Humic Acid Enhances the Soil Amelioration Effect of Biochar on Saline–Alkali Soils in Cotton Fields
Abstract
1. Introduction
2. Materials and Methods
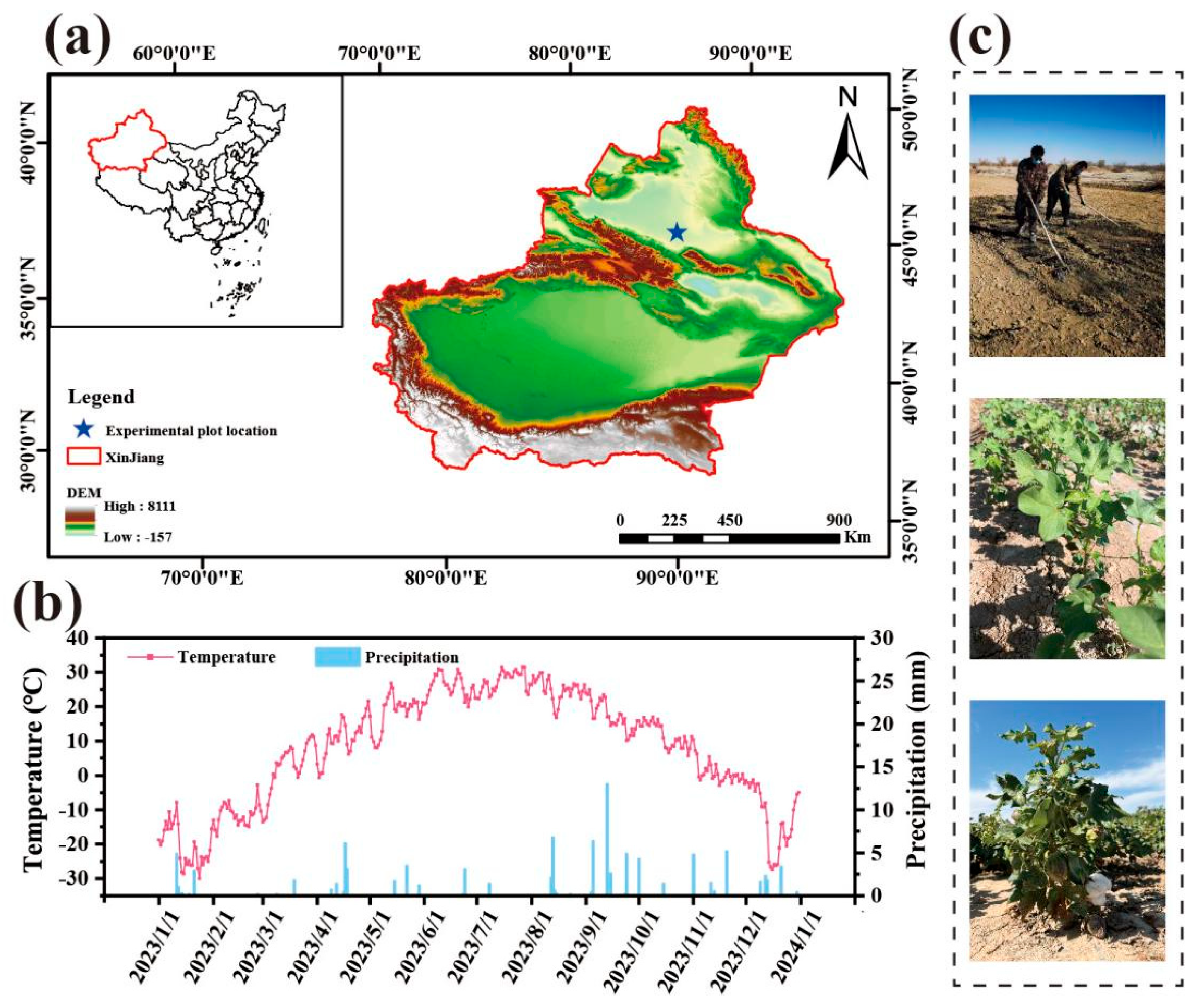
2.1. Site Description
2.2. Experimental Design and Soil Sampling
- (1)
- CK: Control (no amendment);
- (2)
- HUM: HA only (0.6 t ha−1);
- (3)
- SBC: Walnut shell biochar only (12 t ha−1);
- (4)
- MBC: Livestock manure biochar only (12 t ha−1);
- (5)
- H-SBC: Co-application of HA and walnut shell biochar (0.6 t ha−1 + 12 t ha−1);
- (6)
- H-MBC: Co-application of HA and livestock manure biochar (0.6 t ha−1 + 12 t ha−1).
2.3. Sample Analysis
2.4. Statistical Analysis
3. Results
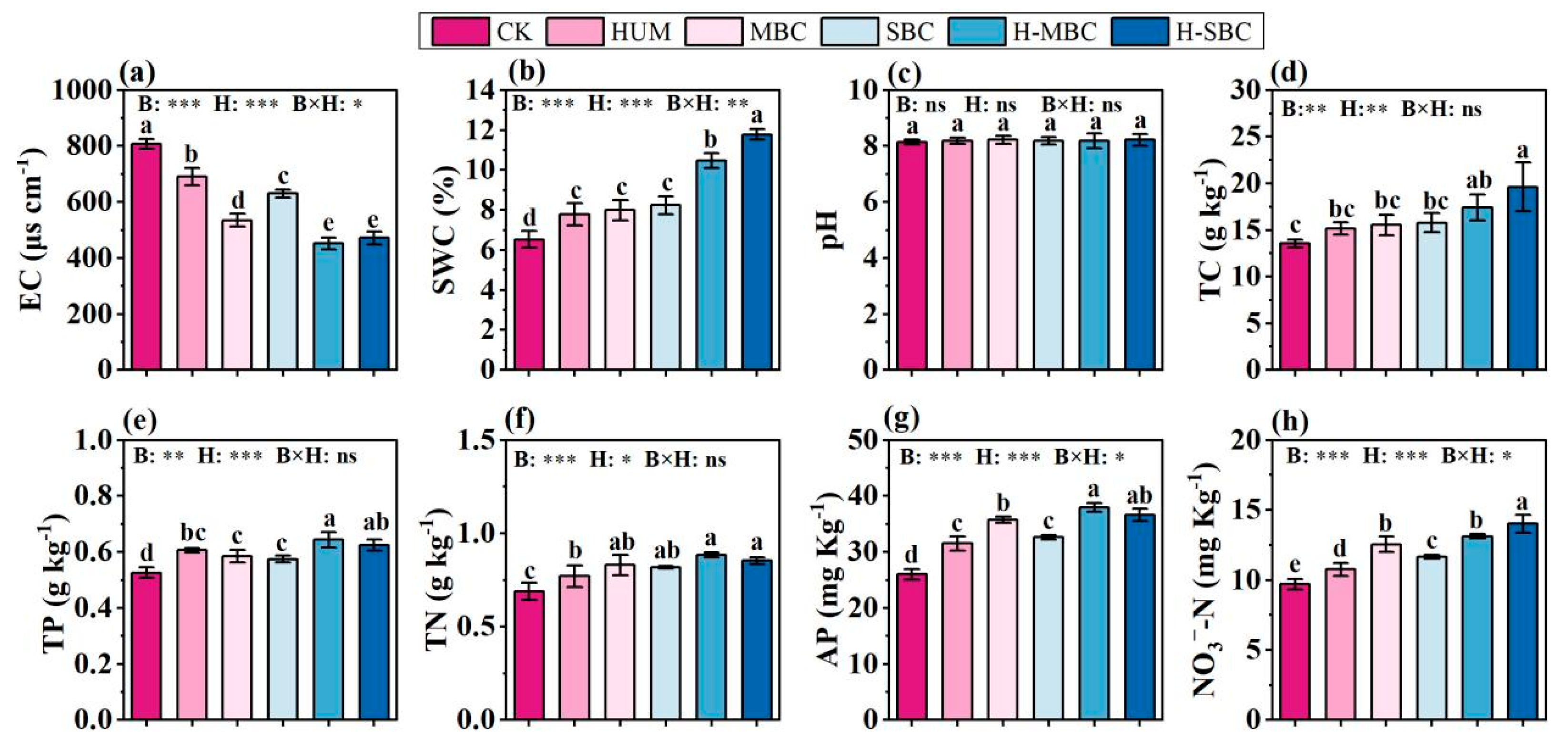
3.1. Response of Soil Physicochemical Properties
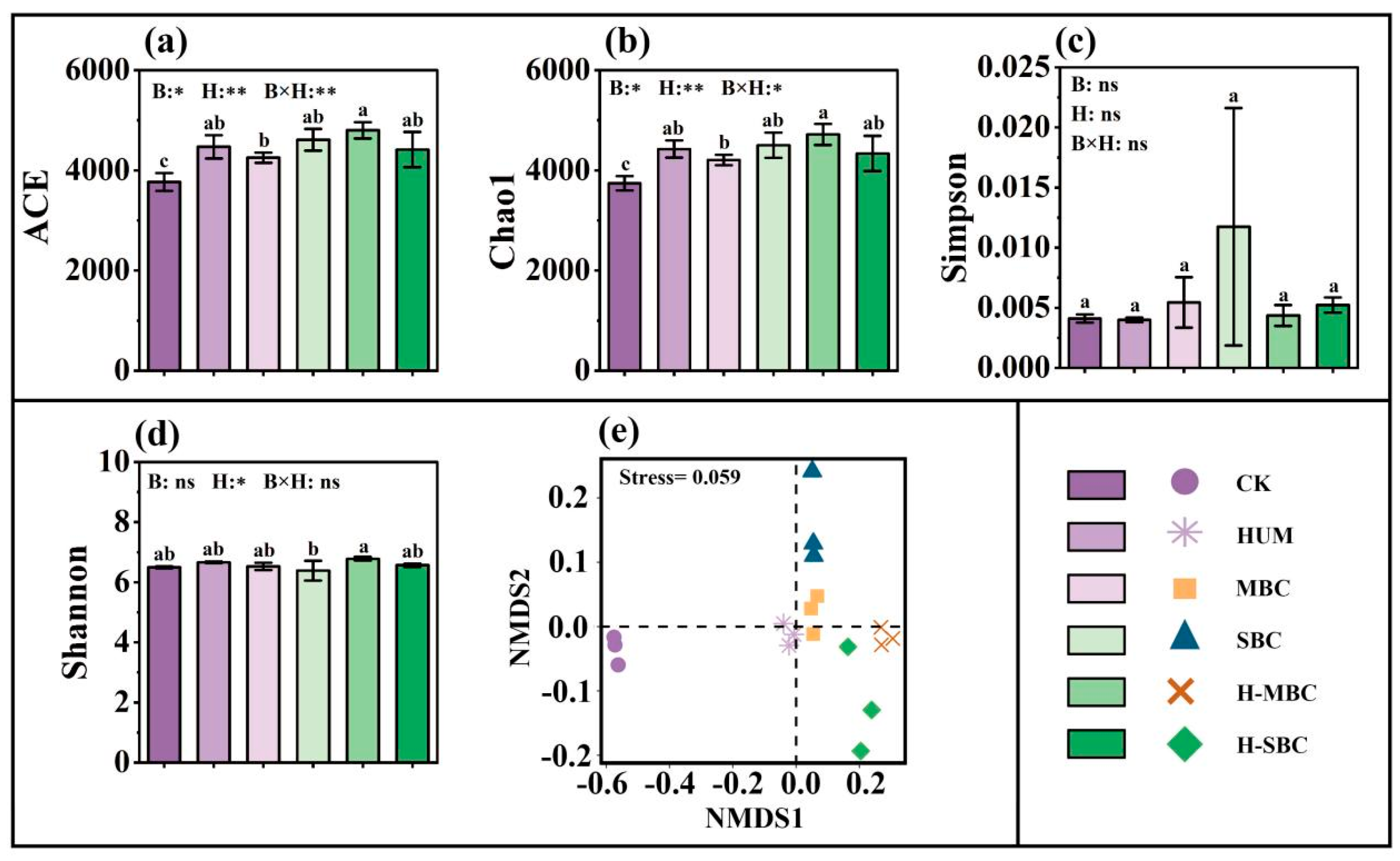
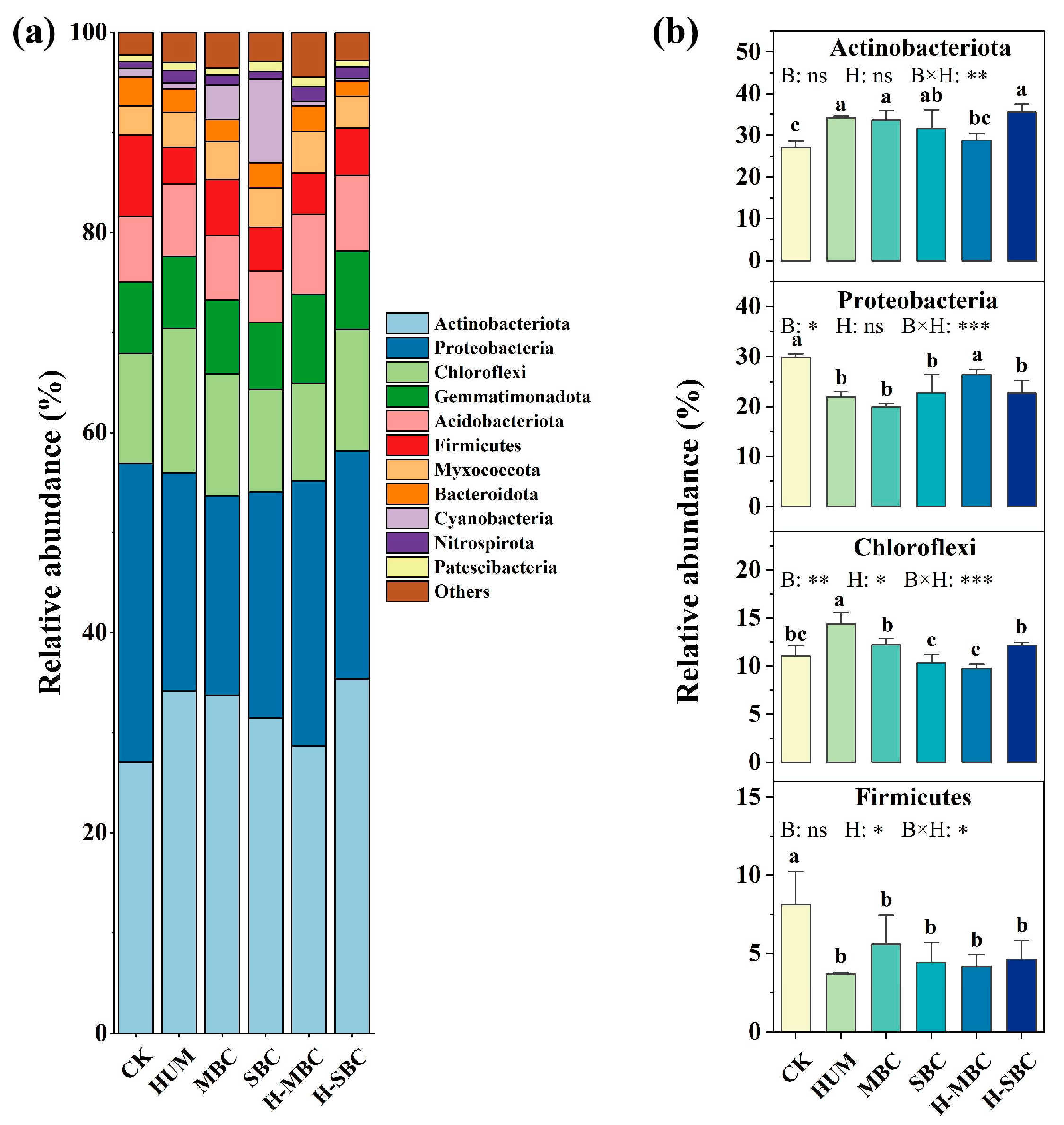
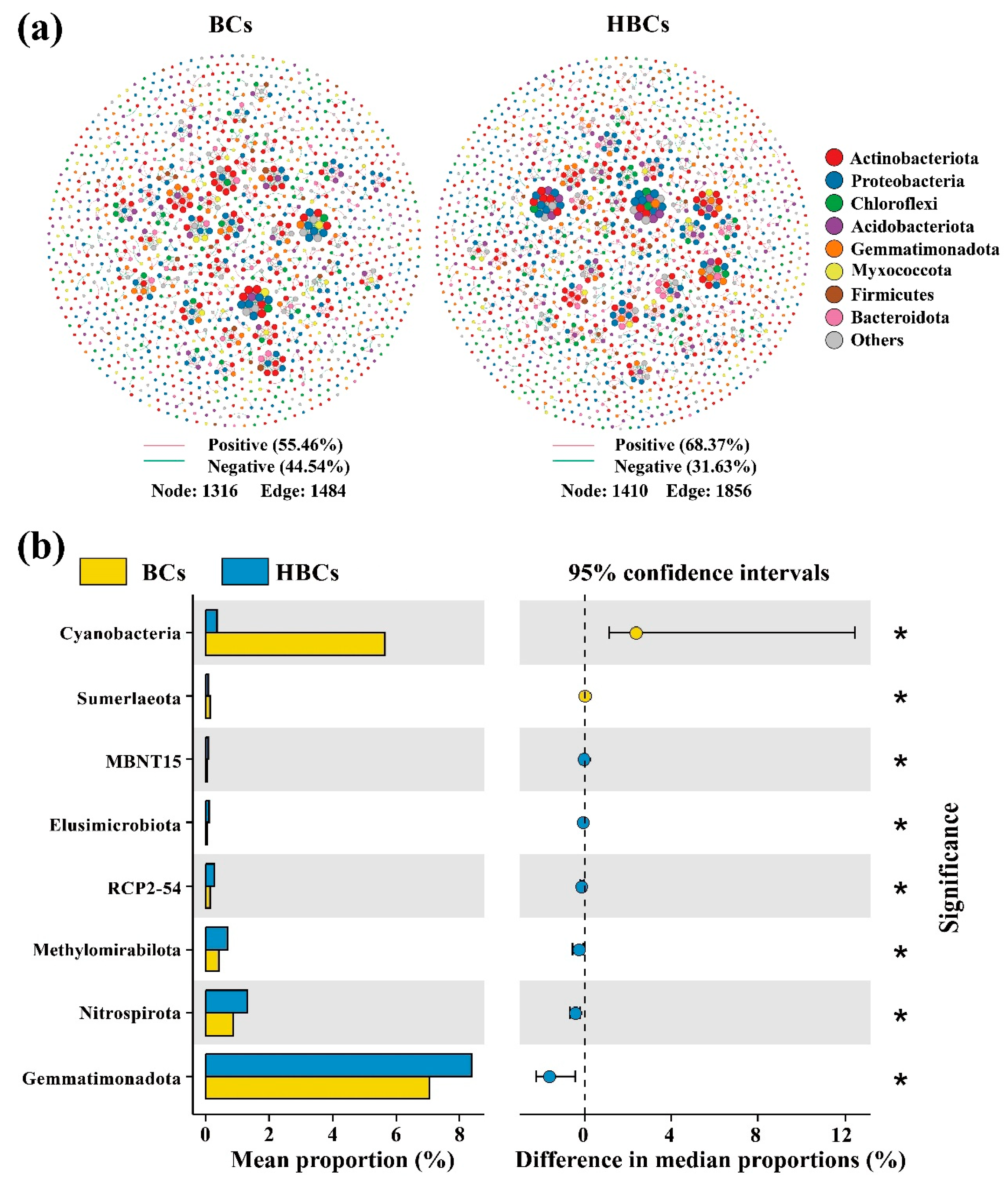
3.2. Changes in Bacterial Community Diversity and Taxa Composition
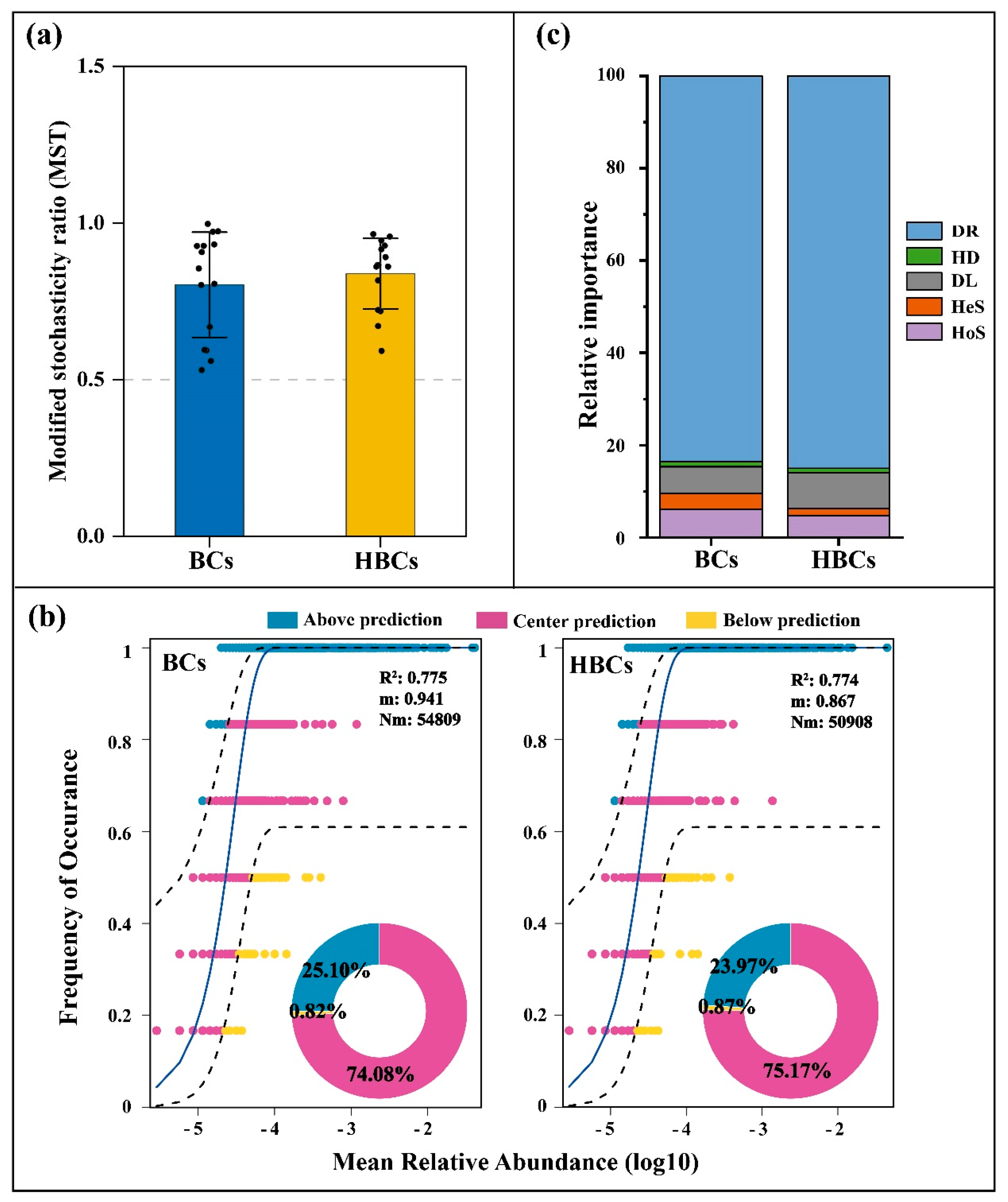
3.3. Characteristics of Bacterial Community Assembly Processes
3.4. Characteristics of the Bacterial Co-Occurrence Network and Keystone Taxa
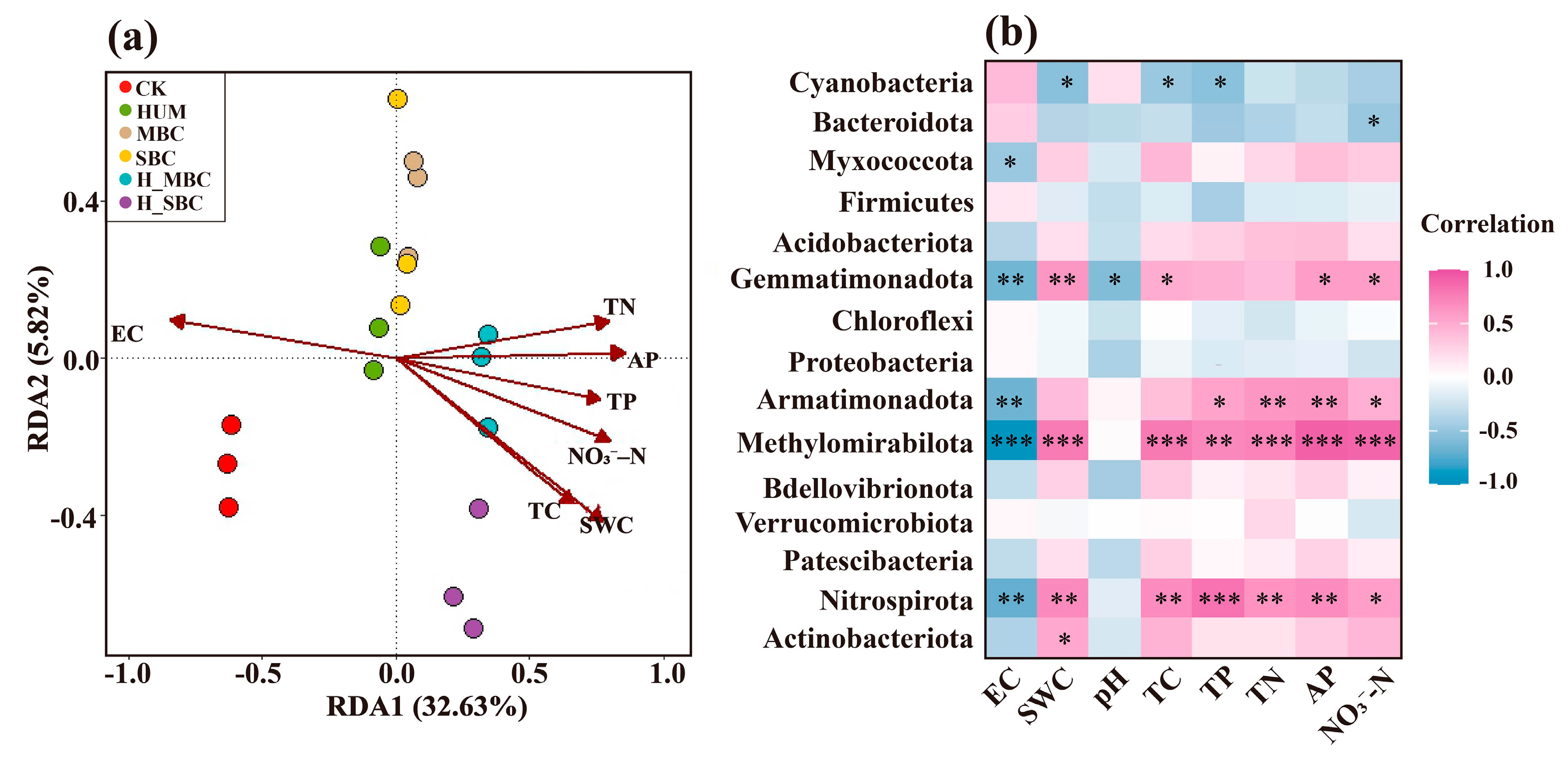
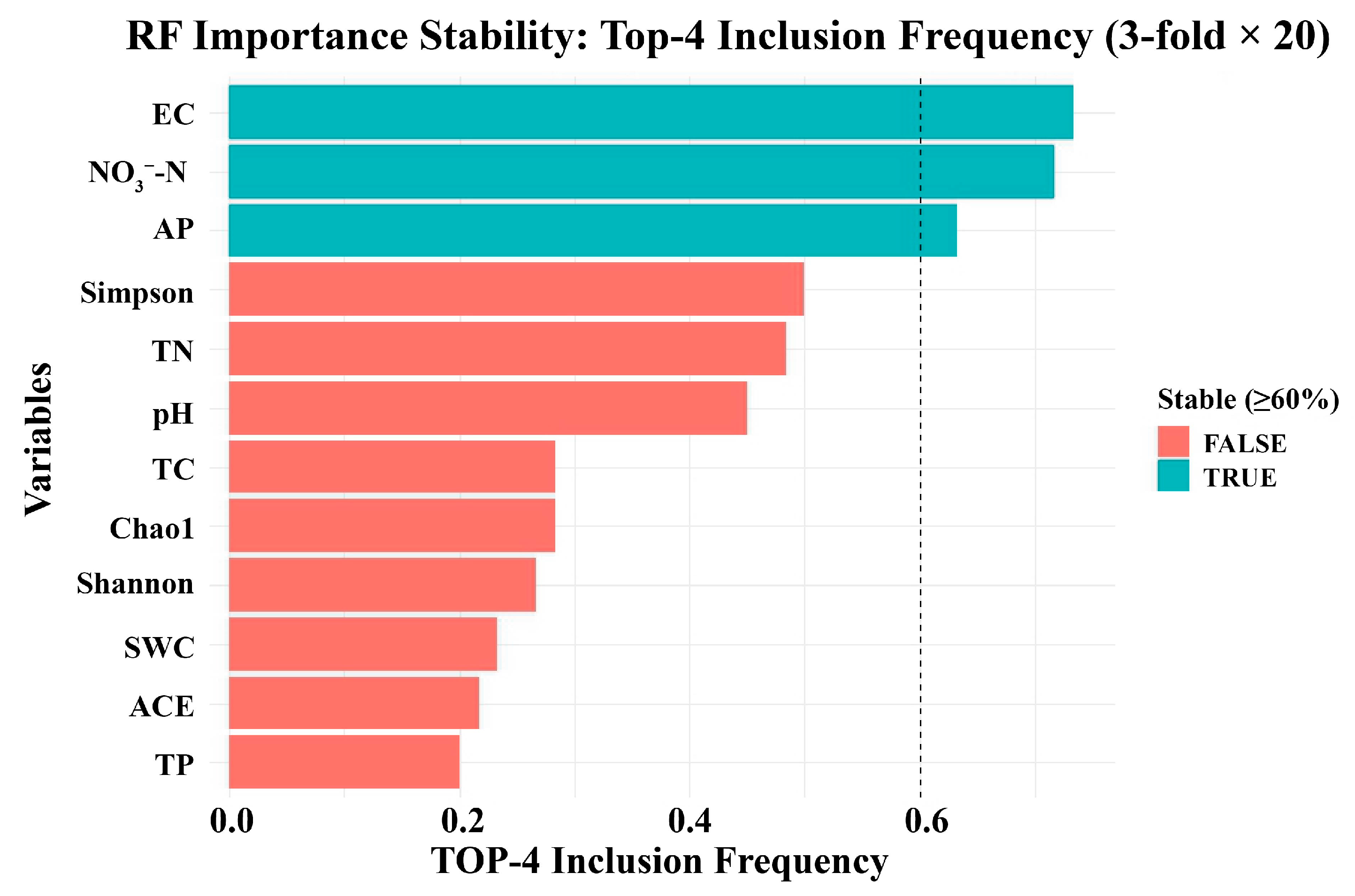
3.5. Key Environmental Drivers of the Bacterial Community
4. Discussion
4.1. Effect of Biochar from Different Feedstock Combined with Humic Acid on Soil Properties
4.2. Regulatory Mechanisms of Soil Amendment Strategies on Microbial Ecological Processes and Co-Occurrence Networks
4.3. Mechanisms of Soil Salinity and Nutrient Supply on Microbial Communities
4.4. Limitations and Implications
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zaman, M.; Shahid, S.A.; Heng, L.; Shahid, S.A.; Zaman, M.; Heng, L. Soil salinity: Historical perspectives and a world overview of the problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer: Cham, Switherland, 2018; pp. 43–53. [Google Scholar]
- Xiong, J.; Ge, X.; Ding, J.; Wang, J.; Zhang, Z.; Zhu, C.; Han, L.; Wang, J. Optimal time-window for assessing soil salinity via Sentinel-2 multitemporal synthetic data in the arid agricultural regions of China. Ecol. Indic. 2025, 176, 113642. [Google Scholar] [CrossRef]
- Khan, M.A.; Wahid, A.; Ahmad, M.; Tahir, M.T.; Ahmed, M.; Ahmad, S.; Hasanuzzaman, M. World cotton production and consumption: An overview. In Cotton Production and Uses: Agronomy, Crop Protection, and Postharvest Technologies; Springer: Singapore, 2020; pp. 1–7. [Google Scholar]
- Zhang, H.; Li, D.; Zhou, Z.; Zahoor, R.; Chen, B.; Meng, Y. Soil water and salt affect cotton (Gossypium hirsutum L.) photosynthesis, yield and fiber quality in coastal saline soil. Agric. Water Manag. 2017, 187, 112–121. [Google Scholar] [CrossRef]
- Ashraf, M. Salt tolerance of cotton: Some new advances. Crit. Rev. Plant Sci. 2002, 21, 1–30. [Google Scholar] [CrossRef]
