Effects of Different Organic Fertilizer Gradients on Soil Nematodes and Physicochemical Properties in Subalpine Meadows of the Qinghai-Tibetan Plateau
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Sampling Procedure
2.3. Determination of Soil Physicochemical Properties
2.4. Soil DNA Extraction, Nematode Sequence Amplification, and Soil Nematode Sequencing
2.5. Statistical Analysis
3. Results
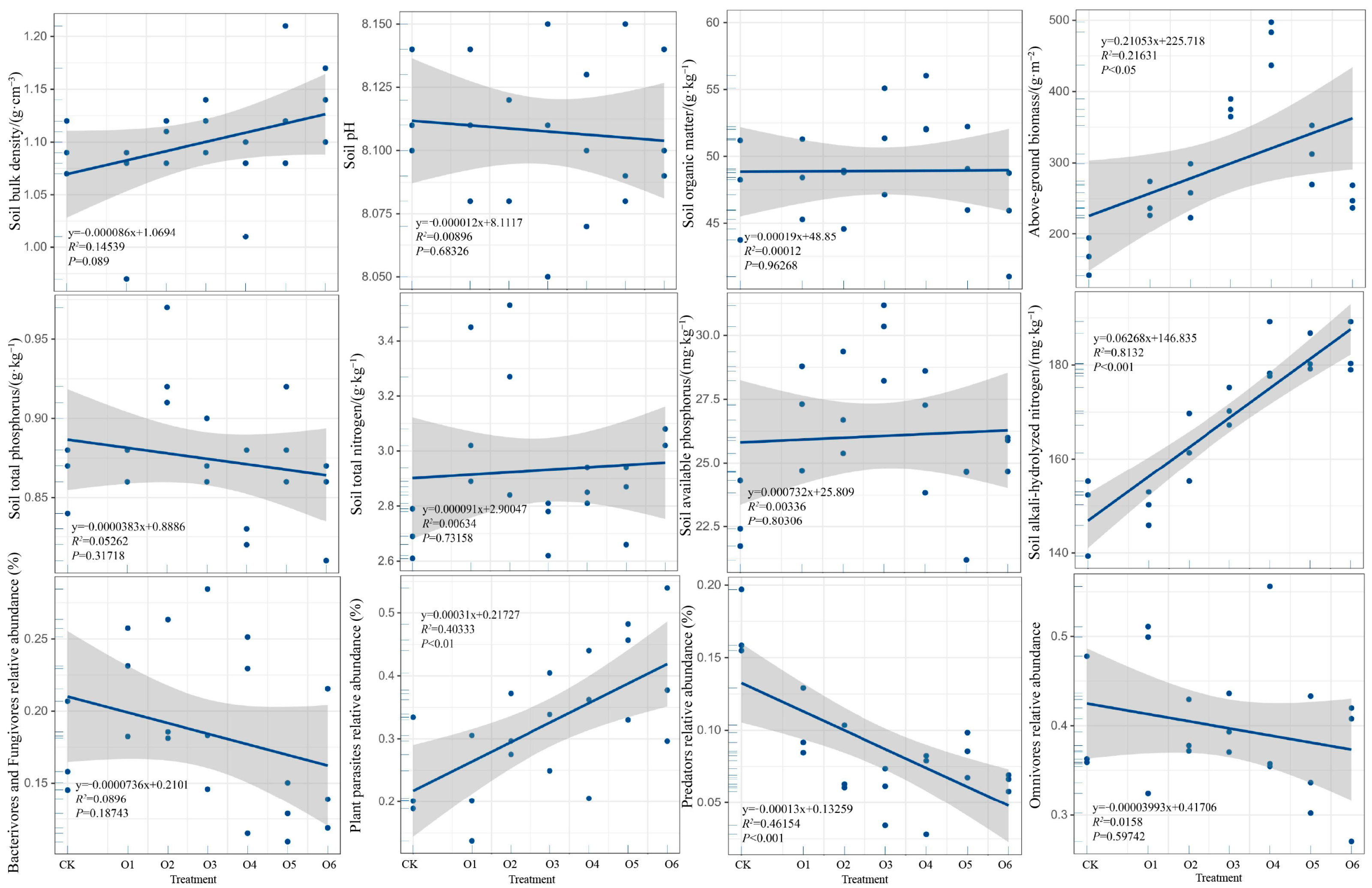
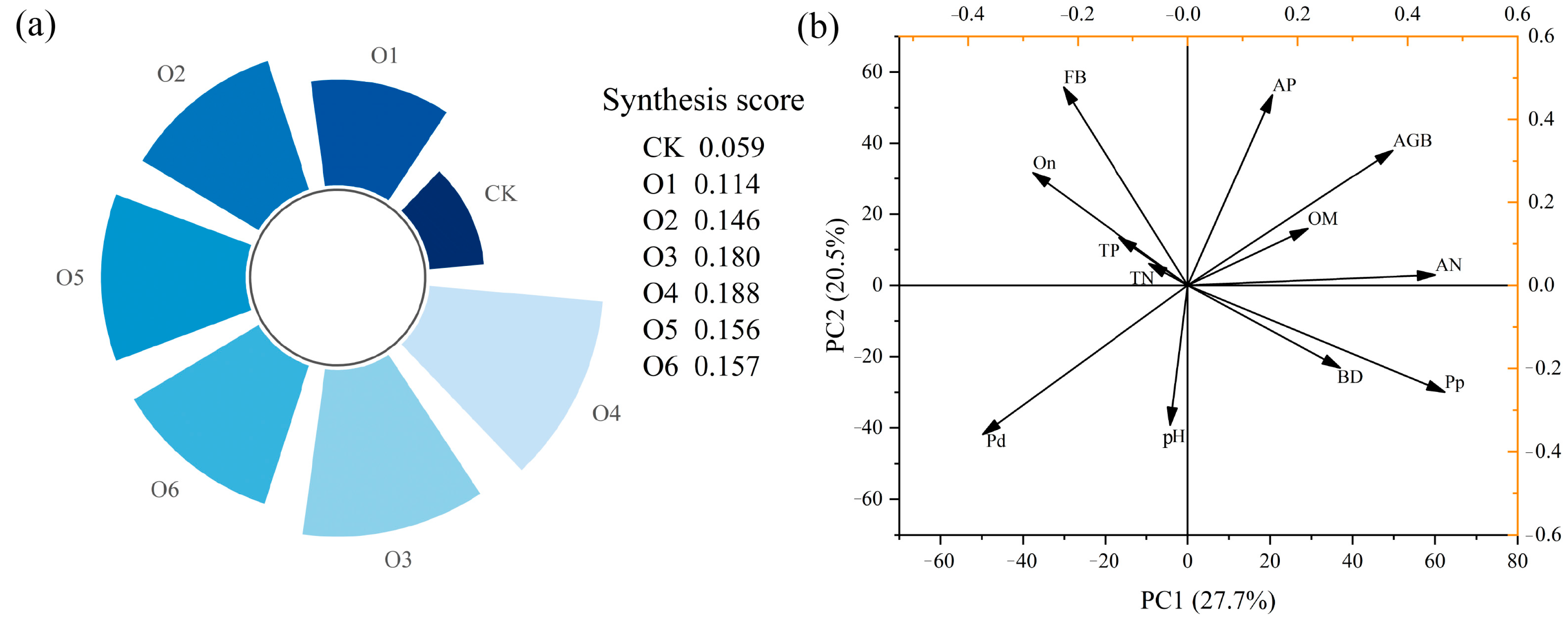
3.1. Effects of Organic Fertilizer on Soil Physicochemical Properties and Aboveground Biomass
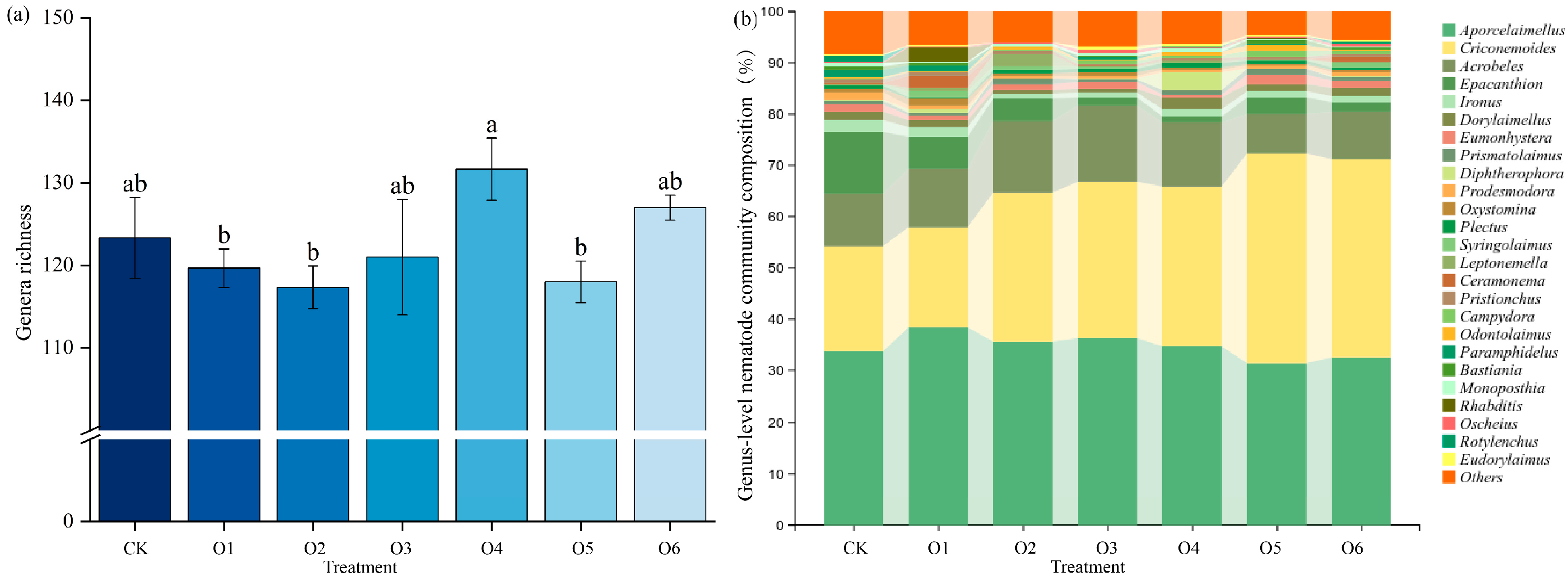
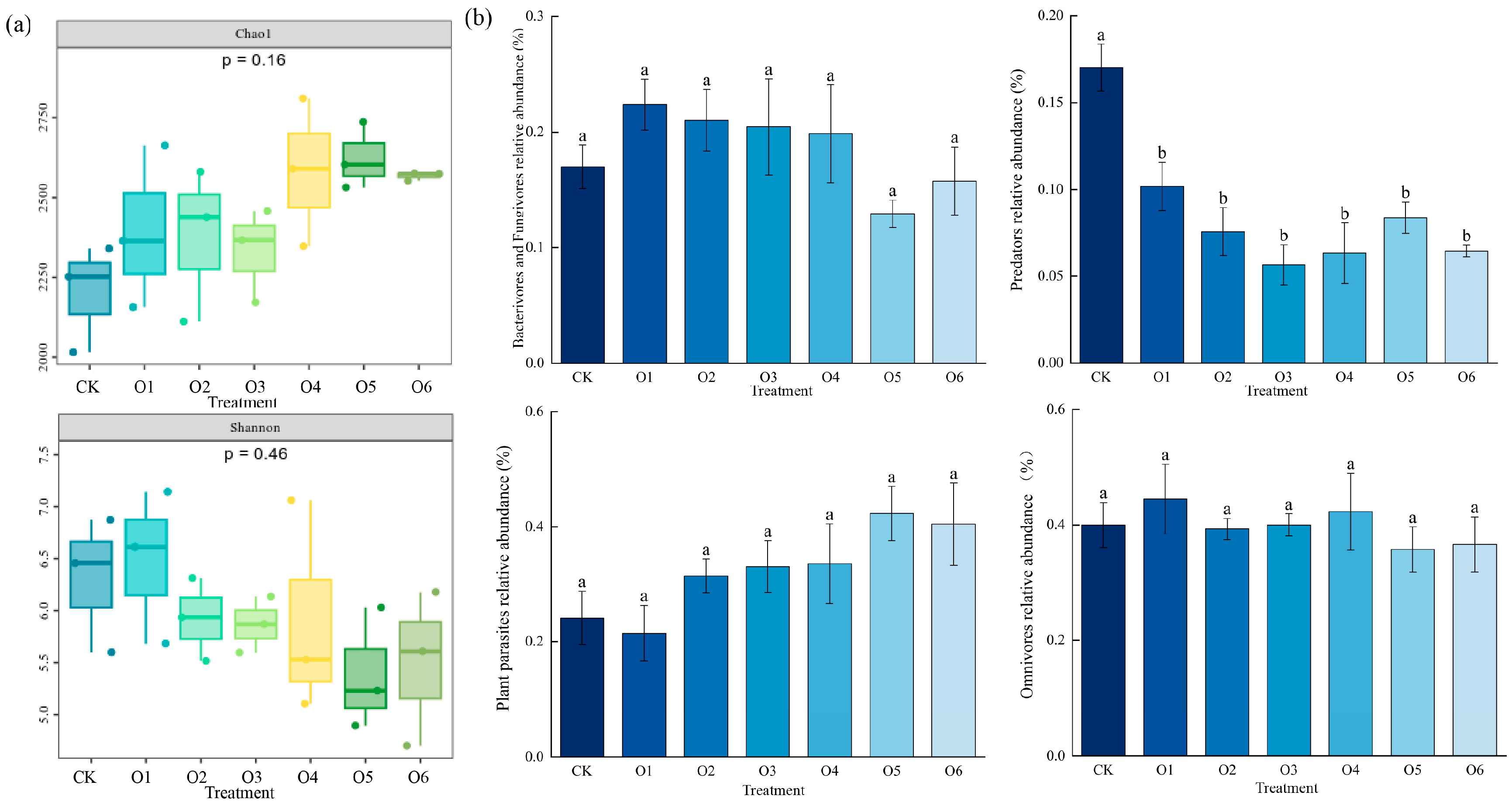
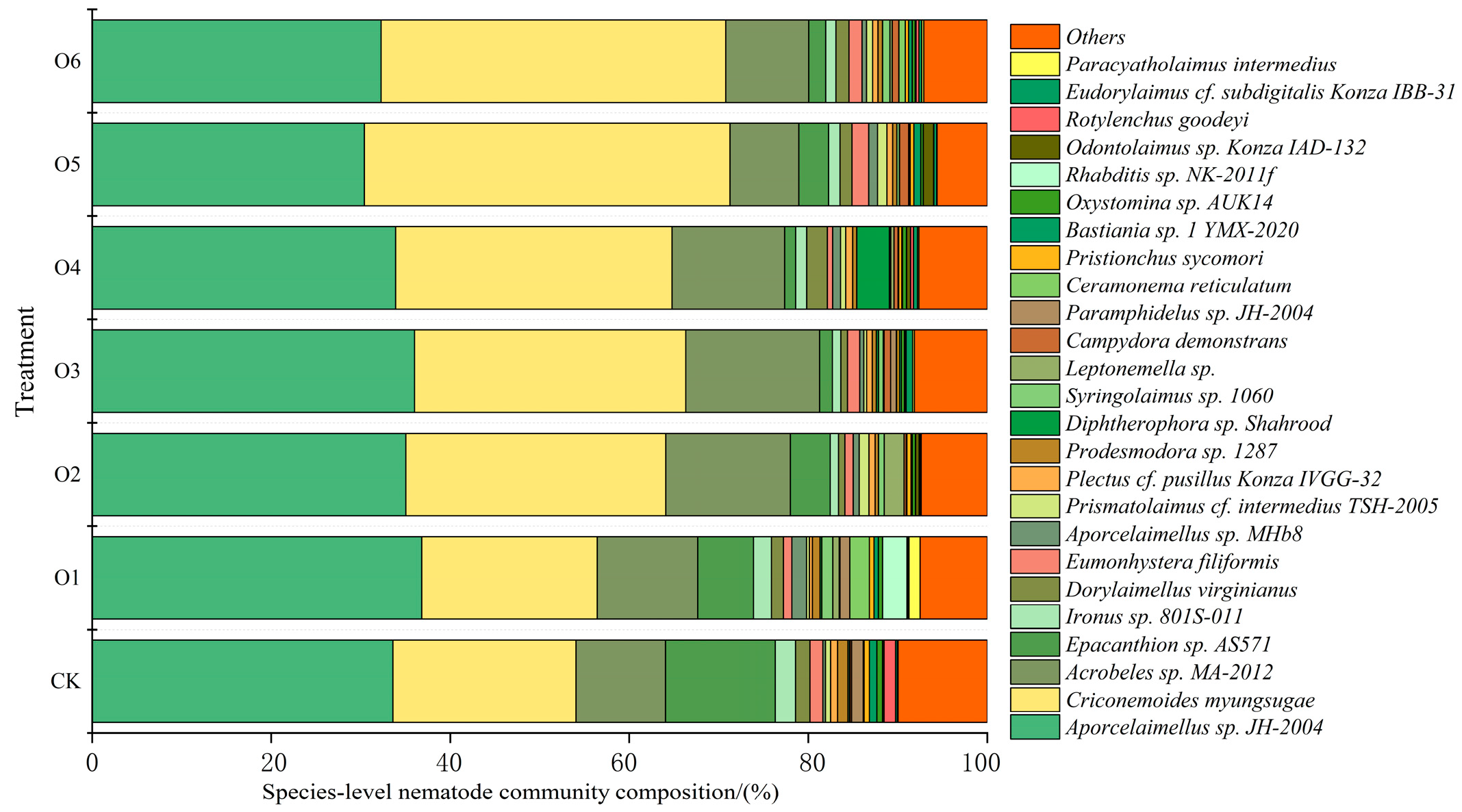
3.2. Effects of Organic Fertilizers on Nematode Diversity
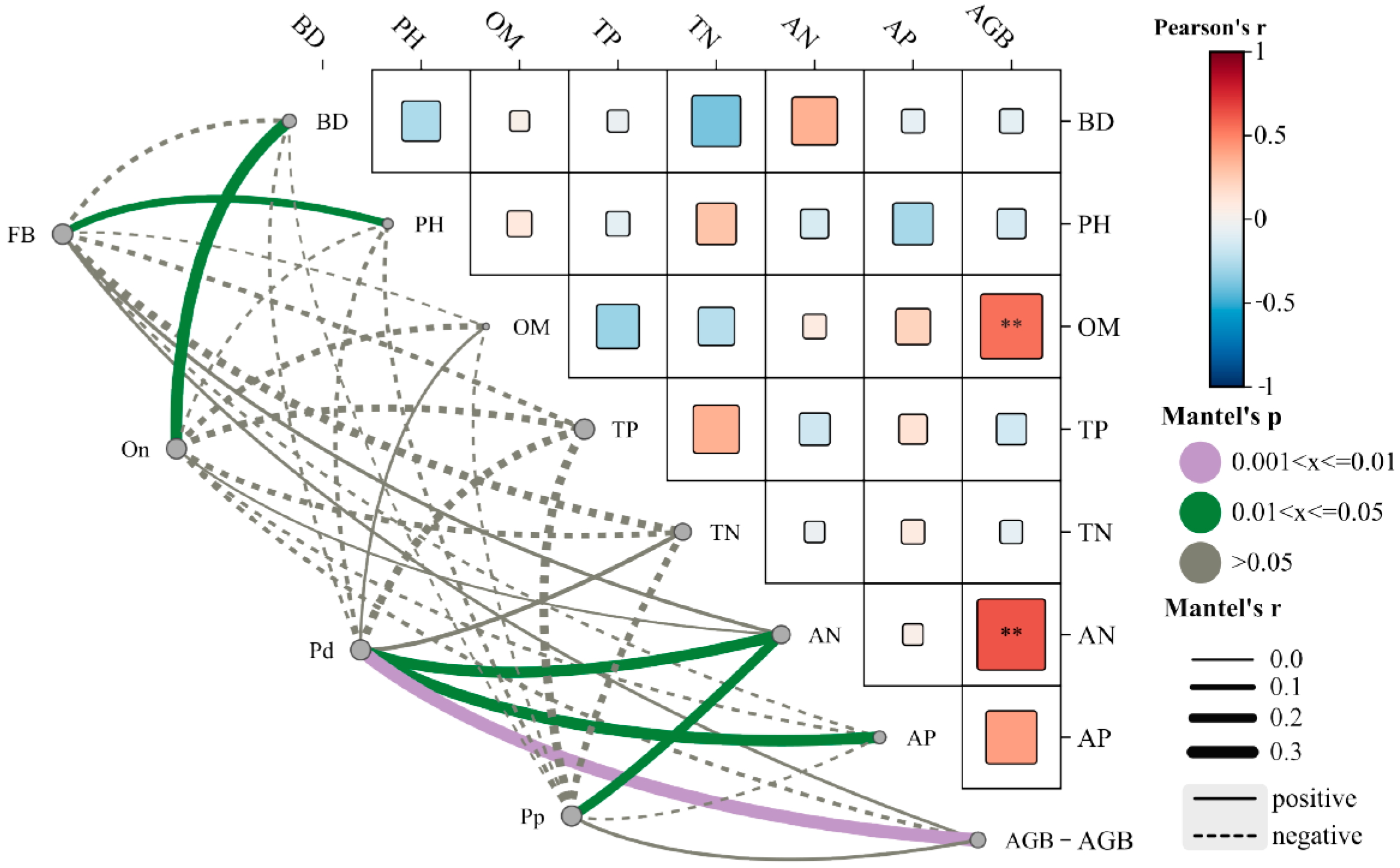
3.3. Relationships Among Grassland Productivity, Soil Physicochemical Properties, and Soil Nematode Communities
4. Discussion
4.1. Effects of Organic Fertilizers on Soil Physicochemical Properties and Aboveground Biomass
4.2. Effects of Organic Fertilizer on Nematode Community Composition
4.3. Relationship Between Grassland Productivity, Soil Physicochemical Properties, and Soil Nematode Community
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miller, D.J. Nomads of the Tibetan Plateau rangelands in western China. I. Pastoral history. Rangel. Arch. 1998, 20, 24–29. [Google Scholar]
- Assessment, M.E. Ecosystems and Human Well-Being: Wetlands and Water; World Resources Institute: Washington, DC, USA, 2005. [Google Scholar]
