Assessment of the Plant Growth-Promoting Potential of Three Pseudomonas and Pantoea Isolates to Promote Pepper Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation and Culture Conditions
2.2. PGP Features In Vitro
2.3. Bacterial Identification and Phylogenetic Tree
2.4. Plant Experiment on Pepper
2.5. Plant Traits Measurements
2.6. Statistical Analysis, PCA and Heatmap
3. Results
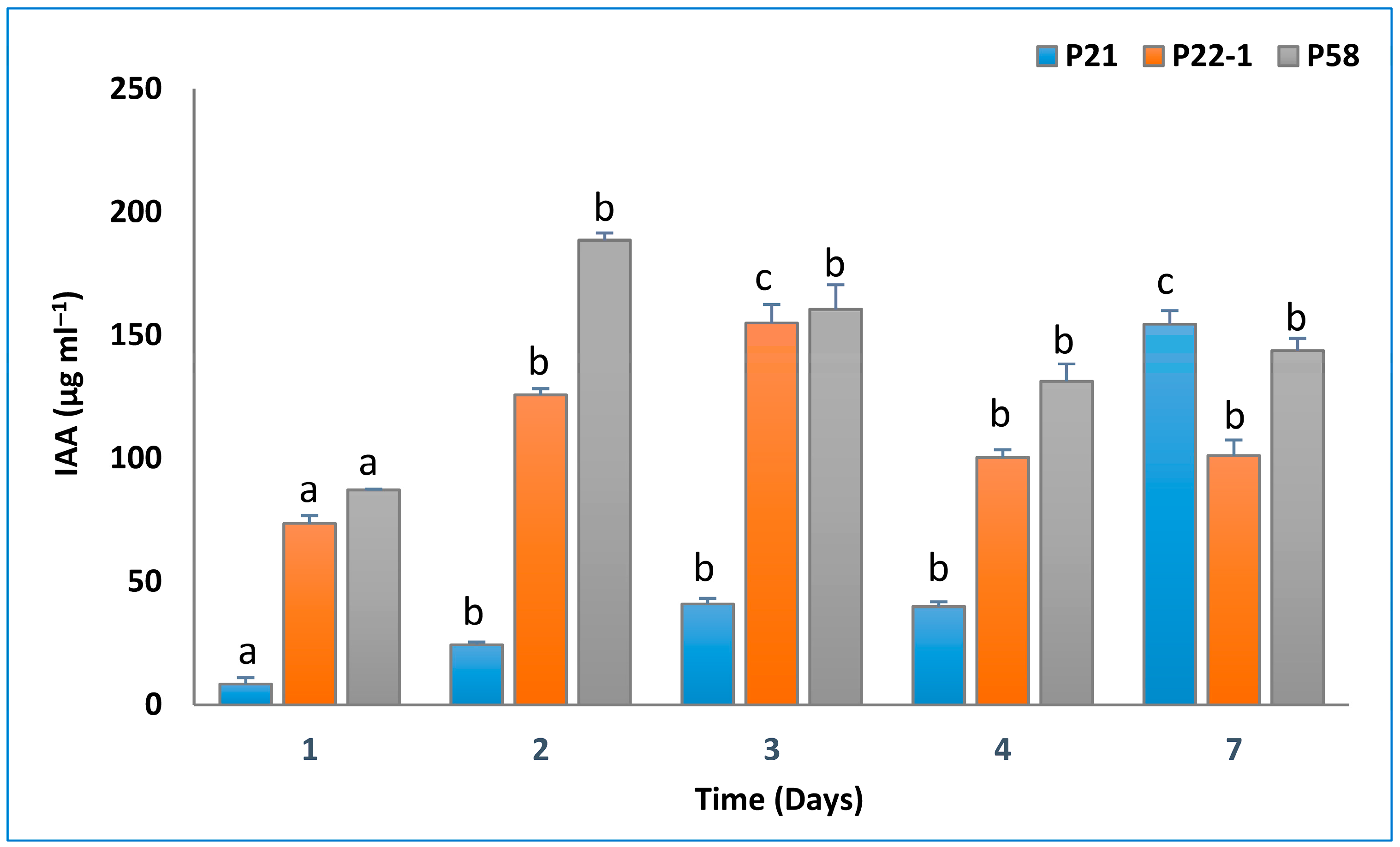
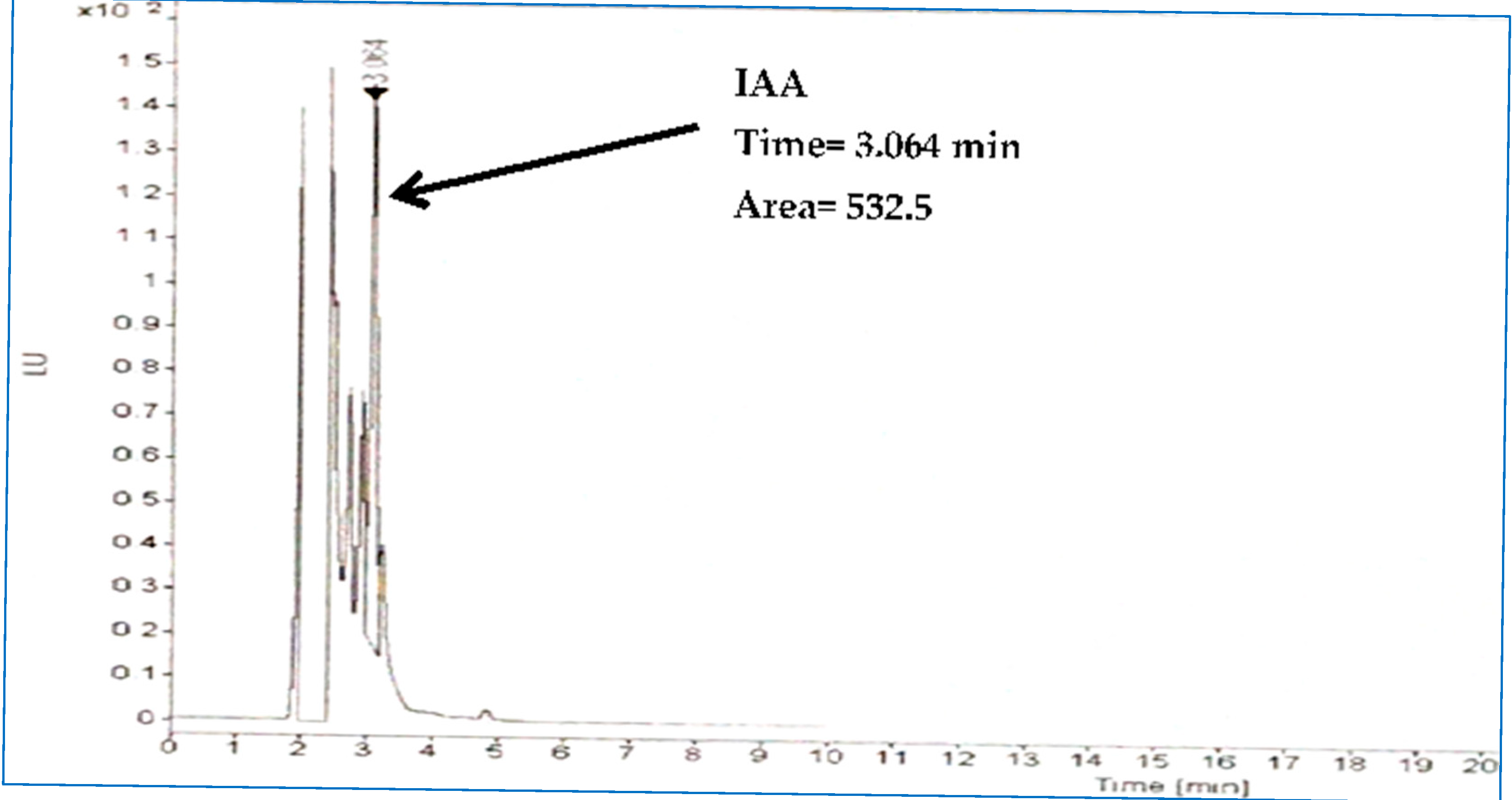
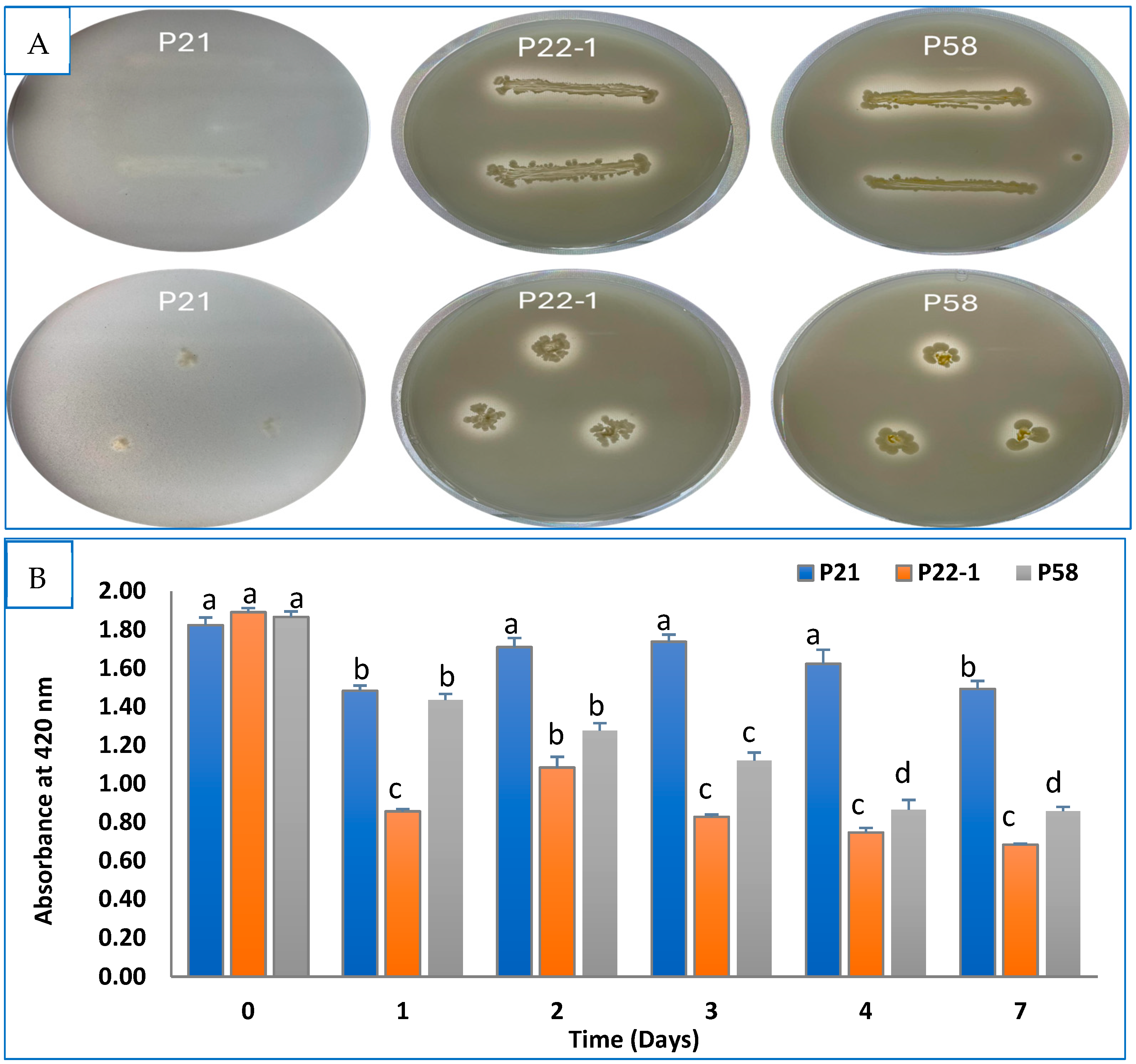
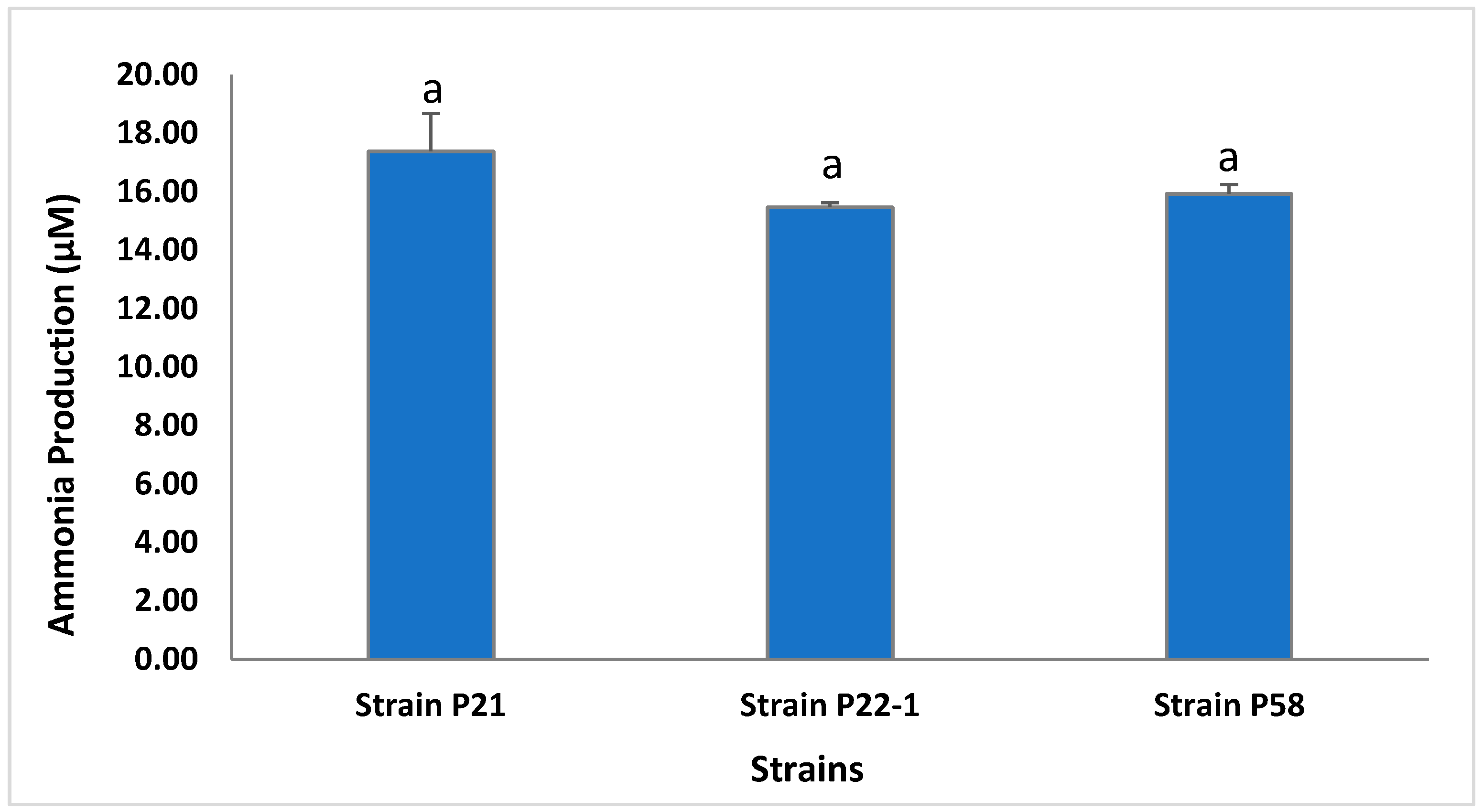
3.1. Assessment of Bacterial PGP In Vitro
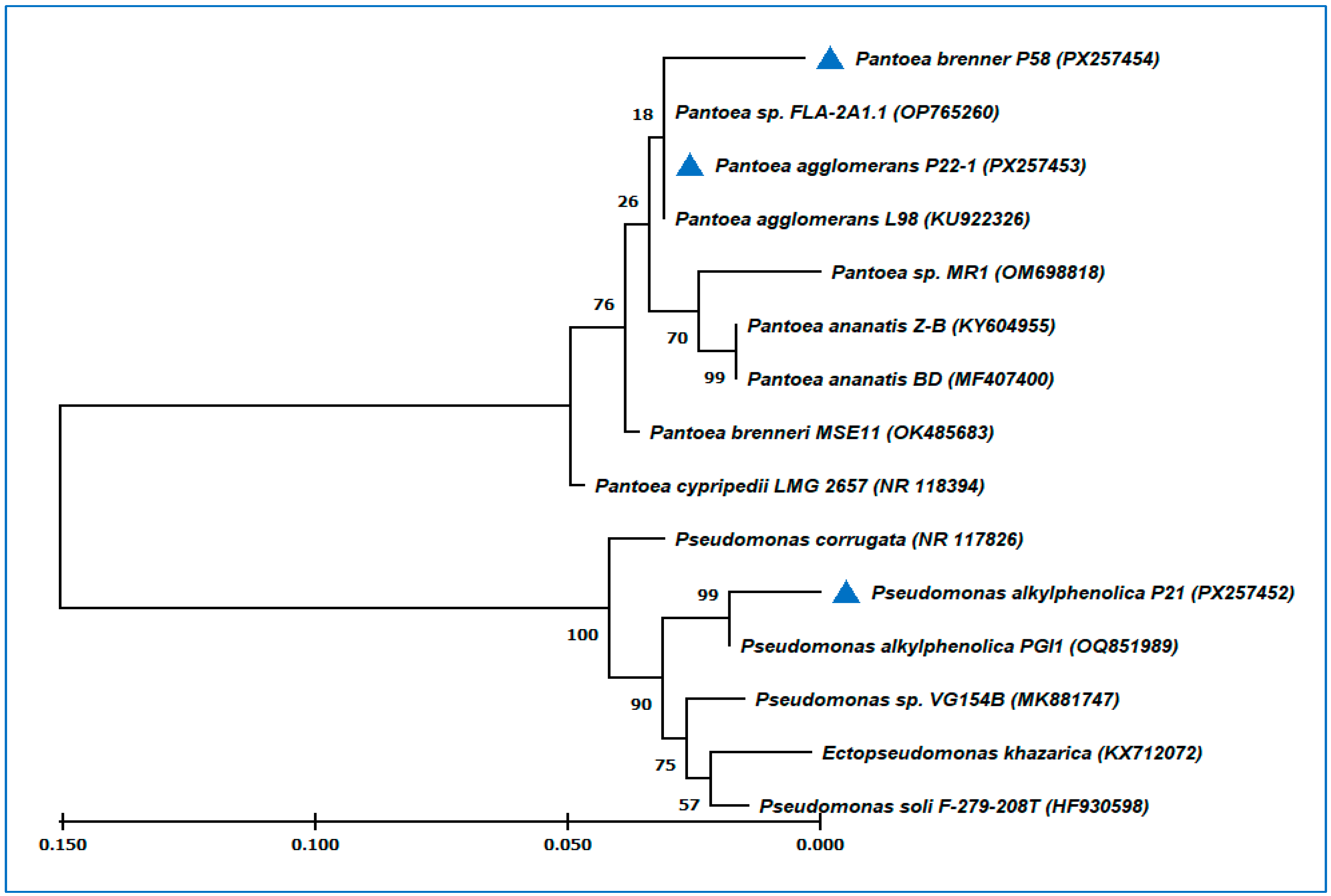
3.2. 16S rRNA Identification and Molecular Phylogentic Tree
3.3. Pepper Traits Evaluation
3.3.1. Vegetative Growth Traits
3.3.2. Plant Phosphorus Contents
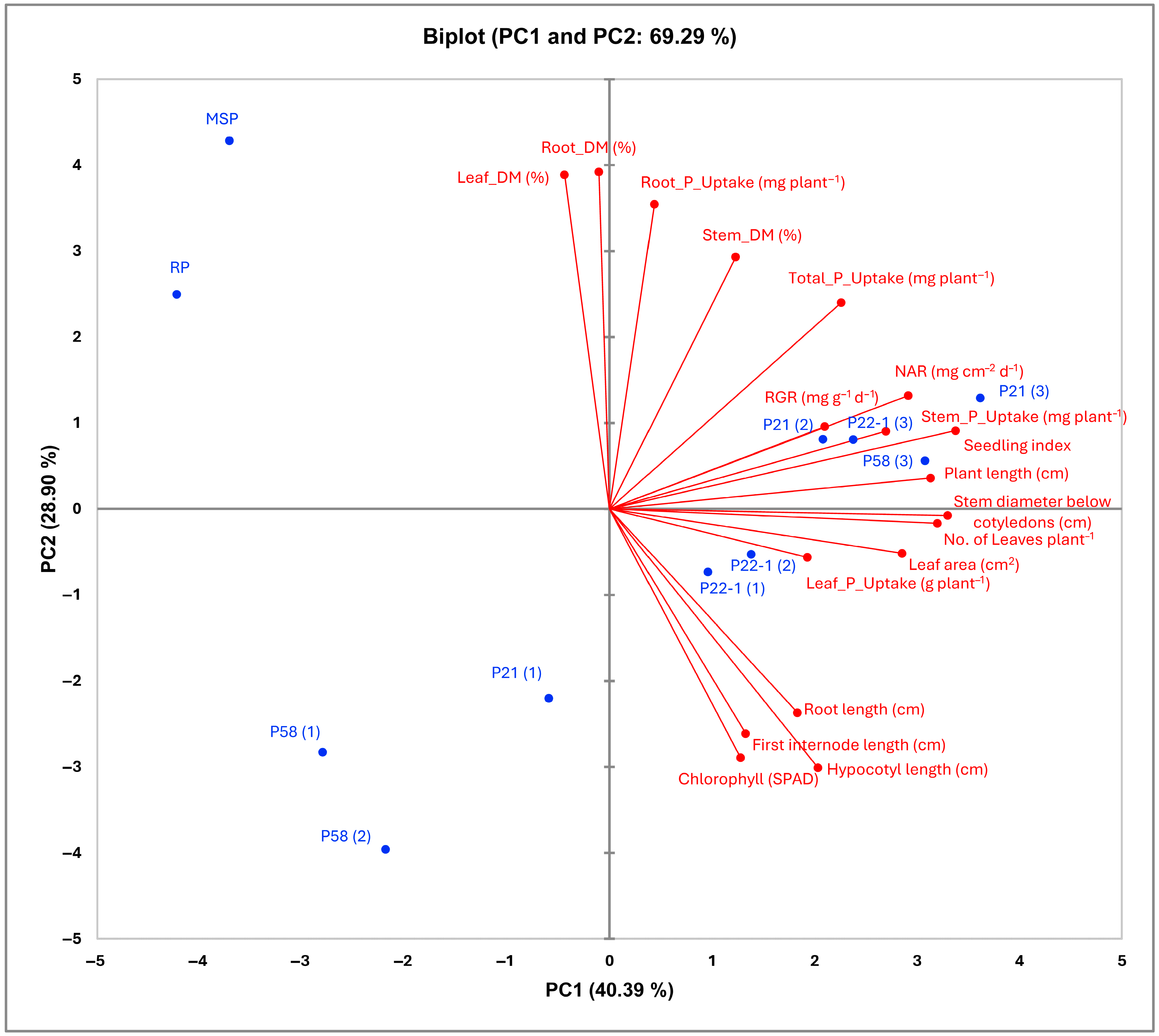
3.4. Principal Component Analysis (PCA)
3.5. Trait Associations and Clustering
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Andrade, L.A.; Santos, C.H.B.; Frezarin, E.T.; Sales, L.R.; Rigobelo, E.C. Plant Growth-Promoting Rhizobacteria for Sustainable Agricultural Production. Microorganisms 2023, 11, 1088. [Google Scholar] [CrossRef]
- Grobelak, A.; Napora, A.; Kacprzak, M. Using Plant Growth-Promoting Rhizobacteria (PGPR) to Improve Plant Growth. Ecol. Eng. 2015, 84, 22–28. [Google Scholar] [CrossRef]
- Basu, A.; Prasad, P.; Das, S.N.; Kalam, S.; Sayyed, R.Z.; Reddy, M.S.; El Enshasy, H. Plant Growth Promoting Rhizobacteria (PGPR) as Green Bioinoculants: Recent Developments, Constraints, and Prospects. Sustainability 2021, 13, 1140. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.-L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Legendre, L.; Wisniewski-Dyé, F.; Prigent-Combaret, C. Plant Growth-Promoting Rhizobacteria and Root System Functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef]
- Omar, A.F.; Rehan, M.; Al-turki, A. Synergistic Effects of Plant Growth Promoting Rhizobacteria in Improvement the Crop Production and Sustainable Agriculture. Fresenius Environ. Bull. 2022, 31, 10563–10574. [Google Scholar]
- Al-Turki, A.; Murali, M.; Omar, A.F.; Rehan, M.; Sayyed, R.Z. Recent Advances in PGPR-Mediated Resilience toward Interactive Effects of Drought and Salt Stress in Plants. Front. Microbiol. 2023, 14, 1214845. [Google Scholar] [CrossRef]
- Gupta, K.; Dubey, N.K.; Singh, S.P.; Kheni, J.K.; Gupta, S.; Varshney, A. Plant Growth-Promoting Rhizobacteria (PGPR): Current and Future Prospects for Crop Improvement. In Current Trends in Microbial Biotechnology for Sustainable Agriculture; Yadav, A.N., Singh, J., Singh, C., Yadav, N., Eds.; Springer: Singapore, 2021; pp. 203–226. ISBN 978-981-15-6949-4. [Google Scholar]
- Tripathi, A.; Pandey, V.K.; Jain, D.; Singh, G.; Brar, N.S.; Taufeeq, A.; Pandey, I.; Dash, K.K.; Samrot, A.V.; Rustagi, S. An Updated Review on Significance of PGPR-Induced Plant Signalling and Stress Management in Advancing Sustainable Agriculture. J. Agric. Food Res. 2024, 16, 101169. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M.P. Plant Growth-Promoting Rhizobacteria (PGPR): Their Potential as Antagonists and Biocontrol Agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef] [PubMed]
- Omar, A.F.; Abdelmageed, A.H.A.; Al-Turki, A.; Abdelhameid, N.M.; Sayyed, R.Z.; Rehan, M. Exploring the Plant Growth-Promotion of Four Streptomyces Strains from Rhizosphere Soil to Enhance Cucumber Growth and Yield. Plants 2022, 11, 3316. [Google Scholar] [CrossRef] [PubMed]
- Rehan, M.; Al-Turki, A.; Abdelmageed, A.H.A.; Abdelhameid, N.M.; Omar, A.F. Performance of Plant-Growth-Promoting Rhizobacteria (PGPR) Isolated from Sandy Soil on Growth of Tomato (Solanum lycopersicum L.). Plants 2023, 12, 1588. [Google Scholar] [CrossRef] [PubMed]
