Abstract
In temperate regions, Fusarium ear rot (FER) caused by Fusarium spp. is among the most important limiting factors to maize yield and kernel quality. The role of genotype and sowing date in mitigating FER risk remains insufficiently explored, particularly under the variable climatic conditions of the Transylvanian Plain, Romania. A three-year field experiment (2021–2023) was conducted to evaluate twelve early to semi-early maize hybrids across four sowing dates (very early—SD1, early—SD2, optimum—SD3, late—SD4). FER incidence and severity were assessed at harvest, and yields were analyzed in relation to genotype, disease pressure, and seasonal climate variability. Mean FER incidence reached 74.9% and severity was 3.4%, with significant variation among years, sowing dates, and hybrids. Early sowings (SD1, SD2) recorded the highest infection levels (up to 83.6% incidence and 4.6% severity). In contrast, the latest sowing (SD4) exhibited the lowest disease pressure (59.1% and 2.5%, respectively) and achieved the highest yield (9.1 t ha−1). Significant differences were noted between hybrids: Turda 332, Turda Star, and Turda 165 were highly susceptible, whereas Turda 380, HST 149, and Turda 2020 displayed higher levels of tolerance. A strong correlation between yield losses and FER severity was observed for very early sowing (r = 0.72, p < 0.01); this relationship was not evident under later sowing. These results indicate that choosing the sowing date according to seasonal climatic conditions, together with the use of the most tolerant hybrids, represents an effective strategy to reduce the risk of FER and to obtain stable maize yields in temperate regions.
1. Introduction
Maize (Zea mays L.) is one of the world’s most important cultivated crops, showing the highest production growth among cereals [1]. This increase is driven by the growing demand for maize-derived products, which are key sources for food and feed, as well as important raw materials for industry and biofuel production [1,2]. Fusarium ear rot (FER) of maize, caused mainly by Fusarium verticillioides, F. subglutinans, F. proliferatum, and F. graminearum, represents one of the most important diseases of maize worldwide [3,4,5]. It significantly affects both yield and grain quality, making it a major concern for maize production [6,7,8,9].
Fusarium species infecting maize primarily use crop residues remaining on the soil as their main sources of inoculum [10,11,12], where the pathogen can survive and produce spores (macroconidia, microconidia, and ascospores in the case of teleomorphic forms) [13,14]. Ear infection occurs predominantly through the silks, but also via wounds caused by insects such as Ostrinia nubilalis, Diabrotica virgifera, and Helicoverpa armigera, which facilitate pathogen entry [15,16,17,18,19,20]. Moreover, systemic transmission from infected seeds to young plants has been reported in the literature as an additional pathway of infection [21,22].
Symptoms observed on maize ears are often associated with the accumulation of fumonisins, deoxynivalenol, zearalenone (Table 1), and other mycotoxins. [11,18,21,23]. These compounds represent major hazards to animal and human health and may persist throughout the entire food chain [10,18,23,24,25]. Chemical methods can reduce the severity of ear rot, but they have disadvantages related to cost and limited effectiveness [16,26]. Research on the effects of triazole fungicides has shown that they may provide some efficacy in limiting infections caused by Fusarium spp. [16]. However, their impact on FER and on fumonisin reduction remains limited, with effectiveness observed only under high infection pressure [16]. While preventive and control measures—including crop rotation, fungicide applications, and insect management—can help reduce disease incidence, the use of resistant hybrids remains the most sustainable and effective strategy [12].

Table 1.
Major maize ear rots: pathogens, mycotoxins, and silk susceptibility period.
The severity of FER and the accumulation of mycotoxins in kernels are determined by complex interactions among genetic factors (genotype, pericarp thickness, husk coverage), environmental conditions (temperature, humidity, rainfall), and insect vector activity [27,28,29]. These interactions explain the high variability observed across years and regions. Among agronomic factors, sowing date plays a critical role: delayed sowing is frequently associated with increased FER incidence and fumonisin levels, while early sowing may reduce the risk depending on hybrid maturity [27,29,30,31,32]. However, there are regional exceptions, as shown by the study conducted in Zambia [33], where early planting was associated with a higher frequency of Fusarium verticillioides kernel infections compared to late planting. This outcome was attributed to the interaction between local climatic conditions, pollination phenology, and insect dynamics [28,34]. In temperate zones, climate change is progressively intensifying the pressure of diseases during key growth stages of maize [35,36,37]. Different studies assessing the influence of sowing dates and hybrid variability have reported significant changes affecting yield parameters [38,39,40], as well as FER incidence [11,27,28,29,30]. Moreover, recent research emphasizes the importance of addressing the Genotype × Environment (G × E) interaction to ensure the stability of hybrid resistance under variable growing conditions [28,34]. Previous studies have shown that variations in sowing date can modify the distribution and interaction of climatic factors such as light, temperature, and moisture throughout the maize growth period, thus affecting crop development and yield formation [40,41,42]. Selecting an appropriate hybrid for the chosen sowing time, in combination with good soil conditions and favorable temperature regimes, plays a crucial role, as optimal sowing timing is essential for achieving maximum yield [39,43].
In the Transylvanian Plain, the effects of climate change have become increasingly evident over the last decades, with direct consequences for maize production [44]. Recent studies highlight rising mean temperatures during the growing season, more frequent heat waves, and increasingly irregular rainfall patterns, with rainfall often concentrated in short and intense episodes alternating with prolonged droughts [45]. These changes negatively affect soil water reserves, especially during critical phenological stages, increase evapotranspiration, and shorten the grain-filling period, thereby reducing yield stability over the years [44,45].
Maize productivity has become highly variable, with favorable years ensuring high yields, whereas hot and dry years result in markedly lower yields [44]. The choice of sowing date plays an essential role in maize adaptation to climate variability. Early sowing may expose crops to low spring temperatures, whereas delayed sowing increases exposure to heat and drought stress during flowering and grain filling, leading to reduced yields and kernel quality [37]. Climate-induced stresses interact with biotic factors: drought and heat weaken plant defenses, while pests such as Ostrinia nubilalis facilitate fungal entry into the plant [18,19]. Altogether, climate change in Transylvania not only reduces maize yield stability but also enhances the risk of FER epidemics and mycotoxin contamination [44]. This underscores the necessity of adapting crop management practices, optimizing sowing dates, and breeding resilient hybrids to sustain maize productivity and safety under changing climatic conditions.
Although numerous studies have examined the management of Fusarium ear rot through chemical control, crop rotation, and breeding for resistance, little is known about how agronomic factors such as sowing date interact with genotype. This interaction may significantly influence both disease incidence and yield formation. Most previous research has examined these factors separately, without assessing their combined effects under variable climatic conditions. This lack of integrated analysis is particularly evident in Central and Eastern Europe, where increasing climatic variability strongly affects maize productivity. Studies focusing on sowing date have shown that early or delayed planting alters the synchronization between crop phenology and climatic conditions, thereby modifying the risk of Fusarium infection and yield potential [27,31,46]. On the other hand, genotype-based research has highlighted considerable variation among hybrids in terms of resistance to Fusarium spp. and mycotoxin accumulation [9,34,47]. Despite these findings, the combined influence of sowing time and genotype under changing climatic conditions remains insufficiently documented, particularly in Central and Eastern Europe, and especially in Romania (Transylvanian Plain). Therefore, the present study aims to fill this research gap by evaluating the combined influence of genotype and sowing time on Fusarium ear rot incidence, severity, and grain yield stability of maize hybrids under the temperate climatic conditions of the Transylvanian Plain, Romania.
This study was designed to test the following hypotheses: (H1) climatic factors, particularly temperature and rainfall, significantly influence the incidence and severity of Fusarium ear rot (FER) in maize; (H2) sowing date affects both maize yield and susceptibility to Fusarium spp. infection; (H3) maize hybrids differ in their resistance to FER and yield stability under variable climatic conditions; (H4) the interaction between genotype, sowing date, and environmental conditions is a key determinant in reducing FER risk and enhancing maize productivity.
2. Materials and Methods
2.1. Biological Material
In this experiment, twelve maize hybrids from early and semi-early maturity groups were evaluated. The hybrids created by the Agricultural Research and Development Station (ARDS) Turda were selected based on their relevance for cultivation in the target area. The main characteristics of these hybrids are summarized in Table 2.

Table 2.
The main characteristics of the biological material (hybrids) used in this experiment [48].
2.2. Experimental Design
A field polifactorial trial with 12 maize hybrids was performed at ARDS Turda in the field located in the Transylvanian Plain, in the north-west of Turda (46°35′12.3″ N, 23°48′40.7″ E), Cluj County, Romania. The research was developed on a chernozem soil chemically characterized as neutral to slightly alkaline pH, neutral to high humus content, well supplied with nitrogen and potassium, and medium supplied with phosphorus [49].
