The Identification, Characterization, and Fungicide Sensitivity of Leptosphaerulina trifolii Causing Didymellaceae Leaf Spot of Elymus Plants in China
Abstract
1. Introduction
2. Materials and Methods
2.1. Pathogen Isolation
2.2. Morphological Identification
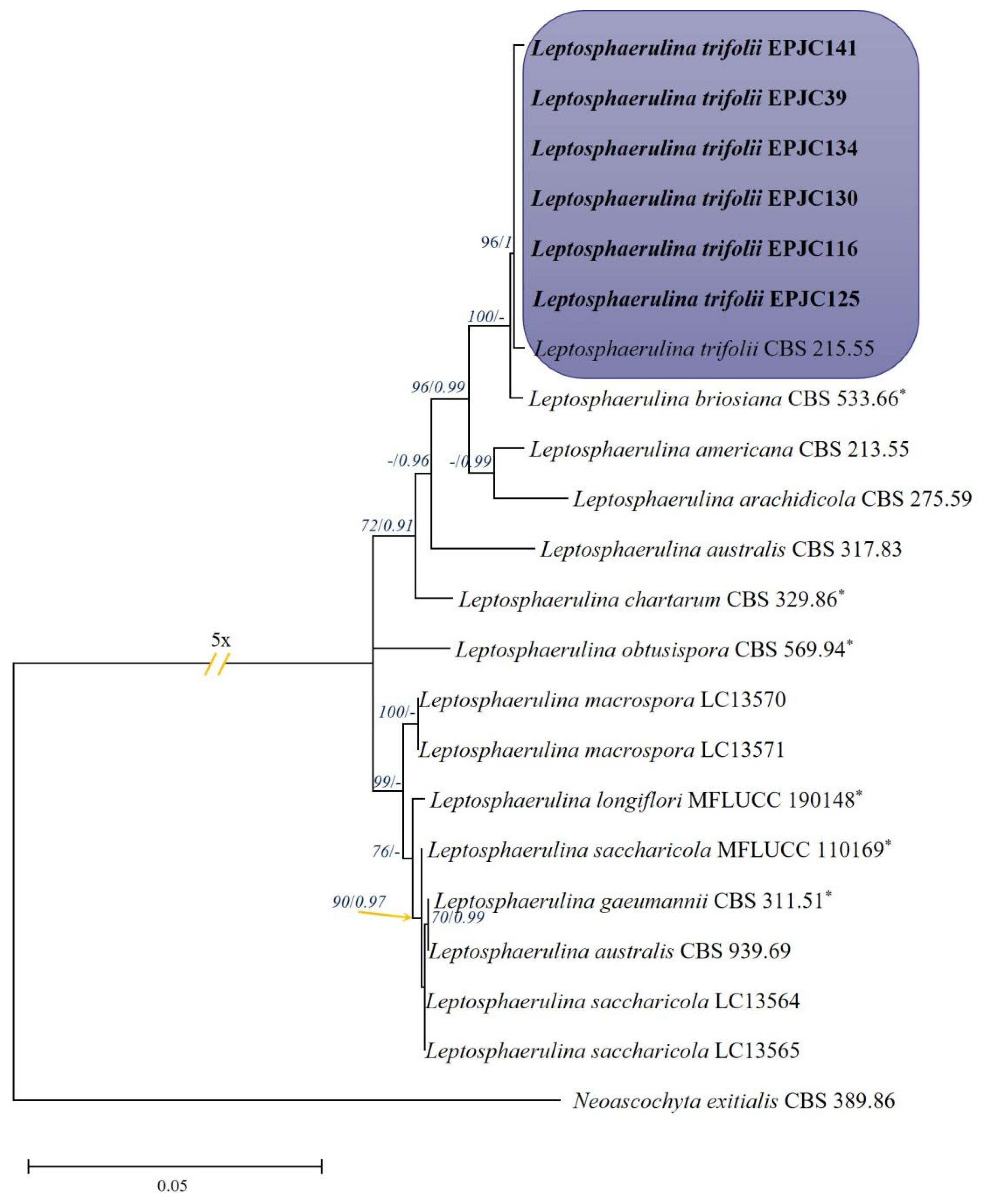
2.3. DNA Extraction, PCR Amplification and Sequencing, and Phylogenetic Analyses
2.4. Cultural Characteristics of Isolates In Vitro
2.5. Pathogenicity Tests
2.6. In Vitro Evaluation of Fungicide Effects on L. trifolii
3. Results
3.1. Disease Symptoms
3.2. Morphological Characterization of the Strains
3.3. Molecular Phylogeny
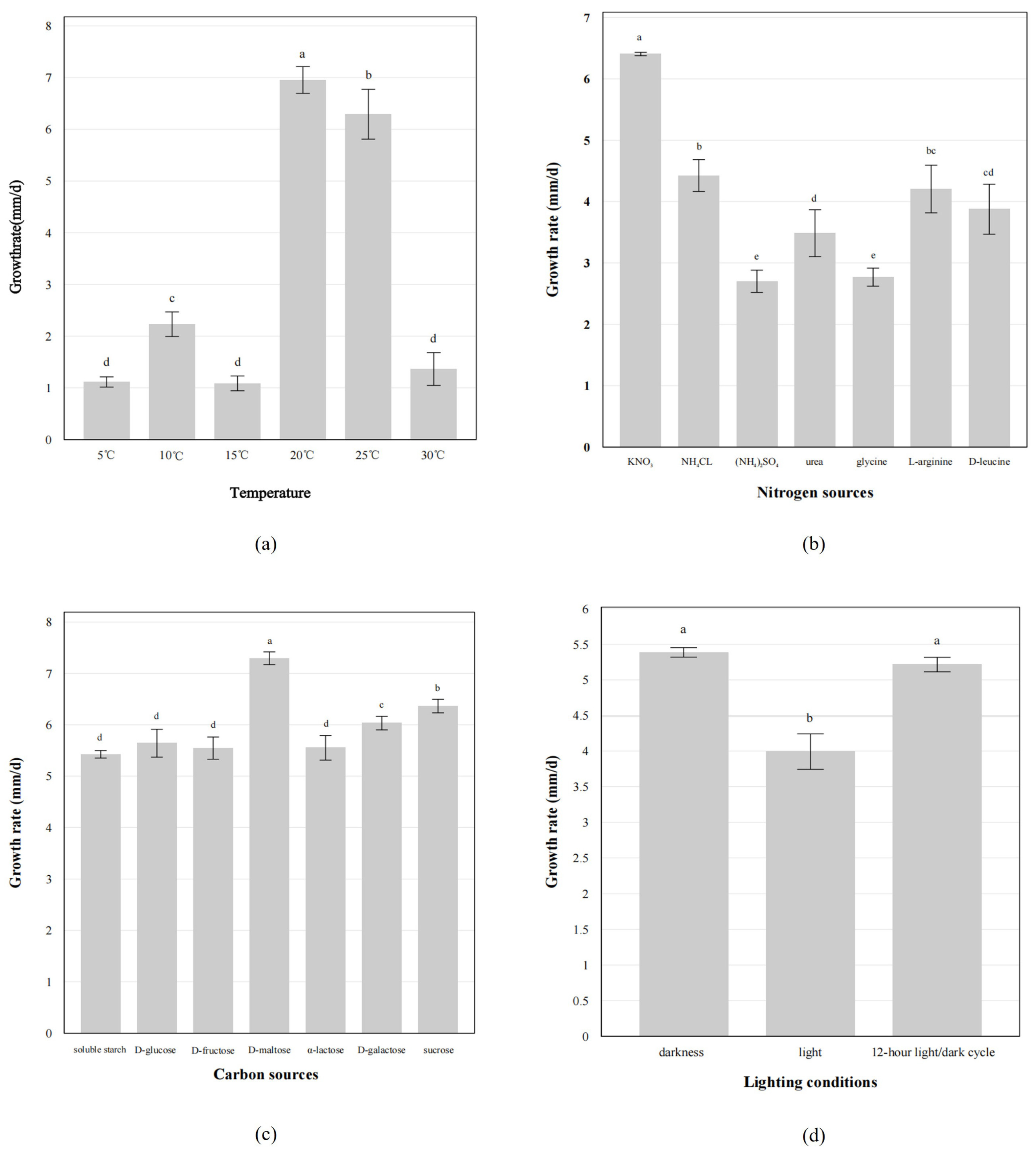
3.4. Biological Characteristics of the Fungus
3.5. The Result of Pathogenicity Tests
3.6. In Vitro Fungicide Sensitivity of L. trifolii
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, M.Q.; Yang, J.; Wag, X.; Li, D.X.; Zhang, C.B.; Tian, Z.H.; You, M.H.; Bai, S.Q.; Lin, H.H. Transcriptome profiles identify the common responsive genes to drought stress in two Elymus species. J. Plant Physiol. 2020, 250, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xue, L.; Li, C. Characterization of Didymellaceae leaf spot associated with Elymus plants in the Tibetan Plateau of China. Plant Dis. 2025. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yang, H.; Yu, X.; Chen, Y.; Yang, C.; He, Y.; Wang, H. Effects of combined application of nitrogen, phosphorus, and potassium fertilizers on seed yield, se0ed quality and economic returns of Elymus nutans in alpine region. BMC Plant Biol. 2025, 25, 130. [Google Scholar] [CrossRef]
- Hou, C.Y.; Wei, X.; Zhou, L.; Ma, J.H.; Ren, X.; Wang, Y.Y.; Wu, P.F. Dynamics of soil nematode community in perennial Gramineae artificial grasslands in Northwest Sichuan. Acta Ecol. Sin. 2023, 43, 10104–10118. [Google Scholar] [CrossRef]