- Subedi, P.; Gattoni, K.; Liu, W.; Lawrence, K.S.; Park, S.-W. Current Utility of Plant Growth-Promoting Rhizobacteria as Biological Control Agents towards Plant-Parasitic Nematodes. Plants 2020, 9, 1167. [Google Scholar] [CrossRef]
- Rezaee Danesh, Y.; Pellegrini, M.; Akköprü, A.; Farda, B.; Boyno, G.; Djebaili, R. Chapter 7-Plant Growth–Promoting Rhizobacteria: Their Potential as Biological Control Agents in Sustainable Agriculture. In Sustainable Agricultural Practices; Kumar, A., White, J.F., Singh, J., Eds.; Plant and Soil Microbiome; Academic Press: Cambridge, MA, USA, 2024; pp. 145–159. ISBN 978-0-443-19150-3. [Google Scholar]
- El-Saadony, M.T.; Saad, A.M.; Soliman, S.M.; Salem, H.M.; Ahmed, A.I.; Mahmood, M.; El-Tahan, A.M.; Ebrahim, A.A.M.; Abd El-Mageed, T.A.; Negm, S.H.; et al. Plant Growth-Promoting Microorganisms as Biocontrol Agents of Plant Diseases: Mechanisms, Challenges and Future Perspectives. Front. Plant Sci. 2022, 13, 923880. [Google Scholar] [CrossRef]
- Danish, M.; Shahid, M.; Altaf, M.; Tyagi, A.; Ali, S. Plant Growth-Promoting Rhizobacteria and Biocontrol Agents Triggered Plant Defence Responses against Phytopathogenic Fungi and Improved Rice Growth. Physiol. Mol. Plant Pathol. 2024, 133, 102337. [Google Scholar] [CrossRef]
- Heo, A.Y.; Koo, Y.M.; Choi, H.W. Biological Control Activity of Plant Growth Promoting Rhizobacteria Burkholderia Contaminans AY001 against Tomato Fusarium Wilt and Bacterial Speck Diseases. Biology 2022, 11, 619. [Google Scholar] [CrossRef]
- Aioub, A.A.A.; Elesawy, A.E.; Ammar, E.E. Plant Growth Promoting Rhizobacteria (PGPR) and Their Role in Plant-Parasitic Nematodes Control: A Fresh Look at an Old Issue. J. Plant Dis. Prot. 2022, 129, 1305–1321. [Google Scholar] [CrossRef]
- Chieb, M.; Gachomo, E.W. The Role of Plant Growth Promoting Rhizobacteria in Plant Drought Stress Responses. BMC Plant Biol. 2023, 23, 407. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Deng, F.; Zeng, F.; Chen, Z.-H.; Qin, Y.; Chen, G. Plant Growth-Promoting Rhizobacteria Improve Drought Tolerance of Crops: A Review. Plant Growth Regul. 2025, 105, 567–581. [Google Scholar] [CrossRef]
- Balci, M.; Arikan-Abdulveli, B.; Yildiztugay, E.; Ozfidan-Konakci, C.; Uysal, A. An Effective Sustainable Strategy: Plant Growth Promoting Rhizobacteria (PGPR), Bacillus Atrophaeus, in Maize Plants against Challenging Environments. J. Environ. Chem. Eng. 2025, 13, 116778. [Google Scholar] [CrossRef]
- Kumar, R.; Swapnil, P.; Meena, M.; Selpair, S.; Yadav, B.G. Plant Growth-Promoting Rhizobacteria (PGPR): Approaches to Alleviate Abiotic Stresses for Enhancement of Growth and Development of Medicinal Plants. Sustainability 2022, 14, 5514. [Google Scholar] [CrossRef]
- Kumawat, K.C.; Sharma, B.; Nagpal, S.; Kumar, A.; Tiwari, S.; Nair, R.M. Plant Growth-Promoting Rhizobacteria: Salt Stress Alleviators to Improve Crop Productivity for Sustainable Agriculture Development. Front. Plant Sci. 2023, 13, 1101862. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of Plant Growth Promoting Rhizobacteria (PGPRs) with Multiple Plant Growth Promoting Traits in Stress Agriculture: Action Mechanisms and Future Prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef]
- Ahmad, H.M.; Fiaz, S.; Hafeez, S.; Zahra, S.; Shah, A.N.; Gul, B.; Aziz, O.; Mahmood-Ur-Rahman; Fakhar, A.; Rafique, M.; et al. Plant Growth-Promoting Rhizobacteria Eliminate the Effect of Drought Stress in Plants: A Review. Front. Plant Sci. 2022, 13, 875774. [Google Scholar] [CrossRef]
- Beshah, A.; Muleta, D.; Legese, G.; Assefa, F. Exploring Stress-Tolerant Plant Growth-Promoting Rhizobacteria from Groundnut Rhizosphere Soil in Semi-Arid Regions of Ethiopia. Plant Signal Behav. 2024, 19, 2365574. [Google Scholar] [CrossRef]
