Measuring Herbage Mass: A Review
Abstract
1. Introduction
2. Techniques to Estimate Herbage Mass
2.1. In Situ Measurement Techniques
- (a)
- Cut and weigh technique
- (b) Visual estimates
- (c) Pasture condition score tool
- (d) Electronic pasture probe
- (e) Sward stick method
- (f) The rising plate meter method
2.2. Remote Sensing and More Recent Technologies for Measuring Herbage Mass
- (a)
- Non-satellite pasture measurements
- (b) Satellite pasture measurements
3. Next Steps to Improve Accuracy and Uptake of Remote Tools
4. Current Challenges Related to Precision Herbage Mass Measurements and the Future of Pasture Monitoring
5. Pasture Data Integration: Opportunities and Challenges
6. Linking Measurement Quality to Economic and Environmental Outcomes
7. Practical Implications
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Artificial intelligence |
| CSH | Compressed sward height |
| CV | Coefficient of variation |
| DEM | Digital elevation model |
| DM | Dry matter |
| DSM | Digital surface model |
| ESA | European Space Agency |
| GHG | Greenhouse gas |
| GPS | Global Positioning System |
| HM | Herbage mass |
| LIC | Livestock Improvement Corporation |
| LiDAR | Light Detection and Ranging |
| N | Nitrogen |
| NASA | National Aeronautics and Space Administration |
| NDVI | Normalised difference vegetation index |
| NZ | New Zealand |
| PFS | Pasture from SpaceTM |
| PM | Pasture meter |
| R2 | Coefficient of determination |
| RMSE | Root mean square error |
| RPE | Relative prediction error |
| RPM | Rising plate meter |
| S.D. | Standard deviation |
| S.E. | Standard error |
| SfM | Structure from a motion |
| SSH | Sward surface height |
| UAV | Unmanned/unoccupied aerial vehicle |
References
- García, S.C.; Clark, C.E.F.; Kerrisk, K.L.; Islam, M.R.; Farina, S.R.; Evans, J. Gaps and variability in pasture utilisation in Australian pasture based dairy systems. In Proceedings of the 22nd International Grassland Congress, Sydney, Australia, 15–19 September 2013; Michalk, D.L., Millar, G.D., Badgery, W.B., Broadfoot, K.M., Eds.; Department of Primary Industries: Sydney, Australia, 2013; pp. 1709–1716. [Google Scholar]
- Ferris, C.P.; Watson, S.; Gordon, A.W.; Barley, J. Physical and economic performance of dairy cows managed within contrasting grassland-based milk production systems over 3 successive lactations. J. Dairy Sci. 2022, 105, 3153–3175. [Google Scholar] [CrossRef]
- Liu, T.; Bruins, R.J.F.; Heberling, M.T. Factors Influencing Farmers’ Adoption of Best Management Practices: A Review and Synthesis. Sustainability 2018, 10, 432. [Google Scholar] [CrossRef]
- Hennessy, D.; Delaby, L.; van den Pol-van Dasselaar, A.; Shalloo, L. Increasing grazing in dairy cow milk production systems in Europe. Sustainability 2020, 12, 2443. [Google Scholar] [CrossRef]
- Thomas, D.T.; Flohr, B.M.; Monjardino, M.; Loi, A.; Llewellyn, R.S.; Lawes, R.A.; Norman, H.C. Selecting higher nutritive value annual pasture legumes increases the profitability of sheep production. Agric. Syst. 2021, 194, 103272. [Google Scholar] [CrossRef]
- Edvan, R.; Bezerra, L.; Marques, C.; Carneiro, M.S.; Oliveira, R.; Ferreira, R. Methods for estimating forage mass in pastures in a tropical climate. Rev. Ciências Agrárias 2016, 39, 36–45. [Google Scholar] [CrossRef]
- ‘t Mannetje, L. Measuring biomass of grassland vegetation. In Field and Laboratory Methods for Grassland and Animal Production Research; ‘t Mannetje, L., Jones, R., Eds.; CABI Publishing: Wallingford, UK, 2000; pp. 151–177. [Google Scholar]
- Frame, J. Herbage mass. In Sward Measurement Handbook, 2nd ed.; Davies, A., Baker, R.D., Grant, S.A., Laidlaw, A.S., Eds.; The British Grassland Society: Berkshire, UK, 1993; pp. 39–68. [Google Scholar]
- López-Díaz, J.E.; Roca-Fernández, A.I.; González-Rodríguez, A. Measuring Herbage Mass by Non- destructive Methods: A Review. J. Agric. Sci. Technol. 2011, 1, 303–314. [Google Scholar]
- Sanderson, M.A.; Rotz, C.A.; Fultz, S.W.; Rayburn, E.B. Estimating forage mass with a commercial Capacitance meter, Rising plate meter, and Pasture ruler. Agron. J. 2001, 93, 1281–1286. [Google Scholar] [CrossRef]
- Paczkowski, A.; Isselstein, J.; Hartmann, S. Prediction of herbage mass in pure stands of lucerne and red clover using a plate meter. In 28th General Meeting of the European Grassland Federation, Helsinki, Finland, 19–22 October 2020; Virkajärvi, P., Hakala, K., Hakojärvi, M., Helin, J., Herzon, I., Jokela, V., Peltonen, S., Rinne, M., Seppänen, M., Uusi-Kämppä, J., Eds.; The Organising Committee of the 28th General Meeting of the European Grassland Federation, Natural Resources Institute Finland: Helsinki, Finland, 2020; Volume 25, pp. 37–39. [Google Scholar]
- Bucci, G.; Bentivoglio, D.; Belletti, M.; Finco, A. Measuring a farm’s profitability after adopting precision agriculture technologies: A case study from Italy. Acta IMEKO 2020, 9, 65–74. [Google Scholar] [CrossRef]