- Bardgett, R.D.; Bullock, J.M.; Lavorel, S.; Manning, P.; Schaffner, U.; Ostle, N.; Chomel, M.; Durigan, G.; Fry, E.L.; Johnson, D.; et al. Combatting global grassland degradation. Nat. Rev. Earth Environ. 2021, 2, 720–735. [Google Scholar] [CrossRef]
- Phukubye, K.; Mutema, M.; Buthelezi, N.; Muchaonyerwa, P.; Cerri, C.; Chaplot, V. On the impact of grassland management on soil carbon stocks: A worldwide meta-analysis. Geoderma Reg. 2022, 2, e00479. [Google Scholar] [CrossRef]
- Yuan, Z.-Q.; Jiang, X.-J.; Liu, G.-J.; Jin, H.-J.; Chen, J.; Wu, Q.B. Responses of soil organic carbon and nutrient stocks to human-induced grassland degradation in a Tibetan alpine meadow. Catena 2019, 178, 40–48. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nature 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Adugna, G. A review on impact of compost on soil properties, water use and crop productivity. Acad. Res. J. Agric. Sci. Res. 2016, 4, 93–104. [Google Scholar] [CrossRef]
- Kurzemann, F.R.; Plieger, U.; Probst, M.; Spiegel, H.; Sandén, T.; Ros, M.; Insam, H. Long-term fertilization affects soil microbiota, improves yield and benefits soil. Agronomy 2020, 10, 1664. [Google Scholar] [CrossRef]
- Zhao, J.; Wan, S.; Li, Z.; Shao, Y.; Xu, G.; Liu, Z.; Zhou, L.; Fu, S. Dicranopteris-dominated understory as major driver of intensive forest ecosystem in humid subtropical and tropical region. Soil Biol. Biochem. 2012, 49, 78–87. [Google Scholar] [CrossRef]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. In Invertebrate Biodiversity as Bioindicators of Sustainable Landscapes; Elsevier: Amsterdam, The Netherlands, 1999; pp. 19–31. [Google Scholar]