- Quan, X.; Qiao, Y.; Chen, M.; Duan, Z.; Shi, H. Comprehensive evaluation of the allelopathic potential of Elymus nutans. Ecol. Evol. 2021, 11, 12389–12400. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.C.; Henk, D.A.; Briggs, C.J.; Brownstein, J.S.; Madoff, L.C.; McCraw, S.L.; Gurr, S.J. Emerging fungal threats to ani-mal, plant and ecosystem health. Nature 2012, 484, 186–194. [Google Scholar] [CrossRef]
- Liu, J.Q.; Bao, G.S.; Li, C.J. First report of brown leaf spot of Elymus nutans caused by Bipolaris sorokiniana in northwestern China. Plant Dis. 2023, 107, 941. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, D.; Jiang, F.; Tian, Z.; Feng, X.; Wu, N.; Hou, F.; Nan, Z.; Chen, T. Long-term heavy grazing increases community-level foliar fungal diseases by shifting plant composition. J. Appl. Ecol. 2022, 59, 791–800. [Google Scholar] [CrossRef]
- Xue, L.H.; Liu, J.Q.; Li, C.J. Progress in research on fungal diseases of the genus Elymus. Acta Prataculturae Sin. 2024, 33, 226–241. (In Chinese). Available online: http://cyxb.magtech.com.cn/CN/10.11686/cyxb2023132 (accessed on 7 September 2025).
- Huo, H.; Huangfu, J.; Song, P.; Zhang, D.; Shi, Z.; Zhao, L.; Li, Z.; Zhou, H. Isolation, identification and characterization of Leptosphaerulina trifolii, the causative agent of alfalfa Leptosphaerulina Leaf spot in Inner Mongolia, China. Agronomy 2024, 14, 1156. [Google Scholar] [CrossRef]
- Hou, L.W.; Groenewald, J.Z.; Pfenning, L.H.; Yarden, O.; Crous, P.W.; Cai, L. The phoma-like dilemma. Stud. Mycol. 2020, 96, 309–396. [Google Scholar] [CrossRef]
