Abstract
To investigate the photosynthetic characteristics and leaf anatomical structures of seedlings from the endangered plants Ormosia olivacea, Ormosia pachycarpa, and Ormosia sericeolucida, this study aimed to elucidate the influence of leaf structure on photosynthetic traits and light requirements among these three Ormosia species, thereby providing reference for their introduction and cultivation. This study measured the light response curves, CO2 response curves, leaf epidermal and anatomical characteristics, and photosynthetic pigment content of the three Ormosia species. Results indicate: 1. All three species exhibit photophilic tendencies, with Ormosia olivacea demonstrating the highest photosynthetic capacity, achieving a maximum net photosynthetic rate (Pmax) of 1.9062 mol m−2 s−1. Ormosia pachycarpa exhibited the highest potential maximum net photosynthetic rate (Amax), demonstrating superior CO2 utilisation capacity. The Amax values for all three species were significantly higher than their Pmax values. 2. Among the three Ormosia species, Ormosia sericeolucida exhibited the thickest leaf structure, with palisade tissue thickness ordered as follows: Ormosia sericeolucida > Ormosia pachycarpa > Ormosia olivacea. 3. Stomata were present on the lower epidermis of all three species. Ormosia sericeolucida possessed the largest individual stomatal area, while Ormosia olivacea exhibited the highest stomatal density. 4. The chlorophyll a content (Chl a) of all three Ormosia species exceeded their chlorophyll b content (Chl b), indicating they are photophilic plants. Ormosia sericeolucida exhibited higher chlorophyll a (Chl a), chlorophyll b (Chl b), and total chlorophyll (Chl) contents than both Ormosia olivacea and Ormosia pachycarpa. Ormosia olivacea possessed the highest carotenoid content (Car). In summary, Ormosia pachycarpa exhibited the highest potential maximum net photosynthetic rate (Amax), demonstrating the strongest CO2 utilisation capacity, followed by Ormosia olivacea, with Ormosia sericeolucida showing the lowest. Appropriately increasing CO2 levels in cultivation sites would benefit photosynthesis and material accumulation in all three Ormosia species, promoting robust growth.
1. Introduction
Ormosia olivacea, Ormosia pachycarpa, and Ormosia sericeolucida are all leguminous Ormosia species endemic to China. Among these, Ormosia olivacea is a tree reaching 20–25 m in height, primarily distributed in northern Guangxi and southern Yunnan; Ormosia pachycarpa is an evergreen tree reaching up to 15 m in height, possessing timber quality comparable to teak, primarily distributed in eastern Guangxi and Guangdong [1]; Ormosia sericeolucida, also known as false hornbeam, is likewise an evergreen tree, commonly found in valley woodlands and streamside forests of Guangxi and Guangdong. It is now rarely seen in Guangdong valleys and has been classified as an endangered plant. These three species, akin to the approximately 130 other Ormosia plants (37 of which occur in China, primarily in Guangdong, Guangxi, Yunnan, and Hainan [2]), possess extensive utilitarian value. They serve as materials for furniture, construction, agricultural implements, and papermaking. Certain species are also employed in landscaping for their aesthetic appeal [3]. Their seeds, with their elegant form and red or bright brown colour, are crafted into jewellery such as necklaces, earrings, and rings. Moreover, the seeds, roots, stems, bark, and leaves of most species contain rich chemical constituents, all of which are used medicinally for their efficacy in promoting blood circulation and unblocking meridians. Ancient practitioners commonly employed them to treat bruises, rheumatic arthritis, and unexplained swellings and pains [4]. However, owing to their intrinsic value and suffering from logging and illegal excavation, these three endemic species face severe resource depletion. Consequently, they were collectively listed as Class II nationally protected wild plants in 2021 [5], with the bright-haired red bean tree (Erythrina lucida) facing particularly critical endangerment. Given their shared genus yet distinct distribution ranges and morphological characteristics, coupled with the current lack of research on their seedling photosynthetic properties and leaf anatomical structures, investigating these species aims to elucidate the influence of leaf structure on photosynthetic traits and their light requirements. This research holds significant scientific value and conservation importance, providing valuable references for their introduction and cultivation.
Photosynthesis is the process by which plants convert solar energy into chemical energy and store it as organic matter, forming the foundation of material cycles and energy balance [6]. Studying the photosynthetic characteristics of plants reveals their adaptability to environmental factors, thereby enabling predictions of their growth requirements and competitive capacity. Investigating the photosynthesis of endangered plants and its influencing factors constitutes a vital method for accurately assessing optimal habitat conditions. This allows determination of the most suitable environmental conditions—such as light and water—required for their growth, facilitating the selection of optimal cultivation sites [7,8]. As the primary site for photosynthesis and transpiration, leaves exhibit extreme sensitivity to environmental fluctuations [9]. Under varying conditions, leaf morphology, thickness, and internal architecture undergo significant alterations. Consequently, elucidating how leaf structure influences photosynthetic traits holds critical significance for understanding plant ecological adaptability.
