Abstract
Biochar application can effectively immobilize Cadmium (Cd) in soil. However, it is largely unknown how the biogeochemical processes of sulfur (S) in biochar affect Cd fixation under conditions of decreasing pe + pH. Using two field-contaminated paddy soils with different Cd concentrations (Shangyu (SY) 0.56 mg kg−1 and Tongling (TL) 2.32 mg kg−1), and rape straw biochars with low S (LB) and high S (HB) contents, we investigated how S-rich biochar regulates Cd solubility in paddy soils that were incubated anaerobically for 40 d. The soluble and extractable Cd content decreased as pe + pH decreased with flooding, and showed a steady trend by day 20. However, Cd was immobilized through different mechanisms in TL and SY soil. The rapid decrease in pe + pH in TL soil enhanced the involvement of S in Cd immobilization and Fe transformation. In SY soil, the delayed reduction in SO42− promoted Cd adsorption onto amorphous Fe oxides. During this process, the liming effect of biochar is critical for Cd immobilization in soil. Furthermore, biochar might promote the biogeochemical processes of S and Fe transformation, thereby enhancing Cd fixation in soils.
1. Introduction
Anthropogenic activities, including mining, agriculture, and textile industries, have induced serious Cd contamination in cultivated land [1,2,3]. Cd contamination in rice paddy soils potentially poses a high risk to food safety, as rice has a strong ability to uptake Cd [4]. An investigation of 183 rice varieties showed that the exceedance rate of Cd content in grains was over 61.7% [5]. The accumulation of Cd in rice grains can cause renal dysfunction, lung injury and dyspnea [6]. Thus, it is urgent to develop effective strategies to reduce Cd mobility in rice paddy soils.
The fractions, transformation, and bioavailability of Cd in rice paddy soils are generally controlled by soil pH and Eh. Fluctuations in pH and Eh drive a series of physical and chemical processes, among which the precipitation/reductive hydrolysis of iron oxides and the redox of sulfides can effectively influence the variation in Cd fate in rice paddy soils [7,8]. It has been suggested that continuous soil flooding is more conducive to Cd immobilization [9]. However, the flooding strategy is not conducive to enhancing soil nutrient availability [10,11]. It is worth noting that, due to its high CEC property, biochar application can release available nutrient elements (e.g., P, K, and Ca) [12,13]. This contributes to both soil nutrient availability and Cd immobilization; thus, biochar has been recommended as an effective and suitable amendment for remediating Cd-contaminated soils [14].
However, the remediation effect of biochar on Cd might be limited due to its poor selectivity. Specifically, S loading can effectively improve the affinity of biochar for Cd [15,16]. Sulfur ligands from metal sulfides, considered soft Lewis bases, can react with Cd2+ ions to form insoluble and stable compounds such as Cd [17,18]. Compared to other crop straws, rape straw is characterized by a higher sulfate content [19,20]. Studies have found that the S content in rape straw biochar is much higher than that in rice straw biochar and corn stalk biochar [21,22]. It has been suggested that a high concentration of sulfur in biochar can promote Cd sorption onto biochar through the deposition of CdS and CdHS+ as well as interaction with organic sulfide [23]. Under reducing conditions, S2- produced from sulfate reduction can react with Fe2+ to form FeS or FeS2 precipitates, which can promote the adsorption or co-precipitation of Cd2+ [24,25]. The application of biochar can promote the reduction in Fe and S during the flooding stage of rice paddies, thereby inhibiting Cd uptake by rice plants [22]. The underlying mechanism is that biochar facilitates the reduction in Fe3+ and SO42− by acting as an electron shuttle through its phenolic, quinone, and condensed aromatic hydrocarbon groups [26].
Studies have proven that biochar can affect metal mobility and transformation through biological pathways by donating, accepting, or transferring electrons in redox reactions [27,28,29]. Considering the participation of microbes in this process, the reduction in sulfate and Fe3+ can be affected by the level of Cd pollution in soils. It has been reported that the inhibitory effect on soil microbial communities intensifies with increasing Cd pollution levels in soil [30,31]. The application of biochar could alleviate the stress on soil microbial communities caused by high Cd content (10.01 mg kg−1) in soil [32]. For soils with light (0.46 mg kg−1) and moderate Cd (4.18 mg kg−1) pollution, biochar could decrease Cd mobility by positively impacting soil properties (e.g., pH, CEC, and SOM).
Taken together, S-rich biochar application might influence Cd mobility by regulating Fe transformation or directly depositing with Cd. However, whether increasing the S content in biochar can boost this effect is rarely addressed. It is hypothesized that S-rich biochar will be more effective for Cd immobilization in soils with low Cd content under reducing pe + pH conditions (pe + pH, where pe = −log(e−) = Eh (mV)/59.2). Using rape straw biochar with low S content (4.21 g kg−1) and high S content (7.01 g kg−1), we investigated the effects of S-rich biochar on Cd immobilization in soils with different Cd pollution levels (0.56 mg kg−1 and 2.32 mg kg−1). The objective of this study is to explore the effects of S-rich biochar on Cd immobilization in soils, especially the mechanisms by which S-rich biochar facilitates Cd immobilization under different soil conditions.
2. Materials and Methods
2.1. Biochar Preparation and Characteristics
Feedstocks of rape straw with different S contents were collected from Huai’an and Nanjing, respectively, in Jiangsu Province. After drying and crushing, the straw feedstocks were pyrolyzed in a muffle furnace at 450 °C for 3 h with a heating rate of 5 °C/min under a N2 atmosphere. The yield of biochar was around 30%. Based on their relative S content levels, the derived biochars were labeled as LB (low S) and HB (high S), respectively. The total S (TS) contents in the corresponding feedstocks were 5.50 g kg−1 and 7.66 g kg−1, respectively. After grinding, the biochars were passed through a 2 mm sieve and stored for later use. The soils used in the experiment were collected from Cd-contaminated fields in Tongling (TL), Anhui Province and Shangyu (SY), Zhejiang Province. These soils are loam in texture and were primary contaminated with Cd from mining activities and sewage irrigation. Surface soils (0–20 cm) from these areas were selected, with impurities removed prior to air-drying under natural conditions. After air-drying, soils were ground and sieved for subsequent analysis. The basic physical and chemical properties of both biochars and soils are shown in Table 1.