- Murphy, D.J.; Murphy, M.D.; O’Brien, B.; O’Donovan, M. A Review of precision technologies for optimising pasture measurement on Irish grassland. Agriculture 2021, 11, 600. [Google Scholar] [CrossRef]
- Shalloo, L.; Byrne, T.; Leso, L.; Ruelle, E.; Starsmore, K.; Geoghegan, A.; Werner, J.; O’Leary, N. A review of precision technologies in pasture-based dairying systems. Ir. J. Agric. Food Res. 2021, 59, 279–291. [Google Scholar] [CrossRef]
- Aquilani, C.; Confessore, A.; Bozzi, R.; Sirtori, F.; Pugliese, C. Review: Precision Livestock Farming technologies in pasture-based livestock systems. Animal 2022, 16, 100429. [Google Scholar] [CrossRef] [PubMed]
- Ogungbuyi, M.G.; Mohammed, C.; Ara, I.; Fischer, A.M.; Harrison, M.T. Advancing skyborne technologies and high-resolution satellites for pasture monitoring and improved management: A review. Remote Sens. 2023, 15, 4866. [Google Scholar] [CrossRef]
- Persson, T.; Murguzur, F.J.A.; Davids, C.; Höglind, M.; Jørgensen, M. Combining satellite-sensed and ground data and the BASGRA model to predict grass yield in high-latitude regions. Field Crops Res. 2024, 318, 109610. [Google Scholar] [CrossRef]
- Pinna, D.; Pezzuolo, A.; Cogato, A.; Pornaro, C.; Macolino, S.; Marinello, F. Applications of satellite platforms and machine learning for mapping and monitoring grasslands and pastures: A systematic and comprehensive review. Smart Agric. Technol. 2024, 9, 100571. [Google Scholar] [CrossRef]
- Vermeire, L.T.; Gillen, R.L. Estimating herbage standing crop with visual obstruction in tallgrass prairie. J. Range Manag. 2001, 54, 57–60. [Google Scholar] [CrossRef]
- Somasiri, S.C.; Kenyon, P.; Morel, P.; Kemp, P.; Morris, S. Alternative Method to Measure Herbage Dry Matter Mass in Plantain and Chicory Mixed Swards Grazed by Lambs; New Zealand Society of Animal Production: Napier, New Zealand, 2014; Volume 74, pp. 115–120. [Google Scholar]
- Catchpole, W.R.; Wheeler, C.J. Estimating plant biomass: A review of techniques. Aust. J. Ecol. 1992, 17, 121–131. [Google Scholar] [CrossRef]
- Murphy, W.M.; Silman, J.P.; Barreto, A.D.M. A comparison of quadrat, capacitance meter, HFRO sward stick, and rising plate for estimating herbage mass in a smooth-stalked, meadowgrass-dominant white clover sward. Grass Forage Sci. 1995, 50, 452–455. [Google Scholar] [CrossRef]
- Stockdale, C.R.; Kelly, K.B. A comparison of a rising-plate meter and an electronic capacitance meter for estimating the yield of pastures grazed by dairy cows. Grass Forage Sci. 1984, 39, 391–394. [Google Scholar] [CrossRef]
- Reddersen, B.; Fricke, T.; Wachendorf, M. A multi-sensor approach for predicting biomass of extensively managed grassland. Comput. Electron. Agric. 2014, 109, 247–260. [Google Scholar] [CrossRef]
- Campbell, N.A.; Arnold, G.W. The visual assessment of pasture yield. Aust. J. Exp. Agric. Anim. Husb. 1973, 13, 263–267. [Google Scholar] [CrossRef]
- Cayley, J.W.D.; Bird, P.R. Techniques for Measuring Pastures; Victoria Dept of Agriculture, Energy and Minerals: Victoria, Australia, 1996. [Google Scholar]
- Cropper, J.B.; Cosgrove, D. Pasture Condition Scoring. In Proceedings of the 37th North American Alfalfa Improvement Conference, Madison, WI, USA, 16–19 July 2000; Phillips, M., Terrill, T., Belesky, D., Berdahl, J., Eds.; American Forage and Grassland Council: Berea, KY, USA, 2000; pp. 141–145. [Google Scholar]
- Sanderson, M.A.; Goslee, S.C.; Gonet, J.; Stout, R. Pasture monitoring at a farm scale with the USDA NRCS pasture condition score system. J. Soil Water Conserv. 2009, 64, 423–433. [Google Scholar] [CrossRef]
- Tucker, C.J.; Vanpraet, C.L.; Sharman, M.J.; Ittersum, G.V. Satellite remote sensing of total herbaceous biomass production in the Senegalese Sahel: 1980–1984. Remote Sens. Environ. 1985, 17, 233–249. [Google Scholar] [CrossRef]
- Stewart, K.E.J.; Bourn, N.A.D.; Thomas, J.A. An evaluation of three quick methods commonly used to assess sward height in ecology. J. Appl. Ecol. 2002, 38, 1148–1154. [Google Scholar] [CrossRef]
- Haultain, J.; Wigley, K.; Lee, J.M. Rising Plate Meters and a Capacitance Probe Estimate the Biomass of Chicory and Plantain Monocultures with Similar Accuracy as for Ryegrass-Based Pasture; New Zealand Grassland Association: Alexandra, New Zealand, 2014; Volume 76, pp. 67–74. [Google Scholar] [CrossRef]
- Rayburn, E. Plate meter calibrations for forage mass follow a continuum of sward basal density. Crop Forage Turfgrass Manag. 2020, 6, 6–11. [Google Scholar] [CrossRef]
- Woodward, S.; Rollo, M. Why Pasture Growth Prediction is Difficult; Agronomy Society of New Zealand: Palmerston North, New Zealand, 2002; Volume 32, pp. 17–26. [Google Scholar]
- Bryant, J.R.; Ogle, G.; Marshall, P.R.; Glassey, C.B.; Lancaster, J.A.S.; García, S.C.; Holmes, C.W. Description and evaluation of the Farmax Dairy Pro decision support model. N. Z. J. Agric. Res. 2010, 53, 13–28. [Google Scholar] [CrossRef][Green Version]