- Dai, J.; Dong, H. Intensive cotton farming technologies in China: Achievements, challenges and countermeasures. Field Crops Res. 2014, 155, 99–110. [Google Scholar] [CrossRef]
- Yang, G.; Li, F.; Tian, L.; He, X.; Gao, Y.; Wang, Z.; Ren, F. Soil physicochemical properties and cotton (Gossypium hirsutum L.) yield under brackish water mulched drip irrigation. Soil Tillage Res. 2020, 199, 104592. [Google Scholar] [CrossRef]
- Paranychianakis, N.; Chartzoulakis, K. Irrigation of Mediterranean crops with saline water: From physiology to management practices. Agric. Ecosyst. Environ. 2005, 106, 171–187. [Google Scholar] [CrossRef]
- Chen, L.; Li, C.; Feng, Q.; Wei, Y.; Zhao, Y.; Zhu, M.; Deo, R.C. Direct and indirect impacts of ionic components of saline water on irrigated soil chemical and microbial processes. Catena 2019, 172, 581–589. [Google Scholar] [CrossRef]
- Xiao, C.; Ji, Q.; Zhang, F.; Li, Y.; Fan, J.; Hou, X.; Yan, F.; Liu, X.; Gong, K. Effects of various soil water potential thresholds for drip irrigation on soil salinity, seed cotton yield and water productivity of cotton in northwest China. Agric. Water Manag. 2023, 279, 108172. [Google Scholar] [CrossRef]
- Sharif, I.; Aleem, S.; Farooq, J.; Rizwan, M.; Younas, A.; Sarwar, G.; Chohan, S.M. Salinity stress in cotton: Effects, mechanism of tolerance and its management strategies. Physiol. Mol. Biol. Plants 2019, 25, 807–820. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.A.; Sarkhosh, A.; Khan, N.; Balal, R.M.; Ali, S.; Rossi, L.; Gómez, C.; Mattson, N.; Nasim, W.; Garcia-Sanchez, F. Insights into the physiological and biochemical impacts of salt stress on plant growth and development. Agronomy 2020, 10, 938. [Google Scholar] [CrossRef]
- Yupeng, W.; Yufei, L.; Yanmeng, B.; Zhenjun, S. Responses of saline soil properties and cotton growth to different organic amendments. Pedosphere 2018, 28, 521–529. [Google Scholar] [CrossRef]
- El-Sayed, M.E.; Hazman, M.; Abd El-Rady, A.G.; Almas, L.; McFarland, M.; Shams El Din, A.; Burian, S. Biochar reduces the adverse effect of saline water on soil properties and wheat production profitability. Agriculture 2021, 11, 1112. [Google Scholar] [CrossRef]
- Kul, R.; Arjumend, T.; Ekinci, M.; Yildirim, E.; Turan, M.; Argin, S. Biochar as an organic soil conditioner for mitigating salinity stress in tomato. Soil Sci. Plant Nutr. 2021, 67, 693–706. [Google Scholar] [CrossRef]
- Ampong, K.; Thilakaranthna, M.S.; Gorim, L.Y. Understanding the role of humic acids on crop performance and soil health. Front. Agron. 2022, 4, 848621. [Google Scholar] [CrossRef]
- Tahoun, A.M.A.; El-Enin, M.M.A.; Mancy, A.G.; Sheta, M.H.; Shaaban, A. Integrative soil application of humic acid and foliar plant growth stimulants improves soil properties and wheat yield and quality in nutrient-poor sandy soil of a semiarid region. J. Soil Sci. Plant Nutr. 2022, 22, 2857–2871. [Google Scholar] [CrossRef]
