Abstract
Red grapes are recognized as valuable sources of phenolic compounds with nutritional and technological importance. Anthocyanins strongly influence the color, stability, and antioxidant activity of wines, thereby contributing to both quality and potential health effects. In this study, berries of nine red grapevine cultivars (Alicante Bouschet, Burgund Mare, Busuioacă de Bohotin, Cabernet Franc, Cabernet Sauvignon, Cadarcă, Malbec, Sangiovese, and Syrah) were examined for their anthocyanin composition, total phenolic and flavonoid content, physicochemical parameters, and fatty acid profiles. Anthocyanins were characterized using High Performance Liquid Chromatography coupled with Mass Spectrometry (HPLC-MS), total polyphenols and flavonoids were quantified spectrophotometrically, and fatty acids were determined by Gas Chromatography coupled with Mass Spectrometry (GC-MS). Substantial variability was observed across cultivars for the analyzed traits, with nine anthocyanins identified (total levels ranging from 70.79 ± 13.84 to 335.75 ± 87.62 mg malvidin-3-O-glucoside equivalents (MGE) per 100 g fresh weight (FW). Total phenolics ranged from 107.51 ± 11.11 to 432.13 ± 42.91 mg gallic acid equivalents (GAE) per 100 g FW, and flavonoids from 34.23 ± 11.45 to 162.51 ± 39.63 mg catechin equivalents (CE) per 100 g FW. Ten fatty acids were identified, with linoleic acid being the most abundant. Alicante Bouschet and Burgund Mare showed the highest levels of total anthocyanins, polyphenols, and flavonoids, while Cabernet Franc, Cabernet Sauvignon, and Sangiovese exhibited the richest profiles of polyunsaturated fatty acids, together highlighting their potential as valuable sources of bioactive and nutritional compounds for functional food applications. Cabernet Franc and Sangiovese, characterized by higher titratable acidity and balanced pH, showed favorable traits for producing stable, high-quality wines. Analysis of the data further grouped the cultivars based on their chemical and lipid profiles. Overall, these findings show the notable biochemical differences among the red grapevine cultivars and their potential uses in food and wine production.
1. Introduction
In recent decades, there has been a growing global trend towards consuming foods rich in bioactive compounds. Grapes have seen a significant rise in both production and economic importance. However, this growth has been uneven, with China, along with Spain, the USA, France, and Italy, leading the way in high production levels [1]. Grapes have been consumed by humans for more than 6000 years in several ways, including as fresh table grapes, juice, wine, and dried and stored as raisins [2]. Moreover, a wide range of additional products, including jam, jelly, vinegar, and seed oil, are made from both Vitis vinifera and non-vinifera grape types [3].
The metabolism of grapes produces a wide range of primary and secondary metabolites that provide the plant with competitive advantages against environmental stress. Plant responses to biotic and abiotic stressors involve a broad class of metabolites known as polyphenols, which are products of secondary metabolism. A wide spectrum of physiological and biological actions is attributed to their distinct chemical structures [4]. Grape berries contain different flavonoids, such as anthocyanins and proanthocyanidins, hydroxycinnamic acids, and stilbenes [5]. Flavonoids, particularly anthocyanins, are among the most studied polyphenols due to their significant health benefits. Anthocyanins are molecules consisting of anthocyanidins and sugar residues [6]. The anthocyanin compounds commonly found in V. vinifera grapes include 3-O-monoglucosides of cyanidin, delphinidin, petunidin, peonidin, and malvidin, while anthocyanins with both 3-O-monoglucosides and 3,5-O-diglucosides derivatives are found in other cultivars, such as V. labrusca, V. rotunfolia, V. rupestris, and their hybrids [7].
Anthocyanins in grapes exhibit a wide range of reported biological activities, including effects on lipid profile [8], cardioprotective properties [9], anti-inflammatory effects [10,11], and strong antioxidant activity [12,13]. Additionally, the consumption of polyphenolic-rich foods has been shown to significantly reduce the incidence of several types of cancer [14,15], highlighting the potential health-promoting properties of grape cultivars with high phenolic content.
Depending on the grape fraction, polyphenol concentrations can differ significantly. The highest concentrations of polyphenols and proanthocyanidins are found in grape seeds. Grape skin contains relatively high amounts of anthocyanins and resveratrol, whereas the pulp has only negligible levels of anthocyanins and other phenolics [7,16]. Additionally, grape seeds are rich in fatty acids [17]. Fatty acids are essential for lipid formation, such as phospholipids or triglycerides [18]. These lipids play an essential role in energy metabolism, cell membrane stability, as well as regulation of several cellular processes [19]. The accumulation of these substances in grapes is influenced by several factors, including environmental conditions, grape cultivar, post-harvest storage, and ripeness. For example, V. vinifera grapes are generally most beneficial to health when harvested at the optimal stage of maturity [20,21].
In addition to bioactive compounds, the physicochemical characteristics of grapes, such as pH and titratable acidity, are important for assessing wine quality. These parameters influence fermentation dynamics, microbial stability, and the sensory attributes of wine [22,23,24]. Moreover, pH can modulate the solubility and stability of phenolic compounds during winemaking, thereby affecting their extraction from grape skins and seeds [25]. Therefore, evaluating these traits enhances our understanding of the technological suitability of different grape cultivars and contributes to the selection of optimal grape cultivars in winemaking practices.
The study comprises well-known red grape cultivars such as Cabernet Sauvignon, Cabernet Franc, Malbec, Sangiovese, Syrah, and Alicante Bouschet, as well as some grape cultivars mainly cultivated in the eastern part of Europe, including Burgund Mare, Cadarca, and Busuioacă de Bohotin. Burgund mare, also known as Blaufraenkisch, is planted in Romania on a surface of 676 ha mainly on the Western and Southern part of the country. The country of origin for Cadarca is Hungary, but it is also planted in the Western part of Romania on a surface of 80 ha [26]. Busuioaca de Bohotin, also known as Muscat rouge de Frontignan, has an uncertain origin in Greece. Still, a population of the cultivar was found 100 years ago in the Eastern part of Romania, near the Bohotin village [27]. In Romania, according to Dușa et al. (2024), the area planted with the Busuioacă de Bohotin grape cultivar increased from 96 to 752 ha over the last 15 years due to the interest of consumers for the wine [26].
Despite extensive research on grape composition, most studies have focused on a limited number of cultivars or specific bioactive components. While phenolic or aromatic profiles of some red grape cultivars have been reported, previous studies were conducted in different regions and under distinct environmental conditions, which can strongly influence grape composition. In Romania and Eastern Europe, several traditional and internationally recognized red grape cultivars are grown, including Burgund Mare, Busuioacă de Bohotin, and Cadarca. However, most existing studies on these cultivars address wine characteristics [28,29], while comprehensive phytochemical data on grapes themselves remain insufficient. Therefore, the present study investigates nine red grape (Vitis vinifera L.) cultivars by assessing their anthocyanin profiles, total flavonoid and polyphenol content, physicochemical properties, and fatty acid composition. This comprehensive characterization provides novel insights into the nutritional and technological potential of these cultivars, particularly in the context of the winemaking industry.
2. Materials and Methods
2.1. Plant Material
In 2024, nine grape cultivars (Vitis vinifera L.)—Alicante Bouschet, Burgund Mare, Busuioacă de Bohotin, Cabernet Franc, Cabernet Sauvignon, Cadarcă, Malbec, Sangiovese, and Syrah were harvested in good sanitary conditions from 11-year-old grapevines, in experimental vineyards of the Faculty of Horticulture and Business in Rural Development located in the Transylvania region. The grapevines were planted as grafted vines and trained on a single trellis system with a 50 cm height double trunk, and spur- and cane-pruned, at a spacing of 1.10 m × 2.0 m. No irrigation was applied, and vineyard management followed consistent protocols for pruning, canopy management, pest and disease control, and harvesting. Grape berry samples (samples of 200 berries in duplicate) were picked randomly from 10 different vine plants. To capture variation in sunlight exposure and berry position, samples were taken from both upper and lower parts of the clusters, as well as from sun-exposed and shaded sides of the canopy. Each cultivar was harvested at its optimal technological maturity, resulting in differences in the exact sampling dates. The overall harvesting period extended from late September to early October 2024, ensuring all cultivars were collected during their optimal ripeness. All samples were transported to the Department of Chemistry and Biochemistry and stored at −80 °C until analysis. Previous findings indicate that certain quality parameters may change during prolonged frozen storage [30]. To minimize this effect, all analyzed parameters, including anthocyanin profiles, total polyphenol and flavonoid contents, physicochemical properties, and fatty acid composition, were measured under the same conditions. The first replication was performed immediately after sample collection, and the following two replicates were carried out within two months. All samples were stored at −80 °C until analysis. This approach ensured that comparisons across cultivars were consistent and not influenced by differential storage effects.
2.2. Climatic Characteristics
Romania has experienced a continuous warming trend from 1901 to 2020, with the annual average temperature increasing by 1.3 °C, particularly in summer, winter, and spring. The frequency and duration of hot extremes, including tropical days, nights, and heatwaves, have also risen. The warmest recorded year was 2019, with an average temperature of 12.1 °C, while the coldest was 1940, with 8.1 °C [31]. Within this context, the Transylvania region, traditionally suited for white wine grapes, has started cultivating red wine grape cultivars in recent years. The 2024 growing season in Transylvania was marked by record high temperatures and variable precipitation, which influenced grape berry development and composition. According to the Romanian National Meteorological Administration (ANM), mean monthly air temperatures for the summer months were 23.1 °C in June, 25.1 °C in July, 24.5 °C in August, and 18.8 °C in September, all significantly above the long-term averages (1991–2020). Precipitation was unevenly distributed, with higher rainfall in June and July, relatively low rainfall in August, and a moderate increase in September. Moderate water stress conditions were observed, with relative humidity estimated at 55–60%. Moreover, the summer of 2024 in Transylvania was marked by extreme weather phenomena, dominated by long heat periods and record temperatures, especially in July and August, with frequent highs between 30 and 35 °C, and short intervals of atmospheric instability [32].