In order to achieve the proposed objectives, the experiment had the following factors: factor A—sowing date with four graduations: sowing date (SD)1—very early; sowing date (SD)2—early; sowing date (SD)3—optimum; sowing date (SD)4—late. These were established according to soil temperature thresholds of 4, 6, 8, and above 10 °C (Table 3), each maintained for three consecutive days; factor B—maize hybrids (early and semi-early) with twelve graduations: Turda 248; Turda 165; Turda 201; Turda Star; Turda 332; Turda 344; Turda 335; Turda 2020; Turda 380; Turda 350; HST 149; Turda 59; factor C—climatic conditions in the experimental years: 2021, 2022, and 2023.

Table 3.
Sowing dates, flowering periods, and harvest dates for the four sowing date variants (SD1–SD4) during the 2021–2023 growing seasons in the Transylvanian Plain.
Fertilization was carried out with 150 kg/ha NPK (20:20:0) at sowing, followed by an additional 200 kg/ha CAN (27%) at the 4–6 leaf stage. The sowing density was 70,000 viable seeds ha−1. The predecessor plant was winter wheat in a rotation of soybean, wheat, and maize. Identical crop technology was implemented in all three experimental years.
Weed management was carried out in two distinct stages, following an integrated chemical control strategy. The first herbicide application was performed pre-emergence, using isoxaflutole 240 g/L at a rate of 0.3 L/ha in combination with glyphosate 360 g/L at 1.0 L/ha, targeting annual monocotyledonous and dicotyledonous weeds by inhibiting seed germination and seedling emergence. The second herbicide application, conducted post-emergence at the 4–6 leaf growth stage of the crop, involved the use of 2,4-D acid 600 g/L at 1.0 L/ha combined with nicosulfuron 40 g/L at 1.0 L/ha, aiming to suppress competition from grass weeds, as well as annual and perennial broadleaf species. In order to assess the response of the genotypes to biotic stress, no chemical treatments for disease or pest control were applied during the experimental period.
The field experiment was established as a two-factor randomized complete block design (RCBD) with three replications. The experimental factors were sowing date (four levels) and hybrid (twelve levels), resulting in a total of 48 treatment combinations and 144 plots. Each experimental plot represented one treatment combination and consisted of 4 rows × 5 m length, with standard row spacing of 70 cm and intra-row spacing of 25 cm. Sowing dates and hybrids were randomized within each block to minimize environmental variation. Buffer zones of 1 m separated the blocks to prevent edge effects. At harvest, 25 ears were randomly collected from each plot and replication For each ear, the percentage of kernels showing Fusarium symptoms was visually assessed according to the severity scale developed by Reid et al. [50]. It should be noted that Fusarium symptoms resulted from natural infections. Mean incidence and severity values were then calculated for each experimental variant.
Disease incidence (I%) = (Total number of assessed ears/Number of infected ears) × 100
Disease severity (S%) = ∑(percentage of diseased kernels per ear)/total number of ears observed.
2.3. Statistical Analysis
The arcsine square root transformation of the proportion (arcsin√%) was applied to stabilize variances. The obtained results were processed statistically by the variance analysis method and establishing the least significant difference—LSD—(5%, 1% and 0.1%) ANOVA (USAMV, Cluj-Napoca, Romania) [51], Past version 4.03 freeware license on Windows and Excel program.
2.4. Climatic Conditions
The average temperatures and rainfall over 3 agricultural years, 2021—2023 (April—October) and the multiannual average (1957–2022) are shown in Figure 1 and Figure 2 (Source: Turda Meteorological Station: longitude 23′47, latitude 46′35′, altitude 427 m) [52].
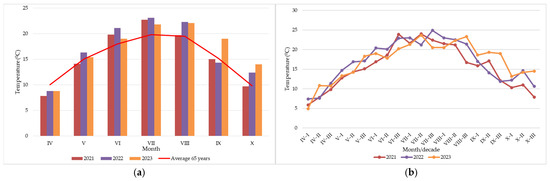
Figure 1.
Thermal regime during 2021–2023: (a) monthly average temperatures compared with the 65-year average, (b) decadal variation in temperatures.
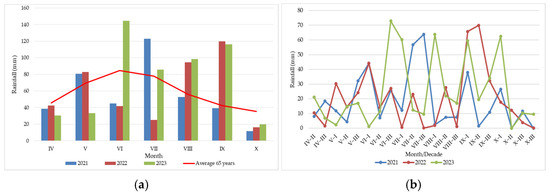
Figure 2.
Rainfall regime during 2021–2023: (a) monthly rainfall (April–October) compared with the 65-year average; (b) decadal amount of rainfall.
For a clearer presentation of climatic data, each month was divided into three 10-day periods (decades), allowing for a more detailed assessment of temperature and rainfall dynamics during the growing season. The analysis of decadal temperature dynamics from April to October during 2021–2023 (Figure 1a) highlights clear differences among years. Spring temperatures increased steadily, with 2022 being warmer than the other years. During summer (July–August), when silking and pollination occur, the maximum values of daily mean temperatures ranged between 20 and 25 °C. In 2022, the highest values were recorded, favoring a more rapid progression to anthesis compared with previous years; however, under simultaneous water deficit, this may have led to kernel abortion. In 2023, temperatures were above the multiannual monthly average in the summer months. In 2021, the temperatures in September and October fell below 15 °C, which accelerated ear drying and restricted Fusarium development. By contrast, in 2022, higher autumn temperatures combined with excessive rainfall created favorable conditions for disease progression.
Several decadal deviations from the general thermal trend were observed across the study years (Figure 1b). In early spring (IV–I, April), 2023, unusually high temperatures were recorded compared with the other years, which should have accelerated the emergence of the plants, but due to the lack of rainfall, emergence was delayed. In 2022, the third decade of July (VII–III) was exceptionally warm (>25 °C), which favored an earlier onset of anthesis, but also increased the risk of stress under water deficit. During the autumn decades of 2023, mean temperatures reached the highest values recorded across the entire study period.
Monthly rainfall from April to October (Figure 2) showed contrasting patterns among years compared with the 65-year average. Regarding the monthly rainfall recorded during the three study years, it can be observed that the values were close to the multiannual average in April; in May 2021 and 2022, they were slightly above the average, while in 2023, a rainfall deficit was recorded (Figure 2a). In the summer months, abundant rainfall was recorded in 2023, with more than 140 mm in June, followed by 2021, which recorded a peak of over 120 mm in July. In the autumn seasons of the three years, 2022 and 2023 had rainfall well above the multiannual average, while 2021 showed a slight deficit.
Decadal rainfall from April to October fluctuated both monthly and annually (Figure 2b). The rainfall most influential to FER occurrence was that recorded during summer and autumn. In this regard, it was observed that in the first decade of July 2022, rainfall was limited, and during the following two decades, almost no rainfall occurred. This situation affected both silk infection and yield formation. In the same year, excessive rainfall starting from the third decade of August and continuing throughout September contributed to the expansion of FER incidence, but it occurred too late to support high-yield production.
The data on monthly rainfall and the number of rainy days (Table 4) show that 2021 had high total rainfall in July (123.1 mm) but with only 8 rainy days, which did not result in a high severity of Fusarium infection. In 2022, August (94.6 mm, 15 days) and September (119.9 mm, 20 days) recorded the highest combination of rainfall and rainy days, which favored a high severity of FER. In 2023, the peak was in June (144.5 mm, 18 days), followed by significant rainfall in August (98.5 mm, 12 days) and September (116.1 mm, 6 days) associated with high temperatures that led to the highest incidence and severity of FER during the entire experimental period.

Table 4.
Total monthly rainfall and number of rainy days.
3. Results
3.1. Incidence and Severity of Fusarium Ear Rot
The results on the influence of climatic conditions on the incidence and severity of Fusarium spp. infection in maize ears (Table 5) highlight that the highest incidence was recorded in 2023 (6.07%), showing a statistically significant increase compared with the three-year average (p < 0.01). On the opposite side, the lowest incidence was observed in 2022 (7.06%), representing a highly significant reduction compared with the control variant (p < 0.001%). Regarding disease severity, the year 2023 showed the highest value (2.38%), significantly higher compared with the three-year average (p < 0.001%). In 2022, severity was also significantly higher than the control (0.43%, p < 0.05). The lowest severity was recorded in 2021 (2.81%), representing a statistically significant reduction compared with the multi-year mean (p < 0.01). The results indicate that the climatic conditions in 2023 were the most favorable for FER development, whereas those in 2021 were unfavorable for disease occurrence and progression.

Table 5.
Influence of climatic conditions, sowing date, and genotype on FER incidence and severity. (2021–2023).
