Foliar Biofortification with Sodium Selenate Enhances Selenium Content in Ocimum basilicum L. Cultivars in a Totally Controlled Environment System
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
Plant Growth Measures
2.2. Selenium Content
2.3. Content of Nitrogen, Carbon and Sulphur
2.4. Physiological Parameters
2.5. Chlorophyll Content
2.6. Extraction and Measurement of Total Phenolic Content
2.7. Nitrate Content
2.8. Statistical Analysis
3. Results
3.1. Plant Yield and Morphological Traits
3.2. Content of Selenium, Nitrogen, Carbon and Sulphur
3.3. Leaf Greenness Index Values and Total Chlorophyll Content
3.4. Gas Exchange and Photosynthetic Efficiency
3.5. Total Phenolics and Nitrate Content
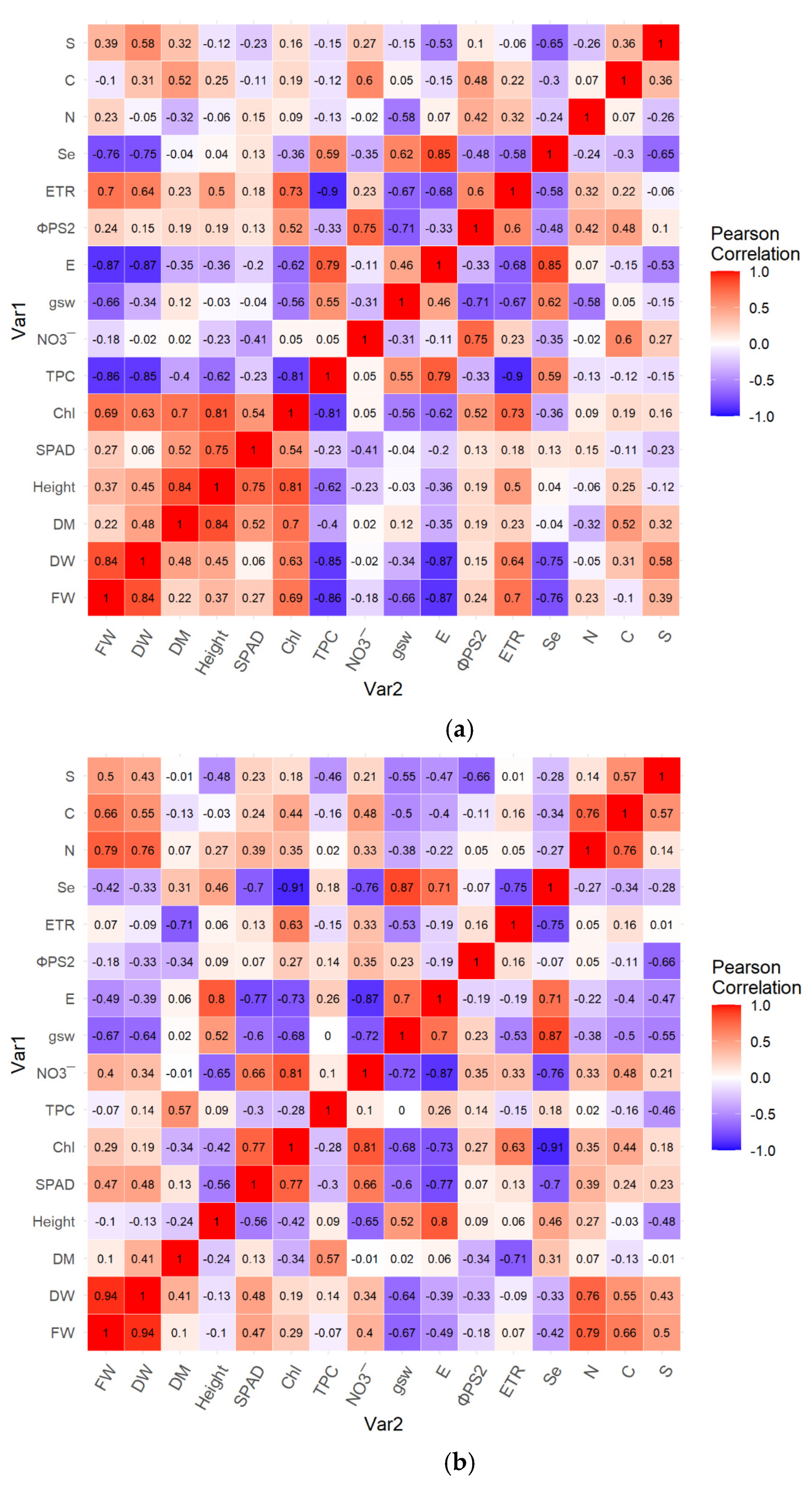
3.6. Cross-Associations of the Treatments and Studied Parameters
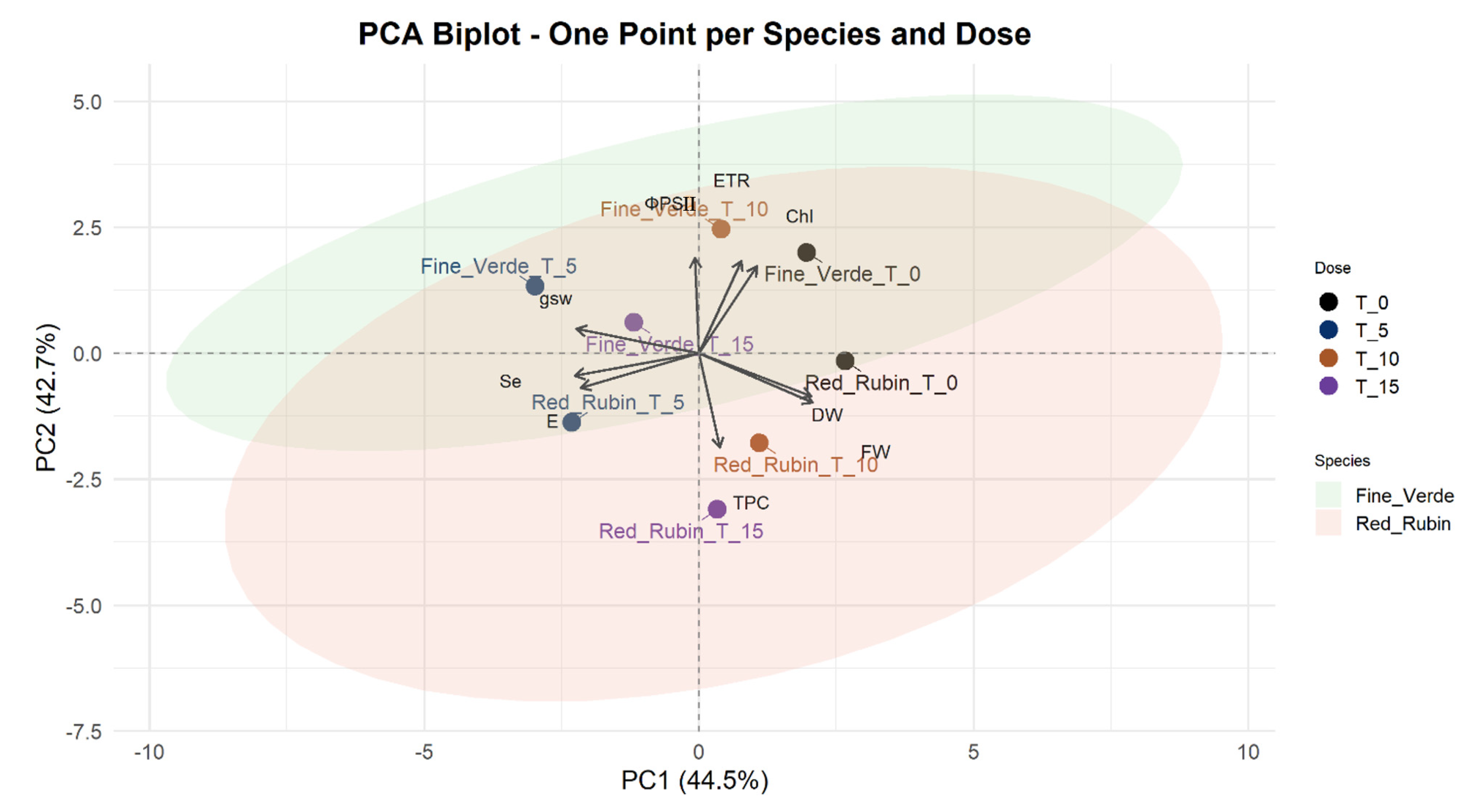
3.7. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sarwar, N.; Akhtar, M.; Kamran, M.A.; Imran, M.; Riaz, M.A.; Kamran, K.; Hussain, S. Selenium Biofortification in Food Crops: Key Mechanisms and Future Perspectives. J. Food Compos. Anal. 2020, 93, 103615. [Google Scholar] [CrossRef]
- Barchielli, G.; Capperucci, A.; Tanini, D. The Role of Selenium in Pathologies: An Updated Review. Antioxidants 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The Argument for Increasing Selenium Intake. Proc. Nutr. Soc. 2002, 61, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Boldrin, P.F.; Faquin, V.; Clemente, A.D.C.S.; De Andrade, T.; Guilherme, L.R.G. Genotypic Variation and Biofortification with Selenium in Brazilian Wheat Cultivars. J. Environ. Qual. 2018, 47, 1371–1379. [Google Scholar] [CrossRef] [PubMed]
- Lara, T.S.; de Lima Lessa, J.H.; de Souza, K.R.D.; Corguinha, A.P.B.; Martins, F.A.D.; Lopes, G.; Guilherme, L.R.G. Selenium Biofortification of Wheat Grain via Foliar Application and Its Effect on Plant Metabolism. J. Food Compos. Anal. 2019, 81, 10–18. [Google Scholar] [CrossRef]
- Alam, A.; Bibi, F.; Deshwal, K.; Sahariya, A.; Bhardwaj, C.; Emmanuel, I. Biofortification of Staple Crops to Eradicate Hidden Hunger: A Review. Nat. Resour. Hum. Health 2021, 2, 91–99. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium Biofortification: Roles, Mechanisms, Responses and Prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef]
- Carucci, F.; Moreno-Martín, G.; Madrid-Albarrán, Y.; Gatta, G.; De Vita, P.; Giuliani, M.M. Selenium Agronomic Biofortification of Durum Wheat Fertilized with Organic Products: Se Content and Speciation in Grain. Agronomy 2022, 12, 2492. [Google Scholar] [CrossRef]
- Ramos, D.P.; Tavares, T.C.D.O.; Sousa, S.A.D.; Nascimento, V.L.; Martinez, R.A.S.; Chagas Junior, A.F.; Fidelis, R.R. Agronomic Biofortification of Cowpea with Selenium by Foliar Fertilization: Effect of Doses in Three Cultivars. J. Plant Nutr. 2020, 43, 538–547. [Google Scholar] [CrossRef]
- Tavan, M.; Wee, B.; Fuentes, S.; Pang, A.; Brodie, G.; Viejo, C.G.; Gupta, D. Biofortification of Kale Microgreens with Selenate-Selenium Using Two Delivery Methods: Selenium-Rich Soilless Medium and Foliar Application. Sci. Hortic. 2024, 323, 112522. [Google Scholar] [CrossRef]
