Antimicrobial Activity and Activation of Defense Genes in Plants by Natural Extracts: Toward Sustainable Plant Health Management
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Culture Media
2.2. Fungal, Bacterial and Viral Strains and Culture Conditions
2.3. Plant Extracts and Other Natural Compounds
2.4. In Vitro Antimicrobial Assays
2.5. In Vivo Evaluation of Effectiveness on Tomato Plants
2.6. Gene Expression Analysis
2.7. Statistical Analysis
3. Results
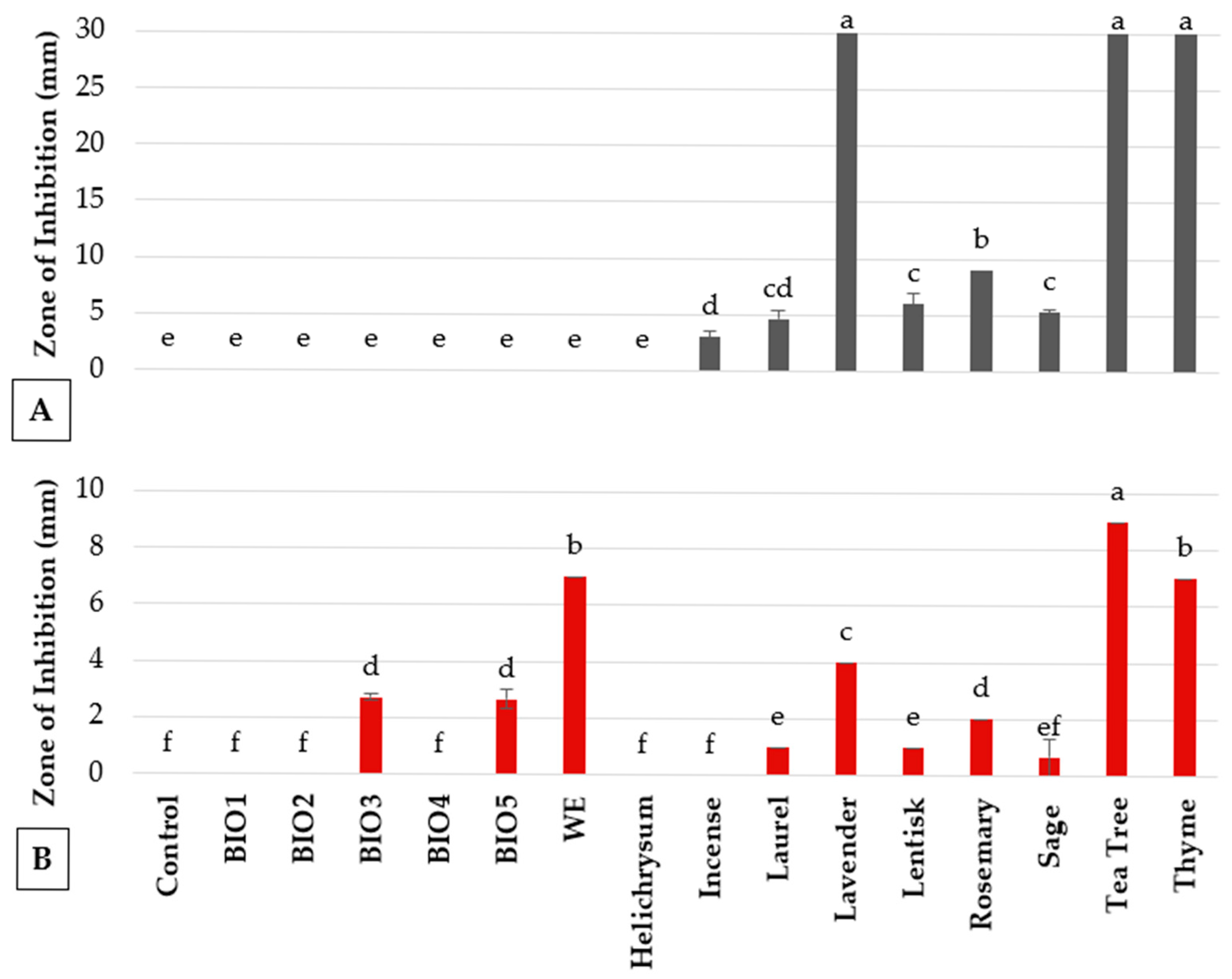
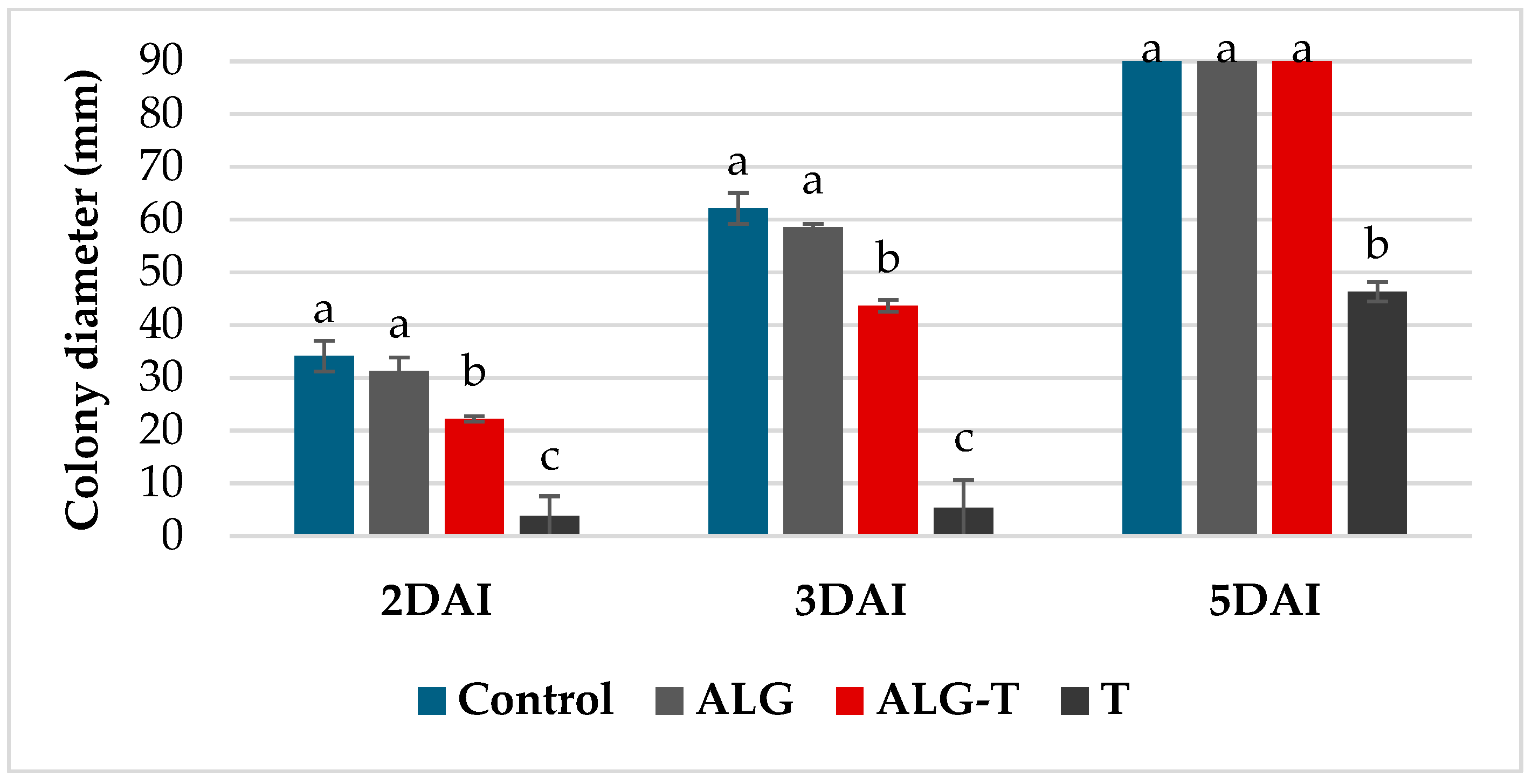
3.1. In Vitro Antimicrobial Activity
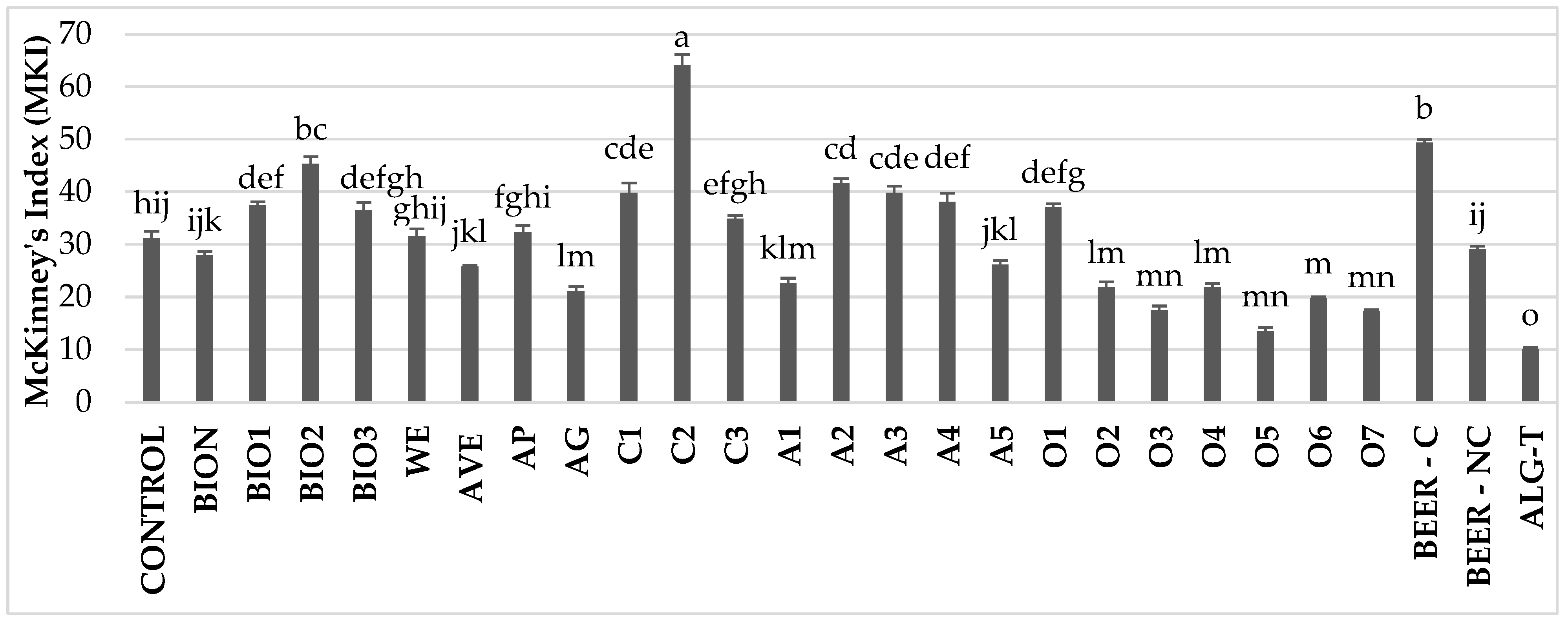
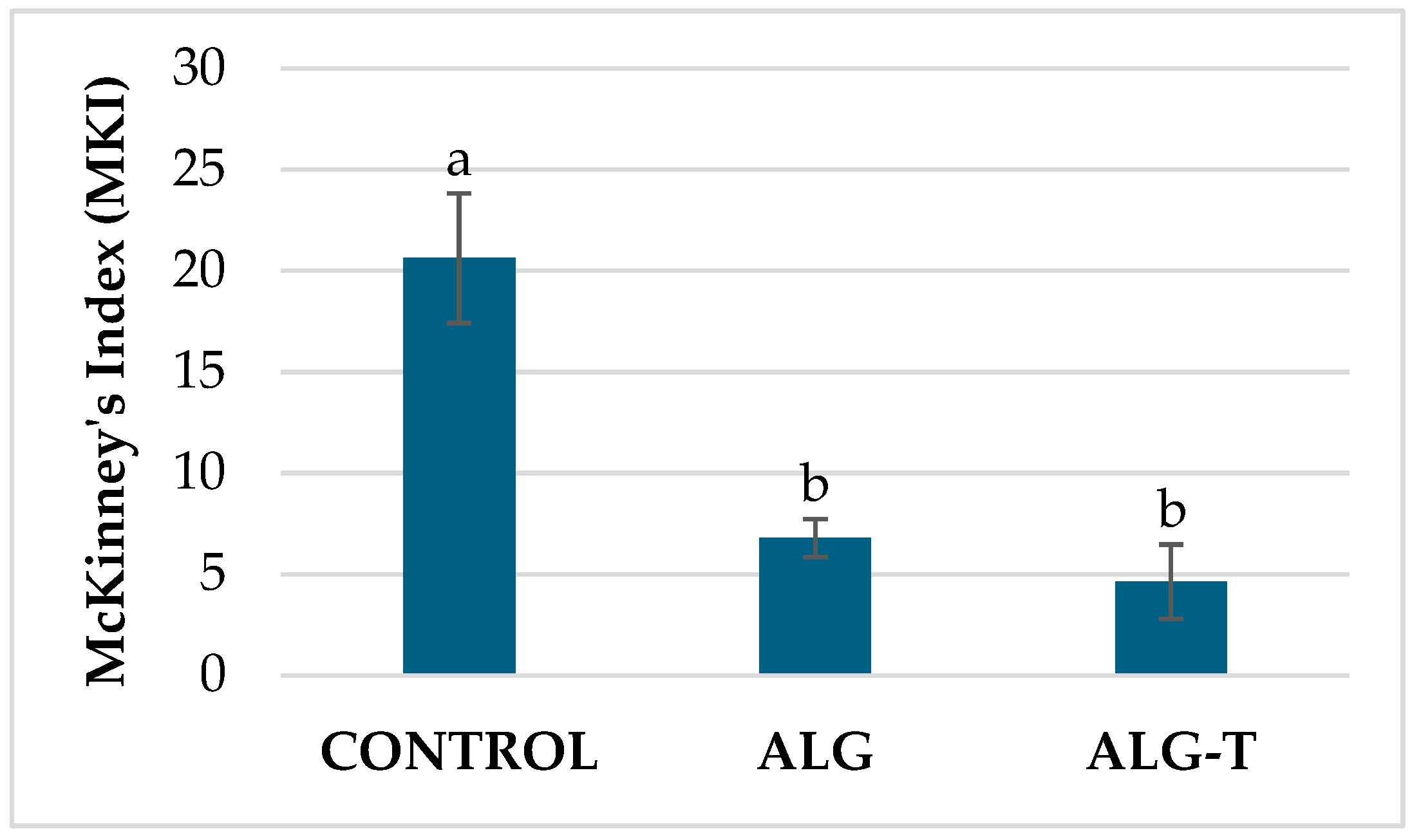
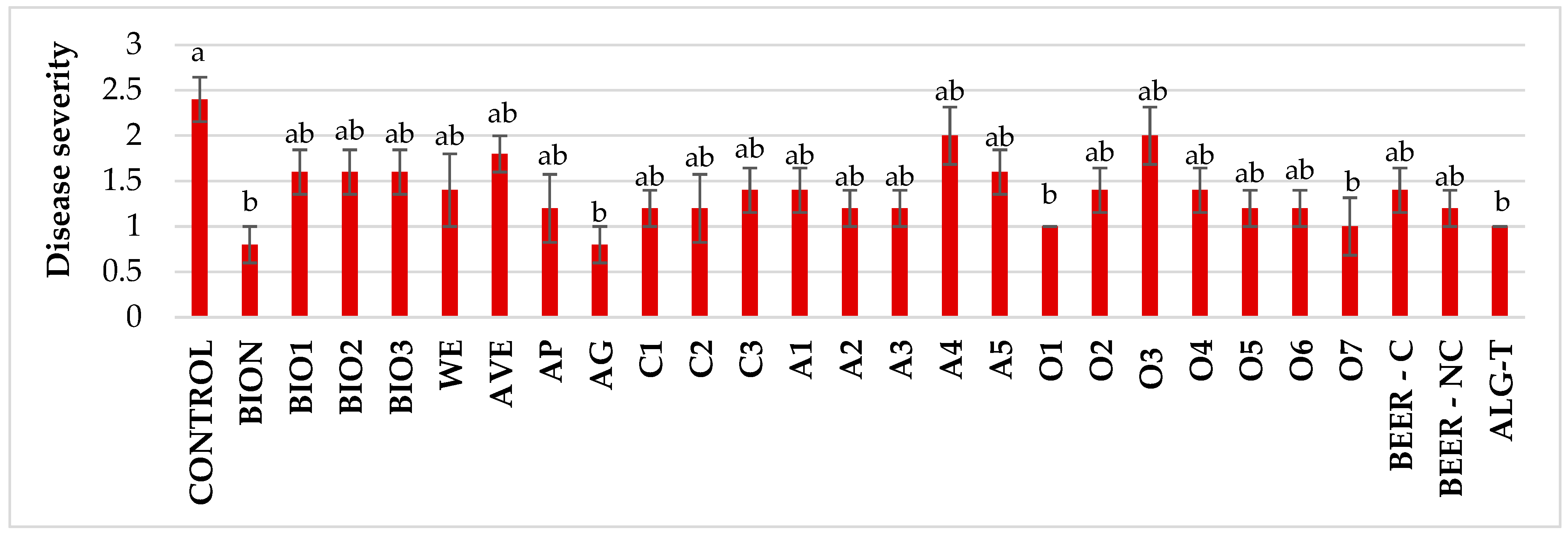
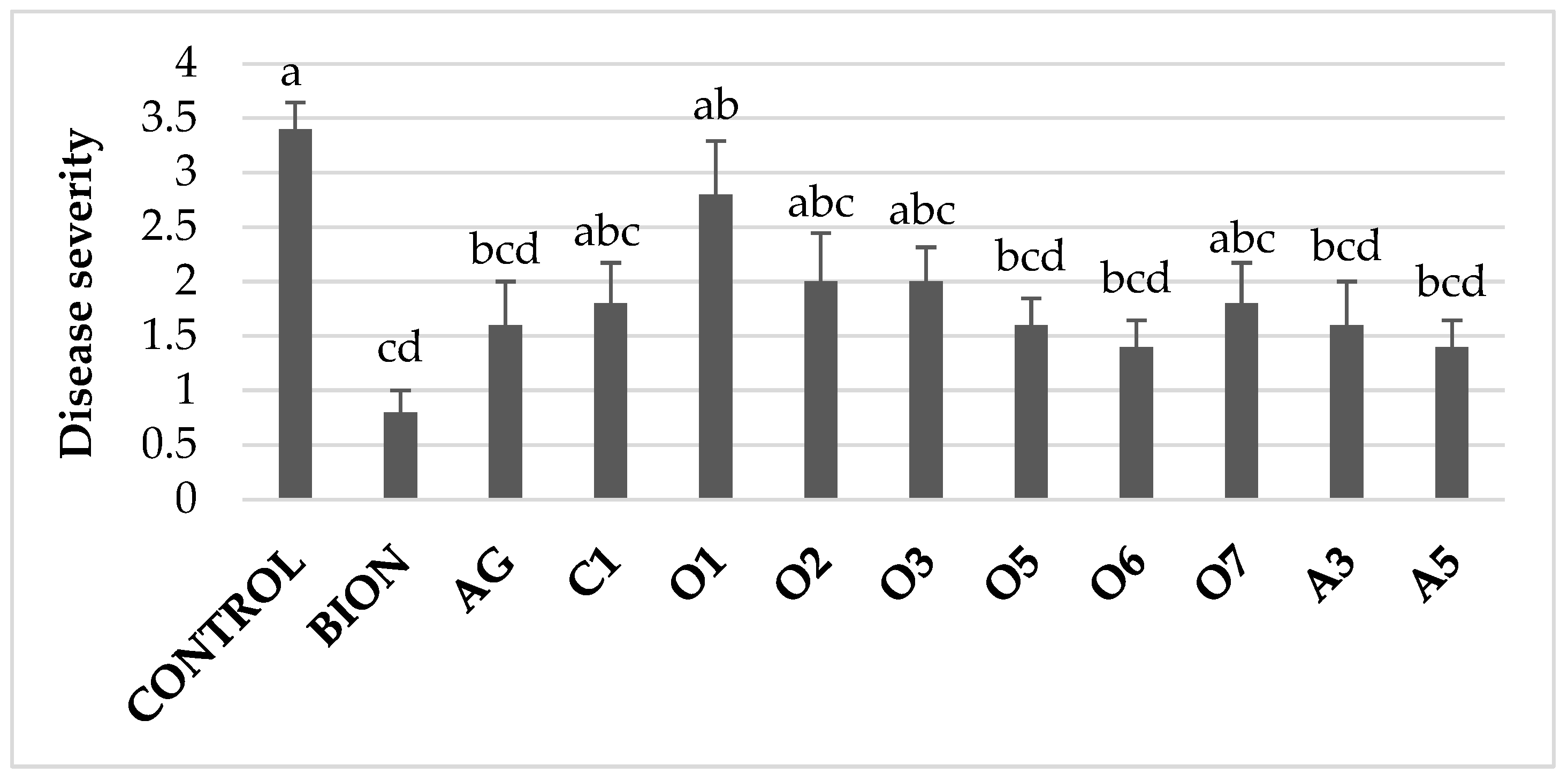
3.2. In Vivo Efficacy Evaluation
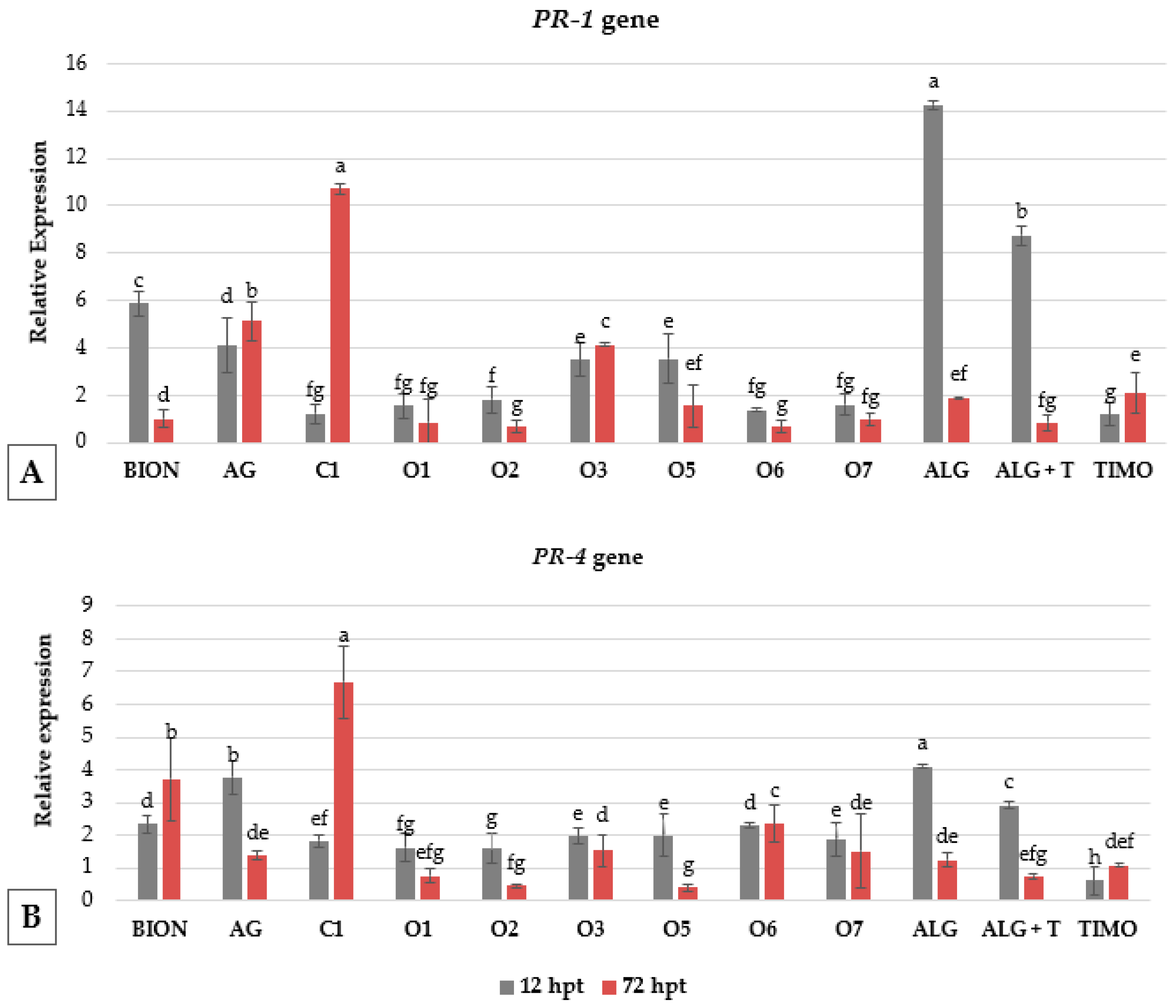