- Khawula, S.; Daniel, A.I.; Nyawo, N.; Ndlazi, K.; Sibiya, S.; Ntshalintshali, S.; Nzuza, G.; Gokul, A.; Keyster, M.; Klein, A.; et al. Optimizing Plant Resilience with Growth-Promoting Rhizobacteria under Abiotic and Biotic Stress Conditions. Plant Stress. 2025, 17, 100949. [Google Scholar] [CrossRef]
- Chen, Q.-B.; Sun, X.-Y.; Zheng, M.-Y.; Liu, Y.-N.; Zhang, J.-X.; Zhou, Q.-F.; Pei, D.-L.; Liu, D.-M.; Chen, Y.-W.; Gao, H.; et al. Transcription Factor CaPHR3 Enhances Phosphate Starvation Tolerance by Up-Regulating the Expression of the CaPHT1;4 Phosphate Transporter Gene in Pepper. Int. J. Biol. Macromol. 2025, 292, 139315. [Google Scholar] [CrossRef]
- Werner, J. Capsaicinoids—Properties and Mechanisms of Pro-Health Action. In Analytical Methods in the Determination of Bioactive Compounds and Elements in Food; Jeszka-Skowron, M., Zgoła-Grześkowiak, A., Grześkowiak, T., Ramakrishna, A., Eds.; Springer International Publishing: Cham, Germany, 2021; pp. 193–225. ISBN 978-3-030-61879-7. [Google Scholar]
- Al-Harbi, A.R.; Obadi, A.; Al-Omran, A.M.; Abdel-Razzak, H. Sweet Peppers Yield and Quality as Affected by Biochar and Compost as Soil Amendments under Partial Root Irrigation. J. Saudi Soc. Agric. Sci. 2020, 19, 452–460. [Google Scholar] [CrossRef]
- Ministry of Environment, Water and Agriculture. Statistical Yearbook; UN iLibrary: Riyadh, Saudi Arabia, 2020. [Google Scholar]
- Kumar, R.; Kumari, P.; Kumar, S. Effect of Irrigation Levels and Frequencies on Yield, Quality and Water Use Efficiency of Capsicum Grown under Protected Conditions. Int. J. Bio-Resour. Stress Manag. 2016, 7, 1290–1296. [Google Scholar] [CrossRef]
- Marín, A.; Ferreres, F.; Tomás-Barberán, F.A.; Gil, M.I. Characterization and Quantitation of Antioxidant Constituents of Sweet Pepper (Capsicum annuum L.). J. Agric. Food Chem. 2004, 52, 3861–3869. [Google Scholar] [CrossRef]
- Igbokwe, G.; Aniakor, G.C.; Anagonye, C.O. Determination of β–Carotene & Vitamin C Content of Fresh Green Pepper (Capsicum annnum), Fresh Red Pepper (Capsicum annum) and Fresh Tomatoes (Solanumly copersicum) Fruits. Biosci. 2013, 1, 89–93. [Google Scholar]
- Mohd Hassan, N.; Yusof, N.A.; Yahaya, A.F.; Mohd Rozali, N.N.; Othman, R. Carotenoids of Capsicum Fruits: Pigment Profile and Health-Promoting Functional Attributes. Antioxidants 2019, 8, 469. [Google Scholar] [CrossRef]
- Palevitch, D.; Craker, L.E. Nutritional and Medical Importance of Red Pepper (Capsicum spp.). J. Herbs Spices Med. Plants 1996, 3, 55–83. [Google Scholar] [CrossRef]
- Bisht, N.; Chauhan, P.S. Excessive and Disproportionate Use of Chemicals Cause Soil Contamination and Nutritional Stress. In Soil Contamination; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Chandini, K.; Ravendra, K.; Prakash, O. The Impact of Chemical Fertilizers on Our Environment and Ecosystem. In Research Trends in Environmental Sciences; Bhumi Publishing: Maharashtra, India, 2019; pp. 69–86. ISBN 978-93-5335-062-8. [Google Scholar]
- Shanmugavel, D.; Rusyn, I.; Solorza-Feria, O.; Kamaraj, S.-K. Sustainable SMART Fertilizers in Agriculture Systems: A Review on Fundamentals to in-Field Applications. Sci. Total Environ. 2023, 904, 166729. [Google Scholar] [CrossRef]
- Khoso, M.A.; Wagan, S.; Alam, I.; Hussain, A.; Ali, Q.; Saha, S.; Poudel, T.R.; Manghwar, H.; Liu, F. Impact of Plant Growth-Promoting Rhizobacteria (PGPR) on Plant Nutrition and Root Characteristics: Current Perspective. Plant Stress 2024, 11, 100341. [Google Scholar] [CrossRef]
- Kumar, S.; Diksha; Sindhu, S.S.; Kumar, R. Biofertilizers: An Ecofriendly Technology for Nutrient Recycling and Environmental Sustainability. Curr. Res. Microb. Sci. 2022, 3, 100094. [Google Scholar] [CrossRef]
- Sahoo, D.; Sahoo, N.R.; Paramjita, D. Effect of Biofertilisers on Yield and Quality of Sweet Pepper (Capsicum annuum cv. Grossum L.). Indian Soc. Coastal Agric. Res. 2017, 35, 15–20. [Google Scholar]