- Du Preez, G.; Daneel, M.; De Goede, R.; Du Toit, M.J.; Ferris, H.; Fourie, H.; Geisen, S.; Kakouli-Duarte, T.; Korthals, G.; Sánchez-Moreno, S.; et al. Nematode-based indices in soil ecology: Application, utility, and future directions. Soil Biol. Biochem. 2022, 169, 108640. [Google Scholar] [CrossRef]
- Boot, C.M.; Hall, E.K.; Denef, K.; Baron, J.S. Long-term reactive nitrogen loading alters soil carbon and microbial community properties in a subalpine forest ecosystem. Soil Biol. Biochem. 2016, 92, 211–220. [Google Scholar] [CrossRef]
- Chen, D.; Xing, W.; Lan, Z.; Saleem, M.; Wu, Y.; Hu, S.; Bai, Y. Direct and indirect effects of nitrogen enrichment on soil organisms and carbon and nitrogen mineralization in a semi-arid grassland. Funct. Ecol. 2019, 33, 175–187. [Google Scholar] [CrossRef]
- Rovira, A.D.; Simon, A. Growth, nutrition and yield of wheat in calcareous sandy loams of South Australia: Effects of soil fumigation, fungicide, nematicide and nitrogen fertilizers. Soil Biol. Biochem. 1985, 17, 279–284. [Google Scholar] [CrossRef]
- Sarathchandra, S.U.; Ghani, A.; Yeates, G.W.; Burch, G.; Cox, N.R. Effect of nitrogen and phosphate fertilisers on microbial and nematode diversity in pasture soils. Soil Biol. Biochem. 2001, 33, 953–964. [Google Scholar] [CrossRef]
- Chen, D.; Lan, Z.; Hu, S.; Bai, Y. Effects of nitrogen enrichment on belowground communities in grassland: Relative role of soil nitrogen availability vs. soil acidification. Soil Biol. Biochem. 2015, 89, 99–108. [Google Scholar] [CrossRef]
