Abstract
Breaking the maize yield plateau necessitates the development of density-tolerant varieties, for which recurrent selection is a key breeding strategy. However, a systematic understanding of how the interaction between selection density (parental screening environment) and evaluation density (variety testing environment) modulates genetic gain remains a critical knowledge gap. This study aimed to systematically elucidate this interaction and its impact on genetic gain and combining ability. We established two F2 base populations from distinct heterotic groups: Zheng 58 × LH196 (Stiff Stalk, SS) and Chang 7-2 × MBUB (Non-Stiff Stalk, NSS). Through bulk selection, we advanced populations for three cycles (C0, C2, C4) under three selection densities: low (60,000 plants/ha), medium (90,000 plants/ha), and high (120,000 plants/ha). Hybrids were generated using a double tester design and evaluated in multi-environment trials at Shijiazhuang in Hebei province and Xinxiang in Henan province in 2023 across matching density gradients. We employed analysis of variance (ANOVA) and general combining-ability (GCA) estimates to assess the genetic gains for yield and combining ability across 14 parental materials and 28 hybrids. Our results demonstrate that density compatibility between selection and evaluation environments is paramount. Genetic gain decreased by 0.89–26.52% with a density discrepancy of >30,000 plants/ha and plummeted by 19.71–77.44% when the discrepancy exceeded 60,000 plants/ha, underscoring the necessity of aligning selection density with the target environment. Under matched densities, population yield increased significantly with escalating density, with the high-density selection regime showing a maximum yield improvement of 53.78% from C0 to C4. Materials selected under high density exhibited superior performance and significantly higher combining ability (averaging a 238.35% increase) and genetic gain (averaging a 263.39% improvement) in medium-to-high-density environments, confirming strong positive selection pressure. Conversely, materials from low-density selection processes were better adapted to environments of ≤60,000 plants/ha. This study provides a crucial theoretical and practical foundation for establishing density-optimized recurrent breeding systems to directionally enhance genetic gain in maize.
1. Introduction
Global maize yield breakthroughs hinge on synergistic optimization of density-tolerant cultivars and planting density, a consensus validated across major production regions [1]. Appropriately increasing planting density is the foundation and important way to achieve high yield, and most high-yield fields at home and abroad are achieved under high planting density conditions [2]. In the United States, for instance, average planting density surged from 30,000 plants/ha in the 1930s to 62,000–104,000 plants/ha today, accompanied by historic yield increases from 1.39 t/ha to 10.99 t/ha [3,4]. Analyses attribute >85% of yield gains to interactions between genetic improvements in density tolerance and optimized planting densities, with <15% contributions from heterosis and per-plant productivity [5,6,7]. This paradigm shift reflects modern maize breeding’s evolution from yield-centric selection to systemic improvements targeting stress resilience [8,9,10].
Density tolerance involves complex physio-ecological regulation. International studies highlight key traits of high-density-tolerant germplasm: synchronized silking to minimize ear-tassel asynchrony, optimized tassel architecture (38–42% fewer branches with 25–30% higher pollen production), and upregulated stress-responsive proteins (e.g., HSP70 and LEA3) [11,12]. Reasonable planting density is beneficial for regulating the leaf area index, stem leaf angle, and leaf orientation of maize plants, thereby constructing an efficient canopy structure, promoting the improvement of collective light energy utilization efficiency, enhancing the production of collective photosynthetic assimilation products and their transportation to grains, increasing the number and weight of grains per ear, and thus improving yield [13,14,15]. However, challenges persist, including density-induced etiolation (12–15% height increase), prolonged anthesis-silking intervals (2.3–3.5 days), and elevated barrenness rates (18.7% spikelet abortion and 9.4% barren plants) [16,17,18]. And with the increase in planting density, the number of grains per ear and the weight of one hundred grains of corn significantly decrease, while the lodging rate significantly increases. Establishing density-stress phenotyping systems for stalk strength, photosynthetic enzyme activity, and osmolyte accumulation is critical to overcoming breeding bottlenecks [19,20,21].