- Kumar, R.; Singh, S.K.; Kumar, N.; Kant Verma, A.; Singh, K. Effect of Biofertilizers on Growth, Yield and Quality of Chilli (Capsicum annuum L.). Pharma Innov. J. 2021, 10, 451–454. [Google Scholar]
- Hariyono, D.; Ali, F.Y.; Nugroho, A. Increasing the Growth and Development of Chili-Pepper under Three Different Shading Condition in Response to Biofertilizers Application. Agrivita 2021, 43, 198–208. [Google Scholar] [CrossRef]
- Camacho-Rodríguez, M.; José Almaraz-Suárez, J.; Vázquez-Vázquez, C.; Angulo-Castro, A.; Ríos-Vega, M.E.; González-Mancilla, A. Effect of Plant Growth-Promoting Rhizobacteria on the Growth and Yield of Jalapeño Pepper. Rev. Mex. De Cienc. Agrícolas 2022, 13, 185–196. [Google Scholar]
- Nejati Sini, H.; Barzegar, R.; Soodaee Mashaee, S.; Ghasemi Ghahsare, M.; Mousavi-Fard, S.; Mozafarian, M. Effects of Biofertilizer on the Production of Bell Pepper (Capsicum annuum L.) in Greenhouse. J. Agric. Food Res. 2024, 16, 101060. [Google Scholar] [CrossRef]
- Tahmasebi, A.; Mirzaalian Dastjerdi, A.; Jamali, B. Microbial-Based Biological Treatments Improved the Nutritional, Nutraceutical and Functional Properties of Greenhouse Sweet Pepper (Capsicum annuum L.). Front. Sustain. Food Syst. 2023, 7, 1145972. [Google Scholar] [CrossRef]
- Zaidi, A.; Ahmad, E.; Khan, M.S.; Saif, S.; Rizvi, A. Role of Plant Growth Promoting Rhizobacteria in Sustainable Production of Vegetables: Current Perspective. Sci. Hortic. 2015, 193, 231–239. [Google Scholar] [CrossRef]
- Lau, E.T.; Tani, A.; Khew, C.Y.; Chua, Y.Q.; Hwang, S.S. Plant Growth-Promoting Bacteria as Potential Bio-Inoculants and Biocontrol Agents to Promote Black Pepper Plant Cultivation. Microbiol. Res. 2020, 240, 126549. [Google Scholar] [CrossRef]
- González-Mancilla, A.; Almaraz-Suárez, J.J.; Ferrera-Cerrato, R.; del Pilar Rodríguez-Guzmán, M.; Taboada-Gaytán, O.R. Photosynthetic Activity and Growth of Poblano Pepper Biofertilized with Plant Growth Promoting Rhizobacteria and Arbuscular Mycorrhizal Fungi. Curr. Res. Microb. Sci. 2024, 7, 100269. [Google Scholar] [CrossRef]
- Al-Dhabaan, F.A.M.; Bakhali, A.H. Analysis of the Bacterial Strains Using Biolog Plates in the Contaminated Soil from Riyadh Community. Saudi J. Biol. Sci. 2017, 24, 901–906. [Google Scholar] [CrossRef]
- Gang, S.; Sharma, S.; Saraf, M.; Buck, M.; Schumacher, J. Analysis of Indole-3-Acetic Acid (IAA) Production in Klebsiellaby LC-MS/MS and the Salkowski Method. Bio Protoc. 2019, 9, e3230. [Google Scholar] [CrossRef]
- Nautiyal, C.S. An Efficient Microbiological Growth Medium for Screening Phosphate Solubilizing Microorganisms. FEMS Microbiol. Lett. 1999, 170, 265–270. [Google Scholar] [CrossRef]
- Manullang, W.; Chuang, H. Streptomyces sp. Mitigates Abiotic Stress Response and Promotes Plant Growth. J. Plant Prot. Res. 2020, 60, 263–274. [Google Scholar] [CrossRef]
- Chaudhary, T.; Gera, R.; Shukla, P. Deciphering the Potential of Rhizobium Pusense MB-17a, a Plant Growth-Promoting Root Endophyte, and Functional Annotation of the Genes Involved in the Metabolic Pathway. Front. Bioeng. Biotechnol. 2021, 8, 617034. [Google Scholar] [CrossRef]
- Cook, A.E.; Meyers, P.R. Rapid Identification of Filamentous Actinomycetes to the Genus Level Using Genus-Specific 16S RRNA Gene Restriction Fragment Patterns. Int. J. Syst. Evol. Microbiol. 2003, 53, 1907–1915. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Jackson, M.L.R. Soil Chemical Analysis; Prentice-Hall: Hoboken, NJ, USA, 1964. [Google Scholar]
- Page, A.L. Methods of Soil Analysis. Part 2. Chemical and Microbiological Properties; John Wiley & Sons: Hoboken, NJ, USA, 1982. [Google Scholar]
- Klute, A.; Dirksen, C. Hydraulic Conductivity and Diffusivity: Laboratory Methods. In Methods of Soil Analysis; SSSA Book Series; John Wiley & Sons: Hoboken, NJ, USA, 1986; pp. 687–734. ISBN 9780891188643. [Google Scholar]
