Weed Resistance to Herbicides in Mexico: A Review
Abstract
1. Introduction
2. Herbicide Resistance in Mexico by Mode of Action
2.1. Acetyl-CoA Carboxylase Inhibitors: Group 1
2.2. Acetolactate Synthase Inhibitors: Group 2
2.3. Auxin Mimics: Group 4
2.4. EPSP Synthase Inhibitors: Group 9
2.5. HPPD Inhibitors: Group 27
3. Weeds Resistant to Multiple Herbicides
3.1. Avena Fatua: Resistance to ACCase (Group 1) and ALS Inhibitors (Group 2)
3.2. Brassica Rapa: Multiple Resistance to ALS Inhibitors (Group 2), Auxin Mimics (Group 4), and EPSPS Inhibitors (Group 9)
4. Practices and Needs in Resistance Management
5. Future Challenges
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACCase | Acetyl-CoA carboxylase |
| ALS | Acetolactate synthase |
| DENs | Phenylpyrazolines |
| DIMs | Cyclohexanediones |
| EPSPS | 5-enolpyruvylshikimate-3-phosphate synthase |
| FOPs | Aryloxyphenoxypropionates |
| HPPD | 4-hydroxyphenylpyruvate dioxygenase |
| IHRWD | International Herbicide-Resistant Weed Database |
| IWM | Integrated weed management |
| MoA | Mode of Action |
| NTSR | Non-target site resistance |
| R | Resistant |
| S | Sensitive |
| T | Tolerant |
| TSR | Target site resistance |
References
- Prather, T.S.; Ditomaso, J.M.; Holt, J.S. Herbicide Resistance: Definition and Management Strategies; UC Agriculture & Natural Resources (ANR): Davis, CA, USA, 2000; pp. 1–14. [Google Scholar] [CrossRef]
- Claridge, M.F.; Den Hollander, J. The Biotype Concept and its Application to Insect Pests of Agriculture. Crop Prot. 1983, 2, 85–95. [Google Scholar] [CrossRef]
- Délye, C.; Jasieniuk, M.; Le Corre, V. Deciphering the Evolution of Herbicide Resistance in Weeds. Trends Genet. 2013, 29, 649–658. [Google Scholar] [CrossRef]
- Manuchehri, M. Herbicide How-To: Understanding Herbicide Mode of Action|Oklahoma State University. Available online: https://extension.okstate.edu/fact-sheets/herbicide-how-to-understanding-herbicide-mode-of-action.html (accessed on 8 May 2025).
- Bagale, S. Modes of Herbicide Action. In New Insights in Herbicide Science; Ferreira Mendes, K., Ed.; IntechOpen: London, UK, 2023; pp. 1–12. [Google Scholar] [CrossRef]
- Gaines, T.A.; Duke, S.O.; Morran, S.; Rigon, C.A.G.; Tranel, P.J.; Küpper, A.; Dayan, F.E. Mechanisms of Evolved Herbicide Resistance. J. Biol. Chem. 2020, 295, 10307–10330. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.P.; Tranel, P.J. Target-Site Mutations Conferring Herbicide Resistance. Plants 2019, 8, 382. [Google Scholar] [CrossRef] [PubMed]
- Oerke, E.C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- FAOSTAT Pesticides Use-FAO-Statistical Database. Available online: https://www.fao.org/faostat/en/#data/RP/visualize (accessed on 18 May 2025).
- Perotti, V.E.; Larran, A.S.; Palmieri, V.E.; Martinatto, A.K.; Permingeat, H.R. Herbicide Resistant Weeds: A Call to Integrate Conventional Agricultural Practices, Molecular Biology Knowledge and New Technologies. Plant Sci. 2020, 290, 110255. [Google Scholar] [CrossRef]
- Ofosu, R.; Agyemang, E.D.; Márton, A.; Pásztor, G.; Taller, J.; Kazinczi, G. Herbicide Resistance: Managing Weeds in a Changing World. Agronomy 2023, 13, 1595. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide Pesticide Usage and Its Impacts on Ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Soltani, N.; Dille, J.A.; Burke, I.C.; Everman, W.J.; VanGessel, M.J.; Davis, V.M.; Sikkema, P.H. Potential Corn Yield Losses from Weeds in North America. Weed Technol. 2016, 30, 979–984. [Google Scholar] [CrossRef]
- Alcántara-de la Cruz, R.; Domínguez-Martínez, P.A.; da Silveira, H.M.; Cruz-Hipólito, H.E.; Palma-Bautista, C.; Vázquez-García, J.G.; Domínguez-Valenzuela, J.A.; De Prado, R. Management of Glyphosate-Resistant Weeds in Mexican Citrus Groves: Chemical Alternatives and Economic Viability. Plants 2019, 8, 325. [Google Scholar] [CrossRef]
- Heap, I. The International Herbicide-Resistant Weed Database. Available online: www.weedscience.org (accessed on 22 September 2025).
