Leaf Spot Disease Caused by Several Pathogenic Species of the Pleosporaceae Family on Agave salmiana and Agave lechuguilla Plants in Mexico, and Their Biocontrol Using the Indigenous Trichoderma asperellum Strain JEAB02
Abstract
1. Introduction
2. Materials and Methods
2.1. The Fungal Strains
2.2. Molecular Characterization
2.3. Pathogenicity Tests for Agave Phytopathogens
2.3.1. Greenhouse Experiment Design and Treatments
2.3.2. Field Pathological Tests
2.4. In Vitro Confrontation Tests
Antagonistic Activity of Trichoderma asperellum Strain JEAB02 Against Pathogenic Fungi of Agave
3. Results and Discussion
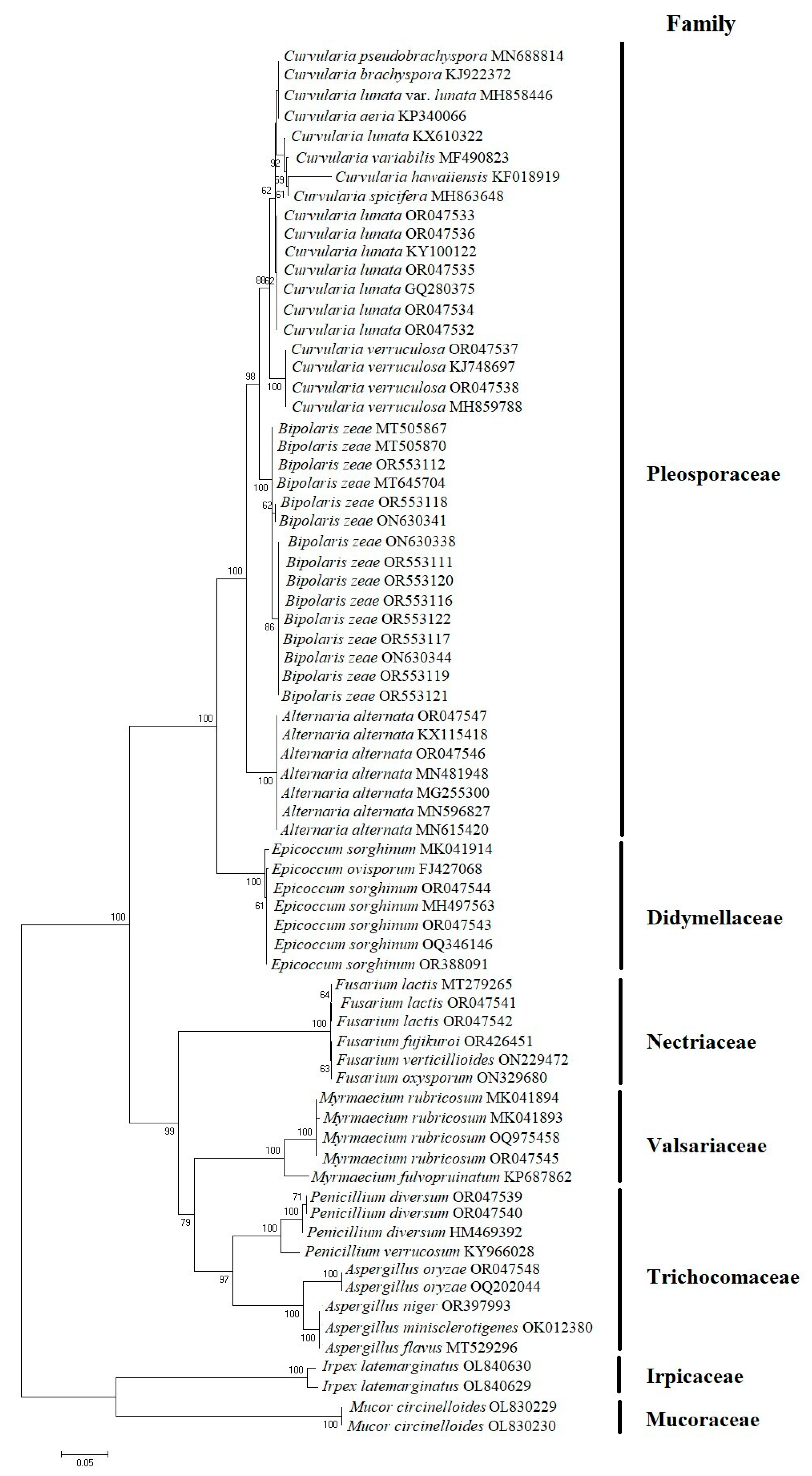
3.1. Molecular Characterization
3.2. Pathogenicity Tests
3.2.1. Greenhouse Tests
3.2.2. Field Tests
3.3. In Vitro Antagonism of Trichoderma Against Pathogenic Fungi in Agave
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castillo, Q.D.; Villarreal, Q.J.A.; Cano, P.A. El género Agave L. bajo cultivo: Taxonomía, distribución y usos. Rev. Cien. For. Mex. 2007, 32, 57–70. [Google Scholar]
- Robles, A.M.A.; Cruz, G.A.E. Agave: Importancia y aplicaciones en México. CienciAcierta 2023, 76, 264–288. [Google Scholar]
- Servicio Nacional de Inspección y Certificación de Semillas. Agave (Agave spp.), Generalidades de la Red Agaváceas. Gobierno de México. 1 August 2017. Available online: https://www.gob.mx/snics/acciones-y-programas/agave-agave-spp (accessed on 13 December 2024).
- García-Mendoza, A.; Cházaro-Basañez, M.J.; Nieto-Sotelo, J.; Sánchez-Teyer, L.; Tapia, E.; Gómez-Leyva, J.; Tamayo-Ordoñez, M.; Narváez-Zapata, J.A.; Rodríguez-Garay, B.; Palomino-Hasbach, G.A.; et al. Panorama del Aprovechamiento de los Agaves en México, 1st ed.; Red Temática Mexicana Aprovechamiento Integral Sustentable y Biotecnología de los Agaves, AGARED: Ciudad de México, México, 2017; pp. 1–49. [Google Scholar]
- Onofre-Sánchez, J.; Testón-Franco, N.; Pinón-Vargas, M. La entomofagia y florifagia en el Valle del Mezquital, Hidalgo México, valor cultural y uso alimentario. Sosquua 2022, 4, 9–21. [Google Scholar] [CrossRef]
- Rodríguez-Juárez, F.A.; Carrasco-Urbina, H.S.; Hernández-Zapata, A. Pulque: Contenido probiótico y potencial en la industria biotecnológica. RD-ICUAP 2021, 7, 95–110. [Google Scholar] [CrossRef]
- Padilla-Camberos, E.; Arrizon, J.; Sandoval, G. Effect of Agave fructan bioconjugates on metabolic syndrome parameters in a murine model. Pharmaceuticals 2023, 16, 412. [Google Scholar] [CrossRef] [PubMed]
- Nava-Cruz, N.Y.; Medina-Morales, M.A.; Martínez, J.L.; Rodríguez, R.; Aguilar, C.N. Agave biotecnology: An overview. Crit. Rev. Biotechnol. 2015, 35, 546–559. [Google Scholar] [PubMed]
- Gonzáles, M.; Ballesteros, A. Crean Muros Ecológicos con Fibra de Agave y Hielo Seco. 17 August 2015. Available online: https://cuaad.udg.mx/?q=noticia/crean-muros-ecologicos-con-fibra-de-agave-y-hielo-seco (accessed on 7 December 2024).
- Carmona, J.E.; Morales-Martínez, T.K.; Mussatto, S.I.; Castillo-Quiroz, D.; Ríos-González, L.J. Propiedades químicas, estructurales y funcionales de la lechuguilla (Agave lechuguilla Torr.). Rev. Mex. Cienc. For. 2017, 8, 100–122. [Google Scholar]
- García-Suárez, M.D.; Serrano, H. Agaves de Ornato Asparagaceae. 7 July 2021. Available online: https://tecnoagro.com.mx/no.149/agaves-de-ornato-asparagaceae (accessed on 7 December 2024).
- Ceja-Ramírez, R.; González-Eguiarte, D.R.; Ruiz-Corral, J.A.; Rendón-Salcido, L.A.; Flores-Garnica, J.G. Detección de restricciones en la producción de agave azul (Agave tequilana Weber var. azul) mediante percepción remota. Terra Latinoam. 2017, 35, 259–268. [Google Scholar]
- Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Diversidad Biológica Magueys. 25 May 2021. Available online: https://www.biodiversidad.gob.mx/diversidad/alimentos/magueyes/diversidadmagueyes (accessed on 8 December 2024).
- Barrientos-Rivera, G.; Esparza-Ibarra, E.L.; Segura-Pacheco, H.R.; Talavera-Mendoza, O.; Sampedro-Rosas, M.L.; Hernández-Castro, E. Caracterización morfológica de Agave angustifolia y su conservación en Guerrero, México. Rev. Mex. Cienc. Agric. 2019, 10, 655–668. [Google Scholar] [CrossRef]
- Domínguez-Arista, D.R. Bacanora, mezcal of Sonora: From clandestinity to denomination of origin. Estud. Sociales. Rev. De Aliment. Contemp. Y Desarro. Reg. 2020, 30, 2–23. [Google Scholar]
- Servicio de Información Agroalimentaria y Pesquera. Maguey Pulquero: El Estado de Hidalgo Destacó en 2017 con 69.6% de la Producción Nacional. 10 April 2018. Available online: https://www.gob.mx/siap/articulos/maguey-pulquero?idiom=es (accessed on 7 December 2024).