- Viltres-Portales, M.; Sánchez-Martín, M.-J.; Llugany, M.; Boada, R.; Valiente, M. Selenium Biofortification of Microgreens: Influence on Phytochemicals, Pigments and Nutrients. Plant Physiol. Biochem. 2024, 206, 108283. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, F.; Cheng, N.; Chen, P.; Ma, Y.; Zhai, H.; Qi, M.; Liu, N.; Liu, Y.; Meng, L.; et al. Soil and Foliar Selenium Application: Impact on Accumulation, Speciation, and Bioaccessibility of Selenium in Wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 13, 988627. [Google Scholar] [CrossRef] [PubMed]
- Puccinelli, M.; Malorgio, F.; Incrocci, L.; Rosellini, I.; Pezzarossa, B. Effects of Individual and Simultaneous Selenium and Iodine Biofortification of Baby-Leaf Lettuce Plants Grown in Two Different Hydroponic Systems. Horticulturae 2021, 7, 590. [Google Scholar] [CrossRef]
- Sheikhi, H.; Nicola, S.; Delshad, M.; Bulgari, R. Sodium Selenate Biofortification, through Seed Priming, on Dill Microgreens Grown in Two Different Cultivation Systems. Front. Plant Sci. 2024, 15, 1474420. [Google Scholar] [CrossRef]
- Poblaciones, M.J.; García-Latorre, C.; Velazquez, R.; Broadley, M.R. Effects of Selenate Application on Growth, Nutrient Bioaccumulation, and Bioactive Compounds in Broccoli (Brassica oleracea var. italica L.). Horticulturae 2024, 10, 808. [Google Scholar] [CrossRef]
- Profico, C.M.; Hassanpour, M.; Hazrati, S.; Ertani, A.; Mollaei, S.; Nicola, S. Sodium Selenate Biofortification of Basil (Ocimum basilicum L.) and Peppermint (Mentha × piperita L.) Plants Grown in a Floating System under Salinity Stress. J. Agric Food Res. 2025, 21, 101842. [Google Scholar] [CrossRef]
- Santos, E.F.; Filho, E.C.; Fontes, L.E.M.F.; Silva, M.A.P.; Silva, G.N.; Oliveira, A.A.; De Moura Rocha, M.; Silva, V.M.; Reis, A.R. Selenium Agronomic Biofortification and Genotypic Variability in Physiological Responses of Cowpea Plants under Field Conditions. Acta Physiol. Plant. 2025, 47, 18. [Google Scholar] [CrossRef]
- Souza, G.A.; Hart, J.J.; Carvalho, J.G.; Rutzke, M.A.; Albrecht, J.C.; Guilherme, L.R.G.; Kochian, L.V.; Li, L. Genotypic Variation of Zinc and Selenium Concentration in Grains of Brazilian Wheat Lines. Plant Sci. 2014, 224, 27–35. [Google Scholar] [CrossRef]
- Banerjee, S.; Roy, P.; Nandi, S.; Roy, S. Advanced Biotechnological Strategies towards the Development of Crops with Enhanced Micronutrient Content. Plant Growth Regul. 2023, 100, 355–371. [Google Scholar] [CrossRef]
- Silva, V.M.; Nardeli, A.J.; Mendes, N.A.C.; Gabriel Filho, L.R.A.; Gabriel, C.P.C.; Reis, A.R.D. Does Selenium Application Increase the Yield of Cowpea Plants? Evidence from 29 Genotypes on Ureides and Sugar Index Affecting the Yield. J. Soil Sci. Plant Nutr. 2023, 23, 5899–5908. [Google Scholar] [CrossRef]
- Ramos, S.J.; Yuan, Y.; Faquin, V.; Guilherme, L.R.G.; Li, L. Evaluation of Genotypic Variation of Broccoli (Brassica oleracea var. italic) in Response to Selenium Treatment. J. Agric. Food Chem. 2011, 59, 3657–3665. [Google Scholar] [CrossRef]
- Šindelářová, K.; Száková, J.; Tremlová, J.; Mestek, O.; Praus, L.; Kaňa, A.; Najmanová, J.; Tlustoš, P. The Response of Broccoli (Brassica oleracea convar. italica) Varieties on Foliar Application of Selenium: Uptake, Translocation, and Speciation. Food Addit. Contam. Part A 2015, 32, 150928143022009. [Google Scholar] [CrossRef]