2.3. Chemical Reagents
Methanol, chloroform, trifluoroacetic acid (TFA), ethyl acetate, ethanol, formic acid, and acetonitrile were purchased from Merck (Darmstadt, Germany). Folin–Ciocalteu’s reagent was purchased from VWR International (Radnor, PA, USA). Malvidin-3-glucoside standard was purchased from Polyphenols (Sandnes, Norway). Amberlite XAD-7, Sephadex LH-20, gallic acid and catechin standards were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
2.4. Extraction and Determination of Anthocyanins
2.4.1. Preparation of Anthocyanin Fraction
The procedure for extracting anthocyanin fractions from grape berries was modified from earlier protocols [33,34]. Briefly, 1 g of berry samples from nine red grape cultivars was homogenized in acidified methanol (0.3% HCl, v/v) using a Miccra D-9 KT Digitronic homogenizer (Bergheim, Germany). Extraction was repeated until the solvent became colorless, followed by an overnight extraction at 4 °C in the dark to maximize pigment recovery. Methanol acidification prevented anthocyanin degradation [34]. The colored extracts were filtered through cotton wool and concentrated under vacuum at 35 °C to remove methanol. To remove polar impurities, the concentrate underwent ethyl acetate–water liquid–liquid extraction. The aqueous phase was subjected to purification using an Amberlite XAD-7 column (1 × 0.5 cm) preconditioned with six volumes of 0.3% aqueous trifluoroacetic acid (TFA). After sample loading, the column was washed with four volumes of 0.3% aqueous TFA to eliminate sugars and other soluble contaminants. Anthocyanins and procyanidins were eluted with methanol containing 0.3% TFA (v/v). Further purification was carried out on a Sephadex LH-20 column (2.5 × 0.5 cm) pre-conditioned and eluted with 10 volumes of a water:methanol (8:2, v/v) solution acidified with 0.3% TFA. The resulting anthocyanin-rich extract was adjusted to a final volume of 5 mL with distilled water, filtered through a 0.45 μm membrane, and analyzed using HPLC-DAD and HPLC-ESI-MS.
2.4.2. HPLC-DAD Analysis of Anthocyanins
High-performance liquid chromatography (HPLC) analysis was conducted using a Shimadzu Prominence system (Shimadzu Corporation, Kyoto, Japan), comprising an LC-20AT binary pump, DGU-20A3 degasser, SPD-M20 diode array detector (DAD), and a UV-Vis detector. Separation was performed on a Luna C18 column (Phenomenex, Torrance, CA, USA; 5 μm, 250 mm × 4.6 mm). The mobile phase consisted of solvent A (4.5% formic acid in distilled water) and solvent B (acetonitrile). A gradient elution was applied, beginning with 10% solvent B for the first 9 min. The proportion of solvent B was then linearly increased to 12% by 17 min and to 25% by 30 min. From 30 to 50 min, the gradient was held at 90% solvent B. The flow rate was maintained at 0.8 mL/min, with the column temperature set at 35 °C. Quantification of anthocyanins was performed using malvidin-3-O-glucoside as standard, calibrated over a concentration range of 2.5–500 μg/mL. Chromatograms were recorded at 520 nm. Total anthocyanin content was determined by summing all identified anthocyanin peaks and expressed as mg malvidin-3-O-glucoside equivalents (MGE) per 100 g fresh weight (FW).
2.4.3. HPLC-ESI-MS Analysis
Mass spectrometric analysis was performed using an Agilent 1200 HPLC system (Agilent Technologies, Santa Clara, CA, USA) equipped with a quaternary pump, degasser, autosampler, UV-VIS diode array detector, and coupled to an Agilent 6110 single-quadrupole mass spectrometer. Compound separation was achieved on an Eclipse XDB C18 column (5 μm, 150 mm × 4.6 mm; Agilent Technologies) using a binary solvent system: solvent C (water containing 0.1% acetic acid) and solvent D (acetonitrile containing 0.1% acetic acid). A gradient elution was employed at a flow rate of 0.5 mL/min over 30 min at 25 °C. The solvent D gradient was programmed as follows: 0–2 min, 5%; 2–18 min, linear increase to 40%; 18–20 min, increased to 90%; held at 90% from 20 to 24 min; returned to 5% by 25 min and re-equilibrated until 30 min. UV spectra were recorded in the range of 200–600 nm, with chromatograms monitored at 280, 340, and 520 nm. Mass spectrometry was carried out in positive electrospray ionization (ESI+) mode under the following conditions: capillary voltage of 3000 V, source temperature 350 °C, nitrogen flow rate of 7 L/min, nebulizer pressure of 35 psi, fragmentor voltage set to 100 eV, and full scan acquisition in the m/z range of 120–1200. Data acquisition and processing were conducted using Agilent ChemStation software (version Rev B.02.01-SR2).
2.5. Determination of Total Flavonoid Content
Total flavonoid content was quantified using a colorimetric assay adapted from Meyers et al. (2003) and Shin (2012) [35,36]. The alcoholic extract was diluted to a final volume of 5 mL with distilled water. To this, 300 μL of 5% sodium nitrite (NaNO2) was added, and the mixture was allowed to stand for 5 min. Subsequently, 300 μL of 10% aluminum chloride (AlCl3) solution was added, followed by 2 mL of 1 N sodium hydroxide (NaOH) after 6 min. The absorbance was measured at 510 nm using a UV-Vis spectrophotometer (Jasco Corporation, Tokyo, Japan). A standard calibration curve was prepared using catechin at concentrations of 100, 150, 200, and 250 mg/L. Total flavonoid content was calculated based on the standard curve and expressed as mg catechin equivalents (CE) per 100 g FW.
2.6. Determination of Total Phenolic Content
Total polyphenol content in grape extracts was measured using the Folin–Ciocalteu colorimetric method, following the procedure described by Meyers et al. (2003) and Shin (2012), with slight modifications [35,36]. A 25 μL aliquot of each extract was diluted in methanol, and further dilutions were performed as needed to ensure absorbance values fell within the linear range of the gallic acid calibration curve (R2 = 0.997). To initiate the reaction, 120 μL of Folin–Ciocalteu reagent was added, followed by the addition of 340 μL of sodium carbonate (Na2CO3) after a 5 min incubation. The mixtures were then left to react for 90 min at room temperature in the dark. Absorbance was measured at 750 nm using a spectrophotometer. Total polyphenol content was determined based on the gallic acid standard curve and expressed as mg gallic acid equivalents (GAE) per 100 g FW.
2.7. Physicochemical Parameters
Physicochemical parameters (pH and titratable acidity) were determined in accordance with International Organisation of Vine and Wine (OIV) [37].
2.8. Extraction and Determination of Fatty Acids
Total lipids were extracted using the method described by Folch et al. (1957) [38]. Extraction was performed using a mixture of chloroform/methanol. 5 g of grape berries were homogenized in 50 mL of methanol for 1 min using a high-power homogenizer (Miccra D-9 KT Digitronic, Bergheim, Germany). Then, 100 mL of chloroform was added, being homogenized for another 2 min. The mixture was filtered, and the solid residue was suspended in chloroform/methanol (2:1, v/v, 150 mL) and homogenized again for 3 min. After filtering the mixture, the residue was washed with 150 mL chloroform/methanol (2:1, v/v). The filtrates and washings were combined and washed with 0.88% aqueous potassium chloride followed by methanol/water (1:1, v/v) solution. The purified lipid layer was filtered and dried over anhydrous sodium sulfate, and the solvent was removed in a rotary evaporator. The recovered oils were transferred to vials with 2 mL chloroform (stock solution) and stored at −18 °C until further analysis.
Fatty Acid Analysis by GC-MS
The fatty acid composition of grape berry extracts was determined by gas chromatography–mass spectrometry (GC–MS) following derivatization to fatty acid methyl esters (FAMEs). FAMEs were prepared from total lipid extracts using an acid-catalyzed transesterification method as described by Dulf (2012) [39]. The resulting esters were extracted twice with hexane, and the combined extracts were dried over anhydrous sodium sulfate and filtered prior to analysis. GC–MS analysis was performed using a PerkinElmer Clarus 600T system equipped with a Supelcowax 10 capillary column (60 m × 0.25 mm i.d., 0.25 μm film thickness; Supelco Inc., Bellefonte, PA, USA). The injector temperature was set to 210 °C. The oven temperature program started at 140 °C, increased at a rate of 7 °C/min to 220 °C, and was held at 220 °C for 23 min. Helium was used as the carrier gas at a flow rate of 0.8 mL/min with a split ratio of 1:24. Electron impact (EI) ionization was carried out at 70 eV, with a trap current of 100 μA and an ion source temperature of 150 °C. Mass spectra were acquired over an m/z range of 22–395 at a scan rate of 0.14 scans/s, with a 0.02 s inter-scan delay. The injection volume was 0.5 μL. FAMEs were identified by comparing retention times with those of a 37-component FAME standard mixture (Supelco, Cat. No. 47885-U) and confirmed by mass spectral matching using the NIST MS Search 2.0 database. Each fatty acid was expressed as a percentage of total identified fatty acids.
2.9. Statistical Analysis
All data are presented as mean ± standard deviation (SD) from three independent replicates per sample. Initial data processing and descriptive statistics (means, medians, and standard deviations) were conducted using Microsoft Excel 365. Statistical analyses were performed using IBM SPSS Statistics (Version 26), XLSTAT (Version 2019.2.2), and GraphPad Prism (Version 10). The Shapiro–Wilk test was applied to assess the normality of the data distribution. Differences among groups were evaluated using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test, with significance set at p < 0.05. Principal Component Analysis (PCA) was used to reduce data dimensionality and explore correlations among biochemical parameters. Heatmap visualization was applied to identify metabolically related patterns across grape cultivars.