Combining ability serves as a crucial indicator for evaluating heterosis. For an extended period, breeders have utilized combining ability to assess the value of parental materials. General combining ability (GCA) reflects the breeding potential of parental materials, while specific combining ability (SCA) indicates the actual performance of particular hybrid combinations and can predict the heterosis between parental lines. However, as a statistically derived trait distinct from directly observable or measurable phenotypic values, the efficient selection of inbred lines with high combining ability has remained a persistent challenge for breeders [22]. In recent years, recurrent selection has been widely adopted as a breeding tool, and when combined with high-density stress, it amplifies genetic gain through a dynamic iteration of “selection pressure, genetic response, and population reorganization”. By creating genotype × environment (G × E) interactions via density gradients, genetic variance expands 2.3–3.1×. Periodic recombination disrupts unfavorable linkages while enriching favorable alleles, enhancing screening efficiency for high-combining-ability inbred lines by 55–60% [23,24,25,26]. Despite global adoption, key parameters—particularly spatiotemporal coordination of selection/evaluation densities and quantitative genetic gain dynamics across heterotic groups—remain underexplored [27,28]. This study employs SS (Zheng 58 × LH196) and NSS (Chang 7-2 × MBUB) heterotic populations subjected to three selection densities (60,000, 90,000, and 120,000 plants/ha) across four cycles of mass selection. Through a dual-location, triple-density evaluation design, we systematically quantify (1) synergistic effects of selection and evaluation density variations on genetic gain, (2) differentiation patterns between heterotic groups during recurrent selection, and (3) mechanisms underlying yield combining ability to respond under varying density pressures. The findings will provide theoretical guidance and empirical support for optimizing high-density recurrent selection systems in maize breeding.
2. Materials and Methods
2.1. Population Development and Selection
In 2017 between May and October at Shunyi, Beijing, China, two hybrid combinations (Zheng 58 × LH196, designated ZL population; Chang 7-2 × MBUB, designated CM population) (Table 1) were developed within the same heterotic group. Zheng 58 and Chang 7-2 are the parental inbred lines of Zhengdan 958, the most widely cultivated maize hybrid in China, and thus possess numerous agronomically superior traits. On this basis, we selected them for further improvement using two American expired proprietary inbred lines. Between October 2018 and February 2019 at Nanbin, Hainan, China, each hybrid combination was planted in two rows (in 4 m rows with 0.6 m between rows) and self-pollinated to generate two F2 selection populations (C0 generation). In 2018 between May and October at Shunyi, Beijing, China, 300 plants per population were cultivated under low (60,000 plants/ha), medium (90,000 plants/ha), and high (120,000 plants/ha) densities (low density: 20 rows; medium density: 14 rows; high density: 10 rows; all plots with 4 m rows with 0.6 m between rows). At each density, 30 superior plants underwent bulk pollination, followed by selection of 10 elite ears for composite threshing post-harvest. Selection criteria followed agronomic traits strongly correlated with density tolerance established in prior research [29], prioritizing earlier-maturing phenotypes while applying moderately relaxed standards for plant height and ear height, thus permitting the selection of individuals slightly above the population mean for C1 ear formation. Through iterative selection cycles, the final materials comprised 6 C4-generation populations, 6 C2-generation populations, and 2 C0-generation populations.

Table 1.
Parental inbred line information.
2.2. Hybrid Evaluation
In 2022 between May and October at Shunyi, Beijing, China, hybrid combinations were developed using the obtained populations: with the ZL population as test lines crossed to testers CA9B1 and Ji B37, and the CM population as test lines crossed to testers CA178 and Ji B37, yielding 28 hybrid combinations in total. In 2023 between May and October, 2 C0-generation populations, 12 C2-C4 generation populations, and 28 hybrid combinations were planted at Shijiazhuang, Hebei, China, and Xinxiang, Henan, China. Three evaluation density gradients were implemented: low (60,000 plants/ha, 0.28 m plant spacing), medium (90,000 plants/ha, 0.19 m plant spacing), and high (120,000 plants/ha, 0.14 m plant spacing). The trial employed an incomplete block design with 2-row plots, two replications, and 4 m row lengths, with 0.6 m between rows and field management practices following standard production protocols. According to local fertilization recommendations, 450 kg/ha of diammonium phosphate before sowing and 150 kg/ha of urea during the pulling period were supplied, and timely manual spraying of chemicals and weed control were conducted in the field for each location.