In recent years, considerable research has been conducted on the photosynthetic characteristics of endangered plants. For instance, studies on endangered Camellia species indicate that camellias are shade-tolerant plants, whereas tea plants exhibit broad light adaptability. Both species demonstrate strong photosynthetic adaptability in new environments, and appropriate shading may enhance the photosynthetic efficiency of cultivated camellias, promoting their growth. This suggests that different plants have varying light requirements [10]. To date, no studies have been published on the photosynthetic characteristics or leaf anatomical structures of seedlings from Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa.
This study investigates the photosynthetic characteristics of the Chinese endemic species Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa. By examining the photosynthetic properties and leaf anatomical structures of seedlings from these three Ormosia species, we explore their photosynthetic traits and the relationships between leaf structure, pigment content, and photosynthetic performance. This research provides a theoretical basis for the introduction and cultivation of Ormosia germplasm resources. Our methodology employed high-precision instruments to determine photosynthetic curves, combined with microscopic observation of leaf structures. Through multi-indicator synchronous analysis and repeated validation, we precisely elucidated the adaptive mechanisms of these species to light and CO2, providing reliable guidance for introduction. The findings clearly established their differing light preferences and CO2 utilisation patterns, holding significant implications for conservation and cultivation.
2. Materials and Methods
2.1. Overview of the Experimental Site
The experimental site is located within the grounds of the Guangxi Institute of Botany, Chinese Academy of Sciences, situated in Guilin City, Guangxi Zhuang Autonomous Region. Positioned at 25°01′ north latitude and 110°17′ east longitude, with an elevation ranging from 180 to 300 m, the area experiences a subtropical monsoon climate. The annual average temperature is approximately 19.2 °C [11]. The site offers favourable environmental conditions conducive to plant growth.
2.2. Materials
All materials comprised introduced cultivated perennial species: Ormosia olivacea, Ormosia pachycarpa, and Ormosia sericeolucida. For each species, three plants were selected for assessment, all sharing identical orientation, exhibiting robust growth, and displaying comparable vigour.
2.3. Method
2.3.1. Determination of the Photosynthesis-Light Response Curve
The photosynthesis-light response curve was determined using a portable photosynthesis meter Li-6800, with measurements conducted on clear mornings. Healthy, intact leaves free from pests and diseases were selected for testing [12]. Prior to measurement, leaves were pre-induced for 15 min under an irradiance of 1000 µmol m−2 s−1. An open gas pathway was employed, with the gas flow rate (Flow) set at 500 µmol s−1. The photosynthetic photon flux density (PFD) was set in a descending gradient: 1600 mol m−2 s−1, 1400 mol m−2 s−1, 1200 mol m−2 s−1, 1000 mol m−2 s−1, 800 mol m−2 s−1, 600 mol m−2 s−1, 400 mol m−2 s−1, 200 mol m−2 s−1, 100 mol m−2 s−1, 50 mol m−2 s−1, 20 mol m−2 s−1, and 0 mol m−2 s−1, totalling 12 gradients. Measurements were taken at each light intensity gradient for 120–180 s. The measured data were then fitted to the Pn_PFD curve using the following equation, plotting the photosynthetic response curve with photosynthetic photon flux density (PFD) on the x-axis and net photosynthetic rate (Pn) on the y-axis. Adaptive testing confirmed satisfactory fitting results. The light saturation point (LSP), maximum net photosynthetic rate (Pmax), and light compensation point (LCP) were calculated using the formulae as follows.
In the formula: Pn denotes net photosynthetic rate; AQY represents apparent quantum efficiency; α, β and r denote coefficients; PFD denotes photosynthetic photon flux density; Rd denotes dark respiration rate.
2.3.2. Determination of the Photosynthetic CO2 Response Curve
The Li-6800 portable photosynthesis meter was employed to determine the photosynthetic CO2 response curve under saturating light intensity, with measurements conducted on clear mornings. Leaves were selected that were intact and free from disease or pest damage. Prior to measurement, leaves were pre-induced for 15 min under 1000 µmol m−2 s−1 light intensity to ensure full stomatal opening. The gas flow rate was set to 500 µmol s−1. The fixed light intensity was set at 1200 µmol m−2 s−1. CO2 concentration gradients were applied at: 400 µmol m−1, 300 µmol m−1, 200 µmol m−1, 100 µmol m−1, 50 µmol m−1, 0 µmol m−1, 400 µmol m−1, 600 µmol m−1, 800 µmol m−1, 1000 µmol m−1, 1200 µmol m−1, 1400 µmol m−1, and 1600 µmol m−1. (The concentration was controlled using a cylinder containing CO2 at 400 µmol m−1. Plot the photosynthetic-CO2 response curve with intercellular CO2 concentration (Ci) on the x-axis and net photosynthetic rate (Pn) on the y-axis. Employ the calculation method from Reference [13] to determine the CO2 compensation point (CCP), CO2 saturation point (CSP), and maximum potential net photosynthetic rate (Amax) using the following formulae:
In the formula: Pn denotes net photosynthetic rate; Ci denotes intercellular CO2 concentration; α denotes the initial carboxylation efficiency of the CO2 response curve; β and r denote coefficients; Rp denotes photorespiration rate.