- Singh Rawat, V.; Kaur, J.; Bhagwat, S.; Arora Pandit, M.; Dogra Rawat, C. Deploying microbes as drivers and indicators in ecological restoration. Restor. Ecol. 2023, 31, e13688. [Google Scholar] [CrossRef]
- Philippot, L.; Chenu, C.; Kappler, A.; Rillig, M.C.; Fierer, N. The interplay between microbial communities and soil properties. Nat. Rev. Microbiol. 2024, 22, 226–239. [Google Scholar] [CrossRef] [PubMed]
- Canfora, L.; Salvati, L.; Benedetti, A.; Francaviglia, R. Is soil microbial diversity affected by soil and groundwater salinity? Evidences from a coastal system in central Italy. Environ. Monit. Assess. 2017, 189, 319. [Google Scholar] [CrossRef]
- Rath, K.M.; Murphy, D.N.; Rousk, J. The microbial community size, structure, and process rates along natural gradients of soil salinity. Soil Biol. Biochem. 2019, 138, 107607. [Google Scholar] [CrossRef]
- Hou, Y.; Zeng, W.; Hou, M.; Wang, Z.; Luo, Y.; Lei, G.; Zhou, B.; Huang, J. Responses of the soil microbial community to salinity stress in maize fields. Biology 2021, 10, 1114. [Google Scholar] [CrossRef]
- Shah, G.; Jan, M.; Afreen, M.; Anees, M.; Rehman, S.; Daud, M.; Malook, I.; Jamil, M. Halophilic bacteria mediated phytoremediation of salt-affected soils cultivated with rice. J. Geochem. Explor. 2017, 174, 59–65. [Google Scholar] [CrossRef]
- Mao, T.; Wang, Y.; Ning, S.; Mao, J.; Sheng, J.; Jiang, P. Assessment of the effects of biochar on the physicochemical properties of saline–alkali soil based on meta-analysis. Agronomy 2024, 14, 2431. [Google Scholar] [CrossRef]
- Mao, X.; Yang, Y.; Guan, P.; Geng, L.; Ma, L.; Di, H.; Liu, W.; Li, B. Remediation of organic amendments on soil salinization: Focusing on the relationship between soil salts and microbial communities. Ecotoxicol. Environ. Saf. 2022, 239, 113616. [Google Scholar] [CrossRef]
- Li, R.; Tao, R.; Ling, N.; Chu, G. Chemical, organic and bio-fertilizer management practices effect on soil physicochemical property and antagonistic bacteria abundance of a cotton field: Implications for soil biological quality. Soil Tillage Res. 2017, 167, 30–38. [Google Scholar] [CrossRef]
- Naz, I.; Bano, A. Biochemical, molecular characterization and growth promoting effects of phosphate solubilizing Pseudomonas sp. isolated from weeds grown in salt range of Pakistan. Plant Soil 2010, 334, 199–207. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Raihan, M.R.H.; Nowroz, F.; Fujita, M. Insight into the mechanism of salt-induced oxidative stress tolerance in soybean by the application of Bacillus subtilis: Coordinated actions of osmoregulation, ion homeostasis, antioxidant defense, and methylglyoxal detoxification. Antioxidants 2022, 11, 1856. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. Diversity of halophilic microorganisms: Environments, phylogeny, physiology, and applications. J. Ind. Microbiol. Biotechnol. 2002, 28, 56–63. [Google Scholar] [CrossRef]