- Zhang, Y.; Schoch, C.L.; Fournier, J.; Crous, P.W.; de Gruyter, J.; Woudenberg, J.H.C.; Hirayama, K.; Tanaka, K.; Pointing, S.B.; Spatafora, J.W.; et al. Multi-locus phylogeny of Pleosporales: A taxonomic, ecological and evolutionary re-evaluation. Stud. Mycol. 2009, 64, 85–102. [Google Scholar] [CrossRef]
- Chen, Q.; Hou, L.W.; Duan, W.J.; Crous, P.W.; Cai, L. Didymellaceae revisited. Stud. Mycol. 2017, 87, 105–159. [Google Scholar] [CrossRef]
- Valenzuela-Lopez, N.; Cano-Lira, J.F.; Guarro, J.; Sutton, D.A.; Wiederhold, N.; Crous, P.W.; Stchigel, A.M. Coelomycetous Dothideomycetes with emphasis on the families Cucurbitariaceae and Didymellaceae. Stud. Mycol. 2018, 90, 177–301. [Google Scholar] [CrossRef]
- Zhang, L.L.; Li, Y.Z. First report of alfalfa leaf spot caused by Leptosphaerulina australis in China. Plant Dis. 2021, 105, 2254. [Google Scholar] [CrossRef]
- Liu, X.P.; Jing, X.M.; Yan, H.X.; Li, G.L.; Luo, Y.H. First report of Leptosphaerulina leaf spot caused by Leptosphaerulina trifolii on alfalfa in Heilongjiang Province, China. Plant Dis. 2019, 103, 2673. [Google Scholar] [CrossRef]
- Victoria, A.A.D.; Furtado, B.E.; Holz, M.T.; Romero-Arenas, O.; Dallagnol, L.J. First report of leptosphaerulina leaf spot caused by Leptosphaerulina trifolii on trifolium repens in brazil. Plant Dis. 2020, 104, 972. [Google Scholar] [CrossRef]
- Sheng, Y.T.; Yu, X.L.; Mao, T.T.; Zhang, J.; Guo, X.T.; Song, Z.Z.; Zhang, H.X. Genome sequence data of Leptosphaerulina arachidicola, a causal agent of peanut scorch spot in China. Plant Dis. 2022, 106, 748–750. [Google Scholar] [CrossRef]
- Phookamsak, R.; Liu, J.K.; Chukeatirote, E.; McKenzie, E.H.; Hyde, K.D. Phylogeny and morphology of Leptosphaerulina saccharicola sp. nov. and Pleosphaerulina oryzae and relationships with Pithomyces. Cryptogam. Mycol. 2013, 34, 303–319. [Google Scholar] [CrossRef]
