Using Flint Maize for Developing New Hybrids: A Case Study in Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material
2.2. Experimental Design and Cultivation Technology
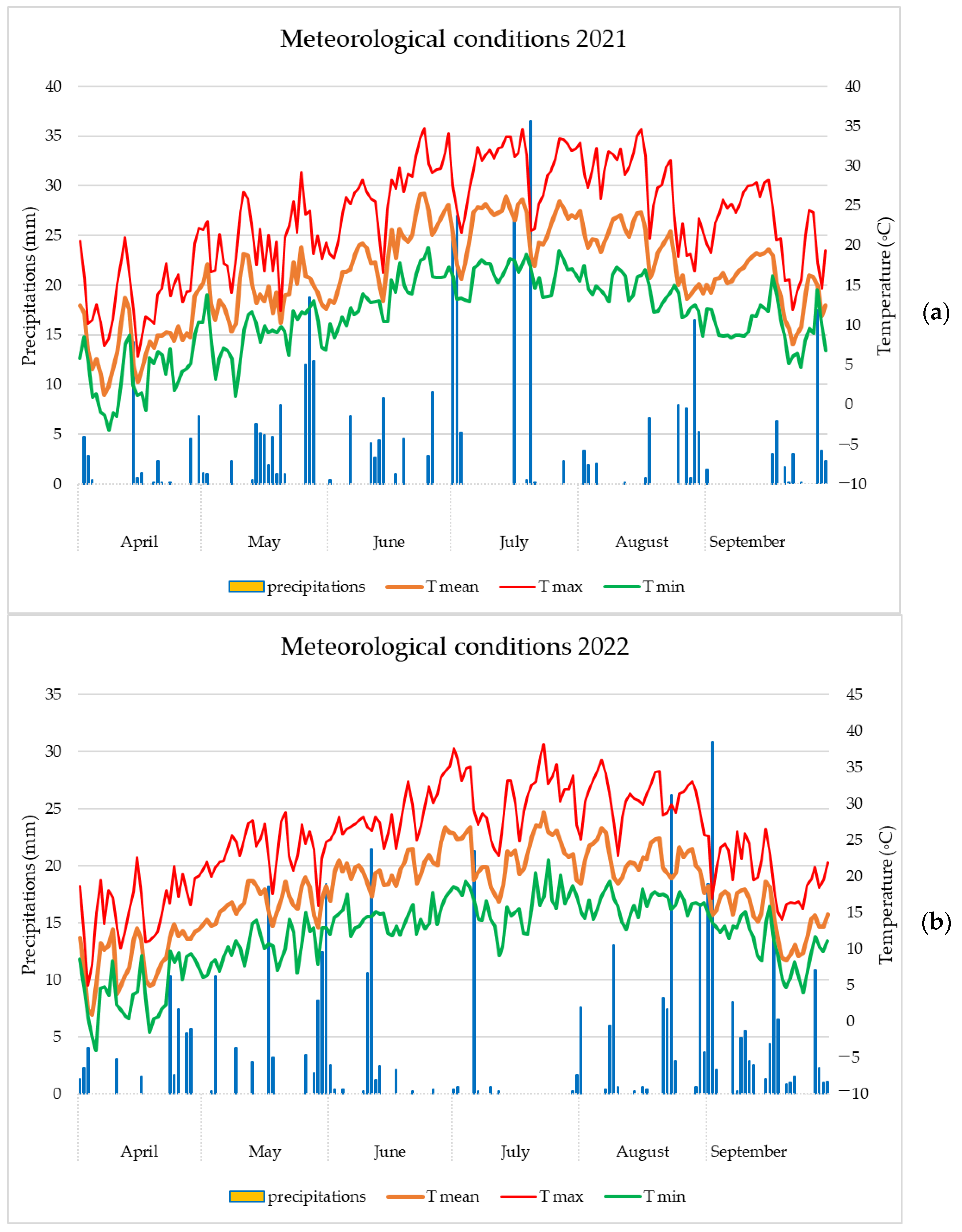
2.3. Meteorological Conditions
2.4. Data Processing and Statistical Methods
3. Results