- Xue, Y.-F.; Li, X.-J.; Yan, W.; Miao, Q.; Zhang, C.-Y.; Huang, M.; Sun, J.-B.; Qi, S.-J.; Ding, Z.-H.; Cui, Z.-L. Biofortification of Different Maize Cultivars with Zinc, Iron and Selenium by Foliar Fertilizer Applications. Front. Plant Sci. 2023, 14, 1144514. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; Kyriacou, M.; Soteriou, G.A.; Graziani, G.; De Pascale, S.; Rouphael, Y. Zinc Biofortification of Hydroponically Grown Basil: Stress Physiological Responses and Impact on Antioxidant Secondary Metabolites of Genotypic Variants. Front. Plant Sci. 2022, 13, 1049004. [Google Scholar] [CrossRef] [PubMed]
- Ciriello, M.; Formisano, L.; Zarrelli, A.; Corrado, G.; Kyriacou, M.C.; De Pascale, S.; Rouphael, Y. Zinc Biofortification of Genovese Basil: Influence on Mineral Profile and Estimated Daily Intake in Adults and Children. Food Res. Int. 2023, 164, 112374. [Google Scholar] [CrossRef] [PubMed]
- Kozai, T.; Niu, G. Role of the Plant Factory with Artificial Lighting (PFAL) in Urban Areas. In Plant Factory; Elsevier: Amsterdam, The Netherlands, 2020; pp. 7–34. ISBN 978-0-12-816691-8. [Google Scholar]
- Hayashi, E. Advances in Plant Factories: New Technologies in Indoor Vertical Farming; Kozai, T., Ed.; Burleigh Dodds Series in Agricultural Science; Burleigh Dodds Science Publishing: Cambridge, UK, 2023; ISBN 978-1-80146-316-4. [Google Scholar]
- Azizah, N.S.; Irawan, B.; Kusmoro, J.; Safriansyah, W.; Farabi, K.; Oktavia, D.; Doni, F.; Miranti, M. Sweet Basil (Ocimum basilicum L.)―A Review of Its Botany, Phytochemistry, Pharmacological Activities, and Biotechnological Development. Plants 2023, 12, 4148. [Google Scholar] [CrossRef] [PubMed]
- Romano, R.; De Luca, L.; Aiello, A.; Pagano, R.; Di Pierro, P.; Pizzolongo, F.; Masi, P. Basil (Ocimum basilicum L.) Leaves as a Source of Bioactive Compounds. Foods 2022, 11, 3212. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Wanichananan, P.; Janta, S.; Toojinda, T.; Darwell, C.T.; Mosaleeyanon, K. The Influence of Different Light Spectra on Physiological Responses, Antioxidant Capacity and Chemical Compositions in Two Holy Basil Cultivars. Sci. Rep. 2022, 12, 588. [Google Scholar] [CrossRef]
- Viršilė, A.; Laužikė, K.; Sutulienė, R.; Brazaitytė, A.; Kudirka, G.; Samuolienė, G. Distinct Impacts of UV-A Light Wavelengths on Nutraceutical and Mineral Contents in Green and Purple Basil Cultivated in a Controlled Environment. Horticulturae 2023, 9, 1168. [Google Scholar] [CrossRef]
- Chutimanukul, P.; Jindamol, H.; Thongtip, A.; Korinsak, S.; Romyanon, K.; Toojinda, T.; Darwell, C.T.; Wanichananan, P.; Panya, A.; Kaewsri, W.; et al. Physiological Responses and Variation in Secondary Metabolite Content among Thai Holy Basil Cultivars (Ocimum tenuiflorum L.) Grown under Controlled Environmental Conditions in a Plant Factory. Front. Plant Sci. 2022, 13, 1008917. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M. Pre-Harvest UV-B Radiation and Photosynthetic Photon Flux Density Interactively Affect Plant Photosynthesis, Growth, and Secondary Metabolites Accumulation in Basil (Ocimum basilicum) Plants. Agronomy 2019, 9, 434. [Google Scholar] [CrossRef]