Table 1.
Basic Physical and Chemical Properties of Soils.
2.2. Experimental Design
The treatments of this study were established as follows: control (blank), low-S biochar, high-S biochar, molybdate, LB + molybdate, and HB + molybdate. These treatments were applied to TL soil and SY soil, with the biochar application rate set at 1%. Table 2 presents the details of the treatments used in this study.

Table 2.
Treatments Applied in This Study.
The details of the incubation process are described in the Supporting Information (SI).
2.3. Sample Collection and Analytical Methods
Pore water and soil collection: serum bottles were collected on the 1st, 4th, 8th, 14th, 20th, 30th, and 40th days of incubation, respectively. The collection method of pore water is summarized in Supplementary Materials. The concentrations of in the pore water were determined. The soil samples collected simultaneously were analyzed for extractable Cd, redox potential (Eh), and pH. The detailed analytical methods of the above parameters can be found in Supplementary Materials.
Soil samples were collected on the 1st, 14th, and 40th days to analyze the contents of amorphous and crystalline iron oxides, as well as the fractions of Cd. On days 0, 4, and 40, the concentrations of available S were determined using the collected soil samples. Additionally, acid-volatile sulfide (AVS) in the soil was analyzed using soil samples collected on day 40. The detailed methods for the aforementioned analyses can be found in the Supplementary Materials.
2.4. Data Processing and Analysis Statistics
All data were presented as the mean ± standard error (SE) of three replicates. Experimental data were statistically analyzed using SPSS 23.0. The data from each treatment were compared using Duncan’s multiple range test (p < 0.05). Pearson’s correlation analysis was used to assess the correlations. Charts were generated using Origin 2024 and Microsoft Word 2021.
The calculation formula for crystalline iron oxide is: Fec = Fed − Feo;
The contribution rate of S to the reduction in Cd content in aqueous solution was calculated as:
Contribution
3. Results
3.1. Changes in Soil pH and Eh
As shown in Figure 1a,b, the pH of both soils exhibited an initial increase followed by stabilization. The TL soil reached peak pH on day 14, while the SY soil peaked on day 20. Compared to the control, biochar application increased pH in all soils. In the TL soil, the pH of TLB treatment was 2.2–3.2% higher than that of the control (TL) on day 4. In the SY soil, the pH of the SLB treatment was 8.6–11.9% higher than that of the control (SY) on day 8. On day 20, the SLB treatment was 1.9–6.5% higher than the control (SY), and the pH of the LB treatment was higher than that of the HB treatment in both soils.
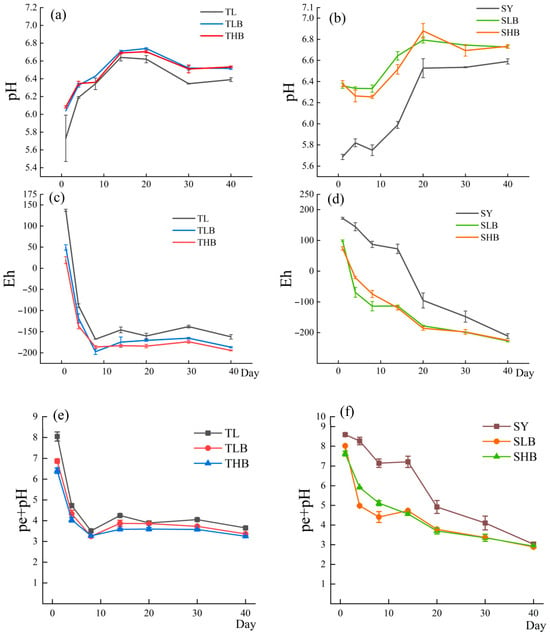
Figure 1.
Changes in soil pH, Eh and pe + pH values over time under different biochar treatments ((a,c,e): TL soil; (b,d,f): SY soil).
The dynamic changes in Eh in the two soils are shown in Figure 1c,d. From day 0 to day 8, the TL soil showed a rapid reduction in Eh, reaching a minimum value of −168 mV. In comparison, the Eh of the SY soil decreased gradually, attaining values between −165 mV and −122 mV by day 30. In the TL soil, the THB treatment had an Eh 14.8–22.1 mV lower than that of the control (TL) on day 8. In the SY soil, the SHB treatment had an Eh 21.5–80.1 mV lower than that of the control (SY) on day 30.
3.2. Changes of SO42− and Fe2+ Contents in Soil Pore Water
The SO42− content in soil pore water during anaerobic incubation is shown in Figure 2. In TL soil, the content of SO42− stabilized after day 20, reaching 45.3–49.6 mg/L on day 20, while in SY soil, it reached 41.0–44.1 mg/L on day 30. On day 14, the SO42− content in TLB and THB was 15.3% and 19.2% higher than that in TL, respectively. Similarly, compared to SY, the concentration of SO42− under SLB and SHB increased by 23.9% and 58.6%, respectively.
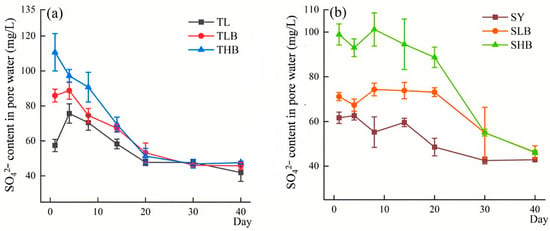
Figure 2.