2.3. Measurement Indicators and Statistical Analysis
Following harvest, all ears from individual plots were threshed and weighed with moisture content recorded, with yields converted to hectare-equivalent yields standardized at 14% moisture content. Data compilation and verification were conducted using Excel 2016, where data were structured by the factorial treatment groups of selection density × evaluation density for subsequent analysis. This framework was used to calculate mean yields for both selection populations across generations and population-tester hybrid combinations, while generating yield and combining-ability trend graphs across planting densities. Our statistical approach encompassed several procedures. Analysis of variance (ANOVA) was carried out with the “aov” function in R (v4.1.2), and independent samples t-tests in SPSS (v26.0) were used for significance determination. In the combining-ability analysis, the general combining ability (GCA) was quantified by the sum of squares attributed to populations and testers, whereas the specific combining ability (SCA) was quantified by the sum of squares of the population × tester interaction. Finally, the GCA and SCA effects themselves were derived from a joint linear mixed model analysis of the crosses, fitted using ASReml-R, considering both individual and combined environments.
Genetic gain was evaluated based on selection and evaluation density-specific yield performance and combining-ability means of selection populations, calculated according to the formula [20]:
where represents the mean of trait i in post-selection populations and the mean of trait i in base populations.
3. Results
3.1. Analysis of Variance
As shown in Table 2, an analysis of variance (ANOVA) on yield performance across generational populations revealed no significant differences among replicates, confirming robust experimental control and high data uniformity. Environment, evaluation density, generational populations, and their interaction effects all reached significant or highly significant levels (p < 0.05/p < 0.01). These results demonstrate authentic yield variations among populations—developed under different selection densities—when evaluated across evaluation density gradients, warranting further detailed analysis.

Table 2.
ANOVA table for yield across different generational populations.
Given the use of distinct testers for combining-ability evaluation across generational populations, hybrid combinations from the ZL and CM heterotic groups underwent separate ANOVAs. Results (Table 3) indicated no significant differences among replicates, reconfirming rigorous experimental control. Although hybrid yields of both groups exhibited highly significant differences (p < 0.01) across environments, most interaction effects with other variation sources were non-significant, demonstrating consistent yield responses to controlled conditions across locations. Significant variations were observed in population general combining ability (GCA) and its interaction with evaluation density, confirming that selection density significantly influences GCA in generational populations—warranting further investigation into underlying mechanisms.

Table 3.
ANOVA table for yield across different hybrid combinations.
3.2. Genetic Gain Dynamics
As illustrated in Figure 1, both selected breeding populations exhibited consistent yield response patterns to selection and evaluation densities. Under low selection density (60,000 plants/ha), peak yields for both populations were achieved at low evaluation densities (ZL: 4.57 t/ha, CM: 5.33 t/ha). After four cycles of selection, yields progressively declined with increasing evaluation density. Notably, the ZL population showed reduced yields in the C4 generation compared to C2 under high evaluation density.
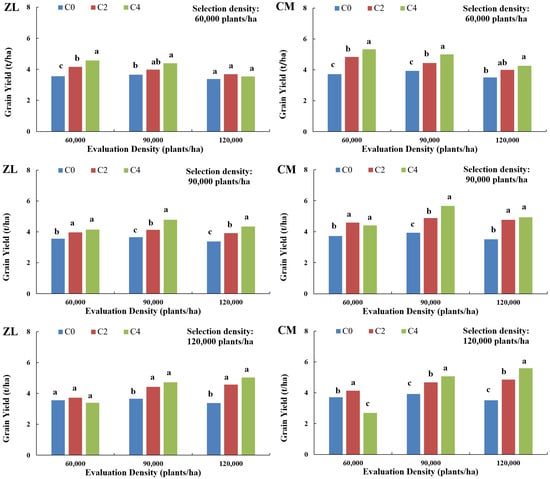
Figure 1.
Relationship between yield of multi-generational selected breeding populations and their selection/evaluation densities (Zheng 58 × LH196, designated ZL population; Chang 7-2 × MBUB, designated CM population). Different lowercase letters indicate significant differences at p < 0.05.
Under medium selection density (90,000 plants/ha), maximum yields for both populations aligned with medium evaluation densities (ZL: 4.78 t/ha, CM: 5.66 t/ha). Following four selection cycles, yields initially increased but subsequently decreased with rising evaluation density, though high-density evaluation still outperformed low-density conditions.