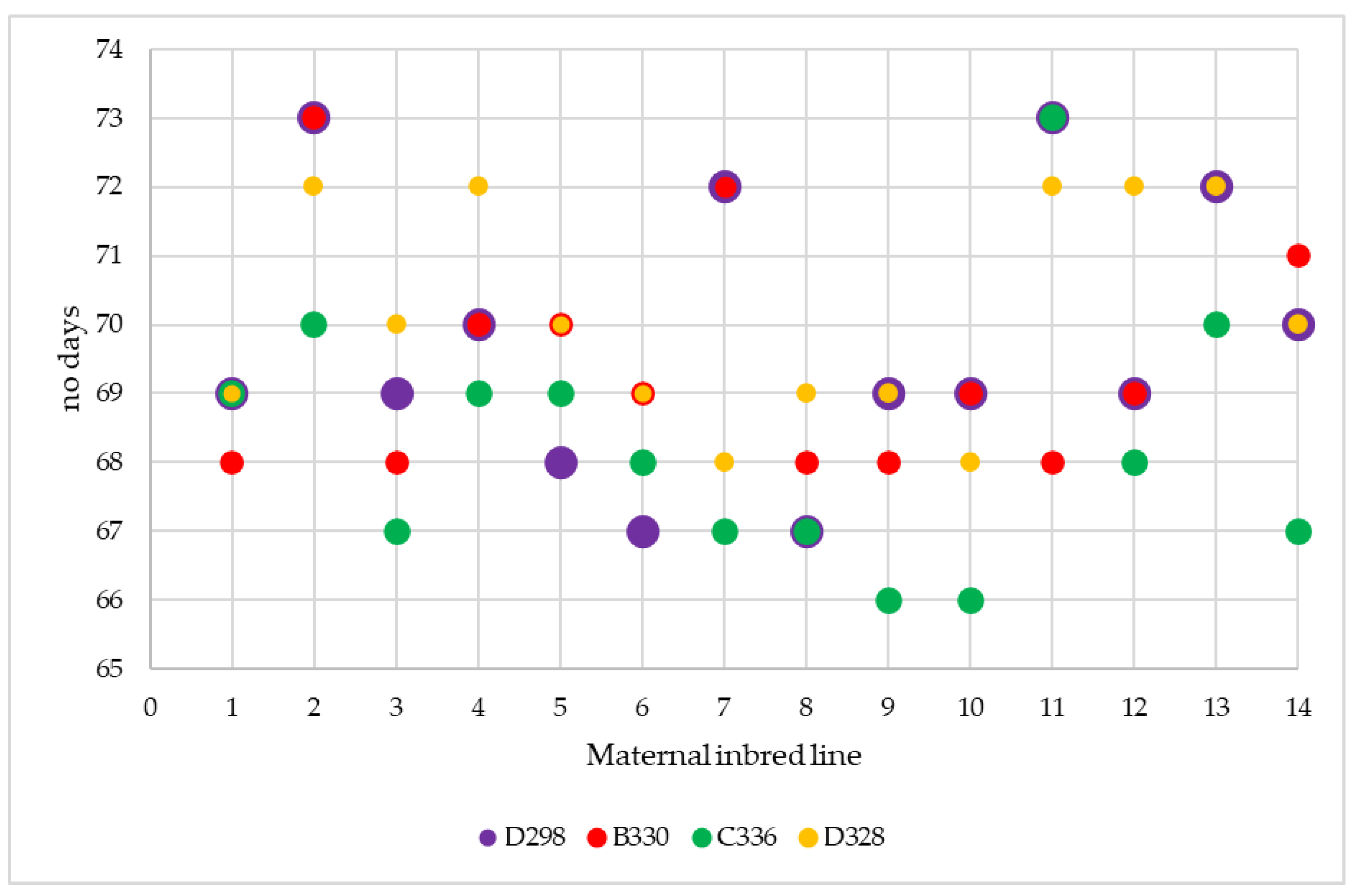
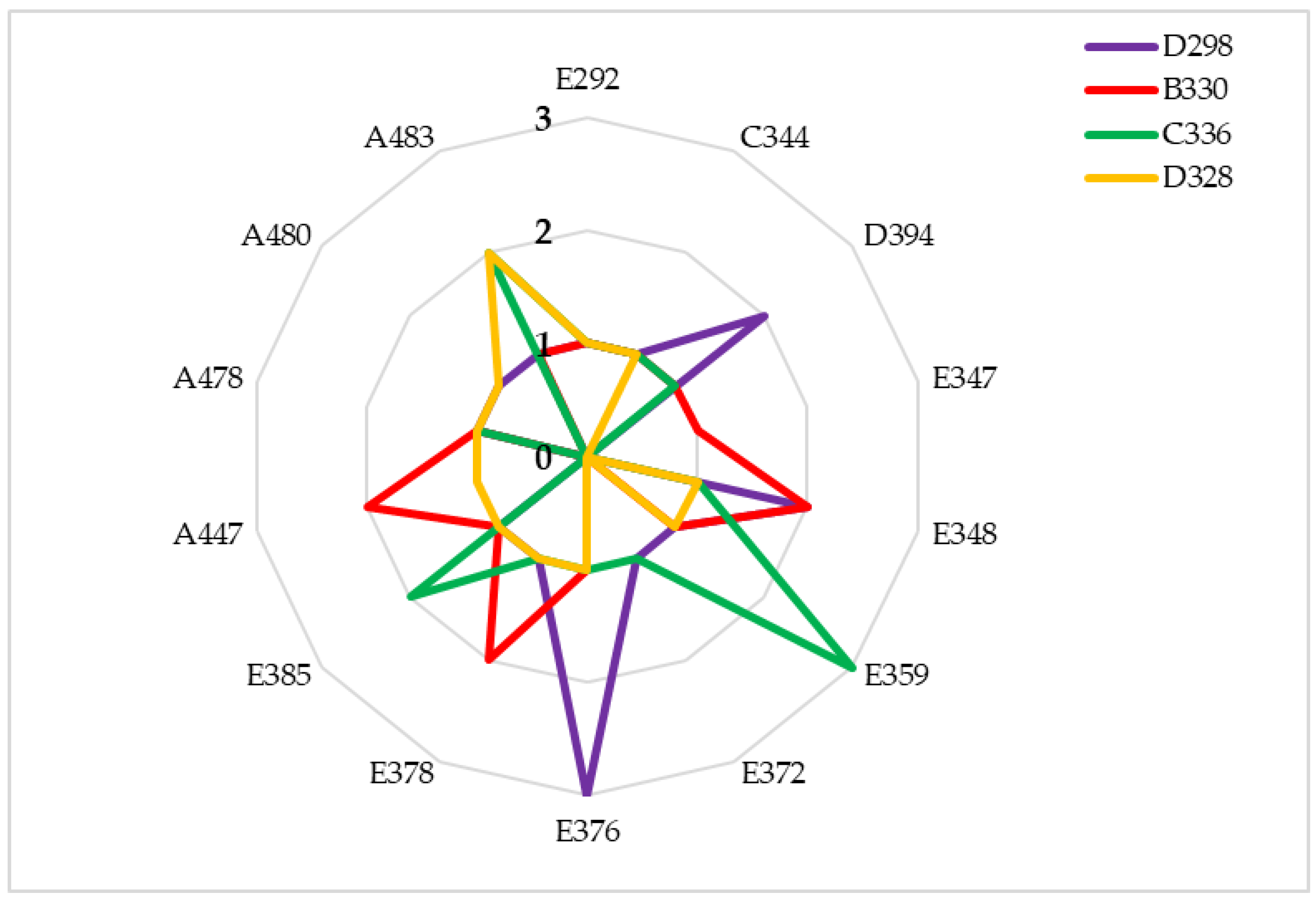
3.1. Plant Phenology
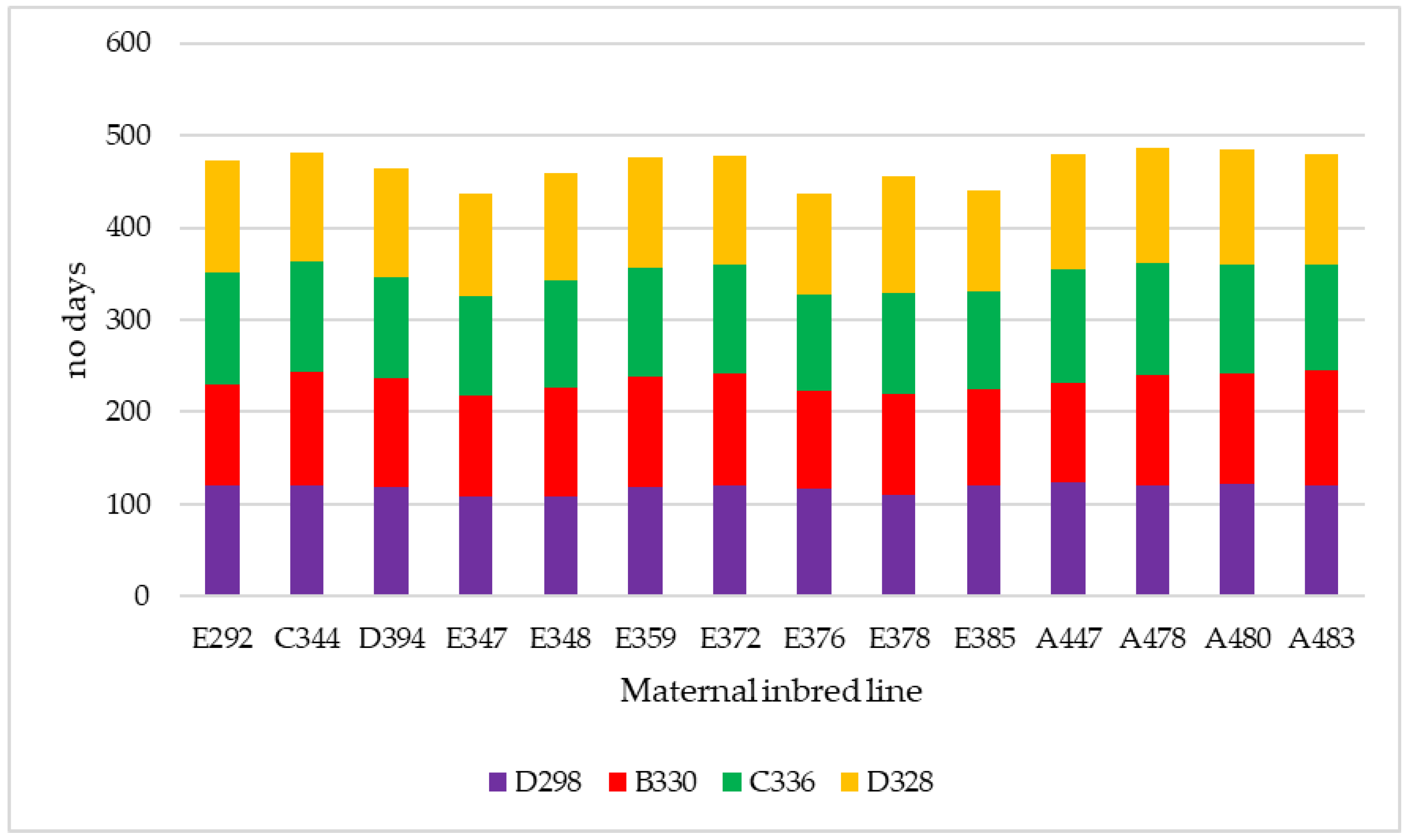
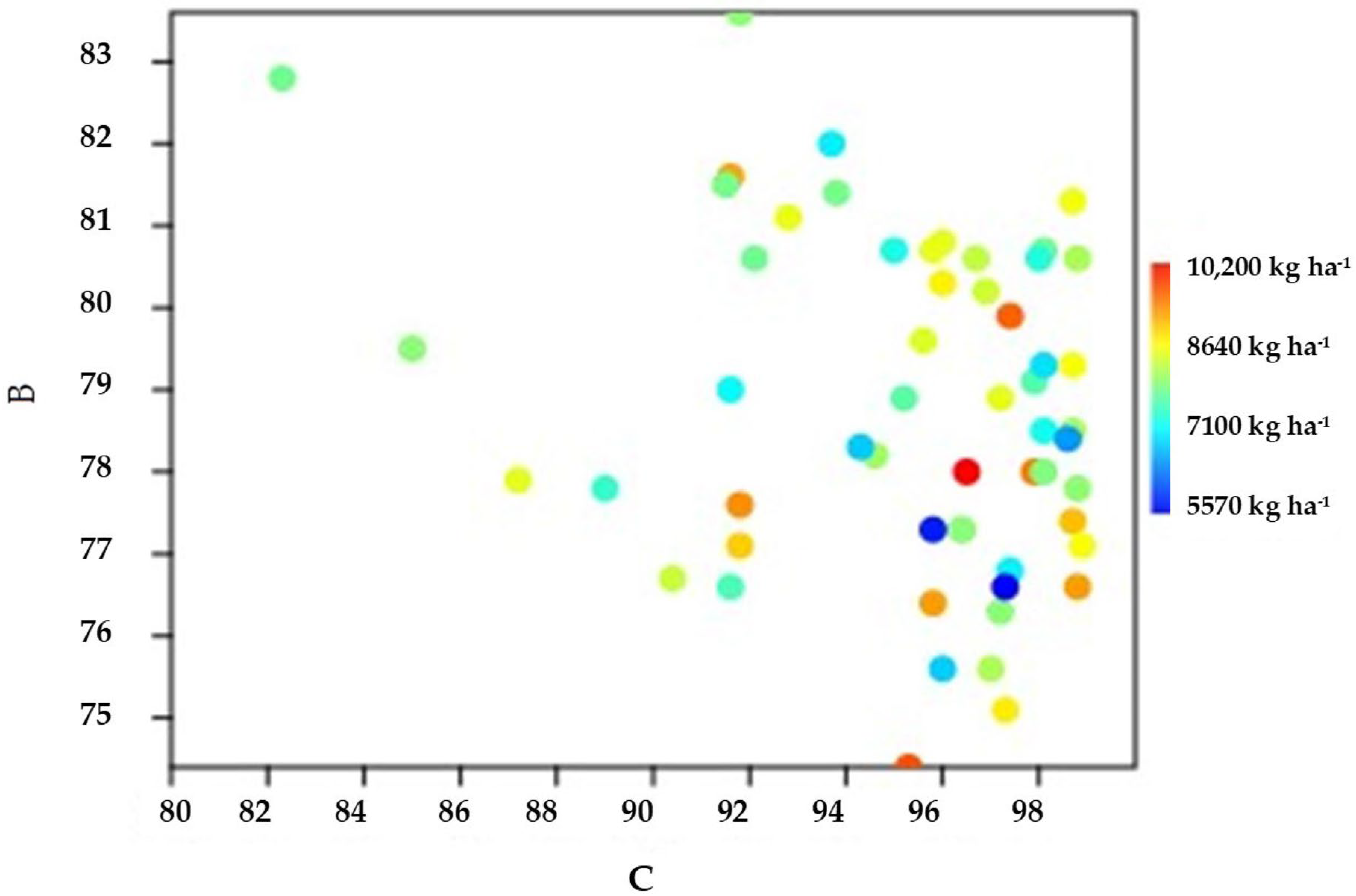
3.2. Hybrid Yield, Dry Matter at Harvest and Unlodged Plants
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARDS | Agricultural Research and Development Station |
| GCA | General combining ability |
| SCA | Specific combining ability |
| UP | Unlodged plant |
| DM | Dry matter |
| ASI | Anthesis-to-silking interval |
References
- FAOSTAT. 2025. Available online: https://www.fao.org/faostat/en/#home (accessed on 30 July 2025).
