Compound Sodium Nitrophenolate (CSN) Improves Photo-Synthesis and Forage Quality in Hemarthria compressa
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Exogenous CSN Treatment
2.3. Measurement of Morphological Traits and Biomass Accumulation
2.4. Determination of Photosynthetic Pigments, Fv/Fm, and Key Carbon Fixation Enzyme Activities
2.5. Determination of Crude Protein, Moisture, Fiber Components, and Ash Content in H. compressa
2.6. Transcriptome Analysis
2.7. Quantitative Real-Time PCR (qRT-PCR) Analysis
3. Results
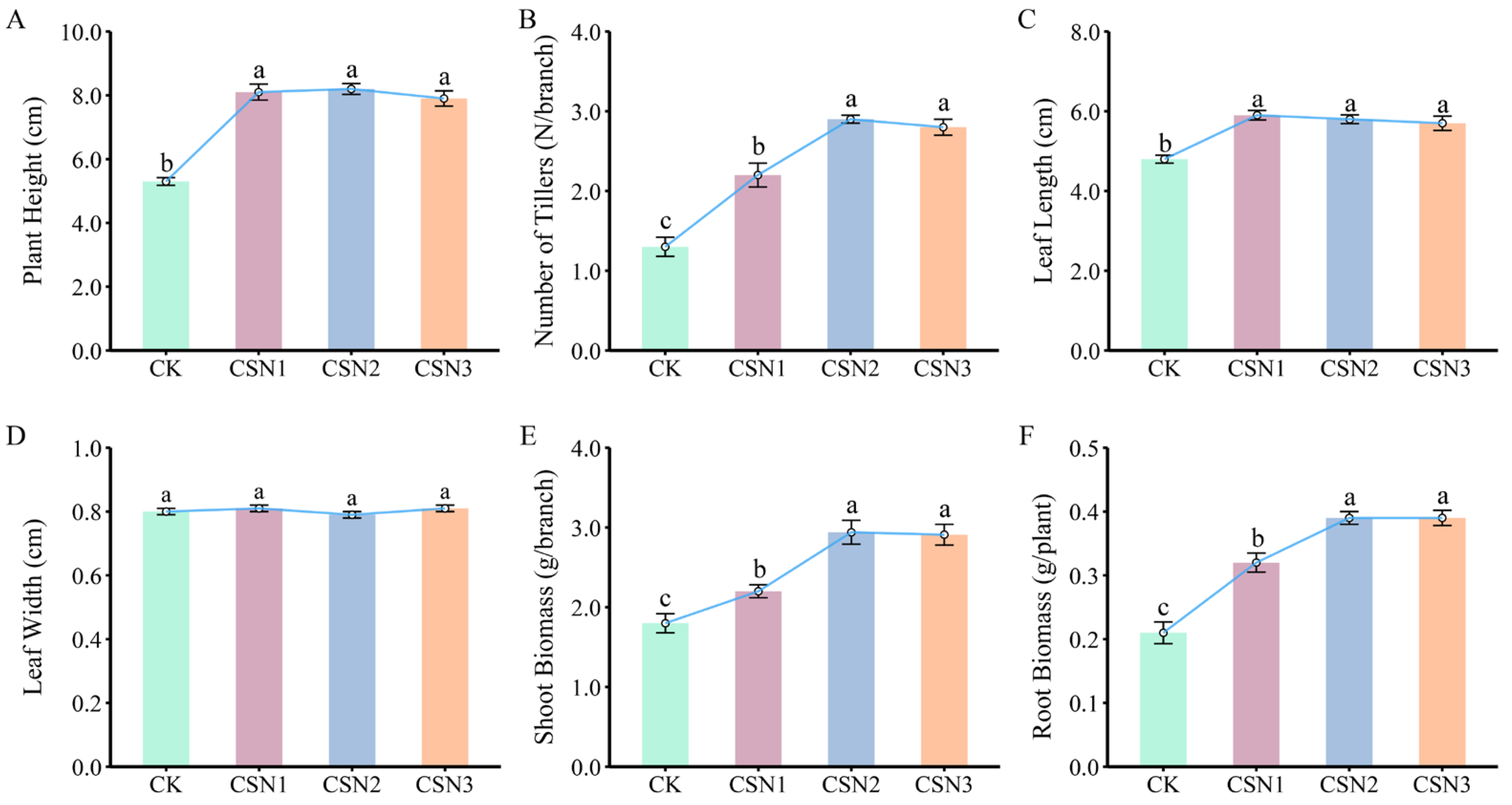
3.1. Effects of Compound Sodium Nitrophenolate (CSN) Concentrations on Morphological Traits of H. compressa
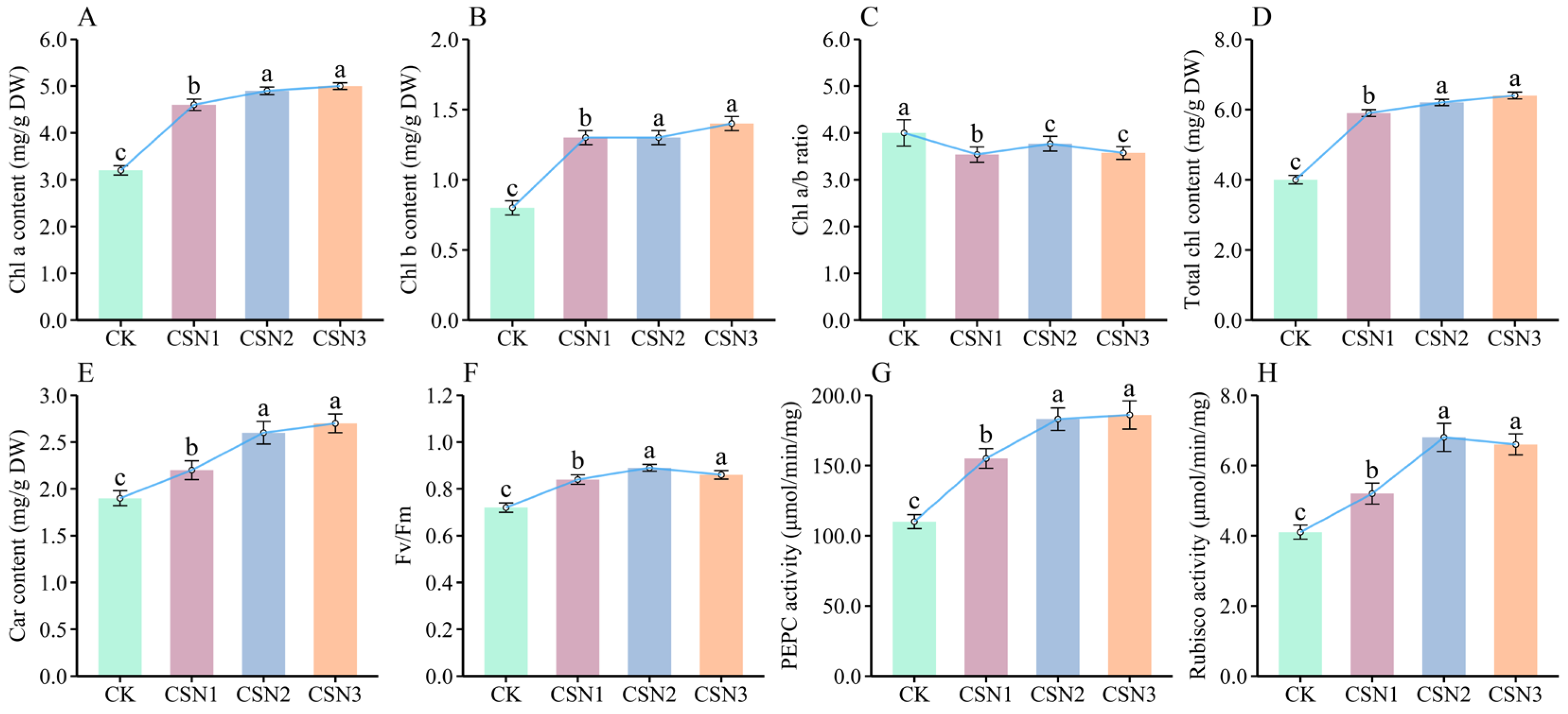
3.2. Effects of Different CSN Concentrations on Photosynthesis in H. compressa
3.3. CSN Modulates Nutritional and Fiber Profiles in H. compressa
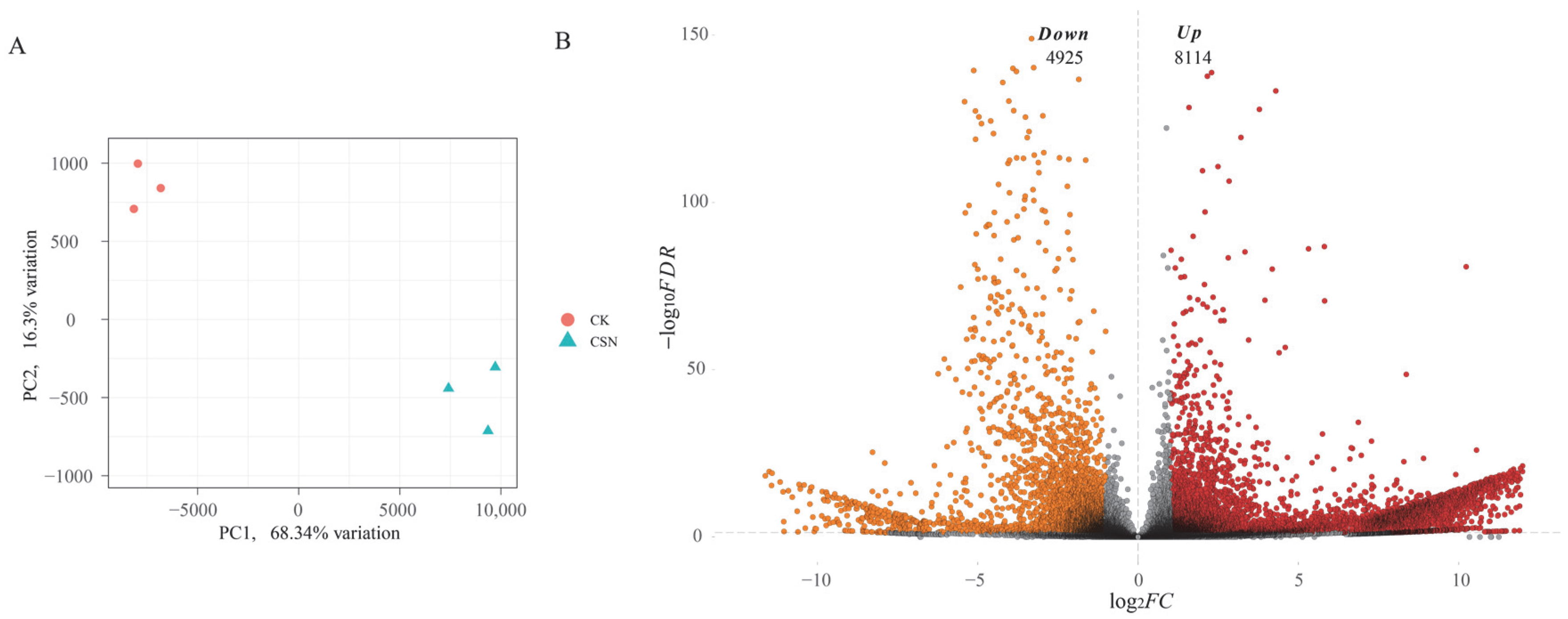
3.4. Transcriptomic Alterations in H. compressa in Response to Compound Sodium Nitrophenolate (CSN) Treatment
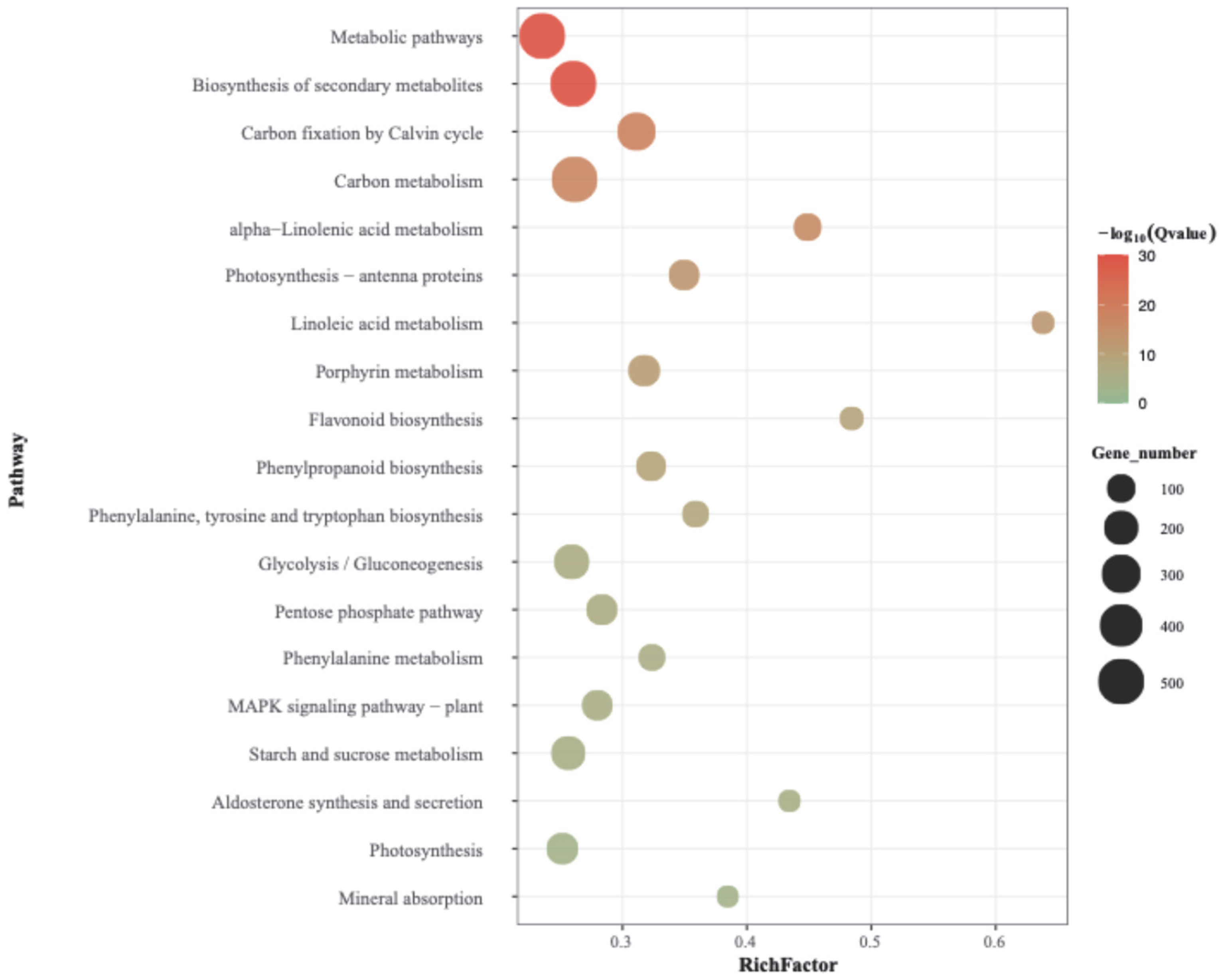
3.5. KEGG Pathway Enrichment Analysis of Differentially Expressed Genes (DEGs)
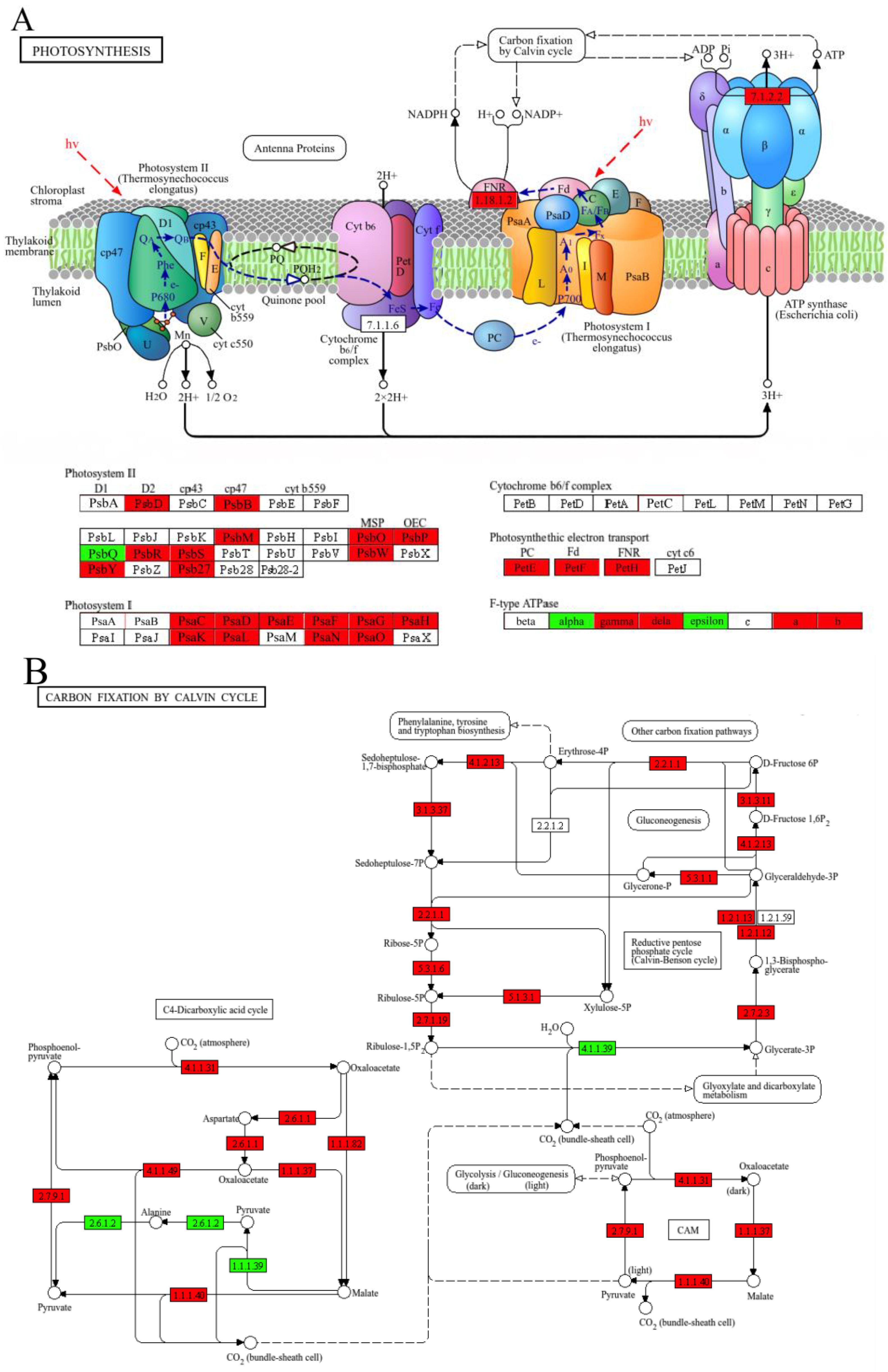
3.6. CSN Treatment Enhances Photosynthetic Light Reactions and Carbon Fixation Pathways in H. compressa
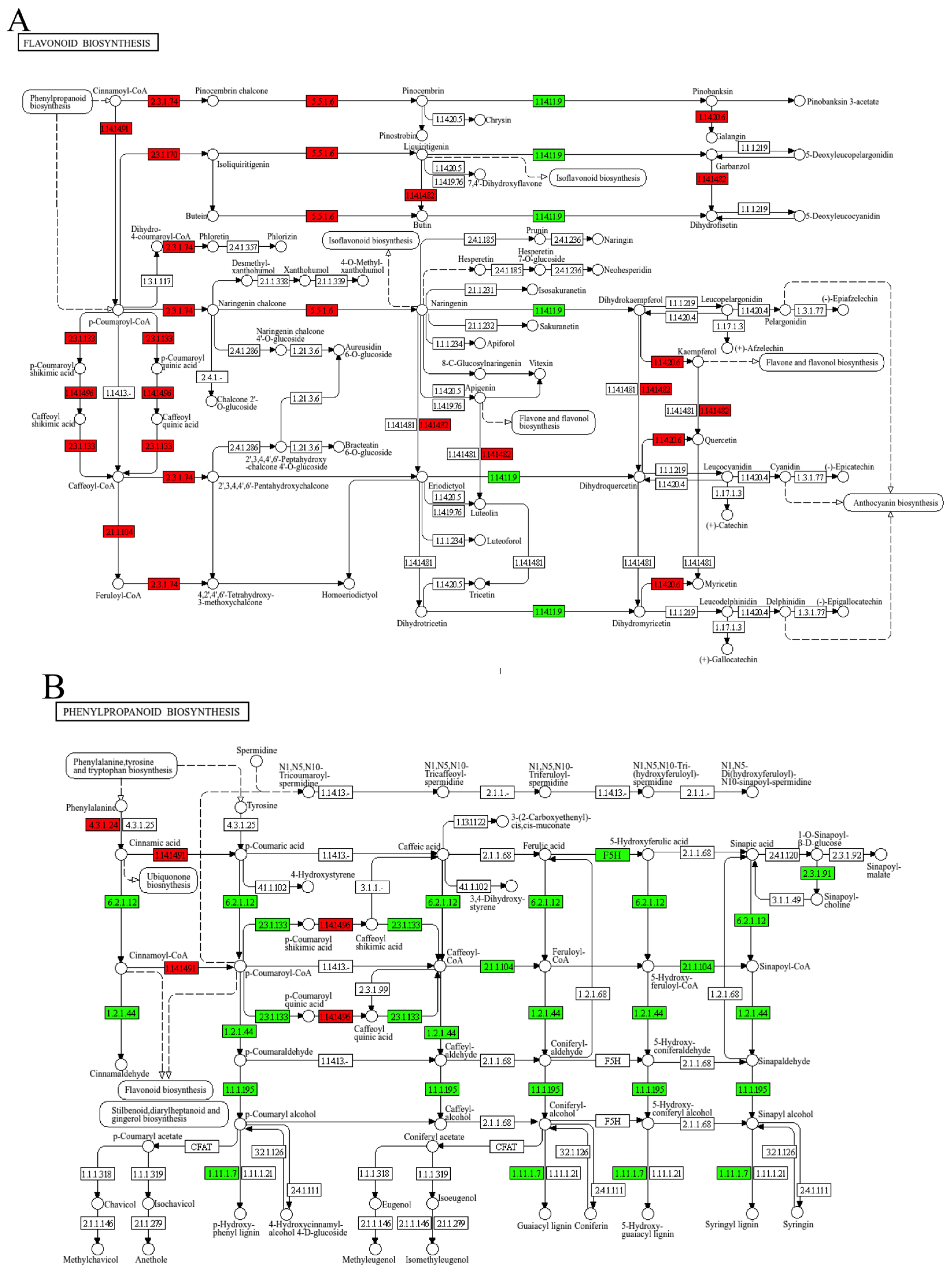
3.7. CSN Regulates the Expression of Flavonoid and Phenylpropanoid Biosynthesis Pathway Genes in H. compressa
3.8. qRT-PCR Validation of Pathway-Representative DEGs
4. Discussion
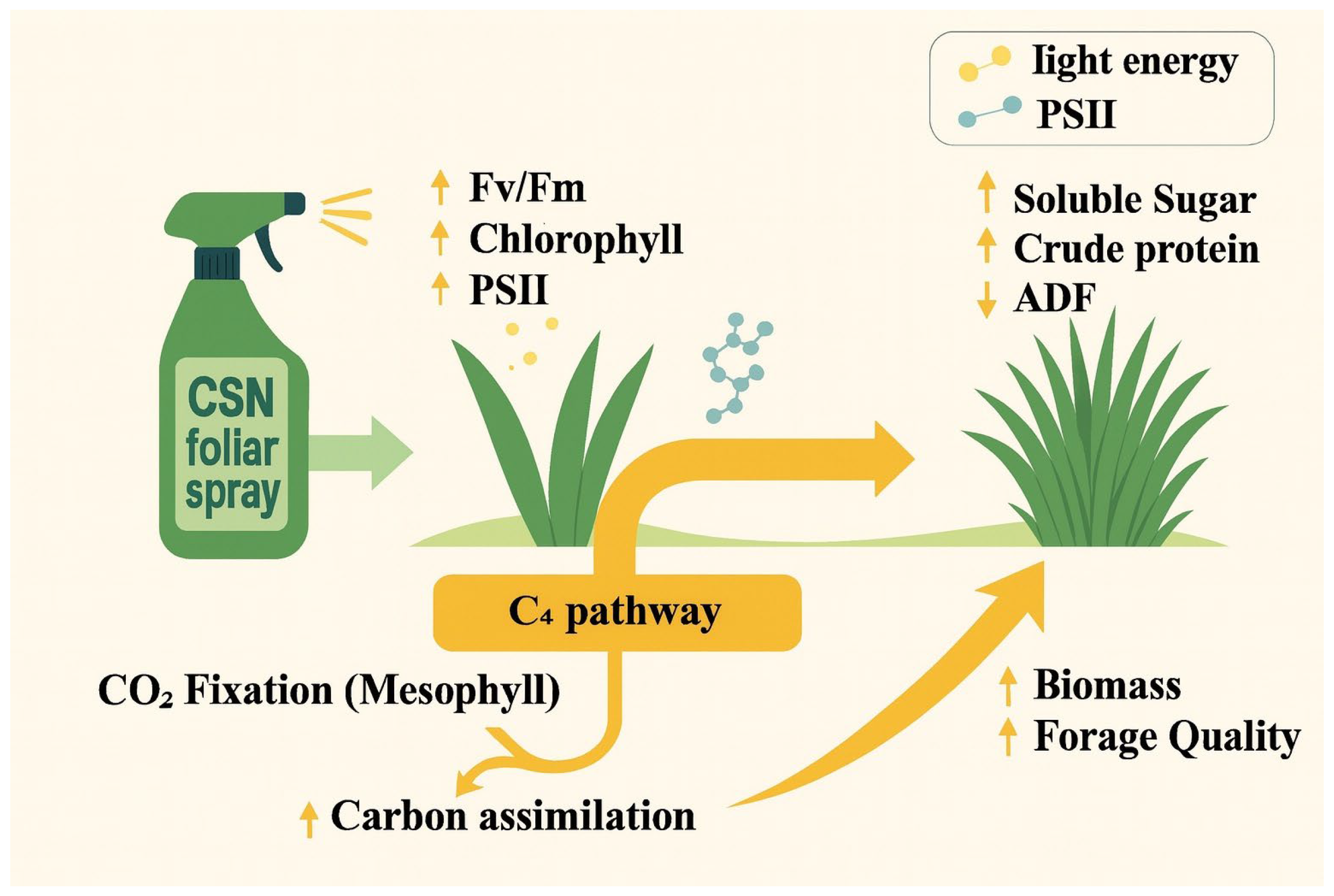
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yan, Y.-H.; Li, J.-L.; Zhang, X.-Q.; Yang, W.-Y.; Wan, Y.; Ma, Y.-M.; Zhu, Y.-Q.; Peng, Y.; Huang, L.-K. Effect of naphthalene acetic acid on adventitious root development and associated physiological changes in stem cutting of Hemarthria compressa. PLoS ONE 2014, 9, e90700. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, X.; Huang, D.-j.; Zhang, Y.; Huang, L.-k.; Lu, L.; Yan, H.-d. Studies on genetic diversity and phylogenetic relationships of limpograss (Hemarthria altissima) and related species based on combined chloroplast DNA intergenic spacer data. Biochem. Syst. Ecol. 2016, 69, 91–100. [Google Scholar] [CrossRef]
