A Framework for Understanding Crop–Weed Competition in Agroecosystems
Abstract
1. Introduction
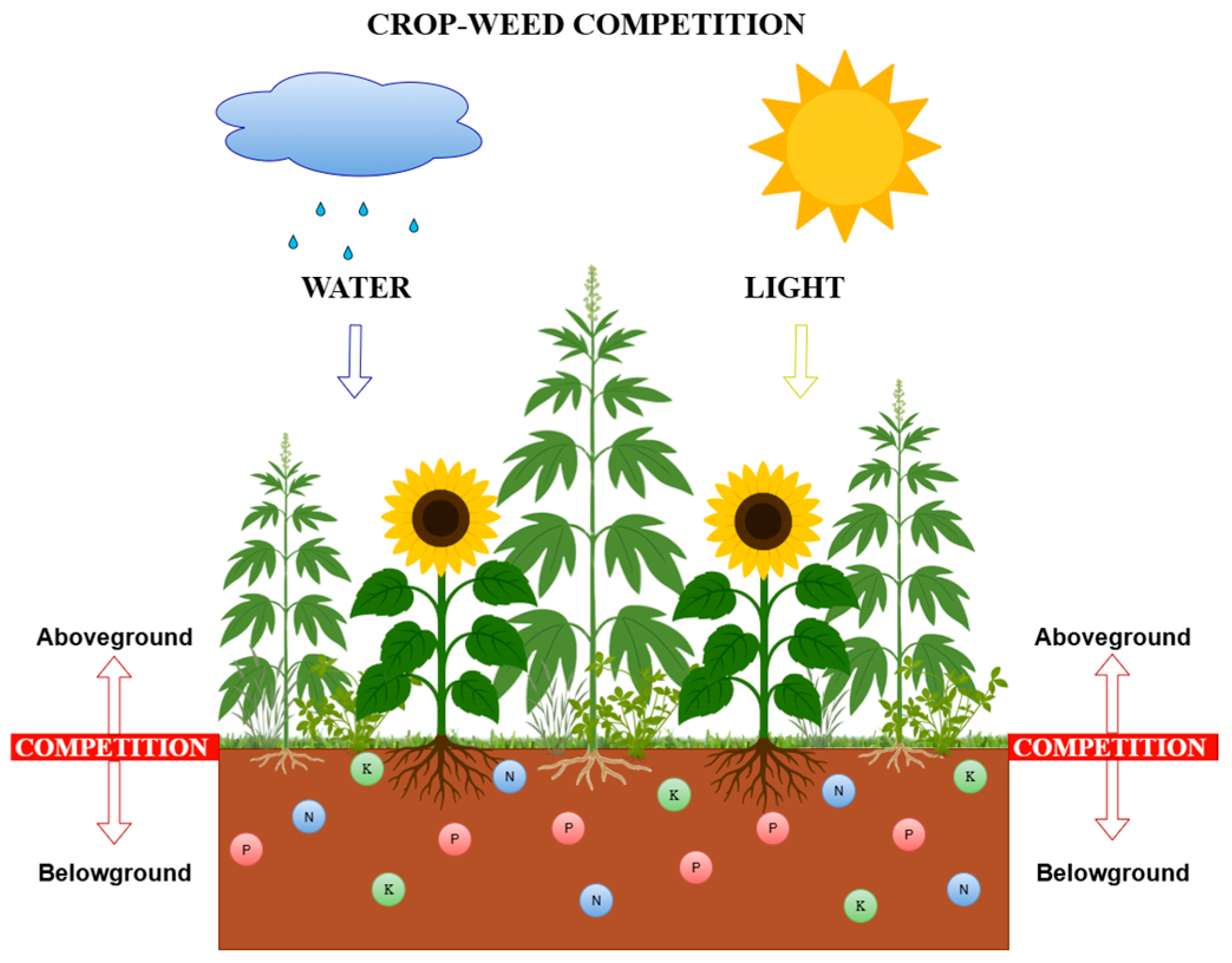
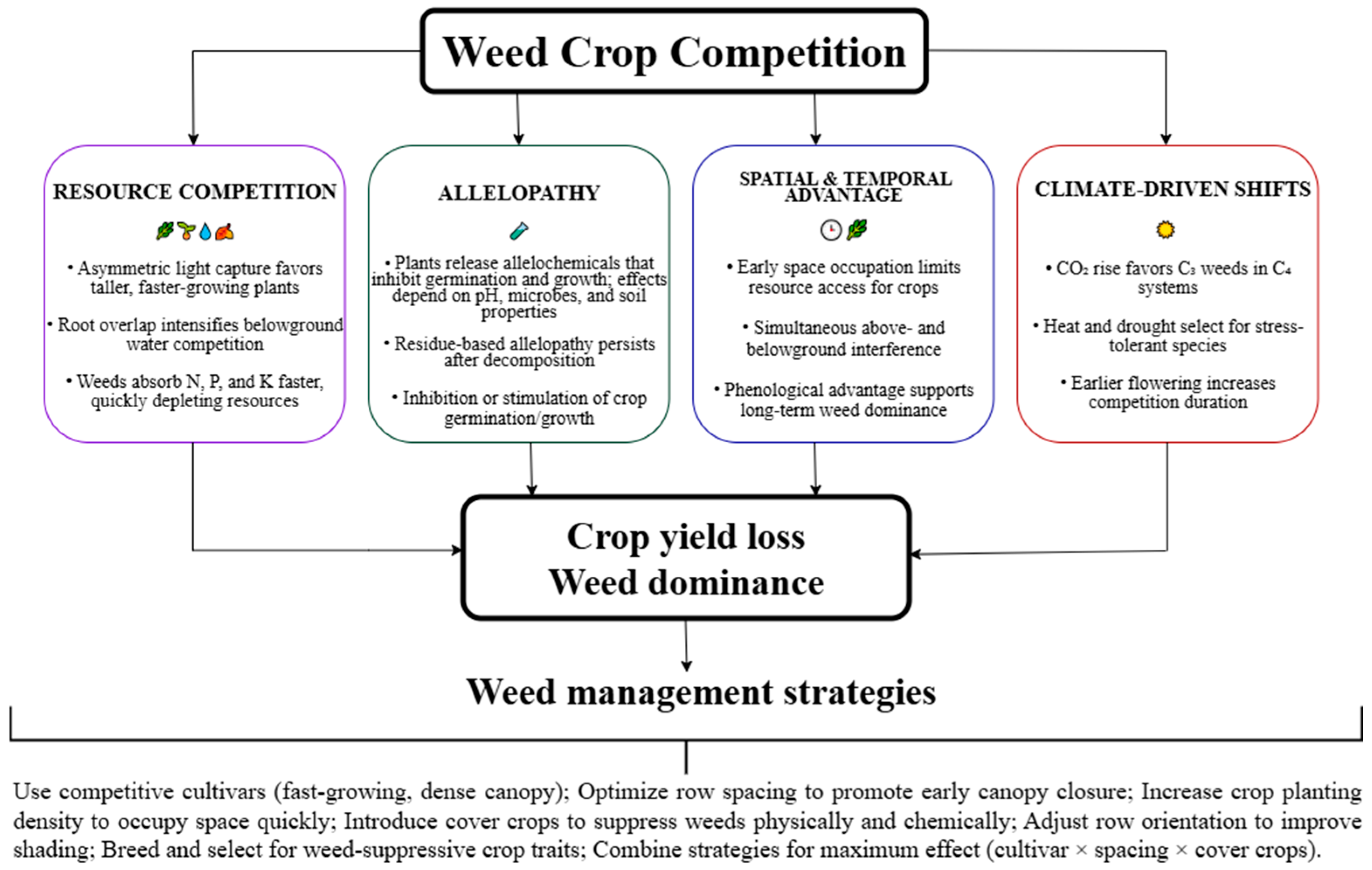
2. Patterns of Crop Weed Competition
2.1. Competition for Space
| Weed Species | Crop | Density (Plants/m2) | Yield Impact (%) | Region | Reference |
|---|---|---|---|---|---|
| A. trifida | Soybean | 2 | 70% | North America | [25] |
| A. trifida | Sunflower | 10 | 60% | Europe | [26] |
| A. artemisiifolia | Soybean | 6 | 90% | North America | [27] |
| A. artemisiifolia | Maize | 25 | 70% | Europe | [27] |
| A. artemisiifolia | Sunflower | 10 | 37% | Europe | [28] |
| Datura stramonium L. | Maize | 10 | 74% | Europe | [29] |
| A. fatua | Wheat | 300 | 70% | North America | [11] |
| Xanthium strumarium L. | Soybean | 0.5–10 | 16–80% | North America | [30,31] |
| X. strumarium | Maize | 4–16 | 30–50% | North America | [32] |
2.2. Competition for Light
2.3. Competition for Moisture
2.4. Competition for Nutrients
2.5. Allelopathy as a Form of Competitive Relationships Between Plants
2.6. Climate-Driven Changes in Weed Species Composition
3. Weed Management Strategies Based on Competition
4. Discussion
5. Future Research Directions
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patterson, D.T. Effects of environmental stress on weed/crop interactions. Weed Sci. 1995, 43, 483–490. [Google Scholar] [CrossRef]
- Kropff, M.J.; van Laar, H.H. Modelling Crop-Weed Interactions; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Savić, A.; Oveisi, M.; Božić, D.; Pavlović, D.; Saulić, M.; Schärer, H.M.; Vrbničanin, S. Competition between Ambrosia artemisiifolia and Ambrosia trifida: Is there a threat of a stronger competitor? Weed Res. 2021, 61, 298–306. [Google Scholar] [CrossRef]
- Liebman, M.; Mohler, C.L.; Staver, C.P. Weed management: A need for ecological approaches. In Ecological Management in Agricultural Weeds; Cambridge University Press: Cambridge, UK, 2001; pp. 1–39. [Google Scholar]
- Thorsted, M.D.; Weiner, J.; Olesen, J.E. Above-and below-ground competition between intercropped winter wheat (Triticum aestivum) and white clover (Trifolium repens). J. Appl. Ecol. 2006, 43, 237–245. [Google Scholar] [CrossRef]
- Singh, R.; Pramanick, B.; Singh, A.P.; Kumar, S.; Kumar, A.; Singh, G. Bio-efficacy of fenoxaprop-p-ethyl for grassy weed control in onion and its residual effect on succeeding maize crop. Indian J. Weed Sci. 2017, 49, 63–66. [Google Scholar]
- Wright, A.; Schnitzer, S.A.; Reich, P.B. Living close to your neighbors: The importance of both competition and facilitation in plant communities. Ecology 2014, 95, 2213–2223. [Google Scholar] [CrossRef]
- Wang, P.; Weiner, J.; Zhou, D.-W. Shoot competition, root competition and reproductive allocation in Chenopodium acuminatum. J. Ecol. 2014, 102, 1681–1689. [Google Scholar] [CrossRef]
- Abul-Fatih, H.A.; Bazzaz, F.A. The biology of Ambrosia trifida L. II. Germination, emergence, growth and survival. New Phytol. 1979, 83, 817–827. [Google Scholar] [CrossRef]
- Harrison, S.K.; Regnier, E.E.; Schmoll, J.T.; Webb, J.E. Competition and fecundity of giant ragweed in corn. Weed Sci. 2001, 49, 224–229. [Google Scholar] [CrossRef]
- Willenborg, C.J.; May, W.E.; Gulden, R.H.; Lafond, G.P.; Shirtliffe, S.J. Influence of wild oat (Avena fatua) relative time of emergence and density on cultivated oat yield, wild oat seed production, and wild oat contamination. Weed Sci. 2005, 53, 342–352. [Google Scholar] [CrossRef]
- Leskovšek, R.; Eler, K.; Zamljen, S.A. Weed suppression and maize yield influenced by cover crop mixture diversity and tillage. Agric. Ecosyst. Environ. 2025, 383, 109530. [Google Scholar] [CrossRef]
- Rasmussen, I.A.; Melander, B.; Askegaard, M.; Kristensen, K.; Olesen, J.E. Elytrigia repens population dynamics under different management schemes in organic cropping systems on coarse sand. Eur. J. Agron. 2014, 58, 18–27. [Google Scholar] [CrossRef]
- Derner, J.D.; Briske, D.D. Intraclonal regulation in a perennial caespitose grass: A field evaluation of above- and below-ground resource availability. J. Ecol. 1999, 87, 737–747. [Google Scholar] [CrossRef]
- Saayman, A.E.J. Influence of intraspecific competition on seed production of Datura stramonium L. S. Afr. J. Plant Soil 2000, 17, 70–73. [Google Scholar] [CrossRef]