2.3.3. Determination of Chlorophyll Content
From plants subjected to photosynthetic measurements, collect three leaves of consistent maturity that correspond to those used in photosynthetic parameter assessments for chlorophyll content determination. Each sample was accurately weighed at 0.2 g and immersed in 25 mL of 95% ethanol. After standing in the dark for 24 h, the absorbance values of the extract were measured at 665 nm, 649 nm, and 470 nm, with each measurement repeated three times. The chlorophyll a (Chl a), chlorophyll b (Chl b), total chlorophyll (Chl), carotenoid (Car) [14] content, the chlorophyll a to chlorophyll b ratio (Chl a/Chl b), and the carotenoid to total chlorophyll ratio (Car/Chl a + b). The specific procedure is as follows:
In the formula: A649, A470, and A665 represent the absorbance values at wavelengths of 649, 470, and 665 nm, respectively.
2.3.4. Determination of Parameters of Leaf Microstructure
Leaf Epidermal Characteristics
Select mature leaves in good condition from plants undergoing photosynthetic measurements, ensuring consistent orientation. Cut a 5 mm × 5 mm section from the midrib to the leaf margin. Fix using a 2.5% glutaraldehyde solution: Progress through sequential ethanol dehydration at concentrations of 30%, 50%, 70%, 80%, 90%, and 100% (with 100% repeated twice), allowing 15 min between each step; Subsequently, the leaf sections underwent critical point drying and gold coating. The upper epidermis, lower epidermis, and stomata were observed using a vacuum scanning electron microscope (ZEISS EVO18) from Carl Zeiss AG, Oberkochen, Germany, with photographs taken and recorded. Ten random fields of view were examined per sample. Axio Vision software was employed for observation [15], with measurements taken for stomatal long axis (SL), stomatal short axis (SW), stomatal density (SD), and single stomatal area (SA), where Π = 3.14.
Determination of Leaf Anatomical Structure
Leaf anatomical structure was examined using paraffin embedding [16] to prepare plant leaf sections. Three well-developed leaves were selected from the same position on each plant. A 10 mm × 10 mm leaf tissue section was excised, fixed in FAA fixative, and dehydrated using ethanol and xylene series. Sections were prepared using the paraffin sectioning method, stained with toluidine blue, and mounted with neutral resin. Sections were observed and photographed under an optical microscope, with microscopic parameters measured using CaseViewer 2.4 image analysis software. Analysed leaf anatomical parameters included: upper epidermal cell thickness (UET), lower epidermal cell thickness (LET), leaf thickness (LT), palisade parenchyma thickness (PPT), spongy parenchyma thickness (SPT), and palisade-to-spongy parenchyma thickness ratio (PPT/SPT). Ten random fields of view were examined for each parameter.
2.4. Data Analysis
The experimental results were processed using Excel 2021. A one-way analysis of variance was conducted with SPSS 21.0 [17], followed by Duncan’s multiple range test for post hoc comparisons. Correlation analyses were performed on photosynthetic parameters, chlorophyll content, leaf anatomical structure, and leaf epidermal characteristics. Graphical representations were generated using Origin 2024 software.
3. Results and Analysis
3.1. Comparative Analysis of Photosynthetic-Photoresponsive Curves in Three Phaseolus Species
As shown in Figure 1A, the net photosynthetic rate (Pn) curves of Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa exhibit broadly consistent trends; The Pn values of Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa all increased with rising PFD. When PFD ranged between 0 and 200 µmol m−2 s−1, the net photosynthetic rates (Pn) of all three species exhibited a linear upward trend. When PFD ranged from 200 to 1200 µmol m−2 s−1, Pn levels of Ormosia olivacea and Ormosia sericeolucida levelled off. At PFD values exceeding 1200 µmol m−2 s−1, Pn of Ormosia olivacea showed a slight increase, whereas Pn of Ormosia pachycarpa exhibited a decreasing trend. Figure 1B: When PFD ranged from 0 to 200 µmol m−2 s−1, Ci decreased with increasing PFD for Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa. Ci exhibited a linear decline trend when PFD ranged from 0 to 200 µmol m−2 s−1. Upon reaching 200 µmol m−2 s−1, Ormosia olivacea’s response gradually levelled off and stabilised. As depicted in Figure 1C,D, the transpiration rates (Emm) and water vapour stomatal conductance (gsw) curves of Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa exhibited broadly consistent trends. Among Emm and gsw, Ormosia pachycarpa exhibited an upward trend, while the transpiration rate (Emm) curves for Ormosia olivacea and Ormosia sericeolucida showed a gentle linear trend. The water vapour stomatal conductance (gsw) curve for Ormosia olivacea exhibited a downward trend.
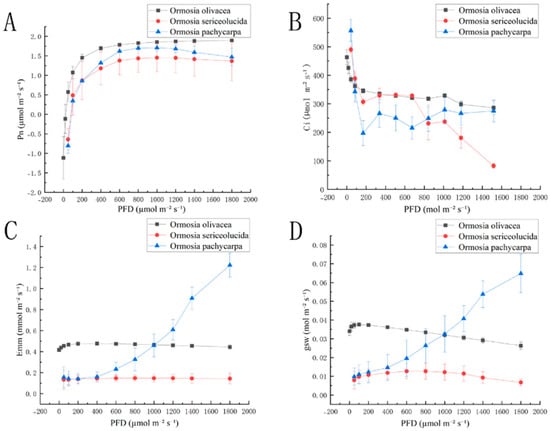
Figure 1.