- Giller, K.E.; Cadisch, G. Driven by Nature: A Sense of Arrival and Departure. In Driven by Nature: Plant Litter Quality and Decomposition; Cadisch, G., Giller, K.E., Eds.; CAB International: Wallingford, UK, 1997; pp. 393–399. [Google Scholar]
- Chiba, A.; Uchida, Y.; Kublik, S.; Vestergaard, G.; Buegger, F.; Schloter, M.; Schulz, S. Soil bacterial diversity is positively correlated with decomposition rates during early phases of maize litter decomposition. Microorganisms 2021, 9, 357. [Google Scholar] [CrossRef]
- Wu, M.; Feng, Q.; Sun, X.; Wang, H.; Gielen, G.; Wu, W. Rice (Oryza sativa L) plantation affects the stability of biochar in paddy soil. Sci. Rep. 2015, 5, 10001. [Google Scholar] [CrossRef]
- Rechberger, M.V.; Kloss, S.; Rennhofer, H.; Tintner, J.; Watzinger, A.; Soja, G.; Lichtenegger, H.; Zehetner, F. Changes in biochar physical and chemical properties: Accelerated biochar aging in an acidic soil. Carbon 2017, 115, 209–219. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef]
- Bao, S. Soil and Agricultural Chemistry Analysis; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Behrendt, L.; Larkum, A.W.; Trampe, E.; Norman, A.; Sørensen, S.J.; Kühl, M. Microbial diversity of biofilm communities in microniches associated with the didemnid ascidian Lissoclinum patella. ISME J. 2012, 6, 1222–1237. [Google Scholar] [CrossRef]
- Ning, D.; Deng, Y.; Tiedje, J.M.; Zhou, J. A general framework for quantitatively assessing ecological stochasticity. Proc. Natl. Acad. Sci. USA 2019, 116, 16892–16898. [Google Scholar] [CrossRef] [PubMed]
- Sloan, W.T.; Lunn, M.; Woodcock, S.; Head, I.M.; Nee, S.; Curtis, T.P. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 2006, 8, 732–740. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Yuan, M.; Wu, L.; Zhang, Y.; Guo, X.; Zhou, X.; Yang, Y.; Arkin, A.P.; Firestone, M.K.; Zhou, J. A quantitative framework reveals ecological drivers of grassland microbial community assembly in response to warming. Nat. Commun. 2020, 11, 4717. [Google Scholar] [CrossRef]
- Li, T.; Wang, S.; Liu, S.; Zhang, X.; Dong, H.; Dai, S.; Chai, L.; Li, H.; Lv, Y.; Li, T. Trade-offs of organic amendment input on soil quality and crop productivity in saline-alkali land globally: A meta-analysis. Eur. J. Agron. 2025, 164, 127471. [Google Scholar] [CrossRef]
- Wang, X.; Ding, J.; Wang, J.; Han, L.; Tan, J.; Ge, X. Ameliorating saline-sodic soils: A global meta-analysis of field studies on the influence of exogenous amendments on crop yield. Land Degrad. Dev. 2024, 35, 3330–3343. [Google Scholar] [CrossRef]
- Malik, Z.; Malik, N.; Noor, I.; Kamran, M.; Parveen, A.; Ali, M.; Sabir, F.; Elansary, H.O.; El-Abedin, T.K.Z.; Mahmoud, E.A. Combined effect of rice-straw biochar and humic acid on growth, antioxidative capacity, and ion uptake in maize (Zea mays L.) grown under saline soil conditions. J. Plant Growth Regul. 2023, 42, 3211–3228. [Google Scholar] [CrossRef]