- Podda, L.; Meloni, F.; Fenu, G.; Iiriti, G.; Bacchetta, G. The vascular flora of the marine protected area of “Capo Carbonara” (SE-Sardinia). Fl. Medit. 2021, 31, 415–449. [Google Scholar]
- Sánchez, J.E.; Torres-Oregón, F.; Pérez-Akaki, P. El mezcal en México: Las tensiones socioculturales con el agroextractivismo. Rev. COPaLa 2020, 9, 143–153. [Google Scholar]
- González, L.H.D.; González, U.D.U. Estimación del rendimiento de biomasa y fibra de Agave lechuguilla Torr. en el norte de Zacatecas. Rev. Mex. Cienc. For. 2023, 14, 97–117. [Google Scholar]
- Mayorga-Hernández, E.; Rössel-Kipping, D.; Ortiz-Laurel, H.; Quero-Carrillo, A.R.; Amante-Orozco, A. Análisis comparativo en la calidad de fibra de Agave lechuguilla Torr., procesada manual y mecánicamente. Agrociencia 2004, 38, 219–225. [Google Scholar]
- Vega, G.M.A.; Álvarez-Rios, G.D.; Figueredo-Urbina, C.J. Sistemas de manejo de agaves pulqueros en el estado de Hidalgo. Pädi Boletín Científico De Cienc. Básicas E Ing. Del ICBI 2023, 10, 92–100. [Google Scholar] [CrossRef]
- García-Mendoza, A. Los Agaves de México. Ciencias 2007, 87, 14–23. [Google Scholar]
- Guillot, O.D.; Van Der, M.P.; Laguna, L.E.; Rosselló, P.J.A. El género Agave L. en la flora alóctona valenciana. Valencia, España. Monogr. De La Rev. Bouteloua 2009, 3, 94. [Google Scholar]
- Domínguez, R.M.S.; González, J.M.L.; González, R.C.; Quiñones, V.C.; Díaz, D.L.; Mireles, O.S.J.; Pérez, M.E. El cultivo in vitro como herramienta para el aprovechamiento, mejoramiento y conservación de especies del género Agave. Investig. Y Cienc. 2008, 41, 53–62. [Google Scholar]
- Cuervo-Parra, J.A.; Pérez-España, V.H.; López, P.P.A.; Morales-Ovando, M.A.; Arce-Cervantes, O.; Aparicio-Burgos, J.E.; Romero-Cortes, T. Scyphophorus acupunctatus (Coleoptera: Dryophthoridae): A weevil threatening the production of Agave in Mexico. Fla. Entomol. 2019, 102, 1–9. [Google Scholar]
- Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA). Plagas Reglamentadas del Agave. 10 February 2020. Available online: https://www.gob.mx/senasica/documentos/plagas-reglamentadas-del-agave-110851 (accessed on 12 December 2024).
- Quiñones-Aguilar, E.E.; Montoya-Martínez, A.C.; Rincón-Enríquez, G.; López-Pérez, L. Inoculación de bulbilos de Agave tequilana con hongos micorrízicos arbusculares: Efecto en el crecimiento y biocontrol contra Fusarium oxysporum. Cienc. Y Tecnol. Agropecu. 2023, 24, e3043. [Google Scholar] [CrossRef]
- Coria-Contreras, J.J.; Mora-Aguilera, G.; Yáñez-Morales, M.J.; Acevedo-Sánchez, G.; Santana-Peñaloza, B.; Mendoza-Ramos, C.; Jiménez-González, L.; Martínez-Bustamante, V.I.; García-Martínez, D.C.; Rubio-Cortés, R. Epidemiología regional aplicada a la caracterización inductive y pronóstico de la mancha gris del agave azul (Cercospora agavicola) en Jalisco, México. Rev. Mex. Fitopatol. 2019, 37, 71–94. [Google Scholar]
- Mancera, A.C.P. Detección de Cercospora en Agave a Través del Análisis de Imágenes Multiespectrales. Master’s Thesis, Instituto Tecnológico de Estudios Superiores de Occidente, Tlaquepaque, Jalisco, Mexico, May 2021. [Google Scholar]
- Ceballos-Álvarez, A.; Chávez-Díaz, I.F.; Zelaya-Molina, L.X.; Cruz-Cárdenas, C.I.; Mercado-Vargas, T.J.; Reséndiz-Venado, Z. Identificación morfológica de hongos filamentosos aislados de diferentes síntomas asociados a Agave tequilero en los Altos de Jalisco. Cienc. Y Tecnol. Agrop. México 2022, 10, 60–69. [Google Scholar]
- Pérez-España, V.H.; Cuervo-Parra, J.A.; Aparicio, B.J.E.; Morales-Ovando, M.A.; Peralta, G.M.; Romero-Cortes, T. Importancia de la capa cuticular durante la colonización del hongo causante de la negrilla en Agave salmiana Otto ex Salm-Dyck ssp. salmiana. Rev. Mex. Cienc. For. 2022, 13, 158–168. [Google Scholar] [CrossRef]
- Romero-Cortes, T.; Cuervo-Parra, J.A.; Pérez, E.V.H.; Pescador, R.J.A.; Rangel, C.E. First Report of Leaf Spot of Agave salmiana Caused by Bipolaris zeae in Mexico. 30 May 2022. Available online: https://www.ncbi.nlm.nih.gov/nuccore/ON630338 (accessed on 29 November 2024).
- Mora-Aguilera, J.A.; Cabrera-Huerta, E.; Nava-Díaz, C.; Vázquez-López, A.; Camacho-Tapia, M.; Hernández-Catro, E. Primer Informe de Phoma sorghina que Causa Manchas de Hojas Necróticas en Agave-Mezcal (Agave angustifolia) en México. 29 January 2023. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OQ346146 (accessed on 26 May 2025).
- Romero-Cortes, T.; Pérez-España, V.H.; Pescador-Rojas, J.A.; Rangel-Cortés, E.; Armendáriz-Ontiveros, M.M.; Cuervo-Parra, J.A. First report of leaf spot disease (“Negrilla”) on Agave salmiana Otto ex Salm-Dyck (ssp. salmiana) plants caused by Bipolaris zeae Zivan in Mexico. Agronomy 2024, 14, 623. [Google Scholar]
- Almazán-Morales, A.; Moreno-Godínez, M.E.; Hernández-Castro, E.; Vázquez-Villamar, M.; Mora-Aguilera, J.A.; Cabrera-Huerta, E.; Alvarez-Fitz, P. Phytochemical profile and in vitro activity of Agave angustifolia and A. cupreata extracts against phytopathogenic fungi. Mex. J. Phytopathol. 2022, 40, 169–187. [Google Scholar] [CrossRef]
- López, G.I.; Cardozo, M.V.A.; Pérez, E.V.H.; Romero-Cortes, T.; Pescador, R.J.A.; Saucedo, G.M. Nanopartículas de plata como control de Bipolaris zeae Zivan en maguey. Ingenio Y Concienc. Boletín Científico De La Esc. Super. Ciudad Sahagún 2025, 12, 78–84. [Google Scholar]
- Cuervo-Parra, J.A.; Pérez, E.V.H.; Zavala-González, E.A.; Peralta-Gil, M.; Aparicio, B.J.E.; Romero-Cortes, T. Trichoderma asperellum strains as potential biological control agents against Fusarium verticillioides and Ustilago maydis in maize. Biocontrol Sci. Technol. 2022, 32, 1–24. [Google Scholar] [CrossRef]
- Ramírez-Cariño, H.F.; Guadarrama-Mendoza, P.C.; Sánchez-López, V.; Cuervo-Parra, J.A.; Ramírez-Reyes, T.; Dunlap, C.A.; Valadez-Blanco, R. Biocontrol of Alternaria alternata and Fusarium oxysporum by Trichoderma asperellum and Bacillus paralicheniformis in tomato plants. Antonie Van Leeuwenhoek 2020, 113, 1247–1261. [Google Scholar] [CrossRef]
- Cuervo-Parra, J.A.; Sánchez-López, V.; Romero-Cortes, T.; Ramírez-Lepe, M. Hypocrea viridescens ITV43 potential biological control agent for Moniliophthora roreri Cif & Par, Phytophthora megasperma and P. capsici. Afr. J. Microbiol. Res. 2014, 8, 1704–1712. [Google Scholar]
- Romero-Cortes, T.; Zavala-González, E.A.; Pérez, E.V.H.; Aparicio-Burgos, J.E.; Cuervo-Parra, J.A. Characterization of Cochliobolus sativus and Pyrenophora teres fungi belonging to the leaf spot complex of barley (Hordeum vulgare) isolated from barley seeds in Mexico. Chil. J. Agric. Anim. Sci. 2021, 37, 277–289. [Google Scholar] [CrossRef]
- Juárez-Hernández, J.; Castillo-Hernández, D.; Pérez-Parada, C.; Nava-Galicia, S.; Cuervo-Parra, J.A.; Surian-Cruz, E.; Díaz-Godínez, G.; Sánchez, C.; Bibbins-Martínez, M. Isolation of fungi from a textile industry effluent and the screening of their potential to degrade industrial dyes. J. Fungi 2021, 7, 805. [Google Scholar] [CrossRef]
- Johnson, M.; Zaretskaya, I.; Raytselis, Y.; Merezhuk, Y.; McGinnis, S.; Madden, T.L. NCBI BLAST: A better web interface. Nucleic Acids Res. 2008, 36, W5–W9. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Reveles, H.R.G.; Maldonado, T.C.; Chávez, R.I.; Muñoz, E.J.J.; Moreno, G.A. Vigencia de los postulados de Koch en la reproducción de Trichinella spiralis en modelo experimental. REDVET 2014, 15, 1–20. [Google Scholar]
- Perelló, A.; Sisterna, M.N.; Cortese, P. Scale for appraising the leaf blight of wheat caused by Alternaria triticimaculans. Cereal Res. Commun. 1998, 26, 189–194. [Google Scholar] [CrossRef]
- Robledo-D’Angelo, O. Enfermedad de damping-off en plántulas de lechuga: Un modelo didáctico-experimental para la enseñanza de los postulados de Koch. REurEDC 2016, 13, 680–685. [Google Scholar]
- Instituto Nacional de Estadística y Geografía (INEGI). Prontuario de Información Geográfica Municipal de los Estados Unidos Mexicanos. Apan, Hidalgo, Clave Geoestadística 13008. 9 December 2009. Available online: https://web.archive.org/web/20170327170244/http://www3.inegi.org.mx/sistemas/mexicocifras/datos-geograficos/13/13008.pdf (accessed on 5 March 2025).