The content of SO42− in soil pore water under different biochar treatments ((a): TL soil; (b): SY soil).
The content of Fe2+ in soil pore water is shown in Figure 3. In TL soil, the Fe2+ content increased rapidly from day 0 to day 14, then slightly decreased and tended to stabilize after day 20. In contrast, in SY soil, the concentration of Fe2+ gradually increased and peaked on day 40. On day 14, the Fe2+ content in TLB and THB was 9.3% and 16.6% higher than that in TL, respectively. Under SLB and SHB treatments, the Fe2+ content increased dramatically by 254.8% and 313.1%, respectively, compared to that under SL treatment.
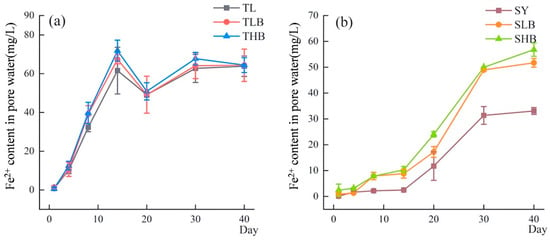
Figure 3.
The Fe2+ content in pore water under different biochar treatments ((a): TL soil; (b): SY soil).
3.3. Changes in Weak Crystalline and Crystalline Iron Oxide Content in Soil
The changes in amorphous iron oxide (Feo) are demonstrated in Figure 4a–c. In TL soil, the Feo content under TLB and THB treatments was 10.0% and 10.3% higher than that under the TL treatment, respectively. In SY soil, on day 14, the Feo content was increased by 10.7% and 15.3% under SLB and SHB treatments, respectively.
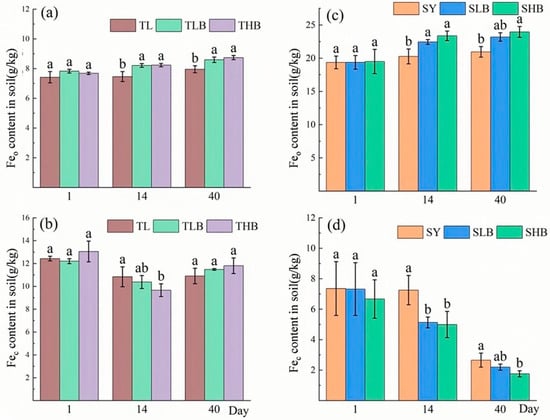
Figure 4.
The content of amorphous and crystalline iron oxides in soil under different biochar treatments ((a,b): TL soil; (c,d): SY soil), Values are means ± SD (n = 3). Different lowercase letters indicate significant differences at p < 0.05 (Duncan’s test).
As shown in Figure 4b–d, biochar application reduced soil crystalline iron oxide (Fec) contents. On day 14, compared to TL, the Fec content in TLB and THB decreased by 10.3% and 13.9%, respectively. By contrast, in SY soil, on day 14, the Fec content under SLB and SHB treatments decreased by 29.3% and 31.1%, respectively, compared with SY. Moreover, On day 40, the concentrations of Fec under SLB and SHB treatments were 17.5% and 33.9% lower than that of SY.
3.4. Changes in Soil Available Sulfur and AVS Content
The dynamic changes in available S content are shown in Figure 5. In TL soil, on the 4th day of incubation, the available S content in TLB and THB was 12.1% and 42.5% higher than that in TL, respectively. On day 40, the content of available S in TLB and THB was 2.1% and 21.9% higher than that in TL, respectively. Similarly, in SY soil, on day 4, compared with SY, SLB and SHB increased soil available S by 15.6% and 23%, respectively. On day 40, the SLB and SHB treatments increased available S content by 15.2% and 27.0%, respectively.
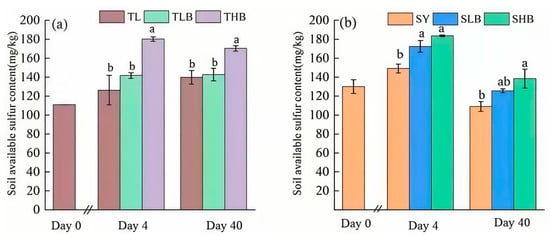
Figure 5.
Soil available sulfur content under different biochar treatments ((a): TL soil, (b): SY soil),Values are means ± SD (n = 3). Different lowercase letters indicate significant differences at p < 0.05 (Duncan’s test).
Results in Figure 6 demonstrate the content of acid-volatile sulfur (AVS) in soil. The AVS content of TL soil was higher than that of SY soil, and HB was more effective than LB in increasing soil AVS content. Compared to SY, the SLB and SHB treatments increased AVS concentrations by 36.1% and 40.4%, respectively.
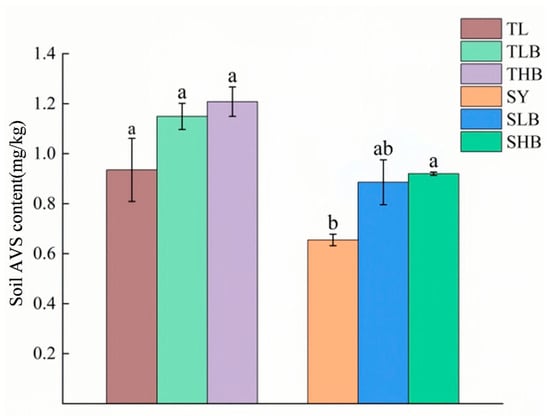
Figure 6.
Soil AVS contents under different biochar treatments, Values are means ± SD (n = 3). Different lowercase letters indicate significant differences at p < 0.05 (Duncan’s test).
