Response of Soil Microbial Biomass and Activity to Cover Crop Incorporation Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Set-Up
2.2. Soil Collection and Analyses
2.3. Statistical Analysis
3. Results and Discussion
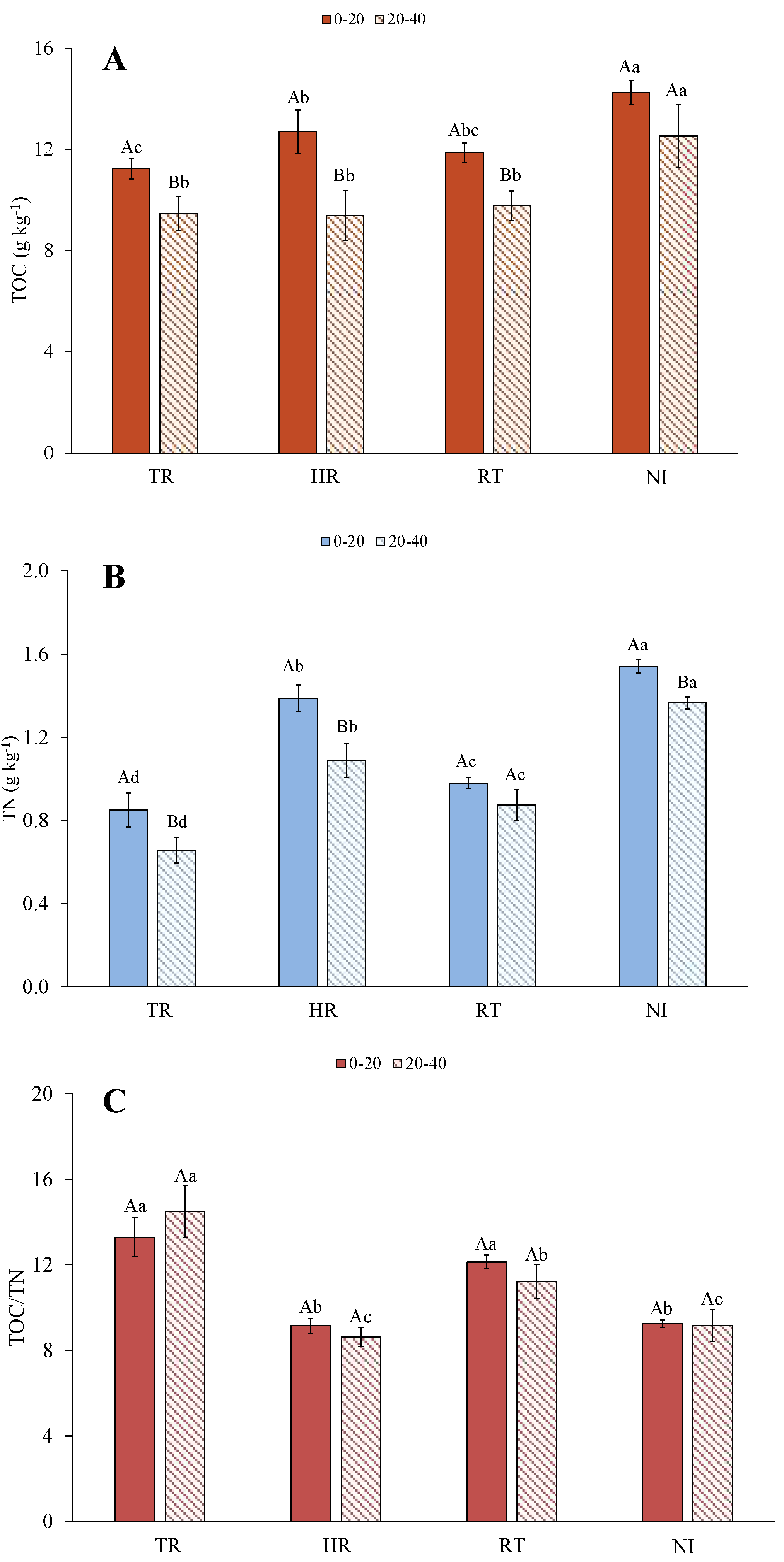
3.1. Total Organic C and N Pool
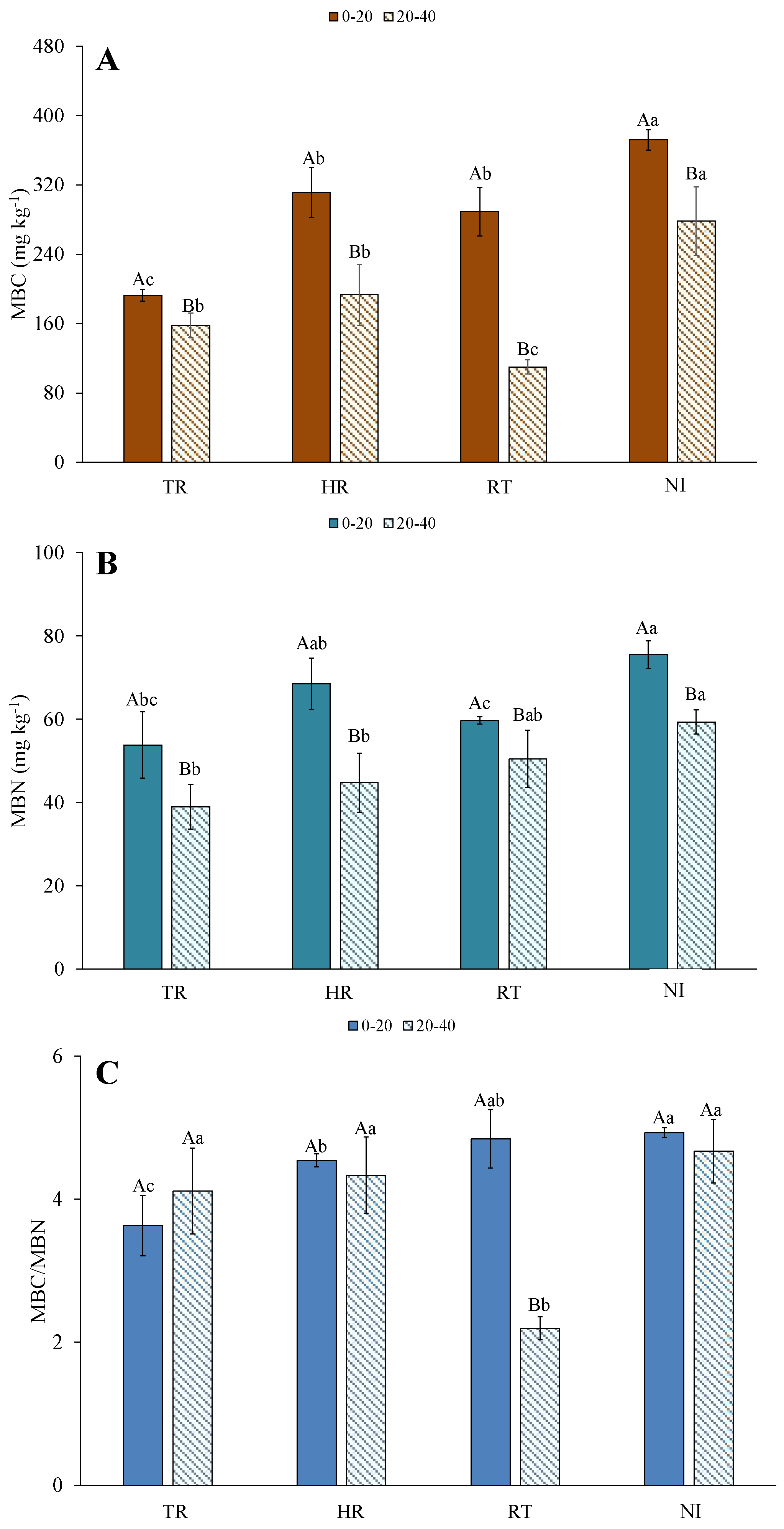
3.2. Microbial Biomass C and N
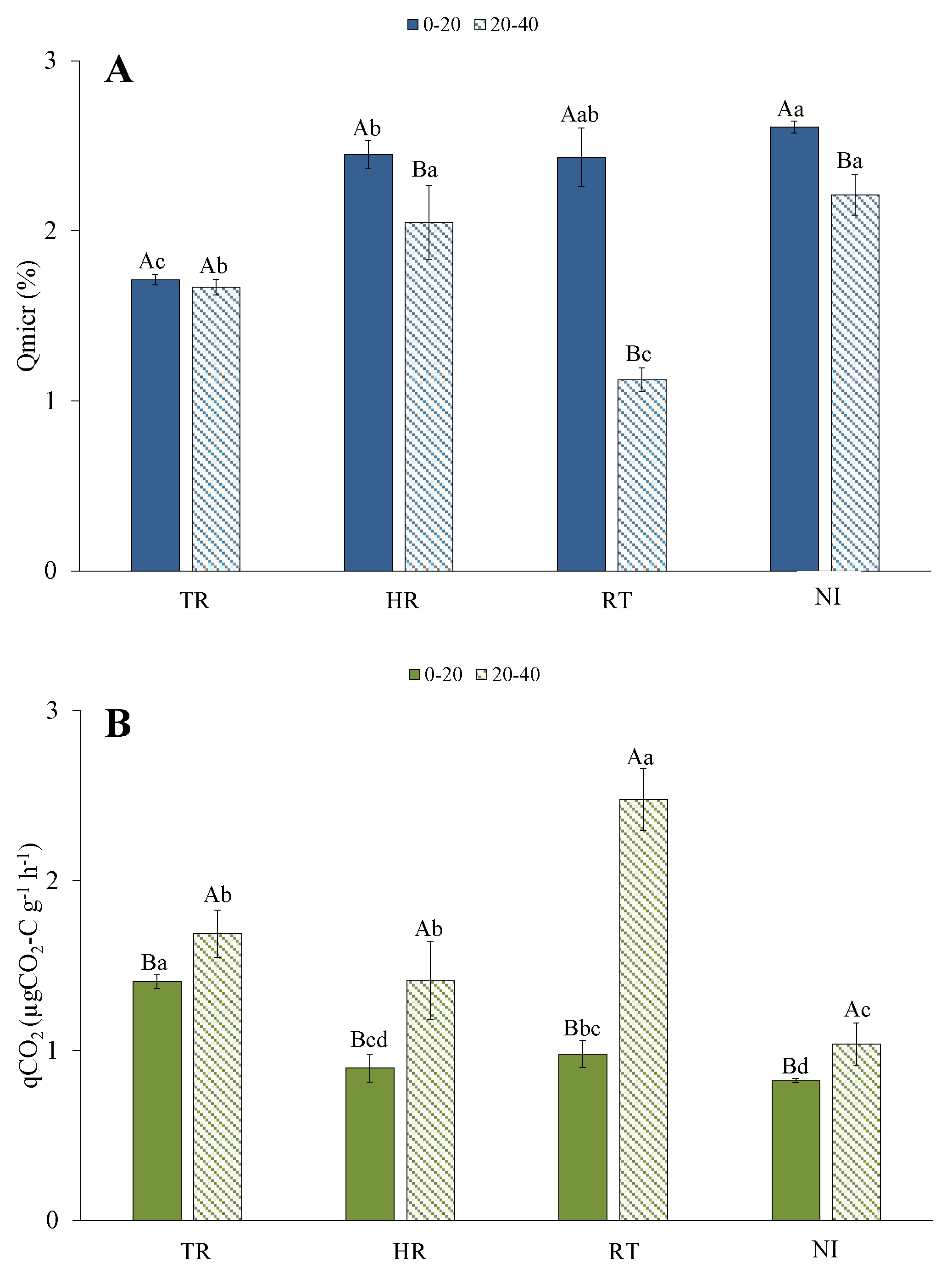
3.3. Microbial Quotients
3.4. Absolute and Specific Enzyme Activities
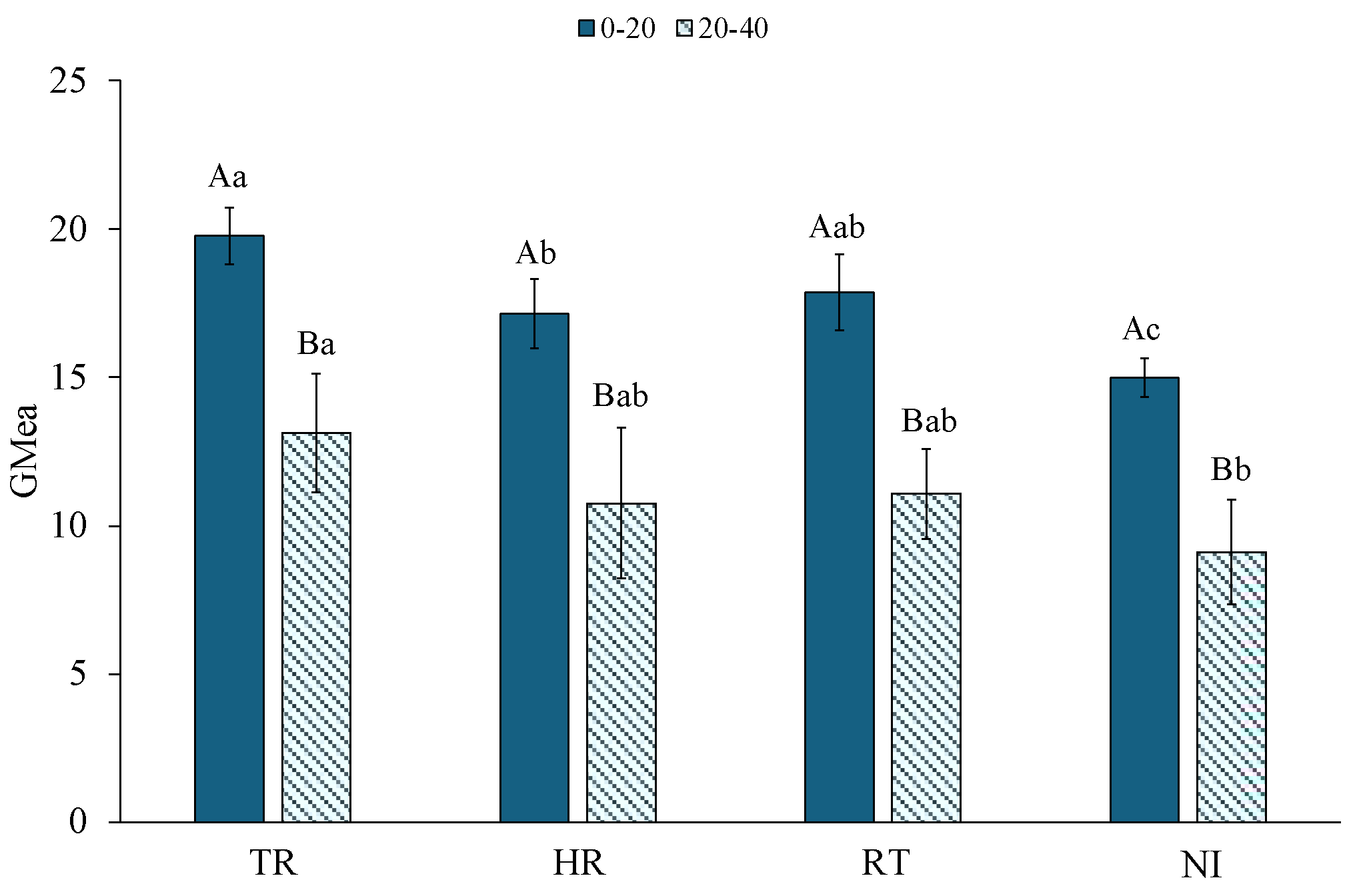
3.5. Geometric Mean of Enzyme Activities (GMea)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Musa, I.O.; Samuel, J.O.; Adams, M.; Abdulsalam, M.; Nathaniel, V.; Maude, A.M.; Adedayo, O.A.; Tiamiyu, A.T. Soil Erosion, Mineral Depletion and Regeneration. In Prospects for Soil Regeneration and Its Impact on Environmental Protection; Aransiola, S.A., Babaniyi, B.R., Aransiola, A.B., Maddela, N.R., Eds.; Earth and Environmental Sciences Library; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Francaviglia, R.; Almagro, M.; Vicente-Vicente, J.L. Conservation Agriculture and Soil Organic Carbon: Principles, Processes, Practices and Policy Options. Soil Syst. 2023, 7, 17. [Google Scholar] [CrossRef]
- Öttl, L.K.; Wilken, F.; Juřicová, A.; Batista, P.V.G.; Fiener, P. A millennium of arable land use—The long-term impact of tillage and water erosion on landscape-scale carbon dynamics. Soil 2024, 10, 281–305. [Google Scholar] [CrossRef]
- Meng, X.; Meng, F.; Chen, P.; Hou, D.; Zheng, E.; Xu, T. A meta-analysis of conservation tillage management effects on soil organic carbon sequestration and soil greenhouse gas flux. Sci. Total Environ. 2024, 954, 176315. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, S.; Guo, R.; Sarwar, M.; Ren, X.; Krstic, D.; Aslam, Z.; Zulifqar, U.; Rauf, A.; Hano, C.; et al. Carbon Sequestration to Avoid Soil Degradation: A Review on the Role of Conservation Tillage. Plants 2021, 10, 2001. [Google Scholar] [CrossRef]
