Biosynthesis of Selenium Nanoparticles from Rosa rugosa Extract: Mechanisms and Applications for Sustainable Crop Protection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Rose Extract and Lyophilized Rose Powder
2.3. Biological Synthesis of SeNPs
2.4. The Characterization of SeNPs
2.4.1. UV–Visible Spectrophotometer
2.4.2. Dynamic Light Scattering (DLS)
2.4.3. Scanning Electron Microscope (SEM) and Transmission Electron Microscope (TEM)
2.4.4. Fourier-Transform Infrared (FTIR) Spectroscopy Assay
2.4.5. The Stability of SeNPs
2.5. Analysis on Tomato Growth and Metabolism
2.5.1. Analysis of Enzymatic and Non-Enzymatic Indicators
2.5.2. Quantification of Phytohormones
2.6. Inhibitory Effect of SeNPs on Plant Pathogen Using the Plate Method
2.7. Data Visualization and Statistical Analysis
3. Results and Discussion
3.1. Optimization of Preparation Parameters of SeNPs
3.1.1. Type of Template
3.1.2. The Ratio of Na2O3Se and VC
3.1.3. The Effect of STPP Amount
3.1.4. The Effect of Reaction Temperature
3.1.5. Amount of Lyophilized Rose Powder
3.2. Characterization of SeNPs
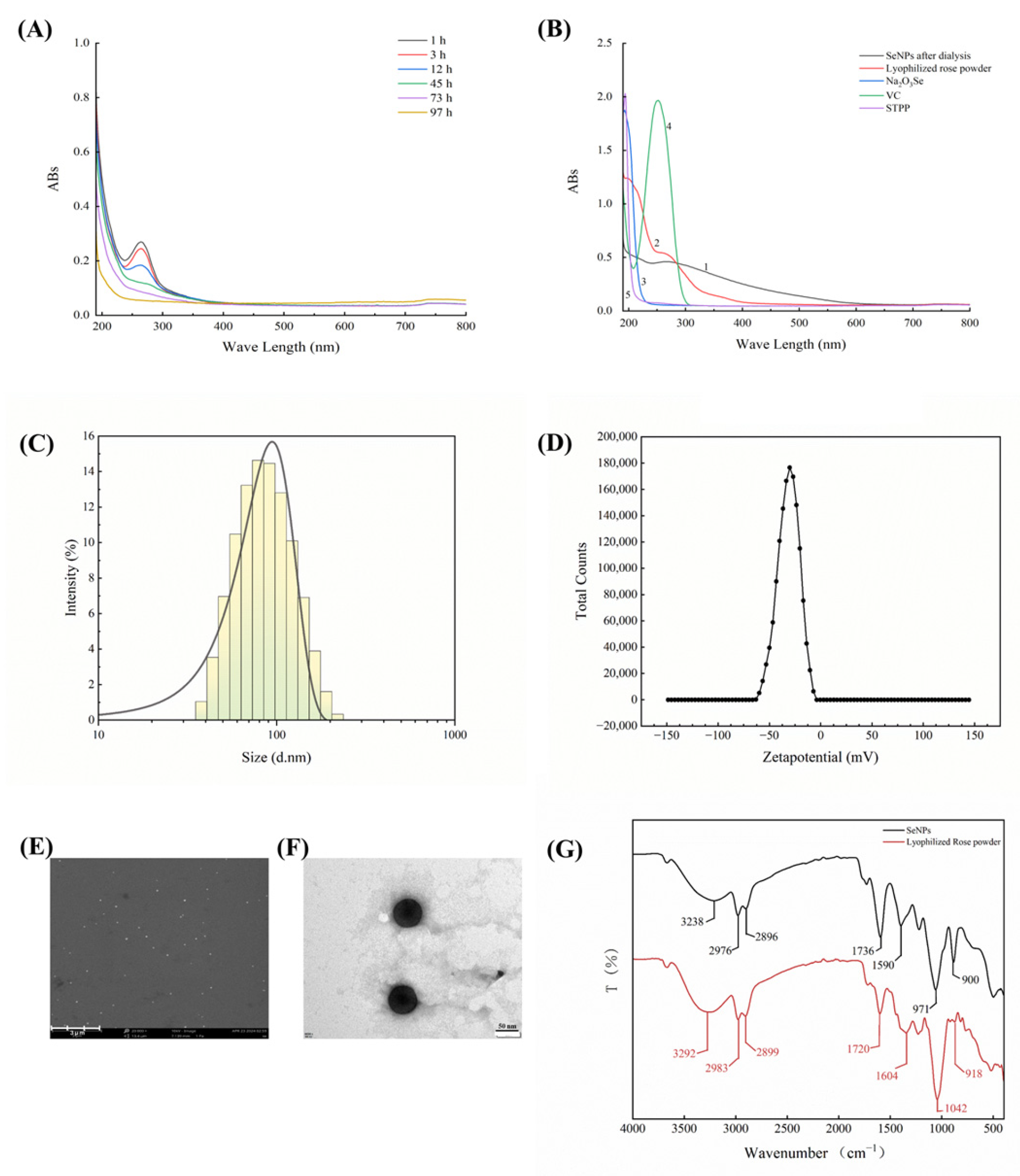
3.2.1. UV-Vis Analysis of SeNPs
3.2.2. Analysis of Particle Size, Zeta Potential, and Morphology of SeNPs
3.2.3. FTIR Analysis of SeNPs
3.2.4. Proposed Formation Mechanism of SeNPs
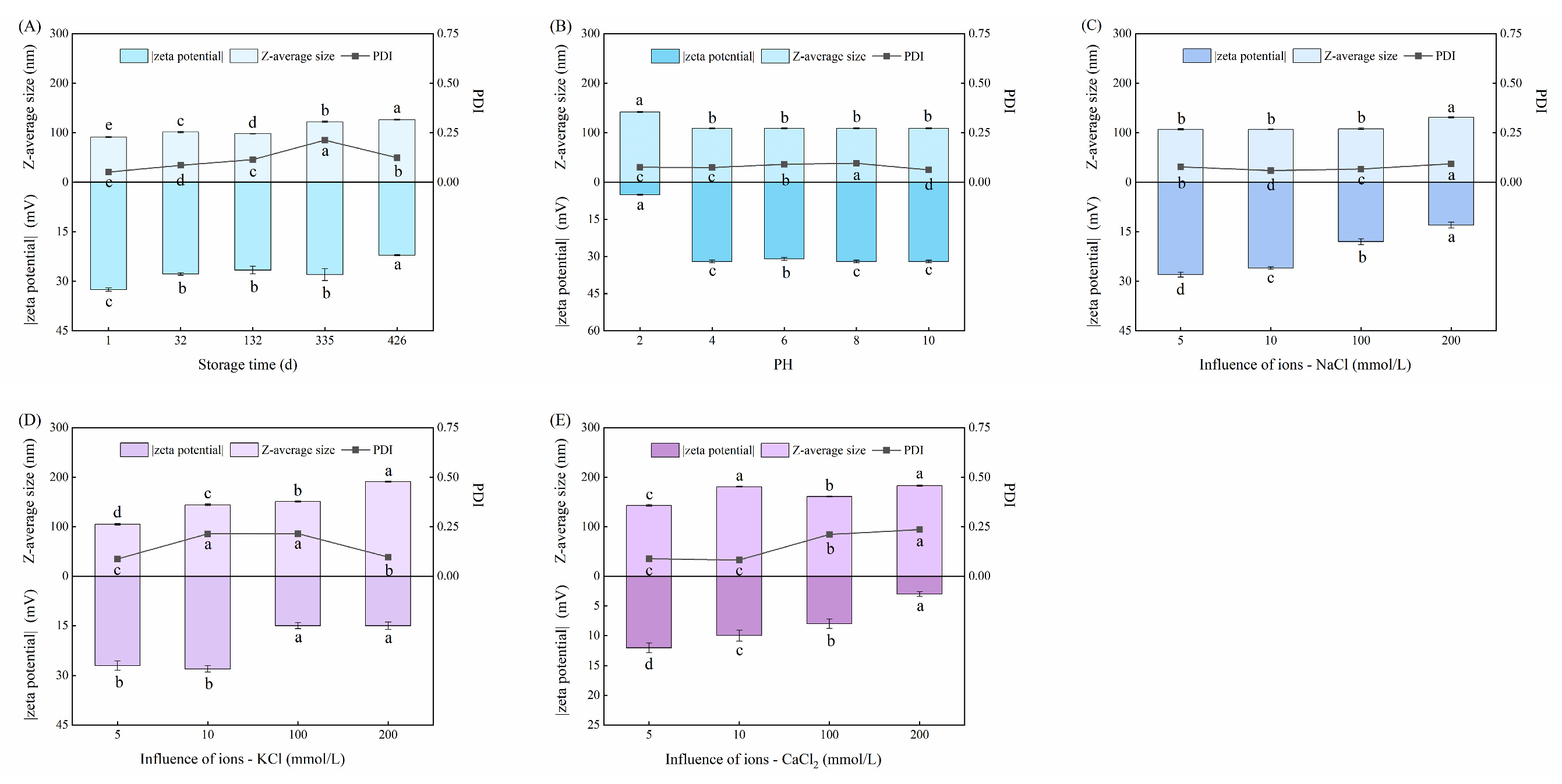
3.3. Stability Test of SeNPs
3.3.1. Effect of Storage Duration on SeNPs
3.3.2. Effect of pH on SeNPs
3.3.3. Effect of Ionic Species on SeNPs
3.4. Application of SeNPs in Crop Health and Disease Control
3.4.1. Effects on Tomato Growth and Metabolism
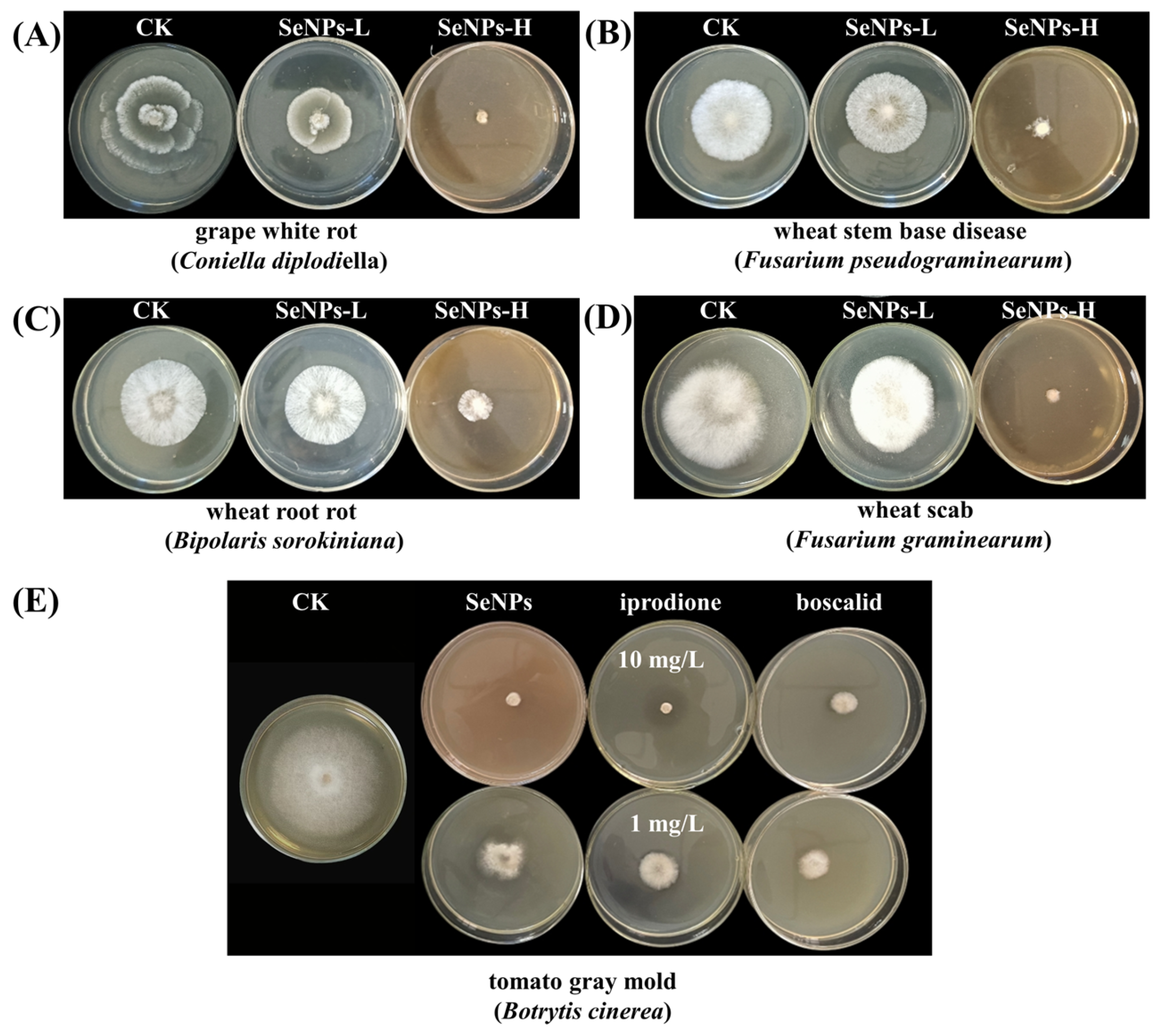
3.4.2. In Vitro Antimicrobial Efficacy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, S.; Liu, X.; Yue, M.; Liu, X.; Yuan, Z.; Xu, F.; Cheng, S.; Rao, S. Comparative Study on Selenium Content and Nutritional Quality of Five Different Varieties of White Tea. Food Chem. X 2025, 26, 102282. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.S.; Srinivasan, S.; Muthuvel, A. Selenium Nanomaterial Is a Promising Nanotechnology for Biomedical and Environmental Remediation: A Detailed Review. Biocatal. Agric. Biotechnol. 2023, 51, 102766. [Google Scholar] [CrossRef]