- Eastwood, C.; Dela Rue, B. Developing decision-support systems for pasture and rangeland management. In Improving Data Management and Decision Support Systems in Agriculture; Burleigh Dodds Series in Agricultural Science; Burleigh Dodds Science Publishing: Sawston, UK, 2020; pp. 279–310. [Google Scholar][Green Version]
- Rennie, G.M.; King, W.M.; Puha, M.R.; Dalley, D.E.; Dynes, R.A.; Upsdell, M.P. Calibration of the C-DAX Rapid Pasturemeter and the rising plate meter for kikuyu-based Northland dairy pastures. Proc. N. Z. Grassl. Assoc. 2009, 71, 49–55. [Google Scholar] [CrossRef]
- McCarthy, A.C.; Raedts, P.; Foley, J.; Hills, J. Improving pasture growth assessment using machine vision. In Australasian Dairy Science Symposium; Australasian Dairy Science: Queensland, Australia, 2022; pp. 103–106. [Google Scholar]
- Obanawa, H.; Yoshitoshi, R.; Watanabe, N.; Sakanoue, S. Portable LiDAR-based method for improvement of grass height measurement accuracy: Comparison with SfM methods. Sensors 2020, 20, 4809. [Google Scholar] [CrossRef] [PubMed]
- Legg, M.; Bradley, S. Ultrasonic arrays for remote sensing of pasture biomass. Remote Sens. 2019, 12, 111. [Google Scholar] [CrossRef]
- Askari, M.S.; McCarthy, T.; Magee, A.; Murphy, D.J. Evaluation of Grass Quality under Different Soil Management Scenarios Using Remote Sensing Techniques. Remote Sens. 2019, 11, 1835. [Google Scholar] [CrossRef]
- Numata, I.; Roberts, D.; Chadwick, O.; Schimel, J.; Galvao, L.; Soares, J. Evaluation of hyperspectral data for pasture estimate in the Brazilian Amazon using field and imaging spectrometers. Remote Sens. Environ. 2008, 112, 1569–1583. [Google Scholar] [CrossRef]
- Edirisinghe, A.; Clark, D.; Waugh, D. Spatio-temporal modelling of biomass of intensively grazed perennial dairy pastures using multispectral remote sensing. Int. J. Appl. Earth Obs. Geoinf. 2012, 16, 5–16. [Google Scholar] [CrossRef]
- Gargiulo, J.; Clark, C.; Lyons, N.; de Veyrac, G.; Beale, P.; Garcia, S. Spatial and Temporal Pasture Biomass Estimation Integrating Electronic Plate Meter, Planet CubeSats and Sentinel-2 Satellite Data. Remote Sens. 2020, 12, 3222. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Goslee, S.C.; Cropper, J.B. Pasture assessment in the Northeast United States. Forage Grazinglands 2005, 3, 1–9. [Google Scholar] [CrossRef]
- Hutchings, N.J. Factors affecting sonic sward stick measurements: The effect of different leaf characteristics and the area of sward sampled. Grass Forage Sci. 1992, 47, 153–160. [Google Scholar] [CrossRef]
- Fricke, T.; Richter, F.; Wachendorf, M. Assessment of forage mass from grassland swards by height measurement using an ultrasonic sensor. Comput. Electron. Agric. 2011, 79, 142–152. [Google Scholar] [CrossRef]
- Fricke, T.; Wachendorf, M. Combining ultrasonic sward height and spectral signatures to assess the biomass of legume–grass swards. Comput. Electron. Agric. 2013, 99, 236–247. [Google Scholar] [CrossRef]
- Beef + Lamb New Zealand. Measuring Pasture Covers Using the Sward Stick—Fact Sheet 2013; Beef + Lamb New Zealand: Wellington, New Zealand, 2013; p. 92. [Google Scholar]
- Ogura, S.; Nagatomo, Y.; Hirata, M. Estimation of herbage mass in a bahia grass (Paspalum notatum) and a centipede grass (Eremochloa ophiuroides) pasture using a capacitance probe, a sward stick and a rising plate. Trop. Grassl. 2005, 39, 22–30. [Google Scholar]
- Martin, R.C.; Astatkie, T.; Cooper, J.M.; Fredeen, A.H. A Comparison of Methods Used to Determine Biomass on Naturalized Swards. J. Agron. Crop Sci. 2005, 191, 152–160. [Google Scholar] [CrossRef]
- Earle, D.F.; McGowan, A.A. Evaluation and calibration of an automated rising plate meter for estimating dry matter yield of pasture. Aust. J. Exp. Agric. Anim. Husb. 1979, 19, 337–343. [Google Scholar] [CrossRef]
- Thomson, N.A.; Upsdell, M.P.; Hooper, R.; Henderson, H.V.; Blackwell, M.B.; McCallum, D.A.; Hainsworth, R.J.; MacDonald, K.A.; Wildermoth, D.D.; Bishop-Hurley, G.J.; et al. Development and evaluation of a standardised means for estimating herbage mass of dairy pastures using the rising plate meter. Hamilton 2001, 63, 149–157. [Google Scholar] [CrossRef]
- Murphy, D.J.; O’ Brien, B.; Hennessy, D.; Hurley, M.; Murphy, M.D. Evaluation of the precision of the rising plate meter for measuring compressed sward height on heterogeneous grassland swards. Precis. Agric. 2020, 22, 922–946. [Google Scholar] [CrossRef]
- McSweeney, D.; Coughlan, N.E.; Cuthbert, R.N.; Halton, P.; Ivanov, S. Micro-sonic sensor technology enables enhanced grass height measurement by a Rising Plate Meter. Inf. Process. Agric. 2019, 6, 279–284. [Google Scholar] [CrossRef]
- Griggs, T.C.; Stringer, W.C. Prediction of Alfalfa Herbage Mass Using Sward Height, Ground Cover, and Disk Technique. Agron. J. 1988, 80, 204–208. [Google Scholar] [CrossRef]
- L’Huillier, P.J.; Thomson, N.A. Estimation of Herbage Mass in Ryegrass/White Clover Dairy Pastures; New Zealand Grassland Association: Matamata, New Zealand, 1988; Volume 49, pp. 117–122. [Google Scholar] [CrossRef]