- Asrotia, P.; Kumari, P.; Malik, K.; Kashyap, P.L.; Kumar, S.; Bhardwaj, A.K.; Singh, G.P. Conservation agriculture based crop management practices impact diversity and population dynamics of the insect-pests and their natural enemies in agroecosystems. Front. Sustain. Food Syst. 2023, 7, 1173048. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Ncube, B.; Mulidzi, R.; Lewu, F.B. Management impact and benefit of cover crops on soil quality: A review. Soil Tillage Res. 2020, 204, 104717. [Google Scholar] [CrossRef]
- Adetunji, A.T.; Ncube, B.; Meyer, A.H.; Olatunji, O.S.; Mulidzi, R.; Lewu, F.B. Soil pH, nitrogen, phosphatase and urease activities in response to cover crop species, termination stage and termination method. Heliyon 2021, 7, e05980. [Google Scholar] [CrossRef] [PubMed]
- Peterson, C.M.; Schomberg, H.H.; Thompson, A.I.; Mirsky, S.B.; Tully, K.L. Cover crop termination method has a limited effect on spring soil moisture and temperature in humid mid-Atlantic U.S. Agric. Water Manag. 2025, 311, 109342. [Google Scholar] [CrossRef]
- Fernando, M.; Shrestha, A. The Potential of Cover Crops for Weed Management: A Sole Tool or Component of an Integrated Weed Management System? Plants 2023, 12, 752. [Google Scholar] [CrossRef]
- Schloter, M.; Nannipieri, P.; Sørensen, S.J.; van Elsas, J.D. Microbial indicators for soil quality. Biol. Fertil. Soils 2018, 54, 1–10. [Google Scholar] [CrossRef]
- Vanden Heuvel, J.; Centinari, M. Under-Vine Vegetation Mitigates the Impacts of Excessive Precipitation in Vineyards. Front. Plant Sci. 2021, 12, 713135. [Google Scholar] [CrossRef]