- Karbin, S.; Hagedorn, F.; Dawes, M.A.; Niklaus, P.A. Treeline soil warming does not affect soil methane fluxes and the spatial micro-distribution of methanotrophic bacteria. Soil Biol. Biochem. 2015, 86, 164–171. [Google Scholar] [CrossRef]
- Chen, X.; Daniell, T.J.; Neilson, R.; O’FLaherty, V.; Griffiths, B.S. Microbial and microfaunal communities in phosphorus limited, grazed grassland change composition but maintain homeostatic nutrient stoichiometry. Soil Biol. Biochem. 2014, 75, 94–101. [Google Scholar] [CrossRef]
- Wei, C.; Zheng, H.; Li, Q.; Lü, X.; Yu, Q.; Zhang, H.; Cheng, Q.; He, N.; Kardol, P.; Liang, W.; et al. Nitrogen addition regulates soil nematode community composition through ammonium suppression. PLoS ONE 2012, 7, e43384. [Google Scholar] [CrossRef]
- Xu, X.; Jiang, R.; Wang, X.; Liu, S.; Dong, M.; Mao, H.; Li, X.; Ni, Z.; Lv, N.; Deng, X.; et al. Protorhabditis nematodes and pathogen-antagonistic bacteria interactively promote plant health. Microbiome 2024, 12, 221. [Google Scholar] [CrossRef]
- Niu, K.; He, J.-S.; Lechowicz, M.J. Foliar phosphorus content predicts species relative abundance in P-limited Tibetan alpine meadows. Perspect. Plant Ecol. Evol. Syst. 2016, 22, 47–54. [Google Scholar] [CrossRef]
- Lu, K. Analytical Methods of Soil and Agricultural Chemistry; China Agricultural Science and Technology Press: Beijing, China, 1999. [Google Scholar]
- Du, X.-F.; Li, Y.-B.; Han, X.; Ahmad, W.; Li, Q. Using high-throughput sequencing quantitatively to investigate soil nematode community composition in a steppe-forest ecotone. Appl. Soil Ecol. 2020, 152, 103562. [Google Scholar] [CrossRef]