- Servició Meteorológico Nacional (SMN) (2010). Estación: 00013002 APAN (DGE) Normales Climatológicas Periodo 1951–2010. 1 December 2010. Available online: https://smn.conagua.gob.mx/es/climatologia/informacion-climatologica/normales-climatologicas-por-estado?estado=hgo (accessed on 5 March 2024).
- Romero-Cortes, T.; Tamayo-Rivera, L.; Morales-Ovando, M.A.; Aparicio, B.J.E.; Pérez, E.V.H.; Peralta-Gil, M.; Cuervo-Parra, J.A. Growth and yield of purple Kculli corn plants under different fertilization schemes. J. Fungi 2022, 8, 1–27. [Google Scholar] [CrossRef]
- Rodríguez, L.L.; Guevara, H.F.; Ovando, C.J.; Marto, G.J.R.; Ortiz, P.R. Crecimiento e índice de cosecha de variedades locales de maíz (Zea mays L.) en comunidades de la región Frailesca de Chiapas, México. Cult. Trop. 2016, 37, 137–145. [Google Scholar]
- Cruz-Vasconcelos, S.T.; Ruiz-Posadas, L.M.; García-Moya, E.; Sandoval-Villa, M.; Cruz-Huerta, N. Growth and CO2 exchange rate of maguey pulquero (Agave salmiana Otto ex Salm-Dyck) obtained by seed. Agrociencia 2020, 54, 911–926. [Google Scholar] [CrossRef]
- Cuervo-Parra, J.A.; López-Pérez, P.A.; Aparicio-Burgos, J.E.; Morales-Ovando, M.A.; Romero-Cortes, T. Chalqueño maize (Zea mays L.) yield under different fertilization schemes in the municipality of Apan, Hidalgo, Mexico. Trop. Subtrop. Agroecosystems 2024, 27, 025. [Google Scholar] [CrossRef]
- Comisión Nacional del Agua. Reporte del Clima en México. Reporte anual 2020. Available online: https://smn.conagua.gob.mx/tools/DATA/Climatología/Diagnóstico%20Atmosférico/Reporte%20del%20Clima%20en%20México/Anual2020.pdf (accessed on 5 March 2025).
- Mata-Santoyo, C.I.; Leyva-Mir, S.G.; Camacho-Tapia, M.; Tovar-Pedraza, J.M.; Huerta-Espino, J.; Villaseñor-Mir, H.E.; García-León, E. Aggressiveness of Bipolaris sorokiniana and Alternaria alternata isolates on wheat cultivars in Mexico. Rev. Mex. Fitopatol. 2018, 36, 432–443. [Google Scholar]
- Analytical Software, Statistix 10, 1st ed.; Analytical Software: Tallahassee, FL, USA, 2017; pp. 7–445.
- Cuervo-Parra, J.A.; Romero-Cortes, T.; Román, A.R.; Valle, H.J.; Aparicio-Burgos, J.E. Identificación morfológica de hongos aislados de plantas de Garcinia mangostana. Rev. Mex. Cienc. Agric. 2024, 15, e3575. [Google Scholar] [CrossRef]
- Mohammadi, A.; Amini, Y. Molecular characterization and identification of Acrostalagmus liteoalbus from Saffron in Iran. Agric. Sci. Dev. 2015, 4, 16–18. [Google Scholar]
- Camacho, C.; Boratyn, G.M.; Joukov, V.; Vera, A.R.; Madden, T.L. ElasticBLAST: Accelerating sequence search via cloud computing. BMC Bioinform. 2023, 24, 117. [Google Scholar] [CrossRef]
- Zhang, Z.; Schwartz, S.; Wagner, L.; Miller, W. A greedy algorithm for aligning DNA sequences. J. Comput. Biol. 2000, 7, 203–214. [Google Scholar] [CrossRef]
- Visagie, C.M.; Bhiya, T.; Kgatle, G. Alternaria Diversity from South Africa. 22 May 2020. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MT505870 (accessed on 25 May 2025).
- Camarena-Pozos, D.A.; Flores-Núñez, V.M.; López, M.G.; Partida-Martínez, L.P. Volatiles Emitted by Fungi Associated with the Phyllosphere of Agaves and Cacti are Diverse and able to Promote Plant Growth. 3 April 2020. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MT279265 (accessed on 20 May 2025).
- Jang, Y.; Huh, N.; Lee, J.; Lee, J.S.; Kim, G.H.; Kim, J.J. Phylogenetic analysis of major molds inhabiting woods and their discoloration characteristics. Part 2. Genus Penicillium. Holzforschung 2011, 65, 265–270. [Google Scholar] [CrossRef]
- Cristobal Alejo, J.; Candelero De La Cruz, J.; Moo Koh, F.A.; Rangel Ortega, A.; Flores Revilla, C. Molecular Identification Phytopathogenic Fungi in Sugar Cane. 5 November 2016. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KY100122 (accessed on 9 May 2025).
- Mora-Aguilera, J.A.; Cabrera-Huerta, E.; Nava-Díaz, C.; Hernández-Castro, E. First Report of Epicoccum sorghinum on Agave Mezcal (Agave angustifolia). 19 June 2018. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MH497563 (accessed on 26 May 2025).
- Zhang, P.; Qiao, Y.L.; Deng, J.X.; Ma, D.F. First Report of Alternaria alternata Causing leaf spot on Kadsura coccinea in China. Plant Dis. 2020, 104, 3073. [Google Scholar] [CrossRef]
- Rodrigues, A.; Mueller, U.G.; Ishak, H.D.; Bacci, M.; Pagnocca, F.C. Ecology of microfungal communities in gardens of fungus-growing ants (Hymenoptera: Formicidae): A year-long survey of three species of attine ants in Central Texas. FEMS Microbiol. Ecol. 2011, 78, 244–255. [Google Scholar] [CrossRef]
- Vu, D.; Groenewald, M.; de Vries, M.; Gehrmann, T.; Stielow, B.; Eberhardt, U.; Al-Hatmi, A.; Groenewald, J.Z.; Cardinali, G.; Houbraken, J.; et al. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Stud. Mycol. 2019, 92, 135–154. [Google Scholar] [CrossRef]
- Campos-Rivero, G.; Sánchez-Teyer, L.F.; De la Cruz-Arguijo, E.A.; Ramírez-González, M.S.; Larralde-Corona, C.P.; Narváez-Zapata, J.A. Bioprospecting for fungi with potential pathogenic activity on leaves of Agave tequilana Weber var. Azul. J. Phytopathol. 2019, 167, 283–294. [Google Scholar] [CrossRef]
- Ma, G.; Wu, X. GenBank Direct Submission. 18 September 2019. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MN481948 (accessed on 26 May 2025).
- Martínez-Pérez, A.; Mares-Rodríguez, F.D.; González-Herrera, S.M.; Muñiz-Márquez, D.B.; Rodríguez-Herrera, R.; Rutiaga-Quiñones, O.M. GenBank Direct Submission. 9 January 2023. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OQ202044 (accessed on 26 May 2025).
- Valencia, Y.T.; Martin, M.R.; Cruz, L.J.R.; Pérez, B.D.; Magaña, A.A.; Cortes, V.A.; Nexticapan, G.A. Identification and Molecular Characterization Phytopathogenic Fungi in Agave tequilana in Mexico. 2 August 2023. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OR388091 (accessed on 26 May 2025).
- Tapia-Tussell, R.; Solis, S.; Pérez-Brito, D. Identification and Molecular Characterization of Ligninolytic Fungi from Yucatan, Mexico. 17 June 2009. Available online: https://www.ncbi.nlm.nih.gov/nuccore/GQ280375.1/ (accessed on 29 November 2024).
- Gómez-Cornelio, S.; De la Rosa-García, S.; Partida-Martínez, L.P. Interactions among Dominant Members of a Fungal Community Isolated from Epilithic Biofilms Limestone-Colonizing. 26 July 2016. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KX610322 (accessed on 29 November 2024).
- Moo, K.F.A.; Cristóbal, A.J.; Reyes, R.A.; Tun, S.J.M.; Sandoval, L.R.; Ramírez, P.J.A. Activity in vitro of an aqueous extract of Bonellia flammea against fungal phytopathogenic. 15 April 2014. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KJ748697 (accessed on 29 November 2024).
- Gutiérrez-Flores, L.M.; López-Reyes, L.; Mauricio-Gutiérrez, A. Control Biológico de Enfermedades Fúngicas en Pinus sp. de Sistemas Forestales de la Sierra Norte de Puebla Como una Alternativa al uso de Plaguicidas. 12 May 2023. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OQ975458 (accessed on 29 May 2025).
- Camacho-Tapia, M.; Leyva-Mir, S.G.; Suaste-Dzul, A.P.; Tovar-Pedraza, J.M. First Report of Alternaria Black Spot of Pomegranate Caused by Alternaria alternata in Mexico. 24 October 2017. Available online: https://www.ncbi.nlm.nih.gov/nuccore/1269612204 (accessed on 29 March 2025).