3.3. Gene Expression and Early Post-Treatment Plant Responses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rockström, J.; Williams, J.; Daily, G.; Noble, A.; Matthews, N.; Gordon, L.; Wetterstrand, H.; DeClerck, F.; Shah, M.; Steduto, P.; et al. Sustainable intensification of agriculture for human prosperity and global sustainability. Ambio 2017, 46, 4–17. [Google Scholar] [CrossRef]
- FAO. The State of the World’s Land and Water Resources for Food and Agriculture (SOLAW)—Systems at Breaking Point; FAO: Rome, Italy, 2021; Available online: https://www.fao.org/3/cb7654en/cb7654en.pdf (accessed on 31 August 2025).
- Pretty, J. Agricultural sustainability: Concepts, principles and evidence. Philos. Trans. R. Soc. B 2008, 363, 447–465. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Challinor, A.J.; Watson, J.; Lobell, D.B.; Howden, S.M.; Smith, D.R.; Chhetri, N. A meta-analysis of crop yield under climate change and adaptation. Nat. Clim. Change 2014, 4, 287–291. [Google Scholar] [CrossRef]
- Lahlali, R.; Taoussi, M.; Laasli, S.-E.; Gachara, G.; Ezzouggari, R.; Belabess, Z.; Aberkani, K.; Assouguem, A.; Meddich, A.; El Jarroudi, M.; et al. Effects of climate change on plant pathogens and host-pathogen interactions. Crop Environ. 2024, 3, 159–170. [Google Scholar] [CrossRef]
- Bilen, C.; El Moujabber, M.; Ben Slimen, A.; Incerti, O.; Jreijiri, F.; Choueiri, E. Detecting new emerging viruses and phytoplasmas of grapevine in Lebanon for developing future adaptation strategies to climate change. Eur. J. Plant Pathol. 2025, 1–17. [Google Scholar] [CrossRef]
- Harman, G.E. Integrated benefits to agriculture with Trichoderma and other endophytic or root-associated microbes. Microorganisms 2024, 12, 1409. [Google Scholar] [CrossRef]
- Bednarek, P. Chemical warfare or modulators of defense responses—The function of secondary metabolites in plant immunity. Curr. Opin. Plant Biol. 2012, 15, 407–414. [Google Scholar] [CrossRef]
