Soluble Phosphate Additives Remodel Microbial Networks to Accelerate Organic Matter Transformation in Food Waste Composting
Abstract
1. Introduction
2. Materials and Methods
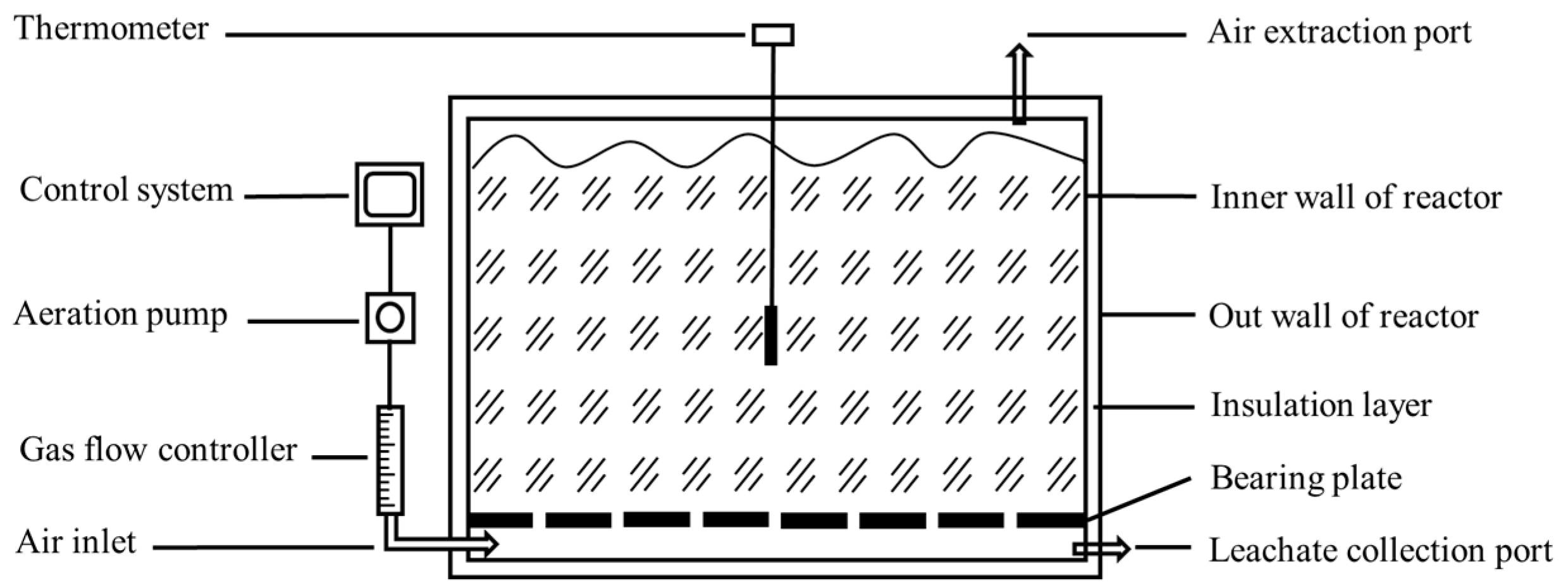
2.1. Experimental Design of Composting
2.2. Determination of Physicochemical Properties and Maturity Indicators
2.3. Analysis of Main Organic Fractions and Phosphorus Forms
2.4. Microbial Community Analysis
2.5. Statistical Analysis
3. Results and Discussion
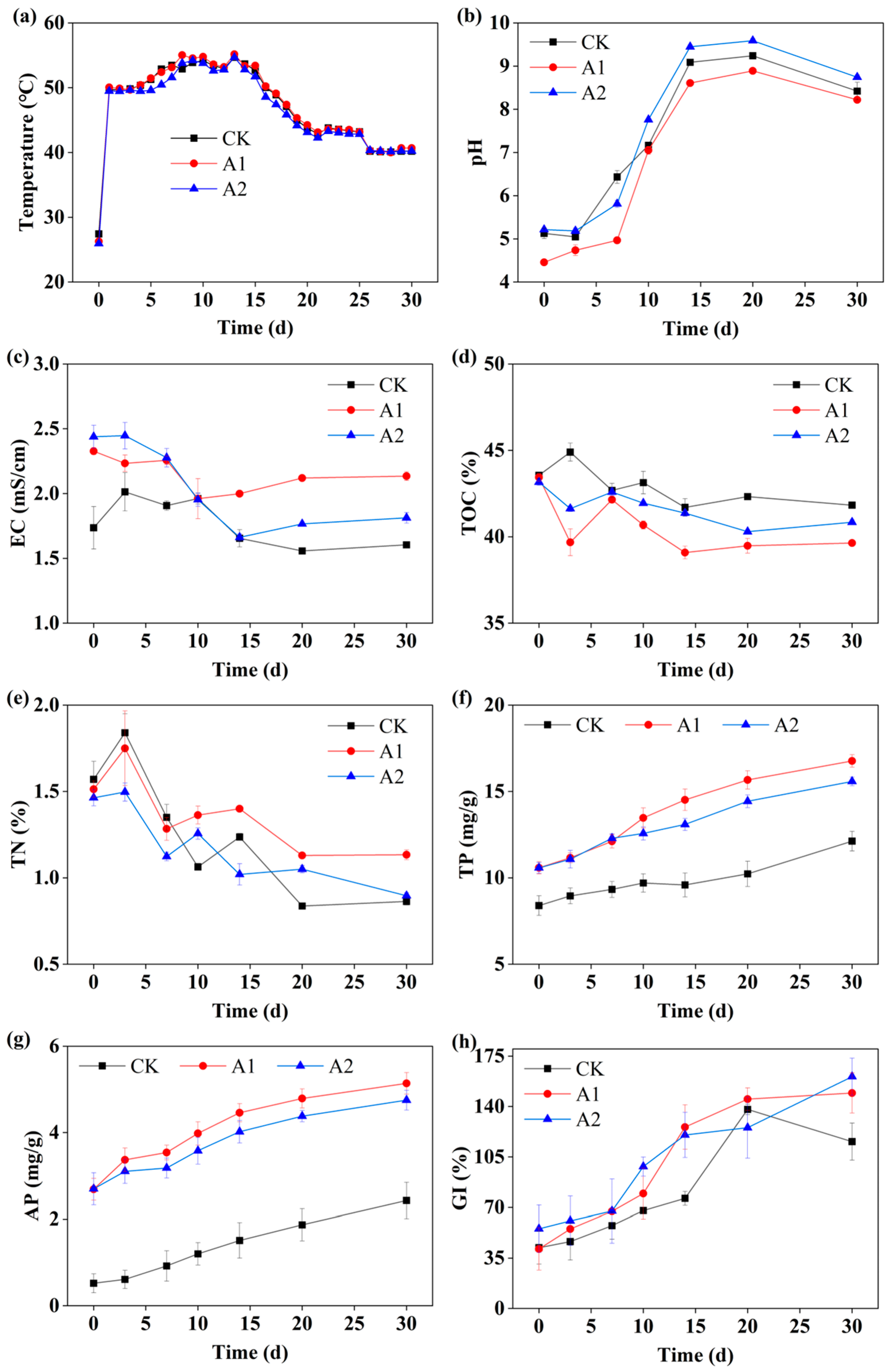
3.1. Changes in Physicochemical Properties and Maturity Indicators
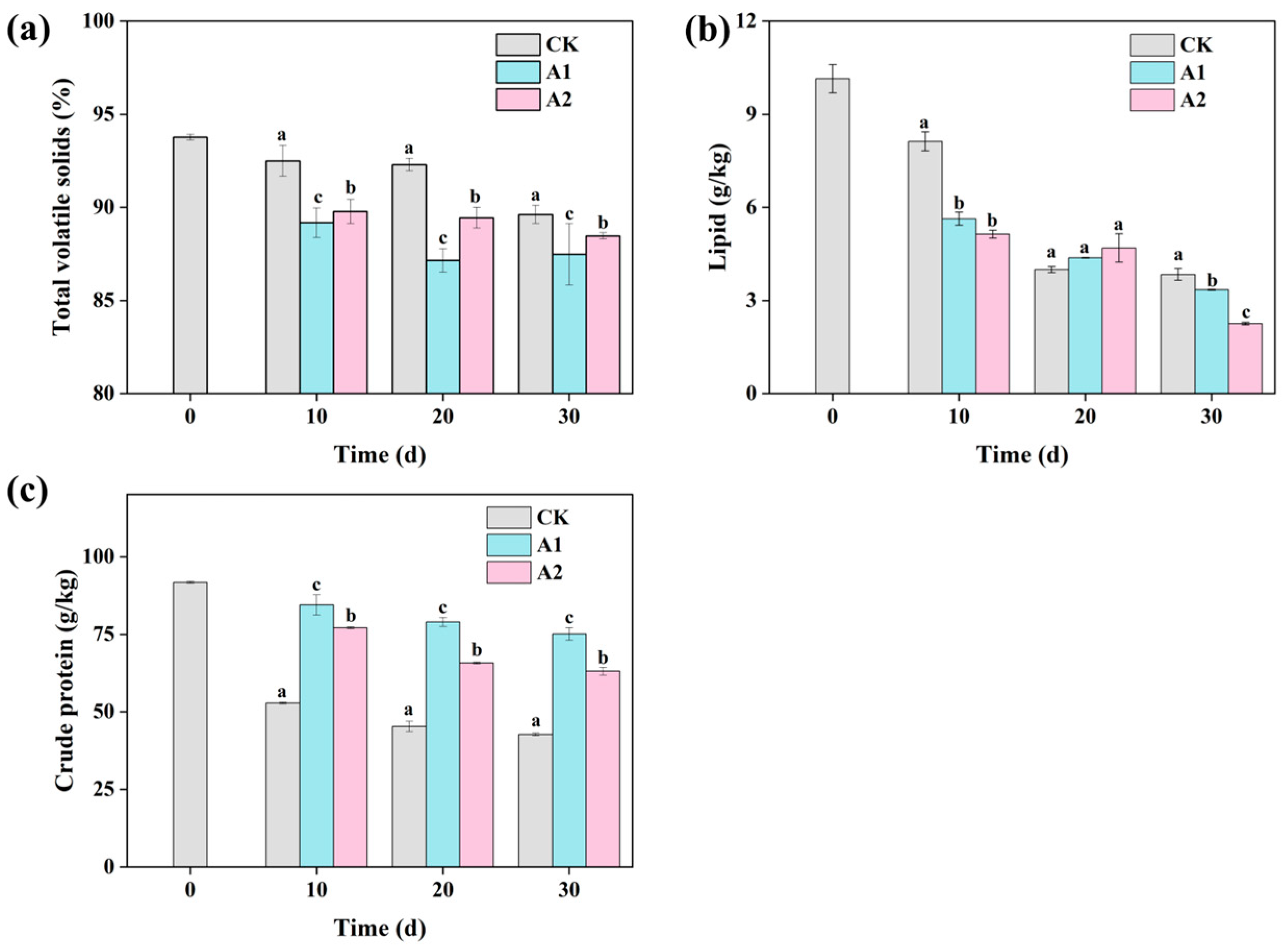
3.2. The Conversion of Organic Fractions
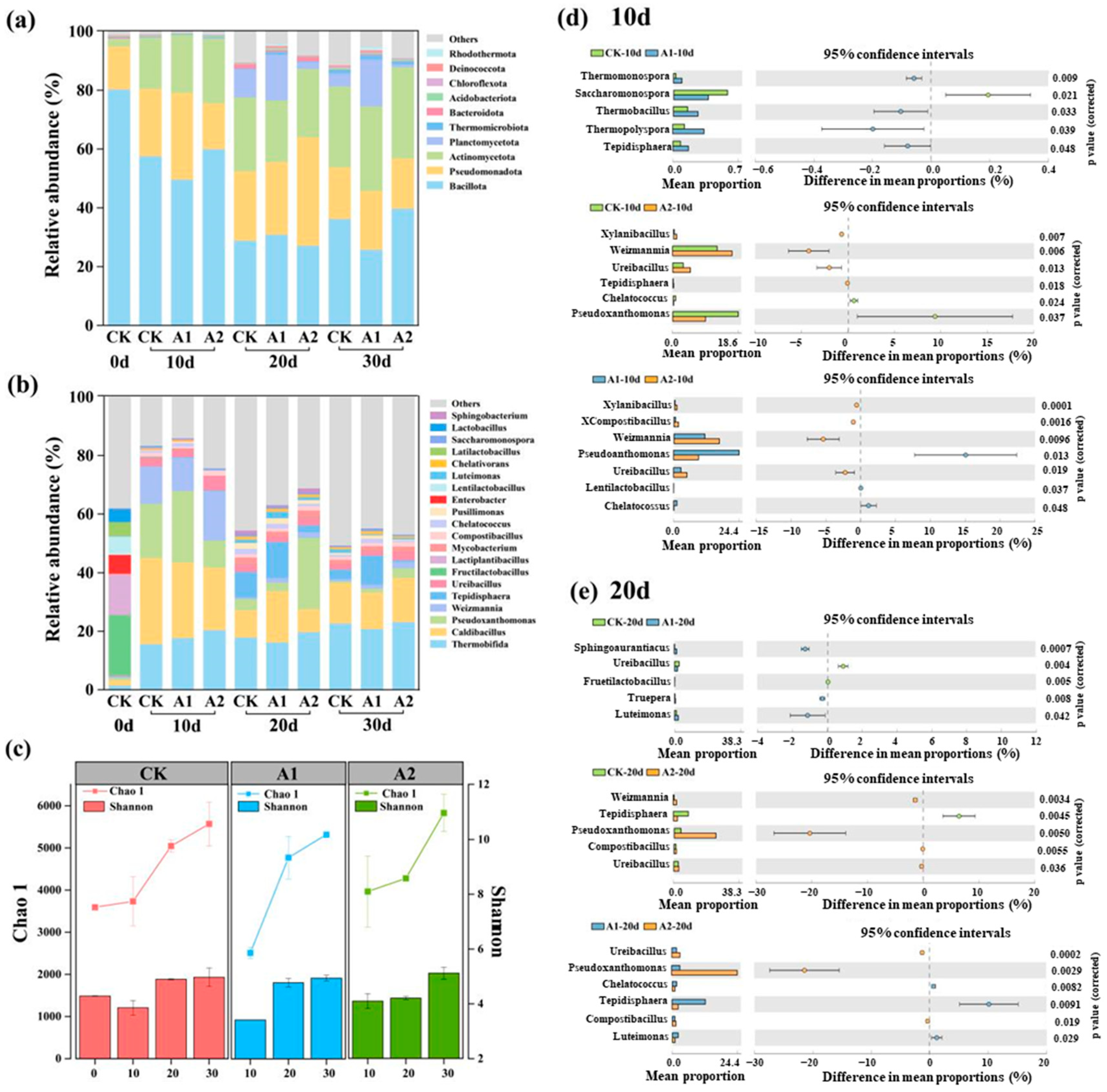
3.3. Microbial Community Composition and Diversity
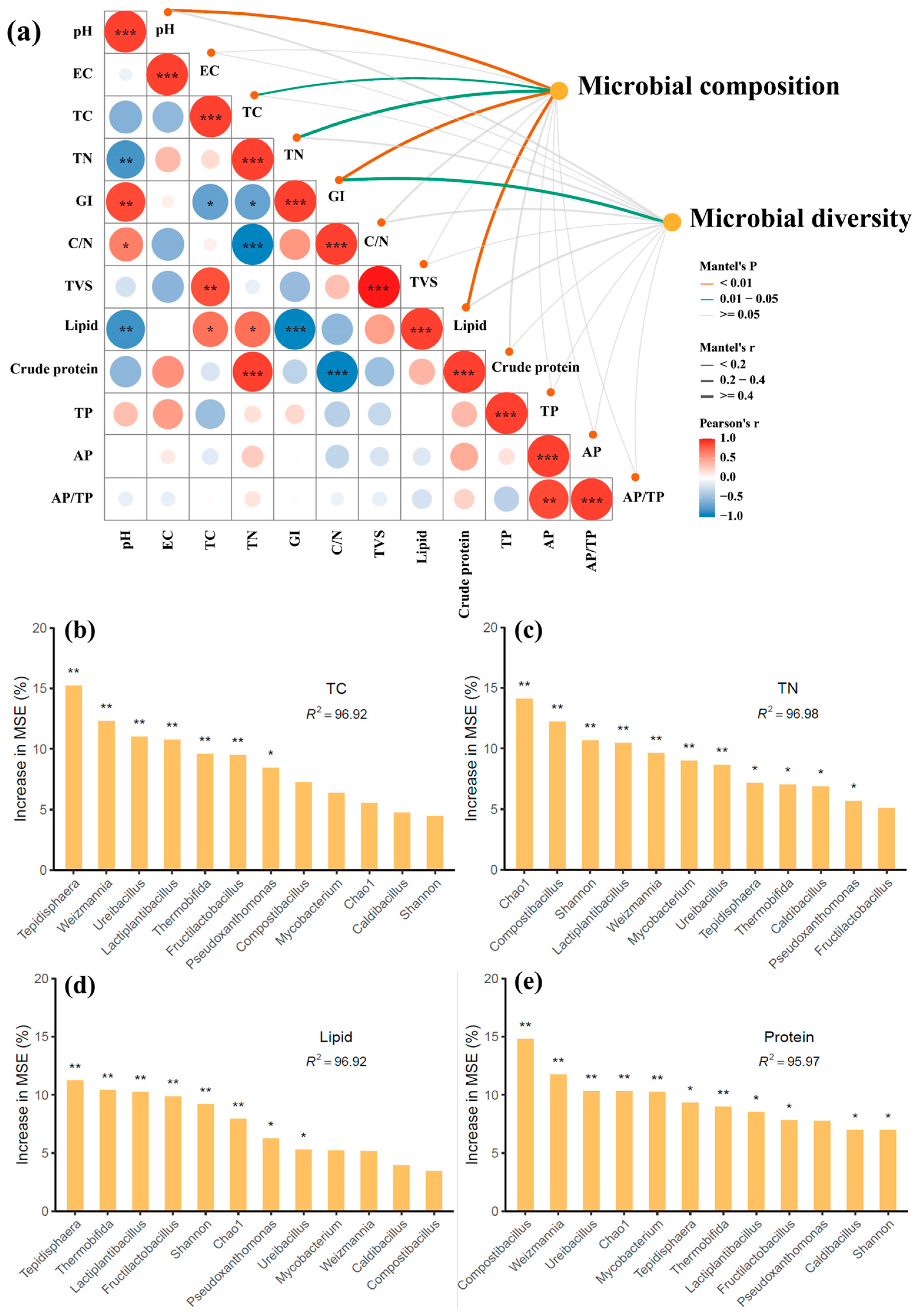
3.4. Effects of Phosphate Addition on Microbial Network and Key Taxa
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EC | Electrical conductivity |
| TOC | Total organic carbon |
| TN | Total nitrogen |
| GI | Germination index |
| TVS | Total volatile solids |
| TP | Total phosphorus |
| AP | Available phosphorus |
References