- Reyes-Tena, A.; Montoya-Martínez, A.C.; Fernández-Pavía, S.P.; Santillán-Mendoza, R.; Jiménez-Villegas, A.; Rodríguez-Alvarado, G. Leaf Blight of Onion Caused by Stemphylium vesicarium and Alternaria alternata in Mexico. 21 October 2019. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MN596827 (accessed on 29 November 2024).
- Romero-Cortes, T.; Pérez, E.V.H.; López, P.P.A.; Rodríguez-Jimenes, G.C.; Robles-Olvera, V.J.; Aparicio, B.J.E.; Cuervo-Parra, J.A. Antifungal activity of vanilla juice and vanillin against Alternaria alternata. J. Food 2019, 17, 375–383. [Google Scholar] [CrossRef]
- Visagie, C.M.; Bhiya, T.; Kgatle, G. Alternaria Diversity from South Africa. 22 May 2020. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MT505867 (accessed on 25 May 2025).
- Chen, T.; Qi, Y.; Li, C. GenBank Direct Submission. 22 June 2020. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MT645704 (accessed on 29 November 2024).
- Manamgoda, D.S.; Cai, L.; McKenzie, E.H.C.; Crous, P.W.; Madrid, H.; Chukeatirote, E.; Shivas, R.G.; Tan, Y.P.; Hyde, K.D. A phylogenetic and taxonomic re-evaluation of the Bipolaris–Cochliobolus–Curvularia Complex. Fungal Divers. 2012, 56, 131–144. [Google Scholar] [CrossRef]
- Azizah, K.; Madihan, N.Z.A.; Shahrizim, Z.; Mohd, T.Y.; Nur, A.; Izzati, M.Z. Morphological and molecular characterization of Curvularia and related species associated with leaf spot disease of rice in Peninsular Malaysia. Rend. Lincei Sci. Fis. Nat. 2016, 27, 205–214. [Google Scholar]
- Cuervo-Parra, J.A.; Romero-Cortes, T.; Ramírez-Lepe, M. Isolation and Molecular identification of Curvularia lunata/Cochliobolus lunatus causal agent of leaf spot disease of cocoa. In Advances in Science, Biotechnology and Safety of Foods, 1st ed.; Santos, G., García, G.H.S., Nevárez-Moorillón, G.V., Eds.; Asociación Mexicana de Ciencias de los Alimentos: Ciudad de México, Mexico, 2015; pp. 327–334. [Google Scholar]
- Manamgoda, D.S.; Rossman, A.Y.; Castlebury, L.A.; Crous, P.W.; Madrid, H.; Chukeatirote, E.; Hyde, K.D. The genus Bipolaris. Stud. Mycol. 2014, 79, 221–288. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Hernández-Restrepo, M.; Crous, P.W. Multi-locus phylogeny of the genus Curvularia and description of ten new species. Mycol. Prog. 2020, 19, 559–588. [Google Scholar] [CrossRef]
- Marin-Felix, Y.; Senwanna, C.; Cheewangkoon, R.; Crous, P.W. New species and records of Bipolaris and Curvularia from Thailand. Mycosphere 2017, 8, 1555–1573. [Google Scholar] [CrossRef]
- Seifert, K.A.; Aoki, T.; Baayen, R.P.; Brayford, D.; Burgess, L.W.; Chulze, S.; Gams, W.; Geiser, D.; de Gruyter, J.; Leslie, J.F.; et al. The name Fusarium moniliforme should no longer be used. Mycol. Res. 2003, 107, 643–644. [Google Scholar] [CrossRef]
- Price, J.L.; Yilmaz, N.; Visagie, C.M. Fungal Diversity Associated with Maize in the Eastern Cape. 11 April 2022. Available online: https://www.ncbi.nlm.nih.gov/nuccore/ON229472 (accessed on 27 May 2025).
- Sandoval, M.M.I.; Zavaleta, M.E.; Leyva, M.S.G.; Osnaya, G.M.M.; Soto, R.L.; Nava, D.C. Fungi Associated to Grain and Seed Discoloration and Leaf Spots of Rice in Campeche, Mexico. 24 April 2022. Available online: https://www.ncbi.nlm.nih.gov/nuccore/ON329680 (accessed on 27 May 2025).
- Roy, B.; Maitra, D.; Mitra, A.K. GenBank Direct Submission. 11 August 2023. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OR426451 (accessed on 27 May 2025).
- Jaklitsch, W.M.; Fournier, J.; Dai, D.Q.; Hyde, K.D.; Voglmayr, H. Valsaria and Valsariales. Fungal Divers. 2015, 73, 159–202. [Google Scholar] [CrossRef]
- Ghosh, S.K. GenBank Direct Submission. 11 April 2017. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KY966028 (accessed on 30 November 2024).
- Li, C. GenBank Direct Submission. 23 May 2020. Available online: https://www.ncbi.nlm.nih.gov/nuccore/MT529296.1/ (accessed on 30 November 2024).
- Omomowo, I.O.; Babalola, O.O. GenBank Direct Submission. 2 September 2021. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OK012380 (accessed on 30 November 2024).
- Namakwa, P.; Mumbi, L.; Ouko, A. Investigation of Aflatoxin-Causing Fungal Isolates Obtained from Soil, Seeds and Diseased Plant Samples. 3 August 2023. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OR397993 (accessed on 30 November 2024).
- Rubio, C.R. Enfermedades del cultivo de agave. In Conocimiento y Prácticas Agronómicas para la Producción de Agave tequilana Weber en la Zona de Denominación de Origen del Tequila, 1st ed.; Rulfo, V.F.O., Pérez, D.J.F., del Real, L.J.I., Byerly, M.K.F., Eds.; Instituto Nacional de Investigaciones Forestales, Agrícolas y Pecuarias, Centro de Investigación Regional del Pacífico Centro: Tepatitlán de Morelos, Jalisco, Mexico, 2007; pp. 169–195. [Google Scholar]
- Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. Medidas de Manejo de Aplicación Contra Alternaria alternata (Fr.) Keissler. 1 January 2023. Available online: https://www.gob.mx/cms/uploads/attachment/file/979054/34._Medidas_de_manejo_Alternaria_alternata.pdf (accessed on 20 June 2025).
- AGQLabs. Requerimientos Técnicos para el Cultivo de Agave. 29 January 2024. Available online: https://agqlabs.mx/2024/01/29/cultivo-de-agave/ (accessed on 8 June 2025).
- Thomma, B.P. Alternaria spp.: From general saprophyte to specific parasite. Mol. Plant Pathol. 2003, 4, 225–236. [Google Scholar] [CrossRef]
- Quezada, S.A.; García, A.C.J.; Florencio, A.J.G.; Hernández, P.S.; Ruiz, G.I.; Bravo, P.D.; Pineda, R.J.M. Marchitez del Agave. Fusarium verticillioides. 2016. Available online: https://www.gob.mx/cms/uploads/attachment/file/244027/Ficha_T_cnica_Fusarium_verticillioides_en_agave_Versi_n_FINAL.pdf (accessed on 10 July 2025).
- Quezada, S.A.; García, A.C.J.; Florencio, A.J.G.; Hernández, P.S.; Ruiz, G.I.; Bravo, P.D.; Pineda, R.J.M. Marchitez del Agave. Fusarium oxysporum. 2003. Available online: https://www.gob.mx/cms/uploads/attachment/file/244026/Ficha_T_cnica_Fusarium_oxysporum_en_agave_Versi_n_FINAL.pdf (accessed on 10 July 2025).
- Bailón-Ortiz, A.Z.; Amábilis-Sánchez, M.J. Identificación de agentes causales de la pudrición seca de Agave potatorum Zucc en vivero. Rev. Mex. De Agroecosistemas 2024, 2, 11–20. [Google Scholar] [CrossRef]
- Van Poucke, K.; Monbaliu, S.; Munaut, F.; Heungens, K.; De Saeger, S.; Van, H.F. Genetic diversity and mycotoxin production of Fusarium lactis species complex isolates from sweet pepper. Int. J. Food Microbiol. 2012, 153, 28–37. [Google Scholar] [CrossRef]
- Imran, M.; Khanal, S.; Zhou, X.G.; Antony-Badu, S.; Atiq, M. First report of leaf spot of rice caused by Epicoccum sorghinum in the United States. Plant Dis. 2022, 106, 2758. [Google Scholar] [CrossRef]
- Liu, L.M.; Zhao, Y.; Zhang, Y.L.; Wang, L.; Hou, Y.X.; Huang, S.W. First report of leaf spot disease on rice caused by Epicoccum sorghinum in China. Plant Dis. 2020, 104, 2735. [Google Scholar] [CrossRef]
- Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria (SENASICA). Monitor Fitosanitario. 26 August 2022. Available online: https://dj.senasica.gob.mx/Contenido/files/2022/agosto/MonitorFitosanitario26082022_3051d5a2-352e-475d-a38d-d622f8d1e833.pdf (accessed on 10 June 2025).