- Langdale, J.A. C4 cycles: Past, present, and future research on C4 photosynthesis. Plant Cell 2011, 23, 3879–3892. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T. The grass leaf developmental gradient as a platform for a systems understanding of the anatomical specialization of C4 leaves. J. Exp. Bot. 2011, 62, 3039–3048. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, L.; Yan, H.; Zhang, X.; Xu, B.; Ma, X. Cloning and characterization of an ABA-independent DREB transcription factor gene, HcDREB2, in Hemarthria compressa. Hereditas 2016, 153, 3. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, X.; Xie, W.; Zhang, J.; Cheng, L.; Yan, H. Molecular diversity and population structure of the forage grass Hemarthria compressa (Poaceae) in south China based on SRAP markers. Genet. Mol. Res. 2012, 11, 2441–2450. [Google Scholar] [CrossRef]
- Huang, X.; Yan, H.-D.; Zhang, X.-Q.; Zhang, J.; Frazier, T.P.; Huang, D.-J.; Lu, L.; Huang, L.-K.; Liu, W.; Peng, Y. De novo transcriptome analysis and molecular marker development of two Hemarthria species. Front. Plant Sci. 2016, 7, 496. [Google Scholar] [CrossRef]
- Guo, Z.-H.; Fu, K.-X.; Zhang, X.-Q.; Bai, S.-Q.; Fan, Y.; Peng, Y.; Huang, L.-K.; Yan, Y.-H.; Liu, W.; Ma, X. Molecular insights into the genetic diversity of Hemarthria compressa germplasm collections native to southwest China. Molecules 2014, 19, 21541–21559. [Google Scholar] [CrossRef]
- Jing, H.; Xue, X.; Zhang, X.; Xu, X.; Tang, Y.; Wang, H.; Zheng, J.; Yang, H.; Han, Y. Metabolomics and microbiome analysis elucidate the detoxification mechanisms of Hemarthria compressa, a low cadmium accumulating plant, in response to cadmium stress. J. Hazard. Mater. 2025, 487, 137226. [Google Scholar] [CrossRef]
- Li, W.; Zhou, X.; Qu, M.; Zheng, Y.; Shen, B.; Zeng, B.; Feng, Y.; Pang, K.; Wu, J.; Zeng, B. WGCNA analysis reveals hub genes in the Hemarthria compressa roots in response to waterlogging stress. Sci. Rep. 2025, 15, 13841. [Google Scholar] [CrossRef]
- Zhang, Q.; Bell, L.W.; Shen, Y.; Whish, J.P. Indices of forage nutritional yield and water use efficiency amongst spring-sown annual forage crops in north-west China. Eur. J. Agron. 2018, 93, 1–10. [Google Scholar]
