Analysis of Soil Nutrients and Microbial Community Characteristics in Rainfed Rice–Potato Cropping Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site Characteristics
2.2. Experimental Materials
2.3. Experimental Design
2.4. Experimental Methodology
2.4.1. Soil Sample Collection Protocol
2.4.2. Analytical Procedures for Soil Properties
2.4.3. Molecular Analysis Procedures
2.5. Statistical Analysis Framework
3. Results
3.1. Effects of Different Rice Cultivation Methods on Soil Nutrients and Enzyme Activity for Subsequent Potato Crops
3.2. Effects of Different Rice Cultivation Methods on Soil Microbial Community Diversity in Subsequent Potato Crops
3.2.1. Soil Microbial Alpha-Diversity Analysis in Rainfed and Waterlogged Rice–Potato Cropping Systems
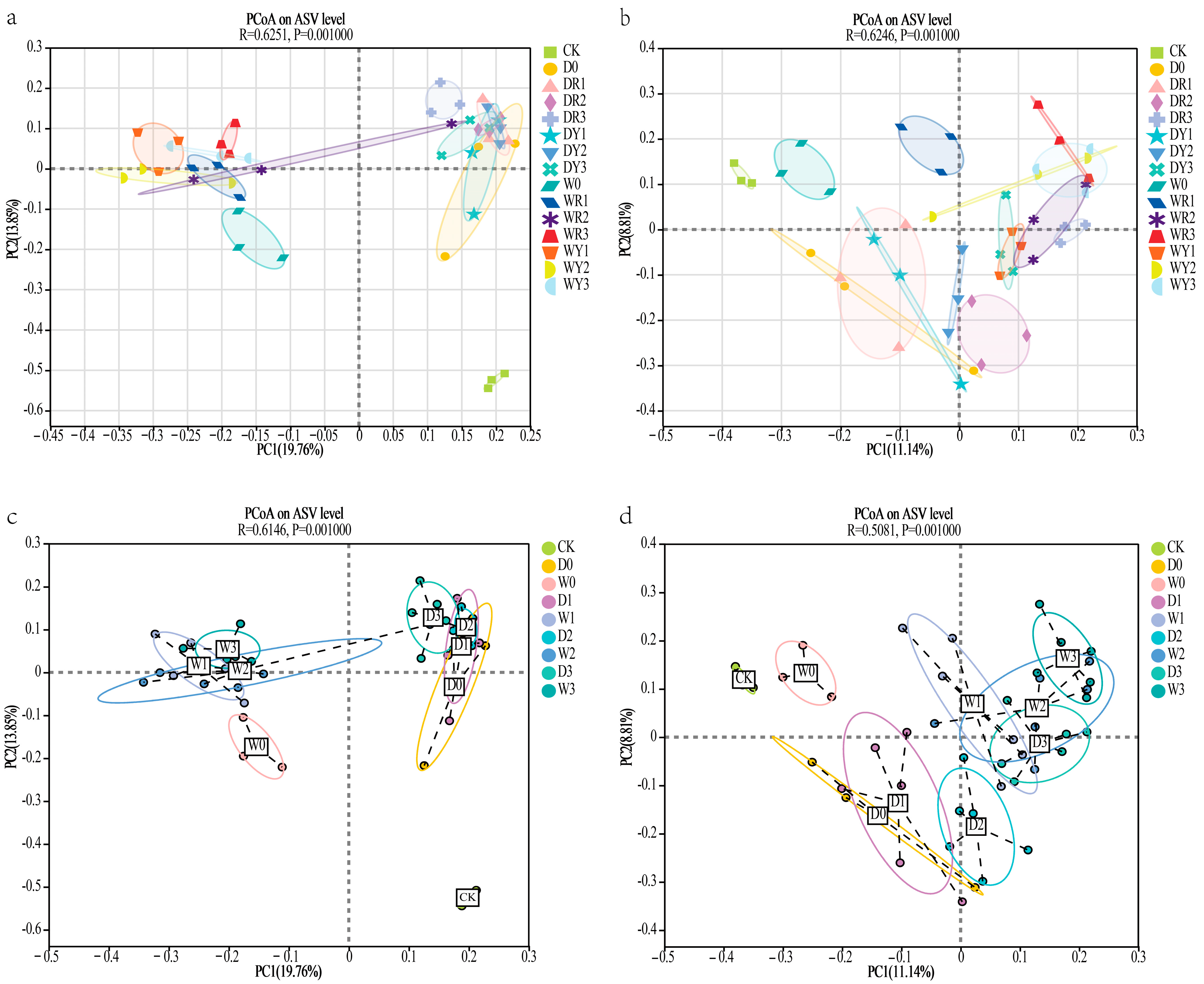
3.2.2. Soil Microbial Beta-Diversity Analysis in Rainfed and Waterlogged Rice–Potato Cropping Systems
3.2.3. Similarity Analysis of Soil Microbial Communities in Dryland/Irrigated Rice–Potato Double Cropping Systems
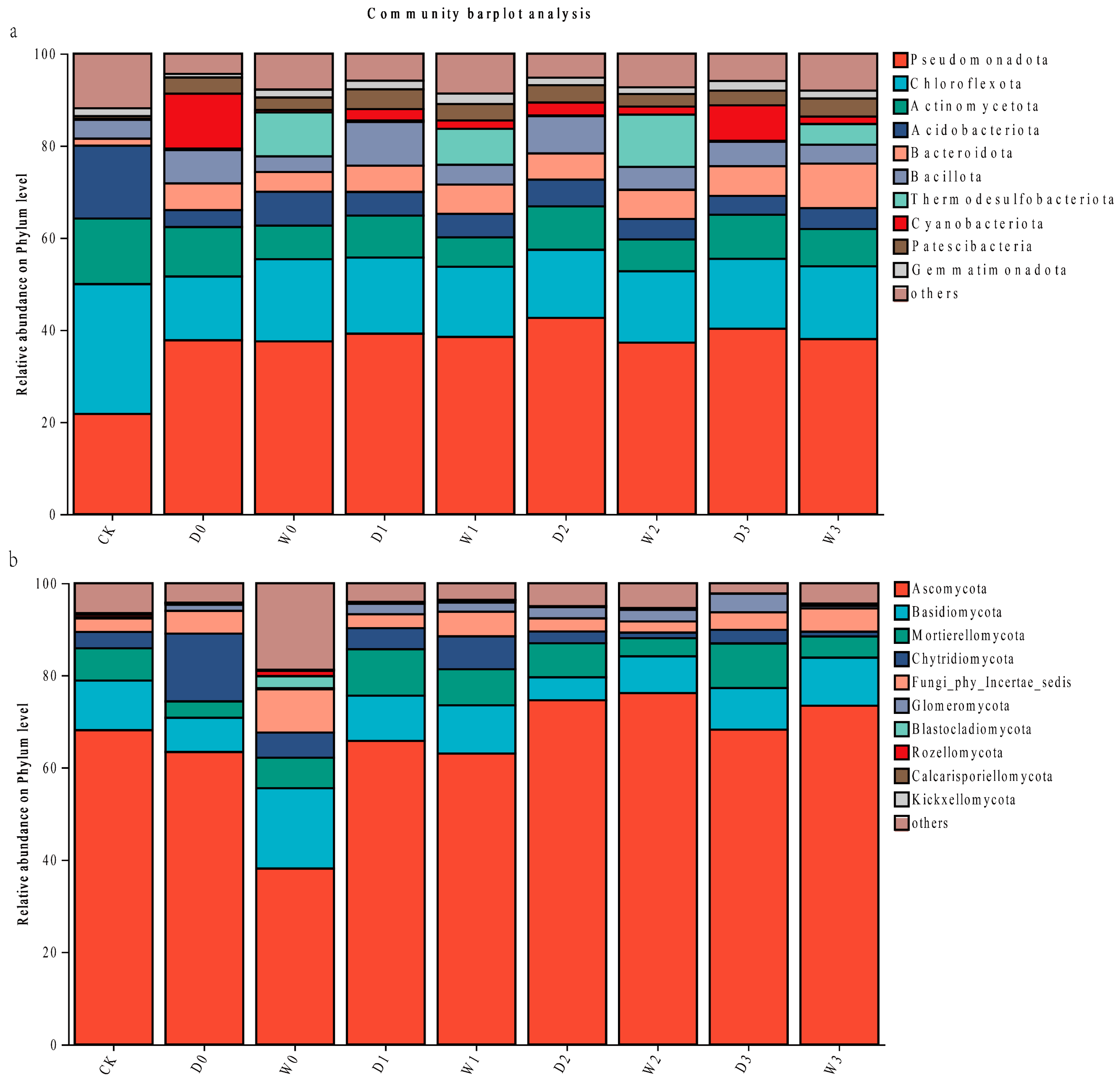
3.2.4. Soil Microbial Community Composition Analysis at Phylum Level
Bacterial Community Structure
Fungal Community Structure
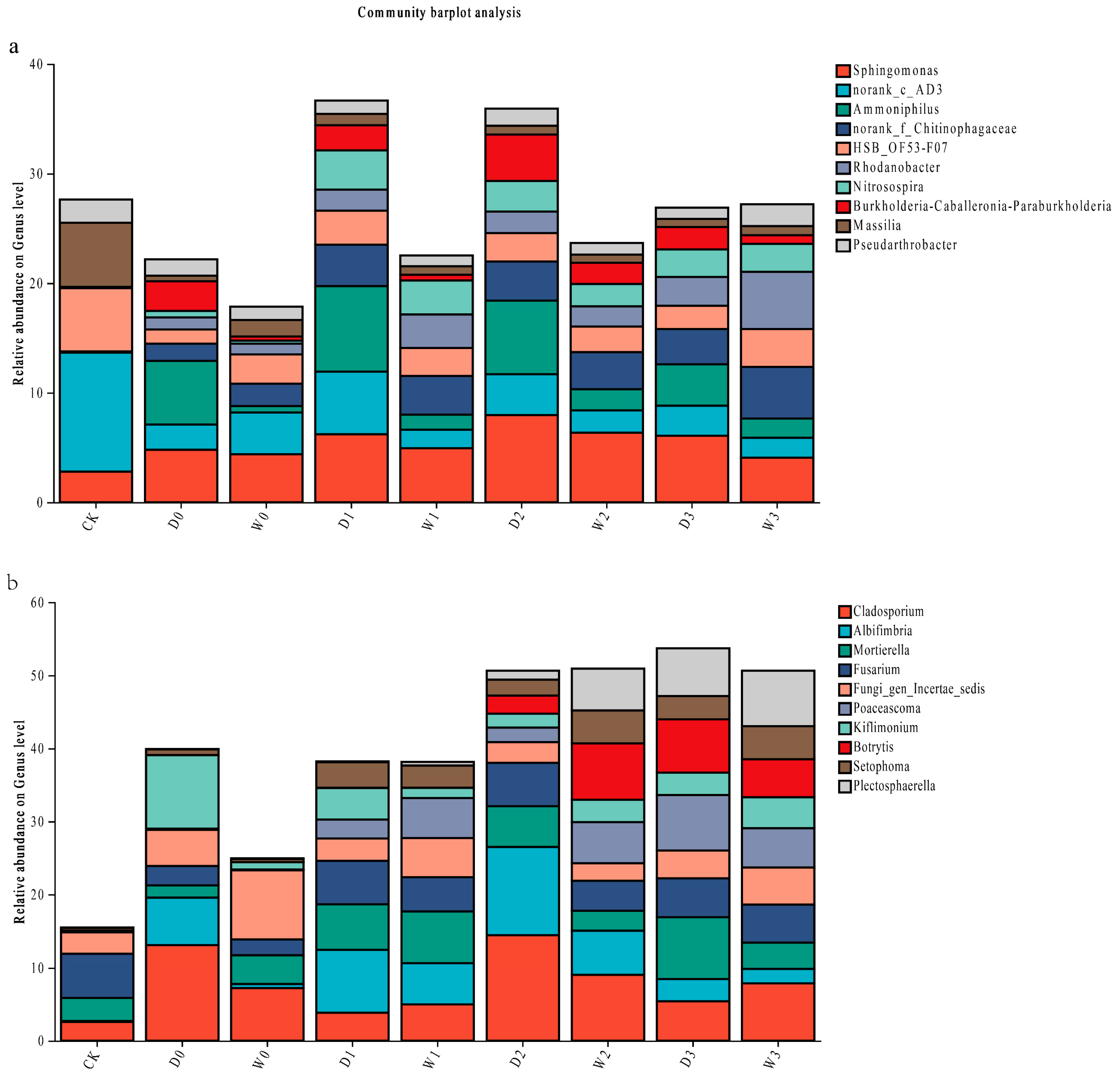
3.2.5. Soil Microbial Community Analysis at Genus Level
Bacterial Community Composition
Fungal Community Composition
3.2.6. Analysis of Differences in Soil Microbial Community Species Composition
Bacterial Community Differentiation
Fungal Community Differentiation
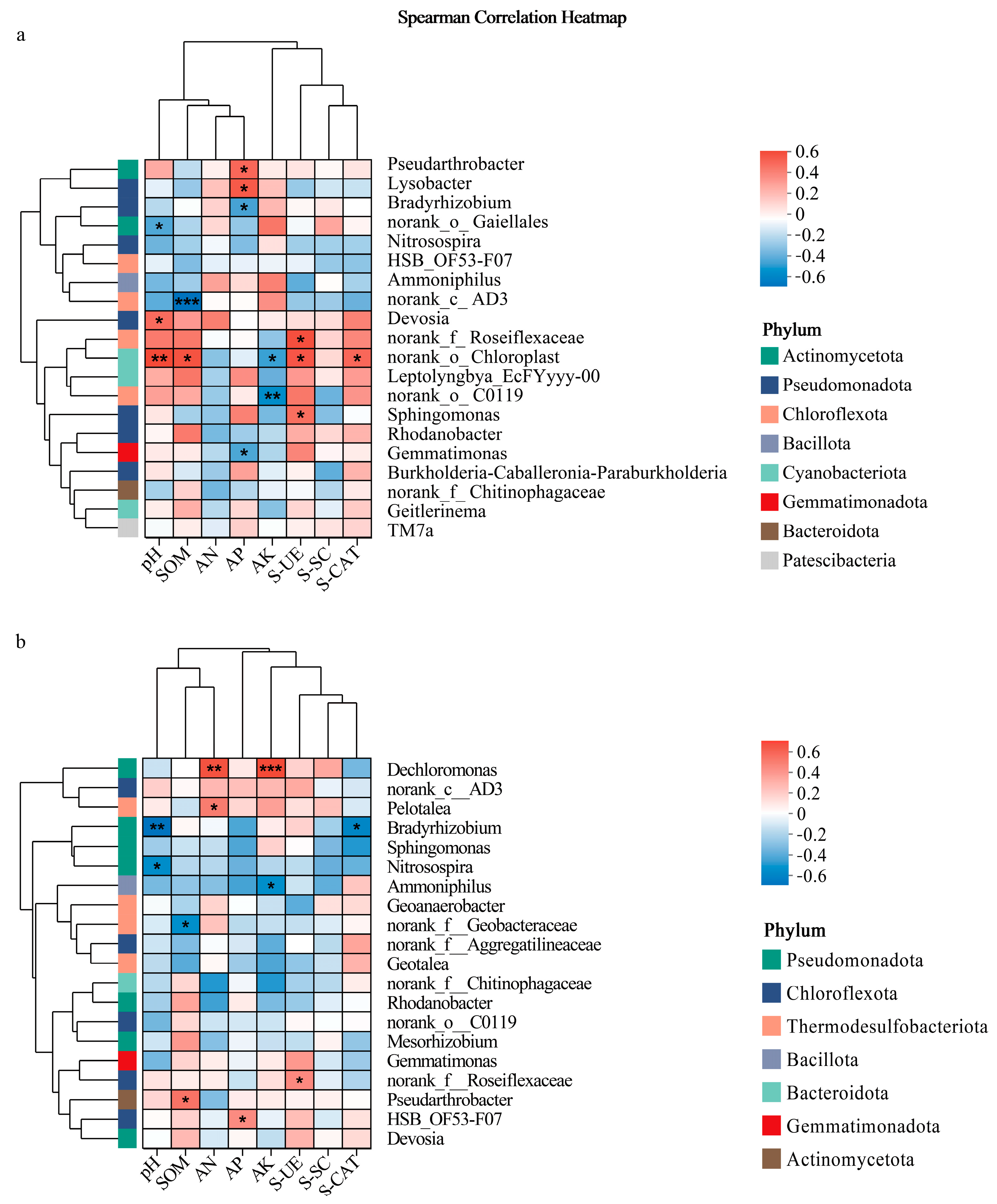
3.3. Correlation Analysis of Soil Physicochemical Properties and Microbial Communities Across Rice Cultivation Systems and Potato Varieties
3.3.1. Rainfed System Bacterial Correlations
3.3.2. Waterlogged System Bacterial Correlations
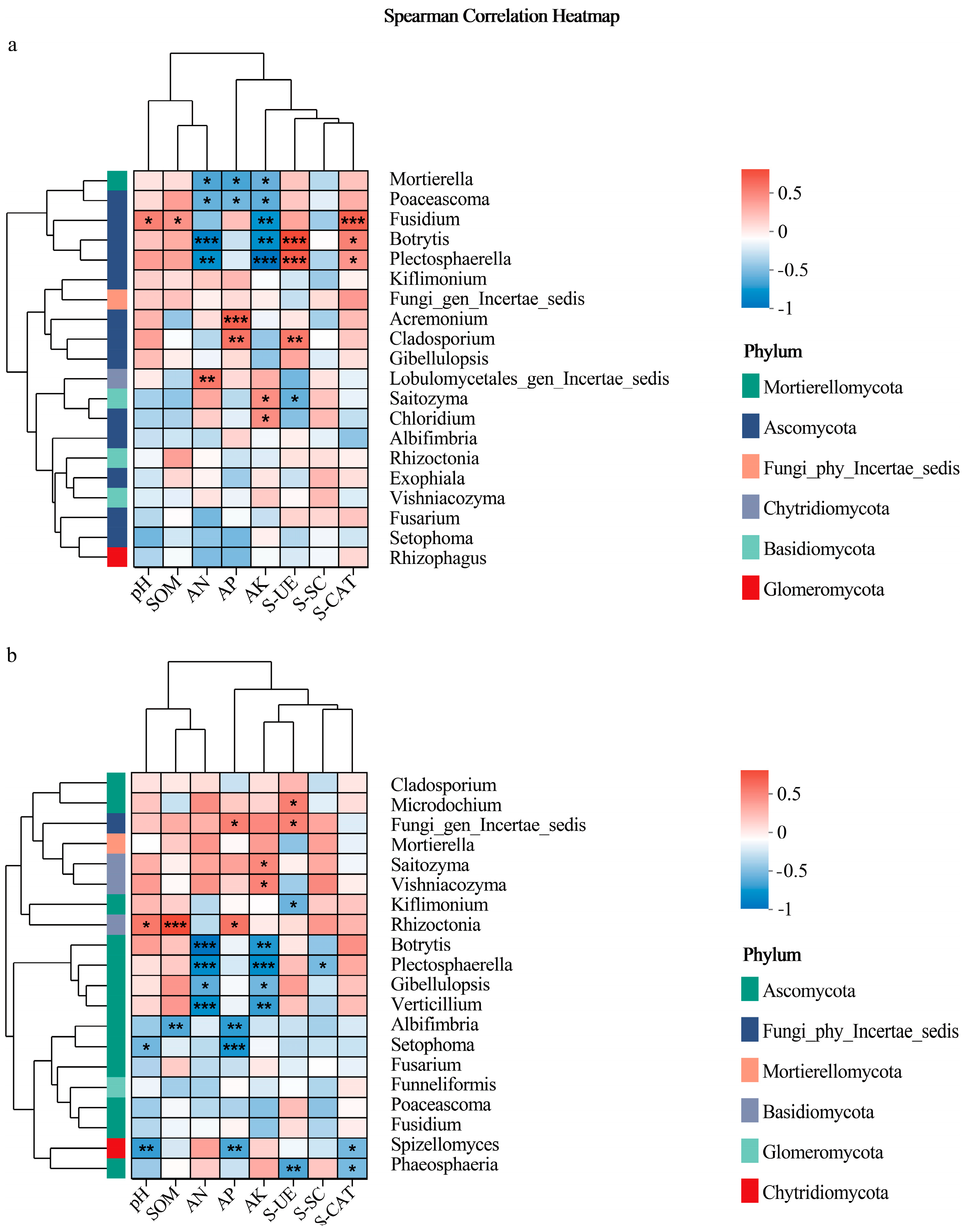
3.3.3. Rainfed System Fungal Correlations
3.3.4. Waterlogged System Fungal Correlations
4. Discussion
4.1. Effects of Rice Cultivation Systems on Soil Physicochemical Properties in Rice–Potato Cropping Sequences
4.2. Microbial Community Dynamics in Rice–Potato Cropping Systems
4.2.1. Bacterial Community Analysis at Phylum Level
4.2.2. Fungal Community Analysis at Phylum Level
4.2.3. Genus-Level Community Analysis
4.3. Environmental–Microbial Correlations at the Genus Level in Rice–Potato Cropping Systems
4.3.1. Bacterial Community Correlations