3.5. Changes in Available Cadmium and Cadmium Speciation in Soil
Results in Figure 7 show the content of CaCl2-extractable Cd (CaCl2-Cd) in soil. The concentration of CaCl2-Cd in soil initially decreased from day 0 to day 20, then tended to stabilize after day 20. On the 40th day of incubation, biochar application decreased CaCl2-Cd by 53.1% (TLB) and 68.3% (THB), respectively. Compared to SY, the reductions in CaCl2-Cd were 66.8% and 71.6% under SLB and SHB treatments, respectively.
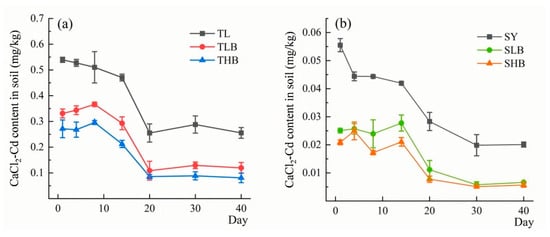
Figure 7.
The content of cadmium content extracted by CaCl2 under different biochar treatments ((a): TL soil, (b): SY soil).
The changes in Cd fractions in soil are displayed in Figure 8. In TL soil, Cd primarily existed as F1-Cd. Comparatively, F2-Cd was the predominant Cd fraction in SY soil. Moreover, in TL soil, biochar application reduced the F1-Cd proportion by 1.9–4.6% on day 14. Compared to TL, the reduction in F1-Cd was 4.6% under the THB treatment. In SY soil, expect on day 14, biochar treatments reduced F1-Cd by 8.3–10.8%. Specifically, compared to SY, SLB decreased the proportion of F1-Cd by 10.8% on day 1. Additionally, biochar application increased the proportion of F2-Cd by 4.7–9.7%. Compared to SY, the increment in F2-Cd proportion under the TLB treatment was 9.7% on day 1. In SY soil, biochar increased the proportion of F2-Cd by 7.2–16.5%, with the SHB treatment increasing F2-Cd by 16.3% compared to SY on day 1. Compared to TL, the increment in F4-Cd under the THB treatment was 38.4% on day 14. In SY soil, SLB increased F4-Cd by 63.7% on day 14, while under the SHB treatment, F4-Cd was 23.7% higher than that in SY on day 40.
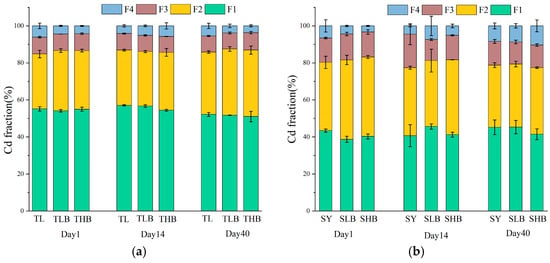
Figure 8.
The cadmium fractions under different biochar treatments: F1: acid extractable, F2: reducible, F3: oxidizable, F4: residual ((a): TL soil, (b): SY soil).
3.6. The Effect of Molybdate on SO42−, Fe2+ and Cd in Pore Water
The changes in SO42− and Fe2+ contents in pore water are summarized in Figure S1. In TL soil, the SO42− content reached 108.2–117.3 mg L−1 on day 20 after treatment, while in SY soil, it reached up to 109.9–122.9 mg L−1 on day 30. In both soils, the SO42− content in HB was higher than that in LB.
The effects of molybdate addition on Fe2+ content in pore water are demonstrated in Figure S2. In TL soil, the Fe2+ content initially increased and then stabilized, while in SY soil, it gradually increased during incubation. The highest Fe2+ content reached 39.8 mg L−1 in TL soil and 8.06 mg L−1 in SY soil, respectively.
As shown in Figure 9, the soluble Cd content in the molybdate-treated group was significantly higher than that in the non-molybdate group. In TL soil, compared to THBM, the THB treatment decreased the Cd content by 53.5%. Correspondingly, the S contribution rate was 38.2% for TLB and 53.8% for THB. In SY soil, compared to SHBM, the SHB treatment decreased the Cd content by 34.1%, with S contribution rates of 33.7% for SLB and 34.5% for SHB.
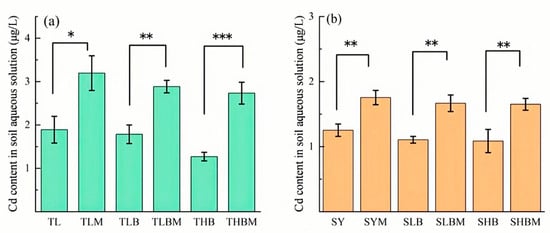
Figure 9.
The cadmium content in water solutions under different biochar additions with molybdate treatment ((a): TL soil, (b): SY soil), Values are means ± SD (n = 3). Significant differences among treatments are indicated by asterisks: * p < 0.05, ** p < 0.01, *** p < 0.001 (one-way ANOVA followed by Duncan’s test).
4. Discussion
The increase in soil pH induced by biochar application might partly contribute to Cd immobilization. Results in this study suggest that from day 0 to day 20, the significant reduction in CaCl2-Cd was accompanied by an increment in soil pH (Figure 1 and Figure 7). This is consistent with previous study, which have shown that an increase in soil pH leads to a reduction in CaCl2-Cd [33]. A meta-analysis indicated that the alkalinity of biochar is critical for Cd immobilization in various soils. In addition, a high soil organic matter (SOM) content can improve the remediation effect of biochar on Cd-contaminated soils [34]. In this study, the pH values of SY soil were higher than those of TL soil (Figure 1). Moreover, SY soil contained significantly more SOM than TL soil (Table 1). These factors might have contributed to the better immobilization effect of biochar in SY soil.