- Capstaff, N.M.; Miller, A.J. Improving the yield and nutritional quality of forage crops. Front. Plant Sci. 2018, 9, 535. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Tang, G.; Wu, D.; Zhang, J. Yield and nutrient composition of forage crops and their effects on soil characteristics of winter fallow paddy in South China. Front. Plant Sci. 2024, 14, 1292114. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yuan, S.; Naser, M.; Zhou, Y.; Jia, H.; Yu, Y.; Yao, X.; Wu, T.; Song, W.; Jiang, B. Evaluation of forage quality in various soybean varieties and high-yield cultivation techniques. Field Crops Res. 2024, 317, 109546. [Google Scholar]
- Rehman, M.A.U.; Zulfiqar, Z. Techniques for Evaluating the Nutritional Profile and Digestibility of Animal Feedstuffs by Different Laboratory Methods. Appl. Anim. Sci. Bull. 2024, 1, 1–10. [Google Scholar]
- Devi, A.; Sharma, M.; Badola, R.; Hussain, S.A. Unveiling the mysteries of Asian herbivores resource partitioning in tropical wet-grassland ecosystem. Glob. Ecol. Conserv. 2024, 54, e03079. [Google Scholar] [CrossRef]
- Shen, B.; Li, W.; Zheng, Y.; Zhou, X.; Zhang, Y.; Qu, M.; Wang, Y.; Yuan, Y.; Pang, K.; Feng, Y. Morphological and molecular response mechanisms of the root system of different Hemarthria compressa species to submergence stress. Front. Plant Sci. 2024, 15, 1342814. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, Y.; Zhang, J.; Zhang, X.; Xie, W.; Jiang, X.; Peng, F.; Yan, Y.; Ma, X.; Liu, W. Genetic stability and DNA fingerprinting of the Hemarthria compressa cultivar “Guangyi”. Biochem. Syst. Ecol. 2014, 55, 310–316. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Chen, H.; Hu, L.; Wang, L.; Wang, S.; Wang, M.L.; Cheng, X. Genetic diversity and a population structure analysis of accessions in the Chinese cowpea [Vigna unguiculata (L.) Walp.] germplasm collection. Crop J. 2017, 5, 363–372. [Google Scholar] [CrossRef]
- Lin, F.; Jing, H.; Xue, X.; Wu, F.; Zhou, H.; Guan, Y.; Wang, H.; Han, Y. Physiology and metabolomics reveal the accumulation patterns and tolerance mechanisms of energy grass Hybrid Pennisetum under elevated Cadmium stress. Ind. Crops Prod. 2025, 232, 121274. [Google Scholar] [CrossRef]
- Meena, D.C.; Birthal, P.S.; Kumara, T.K. Biostimulants for sustainable development of agriculture: A bibliometric content analysis. Discov. Agric. 2025, 3, 2. [Google Scholar] [CrossRef]
- Liao, R.; Zhang, W.; Xu, R.; Li, K.; Wei, W.; Sheng, R. Endophytic microbial communities and functional shifts in Hemarthria compressa grass in response to Silicon and Selenium amendment. BMC Plant Biol. 2025, 25, 169. [Google Scholar] [CrossRef]
