Integrated Approach of Using Biostimulants for Improving Growth, Physiological Traits, and Tolerance to Abiotic Stressors in Rice and Soybean
Abstract
1. Introduction
2. Definition and Concept of Biostimulants
3. Biostimulants Enhance Growth, Yield, and Stress Tolerance in Rice and Soybean Under High-Temperature Stress
3.1. Biostimulants and Rice Tolerance to High-Temperature Stress
3.2. Biostimulants and Soybean Tolerance to High-Temperature Stress
| Crop | Stress Level | Bio Stimulants | Application Methods/ Concentrations | Growth /Yield Effect | Mechanisms | Research Gaps | References |
|---|---|---|---|---|---|---|---|
| Rice | 30–41 °C | Abscisic acid | Foliar (0, 1, 10 and 100 μmol L−1) | Enhanced sugar metabolism, heat shock protein expression, antioxidant activity, and energy balance | Limited to controlled; no field-sale validation | [101] | |
| Rice | 24–40 °C | Cytokinin (CK), Brassinosteroids (BR) | Foliar (CK 1 × 10−5 M, BR 5 × 10−5 M) | Enhanced chlorophyll content, gas exchange, and photosynthetic efficiency; reduced oxidative damage and canopy temperature | Early growth stage focus; yield-related data lacking | [105] | |
| Rice | 40.8 °C | Brassinosteroid (BR)-, Boron (B), Calcium chloride (CaCl2), Salicylic acid (SA), Glycine betaine (GB), Pink-pigmented facultative methylotrophs (PPFM), 1-methyl cyclopropane (1-MCP), (GA3) | Foliar (BR- 5 ppm, B-100 ppm, CaCl2 −0.6%, SA-50 ppm, GB-20 ppm, PPFM-1%, (1-MCP)-50 ppm, GA3-50 ppm) | Pollen viability, spikelet fertility, and yield | Improved physiological traits such as CMSI, photosynthesis, stomatal conductance, and chlorophyll stability | Multi-treatment approach; difficult to isolate effect of individual biostimulants | [106] |
| Rice | 42 °C | Spermidine, Indole-3-acetic acid, Brassinolide, and boron | Seed priming/foliar (Spermidine- 1 mM and 2 mM), (Indole-3-acetic acid 10−3 M, 10−5 M), (Brassinolide 1 mg L−1, 2 mg L−1) and (Boron 50 mg L−1, 100 mg L−1) | Seedling vigor and yield | Enhanced chlorophyll, proline and reduced oxidative damage markers | Needs field trials and reproductive stage validation | [109] |
| Rice | 40.6 °C | Melatonin (MT) | Foliar (200 µM) | Improved chlorophyll content and photosynthesis, especially in thermo-sensitive rice, likely by enhancing antioxidant activity | Only tested in thermosensitive cultivar; broader genotypic validation required | [113] | |
| Rice | 38 °C for 1 day and 26 °C in the light incubator | Melatonin (MT) | Seed-Soaking (20, 100, 500 µM MT) | Germination, shoot and root growth | Increased antioxidant enzyme activity and reduced oxidative damage | Short duration stress; field application unknown | [114] |
| Rice | 38 °C/28 °C days/night | Melatonin (MT) | Foliar (250 mL of 200 µmol L−1 MT) | Yield and grain quality | Increased photosynthetic performance | Yield improvements shown; trial detail missing | [115] |
| Rice | 45 ± 2 °C | Salicylic acid (SA) | Foliar (100 mg L−1) | Growth, fresh and dry biomass | Enhanced organic and inorganic solute concentrations | Lack of detail molecular analysis; limited yield validation missing | [119] |
| Rice | 40 °C | Salicylic acid (SA) | Foliar (0.01, 0.1, 1.0, 10, and 50 mM) | Enhanced pollen viability under heat stress by reducing ROS and tapetum PCD, with H2O2 as key mediator | Mostly physiological study; field and yield validation missing | [120] | |
| Soybean | 24–42 °C | Melatonin (MT) | Root-treated (100 µM MT) | Reduced oxidative stress, enhanced antioxidants, balanced stress hormones, and promoted protective metabolites and gene expression | Needs soil/field validation | [125] | |
| Soybean | 150 mM NaCl + 35 °C | Fulvic acid | Foliar (FA, 2.0 mg L−1) | Increased RWC and activity of SOD, APX and GST. Reduced oxidative damage, H2O2 and MDA content | Lacks growth/yield outcomes | [132] | |
| Soybean | 35 °C | Biostimulant based on lignin derivatives, plant- derived amino acids, and molybdenum | Seed treatment (20 mL biostimulants) | Germination percentage and seedling vigor | Reduced oxidative stress level | Tested only germination stage; lacks growth/yield outcomes | [134] |
| Soybean | 40 °C | Ascophyllum nodosum (L.) seaweed | Foliar (0.25, 0.50, 0.75 and 1 L ha−1) | Yield, reduced leaf temperature | Enhanced antioxidant activity, proline levels, and photosynthetic through improved CO2 assimilation, stomatal conductance, transpiration rate, and carboxylation efficiency | Positive yield response shown, but long-term field consistency unclear | [137] |
4. Biostimulants Enhance Growth, Yield, and Stress Tolerance in Rice and Soybean Under Low-Temperature Stress
4.1. Biostimulants and Rice Tolerance to Low-Temperature Stress
4.2. Biostimulants and Soybean Tolerance to Low-Temperature Stress
| Crop | Stress Level | Bio Stimulants | Application Methods/ Concentrations | Growth /Yield Effect | Mechanisms | Research Gaps | References |
|---|---|---|---|---|---|---|---|
| Rice | 15 °C | Carrot extract | Seed soaking (0, 25, 50, and 100%) | Germination speed and final germination percentage and the growth | Mechanistic basis not explored; only seedling stage tested | [156] | |
| Rice | 10 °C (16 h light/8 h dark) | ZnO NPs | Foliar (25, 50, and 100 mg/L ZnO NPs with 50 mg L−1 TX-10) | Plant growth | Reduced oxidative stress markers (H2O2, MDA, and proline) and enhanced antioxidant enzyme activity (SOD, CAT, and POD) | Lacks yield data | [160] |
| Rice | 12 °C and 20 °C | Zinc (Zn) | Hydroponic (root application) (0.08, 0.15 and 0.31 µM) | Germination and growth | Improved nitrogen metabolism by enhancing Zn and N accumulation and maintaining a higher CTK/IAA ratio | Hydroponic only; field application lacking | [164] |
| Rice | 10 °C | Biochar fast pyrolysis of rice husks, Abscisic acid (ABA) | Foliar (1, 3, 5, 7 and 10% Biochar, 0, 10, 20, and 30 mg of ABA) | Increased ABA and carotenoid levels, gene expression | Interaction effects unclear; long-term soil effects of biochar missing | [167] | |
| Rice | (16 ± 1) °C | Brassinolide (BR) | Foilar (2 mg L−1 BR) | Enhanced antioxidant enzyme activities, nutrient content, chlorophyll and reduced MDA levels | Yield effects not evaluated; short-term response only | [169] | |
| Rice | 15–24 °C | Melatonin (MT) | Seed treatments (150 µmol L−1 MT) | Seed germination | Enhanced GA biosynthesis, reduced ABA and H2O2, and activated OsCAT2 through OsABI5 regulation | Gene-specific results; not validated across cultivars | [170] |
| Rice | 12 °C | Melatonin (MT) | Soaking seed, immersing roots, and spraying (0, 20, or 100 µM MT) | Reduced ROS, MDA, and cell death, improved photosynthesis, and enhanced antioxidant defenses | Mostly physiological data; yield effects not assessed | [172] | |
| Soybean | 4 °C/3 days | Melatonin (MT) | Foliar (100 mmol L−1 MT) | Enhanced antioxidant and hormones levels and activated B3 stress-response genes | Agronomic feasibility unclear | [180] | |
| Soybean | 12 °C | Melatonin (MT) | Foliar (1, 5, 10 and 50 µmol L−1 MT) | Reduced oxidative damage and ROS accumulation and enhanced mineral uptake and antioxidant gene expression | Field trials absent | [182] | |
| Soybean | 6 °C | Exogenous α-oxoglutarate | Foliar (0, 2.5, 5.0 and 7.5 mmol L−1) | Improved soybean cold tolerance by improving key enzymes, proline, and photosynthesis and lowering ammonium | Early-stage study; yield response missing | [191] | |
| Soybean | 8–10 °C | Silicon (Si) | Soil drench (1.0 mM Si) | Plant growth | Reduced oxidative stress, regulated stress genes, and enhanced beneficial microbes | Pot experiments only; multi-season validation needed | [197] |
5. Biostimulants Enhance Growth, Yield, and Stress Tolerance in Rice and Soybean Under Drought Stress
5.1. Biostimulants and Rice Tolerance to Drought Stress
5.2. Biostimulants and Soybean Tolerance to Drought Stress
| Crop | Stress Level | Bio Stimulants | Application Methods/ Concentration | Growth /Yield Effect | Mechanisms | Research Gaps | References |
|---|---|---|---|---|---|---|---|
| Rice | 7 days water withholding | Melatonin (MT) | Foliar (MT-50, 100, 200, and 300 μM) | Growth | Increased RWC, chlorophyll content, antioxidant enzyme activities, and reduced electrolyte leakage, MDA, and H2O2 | Controlled pot study only; field trials absent | [222] |
| Rice | PEG 6000 | Melatonin (MT) | Seed soaking (MT-20, 100, and 500 μM) | Germination, seedling growth, agronomic traits | Enhanced antioxidant enzyme activity, soluble protein content and reduced MDA | Osmotic simulation, not real drought; lacks yield data | [224] |
| Rice | 6-day irrigation withdrawal | Methyl Jasmonate (MJ), salicylic acid (SA), paclobutrazol (PBZ) | Seed-priming (100 μM) | Improved antioxidant activities, increased phenolic and abscisic acid content, modulated NADPH oxidase activity | Lack of field validation or yield-related assessments | [227] | |
| Rice | Severe/moderate drought | Salicylic acid (SA) | Foliar (250, 500, 750 and 1000 µMm2) | Growth and yield components | Limited mechanistic data | [232] | |
| Rice | 100%, 80%, and 60% field capacity | Salicylic acid (SA) | Foliar (SA-100 mg L−1) | Yield | Increased antioxidant capacity and reduced oxidative stress | Lack of multi-location; only two genotypes tested | [233] |
| Rice | 55–60% water holding capacity | Bacillus endophyticus PB3, Bacillus altitudinis PB46, and Bacillus megaterium PB50 | Foliar (15–25 mL of inoculant per plant) | Yield parameters | Improved RWC, key biochemical compounds and stress-responsive gene expression | Pot-scale; needs large-scale agronomic testing | [234] |
| Rice | 5, 10, and 15 days of irrigation interval | Potassium (K) | Soil basal (80,120, 160 kg K2O ha−1) | Yield and harvest index | Increased photosystem II efficiency | Limited mechanistic insight | [241] |
| Rice | 50 kPa | Selenium | Soil (Se-0.5, 1.0 and 2.0 mg kg−1) | Plant height | Enhanced SOD and Reduced H2O2 | Growth only; no yield evaluation | [247] |
| Rice | 29.3–2.8% w/w of soil moisture | Nitrogen | Basal dressing (25, 75, 150 kg N/ha for 2009 and 60, 120, 180 kg N ha−1) | Root plasticity and dry matter production | Limited to short-term season; evaluated only a few genotypes | [249] | |
| Rice | 35%, 70% water holding capacity | ZnO NPs | 24 h seed priming (5, 10, 15, 25, and 50 ppm) | Growth parameters | Increased antioxidant enzyme activities and reduced oxidative stress | Field applicability unknown | [203] |
| Rice | 50, 75, and 100% field capacity | Moringa oleifera | Foliar/ (MLE 3% w/v) | Growth, yield, and grain quality | Increased photosynthesis, pigment content, and antioxidant enzyme activities | Limited to pot; needs field and multi-season trials | [255] |
| Soybean | 30–35% field capacity | Melatonin (MT) | Foliar (MT-50 or 100 μM) | Growth | Improved photosynthesis, hormone balance, and antioxidant activity and reduced oxidative damage | Short-term study; no yield data | [259] |
| Soybean | 40 and 80% field capacity | Methyl jasmonate (MJ) | Foliar (20 µM MJ) | Growth | Enhanced growth, photosynthetic pigments, and biochemical constituents | Growth only; yield untested | [263] |
| Soybean | 35–75% field capacity | Methyl jasmonate (MJ) | Foliar/ (0.5 µM MJ) | Grain yield | Increased antioxidants enzymatic, RWC and reduced lipid peroxidation | Pot-based; Limited field applicability | [206] |
| Soybean | 50%, 75% relative water content | Salicylic acid (SA) | Seed priming (0.5 mM SA) | Yield parameters | Improved energy production, carbon and nitrogen remobilization, redox homeostasis, and antioxidative defense mechanisms | Early-stage; field confirmation missing | [266] |
| Soybean | 45, 65, and 85% field capacity | Salicylic acid (SA) | Foliar (SA, 0.4 and 0.8 mM) | Physiological traits and yield components | Enhanced antioxidant enzyme activity and reduced oxidative damage | Pot-level; needs field-scale testing | [271] |
| Soybean | 30, 50, 70 and 100% field capacity | Exo-GB (glycine betaine) | Foliar (0, 2.5, 5 and 7.5 kg ha−1) | Yield components such as branch number, seed number per plant, and 1000-seed weight | Pod-level, single cultivar; lack of field variability | [272] | |
| Soybean | 40 and 80% field capacity | Red seaweed extract (Gracilaria tenuistipitata var. liui) | Foliar (0.0%, 5.0%, and 10.0% v/v) | Growth and yield | Mechanistic pathways unstudied | [280] | |
| Soybean | Non-irrigated, half-irrigated, and fully irrigated | Nitrogen (N) | Soil (35, and 105 kg ha−1 N) | Physiological traits and yield | No molecular data | [283] | |
| Soybean | PEG-6000 | Nano zinc oxide | Added to Petri dish (0.5, and 1 g L−1) | Germination percentage and rate | Mechanistic pathways unstudied; lacks growth/yield validation | [287] | |
| Soybean | 40, 60 and 80% field capacity | Biochar (pyrolysed at ~400 °C for 5 h) | Soil amendment (0, 25, 50 and 100 t ha−1) | Crop growth rate, total biomass production, and seed yield | Increased water uses efficiency, soil available potassium, and K uptake | Mechanistic pathways unstudied | [292] |
6. Biostimulants Enhance Growth, Yield, and Stress Tolerance in Rice and Soybean Under Salt Stress
6.1. Biostimulants and Rice Tolerance to Salt Stress
6.2. Biostimulants and Soybean Tolerance to Salt Stress
| Crop | Stress Level | Bio Stimulants | Application Methods/ Concentrations | Growth /Yield Effect | Mechanisms | Research Gaps | References |
|---|---|---|---|---|---|---|---|
| Rice | 50–100 mM NaCl | Melatonin (MT) | Foliar (MT-25, 50, 100, 200, 300, and 400 μM) | Growth | Enhanced antioxidant enzyme activity, nutrient accumulation, and ion homeostasis and reduced cellular damage | Pot trials only; yield not tested | [316] |
| Rice | 150 mM NaCl | Melatonin (MT) | Foliar (MT-200 μM) | Dry weight | Reduced ROS accumulation, improving membrane stability, and modulating antioxidant enzyme activity | Pot-based study; no yield traits | [317] |
| Rice | 100 mM NaCl | Melatonin (MT) | Root treatment/ (MT-10, 20, 50, or 100 μM) | Reduced K+ efflux and ROS-induced damage and enhanced K+ retention | Controlled hydroponic study; needs field validation | [318] | |
| Rice | (0, 100, 200, 300 and 400 mM NaCl) | Salicylic acid (SA) | Seed treatment (SA-1.0 mmol L−1) | Germination, and growth | Increased antioxidant enzyme, Na+ and Cl− accumulation | Short-term study; needs field validation | [321] |
| Rice | NaCl 100 mM | Salicylic acid (SA) | Foliar (SA-0.5 mM) | Increased photosynthetic, protein content and gene expression related to antioxidant defense and reduced ROS accumulation and cellular damage | Short-term study; field-level yield and stress tolerance not assessed | [322] | |
| Rice | 0, 40, 120 mM NaCl | Salicylic acid (SA) | Foliar (SA-0.5, 1 and 2 mM) | Growth | Improved RWC, ion balance, and antioxidants and reduced ROS and membrane damage | Needs field validation | [324] |
| Rice | 10 dS/m | Fulvic acid | Seed treatment (0.125, 0.25, 0.5, and 1.0 mL L−1) | Growth parameters | Increased phenolic compounds | Mechanisms limited; yield not reported | [326] |
| Rice | 120 mmol/L NaCl | Glycine betaine (GB) and Iron (Fe) | Applied nutrient solution/Foliar (15 mmol L−1 GB and 10% Fe stock solution) | Enhanced RWC and antioxidative enzyme activities and reduced lipid peroxidation | Short-term hydroponic study; needs field validation | [334] | |
| Rice | 150 mM NaCl | Paclobutrazol | Foliar (15 mg L−1) | Growth | Improved pigment content and antioxidant activity | Yield traits untested | [335] |
| Rice | 0, 25, 50, and 100 mM NaCl | Silicon | Applied nutrient solution (Si-2 mM) | Increased polyamine (PA) levels, reduced polyamine degradation, and modulated GABA metabolism | Hydroponics only; field application missing | [344] | |