- Patel, R.; Memon, J.; Kumar, S.; Patel, D.A.; Sakure, A.A.; Patel, M.B.; Das, A.; Karjagi, C.G.; Patel, S.; Patel, U.; et al. Genetic Diversity and Population Structure of Maize (Zea mays L.) Inbred Lines in Association with Phenotypic and Grain Qualitative Traits Using SSR Genotyping. Plants 2024, 13, 823. [Google Scholar] [CrossRef]
- Sánchez, B.; Rasmussen, A.; Porter, J.R. Temperatures and the Growth and Development of Maize and Rice: A Review. Glob. Change Biol. 2014, 20, 408–417. [Google Scholar] [CrossRef]
- Niu, S.; Du, X.; Wei, D.; Liu, S.; Tang, Q.; Bian, D.; Zhang, Y.; Cui, Y.; Gao, Z. Heat Stress After Pollination Reduces Kernel Number in Maize by Insufficient Assimilates. Front. Genet. 2021, 12, 728166. [Google Scholar] [CrossRef]
- Wang, J.; Vanga, S.K.; Saxena, R.; Orsat, V.; Raghavan, V. Effect of Climate Change on the Yield of Cereal Crops: A Review. Climate 2018, 6, 41. [Google Scholar] [CrossRef]
- Nasser, L.M.; Badu-Apraku, B.; Gracen, V.E.; Mafouasson, H.N.A. Combining Ability of Early-Maturing Yellow Maize Inbreds under Combined Drought and Heat Stress and Well-Watered Environments. Agronomy 2020, 10, 1585. [Google Scholar] [CrossRef]
- Cairns, J.E.; Crossa, J.; Zaidi, P.H.; Grudloyma, P.; Sanchez, C.; Araus, J.L.; Thaitad, S.; Makumbi, D.; Magorokosho, C.; Bänziger, M.; et al. Identification of Drought, Heat, and Combined Drought and Heat Tolerant Donors in Maize. Crop Sci. 2013, 53, 1335–1346. [Google Scholar] [CrossRef]
- Smith, J.S.; Trevisan, W.; McCunn, A.; Huffman, W.E. Global Dependence on Corn Belt Dent Maize Germplasm: Challenges and Opportunities. Crop Sci. 2022, 62, 2039–2066. [Google Scholar] [CrossRef]
- Prasanna, B.M. Diversity in Global Maize Germplasm: Characterization and Utilization. J. Biosci. 2012, 37, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, S.; Greco, I.A.; Almeida, H.; Borrás, L. Physiological Differences in Yield Related Traits between Flint and Dent Argentinean Commercial Maize Genotypes. Eur. J. Agron. 2015, 68, 50–56. [Google Scholar] [CrossRef]
- Singh, N.; Singh, S.; Shevkani, K. Chapter 9—Maize: Composition, Bioactive Constituents, and Unleavened Bread. In Flour and Breads and their Fortification in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: San Diego, CA, USA, 2011; pp. 89–99. ISBN 978-0-12-380886-8. [Google Scholar]
- Murariu, M.; Murariu, D.; Has, V.; Has, I.; Leonte, C.; Placinta, D.D.; Risca, I.; Simioniuc, D. Conservarea Si Utilizarea Germoplasmei Locale de Porumb Din Romania; PIM, IASI: Iași, Romania, 2012; ISBN 978-606-13-1011-1. [Google Scholar]
- Murariu, D.; Plăcintă, D.D.; Simioniuc, V. Assessing Genetic Diversity in Romanian Maize Landraces, Using Molecular Markers. Rom. Agric. Res. 2019, 36, 3–9. [Google Scholar] [CrossRef]
- Rouf Shah, T.; Prasad, K.; Kumar, P. Maize, A Potential Source of Human Nutrition and Health: A Review. Cogent Food Agric. 2016, 2, 1166995. [Google Scholar] [CrossRef]
- Suleiman, R.; Williams, D.; Nissen, A.; Bern, C.J.; Rosentrater, K.A. Is Flint Corn Naturally Resistant to Sitophilus Zeamais Infestation? J. Stored Prod. Res. 2015, 60, 19–24. [Google Scholar] [CrossRef]
- Tenaillon, M.I.; Charcosset, A. A European Perspective on Maize History. Comptes Rendus Biol. 2011, 334, 221–228. [Google Scholar] [CrossRef]
- Unterseer, S.; Pophaly, S.D.; Peis, R.; Westermeier, P.; Mayer, M.; Seidel, M.A.; Haberer, G.; Mayer, K.F.X.; Ordas, B.; Pausch, H.; et al. A Comprehensive Study of the Genomic Differentiation between Temperate Dent and Flint Maize. Genome Biol. 2016, 17, 137. [Google Scholar] [CrossRef] [PubMed]
- Turda Weather Station, Northern Transylvania Regional Meteorological Center Cluj. Available online: https://www.meteoromania.ro/ (accessed on 29 June 2025).
- Bhosale, S.U.; Rymen, B.; Beemster, G.T.S.; Melchinger, A.E.; Reif, J.C. Chilling Tolerance of Central European Maize Lines and Their Factorial Crosses. Ann. Bot. 2007, 100, 1315–1321. [Google Scholar] [CrossRef]
- Boughazi, K.; Wuyts, N.; Muller, O.; Windt, C.W.; Nagel, K.A.; Rascher, U.; Fiorani, F. Doubled Haploid Lines Derived from a European Maize Flint Landrace Contrast in Recovery from Cold Stress. Agronomy 2024, 14, 408. [Google Scholar] [CrossRef]
- Peter, R.; Eschholz, T.W.; Stamp, P.; Liedgens, M. Swiss Flint Maize Landraces—A Rich Pool of Variability for Early Vigour in Cool Environments. Field Crops Res. 2009, 110, 157–166. [Google Scholar] [CrossRef]
- Aroca, R.; Irigoyen, J.J.; Sánchez-Díaz, M. Photosynthetic Characteristics and Protective Mechanisms against Oxidative Stress during Chilling and Subsequent Recovery in Two Maize Varieties Differing in Chilling Sensitivity. Plant Sci. 2001, 161, 719–726. [Google Scholar] [CrossRef]
- Maticiuc, V.; Mistreț, S.; Guzun, L. Realizări În Ameliorarea Porumbului Cu Calităţi Speciale Pentru Utilizare. Akademos 2017, 4, 52–58. [Google Scholar]
- KWS Semințe de Porumb—Produse-KWS SAAT SE & Co. KGaA. Available online: https://www.kws.com/ro/ro/produse/porumb/prezentare-generala-a-hibrizilor-de-porumb/ (accessed on 27 August 2025).
- Donau Saat ROMÂNIA. Available online: https://binealegibineculegi.ro/ (accessed on 27 August 2025).
- MAS Seeds România. Available online: https://www.masseeds.ro/cautator-de-produse/?page_seed=3 (accessed on 27 August 2025).
- Corteva Agriscience. Available online: https://www.corteva.ro/cataloage-corteva-agriscience.html (accessed on 27 August 2025).
- Institutul Național de Statistică. Available online: https://insse.ro/cms/ (accessed on 27 August 2025).
- Șuteu, D.; Băcilă, I.; Haș, V.; Haș, I.; Miclăuș, M. Romanian Maize (Zea mays) Inbred Lines as a Source of Genetic Diversity in SE Europe, and Their Potential in Future Breeding Efforts. PLoS ONE 2013, 8, e85501. [Google Scholar] [CrossRef][Green Version]
- Pedological and Soil Chemistry Office Cluj. Available online: https://ospacluj.ro/ (accessed on 2 April 2025).[Green Version]
- Precision Spaced Planters|WINTERSTEIGER. Available online: https://www.wintersteiger.com/seedmech/en/products/sowing-machines-tool-carriers/precision-spaced-planters (accessed on 30 July 2025).[Green Version]
- AZOGrow NPK 27:13,5:0. Available online: https://www.azomures.com/ingrasaminte-complexe-npk/azogrow/azogrow-npk271350 (accessed on 30 July 2025).[Green Version]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Open Agrar: Berlin, Germany, 2018. [Google Scholar] [CrossRef]
- Bekavac, G.; Stojaković, M.; Ivanović, M.; Jocković, Đ.; Vasić, N.; Purar, B.; Boćanski, J.; Nastasić, A. Relationships of Stay Green Trait in Maize. Genetika 2002, 34, 33–40. [Google Scholar] [CrossRef]
- Plot Combines for Harvesting|WINTERSTEIGER. Available online: https://www.wintersteiger.com/seedmech/en/products/harvesters/plot-combines (accessed on 30 July 2025).
- Umidimetru Bod Întreg Granomat® Plus: Rapid și Precis—Produs|PFEUFFER®. Available online: https://www.pfeuffer.com/ro/produs/granomat-plus (accessed on 27 August 2025).