- Chen, N.; Wei, R.; Cao, X.; Duan, X.; Li, H.; Wang, H. Evaluation of inter-row cover crops effects on the microbial diversity during Cabernet Sauvignon (Vitis vinifera L.) maturation. Food Res. Int. 2022, 162, 112113. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Y.; Li, T.; Chen, L.; Chen, Y.; Sui, P. Changes in soil microbial biomass, diversity, and activity with crop rotation in cropping systems: A global synthesis. Appl. Soil Ecol. 2023, 186, 104815. [Google Scholar] [CrossRef]
- Muhammad, I.; Wang, J.; Sainju, U.M.; Zhang, S.; Zhao, F.; Khan, A. Cover cropping enhances soil microbial biomass and affects microbial community structure: A meta-analysis. Geoderma 2021, 381, 114696. [Google Scholar] [CrossRef]
- Kim, N.; Zabaloy, M.C.; Guan, K.; Villamil, M.B. Do cover crops benefit soil microbiome? A meta-analysis of current research. Soil Biol. Biochem. 2020, 142, 107701. [Google Scholar] [CrossRef]
- Lucadamo, E.E.; Holmes, A.A.; Wortman, S.E.; Yannarell, A.C. Post-termination effects of cover crop monocultures and mixtures on soil inorganic nitrogen and microbial communities on two organic farms in Illinois. Front. Soil Sci. 2022, 2, 824087. [Google Scholar] [CrossRef]
- Kichler, C.M.; Kornecki, T.S.; Torbert, H.A.; Watts, D.B.; Prasad, R. Cover crop termination methods and custom residue manager effects on collard production. Agronomy 2023, 13, 2595. [Google Scholar] [CrossRef]
- Peel, M.C.; Finlayson, B.L.; McMahon, T.A. Updated world map of the Köppen-Geiger climate classification. Hydrol. Earth Syst. Sci. 2007, 11, 1633–1644. [Google Scholar] [CrossRef]
- Gee, G.W.; Bauder, J.W. Particle size analysis. In Methods of Soil Analysis. Part 1, 2nd ed.; Klute, A., Ed.; ASA: Madison, WI, USA; SSSA: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis. Part 3, Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America Book Series No 5; Soil Science Society of America: Madison, WI, USA; American Society of Agronomy: Madison, WI, USA, 1996; pp. 961–1010. [Google Scholar]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis. Part 3, Chemical Methods; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; Volume 9, pp. 1149–1178. [Google Scholar]