4.3.2. Fungal Community Correlations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos, H.; Ortiz, O. The Potato Crop: Its Agricultural, Nutritional and Social Contribution to Humankind; Springer Nature: Cham, Switzerland, 2020. [Google Scholar]
- Jiang, Y.; Ramsay, M.; Meng, F.; Stetson, T. Characterizing potato yield responses to water supply in Atlantic Canada’s humid climate using historical yield and weather data: Implications for supplemental irrigation. Agric. Water Manag. 2021, 255, 107047. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, Q.; Guo, L.; Pu, X.; Wang, C.; Shi, Y.; Gan, Y.; Li, C.; Wang, Y. Pathogenicity and genetic variations in Magnaporthe oryzae isolates from one rice variety planting in paddy and upland fields. Agronomy 2023, 13, 1246. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Yang, X.; Cao, H.; Li, J.; Cao, P.; Guo, L.; Wang, X.; Zhao, J.; Xiang, W. Alternaria spp. associated with leaf blight of maize in Heilongjiang Province, China. Plant Dis. 2022, 106, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Tiemann, L.; Grandy, A.S.; Atkinson, E.; Marin-Spiotta, E.; McDaniel, M. Crop rotational diversity enhances belowground communities and functions in an agroecosystem. Ecol. Lett. 2015, 18, 761–771. [Google Scholar] [CrossRef]
- Behnke, G.; Kim, N.; Zabaloy, M.; Riggins, C.; Rodriguez-Zas, S.; Villamil, M. Soil microbial indicators within rotations and tillage systems. Microorganisms 2021, 9, 1244. [Google Scholar] [CrossRef]
- Dou, Y.; Yu, S.; Liu, S.; Cui, T.; Huang, R.; Wang, Y.; Wang, J.; Tan, K.; Li, X. Crop rotations reduce pathogenic fungi compared to continuous cropping. Rhizosphere 2025, 34, 101074. [Google Scholar] [CrossRef]
- Zhou, W.; Fan, Y.; Jin, C.; Wang, Y.; Yan, F.; Wang, T.; Liu, Q.; Chen, Y.; Deng, F.; Lei, X.; et al. High-yield rice with rich nutrition and low toxicity can be obtained under potato–rice cropping system. J. Sci. Food Agric. 2025, 105, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhou, W.; Yang, Z.; Wang, T.; Fu, Y.; Yue, X.; Xia, H.; Tao, Y.; Deng, F.; Lei, X.; et al. Potato–rice and garlic–rice rotation increases soil phosphorus availability through phosphate-solubiliing bacteria and root exudates in upland–paddy cropping systems. Agric. Ecosyst. Environ. 2025, 390, 109721. [Google Scholar] [CrossRef]
- Kögel-Knabner, I.; Amelung, W.; Cao, Z.; Fiedler, S.; Frenzel, P.; Jahn, R.; Kalbitz, K.; Kölbl, A.; Schloter, M. Biogeochemistry of paddy soils. Geoderma 2010, 157, 1–14. [Google Scholar] [CrossRef]