- Cruz-Hipólito, H.; Domínguez-Valenzuela, J.A.; Osuna, M.D.; De Prado, R. Resistance Mechanism to Acetyl Coenzyme A Carboxylase Inhibiting Herbicides in Phalaris paradoxa Collected in Mexican Wheat Fields. Plant Soil 2012, 355, 121–130. [Google Scholar] [CrossRef]
- Cruz-Hipólito, H.; Fernandez, P.; Alcantara, R.; Gherekhloo, J.; Osuna, M.; De Prado, R. Ile-1781-Leu and Asp-2078-Gly Mutations in ACCase Gene, Endow Cross-Resistance to APP, CHD, and PPZ in Phalaris minor from Mexico. Int. J. Mol. Sci. 2015, 16, 21363–21377. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Hipólito, H.; Osuna, M.D.; Domínguez-Valenzuela, J.A.; Espinoza, N.; De Prado, R. Mechanism of Resistance to ACCase-Inhibiting Herbicides in Wild Oat (Avena fatua) from Latin America. J. Agric. Food Chem. 2011, 59, 7261–7267. [Google Scholar] [CrossRef]
- Tafoya-Razo, J.A.; Mora-Munguía, S.A.; Torres-García, J.R. Diversity of Herbicide-Resistance Mechanisms of Avena fatua L. to Acetyl-CoA Carboxylase-Inhibiting Herbicides in the Bajio, Mexico. Plants 2022, 11, 1644. [Google Scholar] [CrossRef] [PubMed]
- Tafoya-Razo, J.A.; Carrillo-Mejía, R.M. Resistencia de un Biotipo de Sorghum halepense de Isla Veracruz a Herbicidas Inhibidores de la Enzima ALS. In Proceedings of the XXXI Congreso Mexicano de la Ciencia de la Maleza, Cancún, México, 12 November 2010; Tadeo Robledo, M., Esqueda Esquivel, V.A., Cuervo Usán, Y., Sánchez Mendoza, S.M., Monroy Martínez, S., Zita Padilla, G., Eds.; Sociedad Mexicana de la Ciencia de la Maleza: Cancún, México, 2010; p. 105. [Google Scholar]
- Rojano-Delgado, A.M.; Domínguez-Valenzuela, J.A.; Cruz-Hipólito, H.; Toledo, A.; De Prado, R. Resistencia Cruzada a Herbicidas Inhibidores de la ALS en Sorghum halepense Colectado en México. In Proceedings of the Manejo y Control de Malezas en Latinoamérica, Cancún, México, 15 November 2013; Bojórquez-Bojórquez, G.A., Esqueda-Esquivel, V.A., Balbuena-Melgarejo, A., Rosales-Robles, E., Sánchez-Nava, S., Santillanes-Navidad, R., Zita-Padilla, G.A., Eds.; Sociedad Mexicana de la Ciencia de la Maleza: Cancún, México, 2013; pp. 734–738. [Google Scholar]
- Saavedra-Avila, J.I.; Bolaños, A.; Avila, L.A.; Martins, A.A.; Vargas, M. Johnsongrass Resistance to Nicosulfuron. Adv. Weed Sci. 2025, 43, e020250077. [Google Scholar] [CrossRef]
- Pérez-López, M.; González-Torralva, F.; Cruz-Hipólito, H.; Santos, F.; Domínguez-Valenzuela, J.A.; De Prado, R. Characterization of Glyphosate-Resistant Tropical Sprangletop (Leptochloa virgata) and its Alternative Chemical Control in Persian Lime Orchards in Mexico. Weed Sci. 2014, 62, 441–450. [Google Scholar] [CrossRef]
- Alcántara-de la Cruz, R.; Romano, Y.; Osuna-Ruíz, M.D.; Domínguez-Valenzuela, J.A.; Menéndez, J.; De Prado, R. Genetic Relationships Between Tropical Sprangletop (Leptochloa virgata) Populations from Mexico: Understanding Glyphosate Resistance Spread. Weed Sci. 2016, 64, 579–587. [Google Scholar] [CrossRef]