Also, Table 5 presents the influence of sowing date (SD) on the incidence and severity of Fusarium spp. infection in maize ears. At the control variant (SD3), disease incidence was 72% and severity reached 2.90%. Earlier sowing dates (SD1 and SD2) showed the highest incidences (82.50% and 83.60%, respectively; p < 0.001). The lowest incidence was observed in SD4, with a decrease of 7.86% compared with the control (p < 0.001). A similar trend was observed for disease severity: SD1 and SD2 recorded the highest values (4.55% and 3.75%, respectively; p < 0.001), whereas SD IV showed the lowest severity (9.07%, p < 0.001).
The influence of maize genotype on the incidence and severity of FER is presented in Table 5. Calculating the average incidence across the 12 hybrids and four sowing dates resulted in a value of 74.90%. Among the 12 tested hybrids, significant differences were observed. Hybrids with high incidences, such as Turda 332 (90.15%), Turda 165 (82.20%), and Turda Star (87.05), (p < 0.001), stood out, indicating increased susceptibility to Fusarium infection. Several hybrids exhibited reduced susceptibility, including Turda 380 (56.70%), HST 149 (63.70%), and Turda 344 (76.70%), (p < 0.001). Other hybrids, such as Turda 201, Turda 335, Turda 2020, and Turda 59, recorded incidence values close to the mean, with no statistically significant differences. Regarding disease severity, the highest values were observed in Turda 165 (4.70%) and Turda 332 (4.55%) (both p < 0.001), as well as Turda 335 (3.85%, p < 0.01). In contrast, the lowest severity values were recorded for Turda 2020 (2.75%), Turda 380 (2.55%), and HST 149 (2.65%), all with highly significant differences (p < 0.001).
The influence of the interaction between sowing date and genotype is shown in Figure 3. Overall, FER incidence was high, exceeding 60% at most sowing dates and reaching values above 90% in susceptible hybrids such as Turda 332, Turda Star, and Turda 165. Among years, 2023 recorded the highest disease incidence across nearly all hybrids and sowing dates, whereas 2021 showed the lowest values, and 2022 displayed intermediate levels.
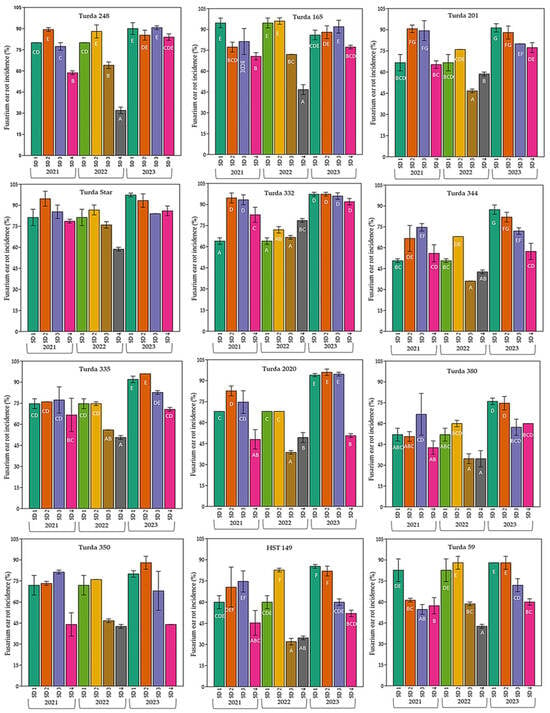
Figure 3.
Variation in FER incidence (%) among twelve maize hybrids sown at four different dates (SD1–SD4) across three growing seasons (2021–2023) under natural infection conditions. Bars represent mean values ± standard error. Different letters above the bars indicate significant differences according to the Duncan test (p < 0.05).
For most hybrids, high values of this indicator were observed at early sowing dates, with the exception of hybrid Turda 332 (SD1 in 2021). Early sowing dates (SD1–SD2) were generally associated with high incidence, while delayed sowing (SD3–SD4) resulted in lower infection levels. However, highly susceptible hybrids, such as Turda 165 and Turda 332, consistently showed a high incidence regardless of sowing date.
These findings highlight that both hybrid selection and sowing date are crucial factors in reducing FER incidence under field conditions.
The severity of FER, similar to incidence, varied significantly among hybrids, years, and sowing dates (Figure 4). Compared to incidence, severity values were much lower, not exceeding 13%. In hybrids such as Turda 165, Turda 332, Turda 248, and Turda 350, higher values were recorded at early sowing dates (SD1 and SD2). In 2023, the highest severity values were recorded in most hybrids, particularly at early sowing dates. In contrast, 2021 exhibited the lowest disease severity across all hybrids and sowing dates, while 2022 showed intermediate values. Hybrids Turda 380, HST 149, Turda 2020, and Turda 59 consistently displayed the lowest severity values, especially at optimum or late sowings (SD3–SD4). By contrast, early sowing dates (SD1–SD2) resulted in increased severity. Highly susceptible hybrids (Turda 165, Turda 332) recorded moderate-to-high severity levels (12–15%), remaining largely unaffected by sowing date. Mean separations (Duncan, p < 0.05) supported clear among-genotype contrasts within and across sowing dates.

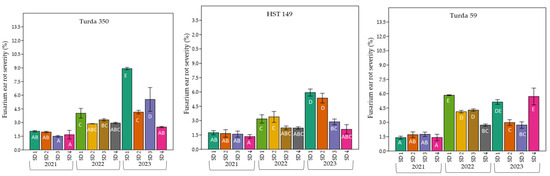
Figure 4.
Variation in FER severity (%) among twelve maize hybrids sown at four different dates (SD1–SD4) across three growing seasons (2021–2023) under natural infection conditions. Bars represent mean values ± standard error. Different letters above the bars indicate significant differences according to the Duncan test (p < 0.05).
3.2. Influence of Genotype and Environmental Factors (Sowing Date, Year) on Maize Grain Yield
Table 6 highlights the impact of climatic conditions during the experimental years on maize grain yield. Examining the data, it can be observed that in 2021, yield was significantly higher than the multi-year average of the 12 hybrids across the four sowing dates, reaching 9999 kg ha−1 (p < 0.001), a result correlated with favorable rainfall distribution and moderate temperatures recorded that year. In contrast, in 2022, yield dropped sharply to 6429 kg ha−1, by nearly 2000 kg ha−1 (p < 0.001), reflecting the combined effect of drought during the flowering and grain-filling stages and the occurrence of FER infections in maize ears. In 2023, yield slightly exceeded the control, reaching 8765 kg ha−1 (p < 0.01), which can be explained by uneven rainfall distribution as well as by the increased incidence of Fusarium infections.

Table 6.
Influence of climatic conditions, sowing date, and genotype on maize grain yield.
The effect of sowing date on maize grain yield is presented in Table 6. Sowing at the optimum time (SD3), considered the control variant, resulted in an average yield of 8771 kg ha−1. In comparison, early sowing (SD1 and SD2) led to much lower yields, 8170 and 8169 kg ha−1, respectively (p < 0.001), most likely due to the exposure of flowering and grain-filling stages to thermal and water stress. In contrast, late sowing (SD4) recorded the highest yield, 9088 kg ha−1 (p < 0.001), suggesting that delaying the sowing date placed the critical developmental stages of maize under more favorable climatic conditions.
The influence of genotype on maize grain yield is presented in Table 6. The overall mean yield of the 12 hybrids across the four sowing dates, considered as the control, was 8398 kg ha−1. Hybrids Turda 59, Turda 350, Turda 248, Turda 2020, and Turda 332 significantly exceeded the control, with yield increases ranging between 446 and 867 kg ha−1 (p < 0.001). Significant yield increases were also recorded for Turda 380 (8694 kg ha−1, p < 0.01). In contrast, hybrids that showed susceptibility to FER, such as Turda 165 (6617 kg ha−1), Turda 201 (6705 kg ha−1), and Turda Star (8037 kg ha−1), exhibited significant yield reductions ranging from 1781 to 361 kg ha−1 (p < 0.001). Other hybrids, including Turda 344, Turda 335, and HST 149, had yields close to the mean, with no statistically significant differences.
Among the 12 hybrids, grain yield varied strongly with growing season and sowing date, with a clear Hybrid × Year × Sowing date interaction (Figure 5). In most hybrids, 2021 provided the highest yields, 2022 the lowest, and 2023 was intermediate. Within each hybrid, sowing date significantly influenced yield (different letters above bars; Duncan, p < 0.05): later sowing (SD4) was frequently associated with higher yields, in contrast to early sowings (SD1–SD2), which reduced yield, especially in 2022. The effect of sowing date was hybrid-dependent: Turda 59, Turda 350, and Turda 248 showed stable, high-yielding responses, whereas Turda 165 and Turda 201 exhibited greater sensitivity to sowing date and year. These results suggest that while optimizing sowing date can partially buffer seasonal stress, cultivar selection remains decisive for ensuring high and stable productivity.