- Niu, Z.; Ng, S.J.; Li, B.; Han, J.; Wu, X.; Huang, Y. Food waste and its embedded resources loss: A provincial level analysis of China. Sci. Total Environ. 2022, 823, 153665. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.Z.; Zhao, Y.; Zhang, A.; Zhao, M.Y.; Zhai, W.H.; Wei, Z.M.; Song, Y.Y.; Tang, X.F.; He, P.P. Factoring distinct materials and nitrogen-related microbes into assessments of nitrogen pollution risks during composting. Bioresour. Technol. 2021, 329, 124896. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, L.; Zhang, Y.; Li, D.; Chen, L.; Wei, Z.; Chen, X.; Pan, C.; Song, Y. The active role of metabolic regulators in nitrogen loss reduction and organic nitrogen transformation during different materials composting. J. Clean. Prod. 2022, 345, 131134. [Google Scholar] [CrossRef]
- Peng, X.Y.; Wang, S.P.; Chu, X.L.; Sun, Z.Y.; Xia, Z.Y.; Xie, C.Y.; Gou, M.; Tang, Y.Q. Valorizing kitchen waste to produce value-added fertilizer by thermophilic semi-continuous composting followed by static stacking: Performance and bacterial community succession analysis. Bioresour. Technol. 2023, 373, 128732. [Google Scholar] [CrossRef]
- Yang, F.; Li, G.; Shi, H.; Wang, Y. Effects of phosphogypsum and superphosphate on compost maturity and gaseous emissions during kitchen waste composting. Waste Manag. 2015, 36, 70–76. [Google Scholar] [CrossRef]
