Improving Air Distribution Within Lettuce Plant Canopy by Employing Double-Channel Ventilation Cultivation System: Simulation and Experiment Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Numerical Simulation
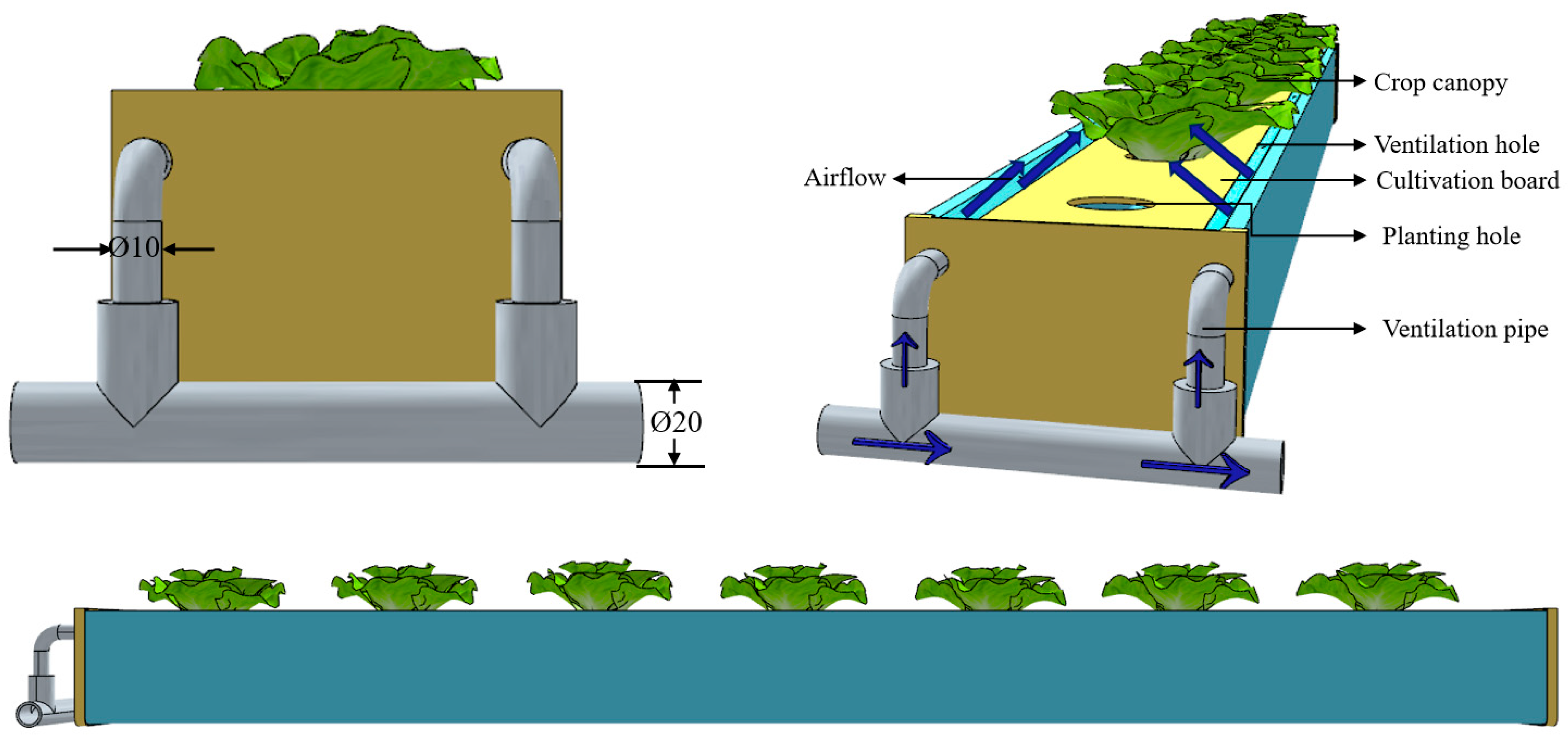
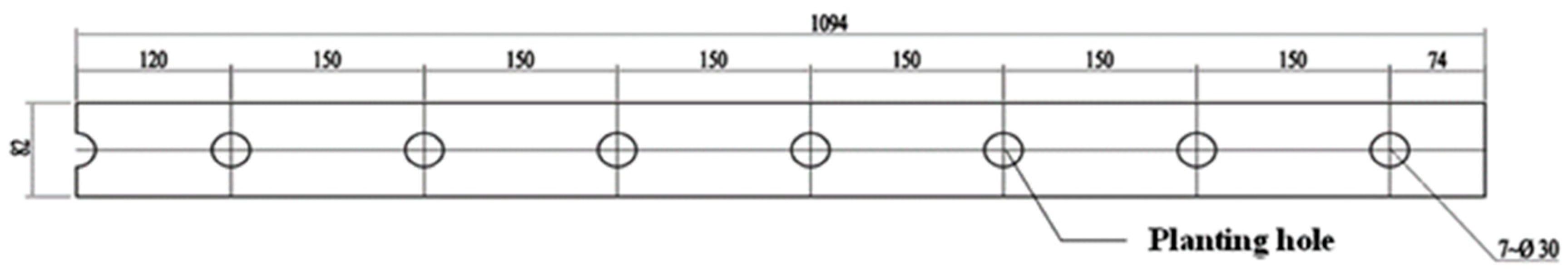
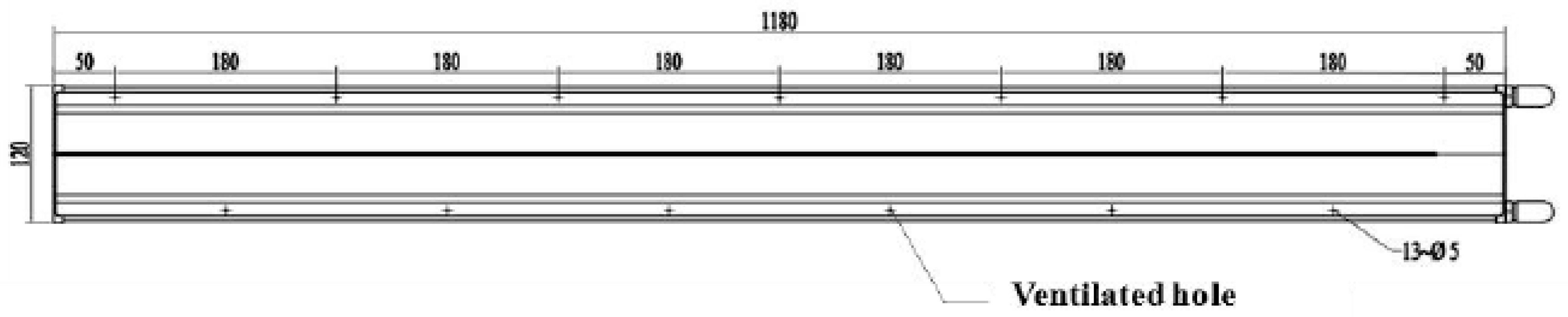
2.1.1. The Model Geometry and Grid Generation
2.1.2. The Numerical Approach
2.1.3. Boundary Conditions
2.1.4. Solver Settings
2.2. Practical Cultivation Experiment Setup
2.2.1. Cultivation Tank Design and Treatment Setup
2.2.2. Plant Preparing
2.2.3. Plant Measurements
2.2.4. Gas Exchange Measurements
2.2.5. Efficiency of Electric Energy Utilization
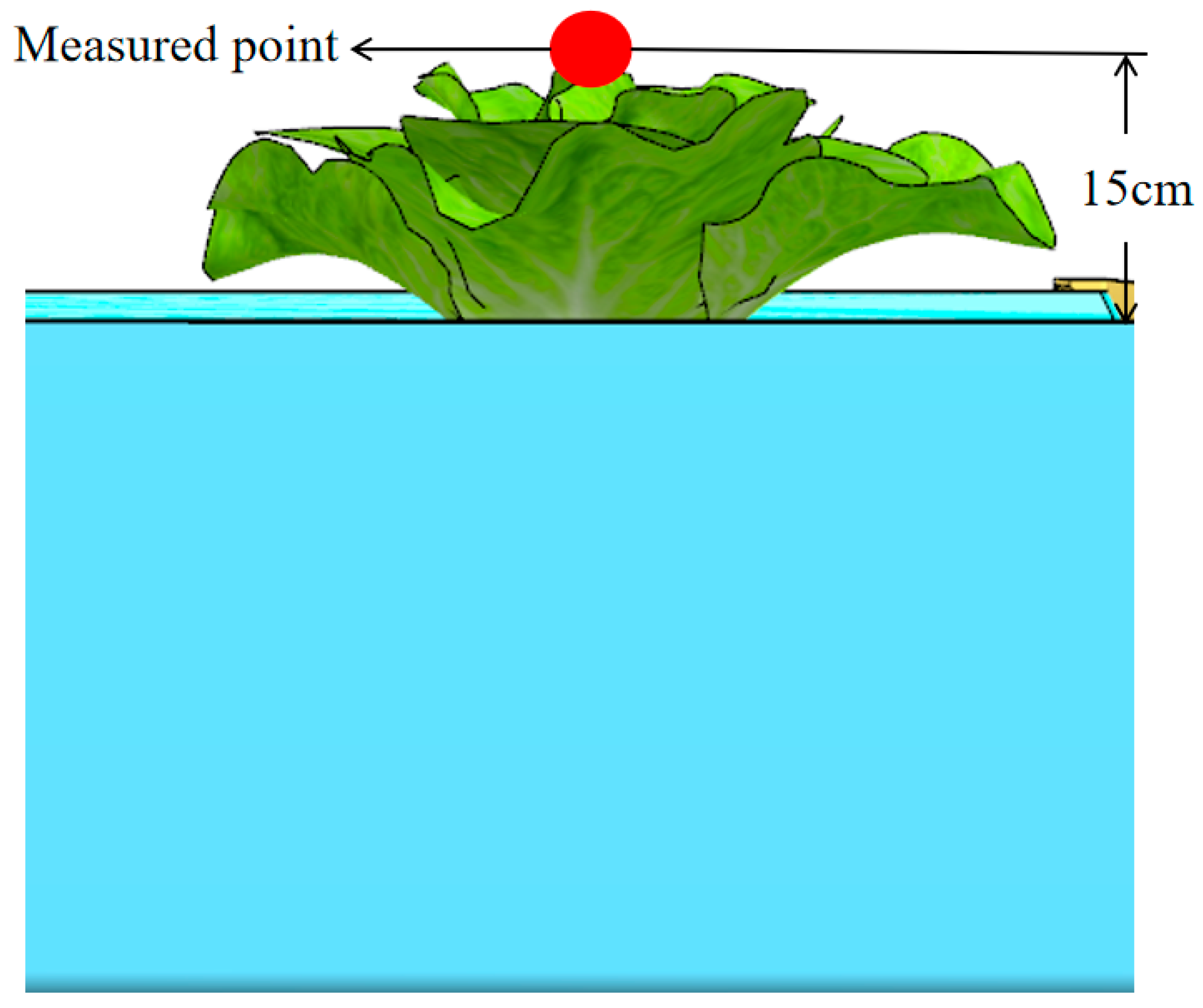
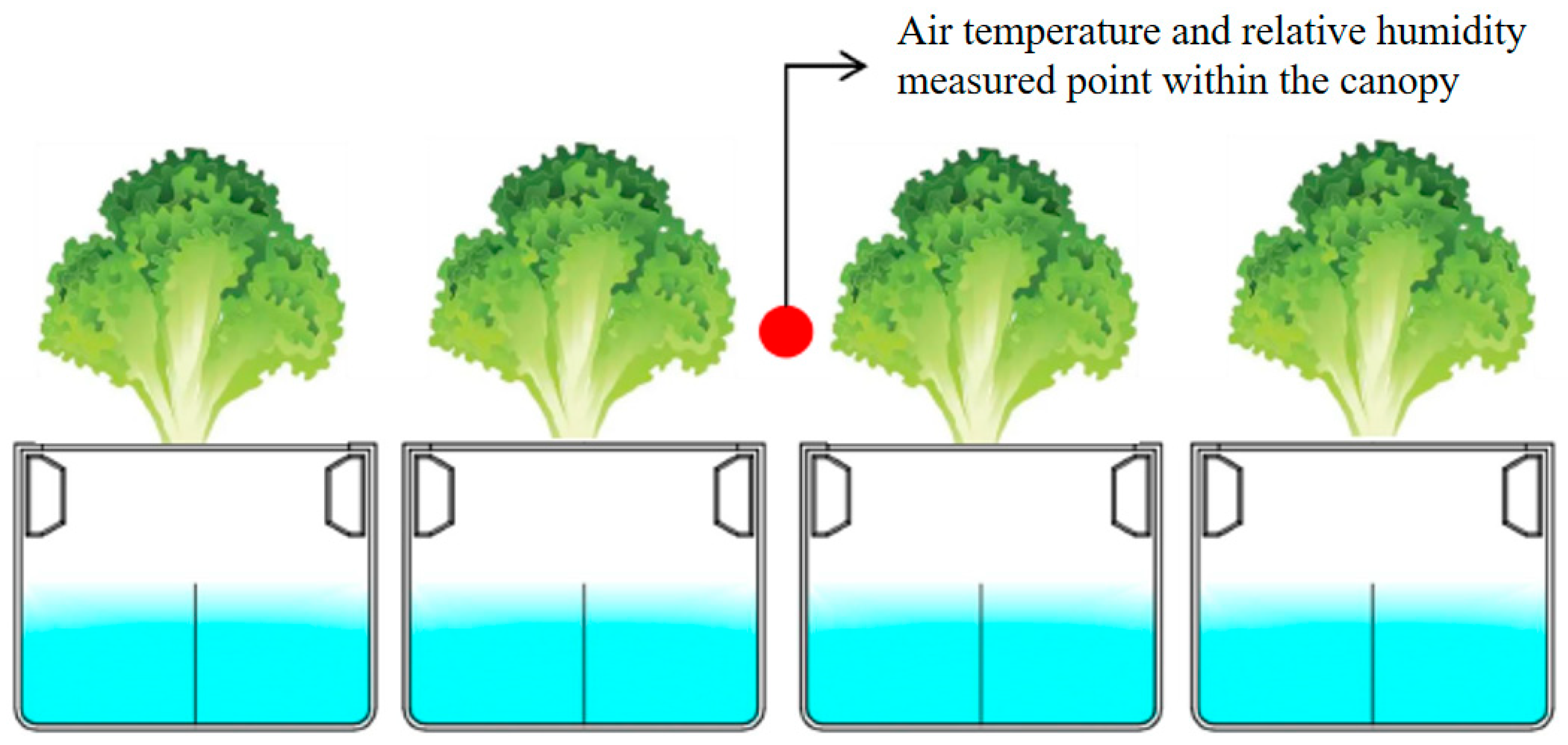
2.2.6. Environmental Parameters Measurements
2.3. Statistical Analysis
3. Results and Discussions
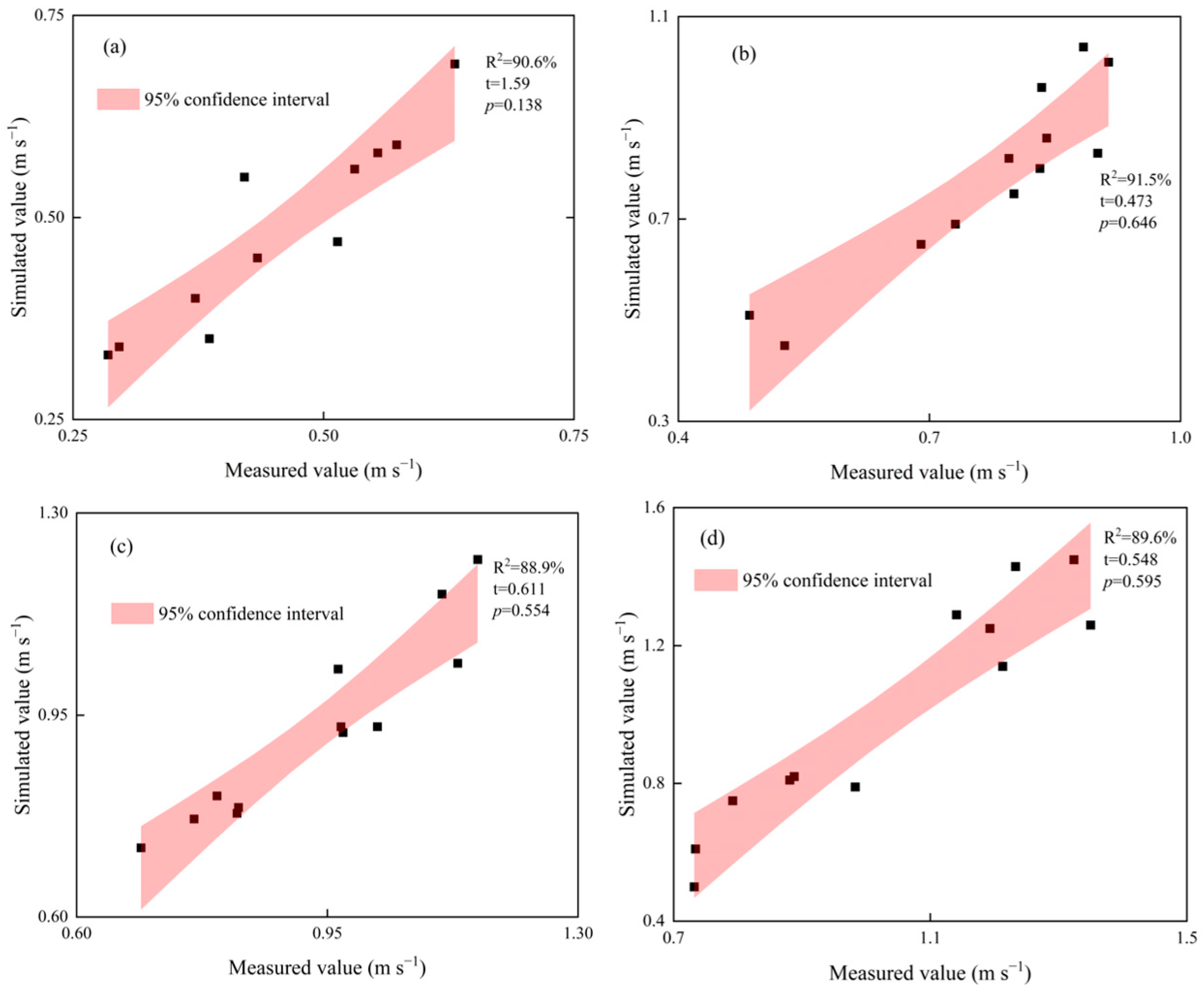
3.1. Numerical Simulation: Model Validation
3.2. Computational Simulation: Design of a Cultivation Tank
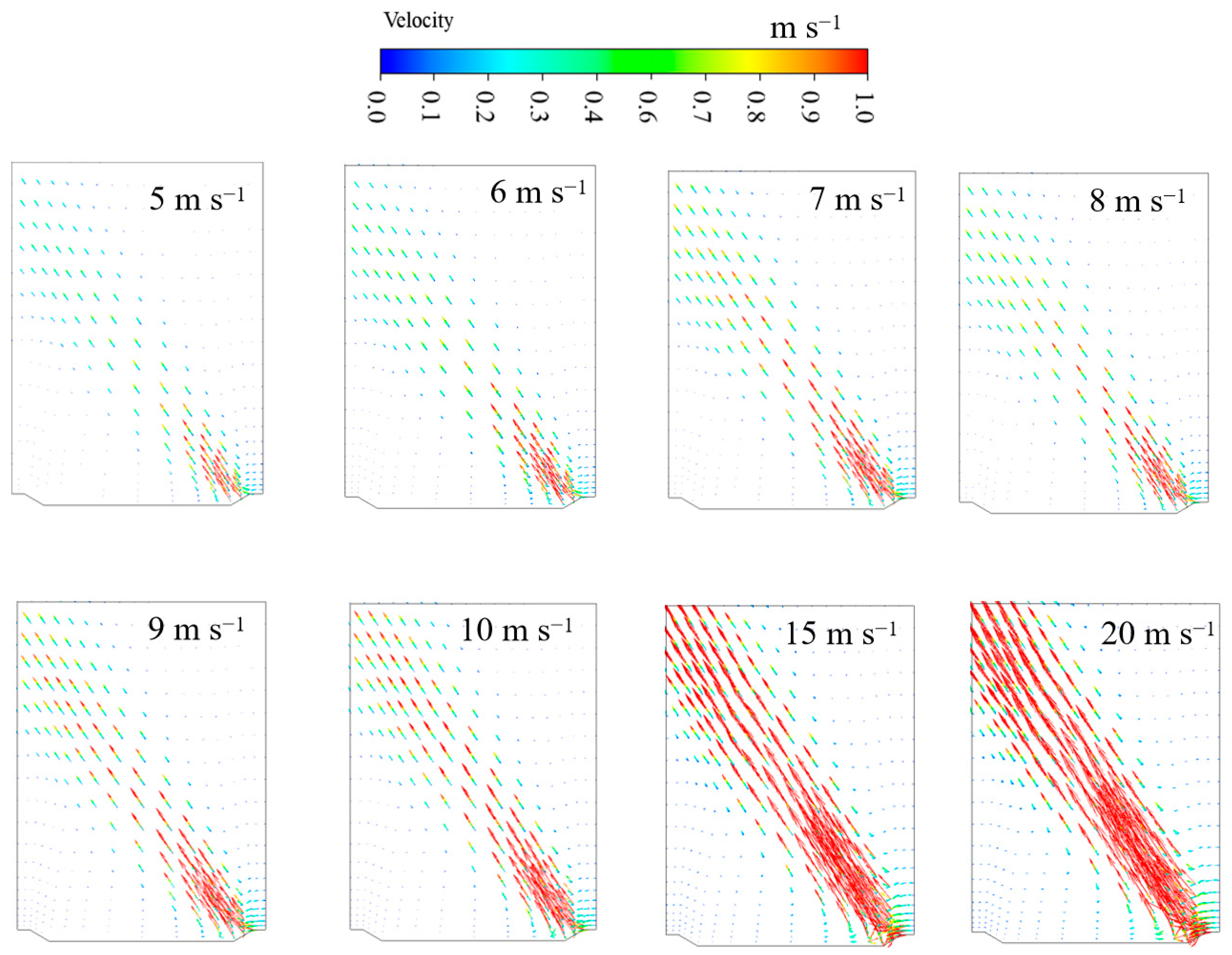
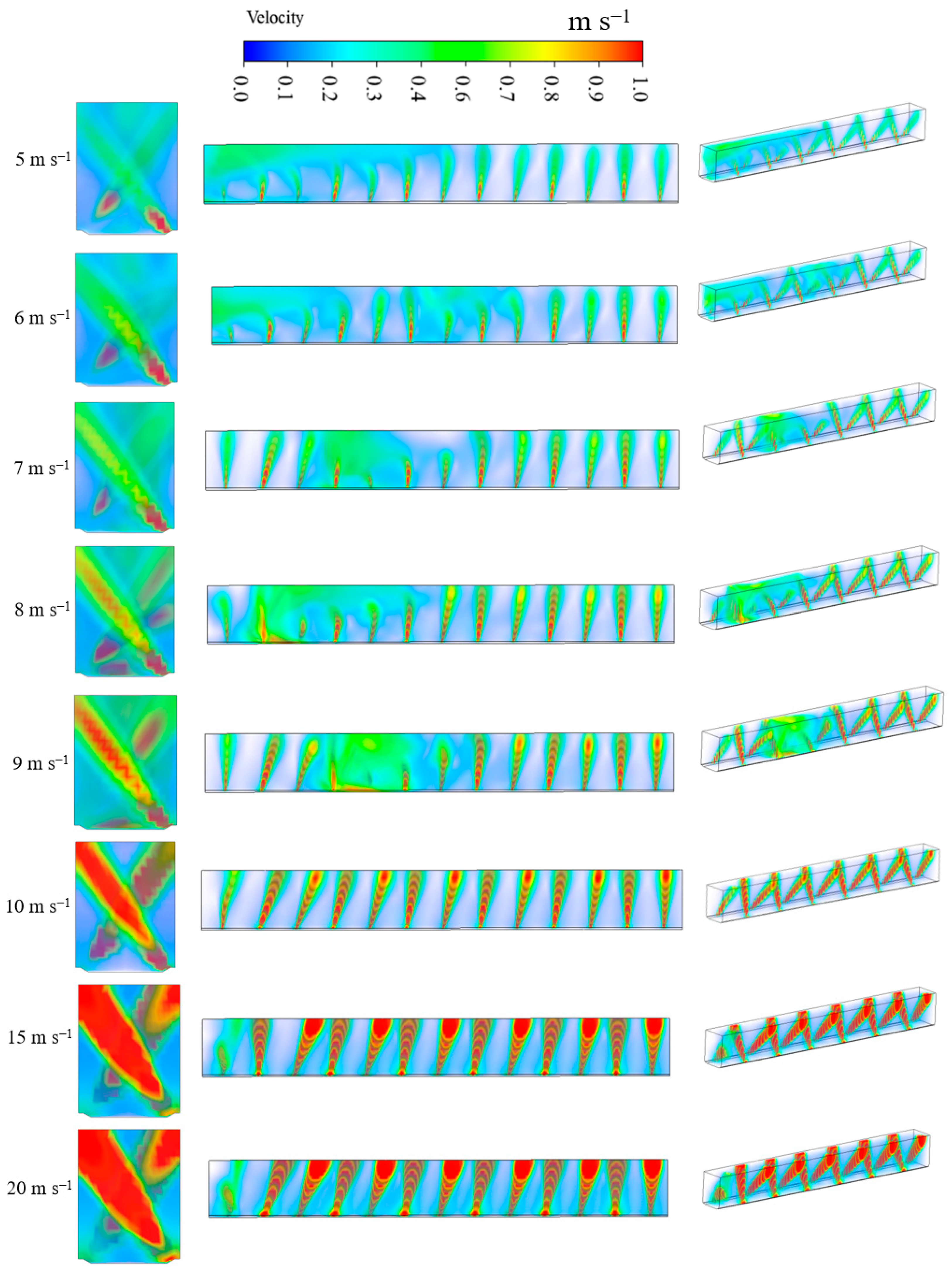
3.2.1. Airflow Patterns with Different Intake Air Velocities
3.2.2. Airflow Distribution in Crop Canopy Interior
3.2.3. The Canopy Airflow Uniformity as Affected by Different Intake Air Velocities
3.3. Practical Plant Cultivation Experiment
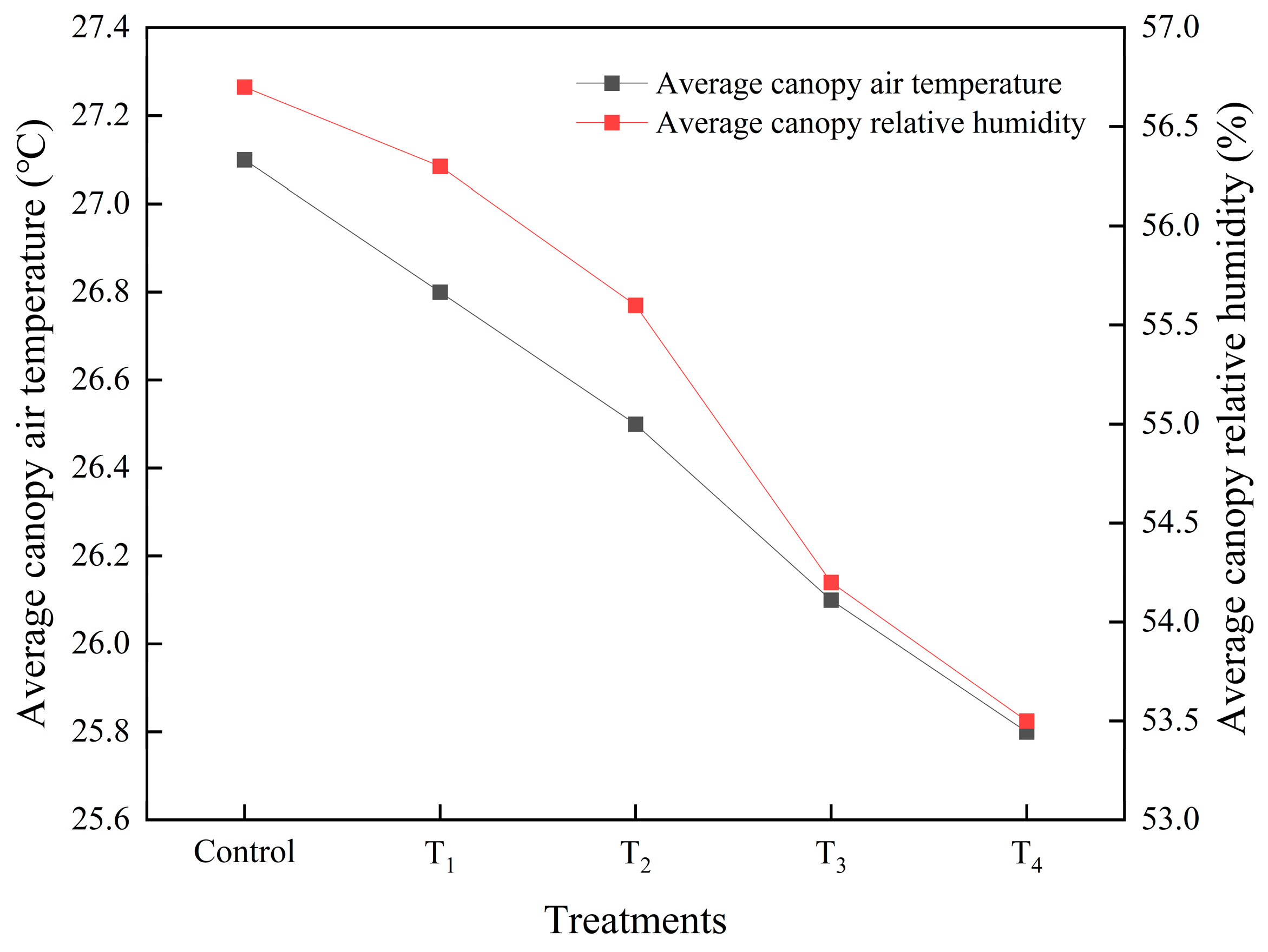
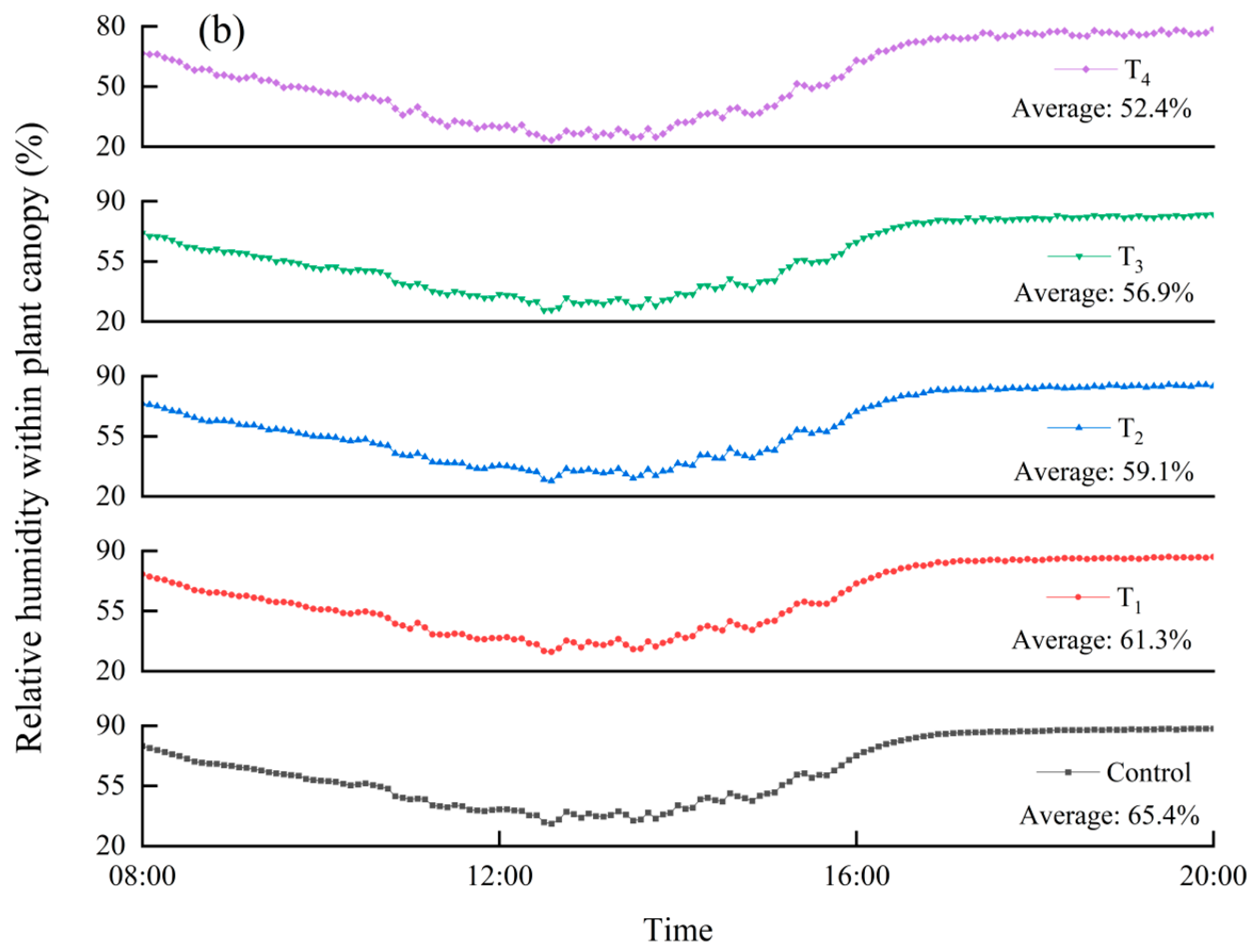
3.3.1. The Canopy Environment as Affected by Different Air Velocities
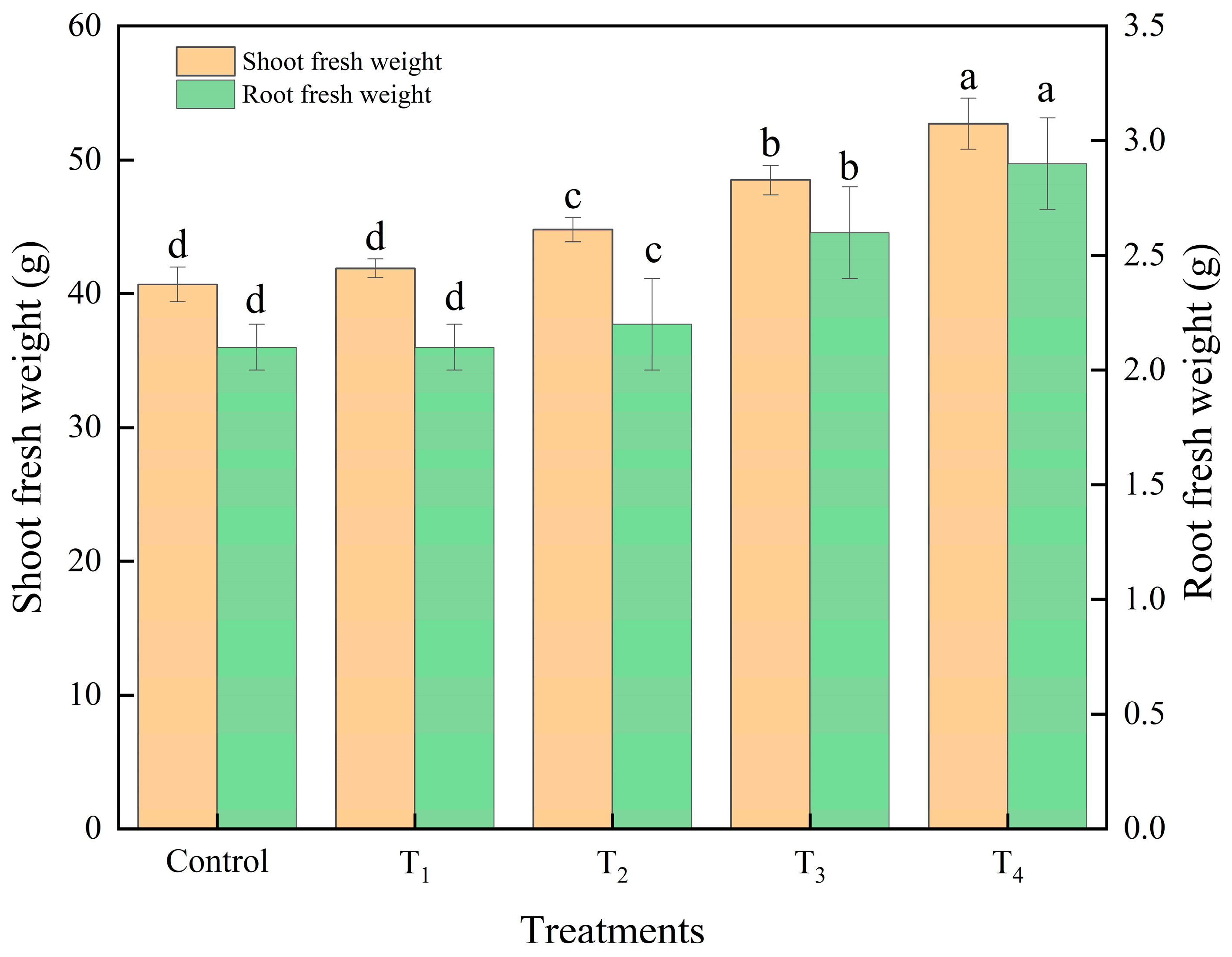
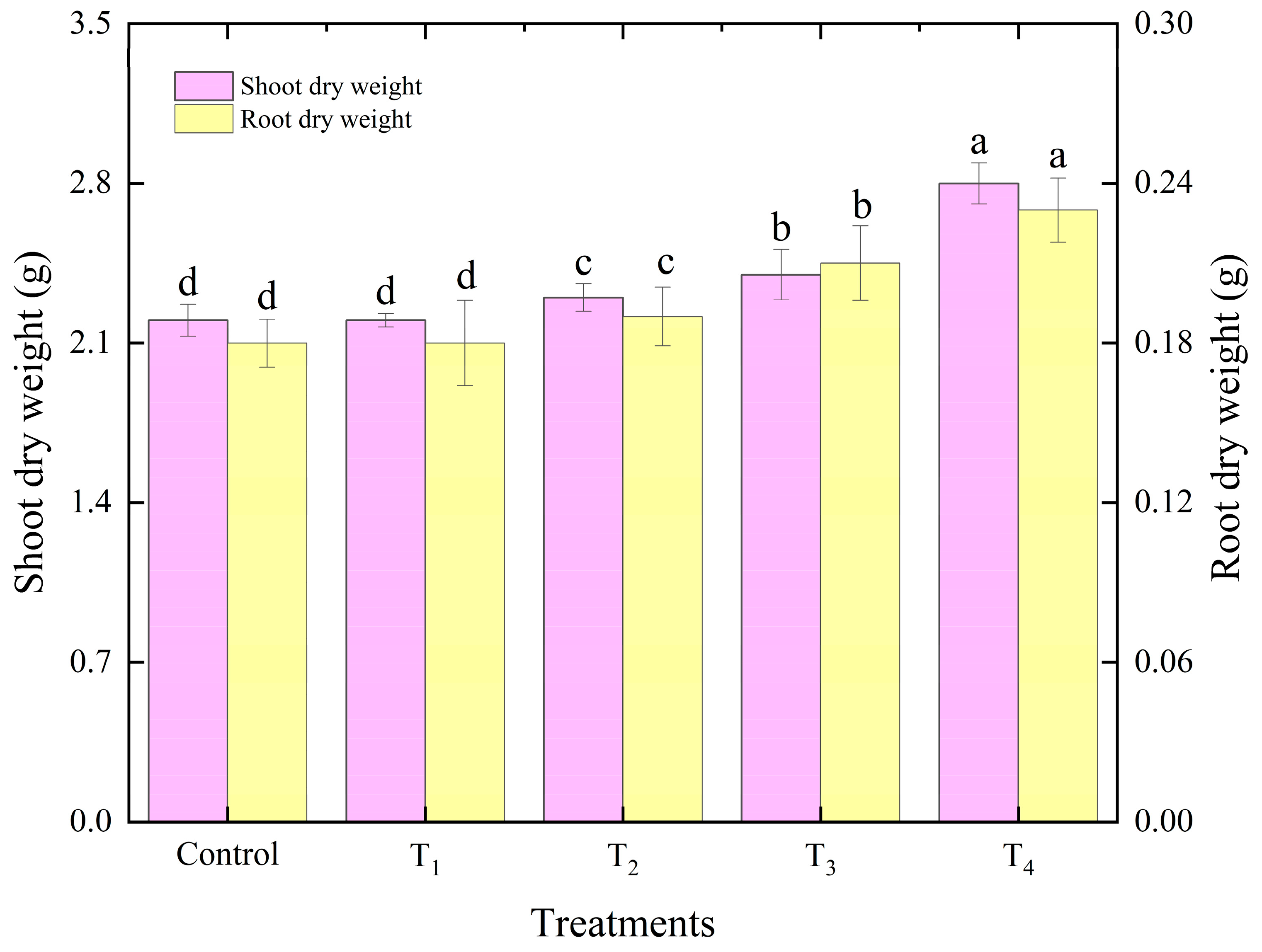
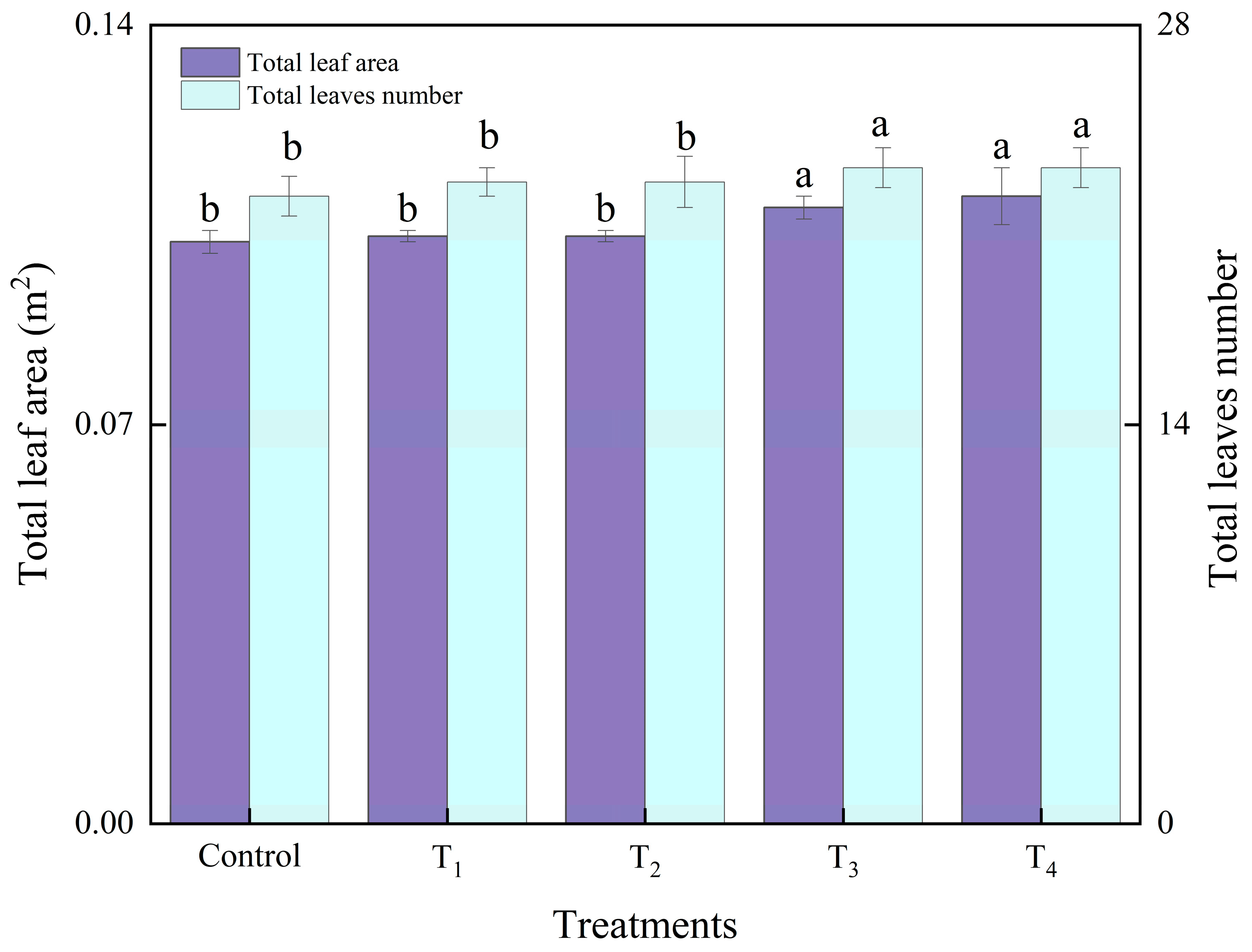
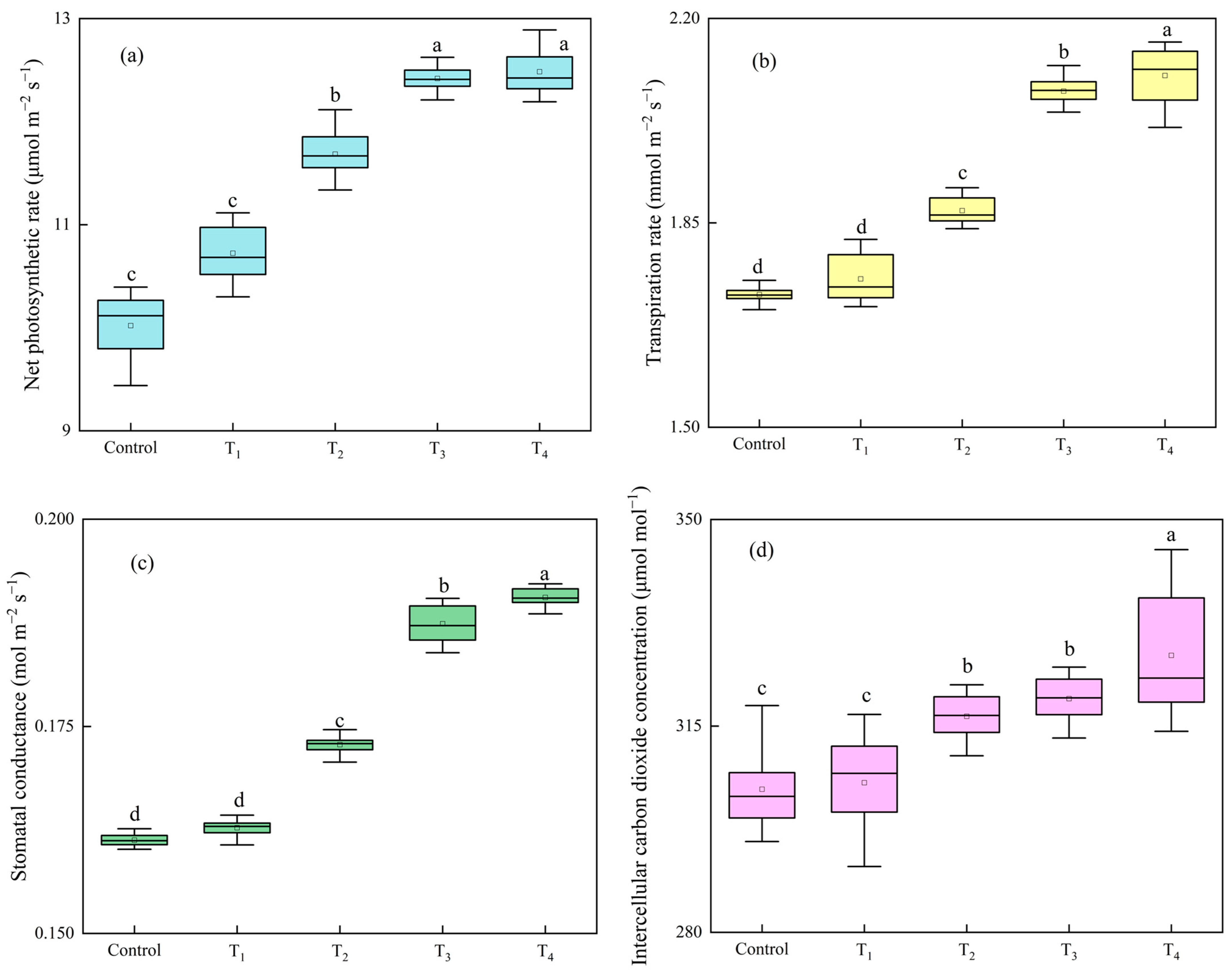
3.3.2. Lettuce Growth and Photosynthesis Analysis as Affected by Different Intake Air Velocities
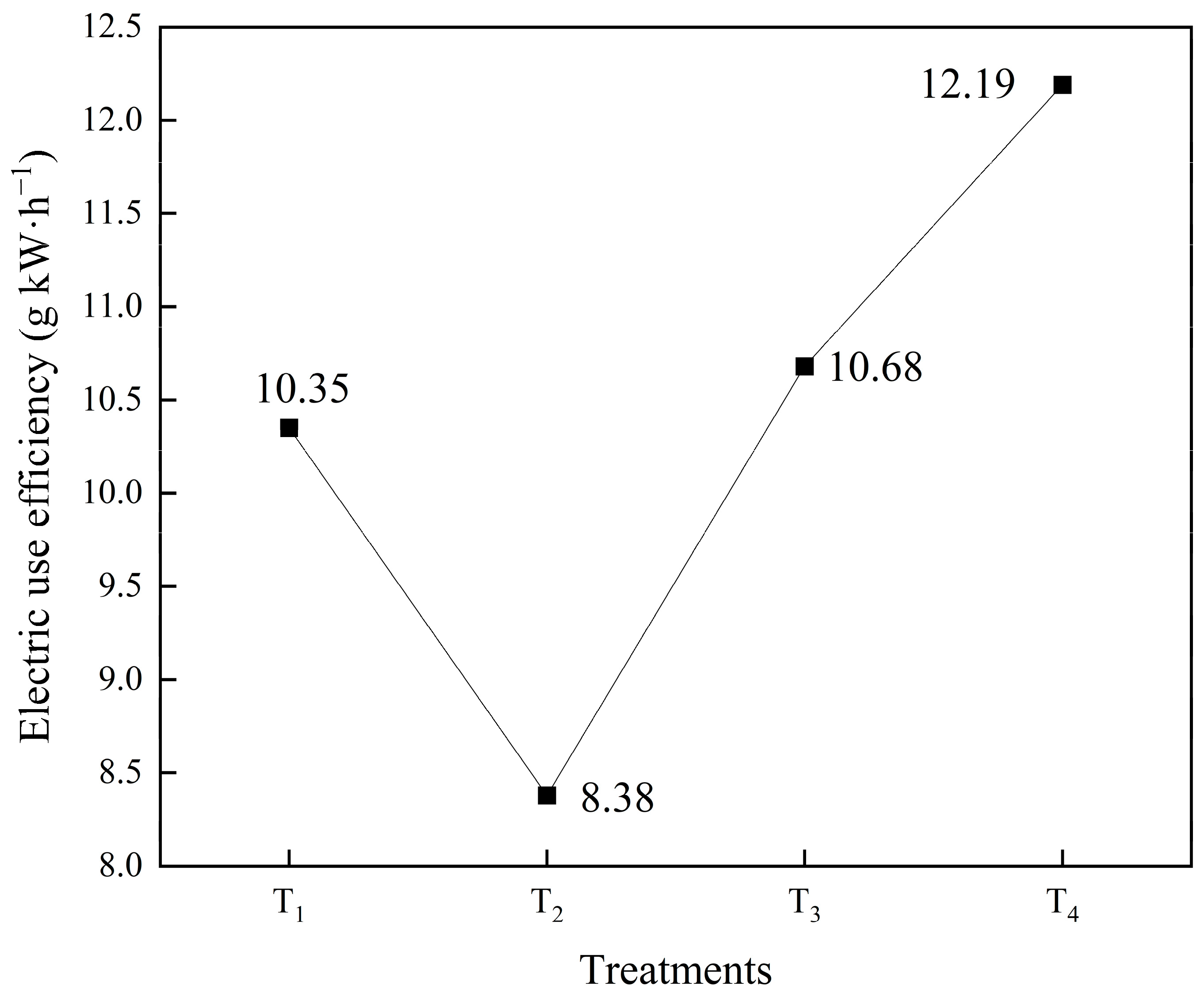