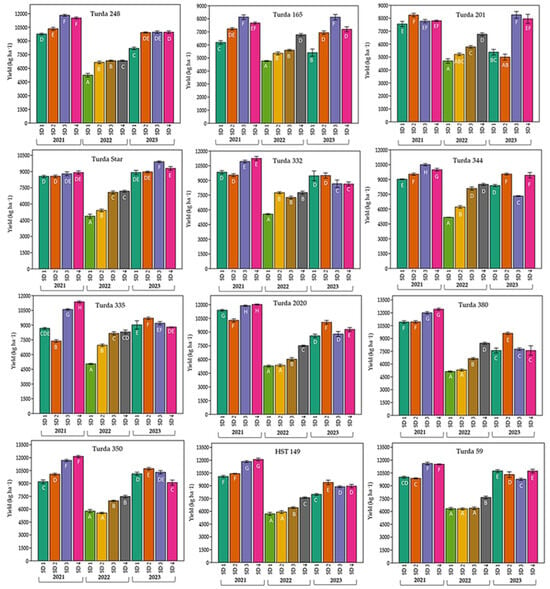
Figure 5.
Variation in grain yield (kg ha−1) of twelve maize hybrids sown at four different dates (SD1–SD4) across three growing seasons (2021–2023). Bars represent mean values ± standard error. Different letters above the bars indicate significant differences according to the Duncan test (p < 0.05).
The grain yield of the 12 studied hybrids varied strongly with year and sowing date, showing a clear Hybrid × Year × Sowing date interaction (Figure 5). For most hybrids, the highest yields were obtained in 2021, the lowest in 2022, and intermediate yields in 2023, reflecting the contrasting climatic conditions across the three years (severe heat and drought in 2022). For each hybrid, sowing date significantly influenced yield (different letters above bars; Duncan, p < 0.05): delayed sowing (SD4) resulted in higher yields, whereas early sowing (SD1–SD2) significantly reduced yield, especially under the unfavorable conditions of 2022. The effect of sowing date was expressed according to the tested hybrid: Turda 59, Turda 350, and Turda 248 achieved high yields with good stability, whereas Turda 165 and Turda 201 showed marked sensitivity to both sowing date and year.
Pearson correlation analysis showed strong and highly significant relationships among sowing temperature, disease parameters, and yield (Table 7). Both disease incidence (r = −0.93 *) and severity (r = −0.99 **) decreased with increasing sowing temperature, while grain yield was positively correlated with sowing temperature (r = 0.99 **). These results indicate that higher soil temperatures at sowing markedly reduced FER intensity and improved yield.

Table 7.
Pearson’s correlation coefficients (r) among sowing temperature, FER incidence, disease severity, and grain yield (means across hybrids and years). * and ** indicate significance at p < 0.05 and p < 0.01, respectively.
Across sowing dates, grain yield tended to decrease with increasing FER severity, but the relationship was sowing date-dependent (Figure 6). A strong and significant correlation was found in SD1 (r = 0.72, R2 = 0.523, p = 0.008), corresponding to an estimated yield loss of 459 kg ha−1 per 1% increase in severity. In SD2, the association was moderate and marginally significant (r = 0.55, R2 = 0.301, p = 0.064), whereas in the optimum and delayed sowings (SD3 and SD4), correlations were weak or non-significant (r = 0.09 and 0.34; p = 0.78 and 0.28, respectively).
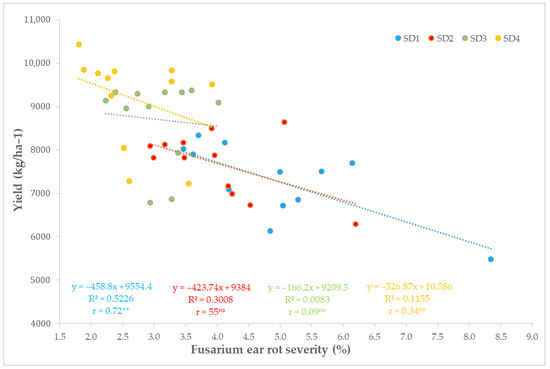
Figure 6.
Linear relationship between FER severity and grain yield across sowing dates (SD1–SD4). ** indicates significance at p < 0.01.
4. Discussion
The present study provides new insights into the impact of sowing date and climatic variability on maize yield and FER incidence and severity in the Transylvanian Plain. Our results showed that sowing date significantly influenced yield stability and disease development, with strong genotype × environment interactions. These findings are consistent with earlier reports highlighting the importance of genotype, crop management, and climatic conditions influencing FER dynamics [11,53,54].
Analysis of temperature and rainfall dynamics shows variation in climatic conditions over the three years with direct consequences for FER development. In 2021, cooler spring and autumn conditions, combined with irregular rainfall and low values in September–October, favored rapid ear drying and limited disease progression. By contrast, 2022 and 2023 were characterized by warmer summers and autumns, together with abundant and frequent rainfall during critical stages (silking and grain filling), which prolonged ear wetness and provided highly favorable conditions for Fusarium infection and mycotoxin accumulation. These results are consistent with earlier reports showing that not only the total rainfall but also its temporal distribution, together with above-average temperatures during flowering and grain filling, strongly influence FER incidence and severity [11,55,56,57,58].
Sowing date had a marked influence on the incidence and severity of FER (Table 5). The control sowing date (SD3) recorded an incidence of 72% and a severity of 2.90%. Earlier sowings (SD1 and SD2) were associated with the highest infection levels, exceeding 82% incidence and recording severity values of 4.55% and 3.75%, respectively (p < 0.001). Delayed sowing (SD4) consistently reduced disease pressure, with a 7.86% lower incidence and the lowest severity values compared with the control (p < 0.001). These results indicate that early sowing dates create favorable conditions for Fusarium infection and disease development, likely due to the coincidence of flowering with periods of higher humidity and insect activity. A similar study that included four sowing dates reported a higher incidence of Ostrinia nubilalis attacks in the early sowing periods [59]. Considering that Fusarium species frequently colonize wounds caused by O. nubilalis, this relationship may explain the higher incidence and severity of Fusarium ear rot observed in early-sown maize. Although most previous studies reported that early sowing reduces the risk of FER by avoiding humid conditions during silking [27,29,31,32,60], in the present study, early sowing dates coincided with silking in July, when climatic conditions were particularly favorable for infection. This could explain the higher incidence of ear rot observed in early sowing dates compared to later ones. If early sowing leads to the synchronization of the silking stage with a period favorable for Fusarium infections, higher disease incidence can be expected compared to later sowing dates. This temporal overlap between maize silking and environmental conditions favorable to pathogen development may therefore explain the increased Fusarium ear rot observed in early-sown maize [21,46,61]. During July 2022 and 2023, high temperatures combined with episodic rainfall events created favorable conditions for spore germination and silk infection. Therefore, early sowings (SD1 and SD2) were exposed to higher infection pressure, while later sowings shifted flowering toward late July–early August, when weather conditions were less favorable for disease development. These findings suggest that the effect of sowing date is strongly dependent on the synchronization between maize phenology and short-term climatic events, particularly rainfall frequency during silking [11,27,57], rather than on total seasonal rainfall [57]. As reported by previous studies [53,56,57,62], maize silk infection exhibits a critical window of approximately six days after silking, during which humidity and rainfall play a decisive role in spore germination and the establishment of infection. Although most studies have reported that early sowing reduces Fusarium infection, our results showed the opposite trend, similar to findings reported in a study conducted in Zambia [33]. In our region, no previous research has examined the influence of sowing date on Fusarium incidence. During the study period (2021–2023), the specific pedoclimatic conditions, combined with increased insect activity, likely contributed to the higher infection levels observed in the early sowing dates. These findings highlight the importance of local environmental factors and pest pressure in shaping the epidemiology of FER.
Across four sowing dates and three years, disease pressure was high (mean FER incidence 74.9%; severity 3.4%), as can be seen in Table 5. Genotype significantly affected both traits. Highly susceptible hybrids included Turda 332 (90.2%), Turda Star (87.1%), and Turda 165 (82.2%), all with high severity (up to 4.7%, p < 0.001). In contrast, Turda 380 (56.7%), HST 149 (63.7%), and Turda 344 (65.4%) exhibited significantly lower incidence and severity (2.5–2.8%, p < 0.001). Several hybrids (Turda 201, Turda 2020, Turda 335, Turda 59) were close to the mean incidence, but differed in severity (e.g., Turda 2020 low vs. Turda 335 high). These results highlight that maize genotype is a decisive factor in FER development, with differentiation between highly susceptible, tolerant, and intermediate hybrids in both incidence and severity. The results also suggest a potential relationship between the germplasm background of the hybrids and their response to FER [9,63,64,65]. Hybrids with a predominant Lancaster and BSSS/Iodent genetic background, such as Turda 165 and Turda 332, showed higher incidence and severity levels, confirming their susceptibility to the disease. By contrast, hybrids that included Oh43 in their pedigree (e.g., Turda 380, Turda 344, HST 149) consistently showed lower infection values, indicating a higher degree of tolerance. Differences were observed among heterotic groups: lines from Lancaster, Iodent, and Stiff Stalk (BSSS) backgrounds tended to be more susceptible, whereas flint materials showed comparatively higher resistance. These results are consistent with previous multi-line screenings and GWAS/QTL studies that highlight the polygenic, germplasm-dependent nature of FER resistance [7,8,28]. Recent studies have highlighted a significant variability in resistance levels among maize hybrids, even within the same growing region. For instance, research conducted in Northeast China identified hybrids with different susceptibility profiles to Fusarium through controlled inoculations [9]. Therefore, the genetic background constitutes an additional determinant of resistance and should be considered alongside sowing date and climatic conditions when developing integrated strategies for Fusarium management.