- Hammerschmiedt, T.; Holatko, J.; Pecina, V.; Huska, D.; Latal, O.; Kintl, A.; Radziemska, M.; Muhammad, S.; Gusiatin, Z.M.; Kolackova, M. Assessing the potential of biochar aged by humic substances to enhance plant growth and soil biological activity. Chem. Biol. Technol. Agric. 2021, 8, 46. [Google Scholar]
- Hasanuzzaman, M.; Nowroz, F.; Raihan, M.R.H.; Siddika, A.; Alam, M.M.; Prasad, P.V. Application of biochar and humic acid improves the physiological and biochemical processes of rice (Oryza sativa L.) in conferring plant tolerance to arsenic-induced oxidative stress. Environ. Sci. Pollut. Res. 2024, 31, 1562–1575. [Google Scholar] [CrossRef]
- Singh, C.; Tiwari, S.; Gupta, V.K.; Singh, J.S. The effect of rice husk biochar on soil nutrient status, microbial biomass and paddy productivity of nutrient poor agriculture soils. Catena 2018, 171, 485–493. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota–a review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Ni, H.; Zhao, J.; Yang, Z. Effects of compound fertilizer decrement and water-soluble humic acid fertilizer application on soil properties, bacterial community structure, and shoot yield in Lei Bamboo (Phyllostachys praecox) plantations in subtropical China. Forests 2024, 15, 400. [Google Scholar] [CrossRef]
- Guo, Y.; Ma, Z.; Ren, B.; Zhao, B.; Liu, P.; Zhang, J. Effects of humic acid added to controlled-release fertilizer on summer maize yield, nitrogen use efficiency and greenhouse gas emission. Agriculture 2022, 12, 448. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, S.; Song, Y.; Wang, X.; Jin, F.J.S. Biochar application reduces saline–alkali stress by improving soil functions and regulating the diversity and abundance of soil bacterial community in highly saline–alkali paddy field. Sustainability 2024, 16, 1001. [Google Scholar] [CrossRef]
- Gabhane, J.W.; Bhange, V.P.; Patil, P.D.; Bankar, S.T.; Kumar, S. Recent trends in biochar production methods and its application as a soil health conditioner: A review. SN Applied Sciences 2020, 2, 1307. [Google Scholar] [CrossRef]
- Yao, T.; Zhang, W.; Gulaqa, A.; Cui, Y.; Zhou, Y.; Weng, W.; Wang, X.; Liu, Q.; Jin, F.; Nutrition, P. Effects of peanut shell biochar on soil nutrients, soil enzyme activity, and rice yield in heavily saline-sodic paddy field. J. Soil Sci. Plant Nutr. 2021, 21, 655–664. [Google Scholar] [CrossRef]
- Sun, J.; He, F.; Shao, H.; Zhang, Z.; Xu, G. Effects of biochar application on Suaeda salsa growth and saline soil properties. Environ. Earth Sci. 2016, 75, 630. [Google Scholar] [CrossRef]
- Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total. Environ. 2018, 625, 320–335. [Google Scholar] [CrossRef]