| Rice | 60.00 mmol·L−1 NaCl | Nano-silicon | Foliar (2.00 mmol L−1) | Root growth | Enhanced photosynthesis, antioxidant defense, beneficial ion uptake, and hormone balance | Field application missing | [309] |
| Rice | 70 mM NaCl | Zinc | Hydroponic root zone application (An-15 mg kg−1) | Growth | Enhanced photosynthesis, antioxidant activity, and ion balance | Hydroponics only; no yield data | [348] |
| Rice | 200 mM NaCl | Seaweed Ascophyllum nodosum | Foliar (2 mL L−1, 0.2% solution) | Shoot and root biomass | Increased pigment content, photosynthesis, and antioxidant defense | Lon-term effects unknown | [349] |
| Soybean | NaCl (3 or 6 dS/m) | Salicylic acid (SA) | Foliar (SA-0.4 or 0.8 mM) | Growth, yield, and biochemical traits | Improved phenol, proline, oil, and protein content and increased unsaturated fatty acid proportion in seed oil | Field trials missing | [355] |
| Soybean | 50–100 mM NaCl | Salicylic acid | Foliar (SA-100 and 200 ppm) | Increased chlorophyll, sugars, starch, proline, and antioxidative enzymes | Physiological only; yield not measured | [356] | |
| Soybean | NaCl (0 and 100 mM | Salicylic acid (SA) and Sodium nitroprusside (NO donor) | Pretreatment root uptake (SA, NO-100 μM) | Reduced Na+ uptake, improved K+ and Ca2+ levels, enhanced antioxidant enzyme activities (PPO, PAL) | Lon-term effects on growth and yield are unknown | [360] | |
| Soybean | NaCl (0 and 100 mM) | Selenium (Se) and salicylic acid (SA) | Foliar (Se-0, 25 and 50 mg L−1, SA-0.5 mM) | Enhanced antioxidant enzyme activities, improved nutrient uptake, and increased protective metabolites | Limited to early growth | [362] | |
| Soybean | 0, 4, 7, and 10 dS/m NaCl | Salicylic acid (SA) and Jasmonic acid (JA) | Foliar (SA-1 mM and JA-0.5 mM) | Improved seed protein yield and essential amino acid content and reduced protein yield but increased levels of specific amino acids | Lon-term effects on growth, yield and phytochemical content are unknown | [365] | |
| Soybean | 5.0 dS/m | Seaweed extract and fulvic acids | Soil (2.5 g pot−1) | Growth parameters | Reduced electrolyte leakage and enhanced chlorophyll content | Pot study; no yield data | [372] |
| Soybean | 150 mM NaCl/35 °C for 2 h for 2 days | Fulvic acid | Foliar (2.0 mg L−1) | Improved water status, antioxidant activity, and regulated stress-responsive gene expression | Field-level validation is needed | [132] | |
| Soybean | 100, 250, and 500 mM NaCl | Bradyrhizobium japonicum, Rhizobium sp. And Hydrogenophaga sp., Amphicarpaea bracteat | Seeds soaking (100 µL bacterial suspension) | Growth and yield | Limited understanding of the full microbial diversity | [376] | |
| Soybean | 6–12 dS m−1 | Potassium chloride and potassium sulfate | Foliar (2.5% solution of potassium sulfate or potassium chloride) | Potassium sulfate improved antioxidant activity and pigment levels more than that of potassium chloride | Limited to greenhouse conditions; no yield data | [378] | |
| Soybean | 8–16 dS m−1 | Ethanol | Foliar (20 mM) | Growth | Enhanced photosynthesis, antioxidant activity, osmotic adjustment, and nutrient uptake and reduced oxidative damage and Na+ accumulation | Pod conditions only; lacks field variability | [382] |
| Soybean | 60 mM | Melatonin (MT) | Seed soaking (MT-100 μM) | Growth | Enhanced antioxidant defense, reduced oxidative damage, and boosted isoflavone biosynthesis | Short-term study; no yield data | [391] |
| Soybean | 0.50, 3.00, and 5.00 dS m−1 | Melatonin (MT) | (MT-0.5, and 1 mM) | Improved chlorophyll b and water status | Short-term study; agronomic feasibility unclear | [393] |
7. Biostimulants Enhance Growth, Yield, and Stress Tolerance in Rice and Soybean Under Waterlogging Stress
7.1. Biostimulants and Rice Tolerance to Waterlogging Stresses
7.2. Biostimulants and Soybean Tolerance to Waterlogging Stress
| Crop | Stress Level | Bio Stimulants | Application Methods/ Concentrations | Growth /Yields Effect | Mechanisms | Research Gaps | References |
|---|---|---|---|---|---|---|---|
| Rice | Irrigation every 3, 6, 9, 12 days | Biostimulant Crop plus products, cytokinin (CK), and abscisic acid | Foliar (0.5, 1.0, 1.5 mL L−1, CK and AA 15, 20, 25 ppm) | Growth and grain quality | Long-term effects unknow; no mechanistic molecular validation | [421] | |
| Rice | Flooding | Silica (Si), phosphorus (P), and nitrogen (N) | Foliar (Urea-0.49 g), Soil basal (single superphosphate-1.14 g), muriate of potash-0.31 g), and (calcium silicate-3.35 g) | Improved photosynthesis and recovery by enhancing chlorophyll and sugars and reducing elongation and leaf senescence | Focused on physiology; yield response missing | [414] | |
| Rice | Cold waterlogged | Bamboo biochar (BB), rice straw biochar (RB), and rice straw (RS) | Soil basal (BB, RB, and RS, 4.5 t C ha−1) | Grain yield | Limited environmental scope; long-term field data absent, unclear mechanisms | [427] | |
| Rice | Cold waterlogged | Straw and biochar | Straw amendment 6 t ha−1, biochar amendment 2 and 40 t ha−1 | Enhanced soil nitrogen and carbon, improved carbon sequestration, and reduced CH4, GWP, and GHG | Short-term field trial; limited variety and biochar evaluation | [429] | |
| Soybean | Flooding | Silver nanoparticles (AgNPs) | Complete submersion of root zone (AgNPs-0.2, 2, and 20 ppm) | Seedling growth | Reduced fermentation-related protein levels in soybean roots, suggesting a shift toward less toxic metabolism | Short-term study; Limited scope | [432] |
| Soybean | Flooding | Aluminum Oxide Nanoparticles (Al2O3 NPs) | Hydroponic exposure (50 ppm) | Root growth | Modulated glycolysis, antioxidant pathways, and ribosomal protein levels | Hydroponics only; field translation unclear | [436] |
| Soybean | Water level maintained at 10–15 cm above the soil surface for 10 days | Ethephon (ETP; donor source of ethylene) | Foliar (50 μM, 100 μM, and 200 μM ETP) | Root growth | Improved photosynthesis, increased endogenous gibberellin and amino acid levels | Short-term study; yield impact not measured | [441] |
| Soybean | Flooding | Melatonin (MT) | Applied via flooding water (MT,10, 50, or 100 μM) | Regulated protein degradation, RNA function, and cell wall lignification | Short-term study; needs yield-level validation | [445] | |
| Soybean | 0, 3, 6, and 9 days waterlogging | Kinetin (KN) and Salicylic acid (SA) | Foliar (KN-0.1 mM and SA-0.5 mM SA) | Reduced oxidative damage and enhanced antioxidant defense and glyoxalase enzyme activities | Pot study only; no field-scale trials | [454] | |
| Soybean | Flooding | Jasmonic acid (JA), salicylic acid (SA) | Applied via flooding water (JA-50, 100, 200, and 300 μM), (SA-50, 100, 200 μM) | Growth | Enhanced oxidative stress, with MDHAR crucial for detoxification | Controlled environmental only; yield outcomes absent | [457] |
8. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Bashir, K.; Matsui, A.; Rasheed, S.; Seki, M. Recent advances in the characterization of plant transcriptomes in response to drought, salinity, heat, and cold stress. F1000Research 2019, 8, 658. [Google Scholar] [CrossRef]
- Di Vittori, L.; Mazzoni, L.; Battino, M.; Mezzetti, B. Pre-harvest factors influencing the quality of berries. Sci. Hortic. 2018, 233, 310–322. [Google Scholar] [CrossRef]
- Atkinson, N.J.; Urwin, P.E. The interaction of plant biotic and abiotic stresses: From genes to the field. J. Exp. Bot. 2012, 63, 3523–3543. [Google Scholar] [CrossRef]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef]
- Skirycz, A.; De Bodt, S.; Obata, T.; De Clercq, I.; Claeys, H.; De Rycke, R.; Andriankaja, M.; Van Aken, O.; Van Breusegem, F.; Fernie, A.R.; et al. Developmental stage specificity and the role of mitochondrial metabolism in the response of Arabidopsis leaves to prolonged mild osmotic stress. Plant Physiol. 2010, 152, 226–244. [Google Scholar] [CrossRef]
- Cramer, G.R.; Urano, K.; Delrot, S.; Pezzotti, M.; Shinozaki, K. Effects of abiotic stress on plants: A systems biology perspective. BMC Plant Biol. 2011, 11, 163. [Google Scholar] [CrossRef] [PubMed]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef]
- Cooke, J.; Leishman, M.R. Consistent alleviation of abiotic stress with silicon addition: A meta-analysis. Funct. Ecol. 2016, 30, 1340–1357. [Google Scholar] [CrossRef]
- Vogel, E.; Donat, M.G.; Alexander, L.V.; Meinshausen, M.; Ray, D.K.; Karoly, D.; Meinshausen, N.; Frieler, K. The effects of climate extremes on global agricultural yields. Environ. Res. Lett. 2019, 14, 054010. [Google Scholar] [CrossRef]
- Mammadov, J.; Buyyarapu, R.; Guttikonda, S.K.; Parliament, K.; Abdurakhmonov, I.Y.; Kumpatla, S.P. Wild relatives of maize, rice, cotton, and soybean: Treasure troves for tolerance to biotic and abiotic stresses. Front. Plant Sci. 2018, 9, 886. [Google Scholar] [CrossRef]
- Ramegowda, V.; Senthil-Kumar, M. The interactive effects of simultaneous biotic and abiotic stresses on plants: Mechanistic understanding from drought and pathogen combination. J. Plant Physiol. 2015, 176, 47–54. [Google Scholar] [CrossRef]
- Maghboli Balasjin, N.; Maki, J.S.; Schläppi, M.R.; Marshall, C.W. Plant growth-promoting activity of bacteria isolated from Asian rice (Oryza sativa L.) depends on rice genotype. Microbiol. Spectr. 2022, 10, e02787-12. [Google Scholar] [CrossRef]
- Normile, D. Reinventing rice to feed the world. Science 2008, 321, 330–333. [Google Scholar] [CrossRef] [PubMed]
- Ray, D.K.; Gerber, J.S.; MacDonald, G.K.; West, P.C. Climate variation explains a third of global crop yield variability. Nat. Commun. 2015, 6, 5989. [Google Scholar] [CrossRef]
- Patra, B.C.; Anilkumar, C.; Chakraborti, M. Rice breeding in India: A journey from phenotype-based pure-line selection to genomics assisted breeding. Agric. Res. J. 2020, 57, 816–825. [Google Scholar] [CrossRef]
- Sun, J.; Mooney, H.; Wu, W.; Tang, H.; Tong, Y.; Xu, Z.; Huang, B.; Cheng, Y.; Yang, X.; Wei, D.; et al. Importing food damages domestic environment: Evidence from global soybean trade. Proc. Natl. Acad. Sci. USA 2018, 115, 5415–5419. [Google Scholar] [CrossRef]
- Staniak, M.; Szpunar-Krok, E.; Kocira, A. Responses of soybean to selected abiotic stresses—Photoperiod, temperature and water. Agriculture 2023, 13, 146. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef]
- Rao, N.K.S.; Laxman, R.H.; Shivashankara, K.S. Physiological and morphological responses of horticultural crops to abiotic stresses. In Abiotic Stress Physiology of Horticultural Crops; Rao, N.K.S., Shivashankara, K.S., Laxman, R.H., Eds.; Springer: New Delhi, India, 2016; pp. 3–7. ISBN 978-81-322-2723-6. [Google Scholar]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 12, 134–136. [Google Scholar] [CrossRef]
- Kusvuran, S.; Kiran, S.; Ellialtioglu, S.S. Antioxidant enzyme activities and abiotic stress tolerance relationship in vegetable crops. In Abiotic and Biotic Stress in Plants—Recent Advances and Future Perspectives; InTechOpen: London, UK, 2016. [Google Scholar]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef]
- Sharma, H.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar] [CrossRef]
- Wang, Y.; Frei, M. Stressed food—The impact of abiotic environmental stresses on crop quality. Agric. Ecosyst. Environ. 2011, 141, 271–286. [Google Scholar] [CrossRef]
- Oh, M.-M.; Carey, E.E.; Rajashekar, C.B. Regulated water deficits improve phytochemical concentration in lettuce. J. Am. Soc. Hortic. Sci. 2019, 135, 223–229. [Google Scholar] [CrossRef]
- Ripoll, J.; Urban, L.; Staudt, M.; Lopez-Lauri, F.; Bidel, L.P.R.; Bertin, N. Water shortage and quality of fleshy fruits—Making the most of the unavoidable. J. Exp. Bot. 2014, 65, 4097–4117. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y. Biostimulants in horticulture. Sci. Hortic. 2015, 196, 1–134. [Google Scholar] [CrossRef]
- Rouphael, Y.; Spichal, L.; Panzarova, K.; Casa, R.; Colla, G. High-throughput plant phenotyping for developing novel biostimulants: From lab to field or from field to lab? Front. Plant Sci. 2018, 9, 1197. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Synergistic biostimulatory action: Designing the next generation of plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P. Plant biostimulants: A new paradigm for the sustainable intensification of crops. In Biostimulants for Sustainable Crop Production; Rouphael, Y., du Jardin, P., Brown, P., De Pascale, S., Colla, G., Eds.; Burleigh Dodds Science Publishing Limited: Cambridge, UK, 2020; pp. 3–29. [Google Scholar]
- De Pascale, S.; Rouphael, Y.; Colla, G. Plant biostimulants: Innovative tool for enhancing plant nutrition in organic farming. Eur. J. Hortic. Sci. 2018, 82, 277–285. [Google Scholar] [CrossRef]
- Basile, B.; Rouphael, Y.; Colla, G.; Soppelsa, S.; Andreotti, C. Appraisal of emerging crop management opportunities in fruit trees, grapevines and berry crops facilitated by the application of biostimulants. Sci. Hortic. 2020, 267, 109330. [Google Scholar] [CrossRef]
- Bradshaw, T.L.; Berkett, L.P.; Griffith, M.C.; Darby, H.M.; Moran, R.E.; Garcia, M.E. Assessment of kelp extract biostimulants on tree growth, yield, and fruit quality in a certified organic apple orchard. In II International Organic Fruit Symposium 1001; International Society for Horticultural Science: Brabant, Belgium, 2013; pp. 191–198. [Google Scholar]
- Rouphael, Y.; Lucini, L.; Miras-Moreno, B.; Colla, G.; Bonini, P.; Cardarelli, M. Metabolomic responses of maize shoots and roots elicited by combinatorial seed treatments with microbial and non-microbial biostimulants. Front. Microbiol. 2020, 11, 664. [Google Scholar] [CrossRef]
- Rouphael, Y.; Kyriacou, M.C. Enhancing quality of fresh vegetables through salinity eustress and biofortification applications facilitated by soilless cultivation. Front. Plant Sci. 2018, 9, 1254. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G. Toward a sustainable agriculture through plant biostimulants: From experimental data to practical applications. Agronomy 2020, 10, 1461. [Google Scholar] [CrossRef]
- Jägermeyr, J. Agriculture’s historic twin-challenge toward sustainable water use and food supply for all. Front. Sustain. Food Syst. 2020, 4, 35. [Google Scholar] [CrossRef]
- du Jardin, P. Plant Biostimulants: Definition, Concept, Main Categories and Regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- European Biostimulant Industry Council. (n.d.). What Are Biostimulants? Available online: https://biostimulants.eu/faqs/what-are-biostimulants (accessed on 19 September 2025).
- Povero, G.; Mejia, J.F.; Di Tommaso, D.; Piaggesi, A.; Warrior, P. A systematic approach to discover and characterize natural plant biostimulants. Front. Plant Sci. 2016, 7, 435. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Moosa, A.; Ali, H.M.; Bermejo, N.F.; Munné-Bosch, S. Biostimulants: A sufficiently effective tool for sustainable agriculture in the era of climate change? Plant Physiol. Biochem. 2024, 211, 108699. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Ciarmiello, L.F.; Woodrow, P.; Corrado, G.; Chiaiese, P.; Rouphael, Y. Enhancing sustainability by improving plant salt tolerance through macro- and micro-algal biostimulants. Biology 2020, 9, 253. [Google Scholar] [CrossRef]
- Hamza, B.; Suggars, A. Biostimulants: Myths and realities. Turfgrass Trends 2001, 10, 6–10. [Google Scholar]
- Bhattacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Chiaiese, P.; Corrado, G.; Colla, G.; Kyriacou, M.C.; Rouphael, Y. Renewable sources of plant biostimulation: Microalgae as a sustainable means to improve crop performance. Front. Plant Sci. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed]
- Mógor, Á.F.; Ördög, V.; Lima, G.P.P.; Molnár, Z.; Mógor, G. Biostimulant properties of cyanobacterial hydrolysate related to polyamines. J. Appl. Phycol. 2018, 30, 453–460. [Google Scholar] [CrossRef]