- Fernandes, R.; Medrano-Padial, C.; Dias-Costa, R.; Domínguez-Perles, R.; Botelho, C.; Fernandes, R.; Barros, A.N. Grape Stems as Sources of Tryptophan and Selenium: Functional Properties and Antioxidant Potential. Food Chem. X 2025, 26, 102260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Song, H.; Guo, Y.; Fan, B.; Huang, Y.; Mao, X.; Liang, K.; Hu, Z.; Sun, X.; Fang, Y.; et al. Benefit–Risk Assessment of Dietary Selenium and Its Associated Metals Intake in China (2017–2019): Is Current Selenium-Rich Agro-Food Safe Enough? J. Hazard. Mater. 2020, 398, 123224. [Google Scholar] [CrossRef]
- Hussain, A.; Lakhan, M.N.; Hanan, A.; Soomro, I.A.; Ahmed, M.; Bibi, F.; Zehra, I. Recent Progress on Green Synthesis of Selenium Nanoparticles—A Review. Mater. Today Sustain. 2023, 23, 100420. [Google Scholar] [CrossRef]
- Nam, N.T.H.; Truong, D.P.; An, T.T.V.; Huong, Q.T.T.; Tuyen, N.N.K.; An, H.; Hai, N.D.; Tinh, N.T.; Linh, N.L.K.; Quynh, N.T.T.; et al. Biological Activity Prospects of Selenium-Decorated Graphene Oxide Composite by Green Synthesis Using Sesbania sesban Flower Extract. Diam. Relat. Mater. 2024, 141, 110563. [Google Scholar] [CrossRef]
- Prasad, K.S.; Patel, H.; Patel, T.; Patel, K.; Selvaraj, K. Biosynthesis of Se Nanoparticles and Its Effect on UV-Induced DNA Damage. Colloids Surf. B Biointerfaces 2013, 103, 261–266. [Google Scholar] [CrossRef]
- Wang, T.; Zhao, H.; Bi, Y.; Fan, X. Preparation and Antioxidant Activity of Selenium Nanoparticles Decorated by Polysaccharides from Sargassum fusiforme. J. Food Sci. 2021, 86, 977–986. [Google Scholar] [CrossRef]