3.3.3. Electric Use Efficiency as Affected by Different Intake Air Velocities
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liao, P.; Liu, J.; Sun, L.; Chang, H. Can the Adoption of Protected Cultivation Facilities Affect Farm Sustainability? Sustainability 2020, 12, 9970. [Google Scholar] [CrossRef]
- Naseer, M.; Persson, T.; Righini, I.; Stanghellini, C.; Maessen, H.; Verheul, M.J. Bio-economic evaluation of greenhouse designs for seasonal tomato production in Norway. Biosyst. Eng. 2021, 212, 413–430. [Google Scholar] [CrossRef]
- Ghani, S.; El-Bialy, E.; Bakochristou, F.; Mohamed, M.; Mohamed, A.; Mohammad, S.; Ben, P. Experimental and numerical investigation of the thermal performance of evaporative cooled greenhouses in hot and arid climates. Sci. Technol. Built Environ. 2020, 26, 141–160. [Google Scholar] [CrossRef]
- Moon, S.; Kwon, S.; Lim, J. Minimization of Temperature Ranges between the Top and Bottom of an Air Flow Controlling Device through Hybrid Control in a Plant Factory. Sci. World J. 2014, 2014, 801590. [Google Scholar] [CrossRef]
- Boulard, T.; Fatnassi, H.; Roy, J.; Lagier, J.; Fargues, J.; Smits, N.; Rougier, M.; Jeannequin, B. Effect of greenhouse ventilation on humidity of inside air and in leaf boundary-layer. Agric. For. Meteorol. 2004, 125, 225–239. [Google Scholar] [CrossRef]
- Yabuki, K.; Miyagawa, H. Studies on the Effect of Wind Speed upon the Photosynthesis (2) The Relation between Wind Speed and Photosynthesis. J. Agric. Meteorol. 1970, 26, 137–141. [Google Scholar] [CrossRef]
- Lee, I.; Short, T. Two-dimensional numerical simulation of natural ventilation in a multi-span greenhouse. Trans. ASAE 2000, 43, 745–753. [Google Scholar] [CrossRef]