Under high selection density (120,000 plants/ha), both populations reached peak yields at high evaluation densities (ZL: 5.04 t/ha, CM: 5.59 t/ha). After four cycles of improvement, the C4 generation demonstrated a 53.78% yield increase over C0, representing the highest gain among all density combinations.
Analysis of genetic gain across evaluation densities (Table 4) revealed distinct patterns: Under low evaluation density (60,000 plants/ha): genetic gains in both populations progressively declined with increasing selection density, culminating in negative cumulative gains at high selection densities (ZL: 4.11%, CM: 23.66%). At medium evaluation density (90,000 plants/ha): all selection densities yielded positive genetic gains across generations, with peak gains observed under medium selection density (ZL: 28.64%, CM: 40.37%). Notably, high selection density marginally outperformed low density. Under high evaluation density (120,000 plants/ha): genetic gains increased with selection density, achieving the highest cumulative gains at high selection density (ZL: 45.86%, CM: 53.78%). However, low selection density resulted in suboptimal performance, with the ZL population exhibiting negative gains from C2 to C4 (−4.33%).

Table 4.
Yield genetic gain (%) of selected breeding populations across evaluation densities.
Critically, maximum yield and genetic gain consistently aligned with matched selection–evaluation density pairs, according to the three selection and evaluation density gradients in this study. Genetic gains declined by 0.89–26.52% when selection–evaluation density mismatches exceeded 30,000 plants/ha and plummeted 19.71–77.44% for >60,000 plants/ha discrepancies, underscoring the necessity for congruence between selection and target evaluation densities. Low-density selected populations showed higher genetic gains under low evaluation densities than under high densities. Conversely, high-density selected populations maximized gains under high evaluation densities. Genetic gains of low-density populations under low evaluation densities were substantially inferior to those of high-density populations under high densities, a trend independent of genetic background. These findings validate the feasibility of high-density selection strategies, demonstrating that optimal genetic gains are achieved when high selection density aligns with high evaluation density.
3.3. Combining Ability Responses
As illustrated in Figure 2, under low selection density (60,000 plants/ha), hybrid combinations from both populations achieved maximum yields (ZL: 7.53 t/ha, CM: 6.43 t/ha) at the C2 generation under low evaluation density (60,000 plants/ha). With successive generational improvement, hybrid yields initially increased but subsequently declined. By the fourth selection cycle (C4), only ZL-derived hybrids exhibited gradual yield increases under medium evaluation density, while both populations showed declining trends under high evaluation density, indicating suboptimal performance of low-density selected hybrids in medium-to-high-density environments.
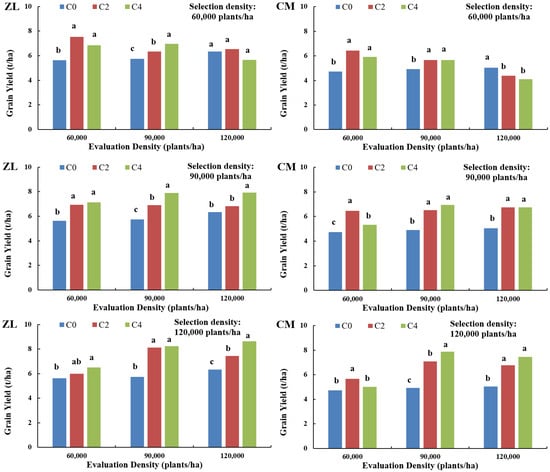
Figure 2.
Relationship between yield of hybrid combinations from multi-generational selected breeding populations and their selection/evaluation densities (Zheng 58 × LH196, designated ZL population; Chang 7-2 × MBUB, designated CM population.). Different lowercase letters indicate significant differences at p < 0.05.
Under medium selection density (90,000 plants/ha), ZL hybrids reached peak yields at the C4 generation under high evaluation density (8.24 t/ha), whereas CM hybrids achieved maximum yields at C4 under medium evaluation density (6.94 t/ha). With the exception of the CM population hybrids, which showed an initial increase followed by a decline under low evaluation density, both the ZL and CM population hybrids exhibited gradually increasing yields across other evaluation densities. This indicates that at the selection density employed, hybrid combinations consistently demonstrated higher yields under medium–high evaluation densities.