- Przybysz, A.; Gawrońska, H.; Gajc-Wolska, J. Biological mode of action of a nitrophenolates-based biostimulant: Case study. Front. Plant Sci. 2014, 5, 713. [Google Scholar] [CrossRef]
- Abood, N.M.; Mirare, S.S. Effect of seed soaking with a growth regulator (atonik) and seeding dates on the vegetative growth characteristics of Sorghum bicolor L. Iraqi J. Agric. Sci. 2024, 55, 951–961. [Google Scholar]
- Batool, Z.; Ishfaq, M.; Akbar, N.; Zulfiqar, U.; Anjum, S.A.; Shafiq, M.; Nazir, S.; Aziz, A. Exogenous application of Atonik (sodium nitrophenolate) under skip irrigation regimes modulated the physiology, growth and productivity of Zea mays L. Arch. Agron. Soil Sci. 2023, 69, 2325–2339. [Google Scholar]
- Yueshan, J.; Sun, M.; Yansu, L.; Xiaojie, F.; Menglu, L.; Aokun, S.; Chaoxing, H.; Yan, Y.; Jun, W.; Xianchang, Y. Sodium nitrophenolate mediates brassinosteroids signaling to enhance cold tolerance of cucumber seedling. Plant Physiol. Biochem. 2024, 206, 108317. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zheng, J.; Zhou, G.; Li, J.; Qian, C.; Lin, G.; Li, Y.; Zuo, Q. Moderate nitrogen application improved salt tolerance by enhancing photosynthesis, antioxidants, and osmotic adjustment in rapeseed (Brassica napus L.). Front. Plant Sci. 2023, 14, 1196319. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Di, Q.; Yan, Y.; He, C.; Wang, J.; Li, Y.; Yu, X.; Sun, M. Multi-omics reveal the molecular mechanisms of Sodium Nitrophenolate in enhancing cold tolerance through hormonal and antioxidant pathways in cucumber. Plant Physiol. Biochem. 2025, 223, 109836. [Google Scholar] [CrossRef]
- Dong, Z.; Huang, J.; Qi, T.; Fu, Q.; Meng, A.; Fu, Y. Effects of plant regulators on the seed germination and antioxidant enzyme activity of cotton under compound salt stress. Plants 2023, 12, 4112. [Google Scholar] [CrossRef]
- Hristov, A.; Mertens, D.; Zaman, S.; Vander Pol, M.; Price, W. Variability in feed and total mixed ration neutral detergent fiber and crude protein analyses among commercial laboratories. J. Dairy Sci. 2010, 93, 5348–5362. [Google Scholar] [CrossRef]
- Jančík, F.; Koukolová, V.; Homolka, P.; Haman, J. Comparison of analyses to predict ruminal fibre degradability and indigestible fibre in temperate grass silages. S. Afr. J. Anim. Sci. 2011, 41, 297–308. [Google Scholar] [CrossRef]