- Gunti, L.; Dass, R.S.; Kalagatur, N.K. Phytofabrication of Selenium Nanoparticles From Emblica officinalis Fruit Extract and Exploring Its Biopotential Applications: Antioxidant, Antimicrobial, and Biocompatibility. Front. Microbiol. 2019, 10, 931. [Google Scholar] [CrossRef]
- Sowndarya, P.; Ramkumar, G.; Shivakumar, M.S. Green Synthesis of Selenium Nanoparticles Conjugated Clausena dentata Plant Leaf Extract and Their Insecticidal Potential against Mosquito Vectors. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1490–1495. [Google Scholar] [CrossRef]
- Satgurunathan, T.; Bhavan, P.S.; Kalpana, R.; Jayakumar, T.; Sheu, J.-R.; Manjunath, M. Influence of Garlic (Allium sativum) Clove-Based Selenium Nanoparticles on Status of Nutritional, Biochemical, Enzymological, and Gene Expressions in the Freshwater Prawn Macrobrachium Rosenbergii (De Man, 1879). Biol. Trace Elem. Res. 2023, 201, 2036–2057. [Google Scholar] [CrossRef]
- D’Amato, R.; Regni, L.; Falcinelli, B.; Mattioli, S.; Benincasa, P.; Dal Bosco, A.; Pacheco, P.; Proietti, P.; Troni, E.; Santi, C.; et al. Current Knowledge on Selenium Biofortification to Improve the Nutraceutical Profile of Food: A Comprehensive Review. J. Agric. Food Chem. 2020, 68, 4075–4097. [Google Scholar] [CrossRef]
- Khai, H.D.; Mai, N.T.N.; Tung, H.T.; Luan, V.Q.; Cuong, D.M.; Ngan, H.T.M.; Chau, N.H.; Buu, N.Q.; Vinh, N.Q.; Dung, D.M.; et al. Selenium Nanoparticles as in Vitro Rooting Agent, Regulates Stomata Closure and Antioxidant Activity of Gerbera to Tolerate Acclimatization Stress. Plant Cell Tiss. Organ. Cult. 2022, 150, 113–128. [Google Scholar] [CrossRef]
- Shi, M.-T.; Zhang, T.-J.; Fang, Y.; Pan, C.-P.; Fu, H.-Y.; Gao, S.-J.; Wang, J. Nano-Selenium Enhances Sugarcane Resistance to Xanthomonas albilineans Infection and Improvement of Juice Quality. Ecotoxicol. Environ. Saf. 2023, 254, 114759. [Google Scholar] [CrossRef]