- Kang, Y.; Zhang, J.; Wan, Q.; Xu, T.; Li, C.; Cao, H. First report of leaf brown spot caused by Epicoccum sorghinum on Digitaria sanguinalis in China. Plant Dis. 2019, 103, 1787. [Google Scholar] [CrossRef]
- Laurel, N.R.; De Torres, R.L.; Mendoza, J.V.S.; Balendres, M.A.O.; De la Cueva, F.M. Identification of Epicoccum sorghinum and its effect on stalk sugar yield. Sugar Tech. 2021, 23, 1383–1392. [Google Scholar] [CrossRef]
- Chen, Q.H.; Li, J.X.; Qi, Y.; Liu, D.H.; Miao, Y.H. First report of leaf spot on white Chrysanthemum (Chrysanthemum morifolium) caused by Epicoccum sorghinum in Hubei province, China. Plant Dis. 2021, 105, 1212. [Google Scholar] [CrossRef]
- Gutiérrez-Flores, L.M.; Mauricio-Gutiérrez, A.; Carcaño-Montiel, M.C.; Portillo-Manzano, E.; Gómez-Velázquez, L.; Sánchez-Alonso, P.; López-Reyes, L. Fungi associated with sick trees of Pinus patula in Tetela de Ocampo, Puebla, Mexico. Arch. Phytopathol. Plant Prot. 2020, 53, 591–611. [Google Scholar] [CrossRef]
- Gutiérrez, F.L.M. Diagnóstico de la Enfermedad Fúngica de Pinus patula y Propuesta de Biocontrol en Tetela de Ocampo, Puebla. Master’s Thesis, Universidad Autónoma de Puebla, Posgrado En Ciencias Ambientales, Heroica Puebla de Zaragoza, Puebla, Mexico, October 2018. [Google Scholar]
- Gutiérrez-Flores, L.M.; López-Reyes, L.; Hipólito-Romero, E.; Torres-Ramírez, E.; Castañeda-Roldán, E.I.; Mauricio-Gutiérrez, A. Biological control perspectives in the pine forest (Pinus spp.), an environmentally friendly alternative to the use of pesticides. Rev. Mex. Fitopatol. 2022, 40, 401–424. [Google Scholar] [CrossRef]
- Castro, C.J.; Umaña, R.G. Poblaciones e identificación de los hongos causantes de mohos poscosecha en el pedúnculo de la piña, en dos zonas de Costa Rica. Agron. Costarric. 2015, 39, 61–77. [Google Scholar]
- Gipuzkoa. Enfermedad, Penicillium (Penicillium ssp.). 1999. Available online: https://www.gipuzkoa.eus/es/web/sagarrondoak/-/peniciliuma (accessed on 19 May 2025).
- Stošić, S.; Ristić, D.; Savković, Z.; Ljaljević, G.M.; Vukojević, J.; Živković, S. Penicillium and Talaromyces species as postharvest Pathogens of pear fruit (Pyrus communis) in Serbia. Plant Dis. 2021, 105, 3510–3521. [Google Scholar] [CrossRef]
- Ephytia. Penicillium expansum Link 1809 y Otras Penicillium spp. 6 October 2021. Available online: https://ephytia.inra.fr/es/C/6100/VID-Podredumbre-por-Penicillium (accessed on 20 May 2025).
- Plantix. Pudrición de la Mazorca por Penicillium. 2025. Available online: https://plantix.net/es/library/plant-diseases/100053/penicillium-ear-rot/ (accessed on 20 May 2025).
- Awuchi, C.G.; Ondari, E.N.; Ogbonna, C.U.; Upadhyay, A.K.; Baran, K.; Okpala, C.O.R.; Korzeniowska, M.; Guiné, R.P.F. Mycotoxins affecting animals, foods, humans, and plants: Types, occurrence, toxicities, action mechanisms, prevention, and detoxification strategies—A revisit. Foods 2021, 10, 1279. [Google Scholar] [CrossRef]
- Daba, G.M.; Mostafa, F.A.; Elkhateeb, W.A. The ancient koji mold (Aspergillus oryzae) as a modern biotechnological tool. Bioresour Bioprocess 2021, 8, 1–17. [Google Scholar] [CrossRef]
- García-Conde, K.B.; Cerna-Chávez, E.; Ochoa-Fuentes, Y.M.; Velázquez-Guerrero, J.J. Aspergillus oryzae: An opportunity for agriculture. Mex. J. Phytopathol. 2024, 42, 1–18. [Google Scholar] [CrossRef]
- Food and Drug Administration. Generally Recognized Safe (GRAS)|FDA. 17 October 2023. Available online: https://www.fda.gov/food/food-ingredients-packaging/generally-recognized-safe-gras (accessed on 19 July 2025).
- Gad, S.E. Generally recognized as safe (GRAS). In Encyclopedia of Toxicology, 1st ed.; Wexler, P., Anderson, B.D., Eds.; Gad Consulting Services, Inc., US National Library of Medicine: Bethesda, MD, USA, 2005; Volume 2, pp. 417–420. [Google Scholar]
- Cardona, R.; Suleima, G.M. Caracterización y patogenicidad de hongos del complejo Helminthosporium asociados al cultivo del arroz en Venezuela. Bioagro 2008, 20, 141–145. [Google Scholar]
- Hernández-Navarro, E.; Agustín-Maravilla, G.A.; Sánchez-Rangel, J.C.; Valadez-Ramírez, P.; Chan-Cupul, W. Evaluación in vitro de fungicidas biológicos contra Curvularia eragrostidis, hongo fitopatógeno del cultivo de piña. Rev. Mex. Fitopatol. 2023, 41, 93–111. [Google Scholar]
- Hernández, J.J.; Hernández, D.E.M.; López, V.E.; Álvarez, C.J. Aislamiento e identificación del fitopatógeno causal de viruela o “negrilla” en Agave salmiana de municipios del estado de Hidalgo, México. Sci. Fungorum 2022, 53, e1425. [Google Scholar] [CrossRef]
- Moparthi, S.; Kleczewski, N. First report of Curvularia leaf spot on Zea mays caused by Curvularia lunata in North Carolina. Plant Dis. 2023, 107, 2242. [Google Scholar] [CrossRef]
- Wang, S.; Lu, Z.; Lang, B.; Wang, X.; Li, Y.; Chen, J. Curvularia lunata and Curvularia leaf spot of maize in China. ACS Omega 2022, 7, 47462–47470. [Google Scholar] [CrossRef]
- Zhou, H.K.; Liu, Y.L.; Tang, J.R.; Zhong, F.T.; Li, Y. First report of leaf spot caused by Curvularia lunata on wild rice in China. Plant Dis. 2021, 105, 3300. [Google Scholar] [CrossRef]
- Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. Ficha Técnica Curvularia lunata, 1st ed.; Secretaría de Agricultura y Desarrollo Rural: Ciudad de México, México, 2018; pp. 1–17. [Google Scholar]
- Estrada, G.; Sandoval, I. Patogenicidad de especies de Curvularia en arroz. Fitosanidad 2004, 8, 23–26. [Google Scholar]
- Manzar, N.; Kashyap, A.S.; Sharma, P.K.; Srivastava, A.K. First report of leaf spot on maize caused by Curvularia verruculosa in India. Plant Dis. 2024, 108, 793. [Google Scholar] [CrossRef]
- Palma, A.A.A.; Ureta, J.C.; Rodríguez, J.D. Caracterización morfológica de hongos fitopatógenos asociados a cuatro cultivares de arroz (Oryza sativa) de Panamá. Investig. Agropecu. 2023, 6, 39–52. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lee, J.S.; Lee, H.B.; Kim, H.J. First report of leaf blight caused by Curvularia verruculosa on Zoysiagrass (Zoysia japonica) in Korea. Plant Dis. 2018, 102, 1173. [Google Scholar] [CrossRef]
- Fernández-Mendiola, J.A.; Silva-Valenzuela, M.; Zita-Padilla, G.A.; Espadas-Reséndiz, M.; Colección de Hongos Fitopatógenos. AgroUNAM. Facultad de Estudios Superiores Cuautitlán. UNAM. 2020. Available online: https://virtual.cuautitlan.unam.mx/agrounam/Fitopatologia/Catalogo_hongos_fitopatogenos/pdf/6-Curvularia_verruculosa.pdf (accessed on 4 December 2024).
- Huang, J.; Zheng, L.; Hsiang, T. First report of leaf spot caused by Curvularia verruculosa on Cynodon sp. in Hubei, China. Plant Pathol. 2025, 54, 253. [Google Scholar] [CrossRef]
- Wei, T.; Luo, M.; Zhang, H.; Jia, W.; Zeng, Y.; Jiang, Y. Curvularia verruculosa as new causal pathogen of common vean leaf spot disease in China. Crop Prot. 2022, 162, 106091. [Google Scholar] [CrossRef]
- Sisterna, M.; Wolcan, S. Bipolaris zeae on Pennisetum clandestinum in Argentina. Summa Phytopathol. 1990, 16, 184–188. [Google Scholar]
- Zibani, A.; Benslimane, H.; Sicora, O.; Marian, M. New Records of Bipolaris Species from Algeria. 10 August 2022. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OP218553 (accessed on 8 July 2025).
- Qi, Z. Bipolaris zeae D759 Wheat Isolate. 23 March 2025. Available online: https://www.ncbi.nlm.nih.gov/nuccore/PV368006 (accessed on 8 July 2025).
- Chen, T.X.; Qi, Y.J.; Wang, L.H.; Li, C.J. First report of leaf spot disease on Fagopyrum esculentum caused by Bipolaris zeae in China. Plant Dis. 2021, 105, 3301. [Google Scholar] [CrossRef]
- Arce-Cervantes, O.; Castañeda-Ovando, A. Diversidad de agaves utilizados para la producción de jarabe de aguamiel en el estado de Hidalgo, México. Polibotánica 2024, 58, 265–290. [Google Scholar]
- Liu, Y.; He, P.H.; He, P.; Munir, S.; Wu, Y.; Tang, P.; He, Y.; Kong, B. Bipolaris zeae isolate BSE1 from Axonopus compressus. 22 May 2023. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OR018922.1/ (accessed on 8 July 2025).