- Wen, Y.C.; Li, H.Y.; Lin, Z.A.; Zhao, B.Q.; Sun, Z.B.; Yuan, L.; Xu, J.K.; Li, Y.Q. Long-term fertilization alters soil properties and fungal community composition in fluvo-aquic soil of the North China Plain. Sci. Rep. 2020, 10, 7198. [Google Scholar] [CrossRef]
- Liu, E.; Yan, C.; Mei, X.; He, W.; Bing, S.H.; Ding, L.; Liu, Q.; Liu, S.; Fan, T. Long-term effect of chemical fertilizer, straw, and manure on soil chemical and biological properties in northwest China. Geoderma 2010, 158, 173–180. [Google Scholar] [CrossRef]
- Six, J.; Conant, R.T.; Paul, E.A.; Paustian, K. Stabilization mechanisms of soil organic matter: Implications for C-saturation of soils. Plant Soil 2002, 241, 155–176. [Google Scholar] [CrossRef]
- Cen, Y.; Guo, L.; Liu, M.; Gu, X.; Li, C.; Jiang, G. Using organic fertilizers to increase crop yield, economic growth, and soil quality in a temperate farmland. PeerJ 2020, 8, e9668. [Google Scholar] [CrossRef]
- Sharpley, A.N.; Smith, S.; Bain, W.R. Nitrogen and phosphorus fate from long-term poultry litter applications to oklahoma soils. Soil Sci. Soc. Am. J. 1993, 57, 1131–1137. [Google Scholar] [CrossRef]
- Tejada, M.; Gonzalez, J.; García-Martínez, A.; Parrado, J. Effects of different green manures on soil biological properties and maize yield. Bioresour. Technol. 2008, 99, 1758–1767. [Google Scholar] [CrossRef]
- Rengasamy, P. World salinization with emphasis on Australia. J. Exp. Bot. 2006, 57, 1017–1023. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Briar, S.S.; Grewal, P.S.; Somasekhar, N.; Stinner, D.; Miller, S.A. Soil nematode community, organic matter, microbial biomass and nitrogen dynamics in field plots transitioning from conventional to organic management. Appl. Soil Ecol. 2007, 37, 256–266. [Google Scholar] [CrossRef]
