y.; Govinden, R.; Luo, J.; Ren, G. Biochar-mediated control of phytophthora blight of pepper is closely related to the improvement of the rhizosphere fungal community. Front. Microbiol. 2020, 11, 1427. [Google Scholar] [CrossRef] [PubMed]
- Caldara, M.; Gullì, M.; Graziano, S.; Riboni, N.; Maestri, E.; Mattarozzi, M.; Bianchi, F.; Careri, M.; Marmiroli, N. Microbial consortia and biochar as sustainable biofertilisers: Analysis of their impact on wheat growth and production. Sci. Total Environ. 2024, 917, 170168. [Google Scholar] [CrossRef]
- Yan, Q.; Tian, H.; Huang, Y.; Mu, X.; Tang, G.; Ma, H.; Megharaj, M.; Xu, W.; He, W. Recycled wheat straw biochar enhances nutrient-poor soil: Enzymatic kinetics of carbon, nitrogen, and phosphorus cycling. J. Environ. Manag. 2025, 380, 124950. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, W.; Sun, X.; Jiang, J.; Li, D.; Tang, G.; Xu, W.; Jia, H. Biochar aged for five years altered Carbon fractions and enzyme activities of Sandy Soil. Land 2023, 12, 1645. [Google Scholar] [CrossRef]
- Yan, H.K.; Zhang, C.C.; Nai, G.J.; Ma, L.; Lai, Y.; Pu, Z.H.; Ma, S.Y.; Li, S. Microbial inoculant GB03 increased the yield and quality of grape fruit under salt-alkali stress by changing rhizosphere microbial communities. Foods 2025, 14, 711. [Google Scholar] [CrossRef]
- Cui, Y.; Ning, Z.; Li, M.; Qin, X.; Yue, X.; Chen, X.; Zhu, C.; Sun, H.; Huang, Y. Microbial network-driven remediation of saline-alkali soils by salt-tolerant plants. Front. Microbiol. 2025, 16, 1565399. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Lemanowicz, J.; Debska, B.; Haddad, S.A.; Tobiasova, E. The application of biochar from waste biomass to improve soil fertility and soil enzyme activity and increase carbon sequestration. Energies 2022, 16, 380. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, Y.; Zhang, J.; Chen, Y.; Yang, L.; Li, H.; Wang, L. Factors influencing soil enzyme activity in China’s forest ecosystems. Plant Ecol. 2018, 219, 31–44. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).