Photosynthesis-light response curves of three species of the genus Phaseolus: (A) is the net photosynthetic rate photosynthesis-light response curve diagram; (B) is the intercellular CO2 concentration photosynthesis-light response curve diagram; (C) is the transpiration rate photosynthesis-light response curve diagram; (D) is the water vapour stomatal conductance photosynthesis-light response curve diagram.Note: PFD is photosynthetic photon flux density; Pn is net photosynthetic rate; Ci is intercellular CO2 concentration; Emm is transpiration rate; gsw is water vapour stomatal conductance.
Table 1 indicates that Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa exhibited significant differences (p < 0.05) in maximum net photosynthetic rate (Pmax), light saturation point (LSP), and light compensation point (LCP). with maximum net photosynthetic rates (Pmax) of 0.1228 mol m−2 s−1, 0.0853 mol m−2 s−1, and 0.0226 mol m−2 s−1, respectively. No significant difference was observed between Ormosia sericeolucida and Ormosia pachycarpa (LSP) (p > 0.05). No significant differences were observed in dark respiration rate (Rd) or apparent quantum yield (AQY) among Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa (p > 0.05).

Table 1.
Comparison of light response parameters of three species of the genus Phaseolus.
3.2. Comparative Analysis of Photosynthetic CO2 Response Parameters in Three Phaseolus Species
As shown in Figure 2, the net photosynthetic rate (Pn) of Ormosia sericeolucida was lower than that of Ormosia olivacea and Ormosia pachycarpa. The response curves of Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa exhibited broadly consistent trends, with overall Pn decreasing as Ci increased.
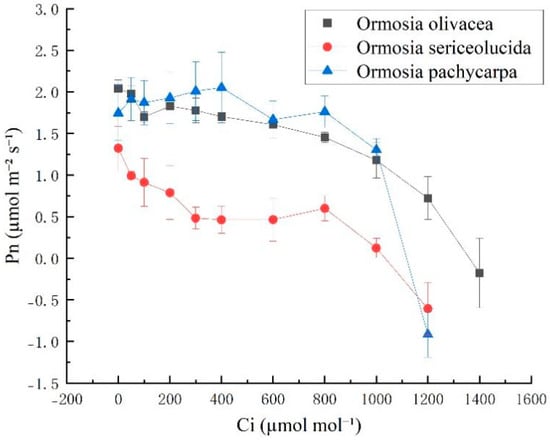
Figure 2.
CO2 response curves of three species of the genus Phaseolus. Note: Pn is the photosynthetic rate; Ci is the intercellular CO2 concentration.
Table 2 indicates that there were no significant differences (p > 0.05) in the maximum net photosynthetic rate (Amax) and CO2 compensation point (CCP) among Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa. Significant differences (p < 0.05) were observed among Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa for the initial carboxylation efficiency (α), photorespiration rate (Rp), and saturation CO2 concentration (CSP). However, no significant difference was found between Ormosia sericeolucida and Ormosia pachycarpa (p > 0.05). The maximum potential net photosynthetic rates (Amax) were 22.6641 mol m−2 s−1, 2.0871 mol m−2 s−1, and 1.35 mol m−2 s−1, respectively.

Table 2.
CO2 response parameters of three species of the genus Phaseolus.
3.3. Comparison of Chlorophyll Content in Three Phaseolus Species
Significant differences (p < 0.05) were observed in the chlorophyll a content (Chl a), chlorophyll b content (Chl b), carotenoid content (Car), total chlorophyll content (Chl), the ratio of chlorophyll a to chlorophyll b (Chl a/Chl b), and the ratio of carotenoids to chlorophyll (Car/Chl) all exhibited significant differences (p < 0.05). Ormosia sericeolucida displayed higher Chl a, Chl b, and Chl levels than both Ormosia olivacea and Ormosia pachycarpa, whilst Ormosia pachycarpa exhibited the lowest values for Chl a, Chl b, Car, and Chl. Ormosia pachycarpa exhibited a higher Chl a/Chl b ratio than both Ormosia olivacea and Ormosia sericeolucida, with Ormosia olivacea displaying the lowest ratio. Ormosia olivacea had a higher Car/Chl ratio than the other two species, while Ormosia sericeolucida had the lowest ratio (Table 3).

Table 3.
Chlorophyll content of three species of Phaseolus plants.
3.4. Comparative Microscopic Structure of Leaves from Three Phaseolus Species
3.4.1. Anatomical Characteristics of Leaf Structure
As shown in Figure 3, the palisade tissue of Ormosia pachycarpa exhibits a relatively dense arrangement, whereas that of Ormosia olivacea and Ormosia sericeolucida is comparatively sparse. Table 4 indicates that significant differences (p < 0.05) exist in leaf thickness (LT) and upper epidermal cell thickness (UET) among Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa. Ormosia sericeolucida exhibited greater leaf thickness (LT) and upper epidermal cell thickness (UET) than both Ormosia olivacea and Ormosia pachycarpa. Ormosia olivacea possessed greater upper epidermal cell thickness (UET) than Ormosia pachycarpa, yet its leaf thickness (LT) was lower than that of Ormosia pachycarpa. The lower epidermal cell thickness (LET) of Ormosia olivacea was greater than that of both Ormosia sericeolucida and Ormosia pachycarpa. No significant difference was observed in lower epidermal cell thickness (LET) between Ormosia sericeolucida and Ormosia pachycarpa (p > 0.05). Significant differences (p < 0.05) were observed in both palisade parenchyma thickness (PPT) and spongy parenchyma thickness (SPT) among Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa. Ormosia sericeolucida exhibited greater phloem parenchyma thickness (PPT) and spongy parenchyma thickness (SPT) than both Ormosia olivacea and Ormosia pachycarpa. However, Ormosia olivacea and Ormosia sericeolucida Ormosia pachycarpa exhibited significant differences in the ratio of palisade to spongy tissue thickness (PPT/SPT) (p < 0.05). However, no significant differences were observed in the PPT/SPT ratio between Ormosia sericeolucida and Ormosia pachycarpa (p > 0.05). with respective ratios of 0.6692, 0.496, and 0.4571.