- Yeates, G.W.; Bongers, T.; De Goede, R.G.; Freckman, D.W.; Georgieva, S.S. Feeding habits in soil nematode families and genera—An outline for soil ecologists. J. Nematol. 1993, 25, 315–331. [Google Scholar]
- Neher, D.A. Role of nematodes in soil health and their use as indicators. J. Nematol. 2001, 33, 161. [Google Scholar]
- Ferris, H.; Bongers, T.; de Goede, R.G. A framework for soil food web diagnostics: Extension of the nematode faunal analysis concept. Appl. Soil Ecol. 2001, 18, 13–29. [Google Scholar] [CrossRef]
- Bongers, T.; Ferris, H. Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol. Evol. 1999, 14, 224–228. [Google Scholar] [CrossRef]
- Zhang, X.; Ferris, H.; Mitchell, J.; Liang, W. Ecosystem services of the soil food web after long-term application of agricultural management practices. Soil Biol. Biochem. 2017, 111, 36–43. [Google Scholar] [CrossRef]
- Ali, A.; Liu, X.; Yang, W.; Li, W.; Chen, J.; Qiao, Y.; Gao, Z.; Yang, Z. Impact of Bio-Organic Fertilizer Incorporation on Soil Nutrients, Enzymatic Activity, and Microbial Community in Wheat–Maize Rotation System. Agronomy 2024, 14, 1942. [Google Scholar] [CrossRef]
- Zhang, J.H.; Li, F.C.; Wang, Y.; Xiong, D.H. Soil organic carbon stock and distribution in cultivated land converted to grassland in a subtropical region of china. Environ. Manag. 2014, 53, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Wardle, D.A.; Bardgett, R.D.; Klironomos, J.N.; Setala, H.; van der Putten, W.H.; Wall, D.H. Ecological linkages between aboveground and belowground biota. Science 2004, 304, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Su, Y.; Zeng, J.; Li, X.; Fan, C.; Lu, J.