- Asefa, M.; Worthy, S.J.; Cao, M.; Song, X.; Lozano, Y.M.; Yang, J. Above- and below-ground plant traits are not consistent in response to drought and competition treatments. Ann. Bot. 2022, 130, 939–950. [Google Scholar] [CrossRef]
- Callaway, R.M. The detection of neighbors by plants. Trends Ecol. Evol. 2002, 17, 104–105. [Google Scholar] [CrossRef]
- Hodge, A. The plastic plant: Root responses to heterogeneous supplies of nutrients. New Phytol. 2004, 162, 9–24. [Google Scholar] [CrossRef]
- Ivanova, L.A.; Khapugin, A.A.; Belozerova, A.A.; Ivanov, L.A. Leaf mesophyll traits of Cirsium arvense and Artemisia vulgaris in relation to their harmfulness in agroecosystems. Weed Res. 2025, 65, e70029. [Google Scholar] [CrossRef]
- Petraki, A.; Kousta, A.; Gerakari, M.; Abraham, E.; Chachalis, D. Weed competitive ability of faba bean (Vicia faba), pea (Pisum sativum), and vetch (Vicia sativa) crops under semi-arid conditions. Not. Bot. Horti Agrobot. Cluj-Napoca 2025, 53, 14302. [Google Scholar] [CrossRef]
- Klima, K.; Kasperczyk, M. Harmful effects of creeping thistle (Cirsium arvense (L.) Scop.) in winter wheat cultivation. Ann. Univ. Paedagog. Cracoviensis Stud. Naturae 2025, 10, 12048. [Google Scholar]
- Webster, T.M.; Loux, M.M.; Regnier, E.E.; Harrison, S.K. Giant ragweed (Ambrosia trifida) canopy architecture and interference studies in soybean (Glycine max). Weed Technol. 1994, 8, 559–564. [Google Scholar] [CrossRef]
- Wilson, B.J.; Wright, K.J.; Brain, P.; Clements, M.; Stephens, E. Predicting the competitive effects of weed and crop density on weed biomass, weed seed production and crop yield in wheat. Weed Res. 1995, 35, 265–278. [Google Scholar] [CrossRef]
- Simić, M.; Stefanović, L. Effects of maize density and sowing pattern on weed suppression and maize grain yield. Pestic. Phytomed. 2007, 22, 93–103. [Google Scholar]
- Begna, S.H.; Hamilton, R.I.; Dwyer, L.M.; Stewart, D.W.; Cloutier, D.; Assemat, L.; Smith, D.L. Weed biomass production response to plant spacing and corn (Zea mays) hybrids differing in canopy architecture. Weed Technol. 2001, 15, 647–653. [Google Scholar] [CrossRef]
- Vrbničanin, S.; Malidža, G.; Božić, D.; Rajković, M.; Pavlović, D.; Sarić, M.; Elezović, I. Kompetitivni odnosi između suncokreta (Helianthus annuus L.) i ambrozije trolisne (Ambrosia trifida L.). In Proceedings of the XIV Simpozijum o zaštiti bilja i IX Kongres o korovima, Zbornik rezimea radova, Zlatibor, Srbija, 26–30 November 2012; pp. 128–129. [Google Scholar]
- Barnes, E.R.; Jhala, A.J.; Knezević, S.Z.; Sikkema, P.H.; Lindquist, J.L. Common ragweed (Ambrosia artemisiifolia L.) interference with soybean in Nebraska. Agron. J. 2018, 110, 646–653. [Google Scholar] [CrossRef]
- Varga, P.; Kazinczi, G.; Béres, I.; Kovács, I. Competition between sunflower and Ambrosia artemisiifolia in additive experiments. Cereal Res. Commun. 2006, 34, 701–704. [Google Scholar] [CrossRef]
- Oljača, S.; Vrbnicanin, S.; Simic, M.; Stefanovic, L.; Dolijanovic, Z. Jimsonweed (Datura stramonium L.) interference in maize. Maydica 2007, 52, 329–333. [Google Scholar]
- Stoller, E.W.; Harrison, S.K.; Wax, L.M.; Regnier, E.E.; Nafziger, E.D. Weed interference in soybeans (Glycine max). Rev. Weed Sci. 1987, 3, 155–181. [Google Scholar]
- Rushing, G.S.; Oliver, L.R. Influence of planting date on common cocklebur (Xanthium strumarium) interference in early-maturing soybean (Glycine max). Weed Sci. 1998, 46, 99–104. [Google Scholar] [CrossRef]
- Karimmojeni, H.; Rahimian Mashhadi, H.; Alizadeh, H.M.; Cousens, R.D.; Beheshtian Mesgaran, M. Interference between maize and Xanthium strumarium or Datura stramonium. Weed Res. 2009, 50, 253–261. [Google Scholar] [CrossRef]
- Iqbal, J.; Wright, D. Effects of nitrogen supply on competition between wheat and three annual weed species. Weed Res. 1997, 37, 391–400. [Google Scholar] [CrossRef]
- Aerts, R. Interspecific competition in natural plant communities: Mechanisms, trade-offs and plant-soil feedbacks. J. Exp. Bot. 1999, 50, 29–37. [Google Scholar] [CrossRef]
- Hautier, Y.; Vojtech, E.; Hector, A. The importance of competition for light depends on productivity and disturbance. Ecol. Evol. 2018, 8, 10655–10661. [Google Scholar] [CrossRef]