3. Results
3.1. Identification and Quantification of Anthocyanins in Grapes
The rich anthocyanin grape berry extracts were analyzed and characterized by high-performance liquid chromatography (HPLC). The analysis revealed the presence of nine individual anthocyanins by comparison with MS data, retention times, and literature reports (see Supplementary Table S1). Two out of nine grape cultivars exhibited all anthocyanin compounds (Alicante Bouschet and Busuioacă de Bohotin). Figure 1 shows the comparative HPLC chromatograms of the anthocyanin-rich fractions obtained from all cultivars. Three main groups can be clearly distinguished: the monoglucosides of five anthocyanins (peaks 1–5), acetylated anthocyanins (peaks 6–7), and the coumaroyl conjugates (peaks 8–9). The anthocyanin compounds with the highest concentration were malvidin-3-O-glucoside, peonidin-3-O-glucoside, petunidin-3-O-glucoside, and delphinidin-3-O-glucoside (Table 1). Malvidin-3-O-glucoside was the most abundant compound in all cultivars, ranging from 32.81 ± 7.63 mg MGE/100 g FW in Syrah to 165.03 ± 46.50 mg MGE/100 g FW in Alicante Bouschet. Peonidin-3-O-glucoside was the second most abundant in most cultivars, except for Cabernet Sauvignon and Syrah, where delphinidin-3-O-glucoside had higher levels. Acetylated anthocyanins (peaks 6–7), particularly malvidin-3-O-acetylglucoside, were detected only in five cultivars, with the highest concentrations in Alicante Bouschet (13.20 ± 1.76 mg MGE/100 g FW) and Burgund Mare (5.90 ± 0.65 mg MGE/100 g FW). Coumaroyl conjugates (peaks 8–9) were also more abundant in Alicante Bouschet and Syrah, whereas completely absent from Sangiovese. In all cultivars, monoglucosides accounted for most anthocyanins detected. However, the proportion of acetylated anthocyanins and their coumaroyl conjugates varied widely, with Alicante Bouschet and Busuioacă de Bohotin showing the most structurally complex profiles. The total anthocyanin content varied between 70.79 ± 13.84 mg MGE/100 g FW (Syrah) and 335.75 ± 87.62 mg MGE/100 g FW (Alicante Bouschet). High concentrations of total anthocyanins were also identified in Burgund Mare cultivar (238.74 ± 75.52 mg MGE/100 g FW) and Cadarcă cultivar (215.91 ± 65.76 mg MGE/100 g FW) with no significant differences between them. Among the cultivars analyzed, Alicante Bouschet and Busuioacă de Bohotin cultivars contained all nine identified individual anthocyanins. However, Busuioacă de Bohotin had a significantly lower total anthocyanin content (160.47 ± 23.64 mg MGE/100 g FW). The results underline slight differences in anthocyanin profiles among the cultivars, with Alicante Bouschet showing both a high concentration and structural diversity, suggesting a strong potential for antioxidant activity.
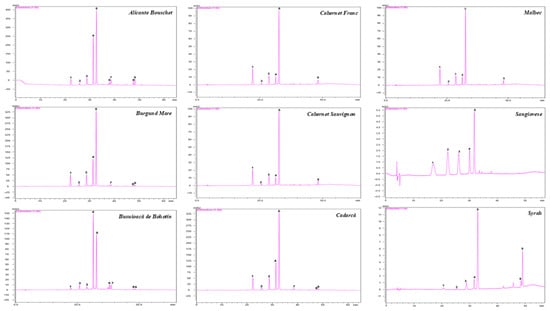
Figure 1.
The HPLC chromatogram for anthocyanin separation in grape (V. vinifera L.) cultivars. 1 = Delphinidin-3-O-glucoside; 2 = Cyanidin-3-O-glucoside; 3 = Petunidin-3-O-glucoside; 4 = Peonidin-3-O-glucoside; 5 = Malvidin-3-O-glucoside; 6 = Peonidin-3-O-acetylglucoside; 7 = Malvidin-3-O-acetylglucoside; 8 = Peonidin-3-O-p-coumaroylglucoside; 9 = Malvidin-3-O-p-coumaroylglucoside (Peak numbers correspond with those from Table S1). Individual anthocyanins were identified based on HPLC-ESI-MS analysis.

Table 1.
Individual anthocyanin content in grape berries, expressed as mg MGE/100 g FW.
3.2. Determination of Total Polyphenols and Total Flavonoids
Total polyphenolic content and total flavonoid content were analyzed through spectrophotometric assays. Alicante Bouschet cultivar had significantly higher total polyphenolic and flavonoid content compared to the other cultivars (Table 2). However, in the case of Burgund Mare cultivar, the difference was not statistically significant for flavonoid content. Additionally, the Burgund Mare and Cadarcă cultivars were found to have high polyphenolic content, while the Syrah cultivar had the lowest concentration, quantified as 107.51 ± 11.11 mg GAE/100 g FW. Reflecting a clear association between polyphenols and flavonoids, Burgund Mare and Cadarcă cultivars also exhibited high concentrations of total flavonoids without significant differences between them, whereas the Syrah cultivar showed the lowest levels.

Table 2.
Total polyphenol and total flavonoid content in grape berries (V. vinifera L.).
3.3. Physicochemical Parameters
The production of wine and assessing their quality are based on physicochemical parameters of grapes. Titratable acidity and pH are critical for their sensorial attributes. Table 3 shows the physicochemical composition of the grape cultivars under study. Alicante Bouschet cultivar recorded the highest pH value (3.46 ± 0.48), without significant difference compared with the other cultivars. However, low pH values were identified in Syrah, Burgund Mare and Cabernet Sauvignon cultivars. In terms of titratable acidity, Sangiovese exhibited the highest level (6.95 ± 0.84 g L−1), while Burgund Mare recorded the lowest value of tritrable acitiy (4.32 ± 0.31 g L−1).

Table 3.
Physicochemical composition of grape berries (V. vinifera L.).
3.4. Identification and Quantification of Fatty Acids in Grapes
The fatty acid composition of whole grape berries oils is listed in Table 4 and Table 5. Figure 2 displays the comparative GC–MS chromatograms of FAMEs obtained from all cultivars, highlighting the differences in their fatty acid composition. Based on fatty acid distribution, the cultivars can be grouped in two distinct categories. The first group (Ali cante Bouschet, Burgund Mare, Busuioacă de Bohotin, Cadarcă, Malbec, and Syrah) exhibited wide variability in fatty acid profiles. In contrast, the second group (Cabernet Sauvignon, Cabernet Franc, and Sangiovese) displayed more homogeneous compositions. The dominating fatty acids were linoleic (C18:2n-6), palmitic (C16:0), and oleic (C18:1n-9) acids. Linoleic acid was predominant in 7 out of the 9 cultivars analyzed, with the highest levels found in Cabernet Franc and Cabernet Sauvignon cultivars (>95%). Exceptions were Burgund Mare and Cadarcă, where palmitic acid was slightly more abundant than linoleic acid. Minor fatty acids, including myristic (C14:0), cis-7-hexadecenoic (C16:1n-9), vaccenic (C18:1n-7), α-linolenic (C18:3n-3), arachidic (C20:0), and behenic acid (C22:0), were detected in all cultivars in trace amounts (<2%).

Table 4.
Fatty acid composition (% weight of total fatty acids) in grape berries (V. vinifera L.) (Part 1). Fatty acid composition (% weight of total fatty acids) in grape berries (V. vinifera L.) (Part 2).

Table 5.
Fatty acid propotions (% weight of total fatty acids) and omega-6/omega-3 ratio in grape berries (V. vinifera L.).
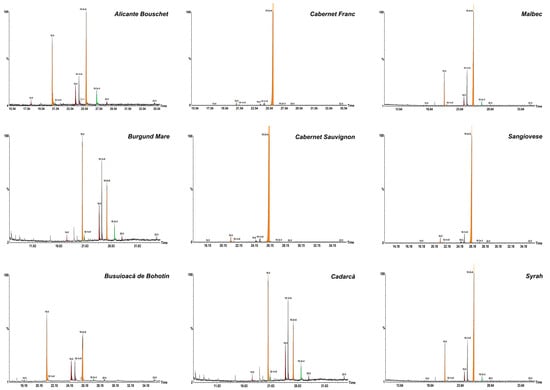
Figure 2.
GC-MS chromatograms of FAME in grape (V. vinifera L.) cultivars. C14:0, myristic; C16:0, palmitic; C16:1n-9, cis-7-hexadecenoic; C18:0, stearic; C18:1n-9, oleic; C18:1n-7, vaccenic; C18:2n-6, linoleic; C18:3n-3, α-linolenic; C20:0, arachidic; C22:0, behenic acid.
On average, polyunsaturated fatty acids (PUFAs) accounted for the largest proportion of total fatty acids across all grape cultivars, followed by saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs). The composition of SFAs varied significantly among the analyzed grape cultivars. The highest total SFA content was recorded in Burgund Mare (49.11%) and Cadarcă (49.00%), largely due to elevated levels of palmitic (C16:0) and stearic acid (C18:0). In contrast, Cabernet Franc and Cabernet Sauvignon exhibited low SFA levels (2.49% and 2.71%, respectively), indicating a potential classification as PUFA-rich cultivars. MUFAs, represented primarily by oleic acid (C18:1n-9), were most abundant in Cadarcă (26.61%) and Burgund Mare (26.71%), with Cabernet Sauvignon and Cabernet Franc showing the lowest MUFA content (2.17% and 2.47%, respectively). Despite variability in total MUFA content, oleic acid (C18:1n-9) remained the principal monounsaturated fatty acid across all cultivars. Cabernet Franc, Cabernet Sauvignon, and Sangiovese cultivars showed the highest proportions of PUFA (>85%), indicating a rich profile in beneficial fatty acids. Conversely, higher saturated fatty acid (SFA) levels were observed in Burgund Mare, Cadarcă, Busuioacă de Bohotin, and Alicante Bouschet. The ω6/ω3 ratio is a key indicator of the nutritional balance of dietary fats. Regarding the ω6/ω3 ratio, Cabernet Franc, Cabernet Sauvignon, and Sangiovese cultivars exhibited very high values, ranging from 704.34 ± 0.34 (Sangiovese) to 1109.77 ± 0.47 (Cabernet Sauvignon). On the other hand, Alicante Bouschet, Burgund Mare and Cadarcă showed relatively low ω6/ω3 ratios (4.44 ± 0.01 to 7.37 ± 0.01), suggesting a healthier balance between omega-6 and omega-3. The PUFA/SFA ratio indicated a predominance of saturated fatty acids in the Burgund Mare, Busuioacă de Bohotin, and Cadarcă cultivars, whereas the Cabernet Franc and Cabernet Sauvignon showed an extremely high ratio (38.21 ± 0.11 and 35.14 ± 0.04, respectively).