- Liang, J.; Shen, Y.; Shou, Z.; Yuan, H.; Dai, X.; Zhu, N. Nitrogen loss reduction by adding KH2PO4-K2HPO4 buffer solution during composting of sewage sludge. Bioresour. Technol. 2018, 264, 116–122. [Google Scholar] [CrossRef]
- Shan, G.; Li, W.; Gao, Y.; Tan, W.; Xi, B. Additives for reducing nitrogen loss during composting: A review. J. Clean. Prod. 2021, 307, 127308. [Google Scholar] [CrossRef]
- Shelly, P.; Neemisha; Sharma, S. Modification in the Composting Environment through Additives. Commun. Soil Sci. Plant Anal. 2022, 53, 2141–2155. [Google Scholar] [CrossRef]
- Li, G.; Chen, W.; Xu, S.; Xiong, S.; Zhao, J.; Liu, D.; Ding, G.; Li, J.; Wei, Y. Role of fungal communities and their interaction with bacterial communities on carbon and nitrogen component transformation in composting with different phosphate additives. Environ. Sci. Pollut. Res. 2023, 30, 44112–44120. [Google Scholar] [CrossRef]
- Su, J.; Zhan, Y.; Chang, Y.; Chang, S.; Luo, Y.; Chen, P.; Tao, X.; Chen, Y.; Yang, L.; Xu, T.; et al. Phosphate additives promote humic acid carbon and nitrogen skeleton formation by regulating precursors and composting bacterial communities. Bioresour. Technol. 2024, 399, 130617. [Google Scholar] [CrossRef]
- Shou, Z.; Zhu, N.; Yuan, H.; Dai, X.; Shen, Y. Buffering phosphate mitigates ammonia emission in sewage sludge composting: Enhanced organics removal coupled with microbial ammonium assimilation. J. Clean. Prod. 2019, 227, 189–198. [Google Scholar] [CrossRef]