It is worth noting that the contribution of S to Cd immobilization was more significant in TL soil than in SY soil (Figure 9). The dynamic changes in pH and Eh showed significant differences between TL and SY soils. In TL soil, the pe + pH value decreased sharply from day 0 to day 8, and stabilized around 3.0 after day 20. When pe + pH was below 3.2, most SO42− in the soil was reduced to S2−, which could effectively induce Cd immobilization through the formation of CdS [9,35,36]. This inference is supported by the results of this study, where the reduction in SO42− was accompanied by a decrease in CaCl2-Cd content (Figure 10). Moreover, the anaerobic reduction in SO42− generates acid volatile sulfide (AVS), which can affect the bioavailability and toxicity of heavy metals [37]. Typically, AVS reacts with freely available divalent heavy metals in sediments and pore water to form relatively stable metal sulfides [38]. In this study, the increment in AVS partly contributed to the reduction in Cd availability (Table S2), which might be attributed to the S supplied by rape straw biochar.
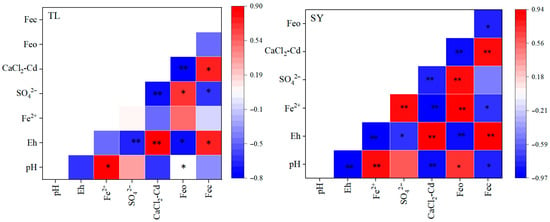
Figure 10.
Pearson’s correlation coefficients among different parameters (with the data obtained on day 14). Significant differences among treatments are indicated by asterisks: * p < 0.05, ** p < 0.01 (one-way ANOVA followed by Duncan’s test).
Besides the contribution of S, the reduction in CaCl2-Cd content can also be attributed to the contribution of Feo in both soils (Figure 9). The reduction in Fe3+ generally occurs when pe + pH decreases from 3.9 to 3.2. Results of this study demonstrated that during the Cd immobilization process, the contribution of Feo to Cd fixation was more significant in SY soil. As reported in previous studies, under low pe + pH conditions, crystalline iron oxides may dissolve or convert into amorphous forms [39,40]. Generally, amorphous iron oxides have a large specific surface area and abundant -OH functional groups, thus providing effective adsorption sites for Cd [36,41,42]. The content of Feo in SY soil was significantly higher than that in TL soil (Figure 4). Furthermore, the increase in soil available S might promote the reduction in Fe3+. Typically, the content of S in soil is much lower than that of Fe [43]. During microbial reduction in S compounds, the generated hydrogen sulfide can indirectly reduce Fe3+ [44]. This can partly explain the positive correlation between AVS and water-soluble Fe2+ (W-Fe2+) in this study. Furthermore, results from the ammonium molybdate treatments showed a decrease in soil W-Fe2+ (Figure S2), which also verifies that sulfate-reducing bacteria involved in Fe3+ reduction. This process would facilitate the absorption of Cd onto Fe oxides.
Moreover, biochar application might promote the transformation of Fe and S. Previous studies have demonstrated that rape straw biochar can immobilize Cd by enhancing S and Fe transformations [19,20,22]. It has been shown that interactions between biochar and soil minerals can result in the formation of redox-active, mesoporous organo-mineral layers [45], which could boost the redox cycling of Fe and other nutrients in the soil environment [46]. In general, biochar serves as an electron shuttle, mediating electron transfer between iron-reducing bacteria and sulfate-reducing bacteria [47,48,49]. However, high soil Cd concentrations generally exhibit a strong inhibitory effect on soil microbial communities, whereas biochar plays a relatively weak role [32,50]. In SY soil, the lower Cd content might have enhanced bacterial mediation in Fe and S transformations. Additionally, the higher content of SOM in SY soil could facilitate Fe3+ reduction. Biochar has been found to promote Fe3+ reduction by acting as an electron shuttle to stimulate electron transfer from Fe3+ oxides to organic matter [51].
5. Conclusions
This study explored the dynamic process and mechanisms of Cd immobilization under anaerobic incubation conditions with the application of S-rich biochar. The application of S-rich biochar might enhance Cd immobilization by inducing the precipitation of Cd and S in soils with high Cd content, low pH, and low SOM content. In contrast, in soils with low Cd concentration, high pH, and high SOM content, S-rich biochar promotes Cd fixation onto amorphous iron oxides (Feo). Overall, the application of S-rich biochar could boost Fe transformation by increasing soil SO42− content and acting as an electron shuttle.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy15102423/s1, Text S1: Experimental procedure; Text S2: Determination of free iron oxides (Fed) and amorphous iron oxides (Feo); Text S3: Determination of CaCl2-extractable Cd; Text S4: Determination of BCR extract cadmium form; Text S5: Reagents and materials; Table S1: Correlation between available S in biochar, soil pe + pH and soil S; Table S2: Correlation between soil AVS and W-Fe2+, CaCl2-Cd; Table S3: Correlation between soil Feo and various forms of cadmium and soluble iron content; Figure S1: Sulfate content in the leachate of molybdate-treated soil solution (a: TL soil; b SY soil); Figure S2: The content of Fe2+ in the aqueous solution after molybdate treatment (a: TL soil; b: SY soil). Refs. [40,43,52] are cited in supplementary materials.