- Liu, Y.; Su, H. Some necessary and sufficient conditions for containment of second-order multi-agent systems with sampled position data. Neurocomputing 2020, 378, 228–237. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, Y.; Zhang, K.; Jeong, J.; Zeng, Z.; Zang, H. Does crop rotation yield more in China? A meta-analysis. Field Crops Res. 2020, 245, 107659. [Google Scholar] [CrossRef]
- Bowles, T.M.; Mooshammer, M.; Socolar, Y.; Calderón, F.; Cavigelli, M.A.; Culman, S.W.; Deen, W.; Drury, C.F.; y Garcia, A.G.; Gaudin, A.C.; et al. Long-term evidence shows that crop-rotation diversification increases agricultural resilience to adverse growing conditions in North America. One Earth 2020, 2, 284–293. [Google Scholar] [CrossRef]
- Hao, P.; Lin, B.; Yi, K.; Xue, B.; Hua, S. Comprehensive illustration of the improvement of soil conditions and rice production through paddy-upland rotations for sustainable agricultural development. Soil Tillage Res. 2025, 248, 106453. [Google Scholar] [CrossRef]
- Meng, Y.; Wang, T.; Zhou, X.; Yang, X.; Hernandez, M.; Zhou, T.; Lv, Q.; Ren, X.; Feng, H.; Pan, H.; et al. Evaluating the Optimal Land Use Pattern for Saline-Sodic Soils from the Perspective of Nitrogen Metabolism. Environ. Technol. Innov. 2025, 40, 104363. [Google Scholar] [CrossRef]
- Han, S.; Ji, X.; Huang, L.; Liu, G.; Ye, J.; Wang, A. Effects of aftercrop tomato and maize on the soil microenvironment and microbial diversity in a long-term cotton continuous cropping field. Front. Microbiol. 2024, 15, 1410219. [Google Scholar] [CrossRef] [PubMed]
- Graham, J.H.; Duda, J.J. The humpbacked species richness-curve: A contingent rule for community ecology. Int. J. Ecol. 2011, 2011, 868426. [Google Scholar] [CrossRef]
- Wei, X.; Hu, Y.; Cai, G.; Yao, H.; Ye, J.; Sun, Q.; Veresoglou, S.D.; Li, Y.; Zhu, Z.; Guggenberger, G.; et al. Organic phosphorus availability shapes the diversity of phoD-harboring bacteria in agricultural soil. Soil Biol. Biochem. 2021, 161, 108364. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Dungait, J.A.; Wei, X.; Ge, T.; Hou, R.; Ouyang, Z.; Zhang, F.; Tian, J. Long-term warming increased microbial carbon use efficiency and turnover rate under conservation tillage system. Soil Biol. Biochem. 2022, 172, 108770. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef]
- Fernandes, C.F.; da Silva Iúdice, T.N.; Bezerra, N.V.; Pontes, A.N. Biodegradation of oil-derived hydrocarbons by marine actinobacteria: A systematic review. Environ. Pollut. 2024, 367, 125509. [Google Scholar] [CrossRef]