- Zhuang, L.; Wang, C.; Hao, H.; Song, W.; Guo, X. Maize Anthesis-Silking Interval Estimation via Image Detection under Field Rail-Based Phenotyping Platform. Agronomy 2024, 14, 1723. [Google Scholar] [CrossRef]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Chibane, N.; Revilla, P.; Yannam, V.R.R.; Marcet, P.; Covelo, E.F.; Ordás, B. Impact of Irrigation, Nitrogen Fertilization, and Plant Density on Stay-Green and Its Effects on Agronomic Traits in Maize. Front. Plant Sci. 2024, 15, 1399072. [Google Scholar] [CrossRef]
- Varga, A.; Calugar, R.E.; Vana, C.D.; Haș, V.; Ceclan, L.A.; Tritean, N.; Racz, I.; Tărău, A.-D.; Ona, A.D.; Fodor, A. Use of Genetic and Phenotypic Variability of Romanian Local Population Maize. Rom. Agric. Res. 2025, 42, 523–532. [Google Scholar] [CrossRef]
- Chetan, F.; Dulf, E.-H.; Rusu, T.; Chetan, C.; Simon, A. Temperature and Precipitation Forecast for Western Transylvanian Plains Using Artificial Intelligence. AgroLife Sci. J. 2024, 13, 41–53. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Narayanan, S.; Erdayani, E.; Prasad, P.V.V. Effects of High Temperature Stress during Anthesis and Grain Filling Periods on Photosynthesis, Lipids and Grain Yield in Wheat. BMC Plant Biol. 2020, 20, 268. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Wang, K.J.; Jiang, G.M.; Liu, M.Z.; Niu, S.L.; Gao, L.M. Post-Anthesis Changes in Photosynthetic Traits of Maize Hybrids Released in Different Years. Field Crops Res. 2005, 93, 108–115. [Google Scholar] [CrossRef]
- Schema de Ajutor de Stat Pentru Fermierii Afectați de Secetă Pentru Culturi Înființate în Primăvara Anului 2022—Ministerul Agriculturii si Dezvoltarii Rurale. Available online: https://madr.ro/comunicare/8171-schema-de-ajutor-de-stat-pentru-fermierii-afectati-de-seceta-pentru-culturi-infiintate-in-primavara-anului-2022.html (accessed on 28 August 2025).
- Maazou, A.-R.S.; Tu, J.; Qiu, J.; Liu, Z. Breeding for Drought Tolerance in Maize (Zea mays L.). Am. J. Plant Sci. 2016, 7, 1858–1870. [Google Scholar] [CrossRef]
- Gao, Z.-W.; Cui, Y.-H.; Li, X.-N.; Bai, M.-Y.; Asseri, T.A.Y.; Hashem, M.; Wang, Z.-J. Genetic Improvement of Drought Stress Tolerance in Maize, Recent Advancements and Future Research Direction. Not. Bot. Horti Agrobot. Cluj-Napoca 2024, 52, 13840. [Google Scholar] [CrossRef]
- Asif, Z.; Jabeen, A.; Ifran, M.; Khatoon, A.; Bashir, A.; Malik, K.A. Stress-Inducible IPT Gene Expression Boosts Antioxidant Defense and Grain Production in Heat-Stressed Maize. Pak. J. Agric. Sci. 2025, 62, 359–375. [Google Scholar] [CrossRef]
- Sattar, S.; Aslam, M.; Ahmad, R.M. Adaptability Magnitude of Double Haploid Lines in Maize for High Temperature Stress. Pak. J. Agric. Sci. 2024, 61, 233–242. [Google Scholar]
- Sa, K.J.; Park, H.; Fu, Z.; Jang, S.J.; Lee, J.-K. Association Study for Drought Tolerance of Flint Maize Inbred Lines Using SSR Markers. Plant Breed. Biotechnol. 2022, 10, 257–271. [Google Scholar] [CrossRef]
- Bolaños, J.; Edmeades, G.O. The Importance of the Anthesis-Silking Interval in Breeding for Drought Tolerance in Tropical Maize. Field Crops Res. 1996, 48, 65–80. [Google Scholar] [CrossRef]
- Valentinuz, O.R.; Tollenaar, M. Vertical Profile of Leaf Senescence during the Grain-Filling Period in Older and Newer Maize Hybrids. Crop Sci. 2004, 44, 827. [Google Scholar] [CrossRef]
- Ding, L.; Wang, K.J.; Jiang, G.M.; Biswas, D.K.; Xu, H.; Li, L.F.; Li, Y.H. Effects of Nitrogen Deficiency on Photosynthetic Traits of Maize Hybrids Released in Different Years. Ann. Bot. 2005, 96, 925–930. [Google Scholar] [CrossRef]
- Chibane, N.; Caicedo, M.; Martinez, S.; Marcet, P.; Revilla, P.; Ordás, B. Relationship between Delayed Leaf Senescence (Stay-Green) and Agronomic and Physiological Characters in Maize (Zea mays L.). Agronomy 2021, 11, 276. [Google Scholar] [CrossRef]

| Inbred Line | Germplasm Group | Kernel Type | Kernel Color | Cob Color | Ear Average Length | No Kernel Rows | No Kernels/Row |
|---|---|---|---|---|---|---|---|
| Maternal inbred lines | |||||||
| E292 | Iodent + Mo17 | Dent | Dark yellow | Red | 16 | 12–14 | 24 |
| C344 | Iodent | Semi-dent | Dark yellow | Red | 18 | 16–18 | 24 |
| D394 | Iodent | Semi-dent | Dark yellow | Red | 13 | 18–20 | 27 |
| E347 | Iodent | Semi-dent | Dark yellow | White | 16 | 16–18 | 29 |
| E348 | Iodent | Semi-dent | Dark yellow | White | 13 | 16–18 | 21 |