- Hunt, R.; Causton, D.R.; Shipley, B.; Askew, A.P. A Modern Tool for Classical Plant Growth Analysis. Ann. Bot. 2002, 90, 485–488. [Google Scholar] [CrossRef]
- Evans, G.C. The Quantitative Analysis of Plant Growth; Univ of California Press: Oakland, CA, USA, 1972; Volume 1, ISBN 0520094328. [Google Scholar]
- Khiddir, S.M. A Statistical Approach in the Use of Parametric Systems Applied to the FAO Framework for Land Evaluation; Ghent University: Ghent, Belgium, 1986. [Google Scholar]
- Addinsoft XLSTAT. Statistical and Data Analysis Solution; Addinsoft XLSTAT: New York, NY, USA, 2019. [Google Scholar]
- Joshi, S.K.; Gauraha, A.K. Global Biofertilizer Market: Emerging Trends and Opportunities. Trends Appl. Microbiol. Sustain. Econ. 2022, 689–697. [Google Scholar]
- Etesami, H. The Dual Nature of Plant Growth-Promoting Bacteria: Benefits, Risks, and Pathways to Sustainable Deployment. Curr. Res. Microb. Sci. 2025, 9, 100421. [Google Scholar] [CrossRef]
- Olanrewaju, O.S.; Glick, B.R.; Babalola, O.O. Mechanisms of Action of Plant Growth Promoting Bacteria. World J. Microbiol. Biotechnol. 2017, 33, 197. [Google Scholar] [CrossRef]
- Ajijah, N.; Fiodor, A.; Pandey, A.K.; Rana, A.; Pranaw, K. Plant Growth-Promoting Bacteria (PGPB) with Biofilm-Forming Ability: A Multifaceted Agent for Sustainable Agriculture. Diversity 2023, 15, 112. [Google Scholar] [CrossRef]
- de Oliveira, D.A.; Ferreira, S.d.C.; Carrera, D.L.R.; Serrao, C.P.; Callegari, D.M.; Barros, N.L.F.; Coelho, F.M.; Souza, C.R.B. Characterization of Pseudomonas Bacteria of Piper tuberculatum Regarding the Production of Potentially Bio-Stimulating Compounds for Plant Growth. Acta Amazon. 2021, 51, 10–19. [Google Scholar] [CrossRef]
- Hyder, S.; Gondal, A.S.; Rizvi, Z.F.; Ahmad, R.; Alam, M.M.; Hannan, A.; Ahmed, W.; Fatima, N.; Inam-ul-Haq, M. Characterization of Native Plant Growth Promoting Rhizobacteria and Their Anti-Oomycete Potential against Phytophthora Capsici Affecting Chilli Pepper (Capsicum annum L.). Sci. Rep. 2020, 10, 13859. [Google Scholar] [CrossRef]
- Ganesh, J.; Hewitt, K.; Devkota, A.R.; Wilson, T.; Kaundal, A. IAA-Producing Plant Growth Promoting Rhizobacteria from Ceanothus Velutinus Enhance Cutting Propagation Efficiency and Arabidopsis Biomass. Front. Plant Sci. 2024, 15, 1374877. [Google Scholar] [CrossRef]
- Lata, D.L.; Abdie, O.; Rezene, Y. IAA-Producing Bacteria from the Rhizosphere of Chickpea (Cicer arietinum L.): Isolation, Characterization, and Their Effects on Plant Growth Performance. Heliyon 2024, 10, e39702. [Google Scholar] [CrossRef]
- Noreen, S.; Ali, B.; Hasnain, S. Growth Promotion of Vigna mungo (L.) by Pseudomonas spp. Exhibiting Auxin Production and ACC-Deaminase Activity. Ann. Microbiol. 2012, 62, 411–417. [Google Scholar] [CrossRef]
- Papade, S.E.; Mohapatra, B.; Phale, P.S. Pseudomonas and Acinetobacter Spp. Capable of Metabolizing Aromatics Displays Multifarious Plant Growth Promoting Traits: Insights on Strategizing Consortium-Based Application to Agro-Ecosystems. Environ. Technol. Innov. 2024, 36, 103786. [Google Scholar] [CrossRef]
- Mirik, M.; Aysan, Y.; Cinar, O. Biological Control of Bacterial Spot Disease of Pepper with Bacillus Strains. Turk. J. Agric. For. 2008, 32, 381–390. [Google Scholar]
- Phares, C.A.; Amoakwah, E.; Danquah, A.; Afrifa, A.; Beyaw, L.R.; Frimpong, K.A. Biochar and NPK Fertilizer Co-Applied with Plant Growth Promoting Bacteria (PGPB) Enhanced Maize Grain Yield and Nutrient Use Efficiency of Inorganic Fertilizer. J. Agric. Food Res. 2022, 10, 100434. [Google Scholar] [CrossRef]
- Kaur, M.; Vyas, P.; Rahi, P.; Sharma, S. Chlorpyrifos- and Carbofuran-Tolerant Phosphate-Solubilising Arthrobacter Oxydans and Bacillus Flexus Improved Growth and Phosphorus Content in Potato in Pesticide-Amended Soils. Potato Res. 2022, 65, 213–231. [Google Scholar] [CrossRef]