Author Contributions
F.S.: Writing—original draft, Formal analysis, Project administration, Funding acquisition. Y.Q.: Investigation, Data curation, Writing-review & editing. J.M.: Software, Validation. L.C.: Supervision, Writing-review & editing. G.Q.: Supervision, Writing-review & editing. J.Y.: Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financed and supported by the National Natural Science Foundation of China (NSFC) (42207547), Postgraduate Research & Practice Innovation Program of Jiangsu Province (SJCX25_XY014), Natural Science Foundation of Jiangsu Province (SBK2022020100).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
We would like to express our sincere gratitude to the Editor of MDPI for efficiently managing the peer review process of this manuscript. We also highly appreciate the constructive comments and valuable suggestions from the anonymous reviewers, which have significantly improved the quality and clarity of this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Guo, Y.Q.; Li, X.Q.; Liang, L.; Lin, Z.; Su, X.T.; Zhang, W.C. Immobilization of cadmium in contaminated soils using sulfidated nanoscale zero-valent iron: Effectiveness and remediation mechanism. J. Hazard. Mater. 2021, 420, 126605. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.J.; Sun, J.; She, J.Y.; Yin, M.L.; Fang, F.; Xiao, T.F.; Song, G.; Liu, J. Geochemical transfer of cadmium in river sediments near a lead-zinc smelter. Ecotoxicol. Environ. Saf. 2020, 196, 110529. [Google Scholar] [CrossRef]
- Xue, D.W.; Jiang, H.; Deng, X.X.; Zhang, X.Q.; Wang, H.; Xu, X.B.; Hu, J.; Zeng, D.L.; Guo, L.B.; Qian, Q. Comparative proteomic analysis provides new insights into cadmium accumulation in rice grain under cadmium stress. J. Hazard. Mater. 2014, 280, 269–278. [Google Scholar] [CrossRef]
- Wang, J.; Yu, L.; Chen, W.S. Low pe + pH inhibits Cd transfer from paddy soil to rice tissues driven by S addition. Chemosphere 2023, 335, 139126. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Cao, C.L.; Ma, Y.B.; Su, D.C.; Li, J.M. Identification of cadmium bioaccumulation in rice (Oryza sativa L.) by the soil-plant transfer model and species sensitivity distribution. Sci. Total Environ. 2019, 692, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.B.; Qin, P.; Yang, X.; Bhatnagar, A.; Shaheen, S.M.; Rinklebe, J.; Wu, F.C.; Xu, S.; Che, L.; Wang, H.L. Sorption of diethyl phthalate and cadmium by pig carcass and green waste-derived biochars under single and binary systems. Environ. Res. 2021, 193, 110594. [Google Scholar] [CrossRef] [PubMed]
- Griethuysen, C.V.; Luitwieler, M.; Joziasse, J.; Koelmans, A.A. Temporal variation of trace metal geochemistry in floodplain lake sediment subject to dynamic hydrological conditions. Environ. Pollut. 2005, 137, 281–294. [Google Scholar] [CrossRef]
- Zhang, C.H.; Ge, Y.; Yao, H.; Chen, X.; Hu, M.K. Iron oxidation-reduction and its impacts on cadmium bioavailability in paddy soils: A review. Front. Environ. Sci. Eng. 2012, 6, 509–517. [Google Scholar] [CrossRef]
- Honma, T.; Ohba, H.; Kaneko-Kadokura, A.; Makino, T.; Nakamura, K.; Katou, H. Optimal Soil Eh, pH, and Water Management for Simultaneously Minimizing Arsenic and Cadmium Concentrations in Rice Grains. Environ. Technol. 2016, 50, 4178–4185. [Google Scholar] [CrossRef]
- Arao, T.; Kawasaki, A.; Baba, K.; Mori, S.; Matsumoto, S. Effects of Water Management on Cadmium and Arsenic Accumulation and Dimethylarsinic Acid Concentrations in Japanese Rice. Environ. Technol. 2009, 43, 9361–9367. [Google Scholar] [CrossRef]
- Li, X.Y.; Peng, P.Q.; Long, J.; Dong, X.; Jiang, K.; Hou, H.B. Plant-induced insoluble Cd mobilization and Cd redistribution among different rice cultivars. J. Clean. Prod. 2020, 256, 102494. [Google Scholar] [CrossRef]
- Lwin, C.S.; Seo, B.H.; Kim, H.U.; Owens, G.; Kim, K.R. Application of soil amendments to contaminated soils for heavy metal immobilization and improved soil quality-a critical review. Soil Sci. Plant Nutr. 2018, 64, 156–167. [Google Scholar] [CrossRef]
- Santorufo, L.; Maglione, G.; Costanzo, G.; De Tommaso, G.; De Paola, F.; Roviello, G.; Chianese, E.; Ferone, C.; Polimeno, F.; Riccardi, M.; et al. Evaluating the capability of tannic acid-modified biochar in sequestering metal ions and in enhancing soil health. Int. J. Environ. Sci. Technol. 2025, 22, 12843–12854. [Google Scholar] [CrossRef]
- Jiao, Z.Q.; Ge, S.J.; Liu, Y.F.; Wang, Y.Z.; Wang, Y.; Wang, Y.Y. Phosphate-enhanced Cd stabilization in soil by sulfur-doped biochar: Reducing Cd phytoavailability and accumulation in Brassica chinensis L. and shaping the microbial community. Environ. Pollut. 2025, 364, 125375. [Google Scholar] [CrossRef]