- European Commission. A Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System; COM(2020) 640 Final European Commission: Brussels, Belgium, 2020. [Google Scholar]
- Osbourn, A.E. Saponins and plant defence—A soap story. Trends Plant Sci. 1996, 1, 4–9. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Vallad, G.E.; Goodman, R.M. Systemic acquired resistance and induced systemic resistance in conventional agriculture. Crop Sci. 2004, 44, 1920–1934. [Google Scholar] [CrossRef]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 2015, 6, 573. [Google Scholar] [CrossRef]
- Saberi Riseh, R.; Gholizadeh Vazvani, M.; Ebrahimi-Zarandi, M.; Skorik, Y.A. Alginate-induced disease resistance in plants. Polymers 2022, 14, 661. [Google Scholar] [CrossRef]
- Spanò, R.; Ferrara, M.; Gallitelli, D.; Mascia, T. The role of grafting in the resistance of tomato to viruses. Plants 2020, 9, 1042. [Google Scholar] [CrossRef]
- Navarro, D.; Díaz-Mula, H.M.; Guillén, F.; Zapata, P.J.; Castillo, S.; Serrano, M.; Valero, D.; Martínez-Romero, D. Reduction of nectarine decay caused by Rhizopus stolonifer, Botrytis cinerea and Penicillium digitatum with Aloe vera gel alone or with the addition of thymol. Int. J. Food Microbiol. 2011, 151, 241–246. [Google Scholar] [CrossRef]
- Khaliq, G.; Abbas, H.T.; Ali, I.; Waseem, M. Aloe vera gel enriched with garlic essential oil effectively controls anthracnose disease and maintains postharvest quality of banana fruit during storage. Hort. Environ. Biotechnol. 2019, 60, 659–669. [Google Scholar] [CrossRef]
- Luiz, C.; Felipini, R.B.; Costa, M.E.B.; Di Piero, R.M. Polysaccharides from Aloe barbadensis reduce the severity of bacterial spot and activate disease-related proteins in tomato. J. Plant Pathol. 2012, 94, 387–393. Available online: https://www.cabidigitallibrary.org/doi/pdf/10.5555/20123315709 (accessed on 30 September 2025).