- Vance, E.D.; Brookes, P.C.; Jenkinson, D.S. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Paliaga, S.; Lucia, C.; Pampinella, D.; Muscarella, S.M.; Badalucco, L.; Palazzolo, E.; Laudicina, V.A. Shifting Long-Term Tillage to Geotextile Mulching for Weed Control Improves Soil Quality and Yield of Orange Orchards. Agriculture 2023, 13, 764. [Google Scholar] [CrossRef]
- Laudicina, V.A.; Palazzolo, E.; Badalucco, L. Natural organic compounds in soil solution: Potential role as soil quality indicators. Curr. Org. Chem. 2013, 17, 2991–2997. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Glucosidases and galactosidases in soils. Soil Biol. Biochem. 1988, 20, 601–606. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Arylsulfatase activity of soils. Soil Sci. Soc. Am. J. 1970, 34, 225–229. [Google Scholar] [CrossRef]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Kandeler, E.; Gerber, H. Short-term assay of soil urease activity using colorimetric determination of ammonium. Biol. Fertil. Soils 1988, 6, 68–72. [Google Scholar] [CrossRef]
- Thalmann, A. Zur Methodik der Bestimmung der Dehydrogenaseaktivität im Boden mittels triphenyltetrazolium-chlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Raiesi, F.; Salek-Gilani, S. The potential activity of soil extracellular enzymes as an indicator for ecological restoration of rangeland soils after agricultural abandonment. Appl. Soil Ecol. 2018, 126, 140–147. [Google Scholar] [CrossRef]
- Hinojosa, M.B.; García-Ruíz, R.; Viñegla, B.; Carreira, J.A. Microbiological rates and enzyme activities as indicators of functionality in soils affected by the Aznalcóllar toxic spill. Soil Biol. Biochem. 2004, 36, 1637–1644. [Google Scholar] [CrossRef]
- Paz-Ferreiro, J.; Gascó, G.; Gutiérrez, B.; Méndez, A. Soil biochemical activities and the geometric mean of enzyme activities after application of sewage sludge and sewage sludge biochar to soil. Biol. Fertil. Soils 2012, 48, 511–517. [Google Scholar] [CrossRef]