- Li, K.G.; Xu, W.J.; Song, H.J.; Bi, F.X.; Li, Y.H.; Jiang, Z.; Tao, Y.; Qu, J.H.; Zhang, Y. Superior reduction and immobilization of Cr(VI) in soil utilizing sulfide nanoscale zero-valent iron supported by phosphoric acid-modified biochar: Efficiency and mechanism investigation. Sci. Total Environ. 2024, 907, 168133. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Wu, Q.J.; Huang, C.; Wang, P.; Cheng, H.; Sun, C.Y.; Zhu, J.; Xu, H.Y.; Ouyang, K.; Guo, J.; et al. Synergistic effect and mechanism of Cd(II) and As(III) adsorption by biochar supported sulfide nanoscale zero-valent iron. Environ. Res. 2023, 231, 116080. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Huang, H.Y.; Fang, Y.R.; Cai, J.M.; Lv, X.Y.; Wu, W.L.; Gu, Q.; Qian, R.; Yu, K.R.; Tang, K.H.D.; et al. Synthesis of magnetic biochar co-doped with sulfur, iron, and manganese via a novel modification method for simultaneous Cd and Pb remediation. Sep. Purif. Technol. 2025, 363, 132025. [Google Scholar] [CrossRef]
- Zhang, K.M.; Cen, R.L.; Moavia, H.; Shen, Y.; Ebihara, A.; Wang, G.J.; Yang, T.X.; Sakrabani, R.; Singh, K.; Feng, Y.F.; et al. The role of biochar nanomaterials in the application for environmental remediation and pollution control. Chem. Eng. J. 2024, 492, 152310. [Google Scholar] [CrossRef]
- Sui, F.F.; Kang, Y.X.; Wu, H.; Li, H.; Wang, J.B.; Joseph, S.; Munroe, P.; Li, L.Q.; Pan, G.X. Effects of iron-modified biochar with S-rich and Si-rich feedstocks on Cd immobilization in the soil-rice system. Ecotoxicol. Environ. Saf. 2021, 225, 112764. [Google Scholar] [CrossRef]
- Yuan, R.; Si, T.R.; Lu, Q.Q.; Liu, C.; Bian, R.J.; Liu, X.Y.; Zhang, X.H.; Zheng, J.F.; Cheng, K.; Joseph, S.; et al. Rape Straw Biochar Application Enhances Cadmium Immobilization by Promoting Formation of Sulfide and Poorly Crystallized Fe Oxide in Paddy Soils. Agronomy 2023, 13, 2693. [Google Scholar] [CrossRef]
- Hu, H.L.; Li, Z.H.; Xi, B.D.; Xu, Q.G.; Tan, W.B. Responses of bacterial taxonomic attributes to mercury species in rhizosphere paddy soil under natural sulphur-rich biochar amendment. Ecotoxicol. Environ. Saf. 2022, 229, 113058. [Google Scholar] [CrossRef]
- Wang, J.B.; Yuan, R.; Zhang, Y.H.; Si, T.R.; Li, H.; Duan, H.T.; Li, L.Q.; Pan, G.X. Biochar decreases Cd mobility and rice (Oryza sativa L.) uptake by affecting soil iron and sulfur cycling. Sci. Total Environ. 2022, 836, 155547. [Google Scholar] [CrossRef]
- Chen, X.; He, H.Z.; Chen, G.K.; Li, H.S. Effects of biochar and crop straws on the bioavailability of cadmium in contaminated soil. Sci. Rep. 2020, 10, 9528. [Google Scholar] [CrossRef]
- Weber, F.A.; Voegelin, A.; Kretzschmar, R. Multi-metal contaminant dynamics in temporarily flooded soil under sulfate limitation. Geochim. Cosmochim. Acta 2009, 73, 5513–5527. [Google Scholar] [CrossRef]
- Furuya, M.; Hashimoto, Y.; Yamaguchi, N. Time-Course Changes in Speciation and Solubility of Cadmium in Reduced and Oxidized Paddy Soils. Soil Sci. Soc. Am. J. 2016, 80, 870–877. [Google Scholar] [CrossRef]
- Zhu, M.; Zhang, L.J.; Zheng, L.W.; Zhuo, Y.; Xu, J.M.; He, Y. Typical Soil Redox Processes in Pentachlorophenol Polluted Soil Following Biochar Addition. Front. Microbiol. 2018, 9, 579. [Google Scholar] [CrossRef]
- Saquing, J.M.; Yu, Y.H.; Chiu, P.C. Wood-Derived Black Carbon (Biochar) as a Microbial Electron Donor and Acceptor. Environ. Sci. Technol. Lett. 2016, 3, 62–66. [Google Scholar] [CrossRef]
- Xu, Z.B.; Xu, X.Y.; Zhang, Y.; Yu, Y.L.; Cao, X.D. Pyrolysis-temperature depended electron donating and mediating mechanisms of biochar for Cr(VI) reduction. J. Hazard. Mater. 2020, 388, 121794. [Google Scholar] [CrossRef]
- Zhong, D.L.; Jiang, Y.; Zhao, Z.Z.; Wang, L.L.; Chen, J.; Ren, S.P.; Liu, Z.H.; Zhang, Y.R.; Tsang, D.C.W.; Crittenden, J.C. pH Dependence of Arsenic Oxidation by Rice-Husk-Derived Biochar: Roles of Redox-Active Moieties. Environ. Sci. Technol. 2019, 53, 9034–9044. [Google Scholar] [CrossRef]
- Zhang, C.; Nie, S.; Liang, J.; Zeng, G.M.; Wu, H.P.; Hua, S.S.; Liu, J.Y.; Yuan, Y.J.; Xiao, H.B.; Deng, L.J.; et al. Effects of heavy metals and soil physicochemical properties on wetland soil microbial biomass and bacterial community structure. Sci. Total Environ. 2016, 557, 785–790. [Google Scholar] [CrossRef]
- Wood, J.L.; Zhang, C.; Mathews, E.R.; Tang, C.; Franks, A.E. Microbial community dynamics in the rhizosphere of a cadmium hyper-accumulator. Sci. Rep. 2016, 6, 36067. [Google Scholar] [CrossRef]
- Xu, M.L.; Dai, W.J.; Zhao, Z.L.; Zheng, J.T.; Huang, F.; Mei, C.; Huang, S.T.; Liu, C.F.; Wang, P.; Xiao, R.B. Effect of rice straw biochar on three different levels of Cd-contaminated soils: Cd availability, soil properties, and microbial communities. Chemosphere 2022, 301, 134551. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Li, X.; Kang, X.R.; Zhang, J.; Sun, M.J.; Yu, J.P.; Wang, H.; Pan, H.; Yang, Q.A.; Lou, Y.H.; et al. Effects of a novel Cd passivation approach on soil Cd availability, plant uptake, and microbial activity in weakly alkaline soils. Ecotoxicol. Environ. Saf. 2023, 253, 114631. [Google Scholar] [CrossRef]