- Hautier, Y.; Niklaus, P.A.; Hector, A. Competition for light causes plant biodiversity loss after eutrophication. Science 2009, 324, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, J.L.; Mortensen, D.A. Tolerance and velvetleaf (Abutilon theophrasti) suppressive ability of two old and two modern corn (Zea mays) hybrids. Weed Sci. 1998, 46, 569–574. [Google Scholar] [CrossRef]
- Enríquez, S.; Sand-Jensen, K. Variation in light absorption properties of Mentha aquatica L. as a function of leaf form: Implications for plant growth. Int. J. Plant Sci. 2003, 164, 125–136. [Google Scholar] [CrossRef]
- Schmitt, J.; Wulff, R.D. Light spectral quality, phytochrome and plant competition. Trends Ecol. Evol. 1993, 8, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Pierik, R.; Mommer, L.; Voesenek, L.A. Molecular mechanisms of plant competition: Neighbour detection and response strategies. Funct. Ecol. 2013, 27, 841–853. [Google Scholar]
- Kurepin, L.V.; Pharis, R.P. Light signaling and the phytohormonal regulation of shoot growth. Plant Sci. 2014, 229, 280–289. [Google Scholar] [CrossRef]
- Håkansson, S. Competition and Production in Short-Lived Crop-Weed Stands; Report; Institutionen för Växtodling, Sveriges Lantbruksuniversitet: Lomma, Sweden, 1983; Volume 127, p. 85. [Google Scholar]
- Huang, A.H.; Matthews, J.W. Influence of light availability and water depth on competition between Phalaris arundinacea and herbaceous vines. Wetlands 2025, 45, 20. [Google Scholar] [CrossRef]
- McLachlan, S.M.; Swanton, C.J.; Weise, S.F.; Tollenaar, M. Effect of corn-induced shading and temperature on rate of leaf appearance in redroot pigweed (Amaranthus retroflexus L.). Weed Sci. 1993, 41, 590–593. [Google Scholar] [CrossRef]
- McLachlan, S.M.; Tollenaar, M.; Swanton, C.J.; Weise, S.F. Effect of corn-induced shading on dry matter accumulation, distribution, and architecture of redroot pigweed (Amaranthus retroflexus). Weed Sci. 1993, 41, 568–573. [Google Scholar] [CrossRef]
- Cudney, D.W.; Jordan, L.S.; Hall, A.E. Effect of wild oat (Avena fatua) infestations on light interception and growth rate of wheat (Triticum aestivum). Weed Sci. 1991, 39, 175–179. [Google Scholar] [CrossRef]
- Stoller, E.W.; Myers, R.A. Response of soybeans (Glycine max) and four broadleaf weeds to reduced irradiance. Weed Sci. 1989, 37, 570–574. [Google Scholar] [CrossRef]
- Andreasen, C.; Litz, A.S.; Streibig, J.C. Growth response of six weed species and spring barley (Hordeum vulgare) to increasing levels of nitrogen and phosphorus. Weed Res. 2006, 46, 503–512. [Google Scholar] [CrossRef]
- Holt, J.S. Plant responses to light: A potential tool for weed management. Weed Sci. 1995, 43, 474–482. [Google Scholar] [CrossRef]
- Weinig, C. Limits to adaptive plasticity: Temperature and photoperiod influence shade-avoidance responses. Am. J. Bot. 2000, 87, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Xiang, L.; Gu, S.; Wang, D. The effects of intraspecific competition and light transmission within the canopy on wheat yield in a wide-precision planting pattern. J. Integr. Agric. 2020, 19, 1577–1585. [Google Scholar] [CrossRef]
- Rajcan, I.; Swanton, C.J. Understanding maize-weed competition: Resource competition, light quality and the whole plant. Field Crops Res. 2001, 71, 139–150. [Google Scholar] [CrossRef]
- Padilla, F.M.; Pugnaire, F.I. Rooting depth and soil moisture control Mediterranean woody seedling survival during drought. Funct. Ecol. 2007, 21, 489–495. [Google Scholar] [CrossRef]
- Ritchie, J.T. Soil water availability. Plant Soil 1981, 58, 327–338. [Google Scholar] [CrossRef]
- Singh, M.; Kukal, M.S.; Irmak, S.; Jhala, A.J. Water use characteristics of weeds: A global review, best practices, and future directions. Front. Plant Sci. 2022, 12, 794090. [Google Scholar] [CrossRef]
- Casper, B.B.; Jackson, R.B. Plant competition underground. Annu. Rev. Ecol. Syst. 1997, 28, 545–570. [Google Scholar] [CrossRef]
- Warwick, S.I.; Black, L.D. The biology of Canadian weeds: 90 Abutilon theophrasti. Can. J. Plant Sci. 1988, 68, 1069–1085. [Google Scholar] [CrossRef]
- Wiese, A.F.; Vandiver, C.W. Soil moisture effects on competitive ability of weeds. Weed Sci. 1970, 18, 518–519. [Google Scholar] [CrossRef]
- Pant, C.; Sah, S.K. Managing plant population and competition in field crops. Acta Sci. Malays. 2020, 4, 57–60. [Google Scholar] [CrossRef]