- Cheng, Y.; Narayanan, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Phosphate-Solubilizing Bacteria: Their Agroecological Function and Optimistic Application for Enhancing Agro-Productivity. Sci. Total Environ. 2023, 901, 166468. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate Solubilizing Microbes: Sustainable Approach for Managing Phosphorus Deficiency in Agricultural Soils. Springerplus 2013, 2, 587. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of Phosphorus and Nitrogen in the Rhizosphere and Plant Growth Promotion by Microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Mma, Y.; Mfm, E. Biofertilizers and Their Role in Management of Plant Parasitic Nematodes. A Review. E3 J. Biotechnol. Pharm. Res. 2014, 5, 1–6. [Google Scholar]
- Zaidi, A.; Khan, M.; Ahemad, M.; Oves, M. Plant Growth Promotion by Phosphate Solubilizing Bacteria. Acta Microbiol. Immunol. Hung. 2009, 56, 263–284. [Google Scholar] [CrossRef]
- Mohamed, A.E.; Nessim, M.G.; Abou-el-seoud, I.I.; Darwish, K.M.; Shamseldin, A. Isolation and Selection of Highly Effective Phosphate Solubilizing Bacterial Strains to Promote Wheat Growth in Egyptian Calcareous Soils. Bull. Natl. Res. Cent. 2019, 43, 203. [Google Scholar] [CrossRef]
- da Silva, A.M.; da Cruz Paula Neves, P.; Costa, S.S.; Silva, A.; Schneider, M.P.C.; das Graças, D.A.; da Silva, J.K.; Baraúna, R.A. Assessment of Plant-Growth Promoting Potential of Bacteria Isolated from Amazonian Black Pepper Roots. J. Soil. Sci. Plant Nutr. 2024, 24, 2825–2837. [Google Scholar] [CrossRef]
- Bachtiar, T.; Syahputra, A.R.; Citraresmini, A.; Nurjayati, R.; Hidawati, H.; Rachmawati, V.; Mulyono, A. Performances of Phosphate-Solubilizing Microorganisms on Soil Chemical Properties under Different Soil Characteristics: A Meta-Analysis. J. Degrad. Min. Lands Manag. 2024, 11, 6351–6366. [Google Scholar] [CrossRef]
- Yagmur, B.; Gunes, A. Evaluation of the Effects of Plant Growth Promoting Rhizobacteria (PGPR) on Yield and Quality Parameters of Tomato Plants in Organic Agriculture by Principal Component Analysis (PCA). Gesunde Pflanz. 2021, 73, 219–228. [Google Scholar] [CrossRef]
- Vinci, G.; Cozzolino, V.; Mazzei, P.; Monda, H.; Savy, D.; Drosos, M.; Piccolo, A. Effects of Bacillus Amyloliquefaciens and Different Phosphorus Sources on Maize Plants as Revealed by NMR and GC-MS Based Metabolomics. Plant Soil 2018, 429, 437–450. [Google Scholar] [CrossRef]
- Abdelsattar, A.M.; El-Esawi, M.A.; Elsayed, A.; Heikal, Y.M. Comparison between Bacterial Bio-Formulations and Gibberellic Acid Effects on Stevia Rebaudiana Growth and Production of Steviol Glycosides through Regulating Their Encoding Genes. Sci. Rep. 2024, 14, 24130. [Google Scholar] [CrossRef]

| Properties | Value | |
|---|---|---|
| Soil | Water | |
| Physical Properties | ||
| Sand (%) | 94.6 | - |
| Silt (%) | 3.2 | - |
| Clay (%) | 2.2 | - |
| Texture | Sand | - |
| Chemical Properties | ||
| 1 pH | 7.84 | 7.25 |
| 2 EC (dS m−1) | 1.12 | 0.93 |
| 3 Nutrients (mg kg−1) | ||
| Total N | 165 | - |
| Available P | 1.13 | - |
| Available K | 69.0 | 39.0 |
| 4 Soluble Ions (meq L−1) | ||
| 1-Soluble Anions (meq L−1) | ||
| Cl− | 7.8 | 7.0 |
| HCO31− + CO32− | 2.4 | 1.3 |
| 2-Soluble Cations (meq L−1) | ||
| Na+ | 7.7 | 6.9 |
| Ca2+ | 3.2 | 1.8 |
| Mg2+ | 1.0 | 0.9 |
| Treatments | Plant Height (cm) | No. of Leaves Plant−1 | Root Length (cm) | Leaf DM (%) | Stem DM (%) | Root DM (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30DAT | 45DAT | 30DAT | 45DAT | 30DAT | 45DAT | 30DAT | 45DAT | 30DAT | 45DAT | 30DAT | 45DAT | |
| RP | 46.00 ± 4.36 bc | 52.00 ± 7.21 def | 48.0 ± 14.1 bc | 63.0 ± 13.9 b | 15.23 ± 1.37 a | 17.00 ± 2.65 c | 13.78 ± 0.32 a | 13.21 ± 0.62 a | 12.29 ± 0.71 bcd | 13.56 ± 0.26 a | 21.41 ± 2.08 ab | 16.55 ± 0.99 ab |