- Tafoya-Razo, J.A.; Carrillo-Mejía, R.M. Resistencia de Simsia amplexicaulis L. Colectada en Cereales del Altiplano al Herbicida 2,4-D. In Proceedings of the XXXIII Congreso Mexicano de la Ciencia de la Maleza, Villahermosa, México, 9 November 2012; Sánchez-Nava, S., Esqueda-Esquivel, V.A., Eds.; Sociedad Mexicana de la Ciencia de la Maleza: Villahermosa, México, 2012; pp. 84–85. [Google Scholar]
- Alcántara-de la Cruz, R.; Fernández-Moreno, P.T.; Ozuna, C.V.; Rojano-Delgado, A.M.; Cruz-Hipólito, H.E.; Domínguez-Valenzuela, J.A.; Barro, F.; De Prado, R. Target and Non-Target Site Mechanisms Developed by Glyphosate-Resistant Hairy Beggarticks (Bidens pilosa L.) Populations from Mexico. Front. Plant Sci. 2016, 7, 220549. [Google Scholar] [CrossRef]
- Dorado-Rangel, S.I.; Conde-Martínez, V.; Uscanga-Mortera, E.; García-Nava, R.; Domínguez-López, A.; Mellado-Arroyo, M. Detección de Resistencia de Ixophorus unisetus (J. Presl) Schltdl a Tembotrione, Glifosato e Imazetapir. In Proceedings of the XXXIV Congreso Nacional de la Ciencia de la Maleza, Aguascalientes, México, 26 October 2018; Delgado-Castillo, J.C., Clemente-Cruz, J., Hernández-Martínez, M.Á., Téllez-Edith, F., Eds.; Sociedad Mexicana de la Ciencia de la Maleza: Aguascalientes, México, 2018; p. 117. [Google Scholar]
- Domínguez-Valenzuela, J.A.; Vázquez-García, J.G.; Castro, P.; Palma-Bautista, C.; Cruz-Hipólito, H.E.; Rey, M.-D.; De Prado, R.; Portugal, J. Asp376Glu Mutation and Enhanced Metabolism Controlling the Resistance to ALS-Inhibiting Herbicides in Ixophorus unisetus (J. Presl) Schltdl from the Bajio, Mexico. Agronomy 2023, 13, 1682. [Google Scholar] [CrossRef]
- Gherekhloo, J.; Fernández-Moreno, P.T.; Alcántara-de la Cruz, R.; Sánchez-González, E.; Cruz-Hipólito, H.E.; Domínguez-Valenzuela, J.A.; De Prado, R. Pro-106-Ser Mutation and EPSPS Overexpression Acting Together Simultaneously in Glyphosate-Resistant Goosegrass (Eleusine indica). Sci. Rep. 2017, 7, 6702. [Google Scholar] [CrossRef]
- Dominguez-Valenzuela, J.A.; Gherekhloo, J.; Fernández-Moreno, P.T.; Cruz-Hipólito, H.E.; Alcántara-de la Cruz, R.; Sánchez-González, E.; De Prado, R. First Confirmation and Characterization of Target and Non-Target Site Resistance to Glyphosate in Palmer Amaranth (Amaranthus palmeri) from Mexico. Plant Physiol. Biochem. 2017, 115, 212–218. [Google Scholar] [CrossRef]
- Bolaños-Jiménez, J.; Uscanga-Mortera, E.; Tafoya-Razo, J.A.; Kohashi-Shibata, J.; Torres-García, J.R. Biological Efficacy of the Inhibitor Herbicides of Acetyl Coenzyme a Carboxylase and Acetolactate Synthase and the Presence of Resistance in Echinochloa crus-galli (L.) Beauv. Agrociencia 2018, 52, 713–723. [Google Scholar]
- Torres-García, J.R.; Tafoya-Razo, J.A.; Velázquez-Márquez, S.; Tiessen, A. Double Herbicide-Resistant Biotypes of Wild Oat (Avena fatua) Display Characteristic Metabolic Fingerprints before and after Applying ACCase- and ALS-Inhibitors. Acta Physiol. Plant 2018, 40, 119. [Google Scholar] [CrossRef]