- Oancea, F.; Velea, S.; Mincea, C.; Ilie, L. Micro-algae based plant biostimulant and its effect on water stressed tomato plants. Rom. J. Plant Prot. 2013, 6, 104–117. [Google Scholar]
- García, A.C.; Santos, L.A.; Izquierdo, F.G.; Sperandio, M.V.L.; Castro, R.N.; Berbara, R.L.L. Vermicompost humic acids as an ecological pathway to protect rice plant against oxidative stress. Ecol. Eng. 2012, 47, 203–208. [Google Scholar] [CrossRef]
- Lamar, R.T. Possible role for electron shuttling capacity in elicitation of PB activity of humic substances on plant growth enhancement. In Chemistry and Biology of Plant Biostimulants; John Wiley & Sons: Hoboken, NJ, USA, 2020; pp. 97–121. [Google Scholar]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Fatima, N.; Jamal, A.; Huang, Z.; Liaquat, R.; Ahmad, B.; Haider, R.; Ali, M.I.; Shoukat, T.; Alothman, Z.A.; Ouladsmane, M.; et al. Extraction and chemical characterization of humic acid from nitric acid treated lignite and bituminous coal samples. Sustainability 2021, 13, 8969. [Google Scholar] [CrossRef]
- Sarlaki, E.; Sharif Paghaleh, A.; Kianmehr, M.H.; Asefpour, V.K. Extraction and purification of humic acids from lignite wastes using alkaline treatment and membrane ultrafiltration. J. Clean Prod. 2019, 235, 712–723. [Google Scholar] [CrossRef]
- Theocharis, A.; Bordiec, S.; Fernandez, O.; Paquis, S.; Dhondt-Cordelier, S.; Baillieul, F.; Clément, C.; Barka, E.A. Burkholderia phytofirmans PsJN primes Vitis vinifera L. and confers a better tolerance to low nonfreezing temperatures. Mol. Plant Microbe Interact. 2012, 25, 241–249. [Google Scholar] [CrossRef]
- Çimrin, K.M.; Türkmen, Ö.; Turan, M.; Tuncer, B. Phosphorus and humic acid application alleviate salinity stress of pepper seedling. Afr. J. Biotechnol. 2010, 9, 5845–5851. [Google Scholar]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Berbara, R.L.L.; García, A.C. Humic substances and plant defense metabolism. In Physiological Mechanisms and Adaptation Strategies in Plants Under Changing Environment; Ahmad, P., Wani, M.R., Eds.; Springer: New York, NY, USA, 2014; pp. 297–319. [Google Scholar]
- Tahir, M.M.; Khurshid, M.; Khan, M.Z.; Abbasi, M.K.; Kazmi, M.H. Lignite-derived humic acid effect on growth of wheat plants in different soils. Pedosphere 2011, 21, 124–131. [Google Scholar] [CrossRef]
- Ayuso, M.; Hernandez, T.; Garcia, C.; Pascual, J.A. Stimulation of barley growth and nutrient absorption by humic substances originating from various organic materials. Bioresour. Technol. 1996, 57, 251–257. [Google Scholar] [CrossRef]
- Celik, H.; Katkat, A.V.; Aşik, B.B.; Turan, M.A. Effect of foliar-applied humic acid to dry weight and mineral nutrient uptake of maize under calcareous soil conditions. Commun. Soil Sci. Plant Anal. 2010, 42, 29–38. [Google Scholar] [CrossRef]
- Jindo, K.; Soares, T.S.; Peres, L.E.P.; Azevedo, I.G.; Aguiar, N.O.; Mazzei, P.; Spaccini, R.; Piccolo, A.; Olivares, F.L.; Canellas, L.P. Phosphorus speciation and high-affinity transporters are influenced by humic substances. J. Plant Nutr. Soil Sci. 2016, 179, 206–214. [Google Scholar] [CrossRef]
- Zhu, K.; Zhou, H.; Qian, H. Antioxidant and free radical-scavenging activities of wheat germ protein hydrolysates (WGPH) prepared with alcalase. Process Biochem. 2006, 41, 1296–1302. [Google Scholar] [CrossRef]
- Colla, G.; Nardi, S.; Cardarelli, M.; Ertani, A.; Lucini, L.; Canaguier, R.; Rouphael, Y. Protein hydrolysates as biostimulants in horticulture. Sci. Hortic. 2015, 196, 28–38. [Google Scholar] [CrossRef]
- Nardi, S.; Pizzeghello, D.; Schiavon, M.; Ertani, A. Plant biostimulants: Physiological responses induced by protein hydrolyzed-based products and humic substances in plant metabolism. Sci. Agric. 2016, 73, 18–23. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 364, 45–158. [Google Scholar] [CrossRef]
- Petrozza, A.; Santaniello, A.; Summerer, S.; Di Tommaso, G.; Di Tommaso, D.; Paparelli, E.; Piaggesi, A.; Perata, P.; Cellini, F. Physiological responses to Megafol® treatments in tomato plants under drought stress: A phenomic and molecular approach. Sci. Hortic. 2014, 174, 185–192. [Google Scholar] [CrossRef]
- Rakkammal, K.; Maharajan, T.; Ceasar, S.A.; Ramesh, M. Biostimulants and their role in improving plant growth under drought and salinity. Cereal Res. Commun. 2023, 51, 61–74. [Google Scholar] [CrossRef]
- Botta, A. Enhancing plant tolerance to temperature stress with amino acids: An approach to their mode of action. Acta Hortic. 2012, 1009, 29–36. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant action of protein hydrolysates: Unraveling their effects on plant physiology and microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Trevisan, S.; Manoli, A.; Quaggiotti, S. A novel biostimulant, belonging to protein hydrolysates, mitigates abiotic stress effects on maize seedlings grown in hydroponics. Agronomy 2019, 9, 28. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Vasseur-Coronado, M.; du Boulois, H.D.; Pertot, I.; Puopolo, G. Selection of plant growth promoting rhizobacteria sharing suitable features to be commercially developed as biostimulant products. Microbiol. Res. 2021, 245, 126672. [Google Scholar] [CrossRef] [PubMed]
- Bhuyan, M.H.M.B.; Mohsin, S.M.; Mahmud, J.A.; Hasanuzzaman, M. Use of biostimulants for improving abiotic stress tolerance in Brassicaceae plants. In The Plant Family Brassicaceae; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020; pp. 497–531. [Google Scholar]
- Pilon-Smits, E.A.H.; Quinn, C.F.; Tapken, W.; Malagoli, M.; Schiavon, M. Physiological functions of beneficial elements. Curr. Opin. Plant Biol. 2009, 12, 267–274. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-De la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and nanomaterials as plant biostimulants. Int. J. Mol. Sci. 2019, 20, 162. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N. Nano-titanium dioxide (Nano-TiO2) mitigates NaCl stress by enhancing antioxidative enzymes and accumulation of compatible solutes in tomato (Lycopersicon esculentum Mill.). J. Plant Sci. 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Kiapour, H.; Moaveni, P.; Habibi, D.; Sani, B. Evaluation of the application of gibberellic acid and titanium dioxide nanoparticles under drought stress on some traits of basil (Ocimum basilicum L.). Int. J. Agron. Agric. Res. 2015, 6, 138–150. [Google Scholar]
- Qi, M.; Liu, Y.; Li, T. Nano-TiO2 improve the photosynthesis of tomato leaves under mild heat stress. Biol. Trace Elem. Res. 2013, 156, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Challinor, A.J.; Watson, J.; Lobell, D.B.; Howden, S.M.; Smith, D.R.; Chhetri, N. A meta-analysis of crop yield under climate change and adaptation. Nat. Clim. Change 2014, 4, 287–291. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W.; Adkins, S.; Saud, S.; et al. Crop production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Mahat, J.; Shrestha, J.; Madhav, K.C.; Paudel, K. Influence of high-temperature stress on rice growth and development: A review. Heliyon 2022, 8, e1265. [Google Scholar] [CrossRef]
- Arora, N.K. Impact of climate change on agriculture production and its sustainable solutions. Environ. Sustain. 2019, 2, 95–96. [Google Scholar] [CrossRef]
- Peraudeau, S.; Lafarge, T.; Roques, S.; Quiñones, C.O.; Clement-Vidal, A.; Ouwerkerk, P.B.F.; Van Rie, J.; Fabre, D.; Jagadish, K.S.V.; Dingkuhn, M. Effect of carbohydrates and night temperature on night respiration in rice. J. Exp. Bot. 2015, 66, 3931–3944. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Anwar, S.; Ashraf, M.Y.; Khaliq, B.; Sun, M.; Hussain, S.; Gao, Z.Q.; Noor, H.; Alam, S. Mechanisms and adaptation strategies to improve heat tolerance in rice: A review. Plants 2019, 8, 508. [Google Scholar] [CrossRef]
- Kotak, S.; Larkindale, J.; Lee, U.; von Koskull-Döring, P.; Vierling, E.; Scharf, K.D. Complexity of the heat stress response in plants. Curr. Opin. Plant Biol. 2007, 10, 310–316. [Google Scholar] [CrossRef]
- Khan, A.H.; Min, L.; Ma, Y.; Zeeshan, M.; Jin, S.; Zhang, X. High-temperature stress in crops: Male sterility, yield loss and potential remedy approaches. Plant Biotechnol. J. 2022, 21, 680–697. [Google Scholar] [CrossRef]
- Liu, B.; Asseng, S.; Müller, C.; Ewert, F.; Elliott, J.; Lobell, D.B.; Martre, P.; Ruane, A.C.; Wallach, D.; Jones, J.W.; et al. Similar estimates of temperature impacts on global wheat yield by three independent methods. Nat. Clim. Change 2016, 6, 1130–1136. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xia, X.J.; Li, X.; Shi, K.; Yu, J.Q.; Zhou, Y.H. Role of brassinosteroid in plant adaptation to abiotic stresses and its interplay with other hormones. Curr. Protein Pept. Sci. 2015, 16, 462–473. [Google Scholar] [CrossRef]
- Bita, C.; Gerats, T. Plant tolerance to high temperature in a changing environment: Scientific fundamentals and production of heat stress-tolerant crops. Front. Plant Sci. 2013, 4, 273. [Google Scholar] [CrossRef]
- Karwa, S.; Bahuguna, R.N.; Chaturvedi, A.K.; Maurya, S.; Arya, S.S.; Chinnusamy, V.; Pal, M. Phenotyping and characterization of heat stress tolerance at reproductive stage in rice (Oryza sativa L.). Acta Physiol. Plant. 2020, 42, 29. [Google Scholar] [CrossRef]
- Zhang, C.X.; Fu, G.F.; Yang, X.Q.; Yang, Y.J.; Zhao, X.; Chen, T.T.; Zhang, X.F.; Jin, Q.Y.; Tao, L.X. Heat stress effects are stronger on spikelets than on flag leaves in rice due to differences in dissipation capacity. J. Agron. Crop Sci. 2016, 202, 394–408. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Roychowdhury, R.; Fujita, M. Physiological, biochemical, and molecular mechanisms of heat stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 9643–9684. [Google Scholar] [CrossRef]
- Locato, V.; de Gara, L. Programmed cell death in plants: An overview. Methods Mol. Biol. 2018, 1743, 1–8. [Google Scholar] [PubMed]
- Zhao, Q.; Zhou, L.; Liu, J.; Cao, Z.; Du, X.; Huang, F.; Pan, G.; Cheng, F. Involvement of CAT in the detoxification of heat-induced ROS burst in rice anther and its relation to pollen fertility. Plant Cell Rep. 2018, 37, 741–757. [Google Scholar] [CrossRef]
- Swift, R.; Denton, M.D.; Melino, V.J. Plant probiotics for nutrient acquisition by agriculturally important grasses: A comprehensive review of the science and the application. Annu. Plant Rev. Online 2018, 2, 537–584. [Google Scholar]
- Akhtar, G.; Faried, H.N.; Razzaq, K.; Ullah, S.; Wattoo, F.M.; Shehzad, M.A.; Sajjad, Y.; Ahsan, M.; Javed, T.; Dessoky, E.S.; et al. Chitosan-induced physiological and biochemical regulations confer drought tolerance in pot marigold (Calendula officinalis L.). Agronomy 2022, 12, 474. [Google Scholar] [CrossRef]
- Quintero-Calderón, E.H.; Sánchez-Reinoso, A.D.; Chávez-Arias, C.C.; Garces-Varon, G.; Restrepo-Díaz, H. Rice seedlings showed a higher heat tolerance through the foliar application of biostimulants. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12120. [Google Scholar] [CrossRef]
- Islam, M.R.; Feng, B.; Chen, T.; Fu, W.; Zhang, C.; Tao, L.; Fu, G. Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol. Plant. 2019, 165, 644–663. [Google Scholar]
- Tang, R.S.; Zheng, J.C.; Jin, Z.Q.; Zhang, D.D.; Huang, Y.H.; Chen, L.G. Possible correlation between high temperature-induced floret sterility and endogenous levels of IAA, GAs and ABA in rice (Oryza sativa L.). Plant Growth Regul. 2008, 54, 37–43. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A.; Inupakutika, M.A.; Mittler, R. ABA is required for the accumulation of APX1 and MBF1c during a combination of water deficit and heat stress. J. Exp. Bot. 2016, 67, 5381–5390. [Google Scholar] [CrossRef] [PubMed]
- Kosakivska, I.V.; Voytenko, L.V.; Vasyuk, V.A.; Shcherbatiuk, M.M. Effect of pre-sowing priming of seeds with exogenous abscisic acid on endogenous hormonal balance of spelt wheat under heat stress. Zemdirb.-Agric. 2022, 109, 21–26. [Google Scholar] [CrossRef]
- Pantoja-Benavides, A.D.; Garces-Varon, G.; Restrepo-Díaz, H. Foliar cytokinins or brassinosteroids applications influence the rice plant acclimatization to combined heat stress. Front. Plant Sci. 2022, 13, 983276. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, M.P.; Beena, R.; Mohan, V.; Viji, M.M.; Manju, R.V.; Stephen, R. High temperature stress mitigation in rice (Oryza sativa L.): Foliar application of plant growth regulators and nutrients. J. Crop Weed 2021, 17, 34–47. [Google Scholar] [CrossRef]
- Kothari, A.; Lachowiec, J. Roles of brassinosteroids in mitigating heat stress damage in cereal crops. Int. J. Mol. Sci. 2021, 22, 2706. [Google Scholar] [CrossRef] [PubMed]
- Thussagunpanit, J.; Jutamanee, K.; Sonjaroon, W.; Kaveeta, L.; Chai-Arree, W.; Pankean, P.; Suksamrarn, A. Effects of brassinosteroid and brassinosteroid mimic on photosynthetic efficiency and rice yield under heat stress. Photosynthetica 2015, 53, 312–320. [Google Scholar] [CrossRef]
- Jhansi Lakshmi, K.P.; Irappa, S.; Raghavendra, C.R.; Basavarajappa, S. High temperature erosive behaviour of plasma sprayed NiCrAlY/B4C/Cenosphere coating on MDN 321 turbine steel. Trans. IMF 2023, 101, 49–56. [Google Scholar] [CrossRef]
- Mostofa, M.G.; Yoshida, N.; Fujita, M.J. Spermidine pretreatment enhances heat tolerance in rice seedlings through modulating antioxidative and glyoxalase systems. Plant Growth Regul. 2014, 73, 31–44. [Google Scholar] [CrossRef]
- Guangwu, Z.; Xuwen, J. Roles of gibberellin and auxin in promoting seed germination and seedling vigor in Pinus massoniana. For. Sci. 2014, 60, 367–373. [Google Scholar] [CrossRef]
- Kim, M.; Seo, H.; Park, C.; Park, W.J. Examination of the auxin hypothesis of phytomelatonin action in classical auxin assay systems in maize. J. Plant Physiol. 2016, 190, 67–71. [Google Scholar] [CrossRef]
- Barman, D.; Ghimire, O.P.; Chinnusamy, V.; Kumar, R.R.; Arora, A. Amelioration of heat stress during reproductive stage in rice by melatonin. Indian J. Agric. Sci. 2019, 89, 1151–1156. [Google Scholar] [CrossRef]
- Yu, Y.; Deng, L.; Zhou, L.; Chen, G.; Wang, Y. Exogenous melatonin activates antioxidant systems to increase the ability of rice seeds to germinate under high temperature conditions. Plants 2022, 11, 886. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhao, J.; Sun, X.; Zhu, Y.; Li, Q.; Zhang, L.; Zhao, D.; Huang, L.; Zhang, C.; Liu, Q. Exogenous melatonin improves the quality performance of rice under high temperature during grain filling. Agronomy 2022, 12, 949. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.R.; Sun, J.; Shu, S.; Wang, Y.; El-Yazied, A.A.; Alabdallah, N.M.; Hikal, M.; Mohamed, M.H.M.; Ibrahim, M.F.M.; et al. Melatonin-mediated photosynthetic performance of tomato seedlings under high-temperature stress. Plant Physiol. Biochem. 2021, 167, 309–320. [Google Scholar] [CrossRef]
- Qi, Z.Y.; Wang, K.X.; Yan, M.Y.; Kanwar, M.K.; Li, D.Y.; Wijaya, L.; Alyemeni, M.N.; Ahmad, P.; Zhou, J. Melatonin alleviates high temperature-induced pollen abortion in Solanum lycopersicum. Molecules 2018, 23, 386. [Google Scholar] [CrossRef]
- Li, X.; Li, M.H.; Deng, W.W.; Ahammed, G.J.; Wei, J.P.; Yan, P.; Zhang, L.P.; Fu, J.Y.; Han, W.Y. Exogenous melatonin improves tea quality under moderate high temperatures by increasing epigallocatechin-3-gallate and theanine biosynthesis in Camellia sinensis L. J. Plant Physiol. 2020, 253, 153273. [Google Scholar] [CrossRef]
- Akasha, A.; Ashraf, M.; Shereen, A.; Mahboob, W.; Faisal, S. Heat tolerance screening studies and evaluating salicylic acid efficacy against high temperature in rice (Oryza sativa L.) genotypes. J. Plant Biochem. Physiol. 2019, 7, 235. [Google Scholar]