- Kitaya, Y.; Tsuruyama, J.; Shibuya, T. Effects of air current speed on gas exchange in plant leaves and plant canopies. Adv. Space Res. 2003, 31, 177–182. [Google Scholar] [CrossRef]
- Nishikawa, T.; Fukuda, H.; Murase, H. Effects of Airflow for Lettuce Growth in the Plant Factory with an Electric Turntable. IFAC Proc. Vol. 2013, 46, 270–273. [Google Scholar] [CrossRef]
- Kitaya, Y. Importance of air movement for promoting gas and heat exchanges between plants and atmosphere under controlled environments. In Plant Responses to Air Pollution and Global Change; Springer: Tokyo, Japan, 2005; pp. 185–193. [Google Scholar]
- Shibata, T.; Iwao, K.; Takano, T. Effect of vertical air flowing on lettuce growing in a plant factory. In Acta Horticulturae; International Society for Horticultural Science (ISHS): Leuven, Belgium, 1995; pp. 175–182. [Google Scholar]
- Kitaya, Y.; Tsuruyama, J.; Shibuya, T.; Kiyota, M. Effects of air current speed, light intensity and CO2 concentration on photosynthesis and transpiration of plant leaves. In Proceedings of the 35th COSPAR Scientific Assembly, Paris, France, 18–25 July 2004. [Google Scholar]
- Shibuya, T.; Tsuruyama, J.; Kitaya, Y.; Kiyota, M. Enhancement of photosynthesis and growth of tomato seedlings by forced ventilation within the canopy. Sci. Hortic. 2006, 109, 218–222. [Google Scholar] [CrossRef]
- Chintakovid, W.; Kubota, C.; Bostick, W.; Kozai, T. Effect of Air Current Speed on Evapotranspiration Rate of Transplant Canopy under Artificial Light. Shokubutsu Kojo Gakkaishi 2002, 14, 25–31. [Google Scholar] [CrossRef]
- Goto, E.; Takakura, T. Promotion of Ca Accumulation in Inner Leaves by Air Supply for Prevention of Lettuce Tipburn. Trans. ASAE 1992, 35, 647–650. [Google Scholar] [CrossRef]