| MSP | 40.33 ± 7.02 cd | 48.67 ± 8.14 ef | 51.3 ± 1.2 abc | 64.0 ± 22.3 b | 14.83 ± 0.76 a | 16.33 ± 1.15 c | 13.82 ± 0.94 a | 12.75 ± 1.24 ab | 13.69 ± 0.49 a | 13.08 ± 1.71 ab | 23.03 ± 8.43 a | 17.62 ± 1.22 a |
| P58(1) | 32.67 ± 0.58 d | 44.67 ± 5.03 f | 36.7 ± 11.0 c | 73.3 ± 7.6 ab | 17.33 ± 3.21 a | 18.67 ± 2.08 bc | 12.94 ± 1.03 a | 10.62 ± 1.19 c | 11.83 ± 0.369 d | 10.24 ± 1.47 c | 18.06 ± 3.94 abc | 7.80 ± 2.60 f |
| P58(2) | 41.33 ± 9.07 bc | 56.33 ± 2.08 cde | 55.3 ± 11.0 ab | 78.7 ± 20.6 ab | 16.00 ± 1.00 a | 22.67 ± 6.43 abc | 13.68 ± 0.84 a | 10.17 ± 1.63 c | 13.76 ± 0.21 a | 11.42 ± 1.00 bc | 18.64 ± 4.56 abc | 8.87 ± 0.78 ef |
| P58(3) | 46.00 ± 1.73 bc | 67.00 ± 11.36 bc | 53.7 ± 7.6 ab | 94.7 ± 2.1 ab | 16.97 ± 0.90 a | 25.33 ± 0.58 ab | 14.20 ± 0.69 a | 11.86 ± 0.54 abc | 13.10 ± 0.58 ab | 12.90 ± 1.13 ab | 16.07 ± 1.70 bc | 14.22 ± 2.82 abc |
| P21(1) | 46.33 ± 2.08 bc | 60.67 ± 2.31 bcd | 55.3 ± 5.1 ab | 73.3 ± 18.8 ab | 17.90 ± 1.01 a | 26.67 ± 1.15 a | 13.97 ± 0.66 a | 10.70 ± 0.28 c | 12.98 ± 0.46 abc | 12.35 ± 1.20 ab | 20.37 ± 3.60 abc | 10.08 ± 1.99 def |
| P21(2) | 56.67 ± 4.16 a | 68.67 ± 1.53 b | 54.7 ± 10.0 ab | 102.0 ± 12.0 a | 18.13 ± 2.58 a | 20.67 ± 4.51 abc | 13.18 ± 0.58 a | 11.69 ± 1.57 abc | 13.06 ± 0.30 ab | 13.27 ± 0.03 ab | 16.77 ± 0.53 abc | 13.56 ± 2.31 bcd |
| P21(3) | 57.00 ± 5.57 a | 84.67 ± 7.57 a | 54.3 ± 7.0 ab | 101.7 ± 30.7 a | 18.60 ± 1.22 a | 20.33 ± 0.58 abc | 13.87 ± 0.67 a | 11.68 ± 0.57 abc | 12.18 ± 0.71 cd | 13.58 ± 0.94 a | 18.08 ± 1.33 abc | 12.08 ± 0.29 cde |
| P22-1(1) | 45.67 ± 2.89 bc | 65.00 ± 1.73 bc | 48.0 ± 6.1 bc | 80.7 ± 7.4 ab | 16.00 ± 1.73 a | 22.67 ± 5.51 abc | 13.17 ± 0.73 a | 10.90 ± 0.43 bc | 12.12 ± 0.41 d | 12.67 ± 0.79 ab | 14.45 ± 1.77 c | 12.67 ± 1.33 bcde |
| P22-1(2) | 47.33 ± 3.06 bc | 71.67 ± 4.93 b | 60.7 ± 5.0 ab | 79.7 ± 16.3 ab | 17.43 ± 3.02 a | 21.33 ± 1.15 abc | 13.46 ± 0.88 a | 11.87 ± 1.25 abc | 12.10 ± 0.17 d | 13.69 ± 0.96 a | 15.20 ± 0.98 bc | 13.52 ± 3.72 bcd |
| P22-1(3) | 50.00 ± 2.65 ab | 63.00 ± 2.65 bc | 66.3 ± 2.1 a | 87.0 ± 15.6 ab | 16.33 ± 3.21 a | 21.00 ± 5.20 abc | 14.31 ± 1.01 a | 11.82 ± 0.48 abc | 11.63 ± 0.35 d | 12.28 ± 0.64 ab | 14.87 ± 1.06 bc | 14.01 ± 2.62 abcd |
| Treatments | Leaf Area (cm2) | Chlorophyll (SPAD) | Seedling Index | Stem Diameter Below Cotyledons (cm) | Hypocotyl Length (cm) | First Internode Length (cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 30DAT | 45DAT | 30DAT | 45DAT | 30DAT | 45DAT | 30DAT | 45DAT | 30DAT | 45DAT | 30DAT | 45DAT | |
| RP | 1239.7 ± 347 ab | 1914.7 ± 182 cd | 58.5 ± 7.04 ab | 70.1 ± 8.55 ab | 0.16 ± 0.07 bc | 0.27 ± 0.08 d | 0.73 ± 0.15 ab | 0.93 ± 0.15 c | 1.63 ± 0.55 bc | 1.00 ± 0.00 c | 2.07 ± 0.12 b | 2.60 ± 0.53 cd |
| MSP | 1237.7 ± 147 ab | 1464.0 ± 140 d | 50.6 ± 3.59 b | 57.5 ± 9.88 b | 0.20 ± 0.03 abc | 0.21 ± 0.04 d | 0.80 ± 0.17 ab | 0.67 ± 0.21 d | 2.77 ± 0.25 ab | 1.00 ± 0.00 c | 2.56 ± 0.40 ab | 2.40 ± 0.53 d |
| P58(1) | 916.3 ± 236 b | 1632.5 ± 156 cd | 53.7 ± 2.47 ab | 68.4 ± 7.15 ab | 0.11 ± 0.05 c | 0.23 ± 0.06 d | 0.63 ± 0.20 b | 0.87 ± 0.06 cd | 1.10 ± 0.17 c | 2.67 ± 0.58 ab | 2.53 ± 0.90 ab | 3.33 ± 0.58 abc |
| P58(2) | 1431.7 ± 402 a | 1976.7 ± 188 c | 57.9 ± 8.77 ab | 78.6 ± 10.44 a | 0.25 ± 0.11 ab | 0.25 ± 0.07 d | 0.80 ± 0.10 ab | 0.90 ± 0.17 cd | 3.10 ± 1.65 a | 2.33 ± 0.58 ab | 3.57 ± 1.24 a | 3.50 ± 0.50 ab |
| P58(3) | 1311.3 ± 103 ab | 2748.5 ± 262 b | 65.6 ± 11.30 a | 69.4 ± 10.55 ab | 0.22 ± 0.04 ab | 0.51 ± 0.09 ab | 0.83 ± 0.06 ab | 1.27 ± 0.23 ab | 2.47 ± 0.42 ab | 2.87 ± 0.23 a | 3.40 ± 0.40 a | 3.60 ± 0.17 a |
| P21(1) | 1341.7 ± 149 ab | 2994.0 ± 286 b | 61.5 ± 7.65 ab | 75.4 ± 4.03 a | 0.19 ± 0.01 abc | 0.34 ± 0.04 abc | 0.77 ± 0.06 ab | 1.00 ± 0.00 bc | 2.87 ± 0.90 ab | 2.33 ± 0.58 ab | 2.87 ± 0.23 ab | 2.83 ± 0.58 abcd |