Figure 3.
Anatomical structure of leaves of three species of the genus Phaseolus. Note: (a) is a cross-section of the leaf of Ormosia olivacea; (b) is a cross-section of the leaf of Ormosia sericeolucida; (c) represents the cross-section of an Ormosia pachycarpa leaf.

Table 4.
Comparison of anatomical structure characteristics of leaf cross-sections of three species of the genus Phaseolus.
3.4.2. Leaf Stomatal Characteristics
The upper epidermis of Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa exhibits no stomatal distribution; all stomata are located on the lower epidermis of the leaves (Figure 4). Significant differences (p < 0.05) were observed in the stomatal long axis (SL) among Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa, with Ormosia olivacea exhibiting a longer stomatal long axis than both Ormosia sericeolucida and Ormosia pachycarpa. Significant differences (p < 0.05) were observed in the single stomatal area (SA) and stomatal density (SD) between Ormosia olivacea and both Ormosia sericeolucida and Ormosia pachycarpa. No significant differences (p > 0.05) were found in SA or SD between Ormosia sericeolucida and Ormosia pachycarpa. Ormosia olivacea exhibited a larger individual stomatal area (SA) than both Ormosia sericeolucida and Ormosia pachycarpa, but a lower stomatal density (SD) than both species. The short axis (SW) of stomata in Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa showed no significant differences (p > 0.05) (Table 5).

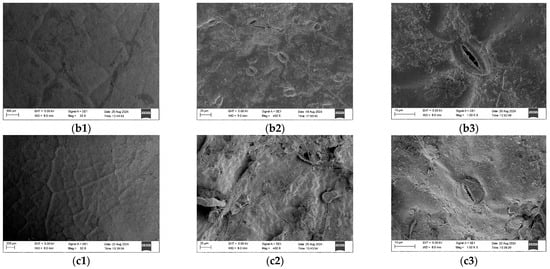
Figure 4.
Epidermal characteristics of three species of the genus Phaseolus. Note: (a1,a2,a3) represent the upper epidermis, lower epidermis and stomata of Ormosia olivacea, respectively; (b1,b2,b3) represent the upper epidermis, lower epidermis and stomata of Ormosia sericeolucida, respectively; (c1,c2,c3) represent the upper epidermis, lower epidermis and stomata of Ormosia pachycarpa, respectively.

Table 5.
Stomatal indices of three species of the genus Phaseolus.
3.5. Correlation Analysis of Leaf Anatomical Characteristics, Chlorophyll Content and Photosynthetic Parameters in Three Phaseolus Species
As shown in Figure 5, there exists a certain correlation between the leaf structural parameters and photosynthetic physiological and ecological parameters of Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa [18]. α exhibited extremely significant correlations (p < 0.001) with Car/Chl, LET, and PPT/SPT, and significant correlations (p < 0.01) with SD, LT, and SPT; Rp exhibited extremely significant correlations with LET and PPT/SPT (p < 0.001), and significant correlations with Car/Chl, SD, LT, and SPT (p < 0.01); Pmax showed extremely significant correlations with Car/Chl, LT, PPT, and SPT (p < 0.001); CSP and LCP showed significant correlations only with Car/Chl (p < 0.05). The photosynthetic physiological and ecological parameters Amax, CCP, Rd, and AQY exhibited no significant correlations with chlorophyll content (Chl a, Chl b, Car, Chl), Chl a/Chl b, Car/Chl, or leaf parameters LT, UET, LET, PPT, SPT, or PPT/SPT.
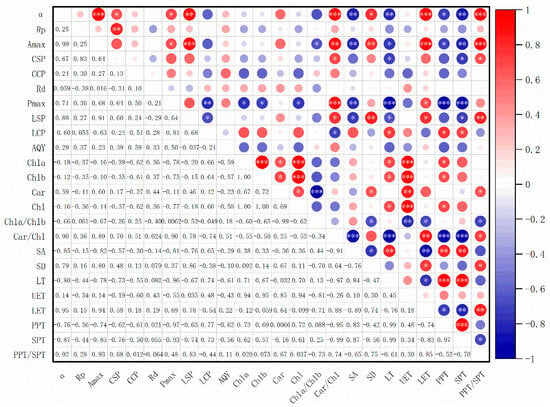
Figure 5.