- Tennakoon, D.S.; Thambugala, K.M.; De Silva, N.I.; Kuo, C.H.; Hyde, K.D. Leaf litter saprobic Didymellaceae (Dothideomycetes): Leptosphaerulina longiflori sp. nov. and Didymella sinensis, a new record from Roystonea regia. Asian J. Mycol. 2019, 2, 87–100. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, S.; Li, X.; Yang, M.; Sun, L.; He, S.; Wu, D.; Fu, Y. Occurrence of maize leaf spot disease caused by Leptosphaerulina australis in Yunnan, China. Plant Dis. 2024, 108, 3407. [Google Scholar] [CrossRef]
- Chen, Q.; Bakhshi, M.; Balci, Y.; Broders, K.D.; Cheewangkoon, R.; Chen, S.F.; Fan, X.L.; Gramaje, D.; Halleen, F.; Jung, M.H.; et al. Genera of phytopathogenic fungi: GOPHY 4. Stud. Mycol. 2022, 101, 417–564. [Google Scholar] [CrossRef]
- Keirnan, E.C.; Tan, Y.P.; Laurence, M.H.; Mertin, A.A.; Liew, E.C.; Summerell, B.A.; Shivas, R.G. Cryptic diversity found in Didymellaceae from Australian native legumes. MycoKeys 2021, 78, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.C.; Tu, Y.Y.; Chen, X.L.; Jiang, H.; Ren, H.Z.; Lu, Q.H.; Wei, C.L.; Lv, W.Y. Didymellaceae species associated with tea plant (Camellia sinensis) in China. MycoKeys 2024, 105, 217–251. [Google Scholar] [CrossRef]
- Nan, Z.B. Establishing sustainable management system for diseases of pasture crops in China. Acta Prataculturae Sin. 2000, 2, 1–9. (In Chinese). Available online: https://kns.cnki.net/kcms2/article/abstract?v=o8vMLOX1CKtXTwECOybpHkymflmFDoPnHyE5VwGYgrO95lkBMExMYbIaluPy1hOi7TbWGq1UgtQ0YIs5PggZPsHcASrGz8JZ7JDPOSM6Mm3iPhxrXTEaMFHPsyhdd-pz_0bt6_Rt5kiSmzaB10S9gymn687yhc0mlHYfwNH87XVhJxXel_6gvA==&uniplatform=NZKPT&language=CHS (accessed on 7 September 2025).