- Xu, L.; Qian, Y.; Xue, L.; Yu, B.; Li, C. First report of Bipolaris zeae causing leaf spot on Setaria viridis in China. Plant Dis. 2023, 107, 2862. [Google Scholar] [CrossRef]
- Xue, L.H.; Liu, Y.; Wu, W.X.; Liang, J.; Zhang, L.; Huang, X.Q.; Johnson, R.D.; Li, C.J.; Wang, J.J.; Hu, J.Q. First report of leaf spot of Hemarthria altissima caused by Bipolaris zeae in China. Plant Dis. 2017, 101, 243. [Google Scholar] [CrossRef]
- Sivanesan, A. Graminicolous Species of Bipolaris, Curvularia, Drechslera, Exserohilum and Their Teleomorphs, 10th ed.; Mycol. Pap., CAB International Mycological Institute: Wallingford, UK, 1987; No. 158; pp. 10–267. [Google Scholar]
- Espinosa, B.L.A. Generalidades e importancia de los agaves en México. Herb. CICY 2015, 7, 161–164. [Google Scholar]
- Prado-Martínez, M.; Anzaldo-Hernández, J.; Becerra-Aguilar, B.; Palacios-Juárez, H.; Vargas-Radillo, J.J.; Rentería-Urquiza, M. Caracterización de hojas de mazorca de maíz y de bagazo de caña para la elaboración de una pulpa celulósica mixta. Madera Y Bosques 2012, 18, 37–51. [Google Scholar] [CrossRef]
- Sun, X.; Qi, X.; Wang, W.; Liu, X.; Zhao, H.; Wu, C.; Chang, X.; Zhang, M.; Chen, H.; Gong, G. Etiology and symptoms of maize leaf spot caused by Bipolaris spp. in Sichuan, China. Pathogens 2020, 9, 229. [Google Scholar] [CrossRef]
- Xiao, S.Q.; Zhang, D.; Zhao, J.M.; Yuan, M.Y.; Wang, J.H.; Xu, R.D.; Li, G.F.; Xue, C.S. First report of leaf spot of maize (Zea mays) caused by Bipolaris setariae in China. Plant Dis. 2019, 104, 582. [Google Scholar] [CrossRef]
- Henrickson, M.; Koehler, A.M. First report of Curvularia lunata causing curvularia leaf spot of corn in Delaware. Plant Dis. 2022, 106, 319. [Google Scholar] [CrossRef]
- GBIF Occurrence Download. Available online: https://doi.org/10.15468/c3kkgh (accessed on 9 July 2025).
- Almaguer, M.; Rojas, T.I.; Dobal, V.; Bastita, A.; Aira, M.J. Efecto de la temperatura sobre el crecimiento y germinación de conidios en especies de Curvularia y Bipolaris aisladas del aire. Aerobiología 2012, 29, 1–8. [Google Scholar]
- Ríos, H.E.N.; Ochoa, F.Y.M.; Cerna, C.E.; Landeros, F.J.; Cepeda, M.S.; Rodríguez, G.R. Fungi associated with the tar spot in maize cultivation in Mexico. Rev. Mex. Cienc. Agric. 2017, 8, 457–462. [Google Scholar]
- LA PIÑORRA. La Importancia de la Lluvia para los Hongos. 2025. Available online: https://www.lapinorravinuesa.com/blog/la-importancia-de-la-lluvia-para-los-hongos/ (accessed on 12 July 2025).
- Noriega-Cantú, D.H.; Toledo-Aguilar, R.; Vazquez-Ortiz, R.; Alejo-Jaimes, A.; Garrido-Ramírez, E.R.; Pereyda-Hernández, J.; González-Mateos, R. Relationship between spore fluctuations, environmental conditions and severity of calyx spot on roselle (Hibiscus sabdariffa). Mex. J. Phytopathol. 2020, 38, 1–124. [Google Scholar] [CrossRef]
- Casadiego, U.M.C. Efecto del Incremento de la Temperatura Sobre Hongos Edáficos en un Bosque Altoandino (Cuenca del río Blanco, Cundinamarca). Bachelor’s Thesis, Pontificia Universidad Javeriana, Bogotá, Colombia, June 2011. [Google Scholar]
- Ramos, R.; Meza, V. Efectos de algunos factores meteorológicos sobre la concentración de esporas de hongos en la plaza San Martín de Lima. Ecol. Apl. 2017, 16, 143–149. [Google Scholar] [CrossRef]
- Priwiratama, H.; Wiyono, S.; Hendrastuti, H.S.; Wening, S.; Toding, T.E. Identification and characterization of Curvularia, the causal agent of leaf spot disease of oil palm seedlings in Indonesia. J. Saudi Soc. Agric. Sci. 2025, in press. [CrossRef]
- Cuervo-Parra, J.A.; Romero-Cortes, T.; Aparicio-Burgos, J.E. Curvularia lunata Strain JCPN21. 25 May 2023. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OR047534 (accessed on 10 July 2025).
- Cuervo-Parra, J.A.; Romero-Cortes, T.; Aparicio-Burgos, J.E. Curvularia verruculosa Strain JCPN30. 25 May 2023. Available online: https://www.ncbi.nlm.nih.gov/nuccore/OR047538 (accessed on 10 July 2025).
- Gonzalez-Chingaté, E.J.; Lievano, K.S.; Cubillos, D.D. Evaluación de la efectividad de antagonismo de Trichoderma sp. sobre diferentes hongos fitopatógenos presentes en el cultivo de maíz (Zea mays) en condiciones in vitro. Rev. Cienc. Agropec. 2019, 6, 19–34. [Google Scholar] [CrossRef]
- Blixt, E.; Olso, Å.; Lindahl, B.; Djurle, A.; Yuen, J. Spatiotemporal variation in the fungal community associated with wheat leaves showing symptoms similar to stagonospora nodorum blotch. Eur. J. Plant Pathol. 2009, 126, 373–386. [Google Scholar] [CrossRef]
- Çelik, O.A.; Karakaya, A. Genetic diversity of barley foliar fungal pathogens. Agronomy 2021, 11, 434. [Google Scholar] [CrossRef]
- Kaul, N.; Kashyap, P.L.; Kumar, S.; Singh, D.; Pratap, S.G. Genetic diversity and population structure of head blight disease causing fungus Fusarium graminearum in northern wheat belt of India. J. Fungi 2022, 8, 820. [Google Scholar] [CrossRef]
- Li, Q.; Li, M.; Jiang, Y.; Wang, S.; Xu, K.; Liang, X.; Niu, J.; Wang, C. Assessing genetic resistance in wheat to black point caused by six species in the Yellow and Huai wheat area of China. Plant Dis. 2020, 104, 3131–3134. [Google Scholar] [CrossRef]
- Pérez, R.M.C.J.; Sánchez, H.G.; Martínez, F.R.; Garza, R.J.L.; Espinosa, R.J. Género Aspergillus Características e Importancia. 2021. Available online: https://masam.cuautitlan.unam.mx/mohos_toxigenos_unigras/aspergillus.html (accessed on 5 December 2024).
- Pérez, R.M.C.J.; Sánchez, H.G.; Martínez, F.R.; Garza, R.J.L.; Espinosa, R.J. Género Penicillium Características e Importancia. 2021. Available online: https://masam.cuautitlan.unam.mx/mohos_toxigenos_unigras/penicillium.html (accessed on 22 May 2025).
- Duke, R.; Fischer, C. m16 Curvularia lunata Scientific Information. January 2022. Available online: https://www.thermofisher.com/phadia/wo/en/resources/allergen-encyclopedia/m16.html (accessed on 19 August 2025).
- Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. Alternaria alternata Mancha Foliar y Tizón del Amaranto, 1st ed.; Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria: Ciudad de México, México, 2023; pp. 1–7. [Google Scholar]
- Servicio Nacional de Sanidad, Inocuidad y Calidad Agroalimentaria. Fusarium spp. (Hypocreales: Nectriaceae) Podredumbre de Raíces, 1st ed.; Secretario de Agricultura y Desarrollo Rural: Ciudad de México, México, 2020; pp. 1–19. [Google Scholar]
- Arunakumar, G.S.; Nisarga, P.M.N.R.; Arya, N.R.; Monika, B.M.; Dolma, C.S.; Anupama, C.; Akhil, S.; Supriya, K.; Supriya, M.; Sruthi, S.; et al. Diversity of fungal pathogens in leaf spot disease of Indian mulberry and its management. Heliyon 2023, 9, e21750. [Google Scholar] [CrossRef]
- El_Komy, M.H.; Saleh, A.A.; Eranthodi, A.; Molan, Y.Y. Characterization of novel Trichoderma asperellum isolates to select effective biocontrol agents against tomato fusarium wilt. Plant Pathol. J. 2015, 31, 50–60. [Google Scholar] [CrossRef]
- Ezziyyani, M.; Pérez, S.C.; Requena, M.E.; Rubio, L.; Candela, M.E. Biocontrol por Streptomyces rochei –Ziyani–, de la podredumbre del pimiento (Capsicum annuum L.) causada por Phytophthora capsici. An. Biol. 2004, 26, 69–78. [Google Scholar]
- Infante, D.; González, N.; Reyes, Y.; Martínez, B. Evaluación de la efectividad de doce cepas de Trichoderma asperellum Samuels sobre tres fitopatógenos en condiciones de campo. Rev. Prot. Veg. 2011, 26, 194–197. [Google Scholar]
- Ramírez-Olier, J.; Trujillo-Salazar, J.; Osorio-Echeverri, V.; Jaramillo-Ciro, M.M.; Botero-Botero, L. Antagonismo in vitro de Trichoderma asperellum contra Colletotrichum gloesporioides, Curvularia lunata, y Fusarium oxysporum. Rev. UIS Ing. 2019, 18, 159–166. [Google Scholar] [CrossRef]
- Silva-Martínez, K.L.; Allende-Molar, R.R.; Vázquez-Luna, D.; González-Cárdenas, J.C.; Murguía-González, J. Antagonismo in vitro de Trichoderma asperellum contra Fusarium sp. agente causal de gomosis en cítricos. Agro Product. 2016, 9, 20–25. [Google Scholar]
- Romero-Cortes, T.; López-Pérez, P.A.; Pérez, E.V.H.; Medina-Toledo, A.K.; Aparicio-Burgos, J.E.; Cuervo-Parra, J.A. Confrontation of Trichoderma asperellum VSL80 against Aspergillus niger via the effect of enzymatic production. Chil. J. Agric. Anim. Sci. 2019, 35, 68–80. [Google Scholar] [CrossRef]
- Infante, M.D.; Martínez, C.B. Antagonismo de seis cepas de Trichoderma asperellum Samuels, Lieckfeldt & Nirenberg sobre Colletotrichum spp. Rev. Prot. Veg. 2020, 35, 1–8. [Google Scholar]
- Iglesias-Velasco, S.J.; Chan-Cupul, W.; Osuna-Castro, J.A.; Hernández-Ortega, H.A.; Centeno-Leija, S. Antagonismo in vitro, actividad ligninolítica y de hidrolasas de pared celular en la interacción de especies de Trichoderma spp. con Curvularia eragrostidis aislado de piña. Sci. Fungorum 2022, 53, e1430. [Google Scholar]
- Arispe, V.J.L.; Sánchez, A.A.; Galindo, C.M.E.; Vázquez, B.M.E.; Oyervides, G.A.; Rodríguez, G.R. Antagonismo de Trichoderma spp. en hongos asociados al daño de Diatraea saccharalis Fabricius (Lepidoptera: Crambidae) en maíz. Bol. Microbiol. 2019, 34, 17–24. [Google Scholar]
- Yassin, M.T.; Mostafa, A.A.F.; Al-Askar, A.A. In vitro antagonistic activity of Trichoderma spp. against fungal pathogens causing black point disease of wheat. J. Taibah Univ. Sci. 2022, 16, 57–65. [Google Scholar] [CrossRef]
- Gallegos-Morales, G.; Espinoza-Ahumada, C.A.; Figueroa-Reyes, J.; Méndez-Aguilar, R.; Rodríguez-Guerra, R.; Salas-Gómez, A.L.; Peña-Ramos, F.M. Compatibilidad de especies de Trichoderma en la producción y biocontrol de marchitez del chile. Ecosist. Recur. Agropec. 2022, 9, e3066. [Google Scholar] [CrossRef]
| Strains | Host | Sample * | Species | Location | Coordinates | Date |
|---|---|---|---|---|---|---|
| JCPN09 | A. salmiana | M1 | Bipolaris zeae | Apan | 19°64′97″ LN, 98°51′93″ LW | 2019 |
| JCPN10 | A. salmiana | M1 | Bipolaris zeae | Apan | 19°64′97″ LN, 98°51′93″ LW | 2020 |
| JCPN11 | A. salmiana | M2 | Bipolaris zeae | Apan | 19°64′96″ LN, 98°51′91″ LW | 2020 |
| JCPN12 | A. salmiana | M3 | Bipolaris zeae | Apan | 19°64′94″ LN, 98°51′88″ LW | 2020 |
| JCPN13 | A. salmiana | M2 | Bipolaris zeae | Apan | 19°64′96″ LN, 98°51′91″ LW | 2021 |
| JCPN14 | A. salmiana | M4 | Bipolaris zeae | Apan | 19°64′90″ LN, 98°51′84″ LW | 2021 |
| JCPN7 | A. salmiana | M4 | Bipolaris zeae | Apan | 19°64′ 90″ LN, 98°51′84″ LW | 2022 |
| JCPN16 | A. salmiana | M5 | Fusarium lactis | Mineral de la Reforma | 20°07′60″ LN, 98°72′43″ LW | 2022 |
| JCPN18 | A. salmiana | M6 | Curvularia lunata | Mineral de la Reforma | 20°07′78″ LN, 98°72′00″ LW | 2022 |
| JCPN19 | A. lechuguilla | M7 | Curvularia lunata | Mineral de la Reforma | 20°07′81″ LN, 98°72′16″ LW | 2022 |
| JCPN22 | A. lechuguilla | M8 | Alternaria alternata | Mineral de la Reforma | 20°07′67″ LN, 98°72′06″ LW | 2022 |
| JCPN24 | A. lechuguilla | M9 | Curvularia verruculosa | Mineral de la Reforma | 20°07′51″ LN, 98°72′50″ LW | 2022 |
| JCPN27 | A. lechuguilla | M10 | Penicillium diversum | Mineral de la Reforma | 20°07′85″ LN, 98°71′57″ LW | 2022 |
| JCPN28 | A. lechuguilla | M11 | Epicoccum sorghinum | Mineral de la Reforma | 20°07′80″ LN, 98°71′66″ LW | 2022 |
| JCPN33 | A. salmiana | M12 | Bipolaris zeae | Mineral de la Reforma | 20°07′93″ LN, 98°71′35″ LW | 2022 |
| JCPN15 | A. salmiana | M13 | Fusarium lactis | Mineral de la Reforma | 20°07′84″ LN, 98°71′78″ LW | 2023 |
| JCPN17 | A. salmiana | M14 | Penicillium diversum | Mineral de la Reforma | 20°07′89″ LN, 98°72′18″ LW | 2023 |
| JCPN20 | A. lechuguilla | M11 | Epicoccum sorghinum | Mineral de la Reforma | 20°07′80″ LN, 98°71′66″ LW | 2023 |
| JCPN21 | A. lechuguilla | M15 | Curvularia lunata | Mineral de la Reforma | 20°07′78″ LN, 98°72′13″ LW | 2023 |
| JCPN23 | A. lechuguilla | M16 | Curvularia lunata | Mineral de la Reforma | 20°07′74″ LN, 98°72′09″ LW | 2023 |
| JCPN25 | A. lechuguilla | M11 | Curvularia lunata | Mineral de la Reforma | 20°07′80″ LN, 98°71′66″ LW | 2023 |
| JCPN26 | A. lechuguilla | M17 | Myrmaecium rubricosum | Mineral de la Reforma | 20°07′83″ LN, 98°71′57″ LW | 2023 |
| JCPN29 | A. lechuguilla | M18 | Alternaria alternata | Mineral de la Reforma | 20°07′61″ LN, 98°72′16″ LW | 2023 |
| JCPN30 | A. salmiana | M19 | Curvularia verruculosa | Mineral de la Reforma | 20°07′53″ LN, 98°72′87″ LW | 2023 |
| JCPN31 | A. salmiana | M20 | Aspergillus oryzae | Mineral de la Reforma | 20°07′63″ LN, 98°72′53″ LW | 2023 |
| JCPN32 | A. salmiana | M21 | Bipolaris zeae | Mineral de la Reforma | 20°07′85″ LN, 98°71′37″ LW | 2023 |
| Key | Treatment * | Inoculation Site |
|---|---|---|
| T1 | A. salmiana control | Without inoculants |
| T2 | A. lechuguilla control | Without inoculants |
| T3 | C. lunata vs. A. salmiana | Leaves and rhizosphere |
| T4 | C. lunata vs. A. lechuguilla | Leaves and rhizosphere |
| T5 | C. verruculosa vs. A. salmiana | Leaves and rhizosphere |
| T6 | C. verruculosa vs. A. lechuguilla | Leaves and rhizosphere |
| T7 | B. zeae vs. A. salmiana | Leaves and rhizosphere |
| T8 | B. zeae vs. A. lechuguilla | Leaves and rhizosphere |
| T9 | A. alternata vs. A. salmiana | Leaves and rhizosphere |
| T10 | A. alternata vs. A. lechuguilla | Leaves and rhizosphere |
| T11 | F. lactis vs. A. salmiana | Leaves and rhizosphere |
| T12 | F. lactis vs. A. lechuguilla | Leaves and rhizosphere |
| T13 | E. sorghinum vs. A. salmiana | Leaves and rhizosphere |
| T14 | E. sorghinum vs. A. lechuguilla | Leaves and rhizosphere |
| T15 | M. rubricosum vs. A. salmiana | Leaves and rhizosphere |
| T16 | M. rubricosum vs. A. lechuguilla | Leaves and rhizosphere |
| T17 | P. diversum vs. A. salmiana | Leaves and rhizosphere |
| T18 | P. diversum vs. A. lechuguilla | Leaves and rhizosphere |
| T19 | A. oryzae vs. A. salmiana | Leaves and rhizosphere |
| T20 | A. oryzae vs. A. lechuguilla | Leaves and rhizosphere |
| Key | Treatment * | Inoculation Site ¥ |