Correlation analysis between leaf structural characteristics, chlorophyll content, and photosynthetic parameters of three species of Phaseolus plants. Note: * indicates p < 0.05; ** indicates p < 0.01; *** indicates p < 0.001.
4. Discussion
Photosynthetic efficiency directly influences a plant’s survival competitiveness and ecological adaptability. In this study, the net photosynthetic rate (Pn) of Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa all exhibited a trend of initial increase followed by stabilisation with increasing photosynthetic flux density (PFD), indicating that these three Ormosia species demonstrate a certain capacity for photosynthetic response under varying light intensities. Analysis of light response curves revealed differences in photosynthetic characteristics among the red bean species under varying light intensities. The relationship between photosynthetic rate and light intensity reflects both the plant’s photosynthetic capacity and its close adaptive capability to environmental conditions.
Ormosia olivacea exhibits a broader light adaptation range, with the greatest difference between its light compensation point (LCP) and light saturation point (LSP) observed in Ormosia olivacea, reaching 3589.7502 mol m−2 s−1. This indicates its capacity to maintain net photosynthesis across a spectrum from low to high light intensities [19]. Furthermore, the olive-green variety exhibited the highest apparent quantum yield (AQY) at 0.0185 mol m−2 s−1. This suggests greater adaptation to low-light environments. However, all three adzuki bean varieties demonstrated relatively low apparent quantum yield (AQY) values, indicating comparatively inefficient utilisation of light energy [20]. Analysis of light response curves revealed differences in photosynthetic characteristics among different Phaseolus species under varying light intensities. The relationship between photosynthetic rate and light intensity reflects a plant’s photosynthetic capacity and its close adaptation to environmental conditions.
This disparity may be related to physiological characteristics such as chlorophyll content and stomatal distribution [21]. For instance, Ormosia olivacea exhibits a higher chlorophyll a (Chl a) content than chlorophyll b (Chl b), consistent with typical photophilic plants, enabling it to maintain a higher maximum net photosynthetic rate (Pmax) under intense light conditions. Conversely, Ormosia sericeolucida exhibits relatively high levels of both chlorophyll a (Chl a) and chlorophyll b (Chl b), demonstrating strong shade tolerance and the capacity to sustain fundamental photosynthetic functions under low-light conditions. In terms of leaf structure, the three Ormosia species exhibit essentially identical leaf architecture, with stomata exclusively distributed on the lower epidermis [22]. The absence of stomata on the upper epidermis reduces water loss and enhances the plants’ drought tolerance [23,24].
CO2 serves as the fundamental raw material for photosynthesis [25], and its concentration directly or indirectly influences plant photosynthesis and associated physiological and biochemical processes [26]. In this study, the potential maximum net photosynthetic rate (Amax) of the three Phaseolus species was significantly higher than their maximum net photosynthetic rate (Pmax), indicating that CO2 concentration is a key factor affecting their photosynthesis. Moreover, the initial carboxylation efficiency (α) serves as a key indicator of a plant’s capacity to utilise low CO2 concentrations [27]. Ormosia olivacea exhibited an initial carboxylation efficiency (α = 1.8126 mol m−2 s−1) was markedly higher than that of Ormosia sericeolucida (α = 0.0545 mol m−2 s−1) and Ormosia pachycarpa (α = 0.2203 mol m−2 s−1). This indicates that elevated CO2 concentrations can promote the growth of all three Ormosia species.
Analysis of light response curves and chlorophyll content revealed differences in photosynthetic characteristics among three Ormosia species under varying light intensities and CO2 concentrations. Ormosia olivacea demonstrated superior performance in light adaptation range, light energy utilisation efficiency, and CO2 utilisation capacity, though its overall light energy utilisation efficiency remained low, indicating stronger adaptation to low-light environments. In contrast, Ormosia sericeolucida and Ormosia pachycarpa exhibited relatively weaker light adaptation ranges and photosynthetic efficiency, though they possessed higher chlorophyll content. These findings provide theoretical foundations for cultivating and propagating Ormosia species across diverse ecological environments, while also directing further research into the adaptive mechanisms of plant photosynthesis.
5. Conclusions
This study analytically revealed differences in photosynthetic characteristics and adaptability among three species of Ormosia: Ormosia olivacea, Ormosia sericeolucida, and Ormosia pachycarpa. From the perspective of the relationship between photosynthetic physiology and leaf structural characteristics, it confirmed that although all three species are photophilic plants, they employ distinct light environment adaptation strategies. Ormosia olivacea exhibits a broad light adaptation range and high quantum efficiency, indicating its ability to maintain robust photosynthetic performance under both low- and high-light conditions. Furthermore, the study identified significant interspecific differentiation in CO2 utilisation capacity and photosynthetic potential. Ormosia pachycarpa exhibited the highest potential maximum net photosynthetic rate (Amax), indicating the greatest potential for photosynthetic enhancement under ample CO2 conditions. Conversely, Ormosia olivacea demonstrated the strongest utilisation capacity at low CO2 concentrations, providing a physiological basis for its competitive advantage in natural CO2 fluctuation environments. This study established the associative mechanism between photosynthetic parameters and leaf structural characteristics. The structural characteristics of chlorophyll a/b ratio and stomata confined to the lower epidermis provide integrated structural and functional evidence explaining interspecific photosynthetic variation, deepening our understanding of photosynthetic adaptation mechanisms in Phaseolus species.