- Wu, C.; Cheng, R.; Fang, H.; Yang, Q.; Zhang, C. Simulation and optimization of air tube ventilation in plant factory based on CFD. J. Chin. Agric. Univ. 2021, 26, 78–87. [Google Scholar]
- Zhang, Y.; Kacira, M.; An, L. A CFD study on improving air flow uniformity in indoor plant factory system. Biosyst. Eng. 2016, 147, 193–205. [Google Scholar] [CrossRef]
- Lim, T.; Kim, H. Analysis of Airflow Pattern in Plant Factory with Different Inlet and Outlet Locations using Computational Fluid Dynamics. J. Biosyst. Eng. 2014, 39, 310–317. [Google Scholar] [CrossRef]
- Li, K.; Zou, Z. Environmental effects of root zone ventilation on canopy and rhizosphere of lettuce in plant factory. Trans. Chin. Soc. Agric. Eng. 2019, 35, 178–187. [Google Scholar]
- Norton, T.; Sun, D.; Grant, J.; Fallon, R.; Dodd, V. Applications of computational fluid dynamics (CFD) in the modelling and design of ventilation systems in the agricultural industry: A review. Bioresour. Technol. 2007, 98, 2386–2414. [Google Scholar] [CrossRef]
- Fang, H.; Li, K.; Wu, G.; Cheng, R.; Zhang, Y.; Yang, Q. A CFD analysis on improving lettuce canopy airflow distribution in a plant factory considering the crop resistance and LEDs heat dissipation. Biosyst. Eng. 2020, 200, 1–12. [Google Scholar] [CrossRef]
- Zhang, C.; Fang, H.; Cheng, R.; Ynag, Q.; Wei, X.; Wu, C. Aerodynamic properties of lettuce canopy based on wind tunnel system. J. China Agric. Univ. 2019, 24, 96–103. [Google Scholar]
- Zhang, Y.; Yasutake, D.; Hidaka, K.; Kitano, M.; Okayasu, T. CFD analysis for evaluating and optimizing spatial distribution of CO2 concentration in a strawberry greenhouse under different CO2 enrichment methods. Comput. Electron. Agric. 2020, 179, 105811. [Google Scholar] [CrossRef]
- Patanker, S. Numerical Heat Transfer and Fluid Flow; CRC Press: Boca Raton, FL, USA, 1980. [Google Scholar] [CrossRef]