- Lombardini, L.; Rossi, L. Ecophysiology of plants in dry environments. In Dryland Ecohydrology; Springer International Publishing: Cham, Switzerland, 2019; pp. 71–100. [Google Scholar]
- Duke, S.O.; Patterson, D.T. Comparative ecophysiology of weeds and crops. In Weed Physiology; CRC Press: Boca Raton, FL, USA, 2018; pp. 101–129. [Google Scholar]
- Peerzada, A.M.; Ali, H.H.; Chauhan, B.S. Weed management in sorghum (Sorghum bicolor L. Moench) using crop competition: A review. Crop Prot. 2017, 95, 74–80. [Google Scholar] [CrossRef]
- Khalil, S.K.; St Hilaire, R.; Khan, A.; Rehman, A.; Mexal, J.G. Growth and physiology of yarrow species ‘Achillea millefolium’ cv. Cerise queen and ‘Achillea filipendulina’ cv. Parker gold at optimum and limited moisture. Aust. J. Crop Sci. 2011, 5, 1698–1706. [Google Scholar]
- Kadmon, R. Plant competition along soil moisture gradients: A field experiment with the desert annual Stipa capensis. J. Ecol. 1995, 83, 253–262. [Google Scholar] [CrossRef]
- Kanatas, P.; Gazoulis, I.; Travlos, I. Irrigation timing as a practice of effective weed management in established alfalfa (Medicago sativa L.) crop. Agronomy 2021, 11, 550. [Google Scholar] [CrossRef]
- Kadmon, R.; Shmida, A. Patterns and causes of spatial variation in the reproductive success of a desert annual. Oecologia 1990, 83, 139–144. [Google Scholar] [CrossRef]
- Vleeshouwers, L.M. Modelling the effect of temperature, soil penetration resistance, burial depth and seed weight on pre-emergence growth of weeds. Ann. Bot. 1997, 79, 553–563. [Google Scholar] [CrossRef]
- Blackshaw, R.E. Influence of soil temperature, soil moisture, and seed burial depth on the emergence of round-leaved mallow (Malva pusilla). Weed Sci. 1990, 38, 518–521. [Google Scholar] [CrossRef]
- Tang, J.; Busso, C.A.; Jiang, D.; Wang, Y.; Wu, D.; Musa, A.; Miao, C. Seed burial depth and soil water content affect seedling emergence and growth of Ulmus pumila var sabulosa in the Horqin Sandy Land. Sustainability 2016, 8, 68. [Google Scholar]
- Fageria, N.K.; Baligar, V.C. Nutrient availability. Encycl. Soils Environ. 2005, 3, 63–71. [Google Scholar]
- Quinn, L.D.; Rauterkus, M.A.; Holt, J.S. Effects of nitrogen enrichment and competition on growth and spread of giant reed (Arundo donax). Weed Sci. 2007, 55, 319–326. [Google Scholar] [CrossRef]
- Rehling, F.; Sandner, T.M.; Matthies, D. Biomass partitioning in response to intraspecific competition depends on nutrients and species characteristics: A study of 43 plant species. J. Ecol. 2021, 109, 2219–2233. [Google Scholar] [CrossRef]
- Mommer, L.; van Ruijven, J.; de Caluwe, H.; Smit-Tiekstra, A.E.; Wagemaker, C.A.; Ouborg, N.J.; de Kroon, H. Unveiling below-ground species abundance in a biodiversity experiment: A test of vertical niche differentiation among grassland species. J. Ecol. 2016, 104, 123–133. [Google Scholar] [CrossRef]
- Teyker, R.H.; Hoelzer, H.D.; Liebl, R.A. Maize and pigweed response to nitrogen supply and form. Plant Soil 1991, 135, 287–292. [Google Scholar] [CrossRef]
- Karlsson, M.; Carrié, R.; Wetterlind, J.; Bergkvist, G.; Ekroos, J.; Smith, H.G. Weed–crop competition under improved nutrient management reveals trade-off between yields and weed diversity in organic farming. Biol. Agric. Hortic. 2025, 41, 201–220. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.Y.; Wen, Q.H.; Mo, F.; Ren, A.T.; Duan, H.X.; Xiong, Y.C. Plant–plant interactions drive the decomposition of soil organic carbon via nutrition competition in dryland. Plant Cell Environ. 2025, 48, 4756–4769. [Google Scholar] [CrossRef]
- Eskelinen, A.; Harpole, W.S.; Jessen, M.T.; Virtanen, R.; Hautier, Y. Light competition drives herbivore and nutrient effects on plant diversity. Nature 2022, 611, 301–305. [Google Scholar] [CrossRef]
- Leskovšek, R.; Eler, K.; Batič, F.; Simončič, A. The influence of nitrogen, water, and competition on the vegetative and reproductive growth of common ragweed (Ambrosia artemisiifolia L.). Plant Ecol. 2012, 213, 769–781. [Google Scholar] [CrossRef]
- Han, P.; Rong, Y.; Liu, W.; Liu, J.; Zhang, L. Nitrate over ammonium: Limited inorganic N niche partitioning between wheat and weeds regardless of fertilization treatment. Rhizosphere 2024, 32, 100962. [Google Scholar] [CrossRef]
- Cahill, J.J.F.; Casper, B.B. Growth consequences of soil nutrient heterogeneity for two old-field herbs, Ambrosia artemisiifolia and Phytolacca americana, grown individually and in combination. Ann. Bot. 1999, 83, 471–478. [Google Scholar] [CrossRef]