- Wang, X.; Ding, J.; Han, L.; Tan, J.; Ge, X.; Nan, Q.J.G. Biochar addition reduces salinity in salt-affected soils with no impact on soil pH: A meta-analysis. Geoderma 2024, 443, 116845. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Solanki, M.K.; Solanki, A.C.; Kumari, B.; Kashyap, B.K.; Singh, R.K. Plant and soil-associated biofilm-forming bacteria: Their role in green agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Biofilms; Elsevier: Amsterdam, The Netherlands, 2020; pp. 151–164. [Google Scholar]
- Joseph, S.; Cowie, A.L.; Van Zwieten, L.; Bolan, N.; Budai, A.; Buss, W.; Cayuela, M.L.; Graber, E.R.; Ippolito, J.A.; Kuzyakov, Y. How biochar works, and when it doesn’t: A review of mechanisms controlling soil and plant responses to biochar. Gcb Bioenergy 2021, 13, 1731–1764. [Google Scholar] [CrossRef]
- Wu, B.; Ding, M.; Zhang, H.; Devlin, A.T.; Wang, P.; Chen, L.; Zhang, Y.; Xia, Y.; Wen, J.; Liu, L. Reduced soil multifunctionality and microbial network complexity in degraded and revegetated alpine meadows. J. Environ. Manag. 2023, 343, 118182. [Google Scholar] [CrossRef]
- Shen, H.; Wang, B.; Jiao, Y.; Zhang, X.; Zhang, Q.; Xiong, Z. Bacteria are more sensitive than fungi to soil fertility in an intensive vegetable field. Appl. Soil Ecol. 2023, 190, 105003. [Google Scholar] [CrossRef]
- Shu, X.; He, J.; Zhou, Z.; Xia, L.; Hu, Y.; Zhang, Y.; Zhang, Y.; Luo, Y.; Chu, H.; Liu, W. Organic amendments enhance soil microbial diversity, microbial functionality and crop yields: A meta-analysis. Sci. Total Environ. 2022, 829, 154627. [Google Scholar] [CrossRef]
- Lu, C.; Zhang, Z.; Guo, P.; Wang, R.; Liu, T.; Luo, J.; Hao, B.; Wang, Y.; Guo, W. Synergistic mechanisms of bioorganic fertilizer and AMF driving rhizosphere bacterial community to improve phytoremediation efficiency of multiple HMs-contaminated saline soil. Sci. Total Environ. 2023, 883, 163708. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, B.; Ning, D.; Zhang, Y.; Dai, T.; Wu, L.; Li, T.; Liu, W.; Zhou, J.; Wen, X. Seasonal dynamics of the microbial community in two full-scale wastewater treatment plants: Diversity, composition, phylogenetic group based assembly and co-occurrence pattern. Water Res. 2021, 200, 117295. [Google Scholar] [CrossRef] [PubMed]
- Gralka, M.; Szabo, R.; Stocker, R.; Cordero, O.X. Trophic Interactions and the Drivers of Microbial Community Assembly. Curr. Biol. 2020, 30, R1176–R1188. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, R.; Yan, W.; Zhong, Y. Divergent response of soil microbes to environmental stress change under different plant communities in the Loess Plateau. Catena 2023, 230, 107240. [Google Scholar] [CrossRef]
- Valverde, A.; Makhalanyane, T.P.; Cowan, D.A. Contrasting assembly processes in a bacterial metacommunity along a desiccation gradient. Front. Microbiol. 2014, 5, 668. [Google Scholar] [CrossRef]