- Squadrone, S.; Brizio, P.; Stella, C.; Prearo, M.; Pastorino, P.; Serracca, L.; Ercolini, C.; Abete, M.C. Presence of Trace Metals in Aquaculture Marine Ecosystems of the Northwestern Mediterranean Sea (Italy). Environ. Pollut. 2016, 215, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Squadrone, S.; Brizio, P.; Battuello, M.; Nurra, N.; Sartor, R.M.; Benedetto, A.; Pessani, D.; Abete, M.C. A First Report Of Rare Earth Elements in Northwestern Mediterranean Seaweeds. Mar. Pollut. Bull. 2017, 122, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of Total Carotenoids and Chlorophylls a and b of Leaf Extracts in Different Solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Arnaldos, T.L.; Ferrer, M.A.; García, A.A.C.; Muńoz, R. Changes in Peroxidase Activity and Isoperoxidase Pattern during Strawberry (Fragaria × ananassa) Callus Development. J. Plant Physiol. 2002, 159, 429–435. [Google Scholar] [CrossRef]
- Meenakshi, S.; Gnanambigai, D.M. Total Flavanoid and In Vitro Antioxidant Activity of Two Seaweeds of Rameshwaram Coast. Glob. J. Pharmacol. 2009, 3, 59–62. [Google Scholar]
- Cataldo, D.A.; Maroon, M.; Schrader, L.E.; Youngs, V.L. Rapid Colorimetric Determination of Nitrate in Plant Tissue by Nitration of Salicylic Acid. Commun. Soil Sci. Plant Anal. 1975, 6, 71–80. [Google Scholar] [CrossRef]
- Danso, O.P.; Asante-Badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X.; Zhu, R. Selenium Biofortification: Strategies, Progress and Challenges. Agriculture 2023, 13, 416. [Google Scholar] [CrossRef]
- Cruz, F.J.R. Selenium in Plants: Biofortification, Toxicity, and Redox State Balance. In The Power of Antioxidants-Unleashing Nature’s Defense Against Oxidative Stress; Novo Barros, A., Cristina Santos Abraão, A., Eds.; IntechOpen: London, UK, 2025; Volume 60, ISBN 978-0-85466-206-7. [Google Scholar]
- Somagattu, P.; Chinnannan, K.; Yammanuru, H.; Reddy, U.K.; Nimmakayala, P. Selenium Dynamics in Plants: Uptake, Transport, Toxicity, and Sustainable Management Strategies. Sci. Total Environ. 2024, 949, 175033. [Google Scholar] [CrossRef]
- Sunic, K.; Spanic, V. Genetic Biofortification of Winter Wheat with Selenium (Se). Plants 2024, 13, 1816. [Google Scholar] [CrossRef]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium Enrichment Enhances the Quality and Shelf Life of Basil Leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The Roles of Selenium in Protecting Plants against Abiotic Stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Bian, Z.; Lei, B.; Cheng, R.; Wang, Y.; Li, T.; Yang, Q. Selenium Distribution and Nitrate Metabolism in Hydroponic Lettuce (Lactuca sativa L.): Effects of Selenium forms and Light Spectra. J. Integr. Agric. 2020, 19, 133–144. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Selenite is More Efficient than Selenate in Alleviation of Salt Stress in Lettuce Plants. Acta Biol. Cracoviensia. Ser. Bot. 2015, 57, 49–54. [Google Scholar] [CrossRef]
- Saffaryazdi, A.; Lahouti, M.; Ganjeali, A.; Bayat, H. Impact of Selenium Supplementation on Growth and Selenium Accumulation on Spinach (Spinacia oleracea L.) Plants. Not. Sci. Biol. 2012, 4, 95–100. [Google Scholar] [CrossRef]
- Mroczek-Zdyrska, M.; Strubińska, J.; Hanaka, A. Selenium Improves Physiological Parameters and Alleviates Oxidative Stress in Shoots of Lead-Exposed Vicia faba L. Minor Plants Grown Under Phosphorus-Deficient Conditions. J. Plant Growth Regul. 2017, 36, 186–199. [Google Scholar] [CrossRef]
- Golubkina, N.; Kekina, H.; Caruso, G. Yield, Quality and Antioxidant Properties of Indian Mustard (Brassica juncea L.) in Response to Foliar Biofortification with Selenium and Iodine. Plants 2018, 7, 80. [Google Scholar] [CrossRef]