Under high selection density (120,000 plants/ha), yield patterns mirrored those observed at medium density: ZL hybrids maximized yields at C4 under high evaluation density (8.62 t/ha), and CM hybrids peaked at C4 under medium evaluation density (7.89 t/ha). These hybrids performed optimally in medium-to-high evaluation densities but underperformed in low-density conditions, reinforcing the critical role of density alignment in hybrid evaluation.
As presented in Table 5, under low selection density (60,000 plants/ha), yield general combining ability (GCA) consistently showed negative effects across generations at medium and high evaluation densities. Conversely, at low evaluation densities, GCA initially increased but subsequently declined with generational advancement. Both the C2 and C4 generations exhibited positive GCA effects, peaking at C2 before decreasing at C4. This confirms superior combining ability for low-density selected materials under matched evaluation conditions. Genetic gain analysis revealed optimal improvement under low evaluation density, where combining ability shifted from negative to positive after four selection cycles (−1.03 to 0.18). Under medium evaluation density, negative effects were mitigated (−1.43 to −0.22), while a high evaluation density exacerbated negative trends (−0.71 to −1.38).

Table 5.
GCA Effects for yield and genetic gain (t/ha) of selected breeding populations under different selection and evaluation densities.
Under medium selection density (90,000 plants/ha), materials selected at a low evaluation density retained negative GCA values. In contrast, medium- and high-density selected materials demonstrated progressive GCA enhancement, with significantly higher GCA under high evaluation density (ZL: 0.87, CM: 0.85). Genetic gains transitioned from negative to positive across all evaluation densities, with improvement efficiency positively correlated with density intensity.
Under high selection density (120,000 plants/ha), GCA patterns aligned with medium-density observations: persistent negative GCA at low evaluation density versus maximized positive GCA at high evaluation density. Genetic gains consistently shifted from negative to positive across all densities, escalating with increasing evaluation density. Critically, high-density selected materials outperformed low-density counterparts in medium-to-high-density environments, exhibiting 238.35% higher yield combining ability and 263.39% greater genetic gain in combining ability, validating the positive regulatory effects of high selection pressure on both yield and combining ability.
4. Discussion
4.1. Response of Yield Genetic Gain to Selection and Planting Densities
Planting density is a critical determinant of maize yield, with increased density serving as a key agronomic strategy for yield enhancement [30]. Elevated planting densities improve grain yield by increasing ear number per unit area [31], yet excessive density without adequate tolerance compromises both yield stability and quality [32]. Yield-density relationships typically follow a parabolic trend—initially increasing before declining—due to reduced ear productivity under high-density stress. Enhancing density tolerance to mitigate per-ear yield loss is therefore pivotal for sustained yield improvement. While previous studies have extensively investigated varietal yield responses to planting density, the dynamics of genetic gain during population selection under density stress remain underexplored.
In this study, under low selection density (60,000 plants/ha), both populations and hybrids exhibited peak yields at the C2 generation, followed by declines at C4, indicating suboptimal improvement when applying uniform selection criteria across low- and medium/high-density regimes. Under medium selection density (90,000 plants/ha), the ZL population showed consistent yield increases across all evaluation densities, with C2 improvements inversely correlated to evaluation density and C4 displaying the opposite trend. The CM population demonstrated stable progress under medium evaluation density but limited gains at low/high densities. High selection density (120,000 plants/ha) materials performed poorly under low evaluation density but excelled in medium/high-density environments. Collectively, higher selection densities significantly enhanced population improvement, with high-density stress selection markedly improving both density tolerance and combining ability. For breeders, this implies that selection nurseries must mirror the target density of commercial production to effectively identify genotypes with optimal phenotypic and physiological adaptations. However, low-density selected materials exhibited superior adaptability in low-density environments, emphasizing the need for precision alignment between selection density and target production conditions. Enhancing maize varietal density tolerance and increasing planting density must be implemented concurrently. From 2020 to 2024, China’s maize planting density has transitioned nationally from 60,000 plants/ha to 80,000–90,000 plants/ha. Thus, selection density should not be abruptly maximized but rather progressively elevated through incremental adjustments aligned with rising production densities. This stepwise approach allows breeding programs to simultaneously accumulate favorable alleles for density tolerance while maintaining genetic variance for continued gain.