- Tang, H.; Shi, L.; Wen, L.; Cheng, K.; Li, C.; Li, W.; Xiao, X. Effects of tillage management on soil organic carbon mineralization under double cropping rice system of southern China. Sci. Rep. 2024, 14, 21146. [Google Scholar] [CrossRef]
- Quintarelli, V.; Radicetti, E.; Allevato, E.; Stazi, S.R.; Haider, G.; Abideen, Z.; Bibi, S.; Jamal, A.; Mancinelli, R. Cover Crops for Sustainable Cropping Systems: A Review. Agriculture 2022, 12, 2076. [Google Scholar] [CrossRef]
- Raffa, D.W.; Antichi, D.; Carlesi, S.; Puig-Sirera, À.; Rallo, G.; Bàrberi, P. Ground Vegetation Covers Increase Grape Yield and Must Quality in Mediterranean Vineyards. Eur. J. Agron. 2022, 136, 126483. [Google Scholar] [CrossRef]
- Balota, E.L.; Filho, A.C.; Andrade, D.S.; Dick, R.P. Long-term tillage and crop rotation effects on microbial biomass and C and N mineralization in a Brazilian Oxisol. Soil Tillage Res. 2004, 77, 137–145. [Google Scholar] [CrossRef]
- Laudicina, V.A.; Badalucco, L.; Palazzolo, E. Effects of compost input and tillage intensity on soil microbial biomass and activity under Mediterranean conditions. Biol. Fertil. Soils 2011, 47, 63–70. [Google Scholar] [CrossRef]
- Kabiri, V.; Raiesi, F.; Ghazavi, M.A. Tillage Effects on Soil Microbial Biomass, SOM Mineralization and Enzyme Activity in a Semi-Arid Calcixerepts. Agric. Ecosyst. Environ. 2016, 232, 73–84. [Google Scholar] [CrossRef]
- Patra, R.; Saha, D.; Neupane, A.; Jagadamma, S. Deep-Rooted Winter Wheat Cover Crop Promotes Subsoil Organic Carbon Storage through Improved Microbial Functional Traits. Appl. Soil Ecol. 2024, 199, 105413. [Google Scholar] [CrossRef]
- Anderson, T.; Domsch, K. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils. Soil Biol. Biochem. 1993, 25, 393–395. [Google Scholar] [CrossRef]