Nevertheless, this study retains certain limitations. The small sample size and exclusive focus on the seedling stage restrict the generalisability of conclusions to populations and different developmental stages; nor did it systematically examine the interactive effects of multiple environmental factors such as water, temperature, and soil nutrients.
Future research should advance in the following directions: expanding sample sizes to include individuals of different age classes, and elucidating the effects of light intensity, CO2 concentration, and other environmental factors on photosynthesis in Phaseolus species. Such work will provide more robust theoretical foundations for the conservation, artificial cultivation, and ecological restoration of Phaseolus species.
Author Contributions
Conceptualization: J.Y. and G.H.; Methodology: J.Y.; Validation: J.Y., S.F. and X.L.; Formal analysis: J.Y., Y.W.; Investigation: G.H. and S.F.; Data curation: X.L. and Y.W.; Writing—original draft: J.Y.; Visualization: J.Y.; Writing—Review and Editing: J.T. and G.H.; Funding acquisition: J.T. and R.Z. Project Administration: R.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Natural Science Foundation of China (32560330); Guangxi Natural Science Foundation (Project Nos. 2024GXNSFAA010452 and 2023GXNSFAA026253); Autonomous Projects of the Guangxi Key Laboratory of Functional Phytochemicals Research and Sustainable Utilisation (Project Nos. ZRJJ2024-3 and ZRJJ2024-11); Hechi Municipal Science and Technology Base and Talent Programme (HeKe AC231113); and the 2022 Guilin Municipal Technology Application and Promotion Programme (20220134-3 and 20230102-3).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research received The successful completion of this research would not have been possible without the support and assistance of my supervisors, senior colleagues, and fellow students. First and foremost, I extend my heartfelt gratitude to He Guohua, Feng Shuo, Li Xi, and Wu Yingying for their meticulous assistance throughout the data processing and analysis stages. Their rigorous approach laid a solid foundation for the accuracy of the findings. Simultaneously, I extend sincere gratitude to Tang Jianmin and Wei Xiao for their professional guidance during the drafting and revision stages of this thesis. Their invaluable suggestions, from refining the research framework to enhancing the logical coherence of the content, have enabled continuous refinement of this work. Here, I offer my most heartfelt thanks to all who have supported this research endeavour.
Conflicts of Interest
The authors declare that no conflict of interest exists.
References
- Liu, M.; Qin, H.Z.; Liang, H.; Tang, J.M.; Wei, X.; Gao, L.M. Research on Quality Grading of Velvet Pod Red Bean Seedlings. Spec. Econ. Plants Anim. 2023, 26, 1–3. [Google Scholar]
- Wang, X.D.; Liu, P.; Liu, M.J.; Xiao, X.Y.; Chen, F.S. Biology and ecology research status of Ormosia species in China. Acta Bot. Sin. 2018, 36, 440–451. [Google Scholar]
- Wu, G.C. Research on the genus Phaseolus in China. Today’s Sci. Technol. 1984, 8. [Google Scholar]
- Zhang, L.J.; Zhou, W.J.; Ni, L.; Huang, M.Q.; Zhang, X.Q.; Xu, H.Y. A review on chemical constituents and pharmacological activities of Ormosia. Chin. Herb. Herbal. Med. 2021, 52, 4433–4442. [Google Scholar]
- Tang, J.M.; Wei, X.; Zou, R.; Ding, T.; Chai, F.S.; Zhu, X.L. Species Diversity Characteristics and Conservation Strategies of National Key Protected Wild Plants in Guangxi ed Wild Plants in Guangxi. Guangxi Sci. 2023, 30, 1025–1036. [Google Scholar]
- Zhang, J.R.; Wang, B.C. Study on leaf anatomical structure and photosynthetic characteristics of two bamboo species in spring greenhouse. Hubei Agric. Sci. 2020, 59, 108–111. [Google Scholar] [CrossRef]
- Xu, A.Z.; Jiang, H.D.; Pu, Q.K.; Wei, X.; Wei, Y.J.; Luo, Y.J.; Chai, S.F. Comparative study on leaf anatomical structures and photosynthetic characteristics of three Geodorum species. Guangxi Plants 2024, 44, 113–125. [Google Scholar]
- Smith, M.; Houpis, J.L.J. Gas exchange responses of the wetland plant Schoenoplectus hallii to irradiance and vapor pressure deficit. Aquat. Bot. 2004, 79, 267–275. [Google Scholar] [CrossRef]