- Feng, B.; Zhang, C.; Chen, T.; Zhang, X.; Tao, L.; Fu, G. Salicylic acid reverses pollen abortion of rice caused by heat stress. BMC Plant Biol. 2018, 18, 245. [Google Scholar] [CrossRef] [PubMed]
- Maestri, E.; Klueva, N.; Perrotta, C.; Gulli, M.; Nguyen, H.T. Molecular genetics of heat tolerance and heat shock proteins in cereals. Plant Mol. Biol. 2002, 48, 667–681. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.R.; Tarpley, L. Impact of high nighttime temperature on respiration, membrane stability, antioxidant capacity, and yield of rice plants. Crop Sci. 2009, 49, 313–322. [Google Scholar] [CrossRef]
- Dat, J.F.; Lopez-Delgado, H.; Foyer, C.H.; Scott, I.M. Parallel changes in H2O2 and catalase during thermotolerance induced by salicylic acid or heat acclimation in mustard seedlings. Plant Physiol. 1998, 116, 1351–1357. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, R.; Zong, X.F.; Wang, S.G.; He, G.H. Effect of salicylic acid on heat resistance of rice seedling under heat stress. Chin. J. Eco-Agric. 2009, 17, 1168–1171. [Google Scholar]
- Imran, M.; Khan, M.A.; Shahzad, R.; Bilal, S.; Khan, M.; Yun, B.-W.; Khan, A.L.; Lee, I.-J. Melatonin ameliorates thermotolerance in soybean seedling through balancing redox homeostasis and modulating antioxidant defense, phytohormones and polyamines biosynthesis. Molecules 2021, 26, 5116. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xiao, S.; Zhang, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Wang, X.; Bai, Z.; Li, C.; Liu, L. Melatonin improves the germination rate of cotton seeds under drought stress by opening pores in the seed coat. PeerJ 2020, 8, e9450. [Google Scholar] [CrossRef]
- Mushtaq, N.; Iqbal, S.; Hayat, F.; Raziq, A.; Ayaz, A.; Zaman, W. Melatonin in Micro-Tom tomato: Improved drought tolerance via the regulation of the photosynthetic apparatus, membrane stability, osmoprotectants, and root system. Life 2022, 12, 1922. [Google Scholar] [CrossRef]
- Sharif, R.; Xie, C.; Zhang, H.; Arnao, M.B.; Ali, M.; Ali, Q.; Muhammad, I.; Shalmani, A.; Nawaz, M.A.; Chen, P.; et al. Melatonin and its effects on plant systems. Molecules 2018, 23, 2352. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.; Weeda, S.; Zhao, B.; Ren, S. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in Cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Raza, A.; Charagh, S.; García-Caparrós, P.; Rahman, M.A.; Ogwugwa, V.H.; Saeed, F.; Jin, W. Melatonin-mediated temperature stress tolerance in plants. GM Crops Food 2022, 13, 196–217. [Google Scholar] [CrossRef] [PubMed]
- Aftab, T.; Masroor, M.; Khan, A.Z.; Idrees, M.; Naeem, M.; Moinuddin. Salicylic acid acts as potent enhancer of growth, photosynthesis and artemisinin production in Artemisia annua L. J. Crop Sci. Biotechnol. 2010, 13, 183–188. [Google Scholar] [CrossRef]
- Dinler, B.S.; Gunduzer, E.; Tekinay, T. Pre-treatment of fulvic acid plays a stimulant role in protection of soybean (Glycine max L.) leaves against heat and salt stress. Acta Biol. Cracov. Ser. Bot. 2016, 58, 29–41. [Google Scholar] [CrossRef]
- Goatley, J.M.; Schmidt, R.E. Seedling Kentucky bluegrass growth response to chelated iron and biostimulator materials. Agron. J. 1990, 82, 901–905. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Mannino, G.; Agliassa, C.; Acquadro, A.; Contartese, V.; Garabello, C.; Bertea, C.M. Transcriptome analyses and antioxidant activity profiling reveal the role of a lignin-derived biostimulant seed treatment in enhancing heat stress tolerance in soybean. Plants 2020, 9, 1308. [Google Scholar] [CrossRef]
- Shuai, H.; Meng, Y.; Luo, X.; Chen, F.; Zhou, W.; Dai, Y.; Qi, Y.; Du, J.; Yang, F.; Liu, J.; et al. Exogenous auxin represses soybean seed germination through decreasing the gibberellin/abscisic acid (GA/ABA) ratio. Sci. Rep. 2017, 7, 12620. [Google Scholar] [CrossRef] [PubMed]
- Bellieny-Rabelo, D.; De Oliveira, E.A.G.; Da Silva Ribeiro, E.; Pessoa Costa, E.; Oliveira, A.E.A.; Venancio, T.M. Transcriptome analysis uncovers key regulatory and metabolic aspects of soybean embryonic axes during germination. Sci. Rep. 2016, 6, 36009. [Google Scholar] [CrossRef]
- Repke, R.A.; Silva, D.M.R.; dos Santos, J.C.C.; Silva, M.A. Increased soybean tolerance to high temperature through biostimulant based on Ascophyllum nodosum (L.) seaweed extract. J. Appl. Phycol. 2022, 34, 3205–3218. [Google Scholar] [CrossRef]
- Zhou, Y.; Sommer, M.L.; Hochholdinger, F. Cold response and tolerance in cereal roots. J. Exp. Bot. 2021, 72, 7474–7481. [Google Scholar] [CrossRef]
- Kodra, E.; Steinhaeuser, K.; Ganguly, A.R. Persisting cold extremes under 21st century warming scenarios. Geophys. Res. Lett. 2011, 38, 8705. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.K. Cold stress regulation of gene expression in plants. Trends Plant Sci. 2007, 12, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Y.; Cai, H.; Guo, M.; Chai, M.; She, Z.; Ye, L.; Cheng, Y.; Wang, B.; Qin, Y. The bZIP transcription factor GmbZIP15 negatively regulates salt- and drought-stress responses in soybean. Int. J. Mol. Sci. 2020, 21, 7778. [Google Scholar] [CrossRef]
- Huang, M.; Jiang, L.; Zou, Y.; Zhang, W. On-farm assessment of effect of low temperature at seedling stage on early-season rice quality. Field Crops Res. 2013, 141, 63–68. [Google Scholar] [CrossRef]
- Shimono, H.; Okada, M.; Kanda, E.; Arakawa, I. Low temperature-induced sterility in rice: Evidence for the effects of temperature before panicle initiation. Field Crops Res. 2007, 101, 221–231. [Google Scholar] [CrossRef]
- Cruz, R.P.D.; Sperotto, R.A.; Cargnelutti, D.; Adamski, J.M.; de FreitasTerra, T.; Fett, J.P. Avoiding damage and achieving cold tolerance in rice plants. Food Energy Secur. 2013, 2, 96–119. [Google Scholar] [CrossRef]
- Zheng, S.; Su, M.; Wang, L.; Zhang, T.; Wang, J.; Xie, H.; Wu, X.; UI Haq, S.I.; Qui, Q.-S. Small signaling molecules in plant response to cold stress. J. Plant Physiol. 2021, 266, 153534. [Google Scholar] [CrossRef]
- Liang, Y.; Xia, J.; Jiang, Y.; Bao, Y.; Chen, H.; Wang, D.; Zhang, D.; Yu, J.; Cang, J. Genome-wide identification and analysis of bZIP gene family and resistance of TaABI5 (TabZIP96) under freezing stress in wheat (Triticum aestivum). Int. J. Mol. Sci. 2022, 4, 235. [Google Scholar] [CrossRef] [PubMed]
- Franzoni, G.; Cocetta, G.; Prinsi, B.; Ferrante, A.; Espen, L. Biostimulants on crops: Their impact under abiotic stress conditions. Horticulturae 2022, 8, 189. [Google Scholar] [CrossRef]
- Yuan, P.; Yang, T.; Poovaiah, B.W. Calcium signaling-mediated plant response to cold stress. Int. J. Mol. Sci. 2018, 19, 3896. [Google Scholar] [CrossRef]
- Jung, J.-H.; Domijan, M.; Klose, C.; Biswas, S.; Ezer, D.; Gao, M.; Khattak, A.K.; Box, M.S.; Charoensawan, V.; Cortijo, S.; et al. Phytochromes function as thermosensors in Arabidopsis. Science 2016, 354, 886–889. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Nawaz, Z.; Kakar, K.U.; Ullah, R.; Yu, S.; Zhang, J.; Shu, Q.-Y.; Ren, X. Genome-wide identification, evolution and expression analysis of cyclic nucleotide-gated channels in tobacco (Nicotiana tabacum L.). Genomics 2019, 111, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Luo, L.; Wei, J.; Chen, Q.; Yang, Y.; Hu, X.; Kong, X. The glutamate receptors AtGLR1.2 and AtGLR1.3 increase cold tolerance by regulating jasmonate signaling in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2018, 506, 895–900. [Google Scholar] [CrossRef]
- Zhang, X.; Yin, J.; Ma, Y.; Peng, Y.; Fenton, O.; Wang, W.; Zhang, W.; Chen, Q. Unlocking the potential of biostimulants derived from organic waste and by-product sources: Improving plant growth and tolerance to abiotic stresses in agriculture. Environ. Technol. Innov. 2024, 34, 103571. [Google Scholar] [CrossRef]
- Wang, H.; Zhi, W.; Qu, H.; Lin, H.; Jiang, Y. Application of α-aminoisobutyric acid and β-aminoisobutyric acid inhibits pericarp browning of harvested longan fruit. Chem. Cent. J. 2015, 9, 54. [Google Scholar] [CrossRef]
- Askari-Khorasgani, O.; Hatterman-Valenti, H.; Flores Pardo, F.B.; Pessarakli, M. Plant and symbiont metabolic regulation and biostimulants application improve symbiotic performance and cold acclimation. J. Plant Nutr. 2019, 42, 2151–2163. [Google Scholar] [CrossRef]
- Teixeira, S.B.; Pires, S.N.; Ávila, G.E.; Silva, B.E.P.; Schmitz, V.N.; Deuner, C.; Armesto, R.S.; Moura, D.S.; Deuner, S. Application of vigor index to evaluate the cold tolerance in rice seeds germination conditioned in plant extract. Sci. Rep. 2021, 11, 11038. [Google Scholar] [CrossRef]
- Bevilacqua, C.B.; Monzon, D.R.; Venske, E.; Basu, S.; Zimmer, P.D. Application of stress indices for low temperature and deep sowing stress screening of rice genotypes. Pak. J. Biol. Sci. 2013, 16, 1618–1622. [Google Scholar] [CrossRef]
- Rodrigues, C.B.; Bevilacqua, C.A.; Bahry, C.A.; Monzon, D.L.R.; Viana, T.P.; Zimmer, P.D.; Fagundes, P.R.R. Application of stress indexes to evaluate cold tolerance of rice cultivars during the initial stage of plant development. Científica 2014, 42, 258–264. [Google Scholar]
- Hernández-Herrera, R.M.; Santacruz-Ruvalcaba, F.; Ruiz-López, M.A.; Norrie, F.; Hernández-Carmona, G. Effect of liquid seaweed extracts on growth of tomato seedlings (Solanum lycopersicum L.). J. Appl. Phycol. 2014, 26, 619–628. [Google Scholar] [CrossRef]
- Song, Y.; Jiang, M.; Zhang, H.; Li, R. Zinc Oxide Nanoparticles alleviate chilling stress in rice (Oryza sativa L.) by regulating antioxidative system and chilling response transcription factors. Molecules 2021, 26, 2196. [Google Scholar] [CrossRef]
- Hussain, A.; Ali, S.; Rizwan, M.; Rehman, M.Z.U.; Javed, M.R.; Imran, M.; Chatha, S.A.S.; Nazir, R. Zinc oxide nanoparticles alter the wheat physiological response and reduce the cadmium uptake by plants. Environ. Pollut. 2018, 242, 1518–1526. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, X.; Liu, Y.; Song, W.; Su, D. The Effect of zinc on the return of cold rice to greening and yield. Chin. J. Soil Sci. 2013, 44, 437–441. [Google Scholar]
- Moradtalab, N.; Ahmed, A.; Geistlinger, J.; Walker, F.; Hoglinger, B.; Ludewig, U.; Neumann, G. Synergisms of microbial consortia, N forms, and micronutrients alleviate oxidative damage and stimulate hormonal cold stress adaptations in maize. Front. Plant Sci. 2020, 11, 396. [Google Scholar] [CrossRef]
- Liu, Z.; Meng, J.; Sun, Z.; Su, J.; Luo, X.; Song, J.; Li, P.; Sun, Y.; Yu, C.; Peng, X. Zinc application after low temperature stress promoted rice tillers recovery: Aspects of nutrient absorption and plant hormone regulation. Plant Sci. 2022, 314, 111104. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management, 2nd ed.; Routledge: London, UK, 2015. [Google Scholar]
- Waqas, M.; Shahzad, R.; Hamayun, M.; Asaf, S.; Khan, A.L.; Kang, S.M.; Yun, S.; Kim, K.-M.; Lee, I.-J.; Paz-Ferreiro, J. Biochar amendment changes jasmonic acid levels in two rice varieties and alters their resistance to herbivory. PLoS ONE 2018, 13, e0191296. [Google Scholar] [CrossRef]
- Yuan, J.; Meng, J.; Liang, X.; Yang, E.; Yang, X.; Chen, W.-F. Biochar’s leacheates affect the abscisic acid pathway in rice seedlings under low temperature. Front. Plant Sci. 2021, 12, 646910. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Meng, J.; Liang, X.; Yang, E.; Yang, X.; Chen, W. Organic molecules from biochar leacheates have a positive effect on rice seedling cold tolerance. Front. Plant Sci. 2017, 8, 1624. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Zhao, H.-H.; Zhao, L.-M.; Gu, C.-M.; Na, Y.-G.; Xie, B.; Cheng, S.-H.; Pan, G.-J. Application of brassinolide alleviates cold stress at the booting stage of rice. J. Integr. Agric. 2020, 19, 975–987. [Google Scholar] [CrossRef]
- Li, R.; Jiang, M.; Song, Y.; Zhang, H. Melatonin alleviates low-temperature stress via ABI5-mediated signals during seed germination in rice (Oryza sativa L.). Front. Plant Sci. 2021, 12, 727596. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.T.; Qian, Y.Q.; Tan, D.X.; Reiter, R.J.; He, C.Z. Melatonin induces the transcripts of CBF/DREB1s and their involvement in both abiotic and biotic stresses in Arabidopsis. J. Pineal Res. 2015, 59, 334–342. [Google Scholar] [CrossRef]
- Han, Q.-H.; Huang, B.; Ding, C.-B.; Zhang, Z.-W.; Chen, Y.-E.; Hu, C.; Zhou, L.-J.; Huang, Y.; Liao, J.-Q.; Yuan, M.; et al. Effects of melatonin on anti-oxidative systems and photosystem II in cold-stressed rice seedlings. Front. Plant Sci. 2017, 8, 785. [Google Scholar] [CrossRef]
- Ahmad, P.; Bhardwaj, R.; Tuteja, N. Plant signaling under abiotic stress environment. In Environmental Adaptations and Stress Tolerance of Plants in the Era of Climate Change; Ahmad, P., Prasad, M., Eds.; Springer: New York, NY, USA, 2012; pp. 297–324. [Google Scholar]
- Kanwar, M.K.; Yu, J.; Zhou, J. Phytomelatonin: Recent advances and future prospects. J. Pineal Res. 2018, 65, e12526. [Google Scholar] [CrossRef]
- Posmyk, M.M.; Kuran, H.; Marciniak, K.; Janas, K.M. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J. Pineal Res. 2008, 45, 24–31. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Ren, S.; Guo, Y. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef]
- Hernández, I.G.; Gomez, F.J.; Cerutti, S.; Arana, M.V.; Silva, M.F. Melatonin in Arabidopsis thaliana acts as plant growth regulator at low concentrations and preserves seed viability at high concentrations. Plant Physiol. Biochem. 2015, 94, 191–196. [Google Scholar] [CrossRef]
- Simlat, M.; Szewczyk, A.; Ptak, A. Melatonin promotes seed germination under salinity and enhances the biosynthesis of steviol glycosides in Stevia rebaudiana Bertoni leaves. PLoS ONE 2020, 15, e0230755. [Google Scholar] [CrossRef]
- Xiao, S.; Liu, L.; Wang, H.; Li, D.; Bai, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Li, C. Exogenous melatonin accelerates seed germination in cotton (Gossypium hirsutum L.). PLoS ONE 2019, 14, e0216575. [Google Scholar] [CrossRef]
- Ren, C.; Wang, H.; Zhou, Z.; Jia, J.; Zhang, Q.; Liang, C.; Li, W.; Zhang, Y.; Yu, G. Genome-wide identification of the B3 gene family in soybean and the response to melatonin under cold stress. Front. Plant Sci. 2023, 13, 1091907. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin and its relationship to plant hormones. Ann. Bot. 2018, 121, 195–207. [Google Scholar] [CrossRef]
- Bawa, G.; Feng, L.; Shi, J.; Chen, G.; Cheng, Y.; Luo, J.; Wu, W.D.; Ngoke, B.; Cheng, P.; Tang, Z.; et al. Evidence that melatonin promotes soybean seedlings growth from low-temperature stress by mediating plant mineral elements and genes involved in the antioxidant pathway. Funct. Plant Biol. 2020, 47, 815–824. [Google Scholar] [CrossRef]
- Li, C.; Liang, B.; Chang, C.; Wei, Z.; Zhou, S.; Ma, F. Exogenous melatonin improved potassium content in Malus under different stress conditions. J. Pineal Res. 2016, 61, 218–229. [Google Scholar] [CrossRef]
- Zhang, R.; Sun, Y.; Liu, Z.; Jin, W.; Sun, Y. Effects of melatonin on seedling growth, mineral nutrition, and nitrogen metabolism in cucumber under nitrate stress. J. Pineal Res. 2017, 62, e12403. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Ahammed, G.J.; Yin, L.; Shi, K.; Xia, X.; Zhou, Y.; Yu, J.; Zhou, J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015, 6, 601. [Google Scholar] [CrossRef]
- Li, M.-Q.; Hasan, M.K.; Li, C.-X.; Ahammed, G.J.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Reiter, R.J.; Yu, J.-Q.; Xu, M.-X. Melatonin mediates selenium-induced tolerance to cadmium stress in tomato plants. J. Pineal Res. 2016, 61, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.-X.; Zhou, Z.; Cruz, M.H.C.; Fuentes-Broto, L.; Galano, A. Phytomelatonin: Assisting plants to survive and thrive. Molecules 2015, 20, 7396–7437. [Google Scholar] [CrossRef]