| P21(2) | 1389.7 ± 61 a | 2900.0 ± 277 b | 58.9 ± 7.48 ab | 73.5 ± 9.81 ab | 0.23 ± 0.03 ab | 0.47 ± 0.13 abc | 0.90 ± 0.10 a | 1.20 ± 0.10 ab | 2.23 ± 0.25 abc | 2.33 ± 0.58 ab | 3.50 ± 0.00 a | 2.70 ± 0.17 bcd |
| P21(3) | 1514.0 ± 99 a | 3147.7 ± 300 b | 53.4 ± 0.21 ab | 71.4 ± 3.51 ab | 0.22 ± 0.05 ab | 0.48 ± 0.02 abc | 0.92 ± 0.03 a | 1.30 ± 0.10 a | 2.17 ± 0.29 abc | 2.07 ± 0.06 b | 2.83 ± 0.29 ab | 3.00 ± 0.00 abcd |
| P22-1(1) | 1356.0 ± 312 ab | 3704.0 ± 482 a | 59.1 ± 5.40 ab | 70.3 ± 7.16 ab | 0.21 ± 0.05 ab | 0.47 ± 0.08 abc | 0.77 ± 0.15 ab | 1.20 ± 0.10 ab | 2.53 ± 0.50 ab | 2.37 ± 0.35 ab | 3.07 ± 1.01 ab | 2.83 ± 0.29 abcd |
| P22-1(2) | 1494.7 ± 126 a | 3260.7 ± 311 ab | 59.2 ± 7.57 ab | 75.3 ± 12.09 a | 0.27 ± 0.06 a | 0.44 ± 0.13 bc | 0.90 ± 0.10 a | 1.23 ± 0.21 ab | 2.07 ± 0.86 abc | 2.43 ± 0.40 ab | 2.77 ± 0.32 ab | 3.27 ± 0.46 abc |
| P22-1(3) | 1346.7 ± 251 ab | 2866.5 ± 273 b | 62.5 ± 2.40 ab | 73.9 ± 5.15 ab | 0.21 ± 0.03 ab | 0.61 ± 0.07 a | 0.90 ± 0.10 a | 1.37 ± 0.06 a | 2.80 ± 0.52 ab | 2.03 ± 0.06 b | 2.53 ± 0.15 ab | 3.30 ± 0.52 abc |
| Treatments | Net Assimilation Rate (NAR) (mg cm2 d−1) | Relative Growth Rate (RGR) (mg g−1 d−1) |
|---|---|---|
| RP | 0.25 ± 0.16 cd | 0.03 ± 0.02 ab |
| MSP | 0.27 ± 0.02 bcd | 0.04 ± 0.01 ab |
| P58(1) | 0.33 ± 0.18 abcd | 0.05 ± 0.03 a |
| P58(2) | 0.14 ± 0.11 d | 0.02 ± 0.02 b |
| P58(3) | 0.51 ± 0.22 abc | 0.05 ± 0.02 a |
| P21(1) | 0.30 ± 0.08 abcd | 0.04 ± 0.01 ab |
| P21(2) | 0.41 ± 0.16 abc | 0.04 ±0.02 ab |
| P21(3) | 0.54 ± 0.15 ab | 0.06 ± 0.01 a |
| P22-1(1) | 0.37 ± 0.07 abcd | 0.05 ± 0.01 ab |
| P22-1(2) | 0.32 ± 0.18 abcd | 0.04 ± 0.02 ab |
| P22-1(3) | 0.56 ± 0.07 a | 0.06 ± 0.01 a |
| Treatment | P-Uptake (mg plant−1) | |||
|---|---|---|---|---|
| Leaf | Stem | Root | Total Uptake | |
| RP | 15.1 ± 0.08 e | 17.6 ± 0.05 e | 16.6 ± 0.12 abc | 49.2 ± 0.19 d |
| MSP | 19.2 ± 0.22 abcd | 22.3 ± 0.33 abcd | 19.4 ± 0.17 ab | 60.8 ± 0.72 ab |
| P58(1) | 19.6 ± 0.26 abcd | 18.7 ± 0.30 de | 12.0 ± 0.42 cde | 50.3 ± 0.90 cd |
| P58(2) | 19.7 ± 0.29 abcd | 20.5 ± 0.14 cde | 10.2 ± 0.07 e | 50.4 ± 0.41 cd |
| P58(3) | 22.3 ± 0.14 a | 22.0 ± 0.23 abcd | 19.7 ± 0.35 a | 64.0 ± 0.25 a |
| P21(1) | 16.7 ± 0.07 de | 22.3 ± 0.25 abcd | 11.6 ± 0.24 de | 50.6 ± 0.54 cd |
| P21(2) | 19.8 ± 0.24 abcd | 25.2 ± 0.05 ab | 14.8 ± 0.22 bcde | 59.8 ± 0.41 abc |
| P21(3) | 18.7 ± 0.12 bcd | 25.6 ± 0.23 a | 15.6 ± 0.06 abcd | 59.9 ± 0.38 abc |
| P22-1(1) | 18.6 ± 0.04 cd | 22.8 ± 0.15 abc | 12.0 ± 0.11 cde | 53.4 ± 0.12 bcd |
| P22-1(2) | 22.1 ± 0.19 ab | 21.4 ± 0.11 bcd | 11.8 ± 0.31 de | 55.3 ± 0.53 abcd |
| P22-1(3) | 20.4 ± 0.12 abc | 22.7 ± 0.16 abc | 17.0 ± 0.35 ab | 60.0 ± 0.62 abc |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, A.F.; Abdelmageed, A.H.A.; Al-Turki, A.; Aggag, A.M.; Rehan, M.; Abdelhameid, N.M. Assessment of the Plant Growth-Promoting Potential of Three Pseudomonas and Pantoea Isolates to Promote Pepper Growth. Agronomy 2025, 15, 2419. https://doi.org/10.3390/agronomy15102419
Omar AF, Abdelmageed AHA, Al-Turki A, Aggag AM, Rehan M, Abdelhameid NM. Assessment of the Plant Growth-Promoting Potential of Three Pseudomonas and Pantoea Isolates to Promote Pepper Growth. Agronomy. 2025; 15(10):2419. https://doi.org/10.3390/agronomy15102419
Chicago/Turabian StyleOmar, Ayman F., Adil H. A. Abdelmageed, Ahmad Al-Turki, Ahmed M. Aggag, Medhat Rehan, and Noha M. Abdelhameid. 2025. "Assessment of the Plant Growth-Promoting Potential of Three Pseudomonas and Pantoea Isolates to Promote Pepper Growth" Agronomy 15, no. 10: 2419. https://doi.org/10.3390/agronomy15102419
APA StyleOmar, A. F., Abdelmageed, A. H. A., Al-Turki, A., Aggag, A. M., Rehan, M., & Abdelhameid, N. M. (2025). Assessment of the Plant Growth-Promoting Potential of Three Pseudomonas and Pantoea Isolates to Promote Pepper Growth. Agronomy, 15(10), 2419. https://doi.org/10.3390/agronomy15102419