- Crits-Christoph, A.; Diamond, S.; Butterfield, C.N.; Thomas, B.C.; Banfield, J.F. Novel soil bacteria possess diverse genes for secondary metabolite biosynthesis. Nature 2018, 558, 440–444. [Google Scholar] [CrossRef]
- Chen, L.-F.; He, Z.-B.; Zhao, W.-Z.; Kong, J.-Q.; Gao, Y. Empirical evidence for microbial regulation of soil respiration in alpine forests. Ecol. Indic. 2021, 126, 107710. [Google Scholar] [CrossRef]
- Gonçalves, O.S.; Fernandes, A.S.; Tupy, S.M.; Ferreira, T.G.; Almeida, L.N.; Creevey, C.J.; Santana, M.F. Insights into plant interactions and the biogeochemical role of the globally widespread Acidobacteriota phylum. Soil Biol. Biochem. 2024, 192, 109369. [Google Scholar] [CrossRef]
- Zhou, Z.; Liao, Y.; Zhang, Q.; Xiong, Z.; Tang, J.; Tian, J.; Chang, X.; Zhang, H.; Xiang, J.; Lin, Z.; et al. Insights into soil carbon metabolism and carbon sequestration capacity under organic fertilizer substitution model. Appl. Soil Ecol. 2025, 213, 106235. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, Y.; Yang, S.; Wang, Y.; Xue, X.; Luo, H. The treatment performance and the removal mechanism of pollutants in vertical flow constructed wetlands combined with magnetic field. J. Water Process Eng. 2025, 71, 107311. [Google Scholar] [CrossRef]
- Fischer, W.W.; Hemp, J.; Johnson, J.E. Evolution of oxygenic photosynthesis. Annu. Rev. Earth Planet. Sci. 2016, 44, 647–683. [Google Scholar] [CrossRef]
- Boer, W.d.; Folman, L.B.; Summerbell, R.C.; Boddy, L. Living in a fungal world: Impact of fungi on soil bacterial niche development. FEMS Microbiol. Rev. 2005, 29, 795–811. [Google Scholar] [CrossRef]
- Hollister, E.B.; Schadt, C.W.; Palumbo, A.V.; Ansley, R.J.; Boutton, T.W. Structural and functional diversity of soil bacterial and fungal communities following woody plant encroachment in the southern Great Plains. Soil Biol. Biochem. 2010, 42, 1816–1824. [Google Scholar] [CrossRef]
- Asaf, S.; Numan, M.; Khan, A.L.; Al-Harrasi, A. Sphingomonas: From diversity and genomics to functional role in environmental remediation and plant growth. Crit. Rev. Biotechnol. 2020, 40, 138–152. [Google Scholar] [CrossRef]
- Woo, H.; Kim, I.; Chhetri, G.; Park, S.; Lee, H.; Yook, S.; Seo, T. Two novel bacterial species, Rhodanobacter lycopersici sp. nov. and Rhodanobacter geophilus sp. nov., isolated from the rhizosphere of Solanum lycopersicum with plant growth-promoting traits. Microorganisms 2024, 12, 2227. [Google Scholar] [CrossRef] [PubMed]