- Ikram, M.; Raja, N.I.; Mashwani, Z.-U.-R.; Omar, A.A.; Mohamed, A.H.; Satti, S.H.; Zohra, E. Phytogenic Selenium Nanoparticles Elicited the Physiological, Biochemical, and Antioxidant Defense System Amelioration of Huanglongbing-Infected ‘Kinnow’ Mandarin Plants. Nanomaterials 2022, 12, 356. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Ma, C.; Li, C.; Cai, Z.; Shen, Y.; Han, L.; Wang, C.; Tran, J.; Elmer, W.H.; White, J.C.; et al. Aloe Vera Extract Gel-Biosynthesized Selenium Nanoparticles Enhance Disease Resistance in Lettuce by Modulating the Metabolite Profile and Bacterial Endophytes Composition. ACS Nano 2023, 17, 13672–13684. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; Wang, Y.; Pan, L.; Liu, Y.; Xu, W.; Zhai, H.; Zhang, Y.; Shao, H.; Tang, G.; Ge, C. Transformation and Accumulation of Selenium Nanoparticles in the Soil-Rice System under Different Water Management. Ecotoxicol. Environ. Saf. 2025, 291, 117880. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Pan, J.; Gao, D.; Feng, Y.; Zhang, B.; Song, L.; Wang, L.; Ma, X.; Liang, L. Bioactive Metabolite Profiles and Quality of Rosa rugosa during Its Growing and Flower-Drying Process. Food Chem. 2024, 450, 139388. [Google Scholar] [CrossRef]
- Li, J.-M.; Wang, W.-J.; Chen, H.; Lin, S.-Y.; Mao, X.-Y.; Yu, J.-M.; Chen, L.-L. Characterization, in Vitro Antioxidant Activity and Stability of Cattle Bone Collagen Peptides-selenium Chelate. Food Chem. X 2024, 23, 101789. [Google Scholar] [CrossRef]
- Rodriguez-Loya, J.; Lerma, M.; Gardea-Torresdey, J.L. Dynamic Light Scattering and Its Application to Control Nanoparticle Aggregation in Colloidal Systems: A Review. Micromachines 2023, 15, 24. [Google Scholar] [CrossRef]
- Kundrat, V.; Bukvisova, K.; Novak, L.; Prucha, L.; Houben, L.; Zalesak, J.; Vukusic, A.; Holec, D.; Tenne, R.; Pinkas, J. W18 O49 Nanowhiskers Decorating SiO2 Nanofibers: Lessons from In Situ SEM/TEM Growth to Large Scale Synthesis and Fundamental Structural Understanding. Cryst. Growth Des. 2024, 24, 378–390. [Google Scholar] [CrossRef]