- Mirade, P.; Daudin, J. Computational fluid dynamics prediction and validation of gas circulation in a cheese-ripening room. Int. Dairy J. 2006, 16, 920–930. [Google Scholar] [CrossRef]
- Zhang, Y.; Yasutake, D.; Hidaka, K.; Okayasu, T.; Kitano, M.; Hirota, T. Crop-localised CO2 enrichment improves the microclimate, photosynthetic distribution and energy utilisation efficiency in a greenhouse. J. Clean. Prod. 2022, 371, 133465. [Google Scholar] [CrossRef]
- Ahmed, H.; Tong, Y.; Li, L.; Sahari, S.; Almogahed, A.; Cheng, R. Integrative Effects of CO2 Concentration, Illumination Intensity and Air Speed on the Growth, Gas Exchange and Light Use Efficiency of Lettuce Plants Grown under Artificial Lighting. Horticulturae 2022, 8, 270. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Q.; Tong, Y. Effects on electric-energy and light use efficiency and quality for lettuce under different light intensities supplied with red light and blue light. J. China Agric. Univ. 2016, 21, 59–66. [Google Scholar]
- Tamimi, E.; Kacira, M.; Yeonsik, C.; An, L. Analysis of Microclimate Uniformity in a Naturally Vented Greenhouse with a High-Pressure Fogging System. Trans. ASABE 2013, 56, 1241–1254. [Google Scholar] [CrossRef]
- Kitaya, Y.; Shibuya, T.; Kozai, T.; Kubota, C. Effects of light intensity and air velocity on air temperature, water vapor pressure, and CO2 concentration inside a plant canopy under an artificial lighting condition. Life Support Biosph. Sci. Int. J. Earth Space 1998, 5, 199–203. [Google Scholar]
- Wells, C.; Amos, N. Design of air Distribution Systems for Closed Greenhouses; International Society for Horticultural Science (ISHS): Leuven, Belgium, 1994; pp. 93–104. [Google Scholar]