- Li, F.; Qian, K.; Wu, J.; Wan, S.X.; Jiang, G.Y.; Zhu, H.B. Influence of applying calcium superphosphate on swine manure composting and phosphorus transformation. J. Plant Nutr. Fertil. 2017, 23, 1037–1044. [Google Scholar]
- Bouhia, Y.; Hafidi, M.; Ouhdouch, Y.; Soulaimani, A.; Zeroual, Y.; Lyamlouli, K. Microbial intervention improves pollutant removal and semi-liquid organo-mineral fertilizer production from olive mill wastewater sludge and rock phosphate. J. Environ. Manag. 2024, 354, 120317. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sheng, Y.; Wang, G.; Zhou, P.; Liao, F.; Mao, H.; Zhang, H.; Qiao, Z.; Wei, Y. Spatiotemporal successions of N, S, C, Fe, and As cycling genes in groundwater of a wetland ecosystem: Enhanced heterogeneity in wet season. Water Res. 2024, 251, 121105. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, R.; Tang, R.; Kong, Y.; Wang, J.; Li, G.; Yuan, J. Effects of phosphate-containing additives and zeolite on maturity and heavy metal passivation during pig manure composting. Sci. Total Environ. 2022, 836, 155727. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Wang, X.D.; Zhou, J.M.; Zheng, J.B. Effects of initial pH values on maturity and nitrogen loss during co-composting of pig manure and edible fungus residue. Acta Agric. Zhejiangensis 2016, 28, 1595. [Google Scholar]