4.2. Response of Combining Ability Genetic Gain to Selection and Planting Densities
Combining ability, a statistical trait critical for evaluating heterosis and parental breeding potential, has been widely studied through mating designs to guide germplasm improvement and hybrid development. However, genetic enhancement of combining ability itself remains underexplored, largely due to its indirect selection challenges.
This study revealed significant density-dependent effects on general combining ability (GCA) improvement. Low selection density minimally enhanced GCA except under low planting density. In contrast, medium/high selection densities progressively elevated GCA across generations, with greater improvements observed at higher planting densities. These findings confirm that high-density stress selection effectively amplifies both combining ability and its genetic gain. The improved GCA under high-density selection likely reflects the enrichment of additive alleles controlling critical stress-adaptive traits such as canopy architecture, root penetration ability, and assimilate partitioning efficiency—attributes essential for hybrid performance in intensive cropping systems. For practical breeding, this underscores the value of evaluating and selecting testcrosses under density conditions that simulate future commercial environments to unlock greater yield potential.
4.3. Feasibility of High-Density Recurrent Breeding Strategies
High-density breeding enhances stress resilience in inbreds and hybrids, including density tolerance, lodging resistance, low-nitrogen adaptability, and kernel setting efficiency [33]. Pioneered by Pioneer Hi-Bred in the 1970s, this approach became central to global maize breeding by the 1980s. In 1990, Mr. Li Jingxiong emphasized the necessity of density-driven breeding in China, aligning with global trends toward intensified planting.
Current U.S. planting densities (90,000 plants/ha) far exceed those in Northeast China (40,500 plants/ha) and the Huang-Huai-Hai region (60,000 plants/ha). U.S. practices—selecting inbreds at double commercial production densities—align with this study’s findings: genetic gains improved with higher selection densities but stagnated when selection–target-density mismatches exceeded practical thresholds. Furthermore, generational responses to selection density varied significantly, highlighting the need to optimize density–genetic-gain relationships across breeding cycles. To implement these findings, breeders should establish multiple parallel selection streams with densities calibrated to different target macro-environments, and introgress known morphological and physiological donors for lodging resistance and canopy efficiency into their base populations. Future research should clarify interactions between population generations and selection densities to refine selection efficiency.
5. Conclusions
This study establishes that precise alignment between selection density and target production environments is the fundamental prerequisite for maximizing genetic gain in maize breeding. This principle is critically demonstrated by the severe yield penalty—up to 77.44%—caused by a density mismatch exceeding 60,000 plants/ha. We show that a density-optimized recurrent selection strategy, where selection is performed under the intended commercial planting density, is the most effective method to develop resilient, high-performing varieties. This approach drives simultaneous improvement in key agronomic traits: it consistently enhances population yield and amplifies combining ability. For breeders, these findings provide a clear and actionable blueprint: implementing parallel selection pipelines calibrated to specific density targets is essential to accelerate the development of next-generation, density-tolerant hybrids and sustainably break the yield plateau.
Author Contributions