- Lindquist, J.L.; Barker, D.C.; Knezevic, S.Z.; Martin, A.R.; Walters, D.T. Comparative nitrogen uptake and distribution in corn and velvetleaf (Abutilon theophrasti). Weed Sci. 2007, 55, 102–110. [Google Scholar] [CrossRef]
- Grant, C.A.; Derksen, D.A.; Blackshaw, R.E.; Entz, T.; Janzen, H.H. Differential response of weed and crop species to potassium and sulphur fertilizers. Can. J. Plant Sci. 2007, 87, 293–296. [Google Scholar] [CrossRef]
- Blackshaw, R.E.; Brandt, R.N.; Janzen, H.H.; Entz, T. Weed species response to phosphorus fertilization. Weed Sci. 2004, 52, 406–412. [Google Scholar] [CrossRef]
- Lehoczky, E.; Reisinger, P. Study on the weed-crop competition for nutrients in maize. Commun. Agric. Appl. Biol. Sci. 2003, 68, 373–380. [Google Scholar] [PubMed]
- Massenssini, A.M.; Bonduki, V.H.A.; Melo, C.A.D.; Tótola, M.R.; Ferreira, F.A.; Costa, M.D. Soil microorganisms and their role in the interactions between weeds and crops. Planta Daninha 2014, 32, 873–884. [Google Scholar] [CrossRef]
- Saberali, S.F.; Mohammadi, K. Organic amendments application downweight the negative effects of weed competition on the soybean yield. Ecol. Eng. 2015, 82, 451–458. [Google Scholar] [CrossRef]
- Menalled, F.D.; Liebman, M.; Buhler, D.D. Impact of composted swine manure and tillage on common waterhemp (Amaranthus rudis) competition with soybean. Weed Sci. 2004, 52, 605–613. [Google Scholar] [CrossRef]
- Vengris, J.; Colby, W.G.; Drake, M. Plant nutrient competition between weeds and corn. Agron. J. 1955, 47, 213–216. [Google Scholar] [CrossRef]
- Carlson, H.L.; Hill, J.E. Wild oat (Avena fatua) competition with spring wheat: Effects of nitrogen fertilization. Weed Sci. 1986, 34, 29–33. [Google Scholar] [CrossRef]
- Liebman, M.; Menalled, F.D.; Buhler, D.D.; Richard, T.L.; Sundberg, D.N.; Cambardella, C.A.; Kohler, K.A. Impacts of composted swine manure on weed and corn nutrient uptake, growth, and seed production. Weed Sci. 2004, 52, 365–375. [Google Scholar] [CrossRef]
- Byrne, M.; Warren, R. Successful allelopathic competition is not limited to nonnative plants. Int. J. Plant Sci. 2025, 186, 332–339. [Google Scholar] [CrossRef]
- Cipollini, D.; Rigsby, C.M.; Barto, E.K. Microbes as targets and mediators of allelopathy in plants. J. Chem. Ecol. 2012, 38, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Todaria, N.P.; Singh, B.; Dhanai, C.S. Allelopathic effects of trees extract, on germination and seedling growth of field crops. Allelopathy J. 2005, 15, 285–294. [Google Scholar]
- Reigosa, M.J.; Sánchez-Moreiras, A.; González, L. Ecophysiological approach in allelopathy. Crit. Rev. Plant Sci. 1999, 18, 577–608. [Google Scholar] [CrossRef]
- Kong, C.H.; Li, H.B.; Hu, F.; Xu, X.H.; Wang, P. Allelochemicals released by rice roots and residues in soil. Plant Soil 2006, 288, 47–56. [Google Scholar] [CrossRef]
- Kong, C.H.; Wang, P. Allelopathy and chemical communication among plants: Questions and reflections. Ying Yong Sheng Tai Xue Bao 2025, 36, 1540–1552. [Google Scholar]
- Šćepanović, M.; Novak, N.; Barić, K.; Ostojić, Z.; Galzina, N.; Goršić, M. Allelopathic effect of two weed species, Abutilon theophrasti Med. and Datura stramonium L. on germination and early growth of corn. Agron. Glas. 2007, 6, 459–472. [Google Scholar]
- Béres, I.; Kazinczi, G.; Narwal, S.S. Allelopathic plants. 4. Common ragweed (Ambrosia elatior L. Syn A. artemisiifolia). Allelopathy J. 2002, 9, 27–34. [Google Scholar]
- Wang, P.; Liang, W.J.; Kong, C.H.; Jiang, Y. Allelopathic potential of volatile allelochemicals of Ambrosia trifida L. on other plants. Allelopathy J. 2005, 15, 131–136. [Google Scholar]
- Kong, C.H.; Wang, P.; Xu, X.H. Allelopathic interference of Ambrosia trifida with wheat (Triticum aestivum). Agric. Ecosyst. Environ. 2007, 119, 416–420. [Google Scholar] [CrossRef]
- Czarnota, M.A.; Paul, R.N.; Dayan, F.E.; Nimbal, C.I.; Weston, L.A. Mode of action, localization of production, chemical nature, and activity of sorgoleone: A potent PSII inhibitor in Sorghum spp. root exudates. Weed Technol. 2001, 15, 813–825. [Google Scholar] [CrossRef]
- Barney, J.N.; Hay, A.G.; Weston, L.A. Isolation and characterization of allelopathic volatiles from mugwort (Artemisia vulgaris). J. Chem. Ecol. 2005, 31, 247–265. [Google Scholar] [CrossRef]