- Risch, A.C.; Zimmermann, S.; Schütz, M.; Borer, E.T.; Broadbent, A.A.D.; Caldeira, M.C.; Davies, K.F.; Eisenhauer, N.; Eskelinen, A.; Fay, P.A.; et al. Drivers of the Microbial Metabolic Quotient across Global Ecosystems. Glob. Ecol. Biogeogr. 2023, 32, 1797–1812. [Google Scholar] [CrossRef]
- Garcia, L.; Krafft, G.; Enard, C.; Bouisson, Y.; Metay, A. Adapting service crop termination strategy in viticulture to increase soil ecosystem functions and limit competition with grapevine. Eur. J. Agron. 2024, 156, 127161. [Google Scholar] [CrossRef]
- Elhaddad, F.; González, J.A.C.; Abdelhamid, S.; Garcia-Ruiz, R.; Chehab, H. Alternative Cover Crops and Soil Management Practices Modified the Macronutrients, Enzymes Activities, and Soil Microbial Diversity of Rainfed Olive Orchards (cv. Chetoui) under Mediterranean Conditions in Tunisia. Sustainability 2024, 16, 5329. [Google Scholar] [CrossRef]
- Badagliacca, G.; Lo Presti, E.; Ferrarini, A.; Fornasier, F.; Laudicina, V.A.; Monti, M.; Preiti, G. Early Effects of No-Till Use on Durum Wheat (Triticum durum Desf.): Productivity and Soil Functioning Vary between Two Contrasting Mediterranean Soils. Agronomy 2022, 12, 3136. [Google Scholar] [CrossRef]
- Zuber, S.M.; Villamil, M.B. Meta-Analysis Approach to Assess Effect of Tillage on Microbial Biomass and Enzyme Activities. Soil Biol. Biochem. 2016, 97, 176–187. [Google Scholar] [CrossRef]
- Ashraf, M.N.; Waqas, M.A.; Rahman, S. Microbial Metabolic Quotient Is a Dynamic Indicator of Soil Health: Trends, Implications and Perspectives (Review). Eurasian Soil Sci. 2022, 55, 1794–1803. [Google Scholar] [CrossRef]
- Laudicina, V.A.; Novara, A.; Barbera, V.; Egli, M.; Badalucco, L. Long-Term Tillage and Cropping System Effects on Chemical and Biochemical Characteristics of Soil Organic Matter in a Mediterranean Semiarid Environment. Land Degrad. Dev. 2015, 26, 45–53. [Google Scholar] [CrossRef]