- Cai, S.-Y.; Zhang, Y.; Xu, Y.-P.; Qi, Z.-Y.; Li, M.-Q.; Ahammed, G.J.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Reiter, R.J. HsfA1a upregulates melatonin biosynthesis to confer cadmium tolerance in tomato plants. J. Pineal Res. 2017, 62, e12387. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cai, S.-Y.; Zhang, Y.; Wang, Y.; Ahammed, G.J.; Xia, X.-J.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Reiter, R.J. Melatonin enhances thermotolerance by promoting cellular protein protection in tomato plants. J. Pineal Res. 2016, 61, 457–469. [Google Scholar] [CrossRef]
- Feher-Juhasz, E.; Majer, P.; Sass, L.; Lantos, C.; Csiszár, J.; Turóczy, Z.; Mihály, R.; Mai, A.; Horváth, G.V.; Vass, I.; et al. Phenotyping shows improved physiological traits and seed yield of transgenic wheat plants expressing the alfalfa aldose reductase under permanent drought stress. Acta Physiol. Plant. 2014, 36, 663–673. [Google Scholar] [CrossRef]
- Gai, Z.; Liu, L.; Zhang, J.; Liu, J.; Cai, L. Effects of exogenous α-oxoglutarate on proline accumulation, ammonium assimilation and photosynthesis of soybean seedling (Glycine max (L.) Merr.) exposed to cold stress. Sci. Rep. 2020, 10, 17017. [Google Scholar] [CrossRef]
- Yuan, Y.; Ou, J.; Wang, Z.; Zhang, C.; Zhou, Z.; Lin, Q. Regulation of carbon and nitrogen metabolisms in rice roots by 2-oxoglutarate at the level of hexokinase. Physiol. Plant. 2007, 129, 296–306. [Google Scholar] [CrossRef]
- Moradtalab, N.; Weinmann, M.; Walker, F.; Höglinger, B.; Ludewig, U.; Neumann, G. Silicon improves chilling tolerance during early growth of maize by effects on micronutrient homeostasis and hormonal balances. Front. Plant Sci. 2018, 9, 420. [Google Scholar] [CrossRef]
- Sharifi, P.; Amirnia, R.; Shirani Bidabadi, S. Role of silicon in mediating heat shock tolerance in soybean. Gesunde Pflanzen 2022, 74, 397–411. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Silicon enhances plant vegetative growth and soil water retention of soybean (Glycine max) plants under water-limiting conditions. Plants 2022, 11, 1687. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Ahmad, W.; Coffman, L.; Weerasooriya, A.D.; Crawford, K.; Khan, A.L. The silicon regulates microbiome diversity and plant defenses during cold stress in Glycine max L. Front. Plant Sci. 2023, 14, 1280251. [Google Scholar] [CrossRef]
- Zhu, Y.; Gong, H. Beneficial effects of silicon on salt and drought tolerance in plants. Agron. Sustain. Dev. 2013, 34, 455–472. [Google Scholar] [CrossRef]
- Guntzer, F.; Keller, C.; Meunier, J.-D. Benefits of plant silicon for crops: A review. Agron. Sustain. Dev. 2012, 32, 201–213. [Google Scholar] [CrossRef]
- Khalvandi, M.; Siosemardeh, A.; Roohi, E.; Keramati, S. Salicylic acid alleviated the effect of drought stress on photosynthetic characteristics and leaf protein pattern in winter wheat. Heliyon 2021, 7, e05908. [Google Scholar] [CrossRef]
- Kim, W.; Iizumi, T.; Nishimori, M. Global patterns of crop production losses associated with droughts from 1983 to 2009. J. Appl. Meteorol. Climatol. 2019, 58, 1233–1244. [Google Scholar] [CrossRef]
- Zhao, T.; Dai, A. The magnitude and causes of global drought changes in the twenty-first century under a low–moderate emissions scenario. J. Clim. 2015, 28, 4490–4512. [Google Scholar] [CrossRef]
- Mazhar, M.W.; Ishtiaq, M.; Hussain, I.; Parveen, A.; Bhatti, K.H.; Azeem, M.; Thind, S.; Ajaib, M. Seed nano-priming with Zinc Oxide nanoparticles in rice mitigates drought and enhances agronomic profile. PLoS ONE 2022, 17, e0264967. [Google Scholar]
- Boretti, A.; Rosa, L. Reassessing the projections of the world water development report. NPJ Clean Water 2019, 2, 15. [Google Scholar] [CrossRef]
- Mazhar, M.; Ali, Q.; Ishtiaq, M.; Ghani, A.; Maqbool, M.; Hussain, T.; Mushtaq, W. Zinc-Aspartate-mediated drought amelioration in maize promises better growth and agronomic parameters than zinc sulfate and L-aspartate. SABRAO J. Breed. Genet. 2021, 53, 290–310. [Google Scholar]
- Anjum, S.A.; Wang, L.; Farooq, M.; Khan, I.; Xue, L. Methyl jasmonate-induced alteration in lipid peroxidation, antioxidative defence system and yield in soybean under drought. J. Agron. Crop Sci. 2011, 197, 296–301. [Google Scholar] [CrossRef]
- Fitton, N.; Alexander, P.; Arnell, N.; Bajzelj, B.; Calvin, K.; Doelman, J.; Gerber, J.S.; Havlik, P.; Hasegawa, T.; Herrero, M.; et al. The vulnerabilities of agricultural land and food production to future water scarcity. Glob. Environ. Change 2019, 58, 101944. [Google Scholar] [CrossRef]
- Lipiec, J.; Doussan, C.; Nosalewicz, A.; Kondracka, K. Effect of drought and heat stresses on plant growth and yield: A review. Int. Agrophys. 2013, 27, 463–477. [Google Scholar] [CrossRef]
- Hussain, H.A.; Men, S.; Hussain, S.; Chen, Y.; Ali, S.; Zhang, S.; Zhang, K.; Li, Y.; Xu, Q.; Liao, C.; et al. Interactive effects of drought and heat stresses on morphophysiological attributes, yield, nutrient uptake and oxidative status in maize hybrids. Sci. Rep. 2019, 9, 3890. [Google Scholar] [CrossRef]
- Samarah, N.H.; Alqudah, A.M.; Amayreh, J.A.; McAndrews, G.M. The effect of late-terminal drought stress on yield components of four barley cultivars. J. Agron. Crop Sci. 2009, 195, 427–441. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought stress responses in plants, oxidative stress, and antioxidant defense. In Climate Change and Plant Abiotic Stress Tolerance; Tuteja, N., Gill, S.S., Eds.; Wiley: New York, NY, USA, 2014; pp. 209–249. [Google Scholar]
- Ali, S.; Hayat, K.; Iqbal, A.; Xie, L. Implications of abscisic acid in the drought stress tolerance of plants. Agronomy 2020, 10, 1323. [Google Scholar] [CrossRef]
- Oladosu, Y.; Rafii, M.Y.; Samuel, C.; Fatai, A.; Magaji, U.; Kareem, I.; Kamarudin, Z.S.; Muhammad, I.; Kolapo, K. Drought resistance in rice from conventional to molecular breeding: A Review. Int. J. Mol. Sci. 2019, 20, 3519. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Sahebi, M.; Hanafi, M.M.; Rafii, M.Y.; Mahmud, T.M.M.; Azizi, P.; Osman, M.; Abiri, R.; Taheri, S.; Kalhori, N.; Shabanimofrad, M.; et al. Improvement of Drought Tolerance in Rice (Oryza sativa L.): Genetics, Genomic Tools, and the WRKY Gene Family. BioMed Res. Int. 2018, 2018, 3158474. [Google Scholar] [CrossRef] [PubMed]
- Lum, M.S.; Hanafi, M.M.; Rafii, Y.M. Effect of drought stress on growth, proline and antioxidant enzyme activities of upland rice. J. Anim. Plant Sci. 2014, 24, 1487–1493. [Google Scholar]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin systemically ameliorates drought stress-induced damage in Medicago sativa plants by modulating nitro-oxidative homeostasis and proline metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef]
- Li, C.; Tan, D.X.; Liang, D.; Chang, C.; Jia, D.F.; Ma, F.W. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Hussan, M.U.; Saleem, M.F.; Hafeez, M.B.; Khan, S.; Hussain, S.; Ahmad, N.; Ramzan, Y.; Nadeem, M. Impact of soil applied humic acid, zinc and boron supplementation on the growth, yield and zinc translocation in winter wheat. Asian J. Agric. Biol. 2021. [Google Scholar] [CrossRef]
- Silalert, P.; Pattanagul, W. Foliar application of melatonin alleviates the effects of drought stress in rice (Oryza sativa L.) seedlings. Not. Bot. Horti Agrobo. 2021, 49, 12417. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.-X.; Naz, S.; Altaf, M.M.; Khan, L.U.; Tiwari, R.K.; Lal, M.K.; Shahid, M.A.; Kumar, R.; et al. Melatonin improves drought stress tolerance of tomato by modulating plant growth, root architecture, photosynthesis, and antioxidant defense system. Antioxidants 2022, 11, 309. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, L.; Yu, Y.; Zeng, H.; Deng, L.; Zhu, L.; Chen, G.; Wang, Y. Melatonin-induced resilience strategies against the damaging impacts of drought stress in rice. Agronomy 2022, 12, 813. [Google Scholar] [CrossRef]
- Khattak, W.A.; He, J.; Abdalmegeed, D.; Hu, W.; Wang, Y.; Zhou, Z. Foliar melatonin stimulates cotton boll distribution characteristics by modifying leaf sugar metabolism and antioxidant activities during drought conditions. Physiol. Plant. 2022, 174, e13526. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Husen, A. Plant response to jasmonates: Current developments and their role in changing environment. Document. Bull. Natl. Res. Centre 2019, 43, 153. [Google Scholar] [CrossRef]
- Sasi, M.; Awana, M.; Samota, M.K.; Tyagi, A.; Kumar, S.; Sathee, L.; Krishnan, V.; Praveen, S.; Singh, A. Plant growth regulator induced mitigation of oxidative burst helps in the management of drought stress in rice (Oryza sativa L.). Environ. Exp. Bot. 2021, 185, 104413. [Google Scholar] [CrossRef]
- Jang, G.; Yoon, Y.; Choi, Y.D. Crosstalk with jasmonic acid integrates multiple responses in plant development. Int. J. Mol. Sci. 2020, 21, 305. [Google Scholar] [CrossRef]
- Khan, N.A.; Nazar, R.; Iqbal, N.; Anjum, N.A. Phytohormones and Abiotic stress Tolerance in Plants; Springer: Berlin, Germany, 2012; pp. 248–276. [Google Scholar]
- Khan, M.R.; Syeed, S.; Nazar, R.; Anjum, N.A. An Insight into the Role of Salicylic Acid and Jasmonic Acid in Salt Stress Tolerance; Springer: Berlin, Germany, 2012; pp. 277–300. [Google Scholar]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Hosain, M.T.; Kamrunnahar; Rahman, M.M.; Munshi, M.H.; Rahman, M.S. Drought stress response of rice yield (Oryza sativa L.) and role of exogenous salicylic acid. Int. J. Biosci. 2020, 16, 222–230. [Google Scholar]
- Asma, I.; Hussain, I.; Ashraf, M.Y.; Saleem, M.H.; Ashraf, M.A.; Ali, B.; Shereen, A.; Farid, G.; Ali, M.; Shirazi, M.U.; et al. Alleviating effects of salicylic acid spray on stage-based growth and antioxidative defense system in two drought-stressed rice (Oryza sativa L.) cultivars. Turk. J. Agric. For. 2023, 47, 79–99. [Google Scholar]
- Devarajan, A.K.; Muthukrishnan, G.; Truu, J.; Truu, M.; Ostonen, I.; Kizhaeral, S.S.; Panneerselvam, P.; Gopalasubramanian, S.K. The foliar application of rice phyllosphere bacteria induces drought-stress tolerance in Oryza sativa (L.). Plants 2021, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Obst, M.; Dynes, J.J.; Lawrence, J.R.; Swerhone, G.D.; Benzerara, K.; Karunakaran, C.; Kaznatcheev, K.; Tyliszczak, T.; Hitchcock, A.P. Precipitation of amorphous CaCO3 (aragonite-like) by cyanobacteria: A STXM study of the influence of EPS on the nucleation process. Geochim. Cosmochim. Acta 2009, 73, 4180–4198. [Google Scholar] [CrossRef]
- Goyal, K.; Walton, L.J.; Tunnacliffe, A. LEA proteins prevent protein aggregation due to water stress. Biochem. J. 2005, 388, 151–157. [Google Scholar] [CrossRef]
- Sami, F.; Yusuf, M.; Faizan, M.; Faraz, A.; Hayat, S. Role of sugars under abiotic stress. Physiol. Biochem. 2016, 109, 54–61. [Google Scholar] [CrossRef]
- Tyerman, S.D.; Niemietz, C.M.; Bramley, H. Plant aquaporins: Multifunctional water and solute channels with expanding roles. Plant Cell Environ. 2002, 25, 173–194. [Google Scholar] [CrossRef]
- Khan, S.; Basra, S.M.A.; Nawaz, M.; Hussain, I.; Foidl, N. Combined application of moringa leaf extract and chemical growth-promoters enhances the plant growth and productivity of wheat crop (Triticum aestivum L.). S. Afr. J. Bot. 2020, 129, 74–81. [Google Scholar] [CrossRef]
- Kumar, A.S.; Sridar, R.; Uthandi, S. Mitigation of drought in rice by a phyllosphere bacterium Bacillus altitudinis FD48. Afr. J. Microbiol. Res. 2017, 11, 1614–1625. [Google Scholar] [CrossRef]
- Mohd Zain, N.A.; Ismail, M.R.; Puteh, A.; Mahmood, M.; Islam, M.R. Drought tolerance and ion accumulation of rice following application of additional potassium fertilizer. Commun. Soil Sci. Plant Anal. 2014, 45, 2295–2309. [Google Scholar] [CrossRef]
- Naser, Z.; Aynehband, A.; Lak, S. Evaluation of physiological trait changes under drought stress and potassium application, and their impact on the yield of mung bean cultivars and promising lines. Adv. Environ. Biol. 2012, 6, 2854–2859. [Google Scholar]
- Djanaguiraman, M.; Prasad, P.V.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef]
- Jiang, C.; Zu, C.; Lu, D.; Zheng, Q.; Shen, J.; Wang, H.; Li, D. Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci. Rep. 2017, 7, 42039. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Bijo, A.J.; Baghel, R.S.; Reddy, C.R.K.; Jha, B. Selenium and spermine alleviate cadmium induced toxicity in the red seaweed Gracilaria dura by regulating antioxidants and DNA methylation. Plant Physiol. Biochem. 2012, 51, 129–138. [Google Scholar] [CrossRef]
- Reis, H.P.G.; Barcelos, J.P.Q.; Junior, E.F.; Santos, E.F.; Silva, V.M.; Moraes, M.F.; Putti, F.F.; Reis, A.R. Agronomic biofortification of upland rice with selenium and nitrogen and its relation to grain quality. J. Cereal Sci. 2018, 79, 508–515. [Google Scholar] [CrossRef]
- Andrade, F.R.; da Silva, G.N.; Guimarães, K.C.; Barreto, H.B.F.; de Souza, K.R.D.; Guilherme, L.R.G.; Faquin, V.; dos Reis, A.R. Selenium protects rice plants from water deficit stress. Ecotoxicol. Environ. Saf. 2018, 164, 562–570. [Google Scholar] [CrossRef]
- Iqbal, M.; Hussain, I.; Liaqat, H.; Ashraf, M.A.; Rasheed, R.; Rehman, A.U. Exogenously applied selenium reduces oxidative stress and induces heat tolerance in spring wheat. Plant Physiol. Biochem. 2015, 94, 95–103. [Google Scholar] [CrossRef]
- Tran, T.T.; Kano-Nakata, M.; Takeda, M.; Menge, D.; Mitsuya, S.; Inukai, Y.; Yamauchi, A. Nitrogen application enhanced the expression of developmental plasticity of root systems triggered by mild drought stress in rice. Plant Soil 2014, 378, 139–152. [Google Scholar] [CrossRef]
- Powell, S.R. The antioxidant properties of zinc. J. Nutr. 2000, 130, 1447S–1454S. [Google Scholar] [CrossRef] [PubMed]
- Tondey, M.; Kalia, A.; Singh, A.; Dheri, G.S.; Taggar, M.S.; Nepovimova, E.; Krejcar, O.; Kuca, K. Seed priming and coating by nano-scale zinc oxide particles improved vegetative growth, yield and quality of fodder maize (Zea mays). Agronomy 2021, 11, 729. [Google Scholar] [CrossRef]
- Itroutwar, P.D.; Govindaraju, K.; Tamilselvan, S.; Kannan, M.; Raja, K.; Subramanian, K.S. Seaweed-based biogenic ZnO nanoparticles for improving agro-morphological characteristics of rice (Oryza sativa L.). J. Plant Growth Regul. 2020, 39, 717–728. [Google Scholar] [CrossRef]
- Khanra, S.; Mamun, M.A.-A.; Ferreira, F.F.; Ghosh, K.; Guha, S. Functionalized self-assembled peptide nanotubes with cobalt ferrite nanoparticles for applications in organic electronics. ACS Appl. Nano Mater. 2018, 1, 1175–1187. [Google Scholar] [CrossRef]
- Haider, M.; Hussain, M.; Farooq, M.; Nawaz, A. Optimizing zinc seed priming for improving the growth, yield and grain biofortification of mungbean (Vigna radiata (L.) Wilczek). J. Plant Nutr. 2020, 43, 1438–1446. [Google Scholar] [CrossRef]
- Khan, S.; Ibrar, D.; Hasnain, Z.; Nawaz, M.; Rais, A.; Ullah, S.; Gul, S.; Siddiqui, M.H.; Irshad, S. Moringa leaf extract mitigates the adverse impacts of drought and improves the yield and grain quality of rice through enhanced physiological, biochemical, and antioxidant activities. Plants 2023, 12, 2511. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.M.; Babar, A. Impacts of plant growth promoters and plant growth regulators on rainfed agriculture. PLoS ONE 2020, 15, e0231426. [Google Scholar] [CrossRef]
- Basra, S.M.A.; Iftikhar, M.N.; Afzal, I. Potential of moringa (Moringa oleifera) leaf extract as priming agent for hybrid maize seeds. Int. J. Agric. Biol. 2011, 13, 1006–1010. [Google Scholar]