- Palma-Bautista, C.; Gherekhloo, J.; Domínguez-Martínez, P.A.; Domínguez-Valenzuela, J.A.; Cruz-Hipolito, H.E.; Alcántara-de la Cruz, R.; Rojano-Delgado, A.M.; De Prado, R. Characterization of Three Glyphosate Resistant Parthenium hysterophorus Populations Collected in Citrus Groves from Mexico. Pestic. Biochem. Physiol. 2019, 155, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bracamonte, E.; da Silveira, H.M.; Alcántara-de la Cruz, R.; Domínguez-Valenzuela, J.A.; Cruz-Hipólito, H.E.; De Prado, R. From Tolerance to Resistance: Mechanisms Governing the Differential Response to Glyphosate in Chloris barbata. Pest Manag. Sci. 2018, 74, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Palma-Bautista, C.; Vázquez-Garcia, J.G.; López-Valencia, G.; Domínguez-Valenzuela, J.A.; Barro, F.; De Prado, R. Reduced Glyphosate Movement and Mutation of the EPSPS Gene (Pro106Ser) Endow Resistance in Conyza canadensis Harvested in Mexico. J. Agric. Food Chem. 2023, 71, 4477–4487. [Google Scholar] [CrossRef]
- Domínguez-Valenzuela, J.A.; Alcántara-de la Cruz, R.; Palma-Bautista, C.; Vázquez-García, J.G.; Cruz-Hipólito, H.E.; De Prado, R. Non-Target Site Mechanisms Endow Resistance to Glyphosate in Saltmarsh Aster (Aster squamatus). Plants 2021, 10, 1970. [Google Scholar] [CrossRef]
- Domínguez-Valenzuela, J.A.; Palma-Bautista, C.; Vazquez-Garcia, J.G.; Yanniccari, M.; Gigón, R.; Alcántara-de la Cruz, R.; De Prado, R.; Portugal, J. Convergent Adaptation of Multiple Herbicide Resistance to Auxin Mimics and ALS- and EPSPS-Inhibitors in Brassica rapa from North and South America. Plants 2023, 12, 2119. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sánchez, L.A.; Palma-Bautista, C.; Domínguez-Valenzuela, J.A. Resistencia de Mostaza (Brassica rapa L.) a Herbicidas en Cultivos de Cereales en Tlaxcala: Evaluación de Niveles de Resistencia. In Proceedings of the XLV Congreso Nacional de la Ciencia de la Maleza, Irapuato, México, 9 October 2024; Rosales-Robles, E., Delgado-Castillo, J.C., Eds.; Sociedad Mexicana de la Ciencia de la Maleza: Irapuato, México, 2024; pp. 231–232. [Google Scholar]
- Caltempa-Sánchez, O.J. Sensibilidad de Brassica rapa L. Procedente de Hidalgo y Tlaxcala a Herbicidas Auxínicos, Inhibidores de ALS y EPSPS. Bachelor’s Thesis, Universidad Autónoma Chapingo, Texcoco, México, 2025. [Google Scholar]
- Domínguez-Valenzuela, J.A.; Palma-Bautista, C.; Vázquez-García, J.G.; López-Valencia, G.; Alcántara-de la Cruz, R.; De Prado, R. Confirmación de la Resistencia del Zacate Pega Ropa (Setaria adhaerens (Forssk.) Chiov.) al Herbicida Tembotrione. In Proceedings of the XLIV Congreso Nacional de la Ciencia de la Maleza, Saltillo, México, 24 November 2023; Rosales-Robles, E., Delgado-Castillo, J.C., Eds.; Sociedad Mexicana de la Ciencia de la Maleza: Saltillo, México, 2023; pp. 162–169. [Google Scholar]
- SOMECIMA-Sociedad Mexicana de la Ciencia de la Maleza. Conference Proceedings. Available online: https://somecima.com/memorias/ (accessed on 23 September 2025).