- Laudicina, V.A.; Palazzolo, E.; Catania, P.; Vallone, M.; Delgado García, A.; Badalucco, L. Soil Quality Indicators as Affected by Shallow Tillage in a Vineyard Grown in a Semiarid Mediterranean Environment. Land Degrad. Dev. 2017, 28, 1038. [Google Scholar] [CrossRef]
- Hallama, M.; Möller, M.; Kätterer, T.; Jörgensen, M.; Leffelaar, P.A.; Berthold, A. Hidden miners—The roles of cover crops and soil microorganisms in phosphorus cycling. Plant Soil 2019, 438, 7–45. [Google Scholar] [CrossRef]
- Mankolo, R.; Reddy, C.; Senwo, Z.; Nyakatawa, E.; Sajjala, S. Soil Biochemical Changes Induced by Poultry Litter Application and Conservation Tillage under Cotton Production Systems. Agronomy 2012, 2, 187–198. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Zhang, Y.; Li, J.; Sun, Z.; Yang, Q. Soil Nutrient, Enzyme Activity, and Microbial Community Dynamics under Eucalyptus Plantations. Forests 2024, 15, 688. [Google Scholar] [CrossRef]
- Silwana, S.; Mulidzi, A.R.; Jovanovic, N. Short-Term Effects of Cover Crop Species and Termination Methods on Soil pH and Key Enzymatic Activities (β-Glucosidase, Phosphatase and Urease Activities) in a Citrus Orchard (Eureka Lemons). Horticulturae 2025, 11, 1014. [Google Scholar] [CrossRef]
- Wen, L.; Peng, Y.; Zhou, Y.; Cai, G.; Lin, Y.; Li, B. Effects of conservation tillage on soil enzyme activities of global cultivated land: A meta-analysis. J. Environ. Manag. 2023, 345, 118904. [Google Scholar] [CrossRef] [PubMed]
| Enzyme Activities | ||||||
| Soil Tillage | Depth | β-Glucosidase | Dehydrogenase | Phosphatase | Arylsulfatase | Urease |
| cm | (mg pNP kg−1 h−1) | (mg TPF kg−1 24 h−1) | (mg pNP kg−1 h−1) | (mg pNP kg−1 h−1) | (mg NH4+-N h−1) | |
| TR | 0–20 | 70.2 Aa | 2.7 Ab | 75.3 Aa | 8.3 Aa | 3.5 Aa |
| 20–40 | 47.2 Ba | 2.4 Aa | 60.4 Ba | 6.7 Ba | 1.6 Ba | |
| HR | 0–20 | 54.6 Ab | 3.3 Aab | 71.1 Ab | 7.0 Ab | 3.2 Aa |
| 20–40 | 29.2 Bb | 2.4 Ba | 53.5 Ba | 5.8 Bab | 1.6 Ba | |