- Geng, X.Y.; Yang, H.Y.; Gao, W.F.; Yue, J.Y.; Mu, D.C.; Wei, Z.M. Greenhouse gas emission characteristics during kitchen waste composting with biochar and zeolite addition. Bioresour. Technol. 2024, 399, 130575. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Gholipour, S.; Mostafaii, G.; Yousefian, F. Biochar-amended food waste compost: A review of properties. Results Eng. 2024, 24, 103118. [Google Scholar] [CrossRef]
- Luo, Y.; Shen, J.Y.; Wang, X.X.; Xiao, H.P.; Yaser, A.Z.; Fu, J. Recent advances in research on microbial community in the composting process. Biomass Convers. Biorefinery 2024, 14, 23319–23333. [Google Scholar] [CrossRef]
- Zhan, Y.B.; Zhang, Z.Y.; Ma, T.T.; Zhang, X.J.; Wang, R.H.; Liu, Y.D.; Sun, B.R.; Xu, T.; Ding, G.C.; Wei, Y.Q.; et al. Phosphorus excess changes rock phosphate solubilization level and bacterial community mediating phosphorus fractions mobilization during composting. Bioresour. Technol. 2021, 337, 125433. [Google Scholar]
- Long, X.E.; Yao, H.Y.; Huang, Y.; Wei, W.X.; Zhu, Y.G. Phosphate levels influence the utilisation of rice rhizodeposition carbon and the phosphate-solubilising microbial community in a paddy soil. Soil Biol. Biochem. 2018, 118, 103–114. [Google Scholar] [CrossRef]