- Cassida, K.; Turner, K.; Foster, J.; Hesterman, O. Comparison of detergent fiber analysis methods for forages high in pectin. Anim. Feed Sci. Technol. 2007, 135, 283–295. [Google Scholar] [CrossRef]
- Liu, J.; Hao, J.; Zhao, M.; Yan, X.; Jia, Y.; Wang, Z.; Ge, G. Effects of different temperature and density on quality and microbial population of wilted alfalfa silage. BMC Microbiol. 2024, 24, 380. [Google Scholar] [CrossRef]
- Zou, Y.; Dong, S.; Du, Y.; Li, S.; Wang, Y.; Cao, Z. Effects of moisture content or particle size on the in situ degradability of maize silage and alfalfa haylage in lactating dairy cows. Anim. Nutr. 2016, 2, 249–252. [Google Scholar] [CrossRef]
- Hall, M.B.; Mertens, D.R. Comparison of alternative neutral detergent fiber methods to the AOAC definitive method. J. Dairy Sci. 2023, 106, 5364–5378. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Cantalapiedra, C.P.; Hernández-Plaza, A.; Letunic, I.; Bork, P.; Huerta-Cepas, J. eggNOG-mapper v2: Functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol. Biol. Evol. 2021, 38, 5825–5829. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- He, Z.; Zhou, M.; Feng, X.; Di, Q.; Meng, D.; Yu, X.; Yan, Y.; Sun, M.; Li, Y. The role of brassinosteroids in plant cold stress response. Life 2024, 14, 1015. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, S.; Ruan, X.; Xu, J.; Cao, D. CSN improves seed vigor of aged sunflower seeds by regulating the fatty acid, glycometabolism, and abscisic acid metabolism. J. Adv. Res. 2021, 33, 1–13. [Google Scholar] [CrossRef]
- Quintero-Calderon, E.H.; Sanchez-Reinoso, A.D.; Chavez-Arias, C.C.; Garces-Varon, G.; Restrepo-Diaz, H. Rice seedlings showed a higher heat tolerance through the foliar application of biostimulants. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12120. [Google Scholar] [CrossRef]
- Banful, B.K.; Attivor, D. Growth and yield response of two hybrid rice cultivars to ATONIK plant growth regulator in a Tropical environment. Environ. Earth Ecol. 2017, 1, 33–45. [Google Scholar] [CrossRef][Green Version]
- Kocira, A.; Kocira, S.; Świeca, M.; Złotek, U.; Jakubczyk, A.; Kapela, K. Effect of foliar application of a nitrophenolate–based biostimulant on the yield and quality of two bean cultivars. Sci. Hortic. 2017, 214, 76–82. [Google Scholar] [CrossRef]