- Uda, M.N.A.; Shaari, N.H.; Said, N.S.; Ibrahim, N.H.; Akhir, M.A.; Hashim, M.K.R.; Gopinath, S.C. Antimicrobial activity of plant extracts from Aloe vera, Citrus hystrix, Sabah snake grass and Zingiber officinale against Pyricularia oryzae that causes rice blast disease in paddy plants. IOP Conf. Ser. Mater. Sci. Eng. 2018, 318, 012009. [Google Scholar] [CrossRef]
- Gammoudi, N.; Nagaz, K.; Ferchichi, A. Potential use of spent coffee grounds and spent tea leaves extracts in priming treatment to promote in vitro early growth of salt- and drought-stressed seedlings of Capsicum annuum L. Waste Biomass Valoriz. 2021, 12, 3341–3353. [Google Scholar] [CrossRef]
- Spanò, R.; Gena, P.; Linsalata, V.; Sini, V.; D’Antuono, I.; Cardinali, A.; Cotugno, P.; Calamita, G.; Mascia, T. Spotlight on secondary metabolites produced by an early-flowering Apulian artichoke ecotype sanitized from virus infection by meristem-tip-culture and thermotherapy. Antioxidants 2024, 13, 852. [Google Scholar] [CrossRef] [PubMed]
- Lopedota, A.A.; Arduino, I.; Lopalco, A.; Iacobazzi, R.M.; Cutrignelli, A.; Laquintana, V.; Racaniello, G.F.; Franco, M.; la Forgia, F.; Fontana, S.; et al. From oil to microparticulate by prilling technique: Production of polynucleate alginate beads loading Serenoa Repens oil as intestinal delivery systems. Int. J. Pharm. 2021, 599, 120412. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-García, S.; Moumni, M.; Romanazzi, G. Antifungal activity of volatile organic compounds from essential oils against the postharvest pathogens Botrytis cinerea, Monilinia fructicola, Monilinia fructigena, and Monilinia laxa. Front. Plant Sci. 2023, 14, 1274770. [Google Scholar] [CrossRef]
- Mascia, T.; Finetti-Sialer, M.M.; Cillo, F.; Gallitelli, D. Biological and molecular characterization of a recombinant isolate of Potato virus Y associated with a tomato necrotic disease occurring in Italy. J. Plant Pathol. 2010, 92, 131–138. [Google Scholar] [CrossRef]
- McKinney, H.H.; Davis, R.J. Influence of soil temperature and moisture on infection of young wheat plants by Ophiobolus graminis. J. Agric. Res. 1925, 31, 827–840. [Google Scholar]
- Molinari, S.; Fanelli, E.; Leonetti, P. Expression of tomato salicylic acid (SA)-responsive pathogenesis-related genes in Mi-1-mediated and SA-induced resistance to root-knot nematodes. Mol. Plant Pathol. 2014, 15, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Dong, C.; Li, B.; Dai, H. A PR-4 gene identified from Malus domestica is involved in the defense responses against Botryosphaeria dothidea. Plant Physiol. Biochem. 2013, 62, 23–32. [Google Scholar] [CrossRef]
- Fuentes, A.; Ortiz, J.; Saavedra, N.; Salazar, L.A.; Meneses, C.; Arriagada, C. Reference gene selection for quantitative real-time PCR in Solanum lycopersicum L. inoculated with the mycorrhizal fungus Rhizophagus irregularis. Plant Physiol. Biochem. 2016, 101, 124–131. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- European Commission. European Green Deal; COM(2019) 640 final; European Commission: Brussels, Belgium, 2019. [Google Scholar]
- Tataridas, A.; Kanatas, P.; Chatzigeorgiou, A.; Zannopoulos, S.; Travlos, I. Sustainable crop and weed management in the era of the EU Green Deal: A survival guide. Agronomy 2022, 12, 589. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Kang, S.; Xu, H.; Lee, S.G.; Baek, K.H.; Kang, S.-C. Potential roles of essential oils on controlling plant pathogenic bacteria Xanthomonas species: A review. Plant Pathol. J. 2011, 27, 207–224. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Gotor-Vila, A.; Teixidó, N.; Usall, J.; Torres, R.; Viñas, I. Antifungal activity of volatile organic compounds from Bacillus amyloliquefaciens CPA-8 against postharvest pathogens of cherry. Int. J. Food Microbiol. 2017, 265, 18–22. [Google Scholar] [CrossRef]
- Pattnaik, S.; Subramanyam, V.R.; Kole, C.R. Antibacterial and antifungal activity of aromatic constituents of essential oils. Microbios 1997, 89, 39–46. [Google Scholar]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Dawood, M.F.A.; Muhammed, M.; Mitra, D.; Mir, M.I.; Thabet, S.G. Alginate-induced immunity: A new frontier in plant health. In Elicitors for Sustainable Crop Production; Abd-Elsalam, K.A., Mohamed, H.I., Eds.; Springer: Singapore, 2025; pp. 251–270. [Google Scholar] [CrossRef]