- Borbély, P.; Molnár, Á.; Valyon, E.; Ördög, A.; Horváth-Boros, K.; Csupor, D.; Fehér, A.; Kolbert, Z. The Effect of Foliar Selenium (Se) Treatment on Growth, Photosynthesis, and Oxidative-Nitrosative Signalling of Stevia rebaudiana Leaves. Antioxidants 2021, 10, 72. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Wang, Q.; Hu, J.; Hu, H.; Li, Y.; Xiang, M.; Wang, D. Integrated Eco-Physiological, Biochemical, and Molecular Biological Analyses of Selenium Fortification Mechanism in Alfalfa. Planta 2022, 256, 114. [Google Scholar] [CrossRef]
- Brito, C.; Andrade, S.; Ferreira, H.; Matos, C.; Martins, S.; Moutinho-Pereira, J. The Synergetic Effect of Light Spectra and Selenium Supplementation on Eruca sativa Mill. Growth and Physiological and Metabolic Responses. Horticulturae 2024, 10, 511. [Google Scholar] [CrossRef]
- Skrypnik, L.; Styran, T.; Savina, T.; Golubkina, N. Effect of Selenium Application and Growth Stage at Harvest on Hydrophilic and Lipophilic Antioxidants in Lamb’s Lettuce (Valerianella locusta L. Laterr.). Plants 2021, 10, 2733. [Google Scholar] [CrossRef]
- Mezeyová, I.; Hegedűsová, A.; Hegedűs, O.; Farkaš, J.; Šlosár, M.; Mezey, J. Effect of Foliar Application of Selenium on Its Uptake and Yields in Basils. AGRFOR Int. J. 2019, 4, 111–119. [Google Scholar] [CrossRef]
- Kiran, A.; Wakeel, A.; Mahmood, K.; Mubaraka, R.; Hafsa; Haefele, S.M. Biofortification of Staple Crops to Alleviate Human Malnutrition: Contributions and Potential in Developing Countries. Agronomy 2022, 12, 452. [Google Scholar] [CrossRef]
- Schiavon, M.; Nardi, S.; dalla Vecchia, F.; Ertani, A. Selenium Biofortification in the 21st Century: Status and Challenges for Healthy Human Nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lu, L.; Shang, Y.; Ran, X.; Liu, Y.; Fang, Y. Can SPAD Values and CIE L*a*b* Scales Predict Chlorophyll and Carotenoid Concentrations in Leaves and Diagnose the Growth Potential of Trees? An Empirical Study of Four Tree Species. Horticulturae 2024, 10, 548. [Google Scholar] [CrossRef]
- Lin, L.; Sun, J.; Cui, T.; Zhou, X.; Liao, M.; Huan, Y.; Yang, L.; Wu, C.; Xia, X.; Wang, Y.; et al. Selenium Accumulation Characteristics of Cyphomandra betacea (Solanum betaceum) Seedlings. Physiol. Mol. Biol. Plants 2020, 26, 1375–1383. [Google Scholar] [CrossRef]
- Trippe, R.C.; Pilon-Smits, E.A.H. Selenium Transport and Metabolism in Plants: Phytoremediation and Biofortification Implications. J. Hazard. Mater. 2021, 404, 124178. [Google Scholar] [CrossRef]
- Sali, A.; Zeka, D.; Fetahu, S.; Rusinovci, I.; Kaul, H.-P. Selenium Supply Affects Chlorophyll Concentration and Biomass Production of Maize (Zea mays L.). J. Land Manag. Food Environ. 2018, 69, 249–255. [Google Scholar] [CrossRef]
- Song, J.; Xin, L.; Gao, F.; Liu, H.; Wang, X. Effects of Foliar Selenium Application on Oxidative Damage and Photosynthetic Properties of Greenhouse Tomato under Drought Stress. Plants 2024, 13, 302. [Google Scholar] [CrossRef]
- Su, L.; Xie, Y.; He, Z.; Zhou, X.; Liu, Y.; Zhang, R.; Li, C. Selenium Mitigates Cd-Induced Oxidative Stress and Photosynthesis Inhibition in Two Cherry Tomato Cultivars. J. Soil Sci. Plant Nutr. 2022, 22, 3212–3227. [Google Scholar] [CrossRef]