- McSweeney, D.; Delaby, L.; O’Brien, B.; Ferard, A.; Byrne, N.; McDonagh, J.; Ivanov, S.; Coughlan, N.E. Dynamic algorithmic conversion of compressed sward height to dry matter yield by a rising plate meter. Comput. Electron. Agric. 2022, 196, 106919. [Google Scholar] [CrossRef]
- Pontes, L.d.S.; Carpinelli, S.; Stafin, G.; Porfírio-da-Silva, V.; Santos, B.R.C.d. Relationship between sward height and herbage mass for integrated crop-livestock systems with trees. Grassl. Sci. 2017, 63, 29–35. [Google Scholar] [CrossRef]
- Serrano, J.M.; Peça, J.O.; Marques da Silva, J.; Shahidian, S. Calibration of a capacitance probe for measurement and mapping of dry matter yield in Mediterranean pastures. Precis. Agric. 2011, 12, 860–875. [Google Scholar] [CrossRef]
- Lile, J.A.; Blackwell, M.B.; Thomson, N.A.; Penno, J.W.; Macdonald, K.A.; Nicholas, P.K.; Lancaster, J.A.S.; Coulter, M. Practical use of the rising plate meter (RPM) on New Zealand dairy farms. Proc. N. Z. Grassl. Assoc. 2001, 63, 159–164. [Google Scholar] [CrossRef]
- Nobilly, F.; Bryant, R.H.; McKenzie, B.A.; Edwards, G.R. Productivity of Rotationally Grazed Simple and Diverse Pasture Mixtures Under Irrigation in Canterbury; New Zealand Grassland Association: Tauranga, New Zealand, 2013; Volume 75, pp. 165–171. [Google Scholar]
- Cárdenas, J.; Balocchi, O.; Calvache, I. Calibration of the rising plate meter for mixed pastures of ryegrass (Lolium perenne L.) and Kikuyu (Cenchrus clandestinus). Chil. J. Agric. Anim. Sci. 2020, 36, 216–223. [Google Scholar] [CrossRef]
- Wilson, R.L.; Bionaz, M.; MacAdam, J.W.; Beauchemin, K.A.; Naumann, H.D.; Ates, S. Milk production, nitrogen utilization, and methane emissions of dairy cows grazing grass, forb, and legume-based pastures. J. Anim. Sci. 2020, 98, skaa220. [Google Scholar] [CrossRef]
- Chapa, J.M.; Pichlbauer, B.; Bobal, M.; Guse, C.; Drillich, M.; Iwersen, M. Field evaluation of a rising plate meter to estimate herbage mass in Austrian pastures. Sensors 2023, 23, 7477. [Google Scholar] [CrossRef]
- Rayburn, E.B.; Rayburn, S.B. A standardized plate meter for estimating pasture mass in on-farm research trials. Agron. J. 1998, 90, 238–241. [Google Scholar] [CrossRef]
- Thomson, N.A.; McCallum, D.A.; Howse, S.; Holmes, C.W.; Matthews, P.N.P.; Matthew, C. Estimation of dairy pastures—The need for standardisation. Proc. N. Z. Grassl. Assoc. 1997, 59, 221–225. [Google Scholar] [CrossRef]
- Aarons, S.R.; Gourley, C.J.P.; Hannah, M.C. Between and within paddock soil chemical variability and forage production gradients in grazed dairy pastures. Nutr. Cycl. Agroecosyst. 2015, 102, 411–430. [Google Scholar] [CrossRef]
- Islam, N.; Rashid, M.M.; Pasandideh, F.; Ray, B.; Moore, S.; Kadel, R. A review of applications and communication technologies for Internet of things (IoT) and Unmanned aerial vehicle (UAV) based sustainable smart farming. Sustainability 2021, 13, 1821. [Google Scholar] [CrossRef]
- French, P.; O’Brien, B.; Shalloo, L. Development and adoption of new technologies to increase the efficiency and sustainability of pasture-based systems. Anim. Prod. Sci. 2015, 55, 931–935. [Google Scholar] [CrossRef]
- Gargiulo, J.I.; Lyons, N.A.; Masia, F.; Beale, P.; Insua, J.R.; Correa-Luna, M.; Garcia, S.C. Comparison of ground-based, unmanned aerial vehicles and satellite remote sensing technologies for monitoring pasture biomass on dairy farms. Remote Sens. 2023, 15, 2752. [Google Scholar] [CrossRef]
- Chen, A.; Xu, C.; Zhang, M.; Guo, J.; Xing, X.; Yang, D.; Xu, B.; Yang, X. Cross-scale mapping of above-ground biomass and shrub dominance by integrating UAV and satellite data in temperate grassland. Remote Sens. Environ. 2024, 304, 114024. [Google Scholar] [CrossRef]
- Ghajar, S.; Tracy, B. Proximal Sensing in Grasslands and Pastures. Agriculture 2021, 11, 740. [Google Scholar] [CrossRef]
- Ogungbuyi, M.G.; Guerschman, J.; Fischer, A.M.; Crabbe, R.A.; Ara, I.; Mohammed, C.; Scarth, P.; Tickle, P.; Whitehead, J.; Harrison, M.T. Improvement of pasture biomass modelling using high-resolution satellite imagery and machine learning. J. Environ. Manag. 2024, 356, 120564. [Google Scholar] [CrossRef]
- Moeckel, T.; Safari, H.; Reddersen, B.; Fricke, T.; Wachendorf, M. Fusion of ultrasonic and spectral sensor data for improving the estimation of biomass in grasslands with heterogeneous sward structure. Remote Sens. 2017, 9, 98. [Google Scholar] [CrossRef]
- Zhang, A.; Hu, S.; Zhang, X.; Zhang, T.; Li, M.; Tao, H.; Hou, Y. A Handheld Grassland Vegetation Monitoring System Based on Multispectral Imaging. Agriculture 2021, 11, 1262. [Google Scholar] [CrossRef]
- Hammad, H.M.; Ahmad, A.; Abbas, F.; Farooque, A.A.; Willkerson, C.; Ahmad, S.; Hoogenboom, G. Handheld Sensor-Based NDVI Measurement as an Alternative to Destructive Sampling for Growth and Yield Assessment in Maize. Int. J. Plant Prod. 2025, 19, 363–376. [Google Scholar] [CrossRef]
- Barrett, P.D.; Laidlaw, A.S.; Mayne, C.S. GrazeGro: A European herbage growth model to predict pasture production in perennial ryegrass swards for decision support. Eur. J. Agron. 2005, 23, 37–56. [Google Scholar] [CrossRef]