- Clarkson, J.; Fawcett, L.; Anthony, S.; Young, C. A Model for Sclerotinia sclerotiorum Infection and Disease Development in Lettuce, Based on the Effects of Temperature, Relative Humidity and Ascospore Density. PLoS ONE 2014, 9, e94049. [Google Scholar] [CrossRef] [PubMed]
- Lyimo, H.; Pratt, R.; Mnyuku, R. An effective integrated crop management strategy for enhanced maize production in tropical agroecosystems prone to gray leaf spot. Crop Prot. 2012, 41, 57–63. [Google Scholar] [CrossRef]
- Kawasaki, Y.; Yoneda, Y. Local Temperature Control in Greenhouse Vegetable Production. Hortic. J. 2019, 88, 305–314. [Google Scholar] [CrossRef]
- Dupont, K.; Den, T.; Zhang, J.; Moene, A.; Vialet-Chabrand, S.R.M. Beyond the boundary: A new road to improve photosynthesis via wind. J. Exp. Bot. 2025, 00, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Korthals, R.; Knight, S.; Christianson, J.; Spomer, L. Chambers for studying the effects of airflow velocity on plant growth. Biotronics 1994, 23, 113–119. [Google Scholar]
- Ahmed, H.A.; Yuxin, T.; Qichang, Y. Lettuce plant growth and tipburn occurrence as affected by airflow using a multi-fan system in a plant factory with artificial light. J. Therm. Biol. 2020, 88, 102496. [Google Scholar] [CrossRef]
- Gruda, N.; Samuolienė, G.; Dong, J.; Li, X. Environmental conditions and nutritional quality of vegetables in protected cultivation. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70139. [Google Scholar] [CrossRef]
- Shibuya, T.; Kozai, T. Effects of Air Current Speed on Net Photosynthetic and Evapotranspiration Rates of a Tomato Plug Sheet under Artificial Light. Environ. Control Biol. 1998, 36, 131–136. [Google Scholar] [CrossRef]