- Zou, Z.; Li, M.; Jia, R.; Zhao, H.; He, P.; Zhang, Y.; Guo, A. Genes Encoding Light-Harvesting Chlorophyll a/b-Binding Proteins in Papaya (Carica papaya L.) and Insight into Lineage-Specific Evolution in Brassicaceae. Gene 2020, 748, 144685. [Google Scholar] [CrossRef] [PubMed]
- Ashenafi, E.L.; Nyman, M.C.; Holley, J.M.; Mattson, N.S.; Rangarajan, A. Phenotypic Plasticity and Nutritional Quality of Three Kale Cultivars (Brassica oleracea L. var. acephala) under Field, Greenhouse, and Growth Chamber Environments. Environ. Exp. Bot. 2022, 199, 104895. [Google Scholar] [CrossRef]
- Vrakas, K.; Florou, E.; Koulopoulos, A.; Zervoudakis, G. Physiological Responses of Ocimum basilicum, Salvia officinalis, and Mentha piperita to Leaf Wounding. Plants 2021, 10, 1019. [Google Scholar] [CrossRef]
- Khan, Z.; Thounaojam, T.C.; Chowdhury, D.; Upadhyaya, H. The Role of Selenium and Nano Selenium on Physiological Responses in Plant: A Review. Plant Growth Regul. 2023, 100, 409–433. [Google Scholar] [CrossRef]
- Sepehri, A.; Gharehbaghli, N. Selenium Alleviate Cadmium Toxicity by Improving Nutrient Uptake, Antioxidative and Photosynthetic Responses of Garlic. Russ. J. Plant Physiol. 2019, 66, 152–159. [Google Scholar] [CrossRef]
- Qi, Z.; Huang, X.; Peng, Y.; Wu, H.; Xu, Z.; Tan, B.; Zhong, Y.; Zhu, P.; Gong, W.; Chen, G.; et al. Identifying Superior Growth and Photosynthetic Traits in Eighteen Oak Varieties for Southwest China. Forests 2024, 15, 2006. [Google Scholar] [CrossRef]
- Sharifi, P.; Amirnia, R.; Torkian, M.; Bidabadi, S.S. Protective Role of Exogenous Selenium on Salinity-Stressed Stachys Byzantine Plants. J. Soil Sci. Plant Nutr. 2021, 21, 2660–2672. [Google Scholar] [CrossRef]
- Yadav, S.; Sharma, S.; Sharma, K.D.; Dhansu, P.; Devi, S.; Preet, K.; Ahlawat, P.; Kamboj, P.; Rani, P.; Rani, B.; et al. Selenium Mediated Alterations in Physiology of Wheat under Different Soil Moisture Levels. Sustainability 2023, 15, 1771. [Google Scholar] [CrossRef]
- Amerian, M.; Palangi, A.; Gohari, G.; Ntatsi, G. Enhancing Salinity Tolerance in Cucumber through Selenium Biofortification and Grafting. BMC Plant Biol. 2024, 24, 24. [Google Scholar] [CrossRef]
- Morales-Espinoza, M.C.; Cadenas-Pliego, G.; Pérez-Alvarez, M.; Hernández-Fuentes, A.D.; Cabrera De La Fuente, M.; Benavides-Mendoza, A.; Valdés-Reyna, J.; Juárez-Maldonado, A. Se Nanoparticles Induce Changes in the Growth, Antioxidant Responses, and Fruit Quality of Tomato Developed under NaCl Stress. Molecules 2019, 24, 3030. [Google Scholar] [CrossRef]
- Santander, C.; Vidal, G.; Ruiz, A.; Vidal, C.; Cornejo, P. Salinity Eustress Increases the Biosynthesis and Accumulation of Phenolic Compounds That Improve the Functional and Antioxidant Quality of Red Lettuce. Agronomy 2022, 12, 598. [Google Scholar] [CrossRef]
- Flanigan, P.M.; Niemeyer, E.D. Effect of Cultivar on Phenolic Levels, Anthocyanin Composition, and Antioxidant Properties in Purple Basil (Ocimum basilicum L.). Food Chem. 2014, 164, 518–526. [Google Scholar] [CrossRef]
- Thongtip, A.; Mosaleeyanon, K.; Janta, S.; Wanichananan, P.; Chutimanukul, P.; Thepsilvisut, O.; Chutimanukul, P. Assessing Light Spectrum Impact on Growth and Antioxidant Properties of Basil Family Microgreens. Sci. Rep. 2024, 14, 27875. [Google Scholar] [CrossRef]
| FW | DW | DM | Shoot Height | |
|---|---|---|---|---|
| g m−2 | g m−2 | % | cm | |
| Basil cultivar | ||||