- Cunliffe, A.M.; Brazier, R.E.; Anderson, K. Ultra-fine grain landscape-scale quantification of dryland vegetation structure with drone-acquired structure-from-motion photogrammetry. Remote Sens. Environ. 2016, 183, 129–143. [Google Scholar] [CrossRef]
- Wigley, K.; Owens, J.L.; Westerschulte, M.; Riding, P.; Fourie, J.; Werner, A. Photogrammetry for assessment of pasture biomass. J. N. Z. Grassl. 2019, 81, 33–40. [Google Scholar] [CrossRef]
- Edirisinghe, A.; Hill, M.J.; Donald, G.E.; Hyder, M. Quantitative mapping of pasture biomass using satellite imagery. Int. J. Remote Sens. 2011, 32, 2699–2724. [Google Scholar] [CrossRef]
- Defalque, G.; Santos, R.; Bungenstab, D.; Echeverria, D.; Dias, A.; Defalque, C. Machine learning models for dry matter and biomass estimates on cattle grazing systems. Comput. Electron. Agric. 2024, 216, 108520. [Google Scholar] [CrossRef]
- Oudshoorn, F.W.; Hansson, S.L.; Hansen, H. Calibration of the C-Dax pasture meter in a Danish grazing system. In Proceedings of the 16th Symposium of the European Grassland Federation, Gumpenstein, Austria, 29–31 August 2011; Pötsch, E.M., Krautzer, B., Hopkins, A., Eds.; Agricultural Research and Education Center: Irdning, Austria, 2011; pp. 166–168. [Google Scholar]
- Schori, F. Sward surface height estimation with a rising plate meter and the C-Dax Pasturemeter. In Grassland Science in Europe; Dasselaar, A.v.d.P.-v., Aarts, H.F.M., Vliegher, A.D., Elgersma, A., Reheul, D., Reijneveld, J.A., Verloop, J., Hopkins, A., Eds.; European Grassland Federation: Wageningen, The Netherlands, 2015; Volume 20, pp. 310–312. [Google Scholar]
- Behmann, J.; Acebron, K.; Emin, D.; Bennertz, S.; Matsubara, S.; Thomas, S.; Bohnenkamp, D.; Kuska, M.T.; Jussila, J.; Salo, H.; et al. Specim IQ: Evaluation of a New, Miniaturized Handheld Hyperspectral Camera and Its Application for Plant Phenotyping and Disease Detection. Sensors 2018, 18, 441. [Google Scholar] [CrossRef]
- Chebrolu, N.; Lottes, P.; Schaefer, A.; Winterhalter, W.; Burgard, W.; Stachniss, C. Agricultural robot dataset for plant classification, localization and mapping on sugar beet fields. Int. J. Robot. Res. 2017, 36, 1045–1052. [Google Scholar] [CrossRef]
- Trotter, T.F.; Frazier, P.S. Objective biomass assessment using an active plant sensor (Crop Circle TM)—Preliminary experiences on a varity of agricultural landscapes. In Proceedings of the 9th International Conference on Precision Agriculture, Denver, CO, USA, 20–23 July 2008; pp. 20–23. [Google Scholar]
- Pullanagari, R.R.; Yule, I.; King, W.; Dalley, D.; Dynes, R. The use of optical sensors to estimate pasture quality. Int. J. Smart Sens. Intell. Syst. 2011, 4, 125–137. [Google Scholar] [CrossRef]
- Lapidus, D.; Salem, M.E.; Beach, R.H.; Zayed, S.; Ortiz-Monasterio, I. Greenhouse gas mitigation benefits and profitability of the GreenSeeker Handheld NDVI sensor: Evidence from Mexico. Precis. Agric. 2022, 23, 2388–2406. [Google Scholar] [CrossRef]
- Kimaro, O.D.; Gebre, S.L.; Hieronimo, P.; Kihupi, N.; Feger, K.-H.; Kimaro, D.N. Handheld NDVI sensor-based rice productivity assessment under combinations of fertilizer soil amendment and irrigation water management in lower Moshi irrigation scheme, North Tanzania. Environ. Earth Sci. 2023, 82, 78. [Google Scholar] [CrossRef]
- Rodriguez, D.; Van Oijen, M.; Schapendonk, A.H.M.C. LINGRA-CC: A sink–source model to simulate the impact of climate change and management on grassland productivity. New Phytol. 2002, 144, 359–368. [Google Scholar] [CrossRef]
- Efremova, N.; Foley, J.C.; Unagaev, A.; Karimi, R. AI for Sustainable Agriculture and Rangeland Monitoring. Ethics Artifcial Intell. Sustain. Dev. Goals 2023, 152, 399–422. [Google Scholar] [CrossRef]
- Gard, S.; Neal, M.; Minnee, E. Pasture performance tools: Current and future state. J. N. Z. Grassl. 2024, 86, 273–279. [Google Scholar] [CrossRef]
- Halter. How It Works. 2023. Available online: https://www.halterhq.com/dairy-overview (accessed on 8 July 2025).
- Proveye. Revolutionising Precision Insights Through Advanced Image Analysis. 2024. Available online: https://www.proveye.io/#platform (accessed on 8 July 2025).
- Aimer_Farming. Aimer Features. 2025. Available online: https://www.aimer-farming.com/features (accessed on 8 July 2025).
- Mata, G.; Clark, D.A.; Edirisinghe, A.; Waugh, D.; Minneé, E.; Gherardi, S.G. Predicting Accurate Paddock-Average Pasture Cover in Waikato Dairy Farms Using Satellite Images; New Zealand Grassland Association: Wairakei, New Zealand, 2007; Volume 69, pp. 23–28. [Google Scholar] [CrossRef]
- Dolling, P.; Curnow, M. Understanding Pastures from Space™ for South West Western Australia|Agriculture and Food. Department of Primary Industries and Regional Development’s Agriculture and Food Division. 2022. Available online: https://www.agric.wa.gov.au/sheep/understanding-pastures-space%E2%84%A2-south-west-western-australia (accessed on 6 March 2024).