- Virginia, T.C.; Néstor, A.J.; Dario, C.A.; Noemí, P.G. Cladosporium species causing “Cladosporium rot” on “Bosc” pear fruit in Argentina. Rev. Argent. Microbiol. 2021, 53, 75–77. [Google Scholar]
- Matić, S.; Gilardi, G.; Gullino, M.L.; Garibaldi, A. Emergence of leaf spot disease on leafy vegetable and ornamental crops caused by Paramyrothecium and Albifimbria species. Phytopathology 2019, 109, 1053–1061. [Google Scholar] [CrossRef]
- Cui, L.; Yang, C.; Yang, L.; Jin, M.; Wei, L. First report of Fusarium equiseti causing fusarium wilt on potato (Solanum tuberosum) in China. Plant Dis. 2021, 105, 2013. [Google Scholar] [CrossRef]
- Christian, C.L.; Rosnow, J.; Woodhall, J.W.; Wharton, P.S.; Duellman, K.M. Pathogenicity of Fusarium Species Associated with Potato Dry Rot in the Pacific Northwest of the United States. Plant Dis. 2025, 109, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Dai, P.; Chen, H.; Yang, B.; Wang, H.; Yang, Q.; Zhang, H.; Chen, W.; Chen, Y.Q. Mortierella alpina feed supplementation enriched hen eggs with DHA and AA. RSC Adv. 2016, 6, 1694–1699. [Google Scholar] [CrossRef]
- Sonnabend, R.; Seiler, L.; Gressler, M. Regulation of the leucine metabolism in Mortierella alpina. J. Fungi 2022, 8, 196. [Google Scholar] [CrossRef]
- Ren, W.; Liu, H.; Mao, T.; Teng, Y.; Zhao, R.; Luo, Y. Enhanced remediation of PAHs-contaminated site soil by bioaugmentation with graphene oxide immobilized bacterial pellets. J. Hazard. Mater. 2022, 433, 128793. [Google Scholar] [CrossRef]
- Chai, J.; Wang, X.; Liu, X.; Li, C.; Han, J.; Yao, T. Inoculation of cold-adapted microbial consortium screened from alpine meadows promotes the growth of mixed grasses by changing soil properties and enzyme activity. Rhizosphere 2023, 28, 100782. [Google Scholar] [CrossRef]
- Zhang, N.; Jin, C.-Z.; Zhuo, Y.; Li, T.; Jin, F.-J.; Lee, H.-G.; Jin, L. Genetic diversity into a novel free-living species of Bradyrhizobium from. Front. Microbiol. 2024, 14, 1295854. [Google Scholar]
- Avontuur, J.R.; Wilken, P.M.; Palmer, M.; Coetzee, M.P.; Stępkowski, T.; Venter, S.N.; Steenkamp, E.T. Complex evolutionary history of photosynthesis in Bradyrhizobium. Microb. Genom. 2023, 9, 001105. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Øverlie Arntzen, M.; Kjos, M.; Bakken, L.R.; Frostegård, Å. Denitrification by Bradyrhizobia under Feast and Famine and the Role of the bc1 Complex in Securing Electrons for N2O Reduction. Appl. Environ. Microbiol. 2023, 89, e0174522. [Google Scholar] [CrossRef]