| FV | 2695.21 ± 130.90 b | 137.18 ± 7.62 b | 4.90 ± 0.17 a | 28.40 ± 0.76 a |
| RR | 3068.66 ± 97.59 a | 156.53 ± 4.80 a | 5.24 ± 0.09 a | 26.25 ± 0.39 b |
| Se Biofortification | ||||
| Se_0 | 3101.73 ± 184.23 a | 157.96 ± 5.58 a | 4.88 ± 0.48 a | 26.56 ± 0.87 a |
| Se_5 | 2975.50 ± 240.25 ab | 155.06 ± 7.50 a | 5.44 ± 0.16 a | 28.25 ± 3.84 a |
| Se_10 | 3011.06 ± 317.78 ab | 151.66 ± 23.95 a | 5.15 ± 0.43 a | 27.67 ± 1.10 a |
| Se_15 | 2439.46 ± 362.79 b | 122.73 ± 17.13 b | 4.81 ± 0.34 a | 26.83 ± 0.94 a |
| Interaction | ||||
| FV_0 | 3047.66 ± 129.00 a | 157.66 ± 5.53 ab | 4.74 ± 0.53 a | 27.25 ± 0.25 b |
| FV_5 | 2822.06 ± 129.13 ab | 150.86 ± 6.06 ab | 5.50 ± 0.10 a | 31.50 ± 0.90 a |
| FV_10 | 2740.53 ± 57.20 ab | 132.00 ± 9.33 bc | 4.81 ± 0.15 a | 28.38 ± 0.88 ab |
| FV_15 | 2170.60 ± 160.06 b | 108.20 ± 0.46 c | 4.54 ± 0.09 a | 26.50 ± 0.50 b |
| RR_0 | 3155.80 ± 168.60 a | 158.26 ± 4.00 ab | 5.02 ± 0.14 a | 25.87 ± 0.37 b |
| RR_5 | 3128.93 ± 151.06 a | 159.26 ± 3.53 ab | 5.37 ± 0.15 a | 25.00 ± 0.50 b |
| RR_10 | 3281.60 ± 42.80 a | 171.33 ± 0.40 a | 5.50 ± 0.14 a | 26.97 ± 0.27 b |
| RR_15 | 2708.33 ± 165.00 ab | 137.26 ± 4.19 bc | 5.07 ± 0.15 a | 27.17 ± 0.92 b |
| Basil Cultivar | ** | *** | ns | ** |
| Se Biofortification | ** | *** | ns | ns |
| Cultivar × Se biof. | * | * | ns | ** |
| Se | N | C | S | |
|---|---|---|---|---|
| mg kg−1 (DW) | % | |||
| Basil Cultivar | ||||
| FV | 24.17 ± 5.52 a | 6.32 ± 0.05 a | 35.20 ± 0.36 a | 0.46 ± 0.04 a |
| RR | 22.05 ± 5.19 a | 6.04 ± 0.10 a | 33.81 ± 0.69 a | 0.42 ± 0.02 a |
| Se Biofortification | ||||
| Se_0 | 0.05 ± 0.02 d | 6.24 ± 0.20 a | 34.94 ± 1.01 a | 0.51 ± 0.17 a |
| Se_5 | 24.36 ± 2.07 c | 6.17 ± 0.24 a | 35.48 ± 0.85 a | 0.45 ± 0.04 a |
| Se_10 | 30.55 ± 3.60 b | 6.28 ± 0.09 a | 34.00 ± 1.16 a | 0.43 ± 0.06 a |
| Se_15 | 37.48 ± 1.35 a | 6.01 ± 0.42 a | 33.61 ± 2.82 a | 0.37 ± 0.02 a |
| Interaction | ||||
| FV_0 | 0.07 ± 0.00 a | 6.37 ± 0.18 a | 35.58 ± 0.80 a | 0.59 ± 0.17 a |
| FV_5 | 25.96 ± 0.60 a | 6.32 ± 0.19 a | 35.97 ± 0.74 a | 0.42 ± 0.01 a |
| FV_10 | 32.75 ± 2.76 a | 6.31 ± 0.10 a | 34.09 ± 0.02 a | 0.44 ± 0.01 a |
| FV_15 | 37.91 ± 0.42 a | 6.27 ± 0.01 a | 35.18 ± 0.79 a | 0.37 ± 0.02 a |
| RR_0 | 0.04 ± 0.01 a | 6.12 ± 0.04 a | 34.30 ± 0.25 a | 0.43 ± 0.06 a |
| RR_5 | 22.75 ± 0.97 a | 6.02 ± 0.09 a | 35.00 ± 0.28 a | 0.48 ± 0.03 a |
| RR_10 | 28.35 ± 1.49 a | 6.26 ± 0.02 a | 33.92 ± 1.42 a | 0.42 ± 0.08 a |
| RR_15 | 37.05 ± 1.48 a | 5.76 ± 0.38 a | 32.05 ± 2.54 a | 0.36 ± 0.00 a |
| Basil Cultivar | ns | ns | ns | ns |
| Se Biofortification | *** | ns | ns | ns |
| Cultivar × Se biof. | ns | ns | ns | ns |
| Leaf Greenness Index | Chla+b Content | gsw | E | ΦPSII | ETR | |
|---|---|---|---|---|---|---|
| SPAD | mg g−1 FW | M m−2 s−1 | mM m−2 s−1 | µM m−2 s−1 | ||
| Basil cultivar | ||||||
| FV | 29.97 ± 0.80 b | 1.31 ± 0.04 a | 0.22 ± 0.01 a | 2.39 ± 0.29 a | 0.72 ± 0.01 a | 64.07 ± 1.37 a |
| RR | 32.50 ± 0.77 a | 1.10 ± 0.05 b | 0.18 ± 0.01 b | 2.57 ± 0.35 a | 0.67 ± 0.01 b | 57.47 ± 1.82 b |
| Se Biofortification | ||||||
| Se_0 | 31.65 ± 3.51 a | 1.32 ± 0.02 a | 0.16 ± 0.03 b | 1.60 ± 0.48 c | 0.71 ± 0.03 a | 65.61 ± 3.06 a |
| Se_5 | 32.20 ± 1.55 a | 1.30 ± 0.21 a | 0.19 ± 0.02 ab | 1.89 ± 0.63 bc | 0.71 ± 0.05 a | 60.89 ± 8.30 ab |
| Se_10 | 31.89 ± 2.85 a | 1.14 ± 0.17 b | 0.20 ± 0.03 ab | 2.93 ± 0.47 ab | 0.66 ± 0.05 a | 57.65 ± 4.66 b |