- Peterson, M.A.; McMaster, S.A.; Riechers, D.E.; Skelton, J.; Stahlman, P.W. 2,4-D Past, Present, and Future: A Review. Weed Technol. 2016, 30, 303–345. [Google Scholar] [CrossRef]
- Ortíz Gutiérrez, T.A. Dosis Respuesta de Parthenium hysterophorus L. a Glifosato y Paraquat. Bachelor’s Thesis, Universidad Autónoma Chapingo, Texcoco, México, 2015. [Google Scholar]
- Njoroge, J.M. Tolerance of Bidens Pilosa L. and Parthenium hysterophorus L. to Paraquat (Gramoxone) in Kenya Coffee. Kenya Coffee 1991, 56, 999–1001. [Google Scholar]
- Van Horn, C.R.; Moretti, M.L.; Robertson, R.R.; Segobye, K.; Weller, S.C.; Young, B.G.; Johnson, W.G.; Schulz, B.; Green, A.C.; Jeffery, T.; et al. Glyphosate Resistance in Ambrosia trifida: Part 1. Novel Rapid Cell Death Response to Glyphosate. Pest Manag. Sci. 2018, 74, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-García, J.G.; Ledesma-Escoba, C.A.; Palma- Bautista, C.; Domínguez-Valenzuela, J.A.; De Prado, R.; Alcántara de la Cruz, R.; Cruz-Hipólito, H.E.; Priego-Capote, F. Caracterización de los Mecanismos de Resistencia de Setaria adhaerens al Herbicida Tembotrione. In Proceedings of the XLIV Congreso Nacional de la Ciencia de la Maleza, Saltillo, México, 24 November 2023; Rosales-Robles, E., Delgado Castillo, J.C., Eds.; Sociedad Mexicana de la Ciencia de la Maleza: Saltillo, México, 2023; pp. 222–223. [Google Scholar]
- Silva, T.S.; Arneson, N.J.; DeWerff, R.P.; Smith, D.H.; Silva, D.V.; Werle, R. Preemergence Herbicide Premixes Reduce the Risk of Soil Residual Weed Control Failure in Corn. Weed Technol. 2023, 37, 410–421. [Google Scholar] [CrossRef]
- Torra, J.; Osuna, M.D.; Merotto, A.; Vila-Aiub, M. Editorial: Multiple Herbicide-Resistant Weeds and Non-Target Site Resistance Mechanisms: A Global Challenge for Food Production. Front. Plant Sci. 2021, 12, 763212. [Google Scholar] [CrossRef]
- Arispe-Vázquez, J.L.; Tamayo-Esquer, L.M.; Toledo-Aguilar, R.; Noriega-Cantú, D.H.; Cadena-Zamudio, D.A.; Ramírez-Sánchez, S.E.; Hernández-Juárez, A.; Mayo-Hernández, J.; Espinoza-Ahumada, C.A. A Review of the Current Panorama of Glyphosate Resistance among Weeds in Mexico and the Rest of the World. Agro Product. 2023, 7, 135–149. [Google Scholar] [CrossRef]
- Alcántara-de la Cruz, R.; Cruz-Hipólito, H.E.; Domínguez-Valenzuela, J.A.; De Prado, R. Glyphosate Ban in Mexico: Potential Impacts on Agriculture and Weed Management. Pest Manag. Sci. 2021, 77, 3820–3831. [Google Scholar] [CrossRef] [PubMed]