- Wu, J.; Chen, W.J.; Zhao, Z.C.; Zhang, K.; Zhan, Y.B.; Wu, J.; Ding, G.C.; Wei, Y.Q.; Li, J. Give priority to abiotic factor of phosphate additives for pig manure composting to reduce heavy metal risk rather than bacterial contribution. Bioresour. Technol. 2021, 341, 125894. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Li, J.J.; Shi, L.H.; Li, Y.Y.; Wang, Y.Y. Role of phosphorous additives on nitrogen conservation and maturity during pig manure composting. Environ. Sci. Pollut. Res. 2021, 28, 17981–17991. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, H.; Zhang, H.; Tao, Y.; Chen, R.; Hang, S.; Ding, X.; Cheng, M.; Ding, G.; Wei, Y.; et al. Synergistic effects of chemical additives and mature compost on reducing H2S emission during kitchen waste composting. J. Environ. Sci. 2024, 139, 84–92. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, K.; Zhang, H.; Zhou, K.; Chang, Y.; Zhan, Y.; Pan, C.; Shi, X.; Zuo, H.; Li, J.; et al. Humic acid and phosphorus fractions transformation regulated by carbon-based materials in composting steered its potential for phosphorus mobilization in soil. J. Environ. Manag. 2023, 325, 116553. [Google Scholar] [CrossRef]
- NY/T 525-2021; Organic Fertilizer. China Agriculture Press: Beijing, China, 2021.