- An, M.; Pratley, J.E.; Haig, T. Phytotoxicity of vulpia residues: III. Biological activity of identified allelochemicals from Vulpia myuros. J. Chem. Ecol. 2001, 27, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Aschehoug, E.T. Invasive plants versus their new and old neighbors: A mechanism for exotic invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef]
- Ridenour, W.M.; Callaway, R.M. The relative importance of allelopathy in interference: The effects of an invasive weed on a native bunchgrass. Oecologia 2001, 126, 444–450. [Google Scholar] [CrossRef]
- Okrushko, S. Allelopathic effect of couch grass (Elymus repens L.) on germination of common wheat seeds. Zemdirbyste 2022, 109, 323–328. [Google Scholar] [CrossRef]
- Singh, H.P.; Batish, D.R.; Kohli, R.K. Allelopathy in agroecosystems: An overview. In Allelopathy in Agroecosystems; Kohli, R.K., Singh, H.P., Batish, D.R., Eds.; The Haworth Press: New York, NY, USA, 2001; pp. 1–41. [Google Scholar]
- Inderjit. Soil environment effects on allelochemical activity. Agron. J. 2001, 93, 79–84. [Google Scholar] [CrossRef]
- Barazani, O.Z.; Friedman, J. Allelopathic bacteria and their impact on higher plants. Crit. Rev. Plant Sci. 1999, 18, 741–755. [Google Scholar] [CrossRef]
- Revillini, D.; David, A.S.; Reyes, A.L.; Knecht, L.D.; Vigo, C.; Allen, P.; Afkhami, M.E. Allelopathy-selected microbiomes mitigate chemical inhibition of plant performance. New Phytol. 2023, 240, 2007–2019. [Google Scholar]
- Monteiro, L.C.P.; Diaz-Gallo, S.A.; de Matos, C.D.C.; da Silva, C.G.; Massenssini, A.M.; de Oliveira Mendes, T.A.; Costa, M.D. Rhizosphere microbial community changes due to weed-weed competition. Eur. J. Soil Biol. 2024, 120, 103594. [Google Scholar] [CrossRef]
- Weston, L.A. Utilization of allelopathy for weed management in agroecosystems. Agron. J. 1996, 88, 860–866. [Google Scholar] [CrossRef]
- Vidotto, F.; Tesio, F.; Ferrero, A. Allelopathic effects of Ambrosia artemisiifolia L. in the invasive process. Crop Prot. 2013, 54, 161–167. [Google Scholar] [CrossRef]
- Peters, K.; Breitsameter, L.; Gerowitt, B. Impact of climate change on weeds in agriculture: A review. Agron. Sustain. Dev. 2014, 34, 707–721. [Google Scholar] [CrossRef]
- Korres, N.E.; Norsworthy, J.K.; Tehranchian, P.; Gitsopoulos, T.K.; Loka, D.A.; Oosterhuis, D.M.; Gealy, D.R.; Moss, S.R.; Bryson, C.T.; Burgos, N.R.; et al. Cultivars to face climate change effects on crops and weeds: A review. Agron. Sustain. Dev. 2016, 36, 12. [Google Scholar] [CrossRef]
- Feeley, K.J.; Bravo-Avila, C.; Fadrique, B.; Perez, T.M.; Zuleta, D. Climate-driven changes in the composition of New World plant communities. Nat. Clim. Change 2020, 10, 965–970. [Google Scholar] [CrossRef]
- Bopp, M.-C.; Kazakou, E.; Metay, A.; Maillet, J.; Quidoz, M.-C.; Genty, L.; Fried, G. Climate and management changes over 40 years drove more stress-tolerant and less ruderal weed communities in vineyards. Ecol. Monogr. 2025, 95, e1631. [Google Scholar]
- Ramesh, K.; Matloob, A.; Aslam, F.; Florentine, S.K.; Chauhan, B.S. Weeds in a changing climate: Vulnerabilities, consequences, and implications for future weed management. Front. Plant Sci. 2017, 8, 95. [Google Scholar] [CrossRef] [PubMed]
- Karthik, M.N.; Parasuraman, P.; Thavaprakaash, N.; Poornimmal, R.; Vincent, S. Resource Conservation Technologies for Mitigating Climate Change Impacts in Agriculture: A Review. Commun. Soil Sci. Plant Anal. 2025, 56, 2088–2104. [Google Scholar] [CrossRef]
- Kaye, J.P.; Quemada, M. Using cover crops to mitigate and adapt to climate change: A review. Agron. Sustain. Dev. 2017, 37, 4–10. [Google Scholar] [CrossRef]
- Clements, D.R.; Ditommaso, A. Climate change and weed adaptation: Can evolution of invasive plants lead to greater range expansion than forecasted? Weed Res. 2011, 51, 227–240. [Google Scholar] [CrossRef]
- Munier-Jolain, N.M.; Collard, A.; Busset, H.; Guyot, S.H.; Colbach, N. Investigating and modelling the morphological plasticity of weeds. Field Crops Res. 2014, 155, 90–98. [Google Scholar] [CrossRef]
- Gaudet, C.L.; Keddy, P.A. Competitive performance and species distribution in shortline plant communities: A comparative approach. Ecology 1995, 76, 280–291. [Google Scholar] [CrossRef]