- Yang, S.; Su, X.; Jiang, Y.; Deng, Y.; Deng, Z.; Luo, X.; Chen, J.; Jiang, J.; Zhu, L.; Xu, R.; et al. Roof runoff pollution control with operating time based on a field-scale assembled bioretention facility: Performance and microbial community dynamics. J. Water Process Eng. 2024, 57, 104697. [Google Scholar] [CrossRef]
- Wang, J.; Lin, C.; Han, Z.; Fu, C.; Huang, D.; Cheng, H. Dissolved nitrogen in salt-affected soils reclaimed by planting rice: How is it influenced by soil physicochemical properties? Sci. Total Environ. 2022, 824, 153863. [Google Scholar] [CrossRef]
- Yue, H.; Miller, A.L.; Khetrapal, V.; Jayaseker, V.; Wright, S.; Du, L. Biosynthesis, regulation, and engineering of natural products from Lysobacter. Nat. Prod. Rep. 2022, 39, 842–874. [Google Scholar] [CrossRef]
- Zhao, W.; Bi, X.; Peng, Y.; Bai, M. Research advances of the phosphorus-accumulating organisms of Candidatus Accumulibacter, Dechloromonas and Tetrasphaera: Metabolic mechanisms, applications and influencing factors. Chemosphere 2022, 307, 135675. [Google Scholar] [CrossRef]
- Das, S.; Rabha, J.; Narzary, D. Assessment of soil yeasts Papiliotrema laurentii S-08 and Saitozyma podzolica S-77 for plant growth promotion and biocontrol of Fusarium wilt of brinjal. J. Appl. Microbiol. 2023, 134, lxad252. [Google Scholar] [CrossRef] [PubMed]
- Rønhede, S.; Jensen, B.; Rosendahl, S.; Kragelund, B.B.; Juhler, R.K.; Aamand, J. Hydroxylation of the herbicide isoproturon by fungi isolated from agricultural soil. Appl. Environ. Microbiol. 2005, 71, 7927–7932. [Google Scholar] [CrossRef] [PubMed]
- Tixier, C.; Sancelme, M.; Sancelme, M.; Bonnemoy, F.; Cuer, A.; Veschambre, H. Degradation products of a phenylurea herbicide, diuron: Synthesis, ecotoxicity, and biotransformation. Environ. Toxicol. Chem. 2001, 20, 1381–1389. [Google Scholar] [CrossRef]


| Sample Time (d) | Treatment | pH | SOM (g/kg) | AN (mg/kg) | AP (mg/kg) | AK (mg/kg) |
|---|---|---|---|---|---|---|
| Before rice planting | CK | 6.07 ± 0.02 | 13.72 ± 1.18 | 57.63 ± 8.47 | 3.28 ± 0.41 | 133.88 ± 4.08 |
| Rice harvest | D | 6.02 ± 0.02 | 22.04 ± 0.61 ** | 61.83 ± 12.72 | 3.13 ± 0.30 | 111.97 ± 2.33 ** |
| W | 6.24 ± 0.04 ** | 16.42 ± 0.51 | 52.27 ± 2.65 | 2.68 ± 0.30 | 71.19 ± 2.65 | |
| Before planting potatoes | D0 | 5.91 ± 0.02 | 22.32 ± 1.41 | 278.83 ± 5.35 * | 5.74 ± 0.78 | 154.64 ± 3.95 |