- Duan, Z.H.; Chen, C.; Ni, C.L.; Xiong, J.; Wang, Z.; Cai, J.X.; Tan, W.F. How different is the remediation effect of biochar for cadmium contaminated soil in various cropping systems? A global meta-analysis. J. Hazard. Mater. 2023, 448, 130939. [Google Scholar] [CrossRef]
- Yang, X.; Pan, H.; Shaheen, S.M.; Wang, H.L.; Rinklebe, J. Immobilization of cadmium and lead using phosphorus-rich animal-derived and iron-modified plant-derived biochars under dynamic redox conditions in a paddy soil. Environ. Int. 2021, 156, 106628. [Google Scholar] [CrossRef]
- Qin, L.Y.; Wang, L.F.; Zhao, S.W.; Sun, X.Y.; Yu, L.; Wang, M.; Chen, S.B. A new insight into Cd reduction by flooding in paddy soil: The different dominant roles of Fe and S on Cd immobilization under fluctuant pe plus pH conditions. Sci. Total Environ. 2022, 847, 157604. [Google Scholar] [CrossRef]
- Leng, Z.R.; Wu, Y.M.; Li, J.; Nie, Z.Y.; Jia, H.; Yan, C.L.; Hong, H.L.; Wang, X.H.; Du, D.L. Phenolic root exudates enhance Avicennia marina tolerance to cadmium under the mediation of functional bacteria in mangrove sediments. Mar. Pollut. Bull. 2022, 185, 114227. [Google Scholar] [CrossRef]
- Leng, Z.R.; Liu, J.; He, C.J.; Wang, Z.Q.; He, S.B.; Du, D.L.; Li, J. Deposition of sulfur by Spartina alterniflora promoted its ecological adaptability in cadmium-polluted coastal wetlands. Bioresour. Technol. 2025, 419, 132069. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Dai, Z.X.; Sun, R.; Zhao, Z.J.; Dong, Y.; Hong, Z.N.; Xu, R.K. Evaluation of ferrolysis in arsenate adsorption on the paddy soil derived from an Oxisol. Chemosphere 2017, 179, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Chen, S.; Wang, M.; Lei, X.; Han, Y. Redistribution of iron oxides in aggregates induced by pe + pH variation alters Cd availability in paddy soils. Sci. Total Environ. 2020, 752, 142164. [Google Scholar] [CrossRef]
- Meng, S.; Liu, H.; Yang, C.H.; Wei, Y.; Hou, D.L. Sorption/desorption differences among three ferrihydrites prepared by different synthesis methods. Appl. Surf. Sci. 2012, 258, 4449–4454. [Google Scholar] [CrossRef]
- Burton, E.D.; Bush, R.T.; Johnston, S.G.; Sullivan, L.A.; Keene, A.F. Sulfur biogeochemical cycling and novel Fe-S mineralization pathways in a tidally re-flooded wetland. Geochim. Cosmochim. Acta 2011, 75, 3434–3451. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.M.; Gu, Y.; Kopittke, P.M.; Zhao, F.J.; Wang, P. Iron-Manganese (Oxyhydro)oxides, Rather than Oxidation of Sulfides, Determine Mobilization of Cd during Soil Drainage in Paddy Soil Systems. Environ. Sci. Technol. 2019, 53, 2500–2508. [Google Scholar] [CrossRef]
- Flynn, T.M.; O’Loughlin, E.J.; Mishra, B.; DiChristina, T.J.; Kemner, K.M. Sulfur-mediated electron shuttling during bacterial iron reduction. Science 2014, 344, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Si, T.R.; Yuan, R.; Qi, Y.J.; Zhang, Y.H.; Wang, Y.; Bian, R.J.; Liu, X.Y.; Zhang, X.H.; Joseph, S.; Li, L.Q.; et al. Enhancing soil redox dynamics: Comparative effects of Fe-modified biochar (N-Fe and S-Fe) on Fe oxide transformation and Cd immobilization. Environ. Pollut. 2024, 347, 123636. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y.; et al. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. Glob. Change Biol. Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Hui, C.; Liu, B.; Du, L.N.; Xu, L.G.; Zhao, Y.H.; Shen, D.S.; Long, Y.Y. Transformation of sulfidized nanoscale zero-valent iron particles and its effects on microbial communities in soil ecosystems. Environ. Pollut. 2022, 306, 119363. [Google Scholar] [CrossRef]
- Wu, Z.; Firmin, K.A.; Cheng, M.L.; Wu, H.; Si, Y.B. Biochar enhanced Cd and Pb immobilization by sulfate-reducing bacterium isolated from acid mine drainage environment. J. Clean. Prod. 2022, 366, 132823. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, Y.F.; Wang, S.S.; Yin, W.Q.; Wang, B.; Yang, R.D.; Wang, X.Z. Effect and mechanism of biochar as a support on immobilization of different heavy metals by iron oxides in a multi-contaminated soil. J. Environ. Chem. Eng. 2023, 11, 109895. [Google Scholar] [CrossRef]
- Pan, X.M.; Zhang, S.R.; Zhong, Q.M.; Gong, G.S.; Wang, G.Y.; Guo, X.; Xu, X.X. Effects of soil chemical properties and fractions of Pb, Cd, and Zn on bacterial and fungal communities. Sci. Total Environ. 2020, 715, 136904. [Google Scholar] [CrossRef]
- Kappler, A.; Wuestner, M.L.; Ruecker, A.; Harter, J.; Halama, M.; Behrens, S. Biochar as an Electron Shuttle between Bacteria and Fe(III) Minerals. Environ. Sci. Technol. Lett. 2014, 1, 339–344. [Google Scholar] [CrossRef]
- Caporale, A.G.; Violante, A. Chemical Processes Affecting the Mobility of Heavy Metals and Metalloids in Soil Environments. Curr. Pollut. Rep. 2016, 2, 15–27. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).