-Z.; Zhou, Z.; Yu, A.; Wang, S.; Scheu, S.; et al. Forest gap regulates soil nematode community through understory plant diversity and soil pH. Geoderma 2024, 451, 117086. [Google Scholar] [CrossRef]
- Kladivko, E.J. Tillage systems and soil ecology. Soil Tillage Res. 2001, 61, 61–76. [Google Scholar] [CrossRef]

| Test Treatment | Application Amount of Organic Fertilizer (kg·ha−1) | N (kg·ha−1) | P2O5 (kg·ha−1) |
|---|---|---|---|
| CK | |||
| O1 | 2250 | 45 | 22.5 |
| O2 | 3750 | 75 | 37.5 |
| O3 | 5250 | 105 | 52.5 |
| O4 | 6750 | 135 | 67.5 |
| O5 | 8250 | 165 | 82.5 |
| O6 | 9750 | 195 | 97.5 |
| Principal Component | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Eigenvalue | 3.60 | 2.44 | 1.71 | 1.55 | 1.32 |
| Variance contribution rate | 27.70 | 18.78 | 13.17 | 11.92 | 10.14 |
| Cumulative variance contribution rate | 27.70 | 46.48 | 59.64 | 71.56 | 81.71 |
| Index | Eigenvector | ||||
| Soil pH (pH) | −0.03 | −0.34 | 0.14 | 0.56 | −0.02 |
| Soil bulk density (BD) | 0.27 | −0.17 | −0.12 | −0.51 | 0.13 |
| Soil organic matter (OM) | 0.18 | 0.15 | −0.37 | 0.41 | 0.33 |
| Aboveground biomass (AGB) | 0.35 | 0.34 | −0.09 | 0.27 | 0.02 |
| Available phosphorus (AP) | 0.11 | 0.47 | 0.16 | 0.02 | 0.34 |
| Available nitrogen (AN) | 0.46 | 0.03 | 0.03 | −0.05 | −0.30 |
| Total nitrogen (TN) | −0.07 | 0.05 | 0.61 | 0.28 | −0.17 |
| Total phosphorus (TP) | −0.12 | 0.10 | 0.52 | −0.18 | 0.02 |