| E359 | Iodent | Semi-dent | Dark yellow | Red | 13 | 14–16 | 22 |
| E372 | Mo17 + C103 | Dent | Dark yellow | Red | 15 | 18–20 | 31 |
| E376 | Iodent | Dent | Dark yellow | Red | 20 | 18–20 | 40 |
| E378 | Iodent | Dent | Dark yellow | Red | 18 | 14–16 | 35 |
| E385 | B73 | Dent | Yellow | Pink | 13 | 18–20 | 22 |
| A447 | Iodent + Oh43 | Dent | Dark yellow | Red | 13 | 16–18 | 25 |
| A478 | Iodent | Semi-dent | Dark yellow | Pink | 12 | 16–18 | 27 |
| A480 | Iodent | Semi-dent | Dark yellow | White | 12 | 18–20 | 30 |
| A483 | Iodent + B73 | Dent | Dark yellow | Pink | 20 | 16–18 | 35 |
| Paternal inbred lines | |||||||
| D298 | Mo17 + C103 | Flint | Dark yellow | Dark red | 18 | 14–16 | 28 |
| B330 | W153 + D105 | Flint | Orange | White | 16 | 12–14 | 17 |
| C336 | W153 + Lo3 | Flint | Yellow | White | 16 | 12–14 | 26 |
| D328 | Mo17 + C103 | Flint | Dark yellow | Red | 13 | 14–16 | 24 |
| Source of Variability | DF | Yield | DM | UP |
|---|---|---|---|---|
| Year (Y) | 1 | 3795 ** | 1421 ** | 364.6 ** |
| Hybrid (H) | 55 | 17.9 ** | 18.3 ** | 1377 ** |
| Maternal genotype (M) | 13 | 31.1 ** | 94.3 ** | 1396 ** |
| Paternal genotype (P) | 3 | 135.4 ** | 37.7 ** | 1319 ** |
| Y × H | 55 | 0.3 | 3.2 ** | 0.1 |
| Y × M | 13 | 0.4 | 1.1 | 0.2 |
| Y × P | 3 | 0.4 | 2.3 ** | 0.2 |
| No. | Maternal Inbred Line | SCA Effects | Maternal Inbred Line GCA | |||
|---|---|---|---|---|---|---|
| Paternal Inbred Line | ||||||
| D298 | B330 | C336 | D328 | |||
| 1 | E292 | 679 | 416 | 651 | 243 | 497 gh |
| 2 | C344 | 45 | −142 | −1019 | −296 | −353 bcd |
| 3 | D394 | −457 | −131 | −818 | 1122 | −71 bc |
| 4 | E347 | 288 | 380 | −764 | −256 | −88 de |
| 5 | E348 | 62 | −3 | −2323 | 86 | −545 bc |
| 6 | E359 | 502 | 538 | −1571 | −425 | −239 cde |
| 7 | E372 | −292 | −658 | −1263 | −199 | −603 b |
| 8 | E376 | 210 | −284 | −123 | 158 | −10 ef |
| 9 | E378 | −944 | −527 | −1129 | −2492 | −1273 a |
| 10 | E385 | 465 | −252 | −1040 | 1166 | 85 ef |
| 11 | A447 | 533 | 854 | −96 | 416 | 427 gh |
| 12 | A478 | 1372 | 1251 | −123 | 2107 | 1152 i |
| 13 | A480 | 955 | 1646 | −1243 | 1525 | 721 h |
| 14 | A483 | 428 | 1164 | −870 | 456 | 295 fg |
| Paternal inbred line GCA | 275 b | 304 b | −838 a | 258 b | ||
| Hybrid | Yield 2021 | Yield 2022 | Average Yield | Rank 2021 | Rank 2022 | Average Rank | |
|---|---|---|---|---|---|---|---|
| E292 × D298 | 11,066 ± 20 | 6415 ± 306 | 8740 | 12 | 11 | 11 | |
| C344 × D298 | 10,565 ± 326 | 5648 ± 346 | 8106 | 25 | 29 | 28 | |
| D394 × D298 | 9963 ± 463 | 5244 ± 275 | 7604 | 43 | 42 | 42 | |
| E347 × D298 | 10,708 ± 364 | 5991 ± 167 | 8350 | 22 | 23 | 22 | |
| E348 × D298 | 10,482 ± 81 | 5765 ± 126 | 8123 | 28 | 27 | 27 | |
| E359 × D298 | 10,855 ± 354 | 6271 ± 141 | 8563 | 18 | 13 | 15 | |
| E372 × D298 | 10,128 ± 241 | 5411 ± 163 | 7770 | 40 | 38 | 39 | |
| E376 × D298 | 10,630 ± 275 | 5913 ± 280 | 8272 | 23 | 24 | 24 | |
| E378 × D298 | 9476 ± 140 | 4759 ± 245 | 7118 | * | 49 | 47 | 48 |
| E385 × D298 | 11,019 ± 487 | 6034 ± 196 | 8526 | 13 | 22 | 16 | |
| A447 × D298 | 10,921 ± 217 | 6268 ± 192 | 8594 | 15 | 14 | 14 | |
| A478 × D298 | 11,725 ± 318 | 7142 ± 273 | 9434 | ** | 5 | 3 | 4 |
| A480 × D298 | 11,375 ± 122 | 6658 ± 93 | 9016 | * | 9 | 9 | 9 |
| A483 × D298 | 10,885 ± 540 | 6094 ± 475 | 8490 | 16 | 19 | 18 | |
| E292 × B330 | 10,836 ± 218 | 6118 ± 318 | 8477 | 20 | 18 | 20 | |
| C344 × B330 | 10,278 ± 337 | 5560 ± 303 | 7919 | 35 | 33 | 34 | |
| D394 × B330 | 10,389 ± 143 | 5472 ± 30 | 7931 | 30 | 35 | 33 | |
| E347 × B330 | 10,800 ± 151 | 6081 ± 431 | 8441 | 21 | 20 | 21 | |
| E348 × B330 | 10,417 ± 422 | 5700 ± 258 | 8058 | 29 | 28 | 29 | |
| E359 × B330 | 10,958 ± 289 | 6240 ± 433 | 8599 | 14 | 15 | 13 | |
| E372 × B330 | 10,193 ± 310 | 4614 ± 304 | 7404 | 37 | 49 | 44 | |
| E376 × B330 | 10,136 ± 430 | 5418 ± 101 | 7777 | 39 | 37 | 38 | |
| E378 × B330 | 9893 ± 183 | 5175 ± 498 | 7534 | 44 | 43 | 43 | |
| E385 × B330 | 10,334 ± 305 | 5284 ± 127 | 7809 | 31 | 40 | 36 | |
| A447 × B330 | 11,274 ± 43 | 6557 ± 488 | 8916 | 10 | 10 | 10 | |
| A478 × B330 | 11,738 ± 380 | 6886 ± 63 | 9312 | ** | 4 | 5 | 5 |
| A480 × B330 | 12,300 ± 354 | 7115 ± 77 | 9707 | *** | 2 | 4 | 2 |
| A483 × B330 | 11,584 ± 397 | 6866 ± 240 | 9225 | * | 7 | 6 | 7 |
| E292 × C336 | 11,071 ± 267 | 6353 ± 304 | 8712 | 11 | 12 | 12 | |