- Roberts, D.; Dolling, P. Pastures from Space™—FOO and PGR for Agricultural Properties in WA. Department of Primary Industries and Regional Development’s Agriculture and Food Division. 2023. Available online: https://www.agric.wa.gov.au/pastures-from-space-wa (accessed on 6 March 2024).
- Anderson, G.; Rawlings, M.; Ogle, G. Mitigation of saturation in satellite pasture measurement via incorporation of a statistical pasture growth model. J. N. Z. Grassl. 2020, 82, 191–198. [Google Scholar] [CrossRef]
- LIC. Space. 2025. Available online: https://www.lic.co.nz/products-and-services/space/ (accessed on 10 July 2025).
- Anderson, G.; McNaughton, L. Validation of a satellite pasture measurement system. In Proceedings of the 8th Australasian Dairy Science Symposium, Palmerston North, New Zealand, 21–23 November 2018; pp. 191–195. [Google Scholar]
- Pasture.io. The Best LIC Space Alternative; Pasture.io: Sandy Bay, Australia, 2019; Volume 2025. [Google Scholar]
- Stevens, D.R.; Thompson, B.R.; Johnson, P.; Welten, B.; Meenken, E.; Bryant, J. Integrating digital technologies to aid grassland productivity and sustainability. Front. Sustain. Food Syst. 2021, 5, 602350. [Google Scholar] [CrossRef]
- Clark, D.; Litherland, A.; Mata, G.; Burling-Claridge, R. Pasture Monitoring from Space. In Proceedings of the SouthIsland Dairy Event, Invercargill, New Zealand, 26–28 June 2006; pp. 108–123. [Google Scholar]
- Smith, R.C.G.; Adams, M.; Gittins, S.; Gherardi, S.; Wood, D.; Maier, S.; Stovold, R.; Donald, G.; Khohkar, S.; Allen, A. Near real-time Feed On Offer (FOO) from MODIS for early season grazing management of Mediterranean annual pastures. Int. J. Remote Sens. 2011, 32, 4445–4460. [Google Scholar] [CrossRef]
- Sharma, V.; Tripathi, A.K.; Mittal, H. Technological revolutions in smart farming: Current trends, challenges & future directions. Comput. Electron. Agric. 2022, 201, 107217. [Google Scholar] [CrossRef]
- Christopher, S.A.; Gupta, P. Satellite remote sensing of particulate matter air quality: The cloud-cover problem. J. Air Waste Manag. Assoc. 2010, 60, 596–602. [Google Scholar] [CrossRef]
- Hollstein, A.; Segl, K.; Guanter, L.; Brell, M.; Enesco, M. Ready-to-Use Methods for the Detection of Clouds, Cirrus, Snow, Shadow, Water and Clear Sky Pixels in Sentinel-2 MSI Images. Remote Sens. 2016, 8, 666. [Google Scholar] [CrossRef]
- Jeppesen, J.H.; Jacobsen, R.H.; Inceoglu, F.; Toftegaard, T.S. A cloud detection algorithm for satellite imagery based on deep learning. Remote Sens. Environ. 2019, 229, 247–259. [Google Scholar] [CrossRef]
- Nazarova, T.; Martin, P.; Giuliani, G. Monitoring Vegetation Change in the Presence of High Cloud Cover with Sentinel-2 in a Lowland Tropical Forest Region in Brazil. Remote Sens. 2020, 12, 1829. [Google Scholar] [CrossRef]
- Sishodia, R.P.; Ray, R.L.; Singh, S.K. Applications of Remote Sensing in Precision Agriculture: A Review. Remote Sens. 2020, 12, 3136. [Google Scholar] [CrossRef]
- Ali, I.; Cawkwell, F.; Green, S.; Dwyer, N. Application of statistical and machine learning models for grassland yield estimation based on a hypertemporal satellite remote sensing time series. In Proceedings of the 2014 IEEE Geoscience and Remote Sensing Symposium, Quebec City, QC, Canada, 13–18 July 2014; pp. 5060–5063. [Google Scholar] [CrossRef]
- Morais, T.G.; Jongen, M.; Tufik, C.; Rodrigues, N.R.; Gama, I.; Serrano, J.; Domingos, T.; Teixeira, R.F.M. Estimation of Annual Productivity of Sown Rainfed Grasslands Using Machine Learning. Grass Forage Sci. 2025, 80, 12707. [Google Scholar] [CrossRef]
- De Rosa, D.; Basso, B.; Fasiolo, M.; Friedl, J.; Fulkerson, B.; Grace, P.R.; Rowlings, D.W. Predicting pasture biomass using a statistical model and machine learning algorithm implemented with remotely sensed imagery. Comput. Electron. Agric. 2021, 180, 105880. [Google Scholar] [CrossRef]
- Correa-Luna, M.; Gargiulo, J.; Beale, P.; Deane, D.; Leonard, J.; Hack, J.; Geldof, Z.; Wilson, C.; Garcia, S. Accounting for minimum data required to train a machine learning model to accurately monitor Australian dairy pastures using remote sensing. Sci. Rep. 2024, 14, 16927. [Google Scholar] [CrossRef] [PubMed]
- Schellberg, J.; Hill, M.J.; Gerhards, R.; Rothmund, M.; Braun, M. Precision agriculture on grassland: Applications, perspectives and constraints. Eur. J. Agron. 2008, 29, 59–71. [Google Scholar] [CrossRef]
- Bretas, I.L.; Dubeux, J.C.B.; Cruz, P.J.R.; Oduor, K.T.; Queiroz, L.D.; Valente, D.S.M.; Chizzotti, F.H.M. Precision livestock farming applied to grazingland monitoring and management—A review. Agron. J. 2023, 116, 1164–1186. [Google Scholar] [CrossRef]
- Eastwood, C.; Dela Rue, B. Identification of operational performance attributes for pasture measuring devices. J. N. Z. Grassl. 2017, 79, 211–216. [Google Scholar] [CrossRef]
- Dalley, D.; Clark, D.; Pairman, D.; Dynes, R.; Yule, I.; King, W.; Mata, G. Technologies for measuring grass/crops. In Proceedings of the South Island Dairy Event, Invercargill, New Zealand, 28–30 January 2009; pp. 134–151. [Google Scholar]
- Dela Rue, B.; Eastwood, C. Technology and Workplace Practices Survey Report 2023; DairyNZ: Hamilton, New Zealand, 2023. [Google Scholar]
- Eastwood, C.; Dela Rue, B.; Kerslake, J. Developing an approach to assess farmer perceptions of the value of pasture assessment technologies. Grass Forage Sci. 2020, 75, 474–485. [Google Scholar] [CrossRef]
- Hall, A.; Turner, L.; Irvine, L.; Kilpatrick, S. Pasture management and extension on Tasmanian dairy farms –who measures up? Rural Ext. Innov. Syst. J. 2017, 13, 32–40. Available online: https://search.informit.org/doi/10.3316/informit.187067789467790 (accessed on 10 January 2025).