- Khan, S.; Basra, S.M.A.; Afzal, I.; Nawaz, M.; Rehman, H.U. Growth promoting potential of fresh and stored Moringa oleifera leaf extracts in improving seedling vigor, growth and productivity of wheat crop. Environ. Sci. Pollut. Res. 2017, 24, 27601–27612. [Google Scholar] [CrossRef]
- Imran, M.; Khan, A.L.; Shahzad, R.; Khan, M.A.; Bilal, S.; Khan, A.; Kang, S.-M.; Lee, I.-J. Exogenous melatonin induces drought stress tolerance by promoting plant growth and antioxidant defence system of soybean plants. AoB Plants 2021, 13, 4. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Reiter, R.J.; Tan, D.X.; Chan, Z. INDOLE-3-ACETIC ACID INDUCIBLE 17 positively modulates natural leaf senescence through melatonin-mediated pathway in Arabidopsis. J. Pineal Res. 2015, 58, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, S.; Qin, B.; Jin, X.; Wang, M.; Ren, C.; Cao, L.; Zhang, Y. Exogenous melatonin reduces the inhibitory effect of osmotic stress on antioxidant properties and cell ultrastructure at germination stage of soybean. PLoS ONE 2020, 15, e0243537. [Google Scholar] [CrossRef]
- Mohamed, H.I.; Latif, H.H. Improvement of drought tolerance of soybean plants by using methyl jasmonate. Physiol. Mol. Biol. Plants 2017, 23, 545–556. [Google Scholar] [CrossRef]
- Karami, A.; Shahbazi, M.; Niknam, V.; Shobbar, Z.; Tafreshi, R.; Abedini, R.; Mabood, H. Expression analysis of dehydrin multigene family across tolerant and susceptible barley (Hordeum vulgare L.) genotypes in response to terminal drought stress. Acta Physiol. Plant. 2013, 35, 2289–2297. [Google Scholar] [CrossRef]
- Walia, H.; Wilson, C.; Condamine, P.; Liu, X.; Ismail, A.M.; Close, T.J. Large-scale expression profiling and physiological characterization of jasmonic acid-mediated adaptation of barley to salinity stress. Plant Cell Environ. 2007, 30, 410–421. [Google Scholar] [CrossRef]
- Yan, Y.; Borrego, E.; Kolomiets, M.V. Jasmonate biosynthesis, perception and function in plant development and stress responses. In Lipid Metabolism; Baez, R.V., Ed.; InTech: Rijeka, Croatia, 2013; pp. 393–442. [Google Scholar]
- Sharma, M.; Gupta, S.K.; Majumder, B.; Maurya, V.K.; Deeba, F.; Alam, A.; Pandey, V. Proteomics unravel the regulating role of salicylic acid in soybean under yield limiting drought stress. Plant Physiol. Biochem. 2018, 130, 529–541. [Google Scholar] [CrossRef]
- Sharafizad, M.; Naderi, A.; Siadat, S.A.; Sakinejad, T.; Lak, S. Effect of salicylic acid pretreatment on yield, its components and remobilization of stored material of wheat under drought stress. J. Agric. Sci. 2012, 4, 2012. [Google Scholar] [CrossRef]
- Sharma, M.; Gupta, S.K.; Majumder, B.; Maurya, V.K.; Deeba, F.; Alam, A.; Pandey, V. Salicylic acid mediated growth, physiological and proteomic responses in two wheat varieties under drought stress. J. Proteom. 2017, 163, 28–51. [Google Scholar] [CrossRef]
- Saruhan, N.; Saglam, A.; Kadioglu, A. Salicylic acid pretreatment induces drought tolerance and delays leaf rolling by inducing antioxidant systems in maize genotypes. Acta Physiol. Plant. 2012, 34, 97–106. [Google Scholar] [CrossRef]
- Kang, G.Z.; Li, G.Z.; Liu, G.Q.; Xu, W.; Peng, X.Q.; Wang, C.Y.; Zhu, Y.J.; Guo, T.C. Exogenous salicylic acid enhances wheat drought tolerance by influence on the expression of genes related to ascorbate-glutathione cycle. Biol. Plant. 2013, 57, 718–724. [Google Scholar] [CrossRef]
- Razmi, N.; Ebadi, A.; Daneshian, J.; Jahanbakhsh, S. Salicylic acid induced changes on antioxidant capacity, pigments and grain yield of soybean genotypes in water deficit condition. J. Plant Interact. 2017, 12, 457–464. [Google Scholar] [CrossRef]
- Rezaei, M.A.; Kaviani, B.; Jahanshahi, H. Application of exogenous glycine betaine on some growth traits of soybean (Glycine max L.) cv. DPX in drought stress conditions. Sci. Res. Essays 2012, 7, 432–436. [Google Scholar]
- Agboma, P.C.; Sinclair, T.R.; Jokinen, K.; Peltonen-Sainio, P.; Pehu, E. An evaluation of the effect of exogenous glycine betaine on the growth and yield of soybean: Timing of application, watering regimes and cultivars. Field Crops Res. 1997, 54, 51–64. [Google Scholar] [CrossRef]
- Makela, P.; Mantila, J.; Hinkkanen, R.; Pehu, E.; Peltonen-Sainio, P. Effects of foliar applications of glycine betaine on stress tolerance, growth, and yield of spring cereals and summer turnip rape in Finland. J. Agron. Crop Sci. 1996, 176, 223–234. [Google Scholar] [CrossRef]
- Iqbal, N.; Ashraf, M.Y.; Ashraf, M. Influence of water stress and exogenous glycine betaine on sunflower achene weight and oil percentage. Int. J. Environ. Sci. Technol. 2005, 2, 155–160. [Google Scholar] [CrossRef]
- Meek, C.; Oosterhuis, D.; Gorham, J. Does foliar applied glycine betaine affect endogenous betaine levels and yield in cotton? Crop Manag. 2003, 2, 1–10. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Dalal, A.; Bourstein, R.; Haish, N.; Shenhar, I.; Wallach, R.; Moshelion, M. Dynamic physiological phenotyping of drought-stressed pepper plants treated with “productivity-enhancing” and “survivability-enhancing” biostimulants. Front. Plant Sci. 2019, 10, 905. [Google Scholar] [CrossRef]
- Xu, C.; Leskovar, D.I. Effects of Ascophyllum nodosum seaweed extracts on spinach growth, physiology, and nutritional value under drought stress. Sci. Hortic. 2015, 183, 39–47. [Google Scholar] [CrossRef]
- Mannan, M.A.; Yasmin, A.; Sarker, U.; Bari, N.; Dola, D.B.; Higuchi, H.; Ercisli, S.; Ali, D.; Alarifi, S. Biostimulant red seaweed (Gracilaria tenuistipitata var. liui) extract spray improves yield and drought tolerance in soybean. PeerJ 2023, 11, e15588. [Google Scholar] [CrossRef]
- Youssef, M.K.; Varsha, S.; Kirshenbaum, G.S.; Atsak, P.; Lass, T.J.; Lieberman, S.R.; Leonardo, E.D.; Dranovsky, A. Ablation of proliferating neural stem cells during early life is sufficient to reduce adult hippocampal neurogenesis. Hippocampus 2018, 28, 586–601. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P.; Xu, L.; Geelen, D. Agricultural functions and action mechanisms of plant biostimulants (PBs): An introduction. In The Chemical Biology of Plant Biostimulants; Wiley: Hoboken, NJ, USA, 2020; pp. 1–30. [Google Scholar]
- Basal, O.; Szabó, A. The combined effect of drought stress and nitrogen fertilization on soybean. Agronomy 2020, 10, 384. [Google Scholar] [CrossRef]
- Fageria, N.; Baligar, V. Enhancing nitrogen use efficiency in crop plants. Adv. Agron. 2005, 88, 97–185. [Google Scholar]
- Salvagiotti, F.; Cassman, K.G.; Specht, J.E.; Walter, D.T.; Weiss, A.; Dobermann, A. Nitrogen uptake, fixation and response to fertilizer N in soybeans: A review. Field Crops Res. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Ray, J.D.; Fritschi, F.B.; Heatherly, L.G. Large applications of fertilizer N at planting affects seed protein and oil concentration and yield in the Early Soybean Production System. Field Crops Res. 2006, 99, 67–74. [Google Scholar] [CrossRef]
- Sedghi, M.; Hadi, M.; Toluie, S.G. Effect of nano zinc oxide on the germination parameters of soybean seeds under drought stress. Ann. West Univ. Timişoara Ser. Biol. 2013, 2, 73–78. [Google Scholar]
- Cakmak, I. Enrichment of cereal grains with zinc: Agronomic or genetic biofortification. Plant Soil 2008, 302, 1–17. [Google Scholar] [CrossRef]
- Paneque, M.; De la Rosa, J.M.; Franco-Navarro, J.D.; Colmenero-Flores, J.M.; Knicker, H. Effect of biochar amendment on morphology, productivity and water relations of sunflower plants under non-irrigation conditions. Catena 2016, 147, 280–287. [Google Scholar] [CrossRef]
- Romdhane, L.; Awad, Y.M.; Radhouane, L.; Dal Cortivo, C.; Barion, G.; Panozzo, A.; Vamerali, T. Wood biochar produces different rates of root growth and transpiration in two maize hybrids (Zea mays L.) under drought stress. Arch. Agron. Soil Sci. 2019, 65, 846–866. [Google Scholar] [CrossRef]
- Omondi, M.O.; Xia, X.; Nahayo, A.; Liu, X.; Korai, P.K.; Pan, G. Quantification of biochar effects on soil hydrological properties using meta-analysis of literature data. Geoderma 2016, 274, 28–34. [Google Scholar] [CrossRef]
- Mannan, M.A.; Mia, S.; Halder, E.; Dijkstra, F.A. Biochar application rate does not improve plant water availability in soybean under drought stress. Agric. Water Manag. 2021, 253, 106940. [Google Scholar] [CrossRef]
- Verheijen, F.; Jeffery, S.; Bastos, A.C.; van der Velde, M.; Diafas, I. Biochar Application to Soils—A Critical Scientific Review of Effects on Soil Properties, PROCESSES, and Functions; EUR 24099 EN.; Office for Official Publications of the European Communities: Luxembourg, 2010; pp. 1–166. [Google Scholar]
- Abel, S.; Peters, A.; Trinks, S.; Schonsky, H.; Facklam, M.; Wessolek, G. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma 2013, 202–203, 183–191. [Google Scholar] [CrossRef]
- Tanure, M.M.C.; da Costa, L.M.; Huiz, H.A.; Fernandes, R.B.A.; Cecon, P.R.; Pereira Junior, J.D.; da Luz, J.M.R. Soil water retention, physiological characteristics, and growth of maize plants in response to biochar application to soil. Soil Tillage Res. 2019, 192, 164–173. [Google Scholar] [CrossRef]
- Ahmad, R.; Hussain, S.; Anjum, M.A.; Khalid, M.F.; Saqib, M.; Zakir, I.; Hassan, A.; Fahad, S.; Ahmad, S. Oxidative stress and antioxidant defense mechanisms in plants under salt stress. In Plant Abiotic Stress Tolerance; Hasanuzzaman, M., Fotopoulos, V., Nahar, K., Fujita, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 191–205. [Google Scholar]
- Jamil, A.; Riaz, S.; Ashraf, M.; Foolad, M.R. Gene expression profiling of plants under salt stress. Crit. Rev. Plant Sci. 2011, 30, 435–458. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- FAO. FAO Statistical Yearbook 2021—World Food and Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- Shah, F.; Wu, W. Soil and crop management strategies to ensure higher crop productivity within sustainable environments. Sustainability 2019, 11, 1485. [Google Scholar] [CrossRef]
- Kromdijk, J.; Long, S.P. One crop breeding cycle from starvation? How engineering crop photosynthesis for rising CO2 and temperature could be one important route to alleviation. Proc. R. Soc. B Biol. Sci. 2016, 283, 20152578. [Google Scholar]
- Shabala, S.; Munns, R. Salinity stress: Physiological constraints and adaptive mechanisms. In Plant Stress Physiology; Shabala, S., Ed.; CABI: Wallingford, UK, 2012; pp. 59–93. [Google Scholar]
- Mariani, L.; Ferrante, A. Agronomic management for enhancing plant tolerance to abiotic stresses—Drought, salinity, hypoxia, and lodging. Horticulturae 2017, 3, 52. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as markers. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant salt stress: Adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Rahnama, A.; James, R.A.; Poustini, K.; Munns, R. Stomatal conductance as a screen for osmotic stress tolerance in durum wheat growing in saline soil. Funct. Plant Biol. 2010, 37, 255–263. [Google Scholar] [CrossRef]
- Xiong, J.; Yang, X.; Sun, M.; Zhang, J.; Ding, L.; Sun, Z.; Feng, N.; Zheng, D.; Zhao, L.; Shen, X. Mitigation effect of exogenous nano-silicon on salt stress damage of rice seedlings. Int. J. Mol. Sci. 2025, 26, 85. [Google Scholar] [CrossRef] [PubMed]
- Alhagdow, M.M.; Qareerah, F.A. Evaluation of salt tolerance of pepper plant (Capsicum annuum L., cv. Jalapino) cultivated in greenhouse. Libyan J. Agric. 2021, 26, 55–65. [Google Scholar]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Plant response to salt stress and role of exogenous protectants to mitigate salt-induced damages. In Ecophysiology and Responses of Plants Under Salt Stress; Ahmad, P., Azooz, M., Prasad, M., Eds.; Springer: New York, NY, USA, 2013; pp. 25–87. [Google Scholar]
- Kaya, C.; Higgs, D.; Sakar, E. Response of two leafy vegetables grown at high salinity to supplementary potassium and phosphorus during different growth stages. J. Plant Nutr. 2002, 25, 2663–2676. [Google Scholar] [CrossRef]
- Taïbi, K.; Taïbi, F.; Ait Abderrahim, L.; Ennajah, A.; Belkhodja, M.; Mulet, J.M. Effect of salt stress on growth, chlorophyll content, lipid peroxidation and antioxidant defence systems in Phaseolus vulgaris L. S. Afr. J. Bot. 2016, 105, 306–312. [Google Scholar] [CrossRef]
- Yarsi, G.; Sivaci, A.; Dasgan, H.Y.; Altuntas, O.; Binzet, R.; Akhoundnejad, Y. Effects of salinity stress on chlorophyll and carotenoid contents and stomata size of grafted and ungrafted galia C8 melon cultivar. Pak. J. Bot. 2017, 49, 421–426. [Google Scholar]
- Campobenedetto, C.; Mannino, G.; Beekwilder, J.; Contartese, V.; Karlova, R.; Bertea, C.M. The application of a biostimulant based on tannins affects root architecture and improves tolerance to salinity in tomato plants. Sci. Rep. 2021, 11, 354. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, H.; Wang, B.; Wu, X.; Lan, R.; Huang, X.; Chen, B.; Chen, G.; Jiang, C.; Wang, J.; et al. Exogenous melatonin improves the growth of rice seedlings by regulating redox balance and ion homeostasis under salt stress. J. Plant Growth Regul. 2022, 41, 2108–2121. [Google Scholar] [CrossRef]
- Yan, F.; Wei, H.; Ding, Y.; Li, W.; Liu, Z.; Chen, L.; Tang, S.; Ding, C.; Jiang, Y.; Li, G. Melatonin regulates antioxidant strategy in response to continuous salt stress in rice seedlings. Plant Physiol. Biochem. 2021, 165, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shabala, S.; Zhang, J.; Ma, G.; Chen, D.; Shabala, L.; Zeng, F.; Chen, Z.-H.; Zhou, M.; Venkataraman, G.; et al. Melatonin improves rice salinity stress tolerance by NADPH oxidase-dependent control of the plasma membrane K+ transporters and K+ homeostasis. Plant Cell Environ. 2020, 43, 2591–2605. [Google Scholar] [CrossRef]
- Jiang, C.; Cui, Q.; Feng, K.; Xu, D.; Li, C.; Zheng, Q. Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol. Plant. 2016, 38, 82. [Google Scholar] [CrossRef]
- Li, X.; Yu, B.; Cui, Y.; Yin, Y. Melatonin application confers enhanced salt tolerance by regulating Na+ and Cl− accumulation in rice. Plant Growth Regul. 2017, 83, 441–454. [Google Scholar] [CrossRef]
- Jin, D.; Joseph, B. Physiological mechanism of salicylic acid for alleviation of salt stress in rice. Rice Sci. 2017, 24, 97–108. [Google Scholar] [CrossRef]
- Khan, M.S.; Akther, T.; Mubarak Ali, D.; Hemalatha, S. An investigation on the role of salicylic acid to alleviate saline stress in rice crop (Oryza sativa L.). Biocatal. Agric. Biotechnol. 2019, 18, 101027. [Google Scholar] [CrossRef]
- Yildirim, E.; Turan, M.; Guvenc, I. Effect of foliar salicylic acid applications on growth, chlorophyll, and mineral content of cucumber grown under salt stress. J. Plant Nutr. 2008, 31, 593–612. [Google Scholar] [CrossRef]
- Pai, R.; Sharma, P.K. Exogenous application of salicylic acid mitigates salt stress in rice seedlings by regulating plant water status and preventing oxidative damage. Environ. Exp. Biol. 2022, 20, 193–204. [Google Scholar] [CrossRef]
- El-Mergawi, R.A.; Abd El-Wahed, M.S. Effect of exogenous salicylic acid or indole acetic acid on their endogenous levels, germination, and growth in maize. Bull. Natl. Res. Cent. 2020, 44, 167. [Google Scholar] [CrossRef]
- Jesmin, A.; Hoang Anh, L.; Nguyen, P.M.; Tran, D.K.; Tran, D.X. Fulvic acid improves salinity tolerance of rice seedlings: Evidence from phenotypic performance, relevant phenolic acids, and momilactones. Plants 2023, 12, 2359. [Google Scholar] [CrossRef]
- Anjum, S.A.; Wang, L.; Farooq, M.; Xue, L.; Ali, S. Fulvic acid application improves the maize performance under well-watered and drought conditions. J. Agron. Crop Sci. 2011, 197, 409–417. [Google Scholar] [CrossRef]