- Wei, Y.; Zhao, Y.; Shi, M.; Cao, Z.; Lu, Q.; Yang, T.; Fan, Y.; Wei, Z. Effect of organic acids production and bacterial community on the possible mechanism of phosphorus solubilization during composting with enriched phosphate-solubilizing bacteria inoculation. Bioresour. Technol. 2018, 247, 190–199. [Google Scholar] [CrossRef]
- Wei, Y.; Wu, D.; Wei, D.; Zhao, Y.; Wu, J.; Xie, X.; Zhang, R.; Wei, Z. Improved lignocellulose-degrading performance during straw composting from diverse sources with actinomycetes inoculation by regulating the key enzyme activities. Bioresour. Technol. 2019, 271, 66–74. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, J.; Li, J.; Xin, Y.; Hao, Z.; Chen, C.; Li, H.; Wang, B.; Ding, M.; Li, W.; et al. Insights into bacterial diversity in compost: Core microbiome and prevalence of potential pathogenic bacteria. Sci. Total Environ. 2020, 718, 137304. [Google Scholar] [CrossRef]
- Zhang, S.; Zhou, Z.; Li, Y.; Meng, F. Deciphering the core fouling-causing microbiota in a membrane bioreactor: Low abundance but important roles. Chemosphere 2017, 195, 108–118. [Google Scholar] [CrossRef]
- Xu, M.; Wei, Y.; Chen, F.; Zhou, B.; Ma, S.; Zhan, Y. Neutral initial pH enhances the formation of humic acid by inhibiting the growth of Lactobacillus in food waste composting. Environ. Technol. Innov. 2025, 39, 104271. [Google Scholar] [CrossRef]
- Lei, L.; Gu, J.; Wang, X.; Song, Z.; Yu, J.; Guo, H.; Xie, J.; Wang, J.; Sun, W. Effects and microbial mechanisms of phosphogypsum and medical stone on organic matter degradation and methane emissions during swine manure composting. J. Environ. Manag. 2022, 315, 115139. [Google Scholar] [CrossRef]
- Ma, J.; Mu, L.; Zhang, Z.; Wang, Z.; Kong, W.; Feng, S.; Li, A.; Shen, B.; Zhang, L. Influence of thermal assistance on the biodegradation of organics during food waste bio-drying: Microbial stimulation and energy assessment. Chemosphere 2021, 272, 129875. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.K.; Wang, Q.; Chen, H.; Wang, M.; Awasthi, S.K.; Ren, X.; Cai, H.; Li, R.; Zhang, Z. In-vessel co-composting of biosolid: Focusing on mitigation of greenhouse gases emissions and nutrients conservation. Renew. Energy 2018, 129, 814–823. [Google Scholar] [CrossRef]
- Bouhia, Y.; Lyamlouli, K.; Fels, L.E.; Youssef, Z.; Ouhdouch, Y.; Hafidi, M. Effect of Microbial Inoculation on Lipid and Phenols Removal During the Co-composting of Olive Mill Solid Sludge with Green Waste in Bioreactor. Waste Biomass Valorization 2021, 12, 1417–1429. [Google Scholar] [CrossRef]
- Li, H.; Zhang, T.; Tsang, D.C.W.; Li, G. Effects of external additives: Biochar, bentonite, phosphate, on co-composting for swine manure and corn straw. Chemosphere 2020, 248, 125927. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, A.; Li, G.; Wei, Y.; He, S.; Lin, Z.; Shen, X.; Wang, Q. Effect of different components of single superphosphate on organic matter degradation and maturity during pig manure composting. Sci. Total Environ. 2019, 646, 587–594. [Google Scholar] [CrossRef]
- Zhao, Y.; Lou, Y.; Qin, W.; Cai, J.; Zhang, P.; Hu, B. Interval aeration improves degradation and humification by enhancing microbial interactions in the composting process. Bioresour. Technol. 2022, 358, 127296. [Google Scholar] [CrossRef]
- Wang, Q.; Li, N.; Jiang, S.; Li, G.; Yuan, J.; Li, Y.; Chang, R.; Gong, X. Composting of post-consumption food waste enhanced by bioaugmentation with microbial consortium. Sci. Total Environ. 2024, 907, 168107. [Google Scholar] [CrossRef]
- Jiang, B.; Zeng, Q.; Liu, J.; Hou, Y.; Xu, J.; Li, H.; Shi, S.; Ma, F. Enhanced treatment performance of phenol wastewater and membrane antifouling by biochar-assisted EMBR. Bioresour. Technol. 2020, 306, 123147. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, M.; Jin, B.; Yang, J.; Li, S. Performance evaluation and microbial community analysis of the biofilter for removing grease and volatile organic compounds in the kitchen exhaust fume. Bioresour. Technol. 2021, 319, 124132. [Google Scholar] [CrossRef]
- Kallscheuer, N.; Jogler, C. The bacterial phylum Planctomycetes as novel source for bioactive small molecules. Biotechnol. Adv. 2021, 53, 107818. [Google Scholar] [CrossRef] [PubMed]