- Wang, Y.-X.; Liu, X.-Y.; Di, H.-H.; He, X.-S.; Sun, Y.; Xiang, S.; Huang, Z.-B. The mechanism of microbial community succession and microbial co-occurrence network in soil with compost application. Sci. Total Environ. 2024, 906, 167409. [Google Scholar] [CrossRef]
- Xu, Z.; Sun, R.; He, T.; Sun, Y.; Wu, M.; Xue, Y.; Meng, F.; Wang, J. Disentangling the impact of straw incorporation on soil microbial communities: Enhanced network complexity and ecological stochasticity. Sci. Total Environ. 2023, 863, 160918. [Google Scholar]
- Zhu, L.; Luan, L.; Chen, Y.; Wang, X.; Zhou, S.; Zou, W.; Han, X.; Duan, Y.; Zhu, B.; Li, Y. Community assembly of organisms regulates soil microbial functional potential through dual mechanisms. Glob. Change Biol. 2024, 30, e17160. [Google Scholar]
- Nemergut, D.R.; Schmidt, S.K.; Fukami, T.; O’Neill, S.P.; Bilinski, T.M.; Stanish, L.F.; Knelman, J.E.; Darcy, J.L.; Lynch, R.C.; Wickey, P. Patterns and processes of microbial community assembly. Microbiol. Mol. Biol. Rev. 2013, 77, 342–356. [Google Scholar] [CrossRef]
- Huo, X.; Ren, C.; Wang, D.; Wu, R.; Wang, Y.; Li, Z.; Huang, D.; Qi, H. Microbial community assembly and its influencing factors of secondary forests in Qinling Mountains. Soil Biol. Biochem. 2023, 184, 109075. [Google Scholar] [CrossRef]
- Sritongon, N.; Sarin, P.; Theerakulpisut, P.; Riddech, N. The effect of salinity on soil chemical characteristics, enzyme activity and bacterial community composition in rice rhizospheres in Northeastern Thailand. Sci. Rep. 2022, 12, 20360. [Google Scholar] [CrossRef] [PubMed]
- Yuan, B.-C.; Li, Z.-Z.; Liu, H.; Gao, M.; Zhang, Y.-Y. Microbial biomass and activity in salt affected soils under arid conditions. Appl. Soil Ecol. 2007, 35, 319–328. [Google Scholar] [CrossRef]
- Chen, H.; Ma, K.; Huang, Y.; Fu, Q.; Qiu, Y.; Yao, Z. Significant response of microbial community to increased salinity across wetland ecosystems. Geoderma 2022, 415, 115778. [Google Scholar] [CrossRef]
- Rath, K.M.; Maheshwari, A.; Rousk, J. Linking microbial community structure to trait distributions and functions using salinity as an environmental filter. mbio 2019, 10, e1607–e1619. [Google Scholar] [CrossRef]
- Galinski, E.A.; Trüper, H.G. Microbial behaviour in salt-stressed ecosystems. FEMS Microbiol. Rev. 1994, 15, 95–108. [Google Scholar] [CrossRef]
- Wang, X.; Xu, L.; Qi, X.; Huang, J.; Han, M.; Wang, C.; Li, X.; Jiang, H. Microbial assembly and stress-tolerance mechanisms in salt-adapted plants along the shore of a Salt Lake: Implications for saline–alkaline soil remediation. Microorganisms 2025, 13, 1942. [Google Scholar] [CrossRef]
- Litchman, E.; Edwards, K.F.; Klausmeier, C.A. Microbial resource utilization traits and trade-offs: Implications for community structure, functioning, and biogeochemical impacts at present and in the future. Front. Microbiol. 2015, 6, 254. [Google Scholar] [CrossRef] [PubMed]