- Sherin, G.; Aswathi, K.R.; Puthur, J.T. Photosynthetic functions in plants subjected to stresses are positively influenced by priming. Plant Stress 2022, 4, 100079. [Google Scholar] [CrossRef]
- Wu, R.; Ran, K.; Zhao, S.; Cheng, F. Genome-wide identification of the light-harvesting chlorophyll A/b binding protein gene family in Pyrus bretschneideri and their transcriptomic features under drought stress. Horticulturae 2023, 9, 522. [Google Scholar] [CrossRef]
- Xiang, P.; Marat, T.; Huang, J.; Cheng, B.; Liu, J.; Wang, X.; Wu, L.; Tan, M.; Zhu, Q.; Lin, J. Response of photosynthetic capacity to ecological factors and its relationship with EGCG biosynthesis of tea plant (Camellia sinensis). BMC Plant Biol. 2025, 25, 199. [Google Scholar] [CrossRef]
- Carvalho, P.; Gomes, C.; Saibo, N.J. C4 Phosphoenolpyruvate Carboxylase: Evolution and transcriptional regulation. Genet. Mol. Biol. 2023, 46, e20230190. [Google Scholar] [CrossRef]
- Chen, J.-H.; Tang, M.; Jin, X.-Q.; Li, H.; Chen, L.-S.; Wang, Q.-L.; Sun, A.-Z.; Yi, Y.; Guo, F.-Q. Regulation of Calvin–Benson cycle enzymes under high temperature stress. Abiotech 2022, 3, 65–77. [Google Scholar]
- Szparaga, A.; Kocira, S.; Kocira, A.; Czerwińska, E.; Świeca, M.; Lorencowicz, E.; Kornas, R.; Koszel, M.; Oniszczuk, T. Modification of growth, yield, and the nutraceutical and antioxidative potential of soybean through the use of synthetic biostimulants. Front. Plant Sci. 2018, 9, 1401. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Klevenhusen, F.; Zebeli, Q. A review on the potentials of using feeds rich in water-soluble carbohydrates to enhance rumen health and sustainability of dairy cattle production. J. Sci. Food Agric. 2021, 101, 5737–5746. [Google Scholar]
- Weimer, P.J. Degradation of cellulose and hemicellulose by ruminal microorganisms. Microorganisms 2022, 10, 2345. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Huasai, S.; Chen, A. Effects of dietary forage to concentrate ratio on nutrient digestibility, ruminal fermentation and rumen bacterial composition in Angus cows. Sci. Rep. 2021, 11, 17023. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Y.; Chang, Z.; Yao, B.; Han, Z.; Wang, T.; Shang, N.; Wang, R. Innovative Lactic Acid Production Techniques Driving Advances in Silage Fermentation. Fermentation 2024, 10, 533. [Google Scholar] [CrossRef]