- Alcántara-de la Cruz, R.; Rojano-Delgado, A.M.; Giménez, M.J.; Cruz-Hipolito, H.E.; Domínguez-Valenzuela, J.A.; Barro, F.; De Prado, R. First Resistance Mechanisms Characterization in Glyphosate-Resistant Leptochloa virgata. Front. Plant Sci. 2016, 7, 220893. [Google Scholar] [CrossRef]
- Martinelli, R.; Rufino, L.R., Jr.; Desiderio, D.R.; Alcántara-de la Cruz, R.; Monquero, P.A.; de Azevedo, F.A. The Impacts of Ecological Mowing Combined with Conventional Mechanical or Herbicide Management on Weeds in Orange Orchards. Weed Res. 2022, 62, 431–445. [Google Scholar] [CrossRef]
- Silwana, S.; Mulidzi, A.R.; Jovanovic, N. Evaluating the Effects and Benefits of Cover Crops in Citrus Orchards: A Review. S. Afr. J. Plant Soil 2023, 40, 117–126. [Google Scholar] [CrossRef]
- Cabrera-Pérez, C.; Llorens, J.; Escolà, A.; Royo-Esnal, A.; Recasens, J. Organic Mulches as an Alternative for Under-Vine Weed Management in Mediterranean Irrigated Vineyards: Impact on Agronomic Performance. Eur. J. Agron. 2023, 145, 126798. [Google Scholar] [CrossRef]
- Gazoulis, I.; Kanatas, P.; Antonopoulos, N.; Tataridas, A.; Travlos, I. False Seedbed for Agroecological Weed Management in Forage Cereal–Legume Intercrops and Monocultures in Greece. Agronomy 2022, 13, 123. [Google Scholar] [CrossRef]
- Bajwa, A.A.; Walsh, M.; Chauhan, B.S. Weed Management Using Crop Competition in Australia. Crop Prot. 2017, 95, 8–13. [Google Scholar] [CrossRef]
- Galon, L.; da Silva, A.M.L.; Franceschetti, M.B.; Müller, C.; Weirich, S.N.; Toso, J.O.; Tonin, R.J.; Perin, G.F. Selectivity and Efficacy of Herbicides Applied on Barley for Weed Control. Bragantia 2023, 82, e20220111. [Google Scholar] [CrossRef]
- Collier, G.R.S.; Spaner, D.M.; Hall, L.M.; Graf, R.J.; Beres, B.L. Fall-Applied Residual Herbicides Improve Broadleaf Weed Management in Ultra-Early Wheat (Triticum aestivum L.) Production Systems on the Northern Great Plains. Can. J. Plant Sci. 2022, 102, 1115–1129. [Google Scholar] [CrossRef]
- Riemens, M.; Sønderskov, M.; Moonen, A.C.; Storkey, J.; Kudsk, P. An Integrated Weed Management Framework: A Pan-European Perspective. Eur. J. Agron. 2022, 133, 126443. [Google Scholar] [CrossRef]
- Scavo, A.; Mauromicale, G. Integrated Weed Management in Herbaceous Field Crops. Agronomy 2020, 10, 466. [Google Scholar] [CrossRef]
- Saavedra-Ávila, J.I. Zacate Jhonson (Sorghum halepense (L.) Pers) Resistente a Nicosulfuron. Master’s Thesis, Universidad Autónoma Chapingo, Texcoco, México, 2020. [Google Scholar]