Across the three seasons and four sowing dates, FER incidence was governed by a strong genotype × sowing date × year interaction (Figure 4). As infection developed under natural field conditions, differences in Fusarium inoculum levels among years may have influenced disease intensity. Such variability, together with climatic fluctuations, could partially explain the interannual differences observed in FER incidence and yield. Overall disease pressure was high—incidence frequently exceeded 60%—and reached near-maximum values in the most susceptible hybrids (Turda 332, Turda Star, Turda 165). Regarding the influence of yearly climatic conditions, incidence was highest in 2023, lowest in 2021, and intermediate in 2022, indicating that interannual climate variability influenced epidemic intensity [66]. Sowing date had exerted a consistent effect on disease, particularly on severity (Figure 5). Importantly, susceptible hybrids such as Turda 165 and Turda 332 remained highly infected regardless of sowing date, suggesting limited plasticity of their response under field pressure. This pattern underscores that sowing date can mitigate—but not fully offset—genetic susceptibility.
Compared with incidence, severity values were lower (<13%) and varied according to sowing date and growing season. The highest severities were recorded in 2023, often peaking at SD1–SD2, whereas the lowest occurred in 2021. Tolerant hybrids (e.g., Turda 380, HST 149) consistently exhibited reduced severity, particularly at SD3–SD4, while several moderately susceptible genotypes (Turda 248, Turda 344, Turda 335, Turda 2020, Turda 350) showed increased severity under early sowing. Notably, Turda Star combined high incidence with only moderate severity, indicating a partial decoupling between incidence and symptom expression and suggesting the presence of distinct genetic components governing infection frequency versus lesion development [65,67]. These results indicate that sowing date has a stronger effect on FER severity than on incidence, suggesting that early sowing, combined with the use of resistant hybrids, may reduce disease-related damage under field conditions. From a management perspective, the choice of a hybrid can play a major role in reducing FER under high disease pressure. Hybrids such as Turda 380 and HST 149 appear promising, whereas Turda 332, Turda Star, and Turda 165 present a higher risk. The pattern reinforces that genetic background is an additional determinant of resistance, to be considered alongside sowing date and climatic conditions [68].
Our data indicate that the rainfall–FER relationship during the silking–ear-filling window is year-dependent, with negative correlations in 2021–2022 and a positive correlation in 2023 (Figure 7). The negative correlation observed may be explained by the uneven distribution of rainfall during the silking–ear filling period. A large proportion of the total precipitation occurred within a few days, resulting in short and intense rainfall events rather than continuous moisture. For example, in July 2021, 123.1 mm of rainfall was recorded over only eight days (Table 4). Such irregular rainfall patterns likely reduced the duration of favorable humidity for fungal infection, thereby limiting FER development despite the overall high precipitation totals. This context-dependence is consistent with prior work showing that infection pressure peaks around silking and is modulated by the interplay of rainfall and temperature during this period [11,57].
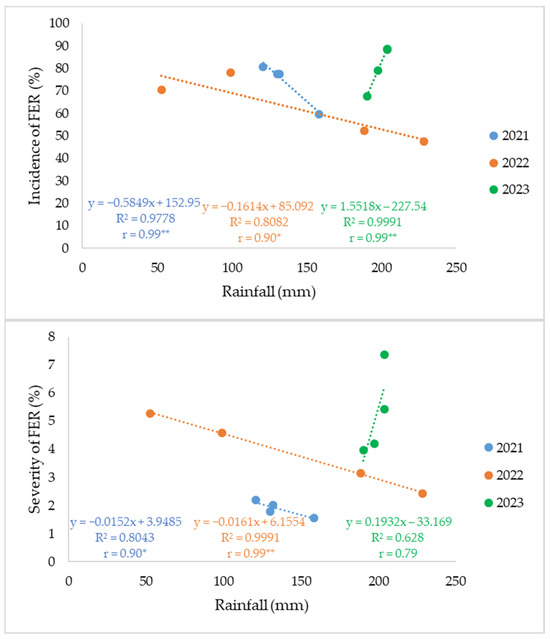
Figure 7.
Relationships between rainfall during the silking–ear-filling period and FER incidence (top) and severity (bottom) in 2021, 2022, and 2023. Each point represents the mean value for one sowing date (n = 4 per year). Linear regression equations, coefficients of determination (R2), and Pearson’s correlation coefficients (r) are shown. *, ** indicate significance at p < 0.05 and p < 0.01, respectively.
Maize grain yield is strongly influenced by climatic conditions, sowing date, and genotype [37,45,69]. Across the three experimental years, maize yield exhibited strong interannual variation (Table 6). The most favorable climatic conditions in 2021 supported the highest yield (9999 kg ha−1, p < 0.001), whereas the combination of severe drought and FER outbreaks in 2022 caused a substantial yield decline (6429 kg ha−1). In 2023, intermediate yields (8765 kg ha−1, p < 0.01) reflected the impact of uneven rainfall distribution and elevated Fusarium incidence.
The correlation analysis (Figure 8) further confirmed that yield variability was closely linked to rainfall dynamics during the reproductive stages. In 2021 and 2022, yield increased with rainfall (r = 0.69 and r = 0.99 **, respectively), indicating that water availability during the silking–ear filling period was a key determinant of grain production. In contrast, in 2023, no significant relationship was observed (r = 0.31), likely due to excessive rainfall and high temperatures that limited pollination efficiency and kernel filling. Based on the regression equation, maize yield increased by approximately 12.3 kg ha−1 for each additional millimeter of rainfall in 2022, but excessive moisture in 2023 no longer benefited yield formation.
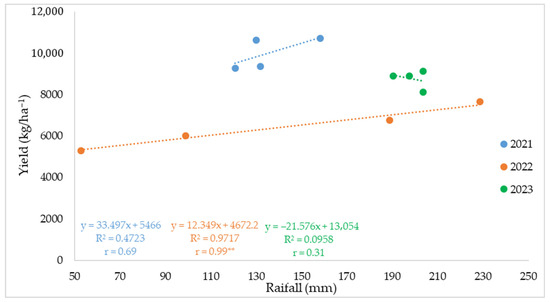
Figure 8.
Relationship between rainfall during the silking–ear filling period and maize yield (kg/ha−1) in 2021, 2022, and 2023. Each point represents the mean value for one sowing date (n − 4 per year). Linear regression equations, coefficients of determination (R2), and Pearson’s correlation coefficients (r) are shown. ** indicates significance at p < 0.01.
Sowing date also played a decisive role (Table 6): early sowing (SD1–SD2) reduced yield to 8170 kg ha−1 (p < 0.001), likely due to stress during flowering and grain filling, whereas delayed sowing (SD4) produced the highest yield (9088 kg ha−1, p < 0.001) by aligning critical growth stages with more favorable climatic conditions. Although most studies report that earlier sowing generally favors higher maize yields [27,39,69,70], our results showed the opposite trend. Similar findings were reported in the Transylvanian region by Simon [71] and Domokos [37], who also observed that delayed sowing resulted in higher yields. This outcome can be explained by the cooler spring conditions during our experimental period compared with earlier studies, which likely slowed early growth and impaired plant development at the earliest sowing dates. Our findings are consistent with previous research from the Transylvanian Plain showing that very early sowing under cold spring conditions (≈6 °C soil temperature) depresses yield, whereas sowing at 8–10 °C improves performance. The rationale is that cooler springs delay early growth and, combined with hotter summers, may shorten the effective grain-filling period; thus, slightly delayed sowing better aligns sensitive stages with more favorable weather conditions.
In addition to climatic and management factors, several biological mechanisms may explain the differences in FER severity observed among sowing dates. The observed differences in FER incidence among sowing dates can be partly explained by microclimatic conditions during flowering. Early sowing exposed plants to cooler and more humid conditions around silking, which may have prolonged silk receptivity and increased the window of infection for Fusarium spores. In contrast, delayed sowing coincided with warmer and drier weather, reducing silk wetness duration and limiting fungal penetration. Differences in plant stress responses may also contribute to this pattern, as high temperatures and drought stress during flowering can impair silk emergence and pollination, indirectly affecting infection dynamics.