- Gong, Y.; Chen, X.; Wu, W. Application of Fourier Transform Infrared (FTIR) Spectroscopy in Sample Preparation: Material Characterization and Mechanism Investigation. Adv. Sample Prep. 2024, 11, 100122. [Google Scholar] [CrossRef]
- Chen, W.; Li, X.; Cheng, H.; Xia, W. Chitosan-Based Selenium Composites as Potent Se Supplements: Synthesis, Beneficial Health Effects, and Applications in Food and Agriculture. Trends Food Sci. Technol. 2022, 129, 339–352. [Google Scholar] [CrossRef]
- Chen, Y.; Stoll, S.; Sun, H.; Liu, X.; Liu, W.; Leng, X. Stability and Surface Properties of Selenium Nanoparticles Coated with Chitosan and Sodium Carboxymethyl Cellulose. Carbohydr. Polym. 2022, 278, 118859. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Wang, L.; Rong, S.; Duan, Y.; Wang, H. Extraction, Purification, Chemical Characterization, and in Vitro Hypoglycemic Activity of Polysaccharides Derived from Rosa laevigata Michx. Int. J. Biol. Macromol. 2024, 279, 135116. [Google Scholar] [CrossRef] [PubMed]
- Hao, H.; Cui, C.; Xing, Y.; Jia, X.; Ma, B.; Kang, W.; Li, T.; Gao, M.; Xu, C. Sulfation of the Extracellular Polysaccharide from the Edible Fungus Stropharia rugosoannulata with Its Antioxidant Activity. J. Future Foods 2023, 3, 37–42. [Google Scholar] [CrossRef]
- Song, J.; Zhou, J.; Li, X.; Li, P.; Tian, G.; Zhang, C.; Zhou, D. Nano-Selenium Stablilized by Konjac glucommannan and Its Biological Activity in Vitro. LWT 2022, 161, 113289. [Google Scholar] [CrossRef]
- Wei, L.; Ji, L.; Rico, C.; He, C.; Shakoor, I.; Fakunle, M.; Lu, X.; Xia, Y.; Hou, Y.; Hong, J. Transcriptomics Reveals the Pathway for Increasing Brassica chinensis L. Yield under Foliar Application of Titanium Oxide Nanoparticles. J. Agric. Food Chem. 2024, 72, 18957–18970. [Google Scholar] [CrossRef]
- Liu, R.; Li, B.; Liu, Y.; Pan, C.; Zhou, Z.; Diao, J.; Zhang, Y. Selenium Nanoparticle Alleviates Penthiopyrad-Induced Oxidative Stress and Restores the Development and Flavor Quality of Tomato Fruit. J. Food Compos. Anal. 2024, 130, 106142. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Kumar, R.; Altaf, M.M.; Kumar, A.; Khan, L.U.; Saqib, M.; Nawaz, M.A.; Saddiq, B.; Bahadur, S.; et al. Phytohormones Mediated Modulation of Abiotic Stress Tolerance and Potential Crosstalk in Horticultural Crops. J. Plant Growth Regul. 2023, 42, 4724–4750. [Google Scholar] [CrossRef]