- Farquhar, G.; Sharkey, T. Stomatal Conductance and Photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Takahashi, Y.; Joo, H.; Pankasem, N.; Hsu, P.; Schroeder, J. Stomatal CO2 sensing in plants: Control of gas exchange and interactions with environmental stimuli. Plant Cell Physiol. 2025, 66, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Goins, G.; Wheeler, R.; Sager, J. Stomatal Conductance of Lettuce Grown Under or Exposed to Different Light Qualities. Ann. Bot. 2004, 94, 691–697. [Google Scholar] [CrossRef]
- Lee, J.; Choi, C.; Jang, Y.; Jang, S.; Lee, S.; Um, Y. Effects of air temperature and air flow rate control on the tipburn occurrence of leaf lettuce in a closed-type plant factory system. Hortic. Environ. Biotechnol. 2013, 54, 303–310. [Google Scholar] [CrossRef]
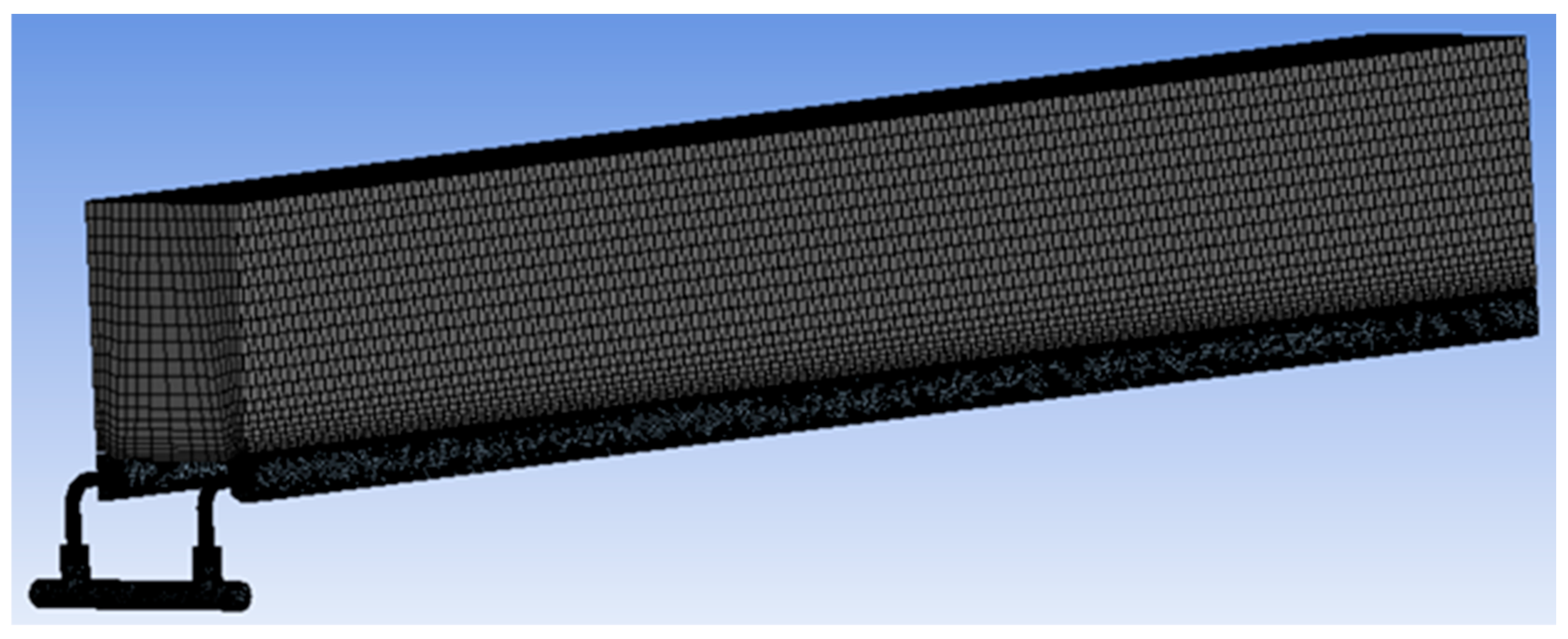

| Max | Min | Average | |
|---|---|---|---|
| Skewness | 0.69 | 2.9 × 10−4 | 0.21 |
| Aspect ratio | 5.5 | 1.1 | 1.8 |
| Orthogonality | 1 | 0.27 | 0.78 |
| Fluid: Air Operating Air Temperature in the Production Domain: 297 K Gravitational Acceleration: 9.81 m s−2 Viscous Model: Realizable K-Epsilon Model with Standard Wall Functions and Full Buoyancy Effect | ||
|---|---|---|
| Parameter | Boundary conditions | Property |
| Air-pipe-inlet | Velocity—inlet | 5/6/7/8/9/10/15/20 m s−1 |
| Two-side-wall-1 | Pressure—outlet | Gauge pressure: 0 Pa |
| Pipe-wall | Wall | Default |
| Two-side-wall-2 | Pressure—outlet | Gauge pressure: 0 Pa |
| Top-side-wall | Pressure—outlet | Gauge pressure: 0 Pa |
| Element | Concentration (mg L−1) |
|---|---|
| Ca(NO3)2·4H2O | 236 |
| KNO3 | 404 |
| NH4H2PO4 | 57 |
| MgSO4·7H2O | 123 |
| H3BO3 | 2.86 |
| MnSO4·4H2O | 2.13 |
| ZnSO4·7H2O | 0.22 |
| CuSO4·5H2O | 0.08 |
| (NH4)6MO7O24·4H2O | 0.02 |
| NaFe-EDTA·3H2O | 32.4 |
| Intake Air Velocity (m s−1) | Percentage (%) | Volume-Weighted Average Velocity (m s−1) | ||