Genotypic variability also had a significant impact on yield performance (Table 6). High-yielding hybrids such as Turda 59, Turda 350, Turda 248, Turda 2020, and Turda 332 exceeded the mean yield by 446–867 kg ha−1 (p < 0.001), while FER-susceptible hybrids (Turda 165, Turda 201, Turda Star) suffered substantial yield losses. These results emphasize that yield formation depends not only on environmental and management factors but also on the genetic potential and adaptability of hybrids to local conditions. Similar patterns were reported by Domokos [37] and Simon [71] for maize hybrids cultivated in the Transylvanian Plain, confirming that genotype × environment interactions play a decisive role under the region’s variable climate. Comparable findings were also obtained in other European environments, where hybrid performance was significantly influenced by both genotype and environmental interactions [72,73]. Therefore, selecting hybrids with stable productivity, such as Turda 59 and Turda 350, is essential for maintaining yield stability under fluctuating climatic conditions.
Grain yield was negatively correlated with FER [29,34]. FER severity reduced yield mainly under early sowing, with significant losses in SD1 and weaker effects in SD2. Under optimum and delayed sowing (SD3–SD4), the relationship was negligible, suggesting that later sowing buffered the yield impact of FER. These findings suggest that sowing time not only affects plant exposure to infection but also determines the crop’s physiological capacity to compensate for yield losses caused by FER.
5. Conclusions
This three-year study demonstrated that both sowing date and genotype strongly influence the incidence, severity, and yield impact of FER in maize under the variable climatic conditions of the Transylvanian Plain. Early sowing dates (SD1–SD2) generally coincided with high infection levels due to frequent rainfall during silking, whereas delayed sowing (SD4) reduced FER severity and was associated with higher and more stable yields. Adjusting the sowing date was insufficient to overcome the effect of genetic susceptibility. Significant genotypic variability was observed, with hybrids such as Turda 380 and HST 149 consistently exhibiting tolerance, while Turda 332, Turda 165, and Turda Star displayed high susceptibility. Yield losses were strongly correlated with FER severity, particularly under early sowing conditions.
The findings highlight that integrating optimum sowing dates with resistant hybrids provides an effective and sustainable strategy for reducing FER risk and ensuring yield stability in temperate maize-growing regions under increasing climatic variability.
The main limitation of this study is the relatively short observation period, as two years exhibited similar climatic trends and one year showed contrasting conditions. Future research should extend the monitoring period and include Fusarium species identification, soil moisture monitoring, and mycotoxin analyses to provide a more comprehensive understanding of disease epidemiology, yield dynamics, and food safety implications.
Author Contributions
Conceptualization, L.Ș. and A.Ș.; methodology, A.Ș. and A.-M.V.; software, L.Ș., A.-M.V. and V.C.; validation, L.S. and A.V.; formal analysis, A.-M.V. and F.R.; investigation, L.S. and A.T.; resources, N.T. and V.C.; data curation, L.Ș. and V.C.; writing—original draft preparation, L.Ș. and A.Ș.; writing—review and editing, A.T., L.S. and F.R.; visualization, A.V. and R.E.C.; supervision, N.T. and R.E.C.; project administration, L.S. and F.V.; funding acquisition, A.Ș., F.V. and F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project “Bioproducts—An alternative for the control of phytopathogenic agents, in the context of reducing the use of pesticides”, No. 1393/27.01.2025, funded by Agroleg Varo SRL.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Acknowledgments
We would like to thank the Agricultural Research and Development Station Turda for organizing research in the experimental field.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global Maize Production, Consumption and Trade: Trends and R&D Implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar] [CrossRef]
- Niu, L.; Liu, L.; Zhang, J.; Scali, M.; Wang, W.; Hu, X.; Wu, X. Genetic Engineering of Starch Biosynthesis in Maize Seeds for Efficient Enzymatic Digestion of Starch during Bioethanol Production. Int. J. Mol. Sci. 2023, 24, 3927. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Wang, X.; Chen, G.; Sun, S.; Yang, Y.; Zhu, Z.; Duan, C. The Major Fusarium Species Causing Maize Ear and Kernel Rot and Their Toxigenicity in Chongqing, China. Toxins 2018, 10, 90. [Google Scholar] [CrossRef] [PubMed]
- Hudson, O.; Meinecke, C.D.; Brawner, J.T. Comparative Genomics of Fusarium Species Causing Fusarium Ear Rot of Maize. PLoS ONE 2024, 19, e0306144. [Google Scholar] [CrossRef]
- Arata, A.F.; Martínez, M.; Castellari, C.; Cristos, D.; Pesquero, N.V.; Dinolfo, M.I. Impact of Fusarium Spp. on Different Maize Commercial Hybrids: Disease Evaluation and Mycotoxin Contamination. Fungal Biol. 2024, 128, 1983–1991. [Google Scholar] [CrossRef]
- Logrieco, A.; Mulè, G.; Moretti, A.; Bottalico, A. Toxigenic Fusarium Species and Mycotoxins Associated with Maize Ear Rot in Europe. Eur. J. Plant Pathol. 2002, 108, 597–609. [Google Scholar] [CrossRef]
- Lanubile, A.; Maschietto, V.; Borrelli, V.M.; Stagnati, L.; Logrieco, A.F.; Marocco, A. Molecular Basis of Resistance to Fusarium Ear Rot in Maize. Front. Plant Sci. 2017, 8, 1774. [Google Scholar] [CrossRef]
- Czembor, E.; Waśkiewicz, A.; Piechota, U.; Puchta, M.; Czembor, J.H.; Stȩpień, Ł. Differences in Ear Rot Resistance and Fusarium Verticillioides-Produced Fumonisin Contamination Between Polish Currently and Historically Used Maize Inbred Lines. Front. Microbiol. 2019, 10, 449. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Wen, S.; Ren, J.; Hui, H.; Huang, Y.; Yang, J.; Zhao, B.; Liu, B.; Gao, Z. Evaluation of Maize Hybrids for Resistance to Ear Rot Caused by Dominant Fusarium Species in Northeast China. Agronomy 2024, 14, 855. [Google Scholar] [CrossRef]
- Tiru, Z.; Mandal, P.; Chakraborty, A.P.; Pal, A.; Sadhukhan, S.; Tiru, Z.; Mandal, P.; Chakraborty, A.P.; Pal, A.; Sadhukhan, S. Fusarium Disease of Maize and Its Management through Sustainable Approach. In Fusarium—An Overview of the Genus; IntechOpen: Gent, Belgium, 2021; ISBN 978-1-83968-736-5. [Google Scholar]
- Pfordt, A.; Ramos Romero, L.; Schiwek, S.; Karlovsky, P.; Von Tiedemann, A. Impact of Environmental Conditions and Agronomic Practices on the Prevalence of Fusarium Species Associated with Ear- and Stalk Rot in Maize. Pathogens 2020, 9, 236. [Google Scholar] [CrossRef]
- Nguyen, T.B.H.; Henri-Sanvoisin, A.; Coton, M.; Le Floch, G.; Picot, A. Shifts in Fusarium Communities and Mycotoxins in Maize Residues, Soils, and Wheat Grains throughout the Wheat Cycle: Implications for Fusarium Head Blight Epidemiology. Microorganisms 2024, 12, 1783. [Google Scholar] [CrossRef]
- Todorović, I.; Moënne-Loccoz, Y.; Raičević, V.; Jovičić-Petrović, J.; Muller, D. Microbial Diversity in Soils Suppressive to Fusarium Diseases. Front. Plant Sci. 2023, 14, 1228749. [Google Scholar] [CrossRef]
- Ajmal, M.; Hussain, A.; Ali, A.; Chen, H.; Lin, H. Strategies for Controlling the Sporulation in Fusarium spp. J. Fungi 2022, 9, 10. [Google Scholar] [CrossRef]
- Frasiński, S.; Czembor, E.; Lalak-Kańczugowska, J. The Impact of Fusarium Ear Rot in Poland and Methods to Reduce Losses Caused by the Disease. Biul. Inst. Hod. Aklim. Roślin 2020, 290, 43–50. [Google Scholar] [CrossRef]