- Tegegne, G.; Pretorius, J.C.; Swart, W.J. Antifungal properties of Agapanthus africanus L. extracts against plant pathogens. Crop Prot. 2008, 27, 1052–1060. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Benavides, S.; Cortés, P.; Parada, J.; Franco, W. Development of alginate microspheres containing thyme essential oil using ionic gelation. Food Chem. 2016, 204, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Saberi Riseh, R.; Ebrahimi-Zarandi, M.; Tarkka, M.T. Actinobacteria as effective biocontrol agents against plant pathogens: An overview on their role in eliciting plant defense. Microorganisms 2022, 10, 1739. [Google Scholar] [CrossRef]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling crop diseases using induced resistance: Challenges for the future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef]
- Sivakumar, D.; Bautista-Baños, S. A review on the use of essential oils for postharvest decay control and maintenance of fruit quality during storage. Crop Prot. 2014, 64, 27–37. [Google Scholar] [CrossRef]
- Jain, D.; Khurana, J.P. Role of pathogenesis-related (PR) proteins in plant defense mechanism. In Molecular Aspects of Plant-Pathogen Interaction; Singh, A., Singh, I.K., Eds.; Springer: Singapore, 2018; pp. 265–285. [Google Scholar] [CrossRef]
- An, C.; Mou, Z. Salicylic acid and its function in plant immunity. J. Integr. Plant Biol. 2011, 53, 412–428. [Google Scholar] [CrossRef]
- Medeiros, F.C.L.; Resende, M.L.V.; Medeiros, F.H.V.; Zhang, H.M.; Paré, P.W. Defense gene expression induced by a coffee-leaf extract formulation in tomato. Physiol. Mol. Plant Pathol. 2009, 74, 175–183. [Google Scholar] [CrossRef]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Esquivel, P.; Jiménez, V.M. Functional properties of coffee and coffee by-products. Food Res. Int. 2012, 46, 488–495. [Google Scholar] [CrossRef]
- Ranatnuge, R.; Hasara, H. Biostimulants in sustainable agriculture: Emerging roles and mechanisms. Discov. Agric. 2024, 3, 56. [Google Scholar] [CrossRef]
- Ben-Jabeur, M.; Ghabri, E.; Myriam, M.; Hamada, W. Thyme essential oil as a defense inducer of tomato against gray mold and Fusarium wilt. Plant Physiol. Biochem. 2015, 94, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Saks, Y.; Barkai-Golan, R. Aloe vera gel activity against plant pathogenic fungi. Postharvest Biol. Technol. 1995, 6, 159–165. [Google Scholar] [CrossRef]
- Saltos-Rezabala, L.A.; Da Silveira, P.R.; Tavares, D.G.; Moreira, S.I.; Magalhães, T.A.; Botelho, D.M.S.; Alves, E. Thyme essential oil reduces disease severity and induces resistance against Alternaria linariae in tomato plants. Horticulturae 2022, 8, 919. [Google Scholar] [CrossRef]
- Tariq, M.; Khan, A.; Asif, M.; Khan, F.; Ansari, T.; Shariq, M.; Siddiqui, M.A. Biological control: A sustainable and practical approach for plant disease management. Acta Agric. Scand. B 2020, 70, 507–524. [Google Scholar] [CrossRef]

| Type | Code | Description | Description | Assay a | Dosage b | Source/Reference |
|---|---|---|---|---|---|---|
| Plant activator | BION | Acibenzolar-S-methyl (BION® 50 WG) | Synthetic resistance inducer | 1, 2, 3 | 50 mg L−1 | Syngenta Crop Protection AG (Basel, Switzerland) |
| Commercial biostimulants | BIO1 | FERTILEADER® | Biostimulants and fertilizers containing seaweed extracts, humified peat, amino acids, and microelements | 1, 2 | 0.3% v/v | TIMAC AGRO International (Saint-Malo, France) |
| BIO2 | FERTIACTYL® | 1, 2 | 0.5% v/v | |||
| BIO3 | VITALFIT® | 1, 2 | 0.3% v/v | |||
| BIO4 | GENAKTIS® 4 | Biostimulator with micronutrient mixture and growth and development stimulator- for grapevines | 1 | na c | ||
| BIO5 | GENAKTIS® 5 | Biostimulator with micronutrient mixture and growth and development stimulator- for olive trees | 1 | na | ||
| Plant strengthener | WE | Wood extract | Steam-extracted from biomass, rich in polyphenols | 1, 2 | 0.2% v/v | Bio-Esperia srl (Civitella in Val di Chiana, AR, Italy); distributed by BASF Italia S.p.A. |
| Essential oils | Curry plant Laurel Lavender Lentisk Rosemary Sage | Helichrysum italicum (Roth) G. Don Laurus nobilis L. Lavandula angustifolia Miller Pistacia lentiscus L. Salvia rosmarinus Spenn. Salvia officinalis L. | Commercial formulations obtained by steam distillation | 1 | na | Hekomia (Ostuni, BR, Italy) |
| Thyme | Thymus vulgaris L. | 1,2,3 | Four droplets of 6 µL plant−1 | |||
| Incense Tea tree | Boswellia sacra Flück.Melaleuca alternifolia Cheel. | 1 | LaborBIO (Collegno, TO, Italy) | |||
| Aloe vera | AVE | Powdered leaf extract | Oven-dried leaves, ethanol extraction | 1, 2 | 10% w/v | Vivai Capitanieo (Monopoli, Italy) [17,18,19,20] |
| AP | Polysaccharide-rich extract | Ethanol precipitation, drying, pH-adjusted and heated | 1, 2 | 0.03% w/v | ||
| AG | Gel extract | Fresh gel manually extracted, acidified (pH 3.75), heat-stabilized (80 °C) | 1, 2, 3 | 10% v/v | ||
| Spent coffee grounds | C1 | Infusion method | Powder added to boiling water, maintained for 10 min | 1, 2, 3 | 4% w/v | Caffè Sartoriale, Bari, Italy [21] |
| C2 | Boiling method | Powder boiled in water for 10 min | 1, 2 | 4% w/v | ||