- Heap, I. The International Survey of Herbicide Resistant Weeds. Available online: http://www.weedscience.org (accessed on 6 August 2025).
- Duke, S.O. Why have no new herbicide modes of action appeared in recent years? Pest. Manag. Sci. 2012, 68, 505–512. [Google Scholar] [CrossRef]
- Jha, P.; Kumar, V.; Godara, R.K.; Chauhan, B.S. Weed management using crop competition in the United States: A review. Crop Prot. 2017, 95, 31–37. [Google Scholar] [CrossRef]
- Shibles, R.; Weber, C.R. Interception of solar radiation and dry matter production by various soybean planting patterns. Crop Sci. 1966, 6, 55–59. [Google Scholar] [CrossRef]
- Koscelny, J.A.; Peeper, T.F.; Solie, J.B.; Solomon, J.G. Seeding date, seeding rate, and row spacing affect wheat (Triticum aestivum) and cheat (Bromus secalinus) competition. Weed Technol. 1990, 4, 487–491. [Google Scholar] [CrossRef]
- Reddy, K.N. Effects of cereal and legume cover crop residues on weeds, weed seedling emergence, and yield of cotton (Gossypium hirsutum). Weed Technol. 2001, 15, 660–668. [Google Scholar] [CrossRef]
- Howe, O.W., III; Oliver, L.R. Influence of soybean (Glycine max) row spacing on pitted morningglory (Ipomoea lacunosa) interference. Weed Sci. 1987, 35, 185–193. [Google Scholar] [CrossRef]
- Spies, J.M.; Warkentin, T.D.; Shirtliffe, S.J. Variation in field pea (Pisum sativum) cultivars for basal branching and weed competition. Weed Sci. 2018, 59, 218–223. [Google Scholar]
- Tharp, B.E.; Kells, J.J. Effect of glufosinate-resistant corn (Zea mays) population and row spacing on light interception, corn yield, and common lambsquarters (Chenopodium album) growth. Weed Technol. 2001, 15, 413–418. [Google Scholar] [CrossRef]
- Oplinger, E.S.; Philbrook, B.D. Soybean planting date, row width, and seeding rate response in three tillage systems. J. Prod. Agric. 1992, 5, 94–99. [Google Scholar] [CrossRef]
- Schroeder, J.; Thomas, S.H.; Murray, L.W. Impacts of crop pests on weeds and weed–crop interactions. Weed Sci. 2005, 53, 918–922. [Google Scholar] [CrossRef]
- Mézière, D.; Lucas, P.; Granger, S.; Colbach, N. Does Integrated Weed Management affect the risk of crop diseases? A simulation case study with blackgrass weed and take-all disease. Eur. J. Agron. 2013, 47, 33–43. [Google Scholar] [CrossRef]
- Kissing Kucek, L.; Mallory, E.B.; Darby, H.M.; Dawson, J.C.; Sorrells, M.E. Breeding wheat for weed-competitive ability: I. Correlated traits. Euphytica 2001, 217, 202. [Google Scholar] [CrossRef]
- Norsworthy, J.K.; Ward, S.M.; Shaw, D.R.; Llewellyn, R.S.; Nichols, R.L.; Webster, T.M.; Barrett, M. Reducing the risks of herbicide resistance: Best management practices and recommendations. Weed Sci. 2012, 60, 31–62. [Google Scholar] [CrossRef]
- Beckie, H.J.; Ashworth, M.B.; Flower, K.C. Herbicide resistance management: Recent developments and trends. Plants 2014, 3, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Mohler, C.L.; Liebman, M.; Staver, C.P. Enhancing the competitive ability of crops. In Ecological Management of Agricultural Weeds; Cambridge University Press: Cambridge, UK, 2001; pp. 269–321. [Google Scholar]
- Weiner, J.; Griepentrong, H.W.; Kristensen, L. Suppression of weeds by spring wheat (Triticum aestivum) increases with crop density and spatial uniformity. J. Appl. Ecol. 2001, 38, 784–790. [Google Scholar] [CrossRef]
- Olsen, J.; Kristensen, L.; Weiner, J.; Griepentrog, H.W. Increased density and spatial uniformity increase weed suppression by spring wheat. Weed Res. 2005, 45, 316–321. [Google Scholar] [CrossRef]
- Korav, S.; Dhaka, A.K.; Singh, R.; Premaradhya, N.; Reddy, G.C. A study on crop weed competition in field crops. J. Pharmacogn. Phytochem. 2018, 7, 3235–3240. [Google Scholar]
- Grotkopp, E.; Rejmánek, M.; Rost, T.L. Toward a causal explanation of plant invasiveness: Seedling growth and life-history strategies of 29 pine (Pinus) species. Am. Nat. 2002, 159, 396–419. [Google Scholar] [CrossRef]
- MacKay, J.; Kotanen, P.M. Local escape of an invasive plant, common ragweed (Ambrosia artemisiifolia L.), from above-ground and below-ground enemies in its native area. J. Ecol. 2008, 96, 1152–1161. [Google Scholar] [CrossRef]
- Sadras, V.O.; Villalobos, F.J.; Fereres, E. Crop development and growth. In Principles of Agronomy for Sustainable Agriculture; Springer International Publishing: Cham, Switzerland, 2017; pp. 141–158. [Google Scholar]