3.5. Metabolic Correlations—Principal Component Analysis and Heatmap Visualization
The principal component analysis (PCA) biplots provide a two-dimensional visualization of the relationships between anthocyanin compounds and fatty acid profiles in the analyzed red grape cultivars (Figure 3 and Figure 4). The first two components explain 61.56% of the variance for anthocyanin compounds (F1 = 40.84%, F2 = 20.72%) and 94.08% for fatty acids (F1 = 86.87%, F2 = 7.21%). Malvidin- and peonidin-derived glycosides including malvidin-3-O-glucoside (M3G), peonidin-3-O-acetylglucoside (Pe3AG), malvidin-3-O-acetylglucoside (M3AG) and peonidin-3-O-glucoside (Pe3G) cluster together, indicating that these compounds are the primary drivers of variation in anthocyanin profiles across cultivars. In contrast, certain monoglucoside anthocyanins, such as cyanidin-3-O-glucoside (C3G), delphinidin-3-O-glucoside (D3G), and petunidin-3-O-glucoside (P3G), are distinctly separated from acylated anthocyanins, suggesting differences in their chemical structures. These structural variations may influence various properties, including stability, antioxidant potential, and bioaccessibility, potentially enhancing or reducing their effectiveness.
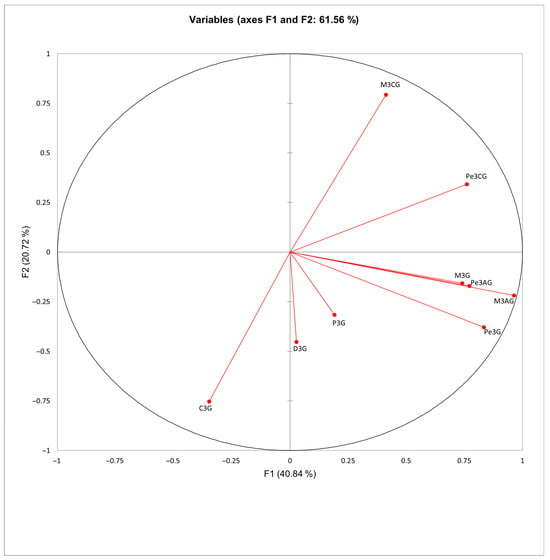
Figure 3.
Principal component analysis (PCA) of anthocyanin compound composition in red grape (Vitis vinifera L.) cultivars. D3G, delphinidin-3-O-glucoside; C3G, cyanidin-3-O-glucoside; P3G, petunidin-3-O-glucoside; Pe3G, peonidin-3-O-glucoside; M3G, malvidin-3-O-glucoside; Pe3AG, peonidin-3-O-acetylglucoside; M3AG, malvidin-3-O-acetylglucoside; Pe3CG, peonidin-3-O-p-coumaroylglucoside; M3CG, malvidin-3-O-p-coumaroylglucoside.
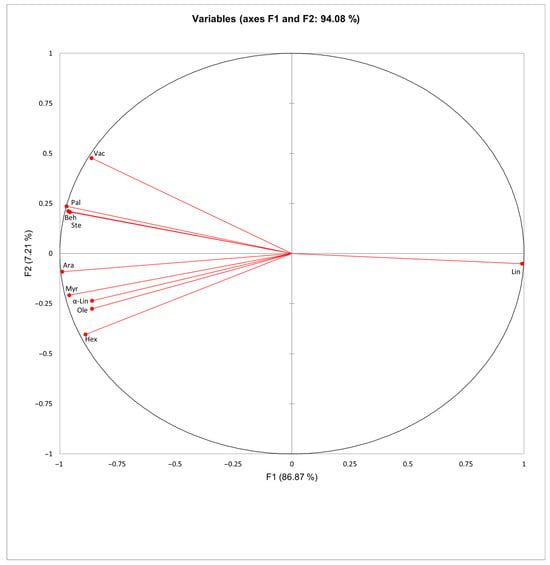
Figure 4.
Principal component analysis (PCA) of lipid composition in red grape (Vitis vinifera L.) cultivars. Myr, myristic; Pal, palmitic; Hex, cis-7-hexadecenoic; Ste, stearic; Ole, oleic; Vac, vaccenic; Lin, linoleic; α-Lin, α-linolenic; Ara, arachidic; Beh, behenic acid.
The biplot regarding fatty acid profiles cluster key fatty acids together such as palmitic (Pal), stearic (Ste), oleic (Ole), behenic (Beh) acids, contributing to the separation of cultivars rich in saturated (SFAs) and monounsaturated fatty acids (MUFAs). Conversely, the most prevalent fatty acid (Lin) in the grape cultivars analyzed in this study loaded in the opposite direction. These results indicate that the primary differentiation among grape cultivars is determined by the relative abundance of linoleic acid versus a group of correlated saturated and monounsaturated fatty acids. Biochemical variations among the analyzed red grape cultivars were also visualized through a heatmap (Figure 5), highlighting relationships among phenolic compounds and fatty acids. Different grape cultivars tend to cluster based on elevated levels of acylated anthocyanins (e.g., Pe3AG) as well as high concentrations of saturated fatty acids (SFAs) and omega-3 polyunsaturated fatty acids (n-3 PUFAs), such as stearic acid (Ste), palmitic acid (Pal), and α-linolenic acid (α-Lin). For example, clear associations were observed between certain cultivars, including Malbec (MA) and Syrah (SYR). Moreover, total flavonoid content (TFC), total phenolic content (TPC), total anthocyanins (TA), and several individual anthocyanins (e.g., M3G, P3G, D3G, and C3G) are strongly correlated among different cultivars.
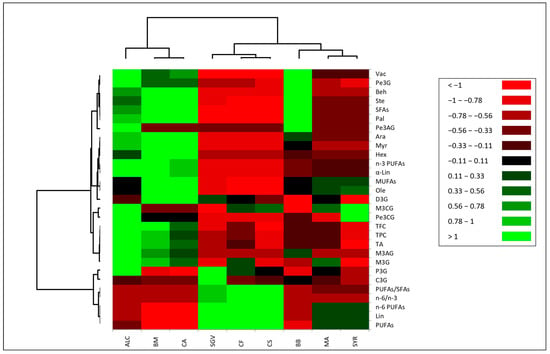
Figure 5.
Heatmap visualization: Biochemical profile of analyzed red grape (V. vinifera L.) cultivars. The heatmap shows correlation coefficients, with red tones indicating negative correlations and green tones positive correlations. The color intensity reflects the correlation strength (scale from −1 to +1). D3G, delphinidin-3-O-glucoside; C3G, cyanidin-3-O-glucoside; P3G, petunidin-3-O-glucoside; Pe3G, peonidin-3-O-glucoside; M3G, malvidin-3-O-glucoside; Pe3AG, peonidin-3-O-acetylglucoside; M3AG, malvidin-3-O-acetylglucoside; Pe3CG, peonidin-3-O-p-coumaroylglucoside; M3CG, malvidin-3-O-p-coumaroylglucoside; Myr, myristic; Pal, palmitic; Hex, cis-7-hexadecenoic; Ste, stearic; Ole, oleic; Vac, vaccenic; Lin, linoleic; α-Lin, α-linolenic; Ara, arachidic; Beh, behenic acid; SFAs, saturated fatty acids; MUFAs, monosaturated fatty acids; n-3 PUFAs, omega-3 polyunsaturated fatty acids; n-6 PUFAs, omega-6 polyunsaturated fatty acids; n-6/n-3, ratio of omega-6 to omega-3 fatty acids; PUFAs/SFAs, ratio of polyunsaturated to saturated fatty acids. ALC, Alicante Bouschet; BM, Burgund Mare; CA, Cadarcă; SGV, Sangiovese; CF, Cabernet Franc; CS, Cabernet Sauvignon; BB, Busuioacă de Bohotin; MA, Malbec; SYR, Syrah.
4. Discussion
The characterization of bioactive compounds from V. vinifera cultivars holds significant importance due to their potential industrial applications and health benefits, closely linked to the presence of large amounts of polyphenols. Through a detailed evaluation of these components, the study sought to expand the understanding of the biochemical composition of nine grape cultivars, offering important insights for both the food and pharmaceutical sectors.
In the grape cultivars analyzed, nine anthocyanin compounds were identified through HPLC analysis. The most abundant anthocyanin compound was malvidin-3-O-glucoside. Even though the most widespread anthocyanin in fruits is cyanidin-3-glucoside [40], the characteristic anthocyanins in red grape and derived products are malvidin glycosides [41]. The results obtained are consistent with most studies on V. vinifera grapes that identified malvidin glycosides as the major anthocyanins [33,42,43,44,45]. Malvidin derivatives are stable compounds and due to their relative resistance to oxidation, they enhance wine stability throughout the winemaking process [46]. Another characteristic of the grapes is the presence of monoglycosides of delphinidin, cyanidin, petunidin, peonidin, and malvidin [47], identified in all grape cultivars analyzed. On the other hand, acylated anthocyanins were found in only a few cultivars in the present study. The acetyl glucoside group, which participates in an intra-molecular copigmentation process leading to an increase in wine color intensity [48], was the minor anthocyanin group. For instance, Sangiovese cultivar did not exhibit any acylated anthocyanins. Silva and Queiroz (2016) identified a profile of seven anthocyanins in the Syrah cultivar that corresponds with the anthocyanins we detected [49]. However, their analysis also revealed the presence of petunidin-3-O-p-coumaroylglucoside, which was not found in this study. Costa et al. (2015) reported the presence of petunidin-3-acetylglucoside in the Alicante Bouschet cultivar, while peonidin-3-acetylglucoside was not detected [43]. Conversely, this study observed the reverse pattern, finding peonidin-3-acetylglucoside but not petunidin-3-acetylglucoside in the same cultivar. Similar patterns were identified in Cabernet Sauvignon, a cultivar that exhibited peonidin-3-O-p-coumaroylglucoside [50,51], but this compound was not found in the cultivars analyzed. Not only the prevalence of monoglycosides over acylated anthocyanins, but also the different anthocyanin profile within the same cultivar could be explained by environmental conditions such as soil composition, light intensity, climate or irrigation [52,53]. The results obtained also revealed differences in the concentrations of total anthocyanins among the cultivars studied. The total anthocyanin content varied from 70.79 ± 13.84 mg MGE/100 g FW in Syrah cultivar to 335.75 ± 87.6 mg MGE/100 g FW in Alicante Bouschet cultivar. These concentrations are consistent with the findings of other studies on the same V. vinifera cultivars [42,54,55]. Some grape cultivars may have a high anthocyanin concentration yet a poor extractability index. Anthocyanins from Cabernet Sauvignon and Syrah cultivars can be extracted easily, according to Romero-Cascales et al. [56]. However, in this study, these cultivars exhibited lower anthocyanin concentrations, and a more limited range of individual anthocyanins compared to the other cultivars. Although only nine anthocyanins were identified in the present study, this result should be interpreted considering the specific environmental and viticultural conditions of the vineyard. Previous studies have demonstrated that anthocyanin biosynthesis is highly sensitive to temperature fluctuations and can be strongly reduced under heat stress conditions [57,58,59,60]. In fact, elevated berry temperatures, which are increasingly frequent under climate change and recurrent summer heatwaves, may irreversibly inhibit anthocyanin accumulation, thereby limiting both their concentration and diversity [61,62]. The reduction in anthocyanin content observed in berries exposed to high temperatures appears to result from a dual effect, namely a decrease in biosynthetic activity and an enhancement of degradation processes, in which peroxidase enzymes are thought to play a central role in anthocyanin catabolism [63].