|---|---|---|
| T1 | A. salmiana control | Without inoculants |
| T2 | A. lechuguilla control | Without inoculants |
| T3 | Z. mays control | Without inoculants |
| T4 | B. zeae vs. A. salmiana | Leaves and rhizosphere |
| T5 | B. zeae vs. A. lechuguilla | Leaves and rhizosphere |
| T6 | B. zeae vs. Z. mays | Leaves and rhizosphere |
| T7 | C. lunata vs. A. salmiana | Leaves and rhizosphere |
| T8 | C. lunata vs. A. lechuguilla | Leaves and rhizosphere |
| T9 | C. lunata vs. Z. mays | Leaves and rhizosphere |
| T10 | C. verruculosa vs. A. salmiana | Leaves and rhizosphere |
| T11 | C. verruculosa vs. A. lechuguilla | Leaves and rhizosphere |
| T12 | C. verruculosa vs. Z. mays | Leaves and rhizosphere |
| Strain | GenBank Accession | Sequence-Based Association | Percent Identity | GenBank Closest Hit | References |
|---|---|---|---|---|---|
| JCPN7 | OR553122 | B. zeae | 99.63% | MT505870 | Visagie et al. [60] |
| JCPN09 | OR553116 | B. zeae | 99.61% | MT505870 | Visagie et al. [60] |
| JCPN10 | OR553117 | B. zeae | 99.60% | MT505870 | Visagie et al. [60] |
| JCPN11 | OR553118 | B. zeae | 99.43% | MT505870 | Visagie et al. [60] |
| JCPN12 | OR553119 | B. zeae | 99.60% | MT505870 | Visagie et al. [60] |
| JCPN13 | OR553120 | B. zeae | 99.60% | MT505870 | Visagie et al. [60] |
| JCPN14 | OR553121 | B. zeae | 99.63% | MT505870 | Visagie et al. [60] |
| JCPN15 | OR047541 | F. lactis | 100% | MT279265 | Camarena-Pozos et al. [61] |
| JCPN16 | OR047542 | F. lactis | 100% | MT279265 | Camarena-Pozos et al. [61] |
| JCPN17 | OR047539 | P. diversum | 98.88% | HM469392 | Jang et al. [62] |
| JCPN18 | OR047532 | C. lunata | 100% | KY100122 | Cristóbal et al. [63] |
| JCPN19 | OR047533 | C. lunata | 99.82% | KY100122 | Cristóbal et al. [63] |
| JCPN20 | OR047543 | E. sorghinum | 100% | MH497563 | Mora-Aguilera et al. [64] |
| JCPN21 | OR047534 | C. lunata | 100% | KY100122 | Cristóbal et al. [63] |
| JCPN22 | OR047547 | A. alternata | 100% | MN615420 | Zhang et al. [65] |
| JCPN23 | OR047535 | C. lunata | 100% | HQ607991 | Rodrigues et al. [66] |
| JCPN24 | OR047537 | C. verruculosa | 99.45% | MH859788 | Vu et al. [67] |
| JCPN25 | OR047536 | C. lunata | 99.82% | KY100122 | Cristóbal et al. [63] |
| JCPN26 | OR047545 | M. rubricosum | 100% | MK041894 | Campos-Rivero et al. [68] |
| JCPN27 | OR047540 | P. diversum | 98.91% | HM469392 | Jang et al. [62] |
| JCPN28 | OR047544 | E. sorghinum | 100% | MH497563 | Mora-Aguilera et al. [64] |
| JCPN29 | OR047546 | A. alternata | 100% | MN481948 | Ma and Wu [69] |
| JCPN30 | OR047538 | C. verruculosa | 99.46% | MH859788 | Vu et al. [67] |
| JCPN31 | OR047548 | A. oryzae | 100% | OQ202044 | Martínez-Pérez et al. [70] |
| JCPN32 | OR553111 | B. zeae | 99.62% | MT505870 | Visagie et al. [60] |
| JCPN33 | OR553112 | B. zeae | 100% | MT505870 | Visagie et al. [60] |
| Key | Treatments * | Damage Percentage ± SE ** |
|---|---|---|
| T1 | A. salmiana control | 0.00 ± 0.00 f |
| T2 | A. lechuguilla control | 0.00 ± 0.00 f |
| T3 | C. lunata vs. A. salmiana | 25.29 ± 0.37 b |
| T4 | C. lunata vs. A. lechuguilla | 24.20 ± 0.18 b |
| T5 | C. verruculosa vs. A. salmiana | 37.29 ± 1.79 a |
| T6 | C. verruculosa vs. A. lechuguilla | 36.36 ± 0.32 a |
| T7 | B. zeae vs. A. salmiana | 34.23 ± 1.27 a |
| T8 | B. zeae vs. A. lechuguilla | 33.38 ± 0.27 a |
| T9 | A. alternata vs. A. salmiana | 36.32 ± 0.29 a |
| T10 | A. alternata vs. A. lechuguilla | 35.64 ± 0.21 a |
| T11 | F. lactis vs. A. salmiana | 33.10 ± 0.11 a |
| T12 | F. lactis vs. A. lechuguilla | 34.53 ± 1.66 a |
| T13 | E. sorghinum vs. A. salmiana | 18.26 ± 0.19 c |
| T14 | E. sorghinum vs. A. lechuguilla | 18.28 ± 0.22 c |
| T15 | M. rubricosum vs. A. salmiana | 11.20 ± 0.23 d |
| T16 | M. rubricosum vs. A. lechuguilla | 11.28 ± 0.08 d |
| T17 | P. diversum vs. A. salmiana | 7.38 ± 1.21 e |
| T18 | P. diversum vs. A. lechuguilla | 7.13 ± 0.19 e |
| T19 | A. oryzae vs. A. salmiana | 0.00 ± 0.00 f |
| T20 | A. oryzae vs. A. lechuguilla | 0.00 ± 0.00 f |
| Key | Treatments * | Damage Percentage ± SE ** |
|---|---|---|
| T1 | A. salmiana control | 0.00 ± 0.00 e |
| T2 | A. lechuguilla control | 0.00 ± 0.00 e |
| T3 | Z. mays control | 0.00 ± 0.00 e |
| T4 | B. zeae vs. A. salmiana | 33.27 ± 0.41 c |
| T5 | B. zeae vs. A. lechuguilla | 31.73 ± 0.11 c |
| T6 | B. zeae vs. Z. mays | 41.50 ± 0.94 a |
| T7 | C. lunata vs. A. salmiana | 23.67 ± 0.61 d |
| T8 | C. lunata vs. A. lechuguilla | 21.07 ± 0.46 d |
| T9 | C. lunata vs. Z. mays | 32.66 ± 1.88 c |
| T10 | C. verruculosa vs. A. salmiana | 36.73 ± 0.61 b |
| T11 | C. verruculosa vs. A. lechuguilla | 34.07 ± 0.30 c |
| T12 | C. verruculosa vs. Z. mays | 38.08 ± 0.35 b |
| Strain | Strain Accession | Specie | Host | Query Cover (%) | Accession Closest Hit | Reference |
|---|---|---|---|---|---|---|
| JCPN34 | PV875568 | C. lunata | A. salmiana | 100 | OR047534 | Cuervo-Parra et al. [159] |
| JCPN35 | PV875569 | C. verruculosa | A. salmiana | 100 | OR047538 | Cuervo-Parra et al. [160] |
| JCPN36 | PV875570 | B. zeae | A. salmiana | 100 | MT505867 | Visagie et al. [79] |
| JCPN37 | PV875624 | C. lunata | A. lechuguilla | 100 | OR047534 | Cuervo-Parra et al. [159] |
| JCPN38 | PV875625 | C. verruculosa | A. lechuguilla | 100 | OR047538 | Cuervo-Parra et al. [160] |
| JCPN39 | PV875626 | B. zeae | A. lechuguilla | 100 | MT505867 | Visagie et al. [79] |
| JCPN40 | PV875637 | C. lunata | Z. mays | 100 | OR047534 | Cuervo-Parra et al. [159] |
| JCPN41 | PV875638 | C. verruculosa | Z. mays | 100 | OR047538 | Cuervo-Parra et al. [160] |
| JCPN42 | PV875639 | B. zeae | Z. mays | 99 | MT505867 | Visagie et al. [79] |
| Pathogenic Fungi | Pathogenic Strain | Trichoderma Strain | BCI € ± SE * |
|---|---|---|---|
| Penicillium diversum | JCPN27 | JEAB02 | 100 ± 0.0 a |
| Curvularia lunata | JCPN18 | JEAB02 | 99.98 ± 0.01 b |
| Bipolaris zeae | JCPN33 | JEAB02 | 99.89 ± 0.08 b |
| Curvularia verruculosa | JCPN24 | JEAB02 | 99.86 ± 0.07 b |
| Alternaria alternata | JCPN29 | JEAB02 | 99.81 ± 0.006 b |
| Aspergillus oryzae | JCPN31 | JEAB02 | 99.81 ± 0.007 b |
| Epicoccum sorghinum | JCPN28 | JEAB02 | 99.81 ± 0.006 b |
| Fusarium lactis | JCPN16 | JEAB02 | 96.01 ± 0.25 c |
| Myrmaecium rubricosum | JCPN26 | JEAB02 | 89.55 ± 0.97 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aparicio-Burgos, J.E.; Romero-Cortes, T.; Armendáriz-Ontiveros, M.M.; Cuervo-Parra, J.A. Leaf Spot Disease Caused by Several Pathogenic Species of the Pleosporaceae Family on Agave salmiana and Agave lechuguilla Plants in Mexico, and Their Biocontrol Using the Indigenous Trichoderma asperellum Strain JEAB02. Agronomy 2025, 15, 2406. https://doi.org/10.3390/agronomy15102406
Aparicio-Burgos JE, Romero-Cortes T, Armendáriz-Ontiveros MM, Cuervo-Parra JA. Leaf Spot Disease Caused by Several Pathogenic Species of the Pleosporaceae Family on Agave salmiana and Agave lechuguilla Plants in Mexico, and Their Biocontrol Using the Indigenous Trichoderma asperellum Strain JEAB02. Agronomy. 2025; 15(10):2406. https://doi.org/10.3390/agronomy15102406
Chicago/Turabian StyleAparicio-Burgos, José Esteban, Teresa Romero-Cortes, María Magdalena Armendáriz-Ontiveros, and Jaime Alioscha Cuervo-Parra. 2025. "Leaf Spot Disease Caused by Several Pathogenic Species of the Pleosporaceae Family on Agave salmiana and Agave lechuguilla Plants in Mexico, and Their Biocontrol Using the Indigenous Trichoderma asperellum Strain JEAB02" Agronomy 15, no. 10: 2406. https://doi.org/10.3390/agronomy15102406
APA StyleAparicio-Burgos, J. E., Romero-Cortes, T., Armendáriz-Ontiveros, M. M., & Cuervo-Parra, J. A. (2025). Leaf Spot Disease Caused by Several Pathogenic Species of the Pleosporaceae Family on Agave salmiana and Agave lechuguilla Plants in Mexico, and Their Biocontrol Using the Indigenous Trichoderma asperellum Strain JEAB02. Agronomy, 15(10), 2406. https://doi.org/10.3390/agronomy15102406