- Craine, J.M.; Dybzinski, R. Mechanisms of plant competition for nutrients, water and light. Funct. Ecol. 2013, 27, 833–840. [Google Scholar] [CrossRef]
- Cerrudo, D.; Page, E.R.; Tollenaar, M.; Stewart, G.; Swanton, C.J. Mechanisms of yield loss in maize caused by weed competition. Weed Sci. 2012, 60, 225–232. [Google Scholar] [CrossRef]
- Grams, T.E.; Andersen, C.P. Competition for resources in trees: Physiological versus morphological plasticity. Prog. Bot. 2007, 68, 356–381. [Google Scholar]
- Lamb, E.G.; Cahill, J.F. Consequences of differing competitive abilities between juvenile and adult plants. Oikos 2006, 112, 502–512. [Google Scholar] [CrossRef]
- Cahill, J.F., Jr. Interactions between root and shoot competition vary among species. Oikos 2002, 99, 101–112. [Google Scholar] [CrossRef]
- Szymura, M.; Szymura, T.H.; Wolski, K.; Świerszcz, S. Can native grass species outcompete invasive goldenrods? Results of a replacement series experiment. Weed Res. 2018, 58, 304–317. [Google Scholar] [CrossRef]
- Molinari, F.A.; Blanco, A.M.; Vigna, M.R.; Chantre, G.R. Towards an integrated weed management decision support system: A simulation model for weed–crop competition and control. Comput. Electron. Agric. 2020, 175, 105597. [Google Scholar] [CrossRef]
- Stępień, A.; Wojtkowiak, K.; Kolankowska, E. Crop Rotation and Weed Control as Factors in the Sustainable Cultivation of Winter Oilseed Rape. Sustainability 2025, 17, 5065. [Google Scholar] [CrossRef]
- Melander, B.; Rasmussen, I.A.; Olesen, J.E. Legacy effects of leguminous green manure crops on the weed seed bank in organic crop rotations. Agric. Ecosyst. Environ. 2020, 302, 107078. [Google Scholar] [CrossRef]
- Jin, T.; Liang, K.; Lu, M.; Zhao, Y.; Xu, Y. WeedsSORT: A weed tracking-by-detection framework for laser weeding applications within precision agriculture. Smart Agric. Technol. 2025, 11, 100883. [Google Scholar] [CrossRef]

| Weed Species | Crop | Density (Plants/m2) | Yield Impact (%) | Region | Ref. |
|---|---|---|---|---|---|
| X. pensylvanicum | Soybean | 2.5 | 54 | North America | [47] |
| A. theophrasti | Soybean | 2.5 | 31 | North America | [47] |
| D. stramonium | Soybean | 2.5 | 40 | North America | [47] |
| X. strumarium | Maize | 4 | 20 | Southwest Asia | [48] |
| X. strumarium | Maize | 8 | 35 | Southwest Asia | [48] |
| X. strumarium | Maize | 12 | 43 | Southwest Asia | [48] |
| X. strumarium | Maize | 16 | 50 | Southwest Asia | [48] |
| D. stramonium | Maize | 4 | 15 | Southwest Asia | [48] |
| D. stramonium | Maize | 8 | 22 | Southwest Asia | [48] |
| D. stramonium | Maize | 12 | 30 | Southwest Asia | [48] |
| D. stramonium | Maize | 16 | 38 | Southwest Asia | [48] |
| Nutrient | Weed Species | Crop | Yield Reduction (%) | Nutrient Uptake (Weed vs. Crop) | Reference |
|---|---|---|---|---|---|
| N | A. retroflexus | Soybean | 58% | Weed 2× more than crop | [85,86] |
| N | Amaranthus rudis L. | Soybean | 40% | Greater uptake in early weed biomass | [87] |
| N, P | A. retroflexus | Maize | 25–40% | Higher total in weed biomass | [88] |
| N | A. fatua | Wheat | 20–50% | Not quantified | [89] |
| N | A. theophrasti | Maize | 30–40% | Higher uptake in weed with compost | [90] |
| P, K | C. album | Maize | 35% | More total P and K in weed than crop | [88] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savić, A.; Popović, A.; Đurović, S.; Pisinov, B.; Ugrinović, M.; Todorović, M.J. A Framework for Understanding Crop–Weed Competition in Agroecosystems. Agronomy 2025, 15, 2366. https://doi.org/10.3390/agronomy15102366
Savić A, Popović A, Đurović S, Pisinov B, Ugrinović M, Todorović MJ. A Framework for Understanding Crop–Weed Competition in Agroecosystems. Agronomy. 2025; 15(10):2366. https://doi.org/10.3390/agronomy15102366
Chicago/Turabian StyleSavić, Aleksandra, Aleksandar Popović, Sanja Đurović, Boris Pisinov, Milan Ugrinović, and Marijana Jovanović Todorović. 2025. "A Framework for Understanding Crop–Weed Competition in Agroecosystems" Agronomy 15, no. 10: 2366. https://doi.org/10.3390/agronomy15102366
APA StyleSavić, A., Popović, A., Đurović, S., Pisinov, B., Ugrinović, M., & Todorović, M. J. (2025). A Framework for Understanding Crop–Weed Competition in Agroecosystems. Agronomy, 15(10), 2366. https://doi.org/10.3390/agronomy15102366