- Sun, J.; Qiu, C.; Ding, Y.; Wang, Y.; Sun, L.; Fan, K.; Gai, Z.; Dong, G.; Wang, J.; Li, X.; et al. Fulvic acid ameliorates drought stress-induced damage in tea plants by regulating the ascorbate metabolism and flavonoids biosynthesis. BMC Genom. 2020, 21, 411. [Google Scholar] [CrossRef] [PubMed]
- Hatami, E.; Shokouhian, A.A.; Ghanbari, A.R.; Naseri, L.A. Alleviating salt stress in almond rootstocks using humic acid. Sci. Hortic. 2018, 237, 296–302. [Google Scholar] [CrossRef]
- Bayat, H.; Shafie, F.; Aminifard, M.H.; Daghighi, S. Comparative effects of humic and fulvic acids as biostimulants on growth, antioxidant activity and nutrient content of yarrow (Achillea millefolium L.). Sci. Hortic. 2021, 279, 109912. [Google Scholar] [CrossRef]
- Gabr, S.; Abouelsaad, I.; Brengi, S.; Gouda, A. Salt stress mitigation of spinach plants as affected by silicon and fulvic acid. J. Adv. Agric. Res. 2022, 27, 26–42. [Google Scholar] [CrossRef]
- Diaz-Zorita, M.; Fernández-Canigia, M.V.; Grosso, G.A. Applications of foliar fertilizers containing glycinebetaine improve wheat yields. J. Agron. Crop Sci. 2001, 186, 209–215. [Google Scholar] [CrossRef]
- Rajasekaran, L.R.; Kriedemann, P.E.; Aspinall, D.; Paleg, L.G. Physiological significance of proline and glycinebetaine: Maintaining photosynthesis during NaCl stress in wheat. Photosynthetica 1997, 34, 357–366. [Google Scholar] [CrossRef]
- Demiral, T.; Turkan, I. Does exogenous glycine betaine affect antioxidative system of rice seedlings under NaCl treatment? J. Plant Physiol. 2004, 161, 1089–1100. [Google Scholar] [CrossRef]
- Khunpon, B. Effects of Paclobutrazol on Growth and Antioxidant Defense System of Rice Seedlings Under Salt Stress. Ph.D. Thesis, Chiang Mai University, Chiang Mai, Thailand, 2017. [Google Scholar]
- Sankar, B.; Jaleel, C.A.; Manivannan, P.; Kishorekumar, A.; Somasundaram, R.; Panneerselvam, R. Effect of paclobutrazol on water stress amelioration through antioxidants and free radical scavenging enzymes in Arachis hypogaea L. Colloids Surf. B Biointerfaces 2007, 60, 229–235. [Google Scholar] [CrossRef]
- Sharma, D.; Dubey, A.K.; Srivastav, M.; Singh, A.K.; Sairam, R.K.; Pandey, R.N.; Dahuja, A.; Kaur, C. Effect of putrescine and paclobutrazol on growth, physiochemical parameters, and nutrient acquisition of salt-sensitive citrus rootstock Karna khatta (Citrus karna Raf.) under NaCl stress. J. Plant Growth Regul. 2011, 30, 301–311. [Google Scholar] [CrossRef]
- Manivannan, P.; Jaleel, C.A.; Kishorekumar, A.; Sankar, B.; Somasundaram, R.; Panneerselvam, R. Protection of Vigna unguiculata (L.) Walp plants from salt stress by paclobutrazol. Colloids Surf. B Biointerfaces 2008, 61, 315–318. [Google Scholar] [CrossRef]
- Almeida, D.M.; Oliveira, M.M.; Saibo, N.J. Regulation of Na+ and K+ homeostasis in plants: Towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 2017, 40, 326–345. [Google Scholar] [CrossRef]
- Pavlovic, J.; Kostic, L.; Bosnic, P.; Kirkby, E.A.; Nikolic, M. Interactions of silicon with essential and beneficial elements in plants. Front. Plant Sci. 2021, 12, 6975–6992. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, H.; Hu, X.; Xu, L.; An, X.; Jin, T.; Ma, R.; Li, Z.; Chen, S.; Du, S.; et al. Comparing the potential of silicon nanoparticles and conventional silicon for salinity stress alleviation in soybean (Glycine max L.): Growth and physiological traits and rhizosphere/endophytic bacterial communities. J. Agric. Food Chem. 2024, 72, 10781–10793. [Google Scholar] [CrossRef]
- Shoukat, A.; Pitann, B.; Hossain, S.; Saqib, Z.A.; Nawaz, A.; Mühling, K.H. Zinc and silicon fertilizers in conventional and nano-forms: Mitigating salinity effects in maize (Zea mays L.). J. Plant Nutr. Soil Sci. 2024, 3, 232–239. [Google Scholar] [CrossRef]
- Taha, R.S.; Seleiman, M.F.; Shami, A.; Alhammad, B.A.; Mahdi, A.H.A. Integrated application of selenium and silicon enhances growth and anatomical structure, antioxidant defense system and yield of wheat grown in salt-stressed soil. Plants 2021, 10, 104. [Google Scholar] [CrossRef]
- Das, P.; Manna, I.; Sil, P.; Bandyopadhyay, M.; Biswas, A.K. Silicon augments salt tolerance through modulation of polyamine and GABA metabolism in two indica rice (Oryza sativa L.) cultivars. Plant Physiol. Biochem. 2021, 166, 41–52. [Google Scholar] [CrossRef]
- Weisany, W.; Razmi, J.; Eshaghabadi, A.H.; Pashang, D. Silicon nanoparticles (SiNP): A novel and sustainable strategy for mitigating environmental stresses in plants. J. Soil Sci. Plant Nutr. 2024, 24, 2167–2191. [Google Scholar] [CrossRef]
- Bouis, H.E.; Saltzman, A. Improving nutrition through biofortification: A review of evidence from HarvestPlus, 2003 through 2016. Glob. Food Sec. 2017, 12, 49–58. [Google Scholar] [CrossRef]
- Cakmak, I.; Kutman, U.B. Agronomic biofortification of cereals with zinc: A review. Eur. J. Soil Sci. 2018, 69, 172–180. [Google Scholar] [CrossRef]
- Jan, M.; Anwar-ul-Haq, M.; Shah, A.N.; Yousaf, M.; Iqbal, J.; Li, X.; Depeng, W.; Fahad, S. Modulation in growth, gas exchange, and antioxidant activities of salt-stressed rice (Oryza sativa L.) genotypes by zinc fertilization. Arab. J. Geosci. 2019, 12, 775. [Google Scholar] [CrossRef]
- Shahzad, R.; Harlinac, P.W.; Gallego, P.P.; Flexas, J.; Ewas, M.; Leiweng, X.; Karuniawan, A. The seaweed Ascophyllum nodosum-based biostimulant enhances salt stress tolerance in rice (Oryza sativa L.) by remodeling physiological, biochemical, and metabolic responses. J. Plant Interact. 2023, 18, 2266514. [Google Scholar] [CrossRef]
- Jithesh, M.N.; Shukla, P.S.; Kant, P.; Joshi, J.; Critchley, A.T.; Prithiviraj, B. Physiological and transcriptomics analyses reveal that Ascophyllum nodosum extracts induce salinity tolerance in Arabidopsis by regulating the expression of stress responsive genes. J. Plant Growth Regul. 2019, 38, 463–478. [Google Scholar] [CrossRef]
- Goñi, O.; Fort, A.; Quille, P.; McKeown, P.C.; Spillane, C.; O’Connell, S. Comparative transcriptome analysis of two Ascophyllum nodosum extract biostimulants: Same seaweed but different. J. Agric. Food Chem. 2016, 64, 2980–2989. [Google Scholar] [CrossRef]
- Salvi, L.; Brunetti, C.; Cataldo, E.; Niccolai, A.; Centritto, M.; Ferrini, F.; Mattii, G.B. Effects of Ascophyllum nodosum extract on Vitis vinifera: Consequences on plant physiology, grape quality and secondary metabolism. Plant Physiol. Biochem. 2019, 139, 21–32. [Google Scholar] [CrossRef]
- Shukla, P.S.; Prithiviraj, B. Ascophyllum nodosum biostimulant improves the growth of Zea mays grown under phosphorus-impoverished conditions. Front. Plant Sci. 2021, 11, 601843. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.; Kandasamy, S.; Zhang, J.; Ji, X.; Kirby, C.; Benkel, B.; Hodges, M.D.; Critchley, A.T.; Hiltz, D.; Prithiviraj, B. Transcriptional and metabolomic analysis of Ascophyllum nodosum-mediated freezing tolerance in Arabidopsis thaliana. BMC Genom. 2012, 13, 643. [Google Scholar] [CrossRef]
- El-Lethy, S.R.; Talaat, I.M.; Tarraf, S.A.; Abdel-Baky, Y.R. Effects of exogenous salicylic acid in soybean plants subjected to salt stress. Middle East J. Appl. Sci. 2017, 7, 956–966. [Google Scholar]
- Jaiswal, A.; Pandurangam, V.; Sharma, S.K. Effect of salicylic acid in soybean (Glycine max L. Meril) under salinity stress. Bioscan 2014, 9, 671–676. [Google Scholar]
- Noreen, S.; Ashraf, M. Alleviation of adverse effects of salt stress on sunflower (Helianthus annuus L.) by exogenous application of salicylic acid: Growth and photosynthesis. Pak. J. Bot. 2008, 40, 1657–1663. [Google Scholar]
- Sharifi, M.; Ghorbanli, M.; Ebrahimzadeh, E. Improved growth of salinity stressed soybean after inoculation with salt pre-treated mycorrhizal fungi. J. Plant Physiol. 2007, 164, 1144–1151. [Google Scholar] [CrossRef]
- Simaei, M.; Khavari-Nejad, R.A.; Saadatmand, S.; Bernard, F.; Fahimi, F. Interactive effects of salicylic acid and nitric oxide on soybean plants under NaCl salinity. Russ. J. Plant Physiol. 2011, 58, 783–790. [Google Scholar] [CrossRef]
- Simaei, M.; Khavari-Nejad, R.A.; Bernard, F. Exogenous application of salicylic acid and nitric oxide on the ionic contents and enzymatic activities in NaCl-stressed soybean plants. Am. J. Plant Sci. 2012, 3, 1495–1503. [Google Scholar] [CrossRef]
- Ardebili, N.O.; Iranbakhsh, A.; Oraghi Ardebili, Z. Efficiency of selenium and salicylic acid protection against salinity in soybean. Iran. J. Plant Physiol. 2019, 9, 2727–2738. [Google Scholar]
- Ardebili, Z.O.; Ardebili, N.O.; Jalili, S.; Safiallah, S. The modified qualities of basil plants by selenium and/or ascorbic acid. Turk. J. Bot. 2015, 39, 401–407. [Google Scholar] [CrossRef]
- Golob, A.; Kavčič, J.; Stibilj, V.; Gaberščik, A.; Vogel-Mikuš, K.; Germ, M. The effect of selenium and UV radiation on leaf traits and biomass production in Triticum aestivum L. Ecotoxicol. Environ. Saf. 2017, 136, 142–149. [Google Scholar] [CrossRef]
- Sun, H.; Dai, H.; Wang, X.; Wan, G. Physiological and proteomic analysis of selenium-mediated tolerance to Cd stress in cucumber (Cucumis sativus L.). Ecotoxicol. Environ. Saf. 2016, 133, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Farhangi-Abriz, S.; Ghassemi-Golezani, K. Improving amino acid composition of soybean under salt stress by salicylic acid and jasmonic acid. J. Appl. Bot. Food Qual. 2016, 89, 243–248. [Google Scholar]
- Pedranzani, H.; Sierra-de-Grado, R.; Vigliocco, A.; Miersch, O.; Abdala, G. Cold and water stresses produce changes in endogenous jasmonates in two populations of Pinus pinaster Ait. Plant Growth Regul. 2007, 52, 111–116. [Google Scholar] [CrossRef]
- Kang, D.J.; Seo, Y.J.; Lee, J.D.; Ishii, R.; Kim, K.U.; Shin, D.H.; Lee, I.J. Jasmonic acid differentially affects growth, ion uptake and abscisic acid concentration in salt-tolerant and salt-sensitive rice cultivars. J. Agron. Crop Sci. 2005, 191, 273–282. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Hamayun, M.; Lee, S.K.; Lee, I.J. Methyl jasmonate alleviated salinity stress in soybean. J. Crop Sci. Biotechnol. 2009, 12, 63–68. [Google Scholar] [CrossRef]
- Asadi, M.; Heidari, M.A.; Kazemi, M.A.; Filinejad, R. Salicylic acid induced changes in some physiological parameters in chickpea (Cicer arietinum L.) under salt stress. J. Agric. Technol. 2013, 9, 311–316. [Google Scholar]
- Horvath, E.; Szalai, G.; Janda, T. Induction of abiotic stress tolerance by salicylic acid signaling. J. Plant Growth Regul. 2007, 26, 290–300. [Google Scholar] [CrossRef]
- Ali, A.Y.A.; Ibrahim, M.E.H.; Zhou, G.; Zhu, G.; Elsiddig, A.M.I.; Suliman, M.S.E.; Elradi, S.B.M.; Salah, E.G.I. Interactive impacts of soil salinity and jasmonic acid and humic acid on growth parameters, forage yield and photosynthesis parameters of sorghum plants. S. Afr. J. Bot. 2022, 146, 293–303. [Google Scholar] [CrossRef]
- da Silva, B.A.; da Silva, J.S.; da Silva, T.I.; da Costa, R.S.; de Castro, C.S.; de Oliveira, L.K.B.; de Sousa, T.R.M.; Rodrigues, C.Y.A.C.; Cardoso, F.B.; Mesquita, R.O. Bioestimulant with Ascophyllum nodosum and fulvic acids as mitigating factors of salinity damage in soybean. Rev. Bras. Eng. Agríc. Ambient. 2024, 28, e278961. [Google Scholar] [CrossRef]
- Martynenko, A.; Shotton, K.; Astatkie, T.; Petrash, G.; Fowler, C.; Neily, W.; Critchley, A.T. Thermal imaging of soybean response to drought stress: The effect of Ascophyllum nodosum seaweed extract. SpringerPlus 2016, 5, 1393. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.S.; Shotton, K.; Norman, E.; Neily, W.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-based biostimulants: Sustainable applications in agriculture for the stimulation of plant growth, stress tolerance, and disease management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [PubMed]
- Yıldıztekin, M.; Tuna, A.L.; Kaya, C. Physiological Effects of the Brown Seaweed (Ascophyllum nodosum) and Humic Substances on Plant Growth and Enzyme Activity of Certain Pepper Plants Grown under Salt Stress. Acta Biol. Hung. 2018, 69, 325–335. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Schwinghamer, T.D.; Smith, D.L. Rhizobacteria from root nodules of an indigenous legume enhance salinity stress tolerance in soybean. Front. Sustain. Food Syst. 2021, 4, 617978. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Jabborova, D.; Rasanen, L.A.; Liao, H. Coordination between Bradyrhizobium and Pseudomonas alleviates salt stress in soybean through altering root system architecture. J. Plant Interact. 2017, 12, 100–107. [Google Scholar] [CrossRef]
- Adhikari, B.; Dhungana, S.K.; Kim, I.-D.; Shin, D.-H. Effect of foliar application of potassium fertilizers on soybean plants under salinity stress. J. Saudi Soc. Agric. Sci. 2020, 19, 261–269. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, J.K. An Arabidopsis mutant that requires increased calcium for potassium nutrition and salt tolerance. Proc. Natl. Acad. Sci. USA 1997, 94, 14960–14964. [Google Scholar] [CrossRef]
- Hu, Y.; Burucs, Z.; Schmidhalter, U. Effect of foliar fertilization application on the growth and mineral nutrient content of maize seedlings under drought and salinity. Soil Sci. Plant Nutr. 2008, 54, 133–141. [Google Scholar] [CrossRef]
- Singh, J.; Singh, M.; Jain, A.; Bhardwaj, S.; Singh, A.; Singh, D.; Bhushan, B.; Dubey, S. An introduction of plant nutrients and foliar fertilization: A review. In Precision Farming: A New Approach; Daya Publishing Co.: New Delhi, India, 2013; pp. 252–320. [Google Scholar]
- Das, A.K.; Anik, T.R.; Rahman, M.M.; Keya, S.S.; Islam, M.R.; Rahman, M.A.; Sultana, S.; Ghosh, P.K.; Khan, S.; Ahamed, T.; et al. Ethanol treatment enhances physiological and biochemical responses to mitigate saline toxicity in soybean. Plants 2022, 11, 272. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Rahman, M.A.; Miah, M.G.; Saha, S.R.; Karim, M.A.; Mostofa, M.G. Mechanistic insight into salt tolerance of Acacia auriculiformis: The importance of ion selectivity, osmoprotection, tissue tolerance, and Na+ exclusion. Front. Plant Sci. 2017, 8, 155. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Manivannan, A.; Ko, C.H.; Muneer, S.; Jeong, B.R. Leaf physiological and proteomic analysis to elucidate silicon-induced adaptive response under salt stress in Rosa hybrida ‘Rock Fire’. Int. J. Mol. Sci. 2017, 18, 1768. [Google Scholar] [CrossRef]
- Tekam, L.M.; Dany, P.M.; Victor, D.T.; Hartmut, S. Changes in plant growth, leaf relative water content and physiological traits in response to salt stress in peanut (Arachis hypogaea L.) varieties. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12049. [Google Scholar] [CrossRef]
- Assaha, D.V.M.; Ueda, A.; Saneoka, H.; Al-Yahyai, R.; Yaish, M.W. The role of Na+ and K+ transporters in salt stress adaptation in glycophytes. Front. Physiol. 2017, 8, 509. [Google Scholar] [CrossRef]
- Benito, B.; Haro, R.; Amtmann, A.; Cuin, T.A.; Dreyer, I. The twins K+ and Na+ in plants. J. Plant Physiol. 2014, 171, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Parida, A.K.; Veerabathini, S.K.; Kumari, A.; Agarwal, P.K. Physiological, anatomical and metabolic implications of salt tolerance in the halophyte Salvadora persica under hydroponic culture condition. Front. Plant Sci. 2016, 7, 351. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a regulatory hub of plant hormone levels and action in stress situations. Plant Biol. 2021, 23, 7–19. [Google Scholar] [CrossRef]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Tian, X.; He, X.; Yang, J.; Yang, Z.; Fang, W. Exogenous melatonin stimulated isoflavone biosynthesis in NaCl-stressed germinating soybean (Glycine max L.). Plant Physiol. Biochem. 2022, 185, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Mukherjee, S.; Baluška, F.; Bhatla, S.C. Regulatory roles of serotonin and melatonin in abiotic stress tolerance in plants. Plant Signal. Behav. 2015, 10, e1049788. [Google Scholar] [CrossRef]