| Se_15 | 29.22 ± 1.24 a | 1.07 ± 0.14 b | 0.24 ± 0.03 a | 3.52 ± 0.20 a | 0.71 ± 0.02 a | 58.93 ± 2.60 ab |
| Interaction | ||||||
| FV_0 | 28.66 ± 0.23 a | 1.33 ± 0.00 ab | 0.19 ± 0.02 a | 1.43 ± 0.07 a | 0.74 ± 0.00 a | 66.53 ± 3.33 ab |
| FV_5 | 31.50 ± 0.90 a | 1.49 ± 0.04 a | 0.21 ± 0.00 a | 2.14 ± 0.68 a | 0.74 ± 0.00 a | 67.78 ± 1.35 a |
| FV_10 | 31.36 ± 2.86 a | 1.27 ± 0.08 abc | 0.23 ± 0.02 a | 2.66 ± 0.16 a | 0.68 ± 0.03 a | 60.88 ± 0.57 ab |
| FV_15 | 28.38 ± 0.88 a | 1.17 ± 0.08 abc | 0.23 ± 0.03 a | 3.35 ± 0.00 a | 0.72 ± 0.01 a | 61.11 ± 0.79 ab |
| RR_0 | 34.63 ± 0.79 a | 1.31 ± 0.02 abc | 0.13 ± 0.00 a | 1.77 ± 0.53 a | 0.69 ± 0.01 a | 64.68 ± 1.10 ab |
| RR_5 | 32.91 ± 1.35 a | 1.12 ± 0.03 bc | 0.17 ± 0.00 a | 1.64 ± 0.05 a | 0.68 ± 0.04 a | 54.01 ± 2.62 b |
| RR_10 | 32.41 ± 1.85 a | 1.00 ± 0.03 bc | 0.18 ± 0.02 a | 3.19 ± 0.41 a | 0.64 ± 0.05 a | 54.43 ± 3.39 b |
| RR_15 | 30.06 ± 0.36 a | 0.98 ± 0.06 c | 0.25 ± 0.01 a | 3.69 ± 0.03 a | 0.69 ± 0.01 a | 56.75 ± 0.20 ab |
| Basil Cultivar | * | ** | * | ns | * | *** |
| Se Biofortification | ns | ** | * | ** | ns | *** |
| Cultivar × Se biof. | ns | * | ns | ns | ns | ** |
| TPC | NO3− | |
|---|---|---|
| mg GE g−1 FW | mg kg−1 FW | |
| Basil cultivar | ||
| FV | 5.49 ± 0.20 b | 1983.82 ± 30.97 a |
| RR | 10.91 ± 0.24 a | 1922.01 ± 39.52 a |
| Se Biofortification | ||
| Se_0 | 7.87 ± 3.23 a | 2031.05 ± 47.39 a |
| Se_5 | 7.97 ± 3.54 a | 2002.53 ± 31.94 a |
| Se_10 | 8.50 ± 3.22 a | 1854.87 ± 43.83 b |
| Se_15 | 8.47 ± 2.66 a | 1923.20 ± 146.81 ab |
| Interaction | ||
| FV_0 | 5.15 ± 0.27 b | 2041.12 ± 36.50 a |
| FV_5 | 4.93 ± 0.25 b | 1989.24 ± 10.70 ab |
| FV_10 | 5.71 ± 0.10 b | 1855.60 ± 32.60 ab |
| FV_15 | 6.19 ± 0.17 b | 2049.30 ± 17.42 a |
| RR_0 | 10.59 ± 0.92 a | 2020.97 ± 42.82 a |
| RR_5 | 11.01 ± 0.49 a | 2015.82 ± 32.60 a |
| RR_10 | 11.29 ± 0.17 a | 1854.14 ± 42.63 ab |
| RR_15 | 10.75 ± 0.45 a | 1797.09 ± 14.99 b |
| Basil Cultivar | *** | ns |
| Se Biofortification | ns | ** |
| Cultivar × Se biof. | ** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Profico, C.M.; Fattahi Siah Kamari, S.; Rabiei, V.; Hazrati, S.; Nicola, S. Foliar Biofortification with Sodium Selenate Enhances Selenium Content in Ocimum basilicum L. Cultivars in a Totally Controlled Environment System. Agronomy 2025, 15, 2368. https://doi.org/10.3390/agronomy15102368
Profico CM, Fattahi Siah Kamari S, Rabiei V, Hazrati S, Nicola S. Foliar Biofortification with Sodium Selenate Enhances Selenium Content in Ocimum basilicum L. Cultivars in a Totally Controlled Environment System. Agronomy. 2025; 15(10):2368. https://doi.org/10.3390/agronomy15102368
Chicago/Turabian StyleProfico, Cosimo M., Saeed Fattahi Siah Kamari, Vali Rabiei, Saeid Hazrati, and Silvana Nicola. 2025. "Foliar Biofortification with Sodium Selenate Enhances Selenium Content in Ocimum basilicum L. Cultivars in a Totally Controlled Environment System" Agronomy 15, no. 10: 2368. https://doi.org/10.3390/agronomy15102368
APA StyleProfico, C. M., Fattahi Siah Kamari, S., Rabiei, V., Hazrati, S., & Nicola, S. (2025). Foliar Biofortification with Sodium Selenate Enhances Selenium Content in Ocimum basilicum L. Cultivars in a Totally Controlled Environment System. Agronomy, 15(10), 2368. https://doi.org/10.3390/agronomy15102368