Anthocyanins are synthesized through the flavonoid biosynthetic pathway, which depends on a series of enzymes regulated by transcription factors such as MYB, bHLH, and WD40 [64]. Exposure to elevated temperatures can disrupt this pathway at multiple levels. For instance, it may suppress the expression of key biosynthetic genes, reduce the activity of specific enzymes like O-methyltransferases and acyltransferases (3AT, AOMT), induce oxidative stress, and limit the availability of energy and carbon substrates. UDP-glucose: flavonoid 3-O-glucosyltransferase (UFGT), the enzyme responsible for the final glycosylation step in anthocyanin biosynthesis, is highly sensitive to heat. Elevated temperatures can suppress the transcription factors that regulate UFGT, reducing its expression and slowing the production of stable anthocyanins, which then become more prone to degradation [65,66,67]. Similarly, inhibition of OMT, 3AT, and AOMT reduces the formation of acylated anthocyanins, simplifying the anthocyanin profile [68]. Heat stress also increases reactive oxygen species (ROS) in berry tissues, which may activate peroxidases that degrade anthocyanins, further decreasing pigment stability [63,69]. Finally, elevated temperatures can alter the distribution of photo assimilates, reducing the flow of sugars into berries. Since sugars act both as substrates and signals for phenolic biosynthesis, this limitation can further contribute to the lower anthocyanin content observed under heat stress [65]. Altitude has also been reported as a determining factor, with low-altitude vineyards often showing lower anthocyanin levels compared to higher-altitude sites due to differences in temperature regimes and solar radiation [70,71]. As noted by Mansour et al. (2022), higher altitudes result in greater UV-B exposure due to the thinner atmospheric column, which can enhance anthocyanin accumulation in grape berries [72]. Anthocyanin biosynthesis pathways rely on a common set of enzymes, yet their regulation is strongly influenced by environmental conditions, with temperature and altitude playing decisive roles. Therefore, the relatively limited anthocyanin profile observed in our study can be attributed to the combination of vineyard location and the exceptionally hot conditions of the harvest year.
Principal component analysis highlighted clear differences between grape cultivars based on their individual anthocyanin compounds, particularly malvidin-3-O-glucoside (M3G) and peonidin-3-O-acetylglucoside (Pe3AG). This suggests that certain anthocyanins play a major role in differentiating cultivars based on their anthocyanin profiles. The distinct separation of certain monoglucoside anthocyanins (C3G, D3G, and P3G) from acylated anthocyanins indicates clear chemical structural differences between these compounds. These structural differences are likely to influence their physicochemical properties, such as solubility, stability, and bioaccessibility. Acylated anthocyanins, typically more complex in structure, may exhibit enhanced stability and bioavailability compared to non-acylated anthocyanins [73,74]. Thus, acylated anthocyanins may impact the sensory characteristics of wine, contributing to more stable color retention and potentially enhancing the complexity of flavor profiles. Given these structural variations, it is important to consider both the type and the concentration of anthocyanins in grape cultivars when selecting grapes for specific winemaking processes. The clustering of metabolic parameters on the vertical axis, as revealed through heatmap analysis, further highlights the relationship among measured compounds, including total flavonoid content (TFC), total phenolic content (TPC), and total anthocyanins (TA) [75,76,77]. This pattern indicates that some compounds tend to coexist in similar proportions within grape cultivars. The individual anthocyanins, such as malvidin-3-O-glucoside (M3G), malvidin-3-O-acetylglucoside (M3AG), peonidin-3-O-p-coumaroylglucoside (Pe3CG), and malvidin-3-O-p-coumaroylglucoside (M3CG) also cluster with these broader phenolic categories. This association highlights that specific anthocyanins contribute not only to the color profile, but also to the antioxidant and sensory properties of grapes and wine. However, some anthocyanins may have a stronger impact on antioxidant activity than others. The findings of Dudek et al. (2022) support this by demonstrating a direct relationship between the structure of anthocyanins and their antioxidant activity [78]. Specifically, anthocyanins with more hydroxyl groups in the B ring, such as those found in certain glucosides, show greater redox potential, suggesting that their structural features directly influence their role in the antioxidant capacity of grapes and wine.
In grapes, pH and titratable acidity are indicators of ripeness and quality, influencing harvest decisions and the potential flavor profile of the wine. The stability and microbiological balance of wine depend on an ideal pH level, which is also closely related to the color and sensory aspects of wine [79]. The grape cultivars studied exhibited pH values between 3.11 ± 0.54 in Burgund Mare cultivar and 3.46 ± 0.48 in Alicante Bouschet cultivar. Costa et al. observed similar pH values in Alicante Bouschet, Cabernet Sauvignon, and Syrah cultivars [43]. On the other hand, in this study, the highest titratable acidity was identified in Sangiovese cultivar (6.95 ± 0.84 g L−1). Pastore et al. reported consistent average values for pH and titratable acidity in the Sangiovese cultivar, based on their study of grapes cultivated during the 2008 and 2009 growing seasons [80]. However, they observed significantly higher values for both pH and titratable acidity in the Cabernet Sauvignon cultivar. These findings align with results from other studies, which reported similar elevated levels for Cabernet Sauvignon [55,81]. The high titratable acidity values may have been attributed to several factors. The ripening process is associated with the degradation of organic acids [82]. Both climate change-related shifts and geographical factors, such as altitude and region, may significantly influence the physicochemical composition of grapes [43,83]. In this context, the reduced titratable acidity values observed in our grape cultivars can be attributed to the combined effect of the extreme heat conditions recorded during the summer of 2024 in Transylvania and the relatively low altitude of the vineyard sites, both of which are known to accelerate ripening and enhance organic acid degradation.
Flavonoids are well-known for their potent antioxidant activity, and these compounds are responsible for all the colors of grape berries and their products, such as wines and juices [84]. They also contribute to organoleptic wine quality as astringency and bitterness [85]. In this study, the Alicante Bouschet cultivar showed the highest levels of both polyphenols and flavonoids, suggesting it could be a valuable source of bioactive compounds. The Burgund Mare and Cadarcă cultivars also had relatively high amounts of these phytochemicals, suggesting that these cultivars may offer substantial health benefits. Costa et al. recorded significantly lower values in Alicante Bouschet (89.4 mg GAE/100 g FW), Syrah (70.7 mg GAE/100 g FW), and Cabernet Sauvignon (47.4 mg GAE/100 g FW) cultivars [43]. Furthermore, the highest total flavonoid content identified in their study was 83.4 mg/100 g FW and 66.1 mg/100 g FW in Alicante Bouschet and Syrah, respectively. However, these values represent averages obtained from the total concentrations from grapes harvested across different years (2008 and 2009) and from various regions (Douro and Dão). In addition to environmental factors, grape skin phenolic composition is strongly influenced by soil characteristics, vineyard management practices, and susceptibility to diseases such as fungal infections [86].
Grapes contain a high concentration of lipophilic elements such as essential fatty acids, phytosterols, and tocopherols, which may contribute to the health benefits associated with the consumption of grapes and their products. In the grape cultivars analyzed, a total of 10 fatty acids were identified. The linoleic acid was the primary fatty acid, followed by palmitic and oleic acids. Al Juhaimi et al. (2017) reported the presence of the same key fatty acids in both Cabernet Sauvignon and Sangiovese cultivars [87]. Nevertheless, the fatty acid profile observed in their study showed some differences compared to the cultivars examined in this research. The differences in lipid profile among various grape cultivars might be correlated with the maturity age of the grape berries [88,89]. Due to its high content of unsaturated fatty acids, grape seed oil has proven to be a high-quality nutritional oil. However, while most of the grape cultivars have a high content of PUFAs, there are significant variations in the balance between omega-3 and omega-6. For instance, Cabernet Franc, Cabernet Sauvignon, and Sangiovese cultivars are rich in PUFAs, but exhibit a pronounced imbalance between omega-6 and omega-3. In contrast, Alicante Bouschet, Burgund Mare and Cadarcă cultivars have a healthier omega-6/omega-3 ratio, but with a lower overall PUFA content. Principal component analysis revealed that saturated fatty acids (SFAs) and monounsaturated fatty acids (MUFAs) were negatively correlated with the same axis. This result indicates that cultivars with higher saturated and monounsaturated fatty acid contents tend to have lower proportions of polyunsaturated fatty acids, suggesting a metabolic balance between saturation and unsaturation processes. The differences observed in the fatty acid profiles among grape cultivars have significant practical implications for viticulture and winemaking. Cultivars rich in polyunsaturated fatty acids (PUFAs), particularly n-6 and n-3 fatty acids, may be more prone to oxidative degradation [90]. Thus, this can potentially lead to loss of aroma compounds and color stability during grape ripening and winemaking [91]. Consequently, these cultivars may require more careful handling and antioxidant protection strategies during vinification. Grapes richer in MUFAs and SFAs may produce different aromatic profiles compared to those abundant in PUFAs. In addition, given the recognized health benefits associated with certain fatty acids, especially n-3 PUFAs [92,93], selecting cultivars with higher n-3 content could support the development of functional wines with added nutritional value. Moreover, as visualized in the generated heatmap, the distribution of fatty acids appeared largely independent of phenolic compound patterns. Cultivars with higher relative phenolic contents were not necessarily those with higher relative fatty acid concentrations, indicating that the biosynthetic regulation of these two groups of metabolites operates through separate pathways. Pérez-Navarro et al. (2019) found that the diversity of lipid composition was not related to berry color [94]. Thus, although phenolic compounds determine the color of the berry, they do not have a direct effect on the distribution of fatty acids. Understanding the distinct accumulation patterns of phenolic compounds and fatty acids offers insights for the winemaking industry seeking to optimize grape selection and practices to enhance specific quality traits.