- Edwards, J.P.; Qasim, M.; Bryant, R.H.; Thomas, C.; Wright-Watson, C.; Zobel, G.; Neal, M.B.; Eastwood, C.R. On-animal sensors may predict paddock level pasture mass in rotationally grazed dairy systems. Comput. Electron. Agric. 2024, 219, 108779. [Google Scholar] [CrossRef]
- Wang, Z.; Ma, Y.; Zhang, Y.; Shang, J. Review of Remote Sensing Applications in Grassland Monitoring. Remote Sens. 2022, 14, 2903. [Google Scholar] [CrossRef]
- Ali, I.; Cawkwell, F.; Dwyer, E.; Barrett, B.; Green, S. Satellite remote sensing of grasslands: From observation to management. J. Plant Ecol. 2016, 9, 649–671. [Google Scholar] [CrossRef]
- Green, J.K.; Zhang, Y.; Luo, X.; Keenan, T.F. Systematic Underestimation of Canopy Conductance Sensitivity to Drought by Earth System Models. AGU Adv. 2024, 5, e2023AV001026. [Google Scholar] [CrossRef]
- Huemmrich, K.F.; Vargas Zesati, S.; Campbell, P.; Tweedie, C. Canopy reflectance models illustrate varying NDVI responses to change in high latitude ecosystems. Ecol. Appl. 2021, 31, e02435. [Google Scholar] [CrossRef]
- Reinermann, S.; Asam, S.; Kuenzer, C. Remote Sensing of Grassland Production and Management—A Review. Remote Sens. 2020, 12, 1949. [Google Scholar] [CrossRef]
- Jones, J.W.; Antle, J.M.; Basso, B.; Boote, K.J.; Conant, R.T.; Foster, I.; Godfray, H.C.J.; Herrero, M.; Howitt, R.E.; Janssen, S.; et al. Toward a new generation of agricultural system data, models, and knowledge products: State of agricultural systems science. Agric. Syst. 2017, 155, 269–288. [Google Scholar] [CrossRef]
- Abiri, R.; Rizan, N.; Balasundram, S.K.; Shahbazi, A.B.; Abdul-Hamid, H. Application of digital technologies for ensuring agricultural productivity. Heliyon 2023, 9, e22601. [Google Scholar] [CrossRef]
- O’Grady, M.J.; Langton, D.; O’Hare, G.M.P. Edge computing: A tractable model for smart agriculture? Artif. Intell. Agric. 2019, 3, 42–51. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Khan, I.H.; Suman, R. Understanding the potential applications of Artificial Intelligence in Agriculture Sector. Adv. Agrochem 2023, 2, 15–30. [Google Scholar] [CrossRef]
- Candido, B.; Mindala, U.; Ebrahimy, H.; Zhang, Z.; Kallenbach, R. Integrating Proximal and Remote Sensing with Machine Learning for Pasture Biomass Estimation. Sensors 2025, 25, 1987. [Google Scholar] [CrossRef]
- Smith, C.; Karunaratne, S.; Badenhorst, P.; Cogan, N.; Spangenberg, G.; Smith, K. Machine Learning Algorithms to Predict Forage Nutritive Value of In Situ Perennial Ryegrass Plants Using Hyperspectral Canopy Reflectance Data. Remote Sens. 2020, 12, 928. [Google Scholar] [CrossRef]
- Kaine, G.W.; Tozer, P.R. Stability, resilience and sustainability in pasture-based grazing systems. Agric. Syst. 2005, 83, 27–48. [Google Scholar] [CrossRef]
- Shalloo, L.; O’Donovan, M.; Leso, L.; Werner, J.; Ruelle, E.; Geoghegan, A.; Delaby, L.; O’Leary, N. Review: Grass-based dairy systems, data and precision technologies. Animal 2018, 12, s262–s271. [Google Scholar] [CrossRef]