- Bello, A.; Han, Y.; Zhu, H.; Deng, L.; Yang, W.; Meng, Q.; Sun, Y.; Egbeagu, U.U.; Sheng, S.; Wu, X.; et al. Microbial community composition, co-occurrence network pattern and nitrogen transformation genera response to biochar addition in cattle manure-maize straw composting. Sci. Total Environ. 2020, 721, 137759. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Ma, R.; Li, G.; Wang, G.; Liu, Y.; Yuan, J. Impact of biochar, calcium magnesium phosphate fertilizer and spent mushroom substrate on humification and heavy metal passivation during composting. Sci. Total Environ. 2022, 824, 153755. [Google Scholar] [CrossRef] [PubMed]
- Estrella-González, M.J.; Suárez-Estrella, F.; Jurado, M.M.; López, M.J.; López-González, J.A.; Siles-Castellano, A.B.; Muñoz-Mérida, A.; Moreno, J. Uncovering new indicators to predict stability, maturity and biodiversity of compost on an industrial scale. Bioresour. Technol. 2020, 313, 123557. [Google Scholar] [CrossRef]
- Lei, L.; Gu, J.; Wang, X.; Song, Z.; Wang, J.; Yu, J.; Hu, T.; Dai, X.; Xie, J.; Zhao, W. Microbial succession and molecular ecological networks response to the addition of superphosphate and phosphogypsum during swine manure composting. J. Environ. Manag. 2021, 279, 111560. [Google Scholar] [CrossRef]
- Ling, N.; Wang, T.; Kuzyakov, Y. Rhizosphere bacteriome structure and functions. Nat. Commun. 2022, 13, 836. [Google Scholar] [CrossRef]
- Li, S.; Gu, X.; Li, H.; Li, M.; Liu, Z.; Xu, Z.; Li, J.; Luo, Y.; Wang, M.; Wan, X. Effects of phosphorus-containing additives on carbon transformation during pig manure composting. Environ. Technol. Innov. 2023, 32, 103290. [Google Scholar]
- Zhang, J.; Wu, Z.; Huang, Y.; Zhan, X.; Zhang, Y.; Cai, C. Industrial-scale composting of swine manure with a novel additive-yellow phosphorus slag: Variation in maturity indicators, compost quality and phosphorus speciation. Bioresour. Technol. 2023, 384, 129356. [Google Scholar] [CrossRef]
- Qiu, W.; Kang, J.; Ding, H.; Sun, R.; Yang, Z.; Ge, J. Aerobic composting of chicken manure with amoxicillin: Alpha diversity is closely related to lipid metabolism, and two-component systems mediating their relationship. Bioresour. Technol. 2022, 360, 127543. [Google Scholar] [CrossRef]
- Zhong, X.Z.; Li, X.X.; Zeng, Y.; Wang, S.P.; Sun, Z.Y.; Tang, Y.Q. Dynamic change of bacterial community during dairy manure composting process revealed by high-throughput sequencing and advanced bioinformatics tools. Bioresour. Technol. 2020, 306, 123091. [Google Scholar] [CrossRef]
- Masuda, Y.; Mise, K.; Xu, Z.; Zhang, Z.; Shiratori, Y.; Senoo, K.; Itoh, H. Global soil metagenomics reveals distribution and predominance of Deltaproteobacteria in nitrogen-fixing microbiome. Microbiome 2024, 12, 1. [Google Scholar] [CrossRef]

| Raw Materials | pH | TOC (g/kg) | TN (g/kg) | Moisture (%) | C/N |
|---|---|---|---|---|---|
| Food waste | 4.62 ± 0.31 | 42.36 ± 1.62 | 3.23 ± 0.06 | 83.64 ± 0.51 | 13.11 ± 0.36 |
| Sawdust | 6.23 ± 0.22 | 45.15 ± 0.51 | 0.26 ± 0.04 | 5.23 ± 0.09 | 173.29 ± 13.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, A.; Chen, Y.; Xu, M.; Liu, B.; Zhang, Z.; Fan, H.; Wei, Y.; Zhan, Y. Soluble Phosphate Additives Remodel Microbial Networks to Accelerate Organic Matter Transformation in Food Waste Composting. Agronomy 2025, 15, 2456. https://doi.org/10.3390/agronomy15112456
Zhang A, Chen Y, Xu M, Liu B, Zhang Z, Fan H, Wei Y, Zhan Y. Soluble Phosphate Additives Remodel Microbial Networks to Accelerate Organic Matter Transformation in Food Waste Composting. Agronomy. 2025; 15(11):2456. https://doi.org/10.3390/agronomy15112456
Chicago/Turabian StyleZhang, Ake, Yunfeng Chen, Min Xu, Bo Liu, Zhi Zhang, Hao Fan, Yuquan Wei, and Yabin Zhan. 2025. "Soluble Phosphate Additives Remodel Microbial Networks to Accelerate Organic Matter Transformation in Food Waste Composting" Agronomy 15, no. 11: 2456. https://doi.org/10.3390/agronomy15112456
APA StyleZhang, A., Chen, Y., Xu, M., Liu, B., Zhang, Z., Fan, H., Wei, Y., & Zhan, Y. (2025). Soluble Phosphate Additives Remodel Microbial Networks to Accelerate Organic Matter Transformation in Food Waste Composting. Agronomy, 15(11), 2456. https://doi.org/10.3390/agronomy15112456