The findings of this study must be considered in light of certain limitations. First, as the grapes were sourced exclusively from the Horticultural Research Station in Cluj, the findings might be region-specific and not applicable to the same grape cultivars grown under different environmental conditions. Second, the analyses focused on specific compound classes (anthocyanins, flavonoids, polyphenols, and fatty acids), while other relevant constituents such as tannins, stilbenes, and aromatic compounds were not considered. Third, the study did not assess potential changes induced by vinification processes, which restrict the direct applicability of the results to wine products. Finally, as the investigation was based on a single harvest year, the findings should be regarded as preliminary and may not reflect seasonal variability.
5. Conclusions
This study offers valuable insights into the phytochemical composition of nine V. vinifera cultivars, with a particular emphasis on anthocyanins and fatty acids. The finding that malvidin-3-O-glucoside represents the most abundant anthocyanin is consistent with the existing literature and highlights its importance in grape-derived products. Nonetheless, the variation in anthocyanin profiles among cultivars, including differences in acylated anthocyanins, underscores the complexity of grape biochemistry and its implications for wine quality and health benefits. The differences in polyphenolic compounds observed among the cultivars indicate that some cultivars may offer greater health benefits and warrant further investigation into their potential applications in product development. The analyses indicated Alicante Bouschet is the most suitable cultivar for both high-quality winemaking and the extraction of bioactive compounds for functional foods. Moreover, its pH and titratable acidity were within optimal ranges, ensuring good stability and a balanced taste, while its high levels of total and individual anthocyanins contribute to its intense color and antioxidant properties, making it ideal for quality wines. Regarding nutritional and functional potential, Alicante Bouschet also stands out with the highest total polyphenol and flavonoid contents and a favorable fatty acid profile, highlighting its value as a source of health-promoting compounds. Alongside Alicante Bouschet, Burgund Mare also exhibited high levels of total anthocyanins, polyphenols, and flavonoids, confirming its strong antioxidant potential. In contrast, Cabernet Franc, Cabernet Sauvignon, and Sangiovese showed the richest profiles of polyunsaturated fatty acids, underlining their importance as valuable sources of bioactive and nutritional compounds for functional food applications. Additionally, Cabernet Franc and Sangiovese, characterized by higher titratable acidity and balanced pH, displayed favorable traits for producing stable, high-quality wines. In addition, the principal component analysis suggested that specific anthocyanins, particularly malvidin-3-O-glucoside and peonidin-3-O-glucoside, could serve as reliable markers for assessing the antioxidant capacity of grape cultivars, given their strong correlation with total anthocyanins, total phenolic content, and total flavonoid content. Moreover, the fatty acid composition in red grape cultivars is tightly regulated, and certain cultivars may be metabolically predisposed to favor the synthesis of specific fatty acid classes. Therefore, understanding the metabolic predisposition of each grape cultivar to specific fatty acid profiles is important for optimizing viticultural practices, identifying the impact of environmental stresses on grape and wine quality and guiding grape selection.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15102443/s1, Table S1: Retention times, UV-VIS and mass spectral data of anthocyanins in the analyzed grape cultivars samples; Figures S1–S9: The HPLC Chromatograms for anthocyanin separation in grape cultivars; Figures S10–S18: The GC-MS Chromatograms for fatty acid separation in grape cultivars; Figures S19–S20: Relationship between eigenvalues and principal components in PCA.
Author Contributions
Conceptualization, C.-A.F., A.B. and D.O.; methodology, C.-A.F., A.B. and D.O.; software, C.-A.F., D.O. and F.-D.B.; validation, C.-A.F., D.O., C.I.B., F.D. and A.B.; formal analysis, C.-A.F., D.O., A.C. and F.-D.B.; investigation, C.-A.F., D.O., C.I.B. and F.D.; resources, C.-A.F., C.I.B., A.C., F.D. and A.B.; data curation, C.-A.F., D.O. and A.C.; writing—original draft preparation, C.-A.F., D.O. and F.-D.B.; writing—review and editing, C.-A.F., C.I.B., F.D. and A.B.; visualization, C.-A.F., D.O., A.C. and F.-D.B.; supervision, C.I.B., F.D. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
The article processing charge was supported by a grant from the Ministry of Education and Research, project number CNFIS-FDI-2025-F-0115.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alston, J.M.; Sambucci, O. Grapes in the World Economy. In The Grape Genome. Compendium of Plant Genomes; Cantu, D., Walker, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Kandylis, P. Grapes and Their Derivatives in Functional Foods. Foods 2021, 10, 672. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Du, B.; Zheng, L.; Li, J. Advance on the Bioactivity and Potential Applications of Dietary Fibre from Grape Pomace. Food Chem. 2015, 186, 207–212. [Google Scholar] [CrossRef]
- Giovinazzo, G.; Grieco, F. Functional Properties of Grape and Wine Polyphenols. Plant Foods Hum. Nutr. 2015, 70, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Gouot, J.C.; Smith, J.P.; Holzapfel, B.P.; Walker, A.R.; Barril, C. Grape Berry Flavonoids: A Review of Their Biochemical Responses to High and Extreme High Temperatures. J. Exp. Bot. 2019, 70, 397–423. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Sabra, A.; Netticadan, T.; Wijekoon, C. Grape Bioactive Molecules, and the Potential Health Benefits in Reducing the Risk of Heart Diseases. Food Chem. X 2021, 12, 100149. [Google Scholar] [CrossRef]
- Lupoli, R.; Ciciola, P.; Costabile, G.; Giacco, R.; Di Minno, M.N.D.; Capaldo, B. Impact of Grape Products on Lipid Profile: A Meta-Analysis of Randomized Controlled Studies. J. Clin. Med. 2020, 9, 313. [Google Scholar] [CrossRef]
- Feringa, H.H.H.; Laskey, D.A.; Dickson, J.E.; Coleman, C.I. The Effect of Grape Seed Extract on Cardiovascular Risk Markers: A Meta-Analysis of Randomized Controlled Trials. J. Am. Diet. Assoc. 2011, 111, 1173–1181. [Google Scholar] [CrossRef] [PubMed]
- Kar, P.; Laight, D.; Rooprai, H.K.; Shaw, K.M.; Cummings, M. Effects of Grape Seed Extract in Type 2 Diabetic Subjects at High Cardiovascular Risk: A Double Blind Randomized Placebo Controlled Trial Examining Metabolic Markers, Vascular Tone, Inflammation, Oxidative Stress and Insulin Sensitivity. Diabet. Med. 2009, 26, 526–531. [Google Scholar] [CrossRef]
- Sarkhosh-Khorasani, S.; Hosseinzadeh, M. The Effect of Grape Products Containing Polyphenols on C-Reactive Protein Levels: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2021, 125, 1230–1245. [Google Scholar] [CrossRef]
- Magrone, T.; Magrone, M.; Russo, M.A.; Jirillo, E. Recent Advances on the Anti-Inflammatory and Antioxidant Properties of Red Grape Polyphenols: In Vitro and In Vivo Studies. Antioxidants 2019, 9, 35. [Google Scholar] [CrossRef]
- Quero, J.; Jiménez-Moreno, N.; Esparza, I.; Osada, J.; Cerrada, E.; Ancín-Azpilicueta, C.; Rodríguez-Yoldi, M.J. Grape Stem Extracts with Potential Anticancer and Antioxidant Properties. Antioxidants 2021, 10, 243. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Kaur, M.; Katnoria, J.K.; Nagpal, A.K. Polyphenols in Food: Cancer Prevention and Apoptosis Induction. Curr. Med. Chem. 2018, 25, 4740–4757. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in Cancer Prevention: New Insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Rebello, L.P.G.; Lago-Vanzela, E.S.; Barcia, M.T.; Ramos, A.M.; Stringheta, P.C.; Da-Silva, R.; Castillo-Muñoz, N.; Gómez-Alonso, S.; Hermosín-Gutiérrez, I. Phenolic Composition of the Berry Parts of Hybrid Grape Cultivar BRS Violeta (BRS Rubea×IAC 1398-21) Using HPLC–DAD–ESI-MS/MS. Food Res. Int. 2013, 54, 354–366. [Google Scholar] [CrossRef]