- Powles, S.B.; Yu, Q. Evolution in Action: Plants Resistant to Herbicides. Annu. Rev. Plant Biol. 2010, 61, 317–347. [Google Scholar] [CrossRef]
- Délye, C. Unravelling the Genetic Bases of Non-target-site-based Resistance (NTSR) to Herbicides: A Major Challenge for Weed Science in the Forthcoming Decade. Pest Manag. Sci. 2013, 69, 176–187. [Google Scholar] [CrossRef]
- Moss, S. Integrated Weed Management (IWM): Why Are Farmers Reluctant to Adopt Non-chemical Alternatives to Herbicides? Pest Manag. Sci. 2019, 75, 1205–1211. [Google Scholar] [CrossRef]
- Schwartz-Lazaro, L.M.; Copes, J.T. A Review of the Soil Seedbank from a Weed Scientists Perspective. Agronomy 2019, 9, 369. [Google Scholar] [CrossRef]
- Kanatas, P.; Travlos, I.S.; Gazoulis, I.; Tataridas, A.; Tsekoura, A.; Antonopoulos, N. Benefits and Limitations of Decision Support Systems (DSS) with a Special Emphasis on Weeds. Agronomy 2020, 10, 548. [Google Scholar] [CrossRef]
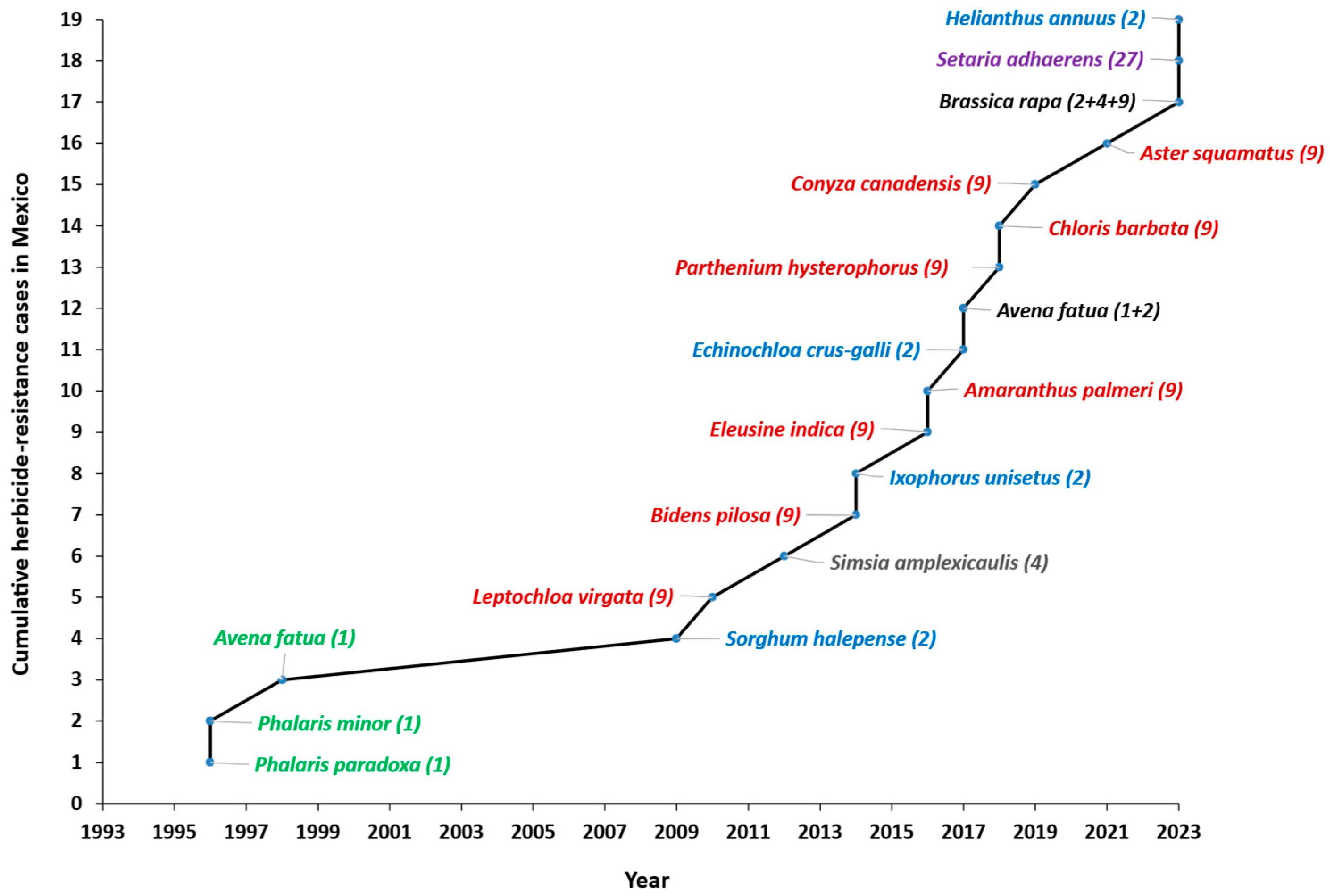
| No. | Year | Species | Common Name | Crops | MoA 1 (HRAC Group) | Resistance Mechanisms | Reference |
|---|---|---|---|---|---|---|---|
| 1 | 1996 | Phalaris paradoxa | Awned canary-grass | Wheat/barley | ACCase; 1 | Point mutations Ile-1781-Leu and Asp-2078-Gly | [15,16] |
| 2 | 1996 | Phalaris minor | Littleseed canarygrass | Wheat/barley | ACCase; 1 | Point mutation Gly-2096-Ser | [15,17] |
| 3 | 1998 | Avena fatua | Wild oat | Wheat/barley | ACCase; 1 | Point mutation Asp-2041-Gly, Ile–1781–Leu, Trp-1999-Cys, Trp-2027-Cys, Ile-2041-Asn, Asp-2078-Gly, Cys-2088-Arg, Gly-2096-Ala and metabolism | [18,19] |
| 4 | 2009 | Sorghum halepense | Johnson grass | Corn | ALS; 2 | Point mutation Asp-376-Glu | [20,21,22] |
| 5 | 2010 | Leptochloa virgata | Tropical sprangletop | Citrus | EPSPS; 9 | Reduced uptake and translocation and point mutation Pro-106-Ser | [23,24] |
| 6 | 2012 | Simsia amplexicaulis | Acahualillo | Barley/wheat | Auxin mimic; 4 | Unknown | [25] |
| 7 | 2014 | Bidens pilosa | Blackjack | Citrus | EPSPS; 9 | Reduced translocation and double mutation Tre-102-Ilo and Pro-106-Ser | [26] |
| 8 | 2014 | Ixophorus unisetus | Mexican grass | Corn | ALS; 2 | Increased metabolism and point mutation Asp-376-Glu | [27,28] |
| 9 | 2016 | Eleusine indica | Goose grass | Citrus | EPSPS; 9 | Reduced retention and translocation and point mutation Pro-106-Ser | [29] |
| 10 | 2016 | Amaranthus palmeri | Palmer amaranth | Cotton | EPSPS; 9 | Reduced translocation and point mutation Pro-106-Ser | [30] |