- Xue, L.; Liu, Y.; Zhou, S.; White, J.F.; Li, C. Characterization of Pyrenophora species causing brown leaf spot on Italian ryegrass (Lolium multiflorum) in southwestern China. Plant Dis. 2020, 104, 1900–1907. [Google Scholar] [CrossRef]
- Xue, L.H.; Xu, Z.T.; Liu, J.Q.; Chen, H.; White, J.F.; Malik, K.l.; Li, C.J. Differences in the characteristics and pathogenicity of Pyrenophora species associated with seeds of Italian ryegrass. Plant Dis. 2023, 107, 758–770. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR Protoc. A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Chen, Q.; Jiang, J.R.; Zhang, G.Z.; Cai, L.; Crous, P.W. Resolving the Phoma enigma. Stud. Mycol. 2015, 82, 137–217. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef]
- Vaidya, G.; Lohman, D.J.; Meier, R. SequenceMatrix: Concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 2011, 27, 171–180. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Bret, L.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Edler, D.; Klein, J.; Antonelli, A.; Silvestro, D. raxmlGUI 2.0: A graphical interface and toolkit for phylogenetic analyses using RAxML. Methods Ecol. Evol. 2021, 12, 373–377. [Google Scholar] [CrossRef]
- Nylander, J.A.A. MrModeltest v2. Program distributed by the author. Evolutionary Biology Centre, Uppsala University.ampignons de l’Équateur (Pugillus IV). Bull. L’Herb. Boissier 2004, 3, 53–74. [Google Scholar]
- Nuin, P. MrMTgui: Cross-Platform Interface for ModelTest and Mr Modeltest 2008. Available online: https://mrmtgui.software.informer.com/ (accessed on 7 September 2025).
- Snow, M.J.; Manley-Harris, M. On the nature of non-peroxide antibacterial activity in New Zealand manuka honey. Food Chem. 2004, 84, 145–147. [Google Scholar] [CrossRef]
- Liang, J.; Li, G.; Hou, L.; Zhao, M.; Cai, L. Leptosphaerulina species isolated from golf turfgrass in China, with description of L. macrospora, sp. nov. Mycologia 2021, 113, 956–967. [Google Scholar] [CrossRef]
- Barbetti, M.J. Resistance in annual Medicago spp. to Phoma medicaginis and Leptosphaerulina trifolii and its relationship to induced production of a phytoestrogen. Plant Dis. 2007, 91, 239–244. [Google Scholar] [CrossRef]
- Karisto, P.; Suffert, F.; Mikaberidze, A. Measuring splash dispersal of a major wheat pathogen in the field. PhytoFrontiers™ 2022, 2, 30–40. [Google Scholar] [CrossRef]
- Penet, L.; Guyader, S.; Pétro, D.; Salles, M.; Bussière, F. Direct splash dispersal prevails over indirect and subsequent spread during rains in Colletotrichum gloeosporioides infecting yams. PLoS ONE 2014, 9, e115757. [Google Scholar] [CrossRef]
- Fitt, B.D.; Brun, H.; Barbetti, M.J.; Rimmer, S.R. World-wide importance of Phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). Eur. J. Plant Pathol. 2006, 114, 3–15. [Google Scholar] [CrossRef]
- Aveskamp, M.M.; de Gruyter, J.; Woudenberg, J.H.C.; Verkley, G.J.M.; Crous, P.W. Highlights of the Didymellaceae: A polyphasic approach to characterise Phoma and related pleosporalean genera. Stud. Mycol. 2010, 65, 1–60. [Google Scholar] [CrossRef] [PubMed]
- Boerema, G.A.; Bollen, G.J. Conidiogenesis and conidial septation as differentiating criteria between Phoma and Ascochyta. Persoonia-Mol. Phylogeny Evol. Fungi 1975, 8, 111–144. Available online: https://repository.naturalis.nl/pub/531868/ (accessed on 7 September 2025).