- Górnaś, P.; Rudzińska, M.; Grygier, A.; Lācis, G. Diversity of Oil Yield, Fatty Acids, Tocopherols, Tocotrienols, and Sterols in the Seeds of 19 Interspecific Grapes Crosses. J. Sci. Food Agric. 2019, 99, 2078–2087. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.Y.; Liu, M.; Portincasa, P.; Wang, D.Q. -H. New Insights into the Molecular Mechanism of Intestinal Fatty Acid Absorption. Eur. J. Clin. Investig. 2013, 43, 1203–1223. [Google Scholar] [CrossRef]
- Glatz, J.F.C.; Luiken, J.J.F.P. Fatty Acids in Cell Signaling: Historical Perspective and Future Outlook. Prostaglandins Leukot. Essent. Fat. Acids 2015, 92, 57–62. [Google Scholar] [CrossRef]
- Perestrelo, R.; Lu, Y.; Santos, S.A.O.; Silvestre, A.J.D.; Neto, C.P.; Câmara, J.S.; Rocha, S.M. Phenolic Profile of Sercial and Tinta Negra Vitis Vinifera L. Grape Skins by HPLC–DAD–ESI-MSn. Food Chem. 2012, 135, 94–104. [Google Scholar] [CrossRef]
- Yang, B.; He, S.; Liu, Y.; Liu, B.; Ju, Y.; Kang, D.; Sun, X.; Fang, Y. Transcriptomics Integrated with Metabolomics Reveals the Effect of Regulated Deficit Irrigation on Anthocyanin Biosynthesis in Cabernet Sauvignon Grape Berries. Food Chem. 2020, 314, 126170. [Google Scholar] [CrossRef]
- Ruiz, J.; Kiene, F.; Belda, I.; Fracassetti, D.; Marquina, D.; Navascués, E.; Calderón, F.; Benito, A.; Rauhut, D.; Santos, A.; et al. Effects on Varietal Aromas during Wine Making: A Review of the Impact of Varietal Aromas on the Flavor of Wine. Appl. Microbiol. Biotechnol. 2019, 103, 7425–7450. [Google Scholar] [CrossRef]
- Mendes Ferreira, A.; Mendes-Faia, A. The Role of Yeasts and Lactic Acid Bacteria on the Metabolism of Organic Acids during Winemaking. Foods 2020, 9, 1231. [Google Scholar] [CrossRef]
- Tian, M.-B.; Hu, R.-Q.; Liu, Z.-L.; Shi, N.; Lu, H.-C.; Duan, C.-Q.; Wang, J.; Sun, Y.-F.; Kong, Q.-S.; He, F. The PH Adjustment of Vitis Amurensis Dry Red Wine Revealed the Evolution of Organic Acids, Volatomics, and Sensory Quality during Winemaking. Food Chem. 2024, 436, 137730. [Google Scholar] [CrossRef] [PubMed]
- Guaita, M.; Bosso, A. Polyphenolic Characterization of Grape Skins and Seeds of Four Italian Red Cultivars at Harvest and after Fermentative Maceration. Foods 2019, 8, 395. [Google Scholar] [CrossRef] [PubMed]
- Dușa, D.Ș.; Heizer, R.T.; Heizer, M.G.; Baniță, S.I.; Călugăr, A.; Constantinescu, D.G.; Dobrei, A.; Bunea, C.I. Romanian Vineyard Areas and the Evolution of Wines with Traceability in the Period 2007–2022, Using European Restructuring/Reconversion Funds. Sci. Papers. Ser. B Hortic. 2024, 68, 283–291. [Google Scholar]
- Stroe, M. Ampelografie; Ceres: Bucuresti, Romania, 2012; ISBN 978-973-40-0943-5. [Google Scholar]
- Geana, I.; Andreea Maria, I.; Roxana, I.; Irina Geana, E.; Maria Iordache, A.; Elena Ionete, R. Assessing the Wine Anthocyanin Profile for Red Grape Varieties Identification. Prog. Cryog. Isot. 2011, 14, 127–133. [Google Scholar]
- Ivanisevic, D.; Kalajdzic, M.; Di Gaspero, G.; Drenjancevic, M.; Korac, N.; Schwander, F.; Braun, U.; Barac, G.; Foria, S. Genetic, Morphological and Chemical Characterisation of the Grape Variety “Probus” (Vitis vinifera L.). Genetika 2019, 51, 1061–1073. [Google Scholar] [CrossRef]
- García, S.; Santesteban, L.G.; Miranda, C.; Royo, J.B. Variety and Storage Time Affect the Compositional Changes That Occur in Grape Samples after Frozen Storage. Aust. J. Grape Wine Res. 2011, 17, 162–168. [Google Scholar] [CrossRef]
- Gnamus, A.; Chioncel, M. Transformative Innovation for Better Climate Change Adaptation—Case Study: Nord-Vest and Cluj-Napoca, Romania; Publications Office of the European Union: Luxembourg, 2025. [Google Scholar]
- Pieptenaru, M.-L.; Stoica, R.-M.; Halada, S.; Milian, N. Temperature Records in the Summer of 2024 in Transylvania. Air Water Compon. Environ. Aerul Compon. Mediu. 2025, 27–41. [Google Scholar]
- Balík, J.; Kumšta, M.; Rop, O. Comparison of Anthocyanins Present in Grapes of Vitis vinifera L. Varieties and Interspecific Hybrids Grown in the Czech Republic. Chem. Pap. 2013, 67, 1285–1292. [Google Scholar] [CrossRef]
- Hariram Nile, S.; Hwan Kim, D.; Keum, Y.-S. Determination of Anthocyanin Content and Antioxidant Capacity of Different Grape Varieties. Ciência Técnica Vitivinícola 2015, 30, 60–68. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and Antiproliferative Activities of Strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y. Correlation between Antioxidant Concentrations and Activities of Yuja (Citrus Junos Sieb Ex Tanaka) and Other Citrus Fruit. Food. Sci. Biotechnol. 2012, 21, 1477–1482. [Google Scholar] [CrossRef]
- International Organization of Vine and Wine. Compendium of International Methods of Wine and Must Analysis. In International Organisation of Vine and Wine; International Organization of Vine and Wine: Paris, France, 2021; Volume 1, pp. 1–520. ISBN 9782850380334. [Google Scholar]
- Folch, J.; Lees, M.; Stanley, G.H.S. A Simple Method for the Isolation of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Dulf, F. V Fatty Acids in Berry Lipids of Six Sea Buckthorn (Hippophae rhamnoides L., Subspecies Carpatica) Cultivars Grown in Romania. Chem. Cent. J. 2012, 6, 106. [Google Scholar] [CrossRef]
- Kong, J.-M.; Chia, L.-S.; Goh, N.-K.; Chia, T.-F.; Brouillard, R. Analysis and Biological Activities of Anthocyanins. Phytochemistry 2003, 64, 923–933. [Google Scholar] [CrossRef]
- Mazza, G. Anthocyanins in Fruits, Vegetables, and Grains; Mazza, G., Miniati, E., Eds.; CRC Press: Boca Raton, FL, USA, 2018; ISBN 9781351069700. [Google Scholar]
- Guerrero, R.F.; Liazid, A.; Palma, M.; Puertas, B.; González-Barrio, R.; Gil-Izquierdo, Á.; García-Barroso, C.; Cantos-Villar, E. Phenolic Characterisation of Red Grapes Autochthonous to Andalusia. Food Chem. 2009, 112, 949–955. [Google Scholar] [CrossRef]
- Costa, E.; da Silva, J.F.; Cosme, F.; Jordão, A.M. Adaptability of Some French Red Grape Varieties Cultivated at Two Different Portuguese Terroirs: Comparative Analysis with Two Portuguese Red Grape Varieties Using Physicochemical and Phenolic Parameters. Food Res. Int. 2015, 78, 302–312. [Google Scholar] [CrossRef]
- Budić-Leto, I.; Mucalo, A.; Ljubenkov, I.; Zdunić, G. Anthocyanin Profile of Wild Grape Vitis vinifera in the Eastern Adriatic Region. Sci. Hortic. 2018, 238, 32–37. [Google Scholar] [CrossRef]
- Kőrösi, L.; Molnár, S.; Teszlák, P.; Dörnyei, Á.; Maul, E.; Töpfer, R.; Marosvölgyi, T.; Szabó, É.; Röckel, F. Comparative Study on Grape Berry Anthocyanins of Various Teinturier Varieties. Foods 2022, 11, 3668. [Google Scholar] [CrossRef] [PubMed]
- Ribeéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Phenolic Compounds. In Handbook of Enology: The Chemistry of Wine Stabilization and Treatments; John Wiley & Sons: Hoboken, NJ, USA, 2021; Volume 2, p. 161. ISBN 9780470010372. [Google Scholar]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Changes in the Detailed Pigment Composition of Red Wine during Maturity and Ageing. Anal. Chim. Acta 2006, 563, 238–254. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Romero-Cascales, I.; López-Roca, J.M.; Ros-García, J.M.; Gómez-Plaza, E. Anthocyanin Fingerprint of Grapes: Environmental and Genetic Variations. J. Sci. Food Agric. 2006, 86, 1460–1467. [Google Scholar] [CrossRef]
- Silva, L.R.; Queiroz, M. Bioactive Compounds of Red Grapes from Dão Region (Portugal): Evaluation of Phenolic and Organic Profile. Asian Pac. J. Trop. Biomed. 2016, 6, 315–321. [Google Scholar] [CrossRef]
- Núñez, V.; Monagas, M.; Gomez-Cordovés, M.C.; Bartolomé, B. Vitis vinifera L. Cv. Graciano Grapes Characterized by Its Anthocyanin Profile. Postharvest Biol. Technol. 2004, 31, 69–79. [Google Scholar] [CrossRef]
- Ju, Y.; Yang, B.; He, S.; Tu, T.; Min, Z.; Fang, Y.; Sun, X. Anthocyanin Accumulation and Biosynthesis Are Modulated by Regulated Deficit Irrigation in Cabernet Sauvignon (Vitis vinifera L.) Grapes and Wines. Plant Physiol. Biochem. 2019, 135, 469–479. [Google Scholar] [CrossRef]
- Adams, D.O. Phenolics and Ripening in Grape Berries. Am. J. Enol. Vitic. 2006, 57, 249–256. [Google Scholar] [CrossRef]
- Conde, C.; Silva, P.; Fontes, N.; Dias, A.C.P.; Tavares, R.M.; Sousa, M.J.; Agasse, A.; Delrot, S.; Gerós, H. Biochemical Changes throughout Grape Berry Development and Fruit and Wine Quality; Special Feature Food; Global Science Books: Hershey, PA, USA, 2007. [Google Scholar]
- Mattivi, F.; Guzzon, R.; Vrhovsek, U.; Stefanini, M.; Velasco, R. Metabolite Profiling of Grape: Flavonols and Anthocyanins. J. Agric. Food Chem. 2006, 54, 7692–7702. [Google Scholar] [CrossRef]