| 11 | 2017 | Echinochloa crus-galli | Barnyard grass | Wheat | ALS; 2 | Unknown | [31] |
| 12 | 2017 | Avena fatua | Wild oat | Wheat | ACCase and ALS; 1 and 2 | Point mutation Ile-1781-Leu and Ser-653-Asn | [32] |
| 13 | 2018 | Parthenium hysterophorus | Parthenium weed | Citrus | EPSPS; 9 | Reduced uptake and translocation and point mutation Pro-106-Ser | [33] |
| 14 | 2018 | Chloris barbata | Purpletop chloris | Citrus | EPSPS; 9 | Reduced translocation and point mutation Pro-106-Ser | [34] |
| 15 | 2019 | Conyza canadensis | Canadian fleabane | Citrus | EPSPS; 9 | Reduced translocation and point mutation Pro-106-Ser | [35] |
| 16 | 2021 | Aster squamatus | Annual saltmarsh aster | Citrus | EPSPS; 9 | Reduced absorption, translocation and increased metabolism | [36] |
| 17 | 2023 | Brassica rapa | Field mustard | Barley/wheat | Auxin mimic, ALS and EPSPS; 2, 4 and 9 | Unknown | [37,38,39] |
| 18 | 2023 | Setaria adhaerens | Bur bristlegrass | Corn | HPPD; 27 | Increased metabolism | [40] |
| 19 | 2023 | Helianthus annuus | Sunflower | Sorghum | ALS; 2 | Unknown | [15] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domínguez-Valenzuela, J.A.; Palma-Bautista, C.; Ruiz-Romero, R.E.; Vázquez-García, J.G.; Delgado-Castillo, J.C.; Cruz-Hipólito, H.E.; Alcántara-de la Cruz, R.; De Prado, R.; Plaza, G. Weed Resistance to Herbicides in Mexico: A Review. Agronomy 2025, 15, 2411. https://doi.org/10.3390/agronomy15102411
Domínguez-Valenzuela JA, Palma-Bautista C, Ruiz-Romero RE, Vázquez-García JG, Delgado-Castillo JC, Cruz-Hipólito HE, Alcántara-de la Cruz R, De Prado R, Plaza G. Weed Resistance to Herbicides in Mexico: A Review. Agronomy. 2025; 15(10):2411. https://doi.org/10.3390/agronomy15102411
Chicago/Turabian StyleDomínguez-Valenzuela, José Alfredo, Candelario Palma-Bautista, Román Eleazar Ruiz-Romero, José G. Vázquez-García, Juan Carlos Delgado-Castillo, Hugo E. Cruz-Hipólito, Ricardo Alcántara-de la Cruz, Rafael De Prado, and Guido Plaza. 2025. "Weed Resistance to Herbicides in Mexico: A Review" Agronomy 15, no. 10: 2411. https://doi.org/10.3390/agronomy15102411
APA StyleDomínguez-Valenzuela, J. A., Palma-Bautista, C., Ruiz-Romero, R. E., Vázquez-García, J. G., Delgado-Castillo, J. C., Cruz-Hipólito, H. E., Alcántara-de la Cruz, R., De Prado, R., & Plaza, G. (2025). Weed Resistance to Herbicides in Mexico: A Review. Agronomy, 15(10), 2411. https://doi.org/10.3390/agronomy15102411