- El-Saadony, M.T.; Saad, A.M.; Najjar, A.A.; Alzahrani, S.O.; Alkhatib, F.M.; Shafi, M.E.; Selem, E.; Desoky, E.-S.M.; Fouda, S.E.E.; El-Tahan, A.M.; et al. The Use of Biological Selenium Nanoparticles to Suppress Triticum aestivum L. Crown and Root Rot Diseases Induced by Fusarium Species and Improve Yield under Drought and Heat Stress. Saudi J. Biol. Sci. 2021, 28, 4461–4471. [Google Scholar] [CrossRef]
- Domínguez, I.; Ferreres, F.; Pascual Del Riquelme, F.; Font, R.; Gil, M.I. Influence of Preharvest Application of Fungicides on the Postharvest Quality of Tomato (Solanum lycopersicum L.). Postharvest Biol. Technol. 2012, 72, 1–10. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, L.; Liang, M.; Wang, Y.; Bian, Y. Biosynthesis of Selenium Nanoparticles from Rosa rugosa Extract: Mechanisms and Applications for Sustainable Crop Protection. Agronomy 2025, 15, 2385. https://doi.org/10.3390/agronomy15102385
Song L, Liang M, Wang Y, Bian Y. Biosynthesis of Selenium Nanoparticles from Rosa rugosa Extract: Mechanisms and Applications for Sustainable Crop Protection. Agronomy. 2025; 15(10):2385. https://doi.org/10.3390/agronomy15102385
Chicago/Turabian StyleSong, Le, Man Liang, Yingxiu Wang, and Yanli Bian. 2025. "Biosynthesis of Selenium Nanoparticles from Rosa rugosa Extract: Mechanisms and Applications for Sustainable Crop Protection" Agronomy 15, no. 10: 2385. https://doi.org/10.3390/agronomy15102385
APA StyleSong, L., Liang, M., Wang, Y., & Bian, Y. (2025). Biosynthesis of Selenium Nanoparticles from Rosa rugosa Extract: Mechanisms and Applications for Sustainable Crop Protection. Agronomy, 15(10), 2385. https://doi.org/10.3390/agronomy15102385