- Guo, X.; Xu, D.; Li, F.; Bai, J.; Su, R. Current approaches on the roles of lactic acid bacteria in crop silage. Microb. Biotechnol. 2023, 16, 67–87. [Google Scholar]
- Wang, D.; Luo, C.; Li, C.; Zhang, S.; Lu, N.; Yang, Z.; Yu, X.; Cao, Z.; Yang, H.; Li, S. Study on the relationship between fermentation-accumulated temperature and nutrient loss of whole-plant corn silage. Agronomy 2022, 12, 2752. [Google Scholar]
- Yan, Y.; Zhao, M.; Sun, P.; Zhu, L.; Yan, X.; Hao, J.; Si, Q.; Wang, Z.; Jia, Y.; Wang, M. Effects of different additives on fermentation characteristics, nutrient composition and microbial communities of Leymus chinensis silage. BMC Microbiol. 2025, 25, 296. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Colla, G. Biostimulants in agriculture. Front. Media SA 2020, 11, 40–42. [Google Scholar]
- Di Sario, L.; Boeri, P.; Matus, J.T.; Pizzio, G.A. Plant biostimulants to enhance abiotic stress resilience in crops. Int. J. Mol. Sci. 2025, 26, 1129. [Google Scholar] [CrossRef]
- Massimi, M.; Radócz, L.; Kabashi, B. The response of chlorophyll content and ionic composition in tomato and pepper seedlings to foliar nutrition in growing chambers. Agronomy 2023, 13, 2234. [Google Scholar] [CrossRef]
- Ehret, D.; Utkhede, R.; Frey, B.; Menzies, J.; Bogdanoff, C. Foliar applications of fertilizer salts inhibit powdery mildew on tomato. Can. J. Plant Pathol. 2002, 24, 437–444. [Google Scholar]
- Horst, R.; Kawamoto, S.; Porter, L. Effect of sodium bicarbonate and oils on the control of powdery mildew and black spot of roses. Plant Dis. 1992, 76, 247–251. [Google Scholar] [CrossRef]
- Shi, J.; Yan, X.; Sun, T.; Shen, Y.; Shi, Q.; Wang, W.; Bao, M.; Luo, H.; Nian, F.; Ning, G. Homeostatic regulation of flavonoid and lignin biosynthesis in phenylpropanoid pathway of transgenic tobacco. Gene 2022, 809, 146017. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, J.; Tschaplinski, T.J.; Tuskan, G.A.; Chen, J.-G.; Muchero, W. Regulation of lignin biosynthesis and its role in growth-defense tradeoffs. Front. Plant Sci. 2018, 9, 1427. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, Y.; Xu, C.; Wang, X.; Wang, P.; Huang, S. Abscisic acid collaborates with lignin and flavonoid to improve pre-silking drought tolerance by tuning stem elongation and ear development in maize (Zea mays L.). Plant J. 2023, 114, 437–454. [Google Scholar] [PubMed]