| RT | 0–20 | 79.2 Aa | 3.3 Aa | 61.8 Ac | 6.5 Ab | 3.2 Aa |
| 20–40 | 46.1 Ba | 2.2 Ba | 54.4 Ba | 5.2 Bbc | 1.2 Bab | |
| NI | 0–20 | 57.1 Ab | 3.5 Aa | 71.4 Ab | 6.9 Ab | 1.8 Ab |
| 20–40 | 51.0 Ba | 2.4 Ba | 54.7 Ba | 3.6 Bc | 0.7 Bb | |
| SEA | ||||||
| Soil tillage | Depth | β-Glucosidase/ MBC | Dehydrogenase/ MBC | Phosphatase/ MBC | Arylsulfatase/ MBC | Urease/ MBC |
| TR | 0–20 | 19.7 Aa | 0.014 Aa | 1.42 Aa | 0.124 Aa | 0.34 Aa |
| 20–40 | 11.6 Bb | 0.015 Ab | 1.57 Aa | 0.122 Aa | 0.18 Ba | |
| HR | 0–20 | 12.0 Ab | 0.010 Ab | 1.04 Bb | 0.089 Ac | 0.29 Ab |
| 20–40 | 6.8 Bc | 0.013 Ab | 1.20 Ab | 0.087 Ac | 0.20 Aa | |
| RT | 0–20 | 16.4 Ba | 0.011 Bb | 1.04 Ab | 0.096 Bb | 0.29 Ab |
| 20–40 | 21.2 Aa | 0.020 Aa | 1.09 Ab | 0.105 Ab | 0.13 Ba | |
| NI | 0–20 | 11.6 Ab | 0.009 Ac | 0.94 Ab | 0.082 Ad | 0.15 Ac |
| 20–40 | 10.9 Bb | 0.009 Ac | 0.92 Ac | 0.048 Bd | 0.07 Bb | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucia, C.; Laudicina, V.A.; Paliaga, S.; Gristina, L.; Muscarella, S.M. Response of Soil Microbial Biomass and Activity to Cover Crop Incorporation Methods. Agronomy 2025, 15, 2504. https://doi.org/10.3390/agronomy15112504
Lucia C, Laudicina VA, Paliaga S, Gristina L, Muscarella SM. Response of Soil Microbial Biomass and Activity to Cover Crop Incorporation Methods. Agronomy. 2025; 15(11):2504. https://doi.org/10.3390/agronomy15112504
Chicago/Turabian StyleLucia, Caterina, Vito Armando Laudicina, Sara Paliaga, Luciano Gristina, and Sofia Maria Muscarella. 2025. "Response of Soil Microbial Biomass and Activity to Cover Crop Incorporation Methods" Agronomy 15, no. 11: 2504. https://doi.org/10.3390/agronomy15112504
APA StyleLucia, C., Laudicina, V. A., Paliaga, S., Gristina, L., & Muscarella, S. M. (2025). Response of Soil Microbial Biomass and Activity to Cover Crop Incorporation Methods. Agronomy, 15(11), 2504. https://doi.org/10.3390/agronomy15112504