- De Gruyter, J.; Aveskamp, M.M.; Woudenberg, J.H.; Verkley, G.J.; Groenewald, J.Z.; Crous, P.W. Molecular phylogeny of Phoma and allied anamorph genera: Towards a reclassification of the Phoma complex. Mycol. Res. 2009, 113, 508–519. [Google Scholar] [CrossRef]
- Morgan-Jones, G. Concerning some species of Microsphaeropsis. Can. J. Bot. 1974, 52, 2575–2579. [Google Scholar] [CrossRef]
- Aveskamp, M.M.; Verkley, G.J.; de Gruyter, J.; Murace, M.A.; Perello, A.; Woudenberg, J.H.; Groenewald, J.Z.; Crous, P.W. DNA phylogeny reveals polyphyly of Phoma section Peyronellaea and multiple taxonomic novelties. Mycologia 2009, 101, 363–382. [Google Scholar] [CrossRef]
- Woudenberg, J.H.; De Gruyter, J.; Crous, P.W.; Zwiers, L.H. Analysis of the mating-type loci of co-occurring and phylogenetically related species of Ascochyta and Phoma. Mol. Plant Pathol. 2012, 13, 350–362. [Google Scholar] [CrossRef]
- Olanya, O.M.; Campbell, C.L. Isolate characteristics and epidemic components of Leptosphaerulina leaf spots on alfalfa and white clover. Phytopathology 1990, 80, 1278–1282. [Google Scholar] [CrossRef]
- Karim, M.M.; Usman, H.M.; Tan, Q.; Hu, J.J.; Fan, F.; Hussain, R.; Luo, C.X. Fungicide resistance in Colletotrichum fructicola and Colletotrichum siamense causing peach anthracnose in China. Pestic. Biochem. Physiol. 2024, 203, 106006. [Google Scholar] [CrossRef]
- Ahonsi, M.O.; Ames, K.A.; Gray, M.E.; Bradley, C.A. Biomass reducing potential and prospective fungicide control of a new leaf blight of Miscanthus× giganteus caused by Leptosphaerulina chartarum. BioEnergy Res. 2013, 6, 737–745. [Google Scholar] [CrossRef]
- Lowe, K.F.; Langdon, P.W.; Bowdler, T.M. Combined effects of leaf spot diseases caused by Stemphylium vesicarium (Wallr.) Simmons and Leptosphaerulina trifolii (Rostr.) Petr. on lucerne cultivars, and the efficacy of some fungicidal control methods. Aust. J. Exp. Agric. 1987, 27, 59–65. [Google Scholar] [CrossRef]
- Crous, P.W.; Schoch, C.L.; Hyde, K.D.; Wood, A.R.; Gueidan, C.; De Hoog, G.S.; Groenewald, J.Z. Phylogenetic lineages in the Capnodiales. Stud. Mycol. 2009, 64, 17–47. [Google Scholar] [CrossRef]
- Gardes, M.; Bruns, T.D. ITS primers with enhanced specificity for basidiomycetes-application to the identification of mycorrhizae and rusts. Mol. Ecol. 1993, 2, 113–118. [Google Scholar] [CrossRef]
- Liu, Y.J.; Whelen, S.; Hall, B.D. Phylogenetic relationships among ascomycetes: Evidence from an RNA polymerse II subunit. Mol. Biol. Evol. 1999, 16, 1799–1808. [Google Scholar] [CrossRef]
- Schoch, C.L.; Seifert, K.A.; Huhndorf, S.; Robert, V.; Spouge, J.L.; Levesque, C.A.; Levesque, A.; Chen, W.; Fungal Barcoding Consortium. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proc. Natl. Acad. Sci. USA 2012, 109, 6241–6246. [Google Scholar] [CrossRef] [PubMed]
- Simon, U.K.; Groenewald, J.Z.; Crous, P.W. Cymadothea trifolii, an obligate biotrophic leaf parasite of Trifolium, belongs to Mycosphaerellaceae as shown by nuclear ribosomal DNA analyses. Persoonia-Mol. Phylogeny Evol. Fungi 2009, 22, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Sung, G.H.; Sung, J.M.; Hywel-Jones, N.L.; Spatafora, J.W. A multi-gene phylogeny of Clavicipitaceae (Ascomycota, Fungi): Identification of localized incongruence using a combinational bootstrap approach. Mol. Phylogenetics Evol. 2007, 44, 1204–1223. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.; Groenewald, M.; De Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef]