| C3 | Room temperature incubation | Powder soaked at room temperature for 15 min | 1, 2 | 4% w/v | ||
| Artichoke by-products | A1 | Dried bracts | Water extracts | 1, 2 | 10% v/v | University of Bari Aldo Moro [22] |
| A2 | ||||||
| A3 | Outer leaf and stem extracts | CO2 extraction in ethanol | ||||
| A4 | Extraction in methanol | |||||
| A5 | ||||||
| Ornamental plants | O1 | Eremophila nivea | Fresh leaves, homogenized in sterile distilled water | 1, 2, 3 | 2.5% w/v | Vivai Capitanio (Monopoli, Italy) and University of Bari Aldo Moro |
| O2 | Centaurea ragusina | |||||
| O3 | Agapanthus umbellatus | |||||
| O4 | Agapanthus africanus | |||||
| O5 | Dymondia margaretae | |||||
| O6 | Arbutus unedo | |||||
| O7 | Limoniastrum monopetalum | |||||
| Beer by-products | BEER-C | Centrifuged extract | Processed beer waste material | 2 | 5% v/v | University of Bari Aldo Moro |
| BEER-NC | Non-centrifuged extract | |||||
| Alginate formulations | ALG | Alginate | Empty 1.8 mm alginate beads | 1, 2, 3 | 4 beads plant−1 | University of Bari Aldo Moro [23] |
| ALG-T | Thyme EO microencapsulated in alginate | 1.8 mm alginate beads containing each 6 µL of thyme EO | 1, 2, 3 |
| Treatment | Inhibition Rate (%) 1 | ||
|---|---|---|---|
| Conidial Germination | Colony Growth | ||
| Biostimulants | BIO1 (10%) | 100.0 ± 0.0 a | 100.0 ± 0.0 a |
| BIO2 (10%) | Nd 2 | 100.0 ± 0.0 a | |
| BIO3 (10%) | Nd | 100.0 ± 0.0 a | |
| BIO4 (1%) | 2.0 ± 2.7 de | 0.0 ± 0.0 d | |
| BIO4 (10%) | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| BIO5 (1%) | 12.1 ± 1.3 bc | 33.9 ± 2.2 cd | |
| BIO5 (10%) | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| Plant strengthener | Wood extract (1%) | 4.7 ± 0.3 cde | 0.0 ± 0.0 d |
| Wood extract (10%) | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| Plant defense activator | BION | 3.1 ± 2.6 de | 0.0 ± 0.0 d |
| Essential oils | Helichrysum | 0.0 ± 0.0 e | 17.1 ± 17.2 de |
| Incense | 0.0 ± 0.0 e | 41.1 ± 2.2 c | |
| Laurel | 8.7 ± 1.8 cd | 100.0 ± 0.0 a | |
| Lavender | 16.4 ± 4.1 b | 100.0 ± 0.0 a | |
| Lentisk | 0.0 ± 0.3 e | 74.4 ± 13.0 b | |
| Rosemary | 0.3± 1.0 e | 100.0 ± 0.0 a | |
| Sage | 0.7 ± 0.7 e | 83.9 ± 16.1 ab | |
| Tea tree | 5.4 ± 3.1 cde | 100.0 ± 0.0 a | |
| Thyme | 100.0 ± 0.0 a | 100.0 ± 0.0 a | |
| Ornamental plants | Agapanthus africanus | 100.0 ± 0.0 a | 100.0 ± 0.0 a |
| Agapanthus umbellatus | 100.0 ± 0.0 a | 32.5 ± 8.1 cd | |
| Arbutus unedo | 0.0 ± 0.3 e | 0.0 ± 0.0 e | |
| Capparis spinosa | 1.0 ± 0.3 e | 0.0 ± 0.0 e | |
| Centaurea cineraria | 0.3 ± 1.0 e | 0.0 ± 0.0 e | |
| Dymodia margaretae | 0.7 ± 0.9 e | 0.0 ± 0.0 e | |
| Eremophyla nivea | 0.0 ± 0.0 e | 13.0 ± 4.1 de | |
| Limoniastrum monopetalum | 0.0 ± 0.7 e | 4.9 ± 0.0 e | |
| Aloe vera extracts | AVE | 1.7 ± 0.7 de | 0.0 ± 0.0 e |
| AG | 0.0 ± 0.7 e | 0.0 ± 0.0 e | |
| AP | 1.7 ± 0.7 de | 0.0 ± 0.0 e | |
| Coffee extracts | C1 | 6.0 ± 1.7 cde | 0.0 ± 0.0 e |
| C2 | 5.7 ± 0.7 cde | 0.0 ± 0.0 e | |
| C3 | 7.0 ± 1.2 cde | 0.0 ± 0.0 e | |
| Artichoke extracts | A1 | 6.5 ± 1.7 cde | 0.0 ± 0.0 e |
| A2 | 6.5 ± 1.7 cde | 0.0 ± 0.0 e | |
| A3 | 2.4 ± 0.7 de | 0.0 ± 0.0 e | |
| A4 | 0.0 ± 0.6 e | 0.0 ± 0.0 e | |
| A5 | 0.0 ± 0.9 e | 0.0 ± 0.0 e | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bilen, C.; Laera, S.; Rotondo, P.R.; Dimaglie, M.; Vaccaro, L.; Marashi, M.; Mascia, T.; Lopedota, A.A.; Spanò, R.; Pollastro, S.; et al. Antimicrobial Activity and Activation of Defense Genes in Plants by Natural Extracts: Toward Sustainable Plant Health Management. Agronomy 2025, 15, 2342. https://doi.org/10.3390/agronomy15102342
Bilen C, Laera S, Rotondo PR, Dimaglie M, Vaccaro L, Marashi M, Mascia T, Lopedota AA, Spanò R, Pollastro S, et al. Antimicrobial Activity and Activation of Defense Genes in Plants by Natural Extracts: Toward Sustainable Plant Health Management. Agronomy. 2025; 15(10):2342. https://doi.org/10.3390/agronomy15102342
Chicago/Turabian StyleBilen, Christine, Sebastiano Laera, Palma R. Rotondo, Matteo Dimaglie, Lorenza Vaccaro, Michela Marashi, Tiziana Mascia, Angela A. Lopedota, Roberta Spanò, Stefania Pollastro, and et al. 2025. "Antimicrobial Activity and Activation of Defense Genes in Plants by Natural Extracts: Toward Sustainable Plant Health Management" Agronomy 15, no. 10: 2342. https://doi.org/10.3390/agronomy15102342
APA StyleBilen, C., Laera, S., Rotondo, P. R., Dimaglie, M., Vaccaro, L., Marashi, M., Mascia, T., Lopedota, A. A., Spanò, R., Pollastro, S., Faretra, F., El Chami, D., & De Miccolis Angelini, R. M. (2025). Antimicrobial Activity and Activation of Defense Genes in Plants by Natural Extracts: Toward Sustainable Plant Health Management. Agronomy, 15(10), 2342. https://doi.org/10.3390/agronomy15102342