Conceptualization, X.L. and H.Y.; methodology, F.Z., Z.Y., M.L., D.Z., Z.Z., Z.X., Z.H., J.W. (Jianfeng Weng) and H.Y.; software, F.Z.; formal analysis, Z.Y., Y.Z. and J.H.; investigation, F.Z., Z.Y., Y.Z., Z.Z. and Z.X.; resources, Z.R., J.W. (Juying Wang), X.L. and H.Y.; data curation, F.Z., Z.Y. and Y.Z.; writing—original draft, F.Z. and Z.Y.; writing—review and editing, F.Z., M.L., D.Z., J.H., Z.H., J.W. (Jianfeng Weng), Z.R., J.W. (Juying Wang), X.L. and H.Y.; visualization, Y.Z. and Z.Y.; project administration, F.Z., X.L. and H.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2022YFD1201000), the Key Research and Development Program of Shandong Province (Agricultural Seed Improvement Project) (ZDYF2023LZGC001), the China Agriculture Research System (CARS-02-02), the Central Guidance for Local Scientific and Technological Development Special Project (ZY04JD05), and the National Natural Science Foundation of China (32261143757).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, G.; Yang, Y.; Liu, W.; Guo, X.; Xie, R.; Ming, B.; Xue, J.; Zhang, G.; Li, R.; Wang, K.; et al. Optimized canopy structure improves maize grain yield and resource use efficiency. Food Energy Secur. 2022, 11, e375. [Google Scholar] [CrossRef]
- Zhang, M.D.; Zhang, G.Q.; Wang, K.R.; Xie, R.Z.; Hou, P.; Ming, B.; Xue, J.; Li, S.K. Effects of planting density and irrigation amount on yield and water use efficiency of spring maize in the west Liaohe Plain. J. Maize Sci. 2023, 31, 116–125. [Google Scholar] [CrossRef]
- Liu, Z.; Sha, Y.; Huang, Y.W.; Hao, Z.H.; Guo, W.Q.; Ke, L.H.; Chen, F.J.; Yuan, L.X.; Mi, G.H. Efficient nitrogen allocation and reallocation into the ear in relation to the superior vascular system in low-nitrogen tolerant maize hybrid. Field Crops Res. 2023, 284, 108580. [Google Scholar] [CrossRef]
- Duvick, D.N. The contribution of breeding to yield advances in maize (Zea mays L.). Adv. Agron. 2005, 86, 83–145. [Google Scholar] [CrossRef]
- Egli, D.B. Comparison of corn and soybean yields in the United States: Historical trends and future prospects. Agron. J. 2008, 100, S-79–S-88. [Google Scholar] [CrossRef]
- Hammer, G.L.; Dong, Z.; McLean, G.; Doherty, A.; Messina, C.; Schussler, J.; Zinselmeier, C.; Paszkiewicz, S.; Cooper, M. Can changes in canopy and/or root system architecture explain historical maize yield trends in the U.S. corn belt? Crop Sci. 2009, 49, 299–312. [Google Scholar] [CrossRef]
- Ci, X.; Li, M.; Liang, X.; Xie, Z.; Zhang, D.; Li, X.; Lu, Z.; Ru, G.; Bai, L.; Xie, C.; et al. Genetic contribution to advanced yield for maize hybrids released from 1970 to 2000 in China. Crop Sci. 2011, 51, 13–20. [Google Scholar] [CrossRef]
- Tollenaar, M.; Wu, J. Yield improvement in temperate maize is attributable to greater stress tolerance. Crop Sci. 1999, 39, 1597–1604. [Google Scholar] [CrossRef]
- Cooper, M.; Gho, C.; Leafgren, R.; Tang, T.; Messina, C. Breeding drought-tolerant maize hybrids for the US corn-belt: Discovery to product. J. Exp. Bot. 2014, 65, 6191–6204. [Google Scholar] [CrossRef]
- Messina, C.D.; Sinclair, T.R.; Hammer, G.L.; Curan, D.; Thompson, J.; Oler, Z.; Gho, C.; Cooper, M. Limited-transpiration trait may increase maize drought tolerance in the US corn belt. Agron. J. 2015, 107, 1978–1986. [Google Scholar] [CrossRef]
- Cao, Y.; Zhong, Z.; Wang, H.; Shen, R. Leaf angle: A target of genetic improvement in cereal crops tailored for high-density planting. Plant Biotechnol. J. 2022, 20, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.M.; Guo, S.L.; Zhang, X.; Wei, L.M.; Zhang, Q.J.; Cao, L.R.; Liu, H.J.; Deng, Y.Z.; Zhang, Z.; Wang, Z.H. Analysis of yield com position of stem lodging resistance related traits of maize under different densities. J. Henan Agric. Sci. 2023, 52, 44–55. [Google Scholar] [CrossRef]
- Abeledo, L.G.; Savin, R.; Slafer, G.A. Maize senescence under contrasting source-sink ratios during the grain filling period. Environ. Exp. Bot. 2020, 180, 104263. [Google Scholar] [CrossRef]
- Li, R.; Zhang, G.; Liu, G.; Wang, K.; Xie, R.; Hou, P.; Ming, B.; Wang, Z.; Li, S. Improving the yield potential in maize by constructing the ideal plant type and optimizing the maize canopy structure. Food Energy Secur. 2021, 10, e312. [Google Scholar] [CrossRef]