| Goods-coverage of soil nematodes (Gc) | −0.32 | 0.06 | 0.05 | 0.03 | 0.61 |
| Omnivores relative abundance (On) | −0.24 | 0.25 | −0.28 | 0.17 | −0.44 |
| Predators’ relative abundance (Pd) | −0.36 | −0.37 | −0.17 | 0.01 | 0.10 |
| Plant parasites relative abundance (Pp) | 0.43 | −0.24 | 0.22 | 0.04 | 0.23 |
| Bacterivores and Fungivores’ relative abundance (FB) | −0.22 | 0.47 | 0.02 | −0.17 | −0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, R.; Liu, S.; Wang, Z.; Zhou, X.; Bai, Y.; Yin, G.; Cao, W. Effects of Different Organic Fertilizer Gradients on Soil Nematodes and Physicochemical Properties in Subalpine Meadows of the Qinghai-Tibetan Plateau. Agronomy 2025, 15, 2403. https://doi.org/10.3390/agronomy15102403
Dai R, Liu S, Wang Z, Zhou X, Bai Y, Yin G, Cao W. Effects of Different Organic Fertilizer Gradients on Soil Nematodes and Physicochemical Properties in Subalpine Meadows of the Qinghai-Tibetan Plateau. Agronomy. 2025; 15(10):2403. https://doi.org/10.3390/agronomy15102403
Chicago/Turabian StyleDai, Rong, Suxing Liu, Zhengwen Wang, Xiayan Zhou, Yajun Bai, Guoli Yin, and Wenxia Cao. 2025. "Effects of Different Organic Fertilizer Gradients on Soil Nematodes and Physicochemical Properties in Subalpine Meadows of the Qinghai-Tibetan Plateau" Agronomy 15, no. 10: 2403. https://doi.org/10.3390/agronomy15102403
APA StyleDai, R., Liu, S., Wang, Z., Zhou, X., Bai, Y., Yin, G., & Cao, W. (2025). Effects of Different Organic Fertilizer Gradients on Soil Nematodes and Physicochemical Properties in Subalpine Meadows of the Qinghai-Tibetan Plateau. Agronomy, 15(10), 2403. https://doi.org/10.3390/agronomy15102403