| C344 × C336 | 9400 ± 262 | 4684 ± 518 | 7042 | * | 50 | 48 | 49 |
| D394 × C336 | 9602 ± 422 | 4885 ± 450 | 7243 | 46 | 45 | 46 | |
| E347 × C336 | 9656 ± 293 | 4937 ± 342 | 7297 | 45 | 44 | 45 | |
| E348 × C336 | 8197 ± 124 | 3279 ± 386 | 5738 | *** | 55 | 55 | 55 |
| E359 × C336 | 8683 ± 248 | 4298 ± 113 | 6490 | *** | 54 | 53 | 54 |
| E372 × C336 | 9157 ± 390 | 4439 ± 481 | 6798 | ** | 53 | 52 | 53 |
| E376 × C336 | 10,297 ± 514 | 5580 ± 305 | 7938 | 34 | 31 | 31 | |
| E378 × C336 | 9291 ± 170 | 4573 ± 233 | 6932 | ** | 52 | 50 | 51 |
| E385 × C336 | 9514 ± 346 | 4528 ± 134 | 7021 | * | 48 | 51 | 50 |
| A447 × C336 | 10,324 ± 188 | 5606 ± 281 | 7965 | 32 | 30 | 30 | |
| A478 × C336 | 10,297 ± 291 | 5579 ± 361 | 7938 | 33 | 32 | 32 | |
| A480 × C336 | 9344 ± 234 | 4293 ± 131 | 6819 | ** | 51 | 54 | 52 |
| A483 × C336 | 9550 ± 99 | 4832 ± 140 | 7191 | 47 | 46 | 47 | |
| E292 × D328 | 10,563 ± 187 | 6045 ± 97 | 8304 | 26 | 21 | 23 | |
| C344 × D328 | 10,124 ± 81 | 5406 ± 181 | 7765 | 41 | 39 | 40 | |
| D394 × D328 | 11,542 ± 471 | 6825 ± 416 | 9184 | * | 8 | 8 | 8 |
| E347 × D328 | 10,164 ± 211 | 5446 ± 419 | 7805 | 38 | 36 | 37 | |
| E348 × D328 | 10,506 ± 240 | 5789 ± 159 | 8148 | 27 | 26 | 26 | |
| E359 × D328 | 9995 ± 185 | 5277 ± 29 | 7636 | 42 | 41 | 41 | |
| E372 × D328 | 10,221 ± 269 | 5504 ± 272 | 7862 | 36 | 34 | 35 | |
| E376 × D328 | 10,578 ± 268 | 5861 ± 102 | 8220 | 24 | 25 | 25 | |
| E378 × D328 | 7928 ± 56 | 3211 ± 380 | 5569 | *** | 56 | 56 | 56 |
| E385 × D328 | 11,616 ± 93 | 6838 ± 210 | 9227 | * | 6 | 7 | 6 |
| A447 × D328 | 10,836 ± 228 | 6118 ± 411 | 8477 | 19 | 17 | 19 | |
| A478 × D328 | 13,161 ± 213 | 7176 ± 107 | 10,168 | *** | 1 | 2 | 1 |
| A480 × D328 | 11,945 ± 327 | 7228 ± 397 | 9586 | ** | 3 | 1 | 3 |
| A483 × D328 | 10,876 ± 185 | 6158 ± 321 | 8517 | 17 | 16 | 17 | |
| CT1 Turda 332 | 10,716 ± 457 | 8315 ± 376 | 9516 | ||||
| CT2 Turda 335 | 11,060 ± 346 | 8172 ± 241 | 9616 | ||||
| Hybrid | DM 2021 | DM 2022 | Average DM | DM Rank | UP 2021 | UP 2022 | Average UP | UP Rank | ||
|---|---|---|---|---|---|---|---|---|---|---|
| E292 × D298 | 74.0 ± 0.3 | 76.1 ± 0.1 | 75.1 | *** | 55 | 97.8 ± 1.3 | 96.8 ± 0.8 | 97.3 | 19 | |
| C344 × D298 | 77.0 ± 0.2 | 80.0 ± 0.4 | 78.5 | 29 | 99.1 ± 0.0 | 98.2 ± 1.8 | 98.7 | 6 | ||
| D394 × D298 | 77.8 ± 1.1 | 80.4 ± 0.3 | 79.1 | 24 | 98.4 ± 0.7 | 97.5 ± 1.6 | 97.9 | 16 | ||
| E347 × D298 | 78.7 ± 0.6 | 81.8 ± 0.3 | 80.2 | 18 | 97.3 ± 0.0 | 96.4 ± 0.8 | 96.9 | 24 | ||
| E348 × D298 | 79.2 ± 0.5 | 82.0 ± 0.3 | 80.6 | * | 15 | 99.2 ± 0.0 | 98.3 ± 1.7 | 98.8 | 4 | |
| E359 × D298 | 79.9 ± 0.7 | 82.6 ± 0.1 | 81.3 | ** | 7 | 99.1 ± 0.0 | 98.2 ± 1.3 | 98.7 | 7 | |
| E372 × D298 | 79.4 ± 0.2 | 82.1 ± 0.3 | 80.7 | * | 10 | 98.5 ± 0.6 | 97.7 ± 1.5 | 98.1 | 12 | |
| E376 × D298 | 79.2 ± 0.3 | 81.9 ± 0.5 | 80.6 | * | 15 | 97.1 ± 1.3 | 96.3 ± 1.3 | 96.7 | 25 | |
| E378 × D298 | 77.5 ± 0.6 | 80.6 ± 0.4 | 79.0 | 25 | 92.0 ± 0.7 | 91.1 ± 2.8 | 91.6 | 50 | ||
| E385 × D298 | 79.0 ± 0.6 | 82.5 ± 0.3 | 80.7 | * | 12 | 96.3 ± 0.7 | 95.3 ± 0.6 | 95.8 | 33 | |
| A447 × D298 | 75.6 ± 0.6 | 78.5 ± 0.3 | 77.1 | * | 45 | 99.2 ± 0.0 | 98.5 ± 1.5 | 98.9 | 1 | |
| A478 × D298 | 76.6 ± 0.5 | 79.4 ± 0.3 | 78.0 | 33 | 98.4 ± 0.7 | 97.5 ± 1.3 | 97.9 | 15 | ||
| A480 × D298 | 74.5 ± 0.6 | 80.2 ± 0.2 | 77.4 | 40 | 100.0 ± 0.0 | 97.5 ± 1.6 | 98.7 | 5 | ||
| A483 × D298 | 79.3 ± 0.4 | 82.3 ± 0.3 | 80.8 | * | 9 | 96.4 ± 0.7 | 95.6 ± 1.0 | 96.0 | 31 | |
| E292 × B330 | 76.4 ± 0.3 | 79.4 ± 0.3 | 77.9 | 36 | 87.7 ± 3.8 | 86.7 ± 2.3 | 87.2 | ** | 54 | |
| C344 × B330 | 75.8 ± 0.3 | 78.8 ± 0.4 | 77.3 | 42 | 96.9 ± 1.4 | 96.0 ± 1.9 | 96.4 | 28 | ||
| D394 × B330 | 78.0 ± 0.6 | 81.0 ± 0.4 | 79.5 | 21 | 85.5 ± 2.7 | 84.6 ± 1.8 | 85.0 | *** | 55 | |
| E347 × B330 | 78.2 ± 0.8 | 81.1 ± 0.6 | 79.6 | 20 | 96.8 ± 1.3 | 94.4 ± 3.2 | 95.6 | 35 | ||
| E348 × B330 | 76.7 ± 0.7 | 79.7 ± 0.3 | 78.2 | 32 | 95.0 ± 3.2 | 94.2 ± 0.8 | 94.6 | 39 | ||
| E359 × B330 | 77.8 ± 0.2 | 80.8 ± 0.6 | 79.3 | 23 | 99.1 ± 0.0 | 98.2 ± 1.4 | 98.7 | 7 | ||
| E372 × B330 | 76.2 ± 0.1 | 79.4 ± 0.3 | 77.8 | 38 | 89.5 ± 3.1 | 88.6 ± 2.2 | 89.0 | * | 53 | |
| E376 × B330 | 81.4 ± 0.6 | 84.1 ± 0.2 | 82.8 | *** | 2 | 83.8 ± 2.3 | 80.7 ± 1.0 | 82.3 | *** | 56 |
| E378 × B330 | 75.1 ± 0.4 | 78.1 ± 0.4 | 76.6 | ** | 48 | 92.0 ± 0.6 | 91.3 ± 1.7 | 91.6 | 48 | |
| E385 × B330 | 80.1 ± 0.0 | 82.7 ± 0.1 | 81.4 | ** | 6 | 95.1 ± 2.6 | 92.4 ± 1.4 | 93.8 | 41 | |