- Orak, H.H. Total Antioxidant Activities, Phenolics, Anthocyanins, Polyphenoloxidase Activities of Selected Red Grape Cultivars and Their Correlations. Sci. Hortic. 2007, 111, 235–241. [Google Scholar] [CrossRef]
- Romero-Cascales, I.; Ortega-Regules, A.; López-Roca, J.M.; Fernández-Fernández, J.I.; Gómez-Plaza, E. Differences in Anthocyanin Extractability from Grapes to Wines According to Variety. Am. J. Enol. Vitic. 2005, 56, 212–219. [Google Scholar] [CrossRef]
- Kliewer, W.M. Influence of Temperature, Solar Radiation and Nitrogen on Coloration and Composition of Emperor Grapes. Am. J. Enol. Vitic. 1977, 28, 96–103. [Google Scholar] [CrossRef]
- Buttrose, M.S.; Hale, C.R.; Kliewer, W.M. Effect of Temperature on the Composition of “Cabernet Sauvignon” Berries. Am. J. Enol. Vitic. 1971, 22, 71–75. [Google Scholar] [CrossRef]
- Spayd, S.E.; Tarara, J.M.; Mee, D.L.; Ferguson, J.C. Separation of Sunlight and Temperature Effects on the Composition of Vitis Vinifera Cv. Merlot Berries. Am. J. Enol. Vitic. 2002, 53, 171–182. [Google Scholar] [CrossRef]
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry Temperature and Solar Radiation Alter Acylation, Proportion, and Concentration of Anthocyanin in Merlot Grapes. Am. J. Enol. Vitic. 2008, 59, 235–247. [Google Scholar] [CrossRef]
- Cohen, S.D.; Tarara, J.M.; Kennedy, J.A. Assessing the Impact of Temperature on Grape Phenolic Metabolism. Anal. Chim. Acta 2008, 621, 57–67. [Google Scholar] [CrossRef]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar] [CrossRef]
- Movahed, N.; Pastore, C.; Cellini, A.; Allegro, G.; Valentini, G.; Zenoni, S.; Cavallini, E.; D’Incà, E.; Tornielli, G.B.; Filippetti, I. The Grapevine VviPrx31 Peroxidase as a Candidate Gene Involved in Anthocyanin Degradation in Ripening Berries under High Temperature. J. Plant Res. 2016, 129, 513–526. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L. Transcriptional Control of Flavonoid Biosynthesis by MYB–BHLH–WDR Complexes. Trends Plant Sci. 2015, 20, 176–185. [Google Scholar] [CrossRef]
- Mori, K.; Sugaya, S.; Gemma, H. Regulatory Mechanism of Anthocyanin Biosynthesis in “Kyoho” Grape Berries Grown under Different Temperature Conditions. Environ. Control. Biol. 2004, 42, 21–30. [Google Scholar] [CrossRef]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of Anthocyanins in Red-Wine Grape under High Temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Yamane, T.; Jeong, S.T.; Goto-Yamamoto, N.; Koshita, Y.; Kobayashi, S. Effects of Temperature on Anthocyanin Biosynthesis in Grape Berry Skins. Am. J. Enol. Vitic 2006, 57, 54–59. [Google Scholar] [CrossRef]
- de Rosas, I.; Deis, L.; Baldo, Y.; Cavagnaro, J.B.; Cavagnaro, P.F. High Temperature Alters Anthocyanin Concentration and Composition in Grape Berries of Malbec, Merlot, and Pinot Noir in a Cultivar-Dependent Manner. Plants 2022, 11, 926. [Google Scholar] [CrossRef]
- Sunil, L.; Shetty, N.P. Biosynthesis and Regulation of Anthocyanin Pathway Genes. Appl. Microbiol. Biotechnol. 2022, 106, 1783–1798. [Google Scholar] [CrossRef]
- Xing, R.-R.; He, F.; Xiao, H.-L.; Duan, C.-Q.; Pan, Q.-H. Accumulation Pattern of Flavonoids in Cabernet Sauvignon Grapes Grown in a Low-Latitude and High-Altitude Region. South Afr. J. Enol. Vitic. 2016, 36, 32–43. [Google Scholar] [CrossRef][Green Version]
- Coklar, H. Antioxidant Capacity and Phenolic Profile of Berry, Seed, and Skin of Ekşikara (Vitis vinifera L.) Grape: Influence of Harvest Year and Altitude. Int. J. Food Prop. 2017, 20, 2071–2087. [Google Scholar] [CrossRef]
- Mansour, G.; Ghanem, C.; Mercenaro, L.; Nassif, N.; Hassoun, G.; Del Caro, A. Effects of Altitude on the Chemical Composition of Grapes and Wine: A Review. OENO One 2022, 56, 227–239. [Google Scholar] [CrossRef]
- Zhao, C.-L.; Yu, Y.-Q.; Chen, Z.-J.; Wen, G.-S.; Wei, F.-G.; Zheng, Q.; Wang, C.-D.; Xiao, X.-L. Stability-Increasing Effects of Anthocyanin Glycosyl Acylation. Food Chem. 2017, 214, 119–128. [Google Scholar] [CrossRef]
- Oliveira, H.; Perez-Gregório, R.; de Freitas, V.; Mateus, N.; Fernandes, I. Comparison of the in Vitro Gastrointestinal Bioavailability of Acylated and Non-Acylated Anthocyanins: Purple-Fleshed Sweet Potato vs Red Wine. Food Chem. 2019, 276, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Orak, H.H.; Yagar, H.; Isbilir, S.S. Comparison of Antioxidant Activities of Juice, Peel, and Seed of Pomegranate (Punica granatum L.) and Inter-Relationships with Total Phenolic, Tannin, Anthocyanin, and Flavonoid Contents. Food. Sci. Biotechnol. 2012, 21, 373–387. [Google Scholar] [CrossRef]
- Muflihah, Y.M.; Gollavelli, G.; Ling, Y.-C. Correlation Study of Antioxidant Activity with Phenolic and Flavonoid Compounds in 12 Indonesian Indigenous Herbs. Antioxidants 2021, 10, 1530. [Google Scholar] [CrossRef]
- Shibata, Y.; Ohara, K.; Matsumoto, K.; Hasegawa, T.; Akimoto, M. Total Anthocyanin Content, Total Phenolic Content, and Antioxidant Activity of Various Blueberry Cultivars Grown in Togane, Chiba Prefecture, Japan. J. Nutr. Sci. Vitaminol. 2021, 67, 201–209. [Google Scholar] [CrossRef]
- Dudek, A.; Spiegel, M.; Strugała-Danak, P.; Gabrielska, J. Analytical and Theoretical Studies of Antioxidant Properties of Chosen Anthocyanins; A Structure-Dependent Relationships. Int. J. Mol. Sci. 2022, 23, 5432. [Google Scholar] [CrossRef]
- Payan, C.; Gancel, A.-L.; Jourdes, M.; Christmann, M.; Teissedre, P.-L. Wine Acidification Methods: A Review. OENO One 2023, 57, 113–126. [Google Scholar] [CrossRef]
- Pastore, C.; Allegro, G.; Valentini, G.; Muzzi, E.; Filippetti, I. Anthocyanin and Flavonol Composition Response to Veraison Leaf Removal on Cabernet Sauvignon, Nero d’Avola, Raboso Piave and Sangiovese Vitis vinifera L. Cultivars. Sci. Hortic. 2017, 218, 147–155. [Google Scholar] [CrossRef]
- Pajovic, R.; Raicevic, D.; Popovic, T.; Sivilotti, P.; Lisjak, K.; Vanzo, A. Polyphenolic Characterisation of Vranac, Kratosija and Cabernet Sauvignon (Vitis vinifera L. Cv.) Grapes and Wines from Different Vineyard Locations in Montenegro. S. Afr. J. Enol. Vitic. 2016, 35, 139–148. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, L.; Jiang, X.; Cherono, S.; Liu, J.; Ogutu, C.; Ntini, C.; Zhang, X.; Han, Y. Assessment of Organic Acid Accumulation and Its Related Genes in Peach. Food Chem. 2021, 334, 127567. [Google Scholar] [CrossRef]
- Mira de Orduña, R. Climate Change Associated Effects on Grape and Wine Quality and Production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef] [PubMed]
- Hornedo-Ortega, R.; Reyes González-Centeno, M.; Chira, K.; Jourdes, M.; Teissedre, P.-L. Phenolic Compounds of Grapes and Wines: Key Compounds and Implications in Sensory Perception. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; IntechOpen: London, UK, 2021. [Google Scholar]
- Bruno, G.; Sparapano, L. Effects of Three Esca-Associated Fungi on Vitis vinifera L.: V. Changes in the Chemical and Biological Profile of Xylem Sap from Diseased Cv. Sangiovese Vines. Physiol. Mol. Plant. Pathol. 2007, 71, 210–229. [Google Scholar] [CrossRef]
- Al Juhaimi, F.; Geçgel, Ü.; Gülcü, M.; Hamurcu, M.; Özcan, M.M. South African Journal for Enology and Viticulture. South Afr. J. Enol. Vitic. 2017, 38, 103–108. [Google Scholar]
- Ohnishi, M.; Hirose, S.; Kawaguchi, M.; Ito, S.; Fujino, Y. Chemical Composition of Lipids, Especially Triacylglycerol, in Grape Seeds. Agric. Biol. Chem. 1990, 54, 1035–1042. [Google Scholar] [CrossRef]
- Baydar, N.G.; Akkurt, M. Oil Content and Oil Quality Properties of Some Grape Seeds. Turk. J. Agric. For. 2001, 25, 163–168. [Google Scholar]
- Tao, L. Oxidation of Polyunsaturated Fatty Acids and Its Impact on Food Quality and Human Health. Adv. Food Technol. Nutr. Sci. Open J. 2015, 1, 135–142. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation Mechanisms Occurring in Wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Swanson, D.; Block, R.; Mousa, S.A. Omega-3 Fatty Acids EPA and DHA: Health Benefits Throughout Life. Adv. Nutr. 2012, 3, 1–7. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Navarro, J.; Da Ros, A.; Masuero, D.; Izquierdo-Cañas, P.M.; Hermosín-Gutiérrez, I.; Gómez-Alonso, S.; Mattivi, F.; Vrhovsek, U. LC-MS/MS Analysis of Free Fatty Acid Composition and Other Lipids in Skins and Seeds of Vitis vinifera Grape Cultivars. Food Res. Int. 2019, 125, 108556. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).