- Shao, H.; Wu, X.; Duan, J.; Zhu, F.; Chi, H.; Liu, J.; Shi, W.; Xu, Y.; Wei, Z.; Mi, G. How does increasing planting density regulate biomass production, allocation, and remobilization of maize temporally and spatially: A global meta-analysis. Field Crops Res. 2024, 315, 109430. [Google Scholar] [CrossRef]
- Sangoi, L.; Gracietti, M.A.; Rampazzo, C.; Bianchetti, P. Response of Brazilian maize hybrids from different eras to changes in plant density. Field Crops Res. 2002, 79, 39–51. [Google Scholar] [CrossRef]
- Tokatlidis, I.S.; Koutroubas, S.D. A review of maize hybrids’ dependence on high plant populations and its implications for crop yield stability. Field Crops Res. 2004, 88, 103–114. [Google Scholar] [CrossRef]
- Maddonni, G.A.; Otegui, M.E. Intra-specific competition in maize: Early establishment of hierarchies among plants affects final kernel set. Field Crops Res. 2004, 85, 1–13. [Google Scholar] [CrossRef]
- Ling, L.; Pei, W.D. Effects of planting density on stem lodging resistance and grain yield of maize at different maturit. J. Maize Sci. 2024, 32, 106–113. [Google Scholar] [CrossRef]
- Zhang, G.; Cui, C.; Lv, Y.; Wang, X.; Wang, X.; Zhao, D.; Hu, F.; Wen, X.; Han, J.; Liao, Y. Is it necessary to increase the maize planting density in China? Eur. J. Agron. 2024, 159, 127235. [Google Scholar] [CrossRef]
- Sergio, F.L.; Alfredo, G.C.; María, E.O. Genetic gains in grain yield and related physiological attributes in Argentine maize hybrids. Field Crops Res. 2006, 95, 383–397. [Google Scholar] [CrossRef]
- Araus, J.L.; Serret, M.D.; Edmeades, G.O. Phenotyping maize for adaptation to drought. Front. Physiol. 2012, 3, 305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.L. Genetic Analysis and Genome-Wide Selection Study on the General Combining Ability of Maize Yield Related Traits. Ph.D. Thesis, Yangzhou University, Yangzhou, China, 2023. [Google Scholar]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, R. Genomewide Selection when Major Genes are Known. Crop Sci. 2014, 54, 68–75. [Google Scholar] [CrossRef]
- Lin, M.; Matschi, S.; Vasquez, M.; Chamness, J.; Kaczmar, N.; Baseggio, M.; Gore, M.A. Genome-wide association study for maize leaf cuticular conductance identifies candidate genes involved in the regulation of cuticle development. G3 Genes Genomes Genet. 2020, 10, 1671–1683. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Zou, C.; Lu, Y.; Olsen, M.S. Enhancing genetic gain in the era of molecular breeding. J. Exp. Bot. 2017, 68, 2641–2666. [Google Scholar] [CrossRef]
- Messina, C.D.; Technow, F.; Tang, T.; Totir, R.; Gho, C.; Cooper, M. Leveraging biological insight and environmental variation to improve phenotypic prediction: Integrating crop growth models (CGM) with whole genome prediction (WGP). Eur. J. Agron. 2018, 100, 151–162. [Google Scholar] [CrossRef]
- Zhang, F.Y. Genetic Gain in Maize Selection Lines in Response to Planting Density. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2018. [Google Scholar]
- Bai, Y.W.; Zhang, H.J.; Zhu, Y.L.; Zheng, X.H.; Yang, M.; Li, C.F.; Zhang, R.H. Responses of canopy light-nitrogen distribution, senescence characteristics, and light energy utilization to planting density in maize with different plant types. Sci. Agric. Sin. 2020, 53, 3059–3070. [Google Scholar]
- Wang, K.; Wang, K.R.; Wang, Y.H.; Zhao, J.; Zhao, R.L.; Wang, X.M.; Li, J.; Liang, M.X.; Li, S.K. Effects of planting density on maize yield (>15,000 kg · hm−2) and its yield components. Sci. Agric. Sin. 2012, 45, 3437–3445. [Google Scholar]
- Yang, J.Y.; Luo, Y.J.; Song, B.; Zhang, J.; Liu, J.; Lu, P.; Ye, K.M.; Luo, S.K. Analysis of density tolerance in different maize varieties and screening of identification indices. Seed 2019, 38, 80–85. [Google Scholar] [CrossRef]
- Li, J.Z. Evaluation of Density Tolerance and Genetic Research in Maize Under Different Density Selection Pressures. Master’s Thesis, Jilin Agricultural University, Changchun, China, 2013. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).