|---|---|---|---|---|
| v < 0.1 m s−1 | 0.1 m s−1 ≤ v ≤ 1 m s−1 | v > 1 m s−1 | ||
| 5 | 53 | 47 | 0 | 0.11 |
| 6 | 43 | 57 | 0 | 0.14 |
| 7 | 55 | 45 | 0 | 0.14 |
| 8 | 45 | 54 | 1 | 0.18 |
| 9 | 40 | 58 | 2 | 0.20 |
| 10 | 34.5 | 63 | 2.5 | 0.22 |
| 15 | 17 | 78 | 5 | 0.30 |
| 20 | 10 | 83 | 7 | 0.37 |
| Intake Air Velocity (m s−1) | Standard Deviation | Volume-Weighted Average Velocity (m s−1) | CV (%) |
|---|---|---|---|
| 5 | 0.09 | 0.11 | 82 |
| 6 | 0.10 | 0.14 | 71 |
| 7 | 0.10 | 0.14 | 71 |
| 8 | 0.13 | 0.18 | 72 |
| 9 | 0.14 | 0.20 | 69 |
| 10 | 0.14 | 0.22 | 64 |
| 15 | 0.24 | 0.30 | 80 |
| 20 | 0.30 | 0.37 | 81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, C.; Fang, H.; Tong, Y. Improving Air Distribution Within Lettuce Plant Canopy by Employing Double-Channel Ventilation Cultivation System: Simulation and Experiment Study. Agronomy 2025, 15, 2326. https://doi.org/10.3390/agronomy15102326
Zhang Y, Chen C, Fang H, Tong Y. Improving Air Distribution Within Lettuce Plant Canopy by Employing Double-Channel Ventilation Cultivation System: Simulation and Experiment Study. Agronomy. 2025; 15(10):2326. https://doi.org/10.3390/agronomy15102326
Chicago/Turabian StyleZhang, Yihan, Can Chen, Hui Fang, and Yuxin Tong. 2025. "Improving Air Distribution Within Lettuce Plant Canopy by Employing Double-Channel Ventilation Cultivation System: Simulation and Experiment Study" Agronomy 15, no. 10: 2326. https://doi.org/10.3390/agronomy15102326
APA StyleZhang, Y., Chen, C., Fang, H., & Tong, Y. (2025). Improving Air Distribution Within Lettuce Plant Canopy by Employing Double-Channel Ventilation Cultivation System: Simulation and Experiment Study. Agronomy, 15(10), 2326. https://doi.org/10.3390/agronomy15102326