| W0 | 6.09 ± 0.05 ** | 24.17 ± 0.97 | 258.30 ± 6.42 | 6.59 ± 0.59 | 176.94 ± 2.17 ** | |
| 40 | D1 | 5.25 ± 0.09 | 19.91 ± 2.03 | 65.57 ± 6.18 | 4.01 ± 0.38 | 159.69 ± 6.37 ** |
| W1 | 5.40 ± 0.07 ** | 19.03 ± 0.64 | 63.47 ± 4.55 | 4.41 ± 0.15 * | 143.49 ± 3.49 | |
| 80 | D2 | 5.69 ± 0.03 | 21.01 ± 0.59 ** | 32.67 ± 2.15 | 5.19 ± 0.34 * | 122.93 ± 3.42 * |
| W2 | 5.70 ± 0.02 | 18.47 ± 0.75 | 30.92 ± 1.50 | 4.37 ± 0.54 | 112.58 ± 9.24 | |
| Potato harvest | D3 | 5.78 ± 0.21 | 25.60 ± 0.72 | 33.25 ± 4.44 | 3.86 ± 0.48 | 117.89 ± 8.82 |
| W3 | 5.76 ± 0.09 | 25.12 ± 0.71 | 29.40 ± 5.05 | 5.06 ± 0.21 ** | 120.05 ± 6.07 |
| Sample Time (d) | Treatment | S-UE (U/g) | S-SC (U/g) | S-CAT (U/g) |
|---|---|---|---|---|
| Before rice planting | CK | 65.96 ± 18.71 | 7.81 ± 0.37 | 15.47 ± 0.45 |
| Before planting potatoes | D0 | 128.69 ± 6.90 | 35.49 ± 0.18 | 17.31 ± 1.05 |
| W0 | 134.98 ± 18.02 | 51.04 ± 4.31 * | 17.66 ± 0.45 | |
| 40 | D1 | 97.15 ± 42.23 | 36.62 ± 14.58 * | 15.87 ± 0.77 |
| W1 | 87.59 ± 15.37 | 20.29 ± 4.63 | 16.51 ± 0.75 | |
| 80 | D2 | 146.29 ± 14.51 | 25.36 ± 6.66 ** | 17.28 ± 0.83 |
| W2 | 136.54 ± 8.64 | 10.90 ± 2.28 | 17.38 ± 0.80 | |
| Potato harvest | D3 | 152.07 ± 16.69 ** | 53.50 ± 35.77 | 17.97 ± 0.50 |
| W3 | 112.92 ± 20.65 | 20.98 ± 6.61 | 17.86 ± 0.57 |
| Type | Sample Time (d) | Treatment | Sobs Value | Shannon Index | Chao Index | Coverage |
|---|---|---|---|---|---|---|
| Bacteria | Before rice planting | CK | 1766.00 ± 69.20 | 6.49 ± 0.11 | 1776.92 ± 73.37 | 99.89% |
| Before planting potatoes | D0 | 1581.67 ± 39.55 * | 5.83 ± 0.26 | 1640.12 ± 83.18 * | 99.70% | |
| W0 | 1425.00 ± 50.48 | 6.07 ± 0.19 | 1437.22 ± 52.51 | 99.89% | ||
| 40 | DR1 | 1369.67 ± 300.85 a | 5.66 ± 0.44 a | 1398.96 ± 314.65 a | 99.83% | |
| WR1 | 1387.67 ± 166.16 a | 6.01 ± 0.19 a | 1397.57 ± 172.78 a | 99.90% | ||
| DY1 | 1553.00 ± 172.27 a | 6.13 ± 0.19 a | 1568.74 ± 172.65 a | 99.87% | ||
| WY1 | 1254.00 ± 292.80 a | 5.68 ± 0.36 a | 1274.11 ± 303.34 a | 99.86% | ||
| 80 | DR2 | 1565.33 ± 76.27 a ** | 6.09 ± 0.22 a | 1591.99 ± 75.61 a ** | 99.83% | |
| WR2 | 1254.00 ± 21.93 ab | 5.83 ± 0.07 a | 1259.72 ± 22.50 b | 99.93% | ||
| DY2 | 1418.67 ± 171.2 ab | 5.94 ± 0.40 a | 1431.34 ± 168.88 ab | 99.89% | ||
| WY2 | 1093.67 ± 272.89 b | 5.63 ± 0.62 a | 1102.77 ± 279.01 b | 99.92% | ||
| Potato harvest | DR3 | 1224.67 ± 196.17 a | 5.84 ± 0.37 a | 1235.14 ± 202.87 a | 99.91% | |