- Dini-Andreote, F.; de Cássia Pereira e Silva, M.; Triado-Margarit, X.; Casamayor, E.O.; Van Elsas, J.D.; Salles, J.F. Dynamics of bacterial community succession in a salt marsh chronosequence: Evidences for temporal niche partitioning. ISME J. 2014, 8, 1989–2001. [Google Scholar] [CrossRef] [PubMed]
- Menge, D.N.; Hedin, L.O.; Pacala, S.W. Nitrogen and phosphorus limitation over long-term ecosystem development in terrestrial ecosystems. PLoS ONE 2012, 7, e42045. [Google Scholar] [CrossRef]
- Wu, W.; Wang, F.; Xia, A.; Zhang, Z.; Wang, Z.; Wang, K.; Dong, J.; Li, T.; Wu, Y.; Che, R. Meta-analysis of the impacts of phosphorus addition on soil microbes. Agric. Ecosyst. Environ. 2022, 340, 108180. [Google Scholar] [CrossRef]
- Liu, L.; Gundersen, P.; Zhang, T.; Mo, J. Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol. Biochem. 2012, 44, 31–38. [Google Scholar] [CrossRef]
- Neher, D. Soil community composition and ecosystem processes: Comparing agricultural ecosystems with natural ecosystems. Agrofor. Syst. 1999, 45, 159–185. [Google Scholar] [CrossRef]
- Albornoz, F.E.; Prober, S.M.; Ryan, M.H.; Standish, R.J. Ecological interactions among microbial functional guilds in the plant-soil system and implications for ecosystem function. Plant Soil 2022, 476, 301–313. [Google Scholar] [CrossRef]
- Li, G.; Shan, Y.; Bai, Y.; Nie, W.; Wang, Q.; Zhang, J.; Liu, H.; Ding, Y.; Wang, X.; Lu, H. Synergistic Effects of Humic Acid, Biochar-Based Microbial Agent, and Vermicompost on the Dry Sowing and Wet Emergence Technology of Cotton in Saline–Alkali Soils, Xinjiang, China. Agronomy 2024, 14, 994. [Google Scholar]
- Zhao, W.; Xiao, J.; Wang, S.; Gai, X.; Chen, G. Bone biochar and humic acid improved soil quality and promoted Olea europaea growth in coastal saline soil by enhancing the stoichiometric homeostasis of nutrient elements. Biochar 2025, 7, 70. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Ding, J.; Wang, J.; Han, L.; Tan, J.; Liu, J.; Ge, X. Humic Acid Enhances the Soil Amelioration Effect of Biochar on Saline–Alkali Soils in Cotton Fields. Agronomy 2025, 15, 2412. https://doi.org/10.3390/agronomy15102412
Wang X, Ding J, Wang J, Han L, Tan J, Liu J, Ge X. Humic Acid Enhances the Soil Amelioration Effect of Biochar on Saline–Alkali Soils in Cotton Fields. Agronomy. 2025; 15(10):2412. https://doi.org/10.3390/agronomy15102412
Chicago/Turabian StyleWang, Xiao, Jianli Ding, Jinjie Wang, Lijing Han, Jiao Tan, Jingming Liu, and Xiangyu Ge. 2025. "Humic Acid Enhances the Soil Amelioration Effect of Biochar on Saline–Alkali Soils in Cotton Fields" Agronomy 15, no. 10: 2412. https://doi.org/10.3390/agronomy15102412
APA StyleWang, X., Ding, J., Wang, J., Han, L., Tan, J., Liu, J., & Ge, X. (2025). Humic Acid Enhances the Soil Amelioration Effect of Biochar on Saline–Alkali Soils in Cotton Fields. Agronomy, 15(10), 2412. https://doi.org/10.3390/agronomy15102412