- Mazzoni, E.; Scandolara, A.; Giorni, P.; Pietri, A.; Battilani, P. Field Control of Fusarium Ear Rot, Ostrinia nubilalis (Hübner), and Fumonisins in Maize Kernels. Pest Manag. Sci. 2011, 67, 458–465. [Google Scholar] [CrossRef]
- Khalaf, E.M.; Shrestha, A.; Rinne, J.; Lynch, M.D.J.; Shearer, C.R.; Limay-Rios, V.; Reid, L.M.; Raizada, M.N. Transmitting Silks of Maize Have a Complex and Dynamic Microbiome. Sci. Rep. 2021, 11, 13215. [Google Scholar] [CrossRef]
- Blandino, M.; Scarpino, V.; Vanara, F.; Sulyok, M.; Krska, R.; Reyneri, A. The Role of the European Corn Borer (Ostrinia nubilalis) on Contamination of Maize with Thirteen Fusarium Mycotoxins. Food Addit. Contam. Part Chem. Anal. Control Expo. Risk Assess. 2014, 32, 533–543. [Google Scholar] [CrossRef]
- Negruț, G.N.; Cotuna, O.; Sărăţeanu, V.; Durău, C.C.; Titus, S. Research Regarding the Relationship among the Pests Ostrinia nubilalis, Helicoverpa armigera and the Fungi Fusarium verticillioides, Aspergillus flavus in Corn in the Climatic Conditions from Lovrin (Timiș County). Res. J. Agric. Sci. 2019, 51, 282–291. [Google Scholar]
- Pintilie, P.L.; Trotuș, E.; Tălmaciu, N.; Irimia, L.M.; Herea, M.; Mocanu, I.; Amarghioalei, R.G.; Popa, L.D.; Tălmaciu, M. European Corn Borer (Ostrinia nubilalis Hbn.) Bioecology in Eastern Romania. Insects 2023, 14, 738. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P. Epidemiology of Fusarium Diseases and Their Mycotoxins in Maize Ears. In Epidemiology of Mycotoxin Producing Fungi; Xu, X., Bailey, J.A., Cooke, B.M., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 705–713. ISBN 978-90-481-6387-8. [Google Scholar]
- Desjardins, A.E.; Plattner, R.D. Fumonisin B1 -Nonproducing Strains of Fusarium Verticillioides Cause Maize (Zea mays) Ear Infection and Ear Rot. J. Agric. Food Chem. 2000, 48, 5773–5780. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, D.; Czubacka, A.; Agacka-Mołdoch, M.; Trojak-Goluch, A.; Księżak, J. The Occurrence of Fungal Diseases in Maize in Organic Farming Versus an Integrated Management System. Agronomy 2022, 12, 558. [Google Scholar] [CrossRef]
- Singh, H.; Kaur, H.; Hunjan, M.S.; Sharma, S. Unveiling Toxigenic Fusarium Species Causing Maize Ear Rot: Insights into Fumonisin Production Potential. Front. Plant Sci. 2025, 16, 1516644. [Google Scholar] [CrossRef] [PubMed]
- Mesterházy, Á.; Oláh, J.; Popp, J. Losses in the Grain Supply Chain: Causes and Solutions. Sustainability 2020, 12, 2342. [Google Scholar] [CrossRef]
- Mesterházy, Á.; Lemmens, M.; Reid, L.M. Breeding for Resistance to Ear Rots Caused by Fusarium spp. in Maize—A Review. Plant Breed. 2012, 131, 1–19. [Google Scholar] [CrossRef]
- Krnjaja, V.; Mandić, V.; Bijelić, Z.; Stanković, S.; Obradović, A.; Caro Petrović, V.; Gogić, M. Influence of Sowing Time on Fusarium and Fumonisin Contamination of Maize Grains and Yield Component Traits. Agriculture 2022, 12, 1042. [Google Scholar] [CrossRef]
- Magarini, A.; Passera, A.; Ghidoli, M.; Casati, P.; Pilu, R. Genetics and Environmental Factors Associated with Resistance to Fusarium graminearum, the Causal Agent of Gibberella Ear Rot in Maize. Agronomy 2023, 13, 1836. [Google Scholar] [CrossRef]
- Parsons, M.W.; Munkvold, G.P. Associations of Planting Date, Drought Stress, and Insects with Fusarium Ear Rot and Fumonisin B1 Contamination in California Maize. Food Addit. Contam. Part A 2010, 27, 591–607. [Google Scholar] [CrossRef]
- Blandino, M.; Saladini, M.A.; Reyneri, A.; Vanara, F.; Alma, A. The Influence of Sowing Date and Insecticide Treatments on Ostrinia nubilalis (Hübner) Damage and Fumonisin Contamination in Maize Kernels. Maydica 2008, 53, 199–206. [Google Scholar]
- Blandino, M.; Reyneri, A.; Vanara, F. Effect of Sowing Time on Toxigenic Fungal Infection and Mycotoxin Contamination of Maize Kernels. J. Phytopathol. 2009, 157, 7–14. [Google Scholar] [CrossRef]
- Blandino, M.; Scarpino, V.; Giordano, D.; Sulyok, M.; Krska, R.; Vanara, F.; Reyneri, A. Impact of Sowing Time, Hybrid and Environmental Conditions on the Contamination of Maize by Emerging Mycotoxins and Fungal Metabolites. Ital. J. Agron. 2017, 12, 928. [Google Scholar] [CrossRef]
- Schjøth, J.E.; Sundheim, L. Epidemic Significance of Planting Time and Hybrid on Fusarium Infection of Maize in Two Agroecological Zones of Zambia. Acta Agric. Scand. Sect. B—Soil Plant Sci. 2013, 63, 153–161. [Google Scholar] [CrossRef]
- Deressa, T.; Adugna, G.; Suresh, L.M.; Bekeko, Z. Resistance of Maize (Zea mays L.) Genotypes against Ear Rot Causing Pathogens in Southern and Western Ethiopia. Phytoparasitica 2025, 53, 67. [Google Scholar] [CrossRef]
- Focker, M.; van Eupen, M.; Verweij, P.; Liu, C.; van Haren, C.; van der Fels-Klerx, H.J. Effects of Climate Change on Areas Suitable for Maize Cultivation and Aflatoxin Contamination in Europe. Toxins 2023, 15, 599. [Google Scholar] [CrossRef]
- Maiorano, A.; Reyneri, A.; Sacco, D.; Magni, A.; Ramponi, C. A Dynamic Risk Assessment Model (FUMAgrain) of Fumonisin Synthesis by Fusarium verticillioides in Maize Grain in Italy. Crop Prot. 2009, 28, 243–256. [Google Scholar] [CrossRef]
- Domokos, Z.; Șimon, A.; Chețan, F.; Ceclan, O.A.; Filip, E.; Călugăr, R.E.; Vâtcă, S.D.; Duda, M.M. The Influence of Sowing Date on the Primary Yield Components of Maize. Agronomy 2024, 14, 2120. [Google Scholar] [CrossRef]
- Partal, E.; Oltenacu, C.V.; Petcu, V. The Influence of Sowing Date and Plant Density on Maize Yield and Quality in the Context of Climate Change in Southern Romania. Sci. Papers. Ser. A. Agron. 2021, LXIV, 508–514. [Google Scholar]
- Liaqat, W.; Akmal, M.; Ali, J. Sowing Dates Effect on Production of High Yielding Maize Varieties. Sarhad J. Agric. 2018, 34, 102–113. [Google Scholar] [CrossRef]
- Lv, X.; Bai, P.; Zhang, W.; Zhu, Y. Analysis on effect of ecological factors on maize dry weight accumulation in different sowing period. J. Shihezi Univ. Sci. 2004, 22, 285–288. [Google Scholar]
- Ping, L.W.; Chen, G.P.; Guo, J.R.; Wang, Z.X.; Rao, C.F. Study on the Source and Sink in Relation to Grain Yield under Different Ecological Areas in Maize (Zea mays L.). Acta Agron. Sin. 1997, 23, 727–733. [Google Scholar]
- Ke, F.; Ma, X. Responses of Maize Hybrids with Contrasting Maturity to Planting Date in Northeast China. Sci. Rep. 2021, 11, 15776. [Google Scholar] [CrossRef]
- Hayat, Z.; Khalil, S. Phenology and Yield of Sweet Corn Landraces Influenced by Planting Dates. Sarhad J. Agric. 2009, 25, 153–157. [Google Scholar]
- Călugăr, R.E.; Varga, A.; Vana, C.D.; Ceclan, L.A.; Racz, I.; Chețan, F.; Șimon, A.; Popa, C.; Tritean, N.; Russu, F.; et al. Influence of Changing Weather on Old and New Maize Hybrids: A Case Study in Romania. Plants 2024, 13, 3322. [Google Scholar] [CrossRef]
- Șimon, A.; Moraru, P.I.; Ceclan, A.; Russu, F.; Chețan, F.; Bărdaș, M.; Popa, A.; Rusu, T.; Pop, A.I.; Bogdan, I. The Impact of Climatic Factors on the Development Stages of Maize Crop in the Transylvanian Plain. Agronomy 2023, 13, 1612. [Google Scholar] [CrossRef]
- Parsons, M.W.; Munkvold, G.P. Effects of Planting Date and Environmental Factors on Fusarium Ear Rot Symptoms and Fumonisin B1 Accumulation in Maize Grown in Six North American Locations. Plant Pathol. 2012, 61, 1130–1142. [Google Scholar] [CrossRef]
- Mesterhazy, A. Food Safety Aspects of Breeding Maize to Multi-Resistance against the Major (Fusarium Graminearum, F. verticillioides, Aspergillus flavus) and Minor Toxigenic Fungi (Fusarium spp.) as Well as to Toxin Accumulation, Trends, and Solutions—A Review. J. Fungi 2024, 10, 40. [Google Scholar] [CrossRef]
- Soiuri, H. Statiunea Cercet. Dezvoltare Agric. Turda 2025. Available online: https://scdaturda.ro/soiurihibrizi/ (accessed on 24 September 2025).