| WR3 | 1062.00 ± 177.39 a | 5.77 ± 0.41 a | 1063.68 ± 177.45 a | 99.97% | ||
| DY3 | 1341.00 ± 156.14 a | 6.07 ± 0.21 a | 1354.65 ± 168.70 a | 99.88% | ||
| WY3 | 1105.67 ± 165.08 a | 5.74 ± 0.41 a | 1115.33 ± 162.32 a | 99.91% | ||
| Fungi | Before rice planting | CK | 479.00 ± 35.00 | 4.70 ± 0.33 | 479.43 ± 34.62 | 99.99% |
| Before planting potatoes | D0 | 438.33 ± 148.19 | 4.03 ± 0.69 | 445.47 ± 144.87 | 99.95% | |
| W0 | 345.33 ± 32.72 | 4.43 ± 0.39 | 345.44 ± 32.84 | 100.00% | ||
| 40 | DR1 | 464.33 ± 59.34 a | 4.08 ± 0.60 ab | 469.19 ± 61.29 a | 99.96% | |
| WR1 | 370.00 ± 115.53 ab | 4.68 ± 0.32 a | 370.25 ± 115.90 ab | 100.00% | ||
| DY1 | 335.67 ± 36.86 ab | 4.06 ± 0.90 ab | 335.98 ± 37.03 ab | 99.99% | ||
| WY1 | 240.00 ± 94.25 b | 3.31 ± 0.39 b | 242.59 ± 94.17 b | 99.98% | ||
| 80 | DR2 | 488.00 ± 85.56 a * | 3.98 ± 0.53 ab | 492.96 ± 87.57 a * | 99.95% | |
| WR2 | 344.67 ± 12.66 b | 3.64 ± 0.32 b | 347.97 ± 12.73 b | 99.98% | ||
| DY2 | 465.33 ± 20.40 a * | 4.46 ± 0.24 a | 469.55 ± 22.77 a * | 99.97% | ||
| WY2 | 350.67 ± 46.92 b | 4.02 ± 0.38 ab | 352.02 ± 46.32 b | 99.98% | ||
| Potato harvest | DR3 | 407.67 ± 29.02 a | 3.99 ± 0.05 a | 420.33 ± 40.13 a | 99.93% | |
| WR3 | 343.67 ± 106.52 a | 3.41 ± 0.69 a | 347.42 ± 106.86 a | 99.97% | ||
| DY3 | 436.67 ± 39.43 a | 4.18 ± 0.29 a | 438.77 ± 40.68 a | 99.97% | ||
| WY3 | 309.67 ± 71.04 a | 3.66 ± 0.37 a | 315.08 ± 74.77 a | 99.96% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, L.; Li, S.; Li, K.; Zhang, X.; Yang, L.; Guo, H. Analysis of Soil Nutrients and Microbial Community Characteristics in Rainfed Rice–Potato Cropping Systems. Agronomy 2025, 15, 2500. https://doi.org/10.3390/agronomy15112500
Liang L, Li S, Li K, Zhang X, Yang L, Guo H. Analysis of Soil Nutrients and Microbial Community Characteristics in Rainfed Rice–Potato Cropping Systems. Agronomy. 2025; 15(11):2500. https://doi.org/10.3390/agronomy15112500
Chicago/Turabian StyleLiang, Longkang, Sunjin Li, Kun Li, Xing Zhang, Longjun Yang, and Huachun Guo. 2025. "Analysis of Soil Nutrients and Microbial Community Characteristics in Rainfed Rice–Potato Cropping Systems" Agronomy 15, no. 11: 2500. https://doi.org/10.3390/agronomy15112500
APA StyleLiang, L., Li, S., Li, K., Zhang, X., Yang, L., & Guo, H. (2025). Analysis of Soil Nutrients and Microbial Community Characteristics in Rainfed Rice–Potato Cropping Systems. Agronomy, 15(11), 2500. https://doi.org/10.3390/agronomy15112500