- Hu, X.L.; Wang, N.; Zhang, H.M.; Li, J. Studies on structure in leaves and photosynthesis of Katy Apricot grown in the greenhouse. J. Henan Agric. Univ. 2010, 44, 411–415. [Google Scholar] [CrossRef]
- Wei, X.; Jiang, Y.S.; Jiang, S.Y.; Qi, X.X.; Xiong, Z.C.; Ye, W.H.; Wang, Z.M. Photosynthetic characteristics of an endangered species Camellia nitidissima and its widespread congener Camellia sinensis. Photosynthetica 2008, 46, 312–314. [Google Scholar] [CrossRef]
- Liu, M.; Qin, H.Z.; Liang, H.; Tang, J.M.; Zou, R.; Wei, X. Research on Quality Grading of Glossy-leaved Red Bean Seedlings. Spec. Econ. Plants Anim. 2023, 26, 51–53. [Google Scholar]
- Pan, L.P.; Tang, J.M.; Chen, T.G.; Zhu, S.J.; Zou, R.; Wei, X. Comparison of Photosynthetic Function and Structure of Leaves between Kmeria septentrionalis Seedlings and Adult Plants. Guangxi Sci. 2023, 30, 1163–1170. [Google Scholar] [CrossRef]
- Ye, Z.P.; Yu, Q. A comparison of response curves of winter wheat photosynthesis to flag leaf inte rcellular and air CO2 concentrations. J. Ecol. 2009, 28, 2233–2238. [Google Scholar]
- Cai, D.S.; Pei, X.X.; Min, X.X.; Du, C.M.; Chen, Q.Q.; Gong, S.F. Comparative Analysis of Anatomical Structure and Photosynthetic Characteristics of Camellia oleifera Leaves in “Sanhua Series”. Anhui Agric. Sci. Bull. 2023, 29, 52–57. [Google Scholar] [CrossRef]
- Chen, N.; Peng, L.H.; Jiang, H.D.; Yang, Z.; Jiang, Q.; Qiu, S.; Xiong, Z.C.; Wei, X.; Chai, F.S. Comparative study on the photosynthetic properties of four rare and endangered Dendrobium species. Guangxi Plants 2025, 45, 1–14. [Google Scholar] [CrossRef]
- Li, W.; Fu, Z.; Hao, X.Z.; Li, Q.Y.; Zhang, C.P. Leaf Anatomical Structure and Photosynthetic Characteristics of Megaskepasma erythrochlamysand Pachystachys lutea in Greenhouse. Bull. Chin. Agric. 2020, 36, 58–61. [Google Scholar] [CrossRef]
- Hu, Y.; Javed, H.H.; Liu, L.; Liu, Y.; Yang, X.; Xu, F.; Liu, Y.; Peng, X.; Wu, Y. Impact of Low Light on Photosynthetic Characteristics, Antioxidant Activity, and Yield of Brassica napus L. Agronomy 2025, 15, 214. Agronomy 2025, 15, 214. [Google Scholar] [CrossRef]
- He, G.H.; Tang, J.M.; Li, X.; Lu, L. Study on the Differences in Photosynthetic Characteristics and Leaf Anatomical Structure Among Various Species of Ormosia Plants. Appl. Ecol. Environ. Res. 2025, 23, 3603–3621. [Google Scholar] [CrossRef]
- Liu, T.; Cui, H.J.; Wu, S.J.; Zhu, J.Y.; Zhou, Z.Q. Response of photosynthetic and fluorescence characteristics of Japanese yew seedlings to different light conditions. J. Beijing For. Univ. 2013, 35, 65–70. [Google Scholar] [CrossRef]
- Dong, L.L.; Li, Y.X.; Quan, Q.M.; Fan, Z.L. Photosynthetic Characteristics of Traditional Chinese Medicine Sambucus chinensis Lind. J. Grassl. Sci. 2013, 21, 816–820. [Google Scholar]
- Sun, Y.; Wang, X.; Ma, C.; Zhang, Q. Effects of nitrogen and phosphorus addition on agronomic characters, photosynthetic performance and anatomical structure of Alfalfa in Northern Xinjiang, China. Agronomy 2022, 12, 1613. [Google Scholar] [CrossRef]
- Luo, S.X.; Zhang, D.X. Leaf Epidermal Morphology of Ormosia Jacks. (Leguminosae) in China. J. Trop. Subtrop. Bot. 2004, 12, 298–308. [Google Scholar]
- Ren, Y.J.; Ma, J.J.; Qin, S.P.; Du, B. Leaf Epidermal Micro-morphology Characteristics of Wild Cerasus humilis (Bge.) Sok. Yanshan Mountains. Plant Res. 2011, 31, 513–517. [Google Scholar]
- Liu, H.N.; Tian, J.F.; Liang, W.Z.; Ren, H.; Xia, L.L.; Zhou, Q. Leaves Anatomical Structure and Drought Resistance Evaluation of 9Germplasm Resources of Averrhoa carambola. Mol. Plant Breed. 2025, 1–25. Available online: https://link.cnki.net/urlid/46.1068.S.20221028.1555.006 (accessed on 22 September 2025).
- Zhang, K.; Zhang, B.; Wang, R.Y.; Wang, H.L.; Zhao, H.; Zhao, F.N.; Qi, Y.; Chen, F. Effects of Elevated CO2 Concentration on the Characteristics of Photosynthesis and Water Physiological-ecological of Spring Wheat in Semi-arid Area in China. J. Ecol. Environ. 2021, 30, 223–232. [Google Scholar] [CrossRef]
- Yu, F.Y.; Xu, Z.X. A Review on Plant Stress Physiology. World For. Res. 2003, 16, 6–11. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Li, Y.X.; Yu, M.L.; Da, W.Y.; Quan, Q.M. Adaptability analysis of photosynthesis models of Oxalis corymbosa. Ecol. Sci. 2018, 37, 18–24. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).