- Oliveira, P.H.A.; de Sá, S.A.; Ribeiro, J.E.S.; da Silva, J.P.P.; de Lima, F.F.; Silva, I.B.M.; da Silveira, L.M.; Barros Júnior, A.P. Exogenous application of melatonin mitigates salt stress in soybean. Rev. Caatinga 2025, 38, e12698. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L.A.C.J. Flooding stress: Acclimations and genetic diversity. Annu. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef]
- Jackson, M.; Colmer, T. Response and adaptation by plants to flooding stress. Ann. Bot. 2005, 96, 501–505. [Google Scholar] [CrossRef]
- Setter, T.; Waters, I. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 2003, 253, 1–34. [Google Scholar] [CrossRef]
- Sun, Q.; Gu, X.; Sun, L.; Yang, G.; Zhou, L.; Guo, W. Dynamic change in rice leaf area index and spectral response under flooding stress. Paddy Water Environ. 2020, 18, 223–233. [Google Scholar] [CrossRef]
- Zhen, B.; Guo, X.; Zhou, X.; Lu, H.; Wang, Z. Effect of the Alternating Stresses of Drought and Waterlogging on the Growth, Chlorophyll Content, and Yield of Rice (Oryza sativa L.). J. Irrig. Drain. Eng. 2019, 145, 04019004. [Google Scholar] [CrossRef]
- Mustafa, G.; Komatsu, S. Quantitative proteomics reveals the effect of protein glycosylation in soybean root under flooding stress. Front. Plant Sci. 2014, 5, 627. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, M.K.; Wang, F.; Chen, J.; Li, X.; Li, Q.; Lin, C.; Lin, X. Effects of open drainage ditch design on bacterial and fungal communities of cold waterlogged paddy soils. Braz. J. Microbiol. 2013, 44, 983–991. [Google Scholar] [CrossRef]
- Xie, K.; Xu, P.; Yang, S.; Lu, Y.; Jiang, R.; Gu, W.; Li, W.; Sun, L. Effects of supplementary composts on microbial communities and rice productivity in cold water paddy fields. J. Microbiol. Biotechnol. 2015, 25, 569–578. [Google Scholar] [CrossRef] [PubMed]
- De San Celedonio, R.P.; Abeledo, L.G.; Miralles, D.J. Identifying the critical period for waterlogging on yield and its components in wheat and barley. Plant Soil 2014, 378, 265–277. [Google Scholar] [CrossRef]
- Herzog, M.; Striker, G.G.; Colmer, T.D.; Pedersen, O. Mechanisms of waterlogging tolerance in wheat—A review of root and shoot physiology. Plant Cell Environ. 2016, 39, 1068–1086. [Google Scholar] [CrossRef] [PubMed]
- Pampana, S.; Masoni, A.; Arduini, I. Grain yield of durum wheat as affected by waterlogging at tillering. Cereal Res. Commun. 2016, 44, 706–716. [Google Scholar] [CrossRef]
- Feyen, L.; Dankers, R.; Bódis, K. Fluvial flood risk in Europe in present and future climates. Clim. Change 2012, 112, 47–62. [Google Scholar] [CrossRef]
- Douglas, I. Climate change, flooding and food security in South Asia. Food Secur. 2009, 1, 127–136. [Google Scholar] [CrossRef]
- Tong, C.; Hill, C.B.; Zhou, G.; Zhang, X.-Q.; Jia, Y.; Li, C. Opportunities for improving waterlogging tolerance in cereal crops—Physiological traits and genetic mechanisms. Plants 2021, 10, 1560. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, G.; Dong, H.; Li, C. Waterlogging stress in cotton: Damage, adaptability, alleviation strategies, and mechanisms. Crop J. 2021, 9, 257–270. [Google Scholar] [CrossRef]
- Dickin, E.; Wright, D. The effects of winter waterlogging and summer drought on the growth and yield of winter wheat (Triticum aestivum L.). Eur. J. Agron. 2008, 28, 234–244. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, D.; Liu, F.; Dai, T.; Jing, Q.; Cao, W. Effects of salt and waterlogging stresses and their combination on leaf photosynthesis, chloroplast ATP synthesis, and antioxidant capacity in wheat. Plant Sci. 2009, 176, 575–582. [Google Scholar] [CrossRef]
- Yu, Q.; Shen, Y.; Wang, Q.; Wang, X.; Fan, L.; Wang, Y.; Zhang, S.; Liu, Z.; Zhang, M. Light deficiency and waterlogging affect chlorophyll metabolism and photosynthesis in Magnolia sinostellata. Trees 2019, 33, 11–22. [Google Scholar] [CrossRef]
- Pan, J.; Sharif, R.; Xu, X.; Chen, X. Mechanisms of waterlogging tolerance in plants: Research progress and prospects. Front. Plant Sci. 2021, 11, 627331. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, J.; Hui, W.; Zhao, F.; Wang, P.; Su, C.; Gong, W. Physiology of plant responses to water stress and related genes: A review. Forests 2022, 13, 324. [Google Scholar] [CrossRef]
- Lal, B.; Gautam, P.; Mohanty, S.; Raja, R.; Tripathi, R.; Shahid, M.; Panda, B.B.; Baig, M.J.; Rath, L.; Bhattacharyya, P.; et al. Combined application of silica and nitrogen alleviates the damage of flooding stress in rice. Crop Pasture Sci. 2015, 66, 679–688. [Google Scholar] [CrossRef]
- Bali, A.S.; Sidhu, G.P.S. Abiotic stress-induced oxidative stress in wheat. In Wheat Production in Changing Environments; Hasanuzzaman, M., Nahar, K., Hossain, M.A., Eds.; Springer: Singapore, 2019; pp. 225–239. [Google Scholar]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Mahmud, J.A.; Nahar, K.; Anne, T.I.; Inafuku, M.; Oku, H.; Fujita, M. Responses, adaptation, and ROS metabolism in plants exposed to waterlogging stress. In Reactive Oxygen Species and Antioxidant Systems in Plants: Role and Regulation Under Abiotic Stress, 1st ed.; Khan, M.I.R., Khan, N.A., Eds.; Springer: Singapore, 2017; pp. 257–281. [Google Scholar]
- Santaniello, A.; Scartazza, A.; Gresta, F.; Loreti, E.; Biasone, A.; Di Tommaso, D.; Piaggesi, A.; Perata, P. Ascophyllum nodosum seaweed extract alleviates drought stress in Arabidopsis by affecting photosynthetic performance and related gene expression. Front. Plant Sci. 2017, 8, 1362. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, H.; Chen, W.; Gao, Y.; Xu, K.; Sun, X.; Huo, L. The role of ethylene in the regulation of plant response mechanisms to waterlogging stress. Plant Cell Rep. 2024, 43, 278. [Google Scholar] [CrossRef]
- Salazar, C.; Hernández, C.; Pino, M.T. Plant water stress: Associations between ethylene and abscisic acid response. Chil. J. Agric. Res. 2015, 75, 71–79. [Google Scholar] [CrossRef]
- Abdel Megeed, T.M.; Elshamey, E.A.; Gharib, H.S.; Hafez, E.M.; El-Sayed, A. Rice grain quality affected by a combined foliar spray of different biostimulated components under different levels of water stress. Appl. Ecol. Environ. Res. 2022, 20, 2095–2112. [Google Scholar] [CrossRef]
- Pan, S.; Rasul, F.; Li, W.; Tian, H.; Mo, Z.; Duan, M.; Tang, X. Roles of plant growth regulators on yield, grain qualities and antioxidant enzyme activities in super hybrid rice (Oryza sativa L.). Rice 2013, 6, 9. [Google Scholar] [CrossRef]
- Kamboj, S.; Mathpal, B. Improving rice grain quality by foliar application of plant growth regulators under various mode of Zn application. Plant Arch. 2019, 19, 2181–2184. [Google Scholar]
- Drake, J.A.; Carrucan, A.; Jackson, W.R.; Cavagnaro, T.R.; Patti, A.F. Biochar application during reforestation alters species present and soil chemistry. Sci. Total Environ. 2015, 514, 359–365. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Biochar–root interactions are mediated by biochar nutrient content and impacts on soil nutrient availability. Eur. J. Soil Sci. 2014, 65, 173–185. [Google Scholar] [CrossRef]
- Sika, M.P.; Hardie, A.G. Effect of pine wood biochar on ammonium nitrate leaching and availability in a South African sandy soil. Eur. J. Soil Sci. 2014, 65, 113–119. [Google Scholar]
- Liu, Y.; Yang, S.; Lu, H.; Wang, Y. Effects of biochar on spatial and temporal changes in soil temperature in cold waterlogged rice paddies. Soil Tillage Res. 2018, 181, 102–109. [Google Scholar] [CrossRef]
- Ventura, F.; Salvatorelli, F.; Piana, S.; Pieri, L.; Pisa, P.R. The effects of biochar on the physical properties of bare soil. Earth Environ. Sci. Trans. R. Soc. 2012, 103, 5–11. [Google Scholar] [CrossRef]
- Cui, Y.-F.; Meng, J.; Wang, Q.-X.; Zhang, W.-M.; Cheng, X.-Y.; Chen, W.-F. Effects of straw and biochar addition on soil nitrogen, carbon, and super rice yield in cold waterlogged paddy soils of North China. J. Integr. Agric. 2017, 16, 1064–1074. [Google Scholar] [CrossRef]
- Han, X.Z.; Zhu, L.Q.; Yang, M.F.; Qi, Y.U.; Bian, X.M. Effects of different amount of wheat straw returning on rice growth, soil microbial biomass and enzyme activity. J. Agro-Environ. Sci. 2012, 31, 2192–2199. [Google Scholar]
- Huo, Z.; Wang, P.; Fu, J.F. Effects of crop residues incorporation and N-fertilizer utilization on the matter production of summer maize. Chin. J. Eco-Agric. 2006, 14, 95–97. [Google Scholar]
- Mustafa, G.; Sakata, K.; Komatsu, S. Proteomic analysis of flooded soybean root exposed to aluminum oxide nanoparticles. J. Proteom. 2015, 128, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Sakata, K.; Nanjo, Y.; Komatsu, S. Analysis of initial changes in the proteins of soybean root tip under flooding stress using gel-free and gel-based proteomic techniques. J. Proteom. 2014, 106, 1–16. [Google Scholar] [CrossRef]
- Salama, H.M.H. Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. Res. J. Biotechnol. 2012, 3, 190–197. [Google Scholar]
- Komatsu, S.; Han, C.; Nanjo, Y.; Altaf-Un-Nahar, M.; Wang, K.; He, D.; Yang, P. Label-free quantitative proteomic analysis of abscisic acid effect in early-stage soybean under flooding. J. Proteome Res. 2013, 12, 4769–4784. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, G.; Komatsu, S. Insights into the response of soybean mitochondrial proteins to various sizes of aluminum oxide nanoparticles under flooding stress. J. Proteome Res. 2016, 15, 4464–4475. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Bowler, C.; Van Montagu, M.; Inzé, D. Superoxide dismutase and stress tolerance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1992, 43, 83–116. [Google Scholar] [CrossRef]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17–MR71. [Google Scholar] [CrossRef] [PubMed]
- Poborilova, Z.; Opatrilova, R.; Babula, P. Toxicity of aluminium oxide nanoparticles demonstrated using a BY-2 plant cell suspension culture model. Environ. Exp. Bot. 2013, 91, 1–11. [Google Scholar] [CrossRef]
- Kim, Y.; Seo, C.-W.; Khan, A.L.; Mun, B.-G.; Shahzad, R.; Ko, J.-W.; Yun, B.-W.; Park, S.-K.; Lee, I.-J. Exo-ethylene application mitigates waterlogging stress in soybean (Glycine max L.). BMC Plant Biol. 2018, 18, 254. [Google Scholar] [CrossRef]
- Steffens, B.; Wang, J.; Sauter, M. Interactions between ethylene, gibberellin and abscisic acid regulate emergence and growth rate of adventitious roots in deepwater rice. Planta 2006, 223, 604–612. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Hwang, S.-J.; Waqas, M.; Khan, A.L.; Lee, J.-H.; Lee, J.-D.; Nguyen, H.T.; Lee, I.-J. Comparative analysis of endogenous hormone levels in two soybean (Glycine max L.) lines differing in waterlogging tolerance. Front. Plant Sci. 2015, 6, 714. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.-K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, F.; Chen, Z.; Yang, B.; Komatsu, S.; Zhou, S. Proteomic analysis reveals the effects of melatonin on soybean root tips under flooding stress. J. Proteom. 2021, 232, 104064. [Google Scholar] [CrossRef]
- Hashiguchi, A.; Sakata, K.; Komatsu, S. Proteome analysis of early-stage soybean seedlings under flooding stress. J. Proteome Res. 2009, 8, 2058–2069. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.N.; Sakata, K.; Hiraga, S.; Komatsu, S. Quantitative proteomics reveals that peroxidases play key roles in post-flooding recovery in soybean roots. J. Proteome Res. 2014, 13, 5812–5828. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin promotes adventitious- and lateral root regeneration in etiolated hypocotyls of Lupinus albus L. J. Pineal Res. 2007, 42, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Sun, Q.; Li, H.; Li, X.; Cao, Y.; Zhang, H.; Li, S.; Zhang, L.; Qi, Y.; Ren, S.; et al. Melatonin Improved Anthocyanin Accumulation by Regulating Gene Expressions and Resulted in High Reactive Oxygen Species Scavenging Capacity in Cabbage. Front. Plant Sci. 2016, 7, 197. [Google Scholar] [CrossRef]
- Kapoor, R.T.; Hasanuzzaman, M. Exogenous kinetin and putrescine synergistically mitigate salt stress in Luffa acutangula by modulating physiology and antioxidant defense. Physiol. Mol. Biol. Plants 2020, 26, 2125–2137. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Mishra, D.S.; Chakraborty, B.; Kumar, P. Pericarp browning and quality management of litchi fruit by antioxidants and salicylic acid during ambient storage. J. Food Sci. Technol. 2013, 50, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Alyemeni, M.N.; Wijaya, L.; Alamri, S.A.; Alam, P.; Ashraf, M.; Ahmad, P. Potential of exogenously sourced kinetin in protecting Solanum lycopersicum from NaCl-induced oxidative stress through up-regulation of the antioxidant system, ascorbate-glutathione cycle and glyoxalase system. PLoS ONE 2018, 13, e0202175. [Google Scholar] [CrossRef] [PubMed]
- Pineda Rodó, A.; Brugière, N.; Vankova, R.; Malbeck, J.; Olson, J.M.; Haines, S.C.; Martin, R.C.; Habben, J.E.; Mok, D.W.; Mok, M.C. Over-expression of a zeatin O-glucosylation gene in maize leads to growth retardation and tassel seed formation. J. Exp. Bot. 2008, 59, 2673–2686. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Ahmed, N.; Saha, T.; Rahman, M.; Rahman, K.; Alam, M.M.; Rohman, M.M.; Nahar, K. Exogenous salicylic acid and kinetin modulate reactive oxygen species metabolism and glyoxalase system to confer waterlogging stress tolerance in soybean (Glycine max L.). Plant Stress 2022, 3, 100057. [Google Scholar] [CrossRef]
- Abdelaal, K.A.; Attia, K.A.; Alamery, S.F.; El-Afry, M.M.; Ghazy, A.I.; Tantawy, D.S.; AlDoss, A.A.; El-Shawy, E.S.M.; Abu-Elsaoud, A.; Hafez, Y.M. Exogenous application of proline and salicylic acid can mitigate the injurious impacts of drought stress on barley plants associated with physiological and histological characters. Sustainability 2020, 12, 1736. [Google Scholar] [CrossRef]
- Tamaoki, D.; Seo, S.; Yamada, S.; Kano, A.; Miyamoto, A.; Shishido, H.; Miyoshi, S.; Taniguchi, S.; Akimitsu, K.; Gomi, K. Jasmonic acid and salicylic acid activate a common defense system in rice. Plant Signal. Behav. 2013, 8, e24260. [Google Scholar] [CrossRef]
- Kamal, A.H.M.; Komatsu, S. Jasmonic acid induced protein response to biophoton emissions and flooding stress in soybean. J. Proteom. 2016, 133, 33–47. [Google Scholar] [CrossRef]
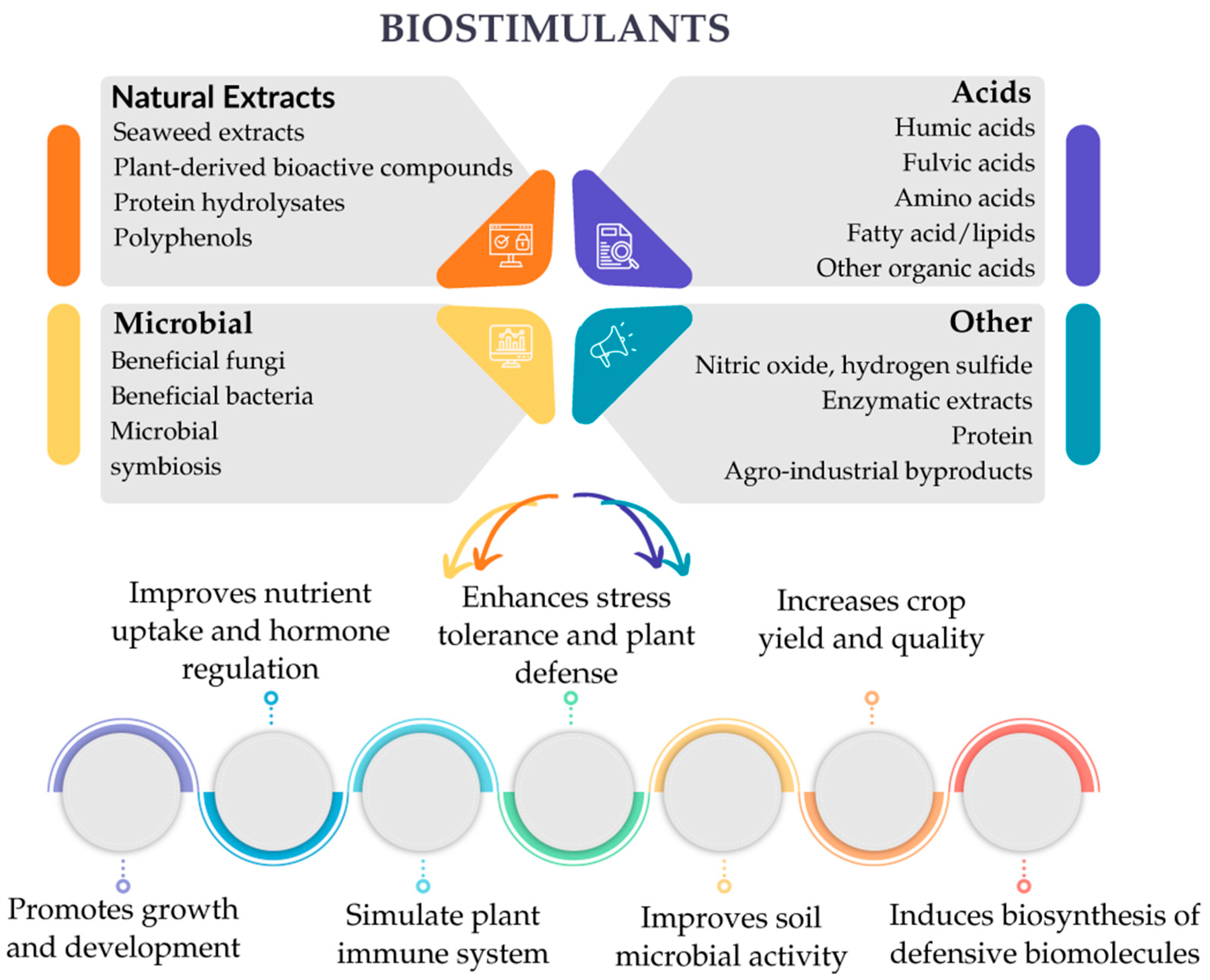
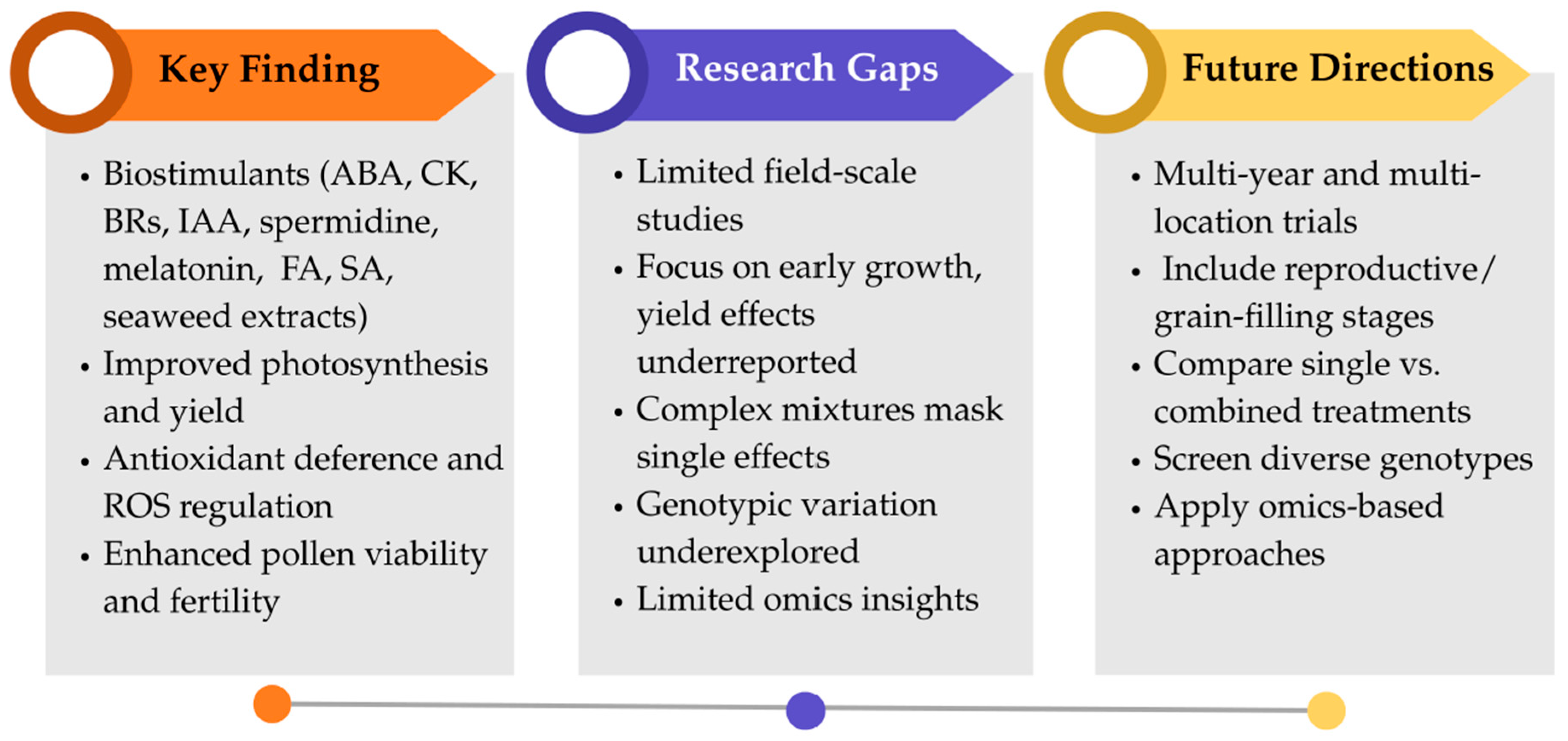
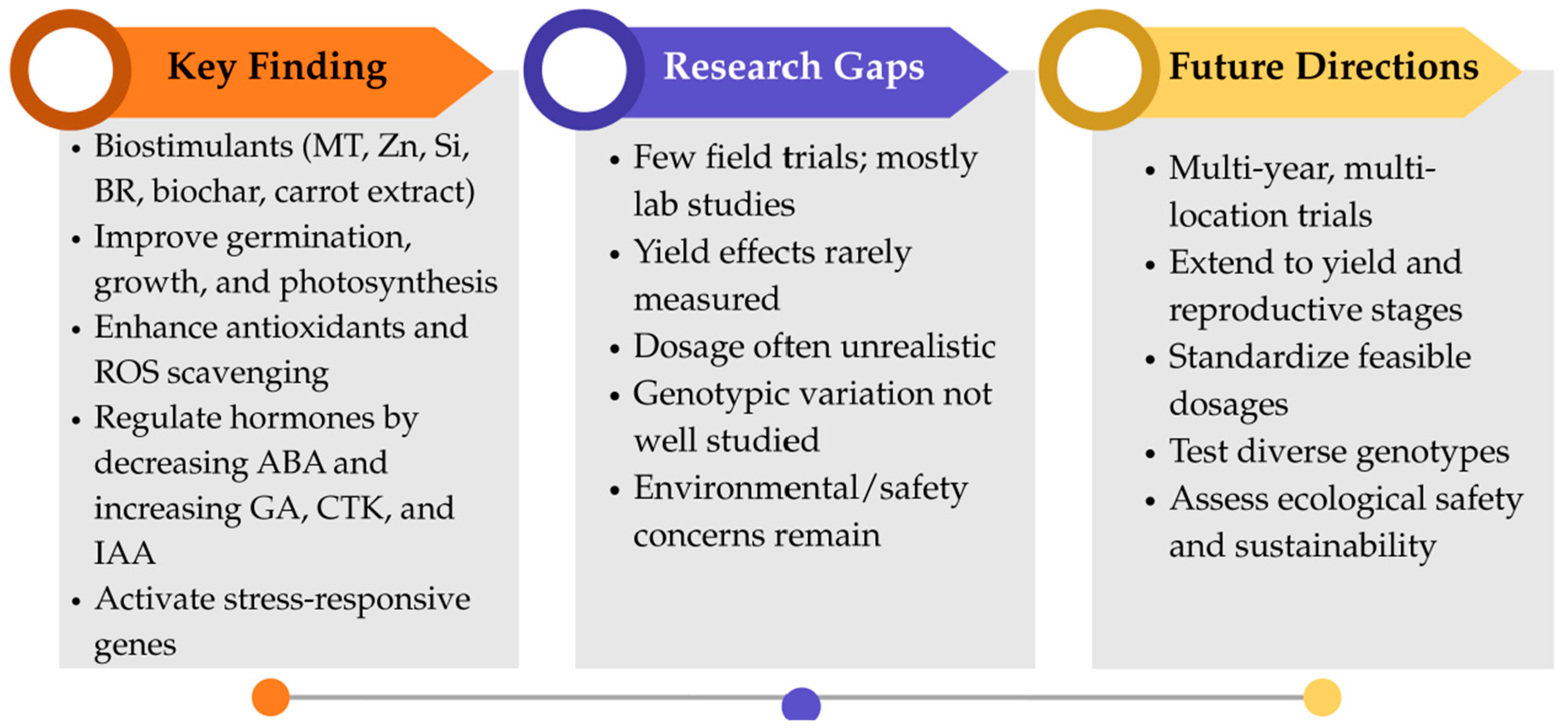
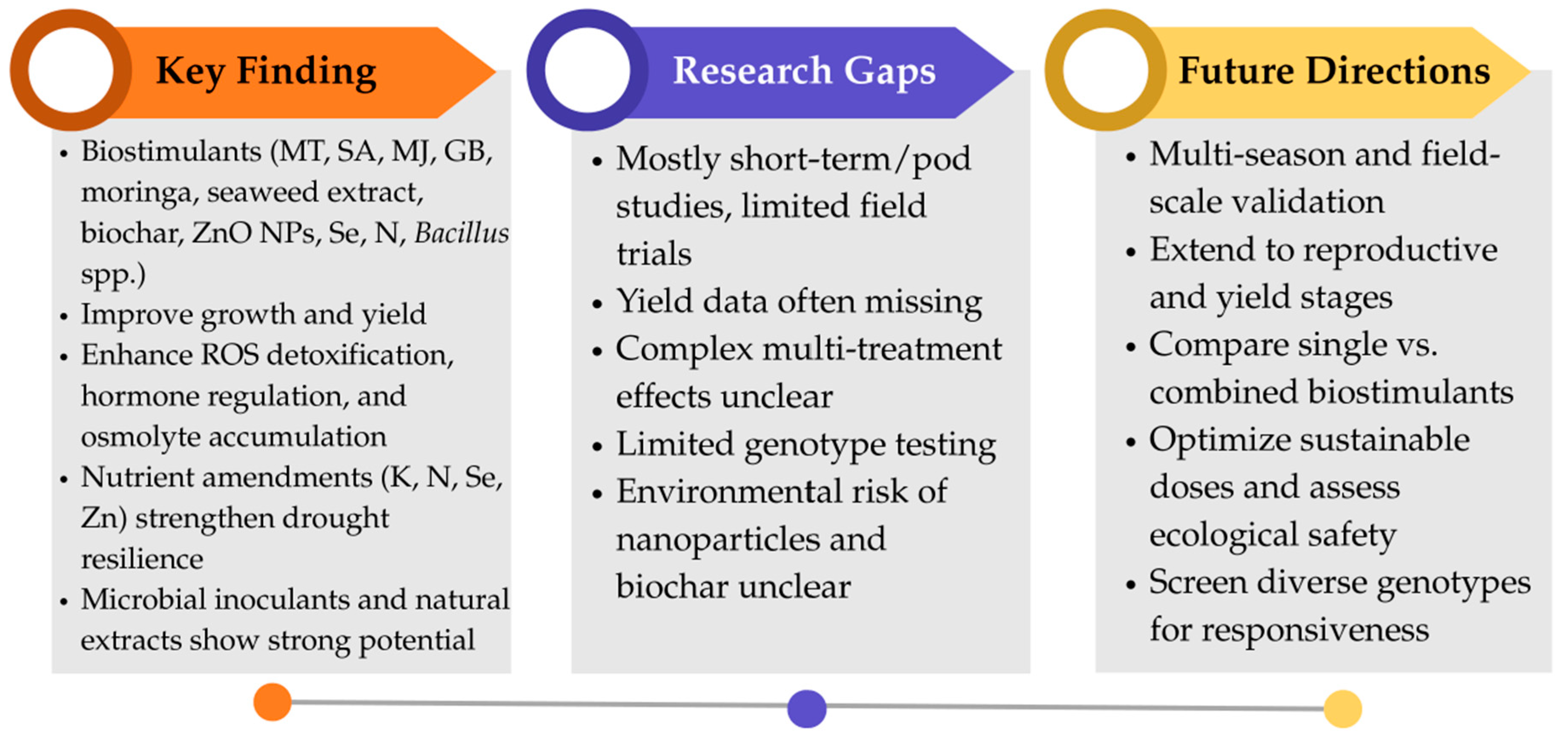
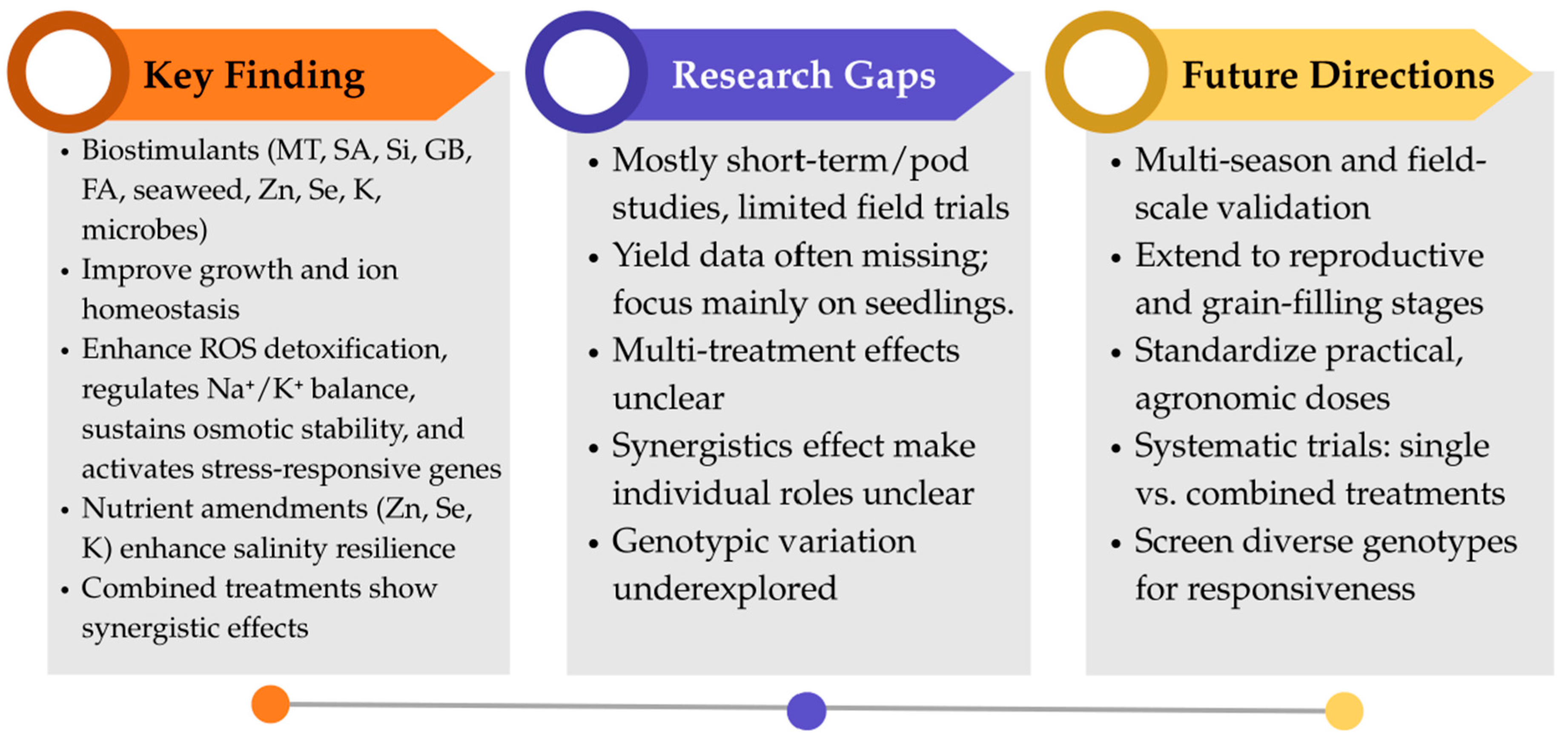
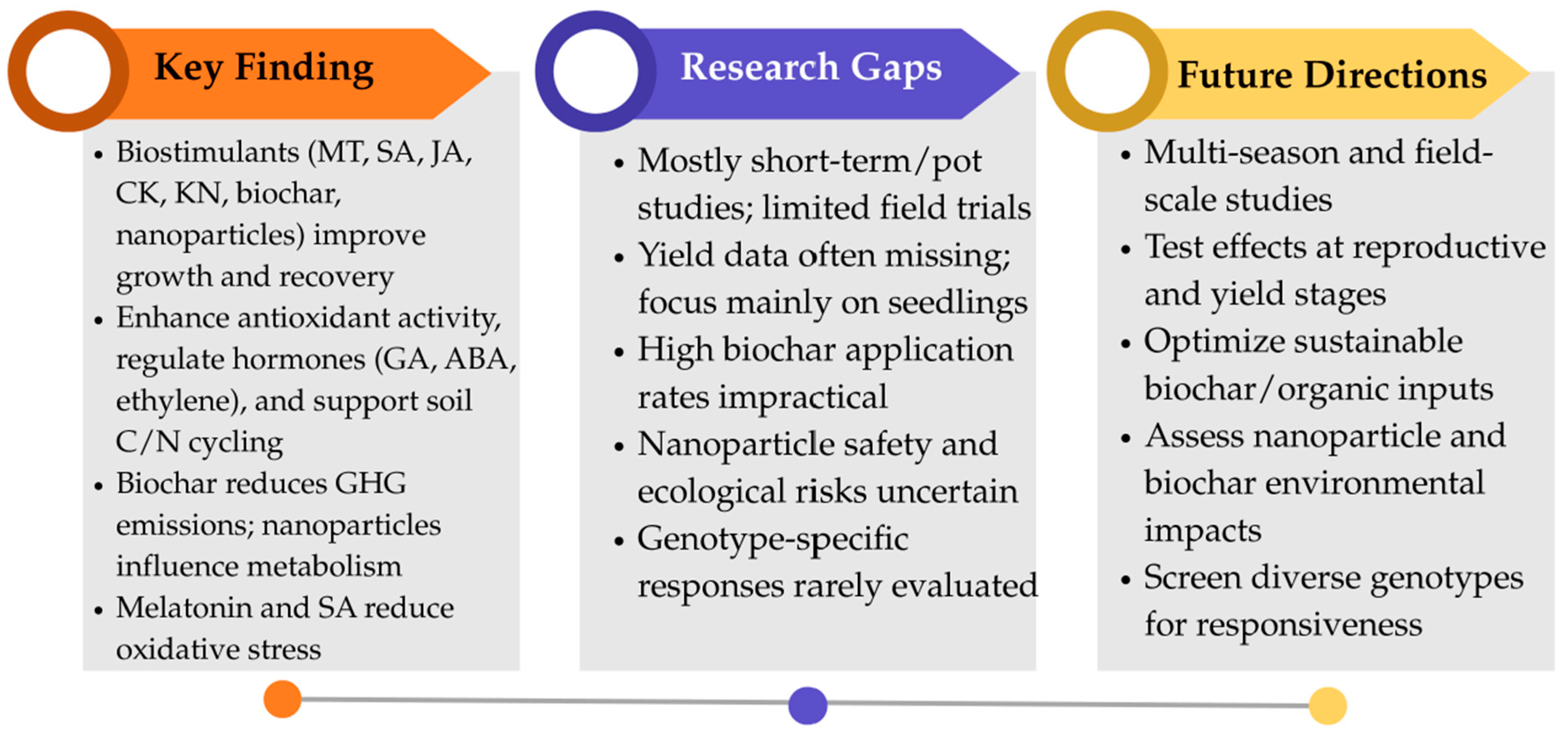
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Win, P.P.; Park, H.-H.; Kuk, Y.-I. Integrated Approach of Using Biostimulants for Improving Growth, Physiological Traits, and Tolerance to Abiotic Stressors in Rice and Soybean. Agronomy 2025, 15, 2265. https://doi.org/10.3390/agronomy15102265
Win PP, Park H-H, Kuk Y-I. Integrated Approach of Using Biostimulants for Improving Growth, Physiological Traits, and Tolerance to Abiotic Stressors in Rice and Soybean. Agronomy. 2025; 15(10):2265. https://doi.org/10.3390/agronomy15102265
Chicago/Turabian StyleWin, Pyae Pyae, Hyun-Hwa Park, and Yong-In Kuk. 2025. "Integrated Approach of Using Biostimulants for Improving Growth, Physiological Traits, and Tolerance to Abiotic Stressors in Rice and Soybean" Agronomy 15, no. 10: 2265. https://doi.org/10.3390/agronomy15102265
APA StyleWin, P. P., Park, H.-H., & Kuk, Y.-I. (2025). Integrated Approach of Using Biostimulants for Improving Growth, Physiological Traits, and Tolerance to Abiotic Stressors in Rice and Soybean. Agronomy, 15(10), 2265. https://doi.org/10.3390/agronomy15102265