| Gene Name | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| LHCB3 | GCCATGGGACGTGCTGCTGT | ACGAGGACGAGGTGCCCAGC |
| PETE | CGAGCCCAGCGAGTTCAC | AGGAGTACCTCAACGCGCGG |
| psaC | TTCAAGAACAACTTCGGG | CGGCAAGGTCACCGTCAACC |
| SBPase | CACCCATGTAGCTCCACTC | TGATTTGTGTCCATGTATGTA |
| 4CL1 | GAAGGTAAATGGCAGCAT | ACCATTGGAGAAGGGAAGA |
| CHS1 | AGGGCAACATTCGATAAC | GGAGGAATGGTTCCCGATGT |
| F3′H | AGCTCATCAACTACTACGT | AGGTGGCGCCTCTGGGCTTGT |
| Terms | CK | CSN1 | CSN2 | CSN3 |
|---|---|---|---|---|
| Crude protein (mg/g DW) | 147.3 ± 2.5 c | 163.5 ± 3.0 b | 177.6 ± 2.8 a | 172.5 ± 2.3 a |
| Moisture content (% initial FW) | 85.73 ± 0.2 | 84.23 ± 0.3 | 85.78 ± 0.3 | 86.20 ± 0.2 |
| Lignin (mg/g DW) | 130.53 ± 1.5 a | 106.3 ± 1.4 b | 110.20 ± 1.3 b | 109.51 ± 1.6 b |
| Hemicellulose (mg/g DW) | 217.52 ± 3.2 | 215.94 ± 3.1 | 211.07 ± 2.9 | 223.32 ± 3.0 |
| NDF (% DW) | 70.59 ± 1.2 a | 60.59 ± 1.3 b | 61.40 ± 1.1 b | 62.53 ± 1.2 b |
| ADF (% DW) | 38.31 ± 1.0 a | 33.92 ± 1.1 b | 32.88 ± 1.0 b | 33.81 ± 1.0 b |
| Ash content (% DW) | 8.53 ± 0.5 c | 9.88 ± 0.4 b | 11.48 ± 0.3 a | 11.22 ± 0.4 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Han, P.; Zhao, R.; Wu, Y.; Wei, W.; He, F.; Dong, C. Compound Sodium Nitrophenolate (CSN) Improves Photo-Synthesis and Forage Quality in Hemarthria compressa. Agronomy 2025, 15, 2526. https://doi.org/10.3390/agronomy15112526
Liu Z, Han P, Zhao R, Wu Y, Wei W, He F, Dong C. Compound Sodium Nitrophenolate (CSN) Improves Photo-Synthesis and Forage Quality in Hemarthria compressa. Agronomy. 2025; 15(11):2526. https://doi.org/10.3390/agronomy15112526
Chicago/Turabian StyleLiu, Zhongpeng, Peng Han, Ruijie Zhao, Yuanyuan Wu, Wenxuan Wei, Fahui He, and Chenfei Dong. 2025. "Compound Sodium Nitrophenolate (CSN) Improves Photo-Synthesis and Forage Quality in Hemarthria compressa" Agronomy 15, no. 11: 2526. https://doi.org/10.3390/agronomy15112526
APA StyleLiu, Z., Han, P., Zhao, R., Wu, Y., Wei, W., He, F., & Dong, C. (2025). Compound Sodium Nitrophenolate (CSN) Improves Photo-Synthesis and Forage Quality in Hemarthria compressa. Agronomy, 15(11), 2526. https://doi.org/10.3390/agronomy15112526