| A447 × B330 | 75.8 ± 0.5 | 78.4 ± 0.2 | 77.1 | * | 44 | 93.1 ± 0.4 | 90.4 ± 1.5 | 91.8 | 47 | |
| A478 × B330 | 74.9 ± 0.2 | 80.2 ± 0.6 | 77.6 | 39 | 92.3 ± 0.6 | 91.3 ± 0.7 | 91.8 | 46 | ||
| A480 × B330 | 73.9 ± 0.4 | 75.0 ± 0.2 | 74.4 | *** | 56 | 96.6 ± 1.3 | 94.1 ± 2.0 | 95.3 | 36 | |
| A483 × B330 | 75.1 ± 0.5 | 77.7 ± 0.6 | 76.4 | ** | 51 | 97.1 ± 1.8 | 94.4 ± 3.6 | 95.8 | 33 | |
| E292 × C336 | 77.6 ± 0.6 | 83.0 ± 0.3 | 80.3 | * | 17 | 96.4 ± 0.6 | 95.6 ± 1.7 | 96.0 | 29 | |
| C344 × C336 | 75.3 ± 0.7 | 78.3 ± 0.1 | 76.8 | ** | 46 | 97.9 ± 1.3 | 97.0 ± 0.7 | 97.4 | 17 | |
| D394 × C336 | 79.2 ± 0.9 | 82.3 ± 0.4 | 80.7 | * | 10 | 95.5 ± 1.2 | 94.6 ± 1.5 | 95.0 | 38 | |
| E347 × C336 | 79.1 ± 0.4 | 82.1 ± 0.4 | 80.6 | * | 13 | 98.5 ± 0.6 | 97.6 ± 1.6 | 98.0 | 14 | |
| E348 × C336 | 75.9 ± 0.2 | 78.8 ± 0.4 | 77.3 | * | 41 | 96.3 ± 1.4 | 95.4 ± 1.3 | 95.8 | 32 | |
| E359 × C336 | 76.8 ± 0.2 | 80.0 ± 0.3 | 78.4 | 30 | 100.0 ± 0.0 | 97.2 ± 1.4 | 98.6 | 9 | ||
| E372 × C336 | 76.5 ± 0.3 | 80.0 ± 0.3 | 78.3 | 31 | 94.7 ± 1.7 | 93.9 ± 3.1 | 94.3 | 40 | ||
| E376 × C336 | 82.2 ± 0.2 | 84.9 ± 0.3 | 83.6 | *** | 1 | 92.2 ± 0.7 | 91.4 ± 1.3 | 91.8 | 45 | |
| E378 × C336 | 78.0 ± 1.4 | 80.6 ± 0.4 | 79.3 | 22 | 98.5 ± 0.7 | 97.7 ± 1.4 | 98.1 | 11 | ||
| E385 × C336 | 80.6 ± 0.7 | 83.4 ± 0.2 | 82.0 | *** | 3 | 94.1 ± 3.4 | 93.3 ± 0.5 | 93.7 | 42 | |
| A447 × C336 | 76.5 ± 0.6 | 79.1 ± 0.2 | 77.8 | 37 | 99.2 ± 0.0 | 98.4 ± 0.9 | 98.8 | 2 | ||
| A478 × C336 | 75.9 ± 1.0 | 76.7 ± 0.7 | 76.3 | *** | 52 | 97.7 ± 1.4 | 96.8 ± 0.9 | 97.2 | 22 | |
| A480 × C336 | 73.9 ± 1.0 | 77.3 ± 0.4 | 75.6 | *** | 53 | 96.4 ± 0.7 | 95.6 ± 1.8 | 96.0 | 30 | |
| A483 × C336 | 77.2 ± 0.2 | 79.9 ± 0.4 | 78.5 | 28 | 98.5 ± 0.6 | 97.7 ± 0.2 | 98.1 | 12 | ||
| E292 × D328 | 74.9 ± 0.6 | 78.4 ± 0.3 | 76.7 | * | 47 | 90.8 ± 1.3 | 90.0 ± 1.8 | 90.4 | * | 52 |
| C344 × D328 | 79.1 ± 0.4 | 82.1 ± 0.1 | 80.6 | 14 | 92.6 ± 3.6 | 91.7 ± 1.4 | 92.1 | 44 | ||
| D394 × D328 | 80.1 ± 0.5 | 83.1 ± 0.5 | 81.6 | ** | 4 | 92.0 ± 0.5 | 91.2 ± 1.5 | 91.6 | 49 | |
| E347 × D328 | 77.2 ± 0.3 | 80.6 ± 1.1 | 78.9 | 26 | 97.7 ± 0.7 | 96.8 ± 1.3 | 97.2 | 21 | ||
| E348 × D328 | 76.7 ± 0.4 | 79.3 ± 0.2 | 78.0 | 33 | 97.2 ± 0.7 | 95.8 ± 2.2 | 96.5 | 26 | ||
| E359 × D328 | 78.5 ± 0.4 | 81.2 ± 0.2 | 79.9 | 19 | 97.8 ± 0.6 | 96.9 ± 2.4 | 97.4 | 18 | ||
| E372 × D328 | 79.8 ± 0.5 | 82.5 ± 0.3 | 81.1 | ** | 8 | 94.0 ± 2.2 | 91.6 ± 1.0 | 92.8 | 43 | |
| E376 × D328 | 80.0 ± 0.0 | 83.1 ± 0.2 | 81.5 | ** | 5 | 92.2 ± 0.7 | 90.7 ± 2.0 | 91.5 | 51 | |
| E378 × D328 | 74.2 ± 0.8 | 76.9 ± 0.5 | 75.6 | *** | 54 | 98.3 ± 1.7 | 95.8 ± 1.1 | 97.0 | 23 | |
| E385 × D328 | 76.9 ± 0.1 | 80.9 ± 0.4 | 78.9 | 27 | 96.8 ± 0.0 | 93.6 ± 1.3 | 95.2 | 37 | ||
| A447 × D328 | 76.6 ± 0.5 | 79.4 ± 0.3 | 78.0 | 35 | 99.5 ± 0.7 | 96.8 ± 2.2 | 98.1 | 10 | ||
| A478 × D328 | 76.1 ± 0.2 | 78.5 ± 0.2 | 77.3 | 42 | 96.9 ± 2.3 | 96.0 ± 2.5 | 96.4 | 27 | ||
| A480 × D328 | 73.6 ± 0.3 | 79.6 ± 0.3 | 76.6 | * | 49 | 97.7 ± 0.7 | 96.8 ± 1.7 | 97.3 | 20 | |
| A483 × D328 | 74.9 ± 0.1 | 78.3 ± 0.1 | 76.6 | * | 50 | 99.2 ± 0.0 | 98.4 ± 0.9 | 98.8 | 3 | |
| CT1 Turda 332 | 78.0 ± 0.8 | 81.8 ± 0.5 | 79.9 | 97.1 ± 1.8 | 97.0 ± 1.5 | |||||
| CT2 Turda 335 | 78.9 ± 0.7 | 81.4 ± 0.7 | 80.2 | 100 ± 0.0 | 97.7 ± 1.2 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Călugăr, R.E.; Varga, A.; Vana, C.D.; Ceclan, L.A.; Chețan, F.; Fodor, A.; Tritean, N. Using Flint Maize for Developing New Hybrids: A Case Study in Romania. Agronomy 2025, 15, 2215. https://doi.org/10.3390/agronomy15092215
Călugăr RE, Varga A, Vana CD, Ceclan LA, Chețan F, Fodor A, Tritean N. Using Flint Maize for Developing New Hybrids: A Case Study in Romania. Agronomy. 2025; 15(9):2215. https://doi.org/10.3390/agronomy15092215
Chicago/Turabian StyleCălugăr, Roxana Elena, Andrei Varga, Carmen Daniela Vana, Loredana Ancuța Ceclan, Felicia Chețan, Andras Fodor, and Nicolae Tritean. 2025. "Using Flint Maize for Developing New Hybrids: A Case Study in Romania" Agronomy 15, no. 9: 2215. https://doi.org/10.3390/agronomy15092215
APA StyleCălugăr, R. E., Varga, A., Vana, C. D., Ceclan, L. A., Chețan, F., Fodor, A., & Tritean, N. (2025). Using Flint Maize for Developing New Hybrids: A Case Study in Romania. Agronomy, 15(9), 2215. https://doi.org/10.3390/agronomy15092215