- Rusu, M.; Mihai, M.; Mihai, V.C.; Moldovan, L.; Ceclan, O.A.; Toader, C. Areas of Agrochemical Deepening Resulting from Long-Term Experiments with Fertilizers—Synthesis Following 20 Years of Annual and Stationary Fertilization. Agriculture 2023, 13, 1503. [Google Scholar] [CrossRef]
- Reid, L.M.; Hamilton, R.I.; Mather, D.E. Screening Maize for Resistance to Gibberella Ear Rot; Research Branch, Agriculture and Agri-Food: Ottawa, ON, Canada, 1996; ISBN 978-0-662-24595-7. [Google Scholar]
- Poly Fact. ANOVA and Duncan’s Test PC Program for Variant Analyses Made for Completely Randomized Polyfactorial Experiences; USAMV: Cluj-Napoca, Romania, 2015; Available online: https://scholar.google.com/scholar_lookup?title=ANOVA+and+Duncan%E2%80%99s+Test+PC+Program+for+Variant+Analyses+Made+for+Completely+Randomized+Polyfactorial+Experiences&author=PoliFact+2020&publication_year=2020 (accessed on 25 August 2025).
- Turda Weather Station. Northern Transylvania Regional Meteorological Center Cluj. Available online: https://www.meteoromania.ro/ (accessed on 20 August 2025).
- Czembor, E.; Frasiński, S.; Urbaniak, M.; Waśkiewicz, A.; Czembor, J.H.; Stępień, Ł. Fusarium Species Shifts in Maize Grain as a Response to Climatic Changes in Poland. Agriculture 2024, 14, 1793. [Google Scholar] [CrossRef]
- Doohan, F.; Brennan, J.M.; Cooke, B.M. Influence of Climatic Factors on Fusarium Species Pathogenic to Cereals. Eur. J. Plant Pathol. 2003, 109, 755–768. [Google Scholar] [CrossRef]
- Cao, A.; Santiago, R.; Ramos, A.J.; Souto, X.C.; Aguín, O.; Malvar, R.A.; Butrón, A. Critical Environmental and Genotypic Factors for Fusarium verticillioides Infection, Fungal Growth and Fumonisin Contamination in Maize Grown in Northwestern Spain. Int. J. Food Microbiol. 2014, 177, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Czembor, E.; Stępień, Ł.; Waśkiewicz, A. Effect of Environmental Factors on Fusarium Species and Associated Mycotoxins in Maize Grain Grown in Poland. PLoS ONE 2015, 10, e0133644. [Google Scholar] [CrossRef]
- Thompson, M.E.H.; Raizada, M.N. Fungal Pathogens of Maize Gaining Free Passage Along the Silk Road. Pathogens 2018, 7, 81. [Google Scholar] [CrossRef]
- Dalla Lana, F.; Madden, L.V.; Paul, P.A. Natural Occurrence of Maize Gibberella Ear Rot and Contamination of Grain with Mycotoxins in Association with Weather Variables. Plant Dis. 2021, 105, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Tărău, A.-D.; Urdă, C.; Vălean, A.-M.; Şopterean, L.; Suciu, L.; Șimon, A.; Russu, F.; Varga, A.; Călugăr, R. The Impact of the Sowing Time on the European Corn Borer (Ostrinia nubilalis Hubner) Attack on Some Romanian Maize Hybrids. Rom. Agric. Res. 2025, 42, 215–223. [Google Scholar] [CrossRef]
- Berghetti, J.; Casa, R.T.; Ferreira, E.Z.; Zanella, E.J.; Scheidt, B.T.; Sangoi, L. Incidence of Stalk Rots in Corn Hybrids Influenced by Sowing Time and Nitrogen Rates. Bragantia 2019, 78, 371–378. [Google Scholar] [CrossRef]
- Reid, L.M.; Nicol, R.W.; Ouellet, T.; Savard, M.; Miller, J.D.; Young, J.C.; Stewart, D.W.; Schaafsma, A.W. Interaction of Fusarium graminearum and F. moniliforme in Maize Ears: Disease Progress, Fungal Biomass, and Mycotoxin Accumulation. Phytopathology 1999, 89, 1028–1037. [Google Scholar] [CrossRef]
- Singh, M.P.; DiFonzo, C.D.; Fusilier, K.M.; Kaur, H.; Chilvers, M.I. Insect Ear-Feeding Impacts Gibberella Ear Rot and Deoxynivalenol Accumulation in Corn Grain. Crop Forage Turfgrass Manag. 2024, 10, e20258. [Google Scholar] [CrossRef]
- Ayesiga, S.B.; Rubaihayo, P.; Sempiira, J.B.; Adjei, E.A.; Dramadri, I.O.; Oloka, B.M.; Sserumaga, J.P. Combining Ability and Gene Action for Resistance to Fusarium Ear Rot in Tropical Maize Hybrids. Front. Plant Sci. 2025, 16, 1509859. [Google Scholar] [CrossRef]
- Neupane, S.P.; Stagnati, L.; Dell’Acqua, M.; Busconi, M.; Lanubile, A.; Pè, M.E.; Caproni, L.; Marocco, A. Genetic Basis of Fusarium Ear Rot Resistance and Productivity Traits in a Heterozygous Multi-Parent Recombinant Inbred Intercross (RIX) Maize Population. BMC Plant Biol. 2025, 25, 639. [Google Scholar] [CrossRef]
- Cao, A.; de la Fuente, M.; Gesteiro, N.; Santiago, R.; Malvar, R.A.; Butrón, A. Genomics and Pathways Involved in Maize Resistance to Fusarium Ear Rot and Kernel Contamination With Fumonisins. Front. Plant Sci. 2022, 13, 866478. [Google Scholar] [CrossRef]
- Nagy, E.; Haş, V.; Haş, I.; Suciu, A.; Florian, V. The Influence of Fusarium Ear Infection on the Maize Yield and Mycotoxin Content (Transylvania-Romania). Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Agric. 2009, 66, 549. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Z.; Lu, P.; Li, R.; Ma, P.; Wu, J.; Li, T.; Zhang, H. Increasing Fusarium verticillioides Resistance in Maize by Genomics-Assisted Breeding: Methods, Progress, and Prospects. Crop J. 2023, 11, 1626–1641. [Google Scholar] [CrossRef]
- Guo, Z.; Zou, C.; Liu, X.; Wang, S.; Li, W.-X.; Jeffers, D.; Fan, X.; Xu, M.; Xu, Y. Complex Genetic System Involved in Fusarium Ear Rot Resistance in Maize as Revealed by GWAS, Bulked Sample Analysis, and Genomic Prediction. Plant Dis. 2020, 104, 1725–1735. [Google Scholar] [CrossRef]
- Wu, W.; Yue, W.; Bi, J.; Zhang, L.; Xu, D.; Peng, C.; Chen, X.; Wang, S. Influence of Climatic Variables on Maize Grain Yield and Its Components by Adjusting the Sowing Date. Front. Plant Sci. 2024, 15, 1411009. [Google Scholar] [CrossRef]
- Varma, V.; Kanaka Durga, K.; Neelima, P. Effect of Sowing Date on Maize Seed Yield and Quality: A Review. Rev. Plant Stud. 2014, 1, 26–38. [Google Scholar] [CrossRef]
- Simon, A.; Ceclan, A.; Has, V.; Varga, A.; Russu, F.; Chetan, F.; Bardas, M. Evaluation of the Impact of Sowing Season and Weather Conditions on Maize Yield. AgroLife Sci. J. 2023, 12, 207–214. [Google Scholar] [CrossRef]
- Bocianowski, J.; Nowosad, K.; Rejek, D. Genotype-Environment Interaction for Grain Yield in Maize (Zea mays L.) Using the Additive Main Effects and Multiplicative Interaction (AMMI) Model. J. Appl. Genet. 2024, 65, 653–664. [Google Scholar] [CrossRef]
- Katsenios, N.; Sparangis, P.; Chanioti, S.; Giannoglou, M.; Leonidakis, D.; Christopoulos, M.V.; Katsaros, G.; Efthimiadou, A. Genotype × Environment Interaction of Yield and Grain Quality Traits of Maize Hybrids in Greece. Agronomy 2021, 11, 357. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).