| Strains | Number of Isolated Strain (n) | Host | Location | GenBank Accession | ||
|---|---|---|---|---|---|---|
| ITS | LSU | RPB2 | ||||
| EPJC39 | 8 | Elymus nutans | Maqu, Gannan, Gansu | PP343084 | PX099127 | PV837467 |
| EPJC116 | 4 | E. nutans | Maqu, Gannan, Gansu | PX099222 | PX099128 | PV837468 |
| EPJC125 | 5 | E. sibiricus | Haiyan, Haibei, Qinghai | PX099223 | PX099129 | PV837469 |
| EPJC130 | 2 | E. nutans | Maqu, Gannan, Gansu | PX108633 | PX108636 | PX111706 |
| EPJC134 | 2 | E. nutans | Maqu, Gannan, Gansu | PX108634 | PX108637 | PX111707 |
| EPJC141 | 4 | E. sibiricus | Haiyan, Haibei, Qinghai | PX108635 | PX108638 | PX111708 |
| Fungicide | Formulation | Manufacturer |
|---|---|---|
| 30% Benzalconazole | Suspo-emulsion | Sichuan Guoguang Agrochemical Co., Ltd., Chengdu, China |
| 70% Propineb | Wettable powder | Bayer AG, North Rhine-Westphalia, Germany |
| 47% Spring Thunder King Copper | Wettable powder | Beijing Green Agricultural Science and Technology Group Co., Ltd., Beijing, China |
| 40% Isoprothiolane | Emulsifiable concentrate | Beijing Green Agricultural Science and Technology Group Co., Ltd., Beijing, China |
| 50% Carbendazim | Wettable powder | Hebei Dewoduo Biotechnology Co., Ltd., Hengshui, China |
| Fungicide | Concentration Gradient of Active Constituent (mg/L) |
|---|---|
| 30% Benzalconazole | 0.15, 0.75, 1.5, 3, 6, 30, 150, 300 |
| 70% Propineb | 0.35, 1.75, 3.5, 7, 14, 70, 350, 850 |
| 47% Spring Thunder King Copper | 0.235, 1.175, 2.35, 4.7, 9.4, 47, 470, 940 |
| 40% Isoprothiolane | 0.25, 1.25, 2.5, 5, 10, 50, 250, 500 |
| 50% Carbendazim | 0.2, 1, 2, 4, 8, 40, 500, 1000 |
| Fungicides | Regression Equation | EC50 (mg/L) | Correlation Index (r2) | 95% Confidence Intervals |
|---|---|---|---|---|
| 30% Benzalconazole | Y = 26.46X + 21.80 | 11.76 | 0.97 | 21.80–31.11 |
| 70% Propineb | Y = 24.72X + 2.182 | 87.10 | 0.88 | 15.72–33.72 |
| 47% Spring Thunder King Copper | Y = 20.07X + 4.866 | 177.83 | 0.91 | 13.72–26.42 |
| 40% Isoprothiolane | Y = 21.92X + 12.58 | 51.29 | 0.95 | 16.81–27.02 |
| 50% Carbendazim | Y = 17.31X + 9.372 | 223.87 | 0.95 | 13.49–21.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Xue, L.; Li, C. The Identification, Characterization, and Fungicide Sensitivity of Leptosphaerulina trifolii Causing Didymellaceae Leaf Spot of Elymus Plants in China. Agronomy 2025, 15, 2502. https://doi.org/10.3390/agronomy15112502
Liu J, Xue L, Li C. The Identification, Characterization, and Fungicide Sensitivity of Leptosphaerulina trifolii Causing Didymellaceae Leaf Spot of Elymus Plants in China. Agronomy. 2025; 15(11):2502. https://doi.org/10.3390/agronomy15112502
Chicago/Turabian StyleLiu, Jiaqi, Longhai Xue, and Chunjie Li. 2025. "The Identification, Characterization, and Fungicide Sensitivity of Leptosphaerulina trifolii Causing Didymellaceae Leaf Spot of Elymus Plants in China" Agronomy 15, no. 11: 2502. https://doi.org/10.3390/agronomy15112502
APA StyleLiu, J., Xue, L., & Li, C. (2025). The Identification, Characterization, and Fungicide Sensitivity of Leptosphaerulina trifolii Causing Didymellaceae Leaf Spot of Elymus Plants in China. Agronomy, 15(11), 2502. https://doi.org/10.3390/agronomy15112502