- Liu, M.; Ouyang, S.; Tian, Y.; Wen, S.; Zhao, Y.; Li, X.; Baoyin, T.-T.; Kuzyakov, Y.; Xu, X. Effects of rotational and continuous overgrazing on newly assimilated C allocation. Biol. Fertil. Soils 2020, 57, 193–202. [Google Scholar] [CrossRef]
- Piñeiro, G.; Paruelo, J.M.; Oesterheld, M.; Jobbágy, E.G. Pathways of grazing effects on soil organic carbon and nitrogen. Rangel. Ecol. Manag. 2010, 63, 109–119. [Google Scholar] [CrossRef]
- Lai, L.; Kumar, S. A global meta-analysis of livestock grazing impacts on soil properties. PLoS ONE 2020, 15, e0236638. [Google Scholar] [CrossRef]
- Wang, Y.; Ren, Z.; Ma, P.; Wang, Z.; Niu, D.; Fu, H.; Elser, J.J. Effects of grassland degradation on ecological stoichiometry of soil ecosystems on the Qinghai-Tibet Plateau. Sci. Total Environ. 2020, 722, 137910. [Google Scholar] [CrossRef]
- Papadopoulos, G.; Arduini, S.; Uyar, H.; Psiroukis, V.; Kasimati, A.; Fountas, S. Economic and environmental benefits of digital agricultural technologies in crop production: A review. Smart Agric. Technol. 2024, 8, 100441. [Google Scholar] [CrossRef]
- Leddin, C.; Morse-McNabb, E.; Smith, K.; Ho, C.; Jacobs, J. How can improved farmer decisions and farm system impacts resulting from the use of digital forage measurement technologies on dairy farms be valued? Agricultural Systems 2023, 212, 103755. [Google Scholar] [CrossRef]

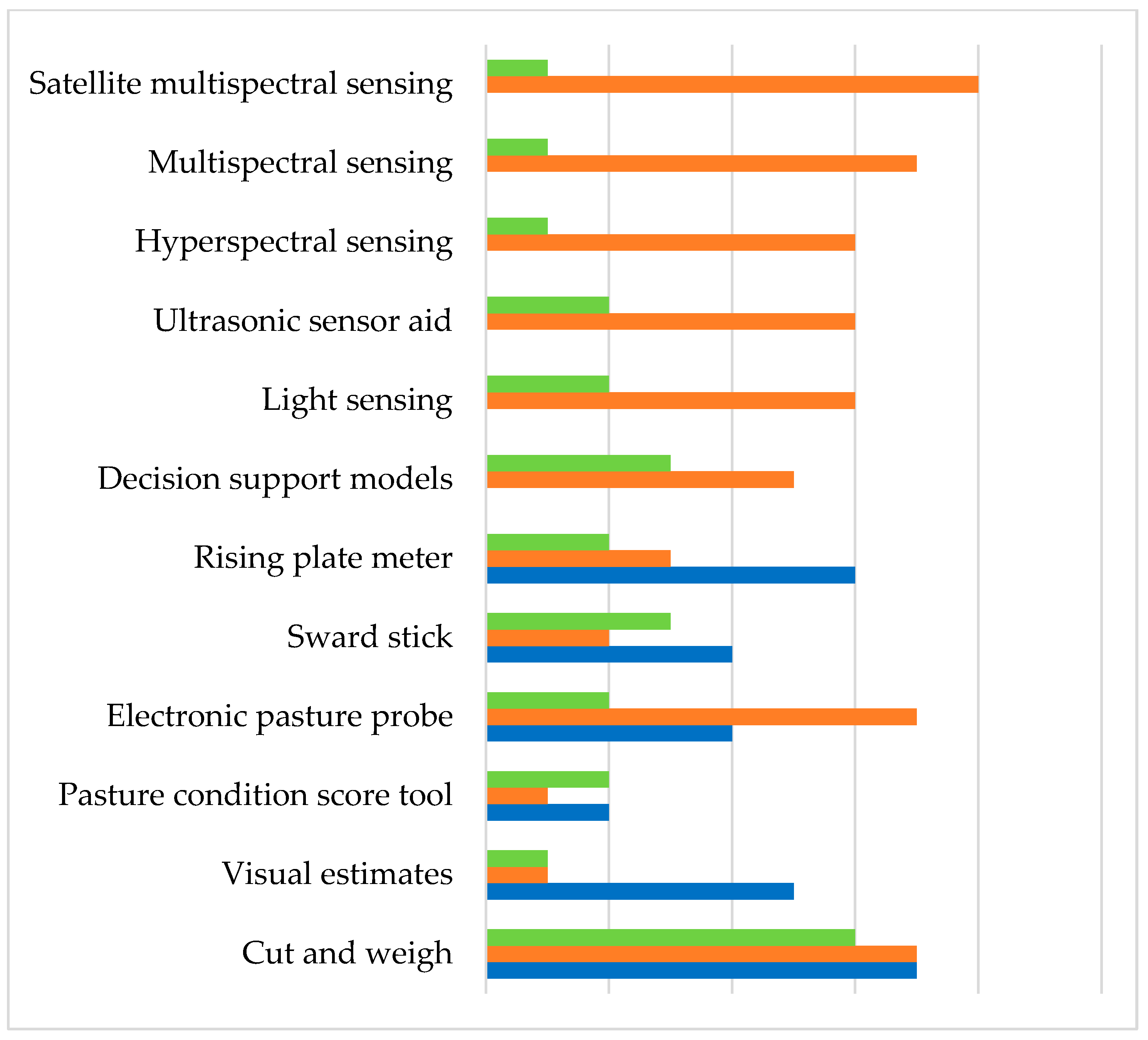
| Assessment Techniques and Tools | Measure | Accuracy | Calibration | Destructive or Non-Destructive | Synergies | Trade-Offs | References |
|---|---|---|---|---|---|---|---|
| Cut and weigh | Weight | High | No | Destructive | Direct method, and accurate for a particular sampling point | Expensive, time-consuming, and labour-intensive Accuracy reduces when generalising to a large area | [21,22,23,24] |
| Visual estimates | SSH and density | Highly variable | Yes | Non-destructive | Quick method, low expense, can assess a large area and is more suited for simple sward | Preliminary training is essential, variation among operators | [8,25,26] |
| Pasture condition score tool | SSH and density | Low | Yes | Non-destructive | Provide timely resource management recommendations | Variation among operators in scoring | [27,28] |
| Electronic pasture probe | SSH | Low | Yes | Non-destructive | Quick and simple method for homogeneous vegetation canopies | Readings are affected by moisture in the vegetation, sward type, and ratio of living to dead material | [8,9,10,23,29] |
| Sward stick | SSH | Low | Yes | Non-destructive | Simple and suitable for recording the sward surface architecture, and best for hill counties | Less accurate with stemmy material and very tall or lodged grass, time-consuming and labour-demanding | [7,9,10,22,30] |
| Rising plate meter | CSH | Moderate | Yes | Non-destructive | Quick and cost-effective Suitable for pure or mixed pastures | Regular calibration is essential, different calibration relationships for various seasons, different species composition and labour demand | [7,8,10,22,31,32] |
| Decision support models | Farm records | - | Yes | Non-destructive | Quick and computer-based method | Complex to use and needs training and demonstrations | [33,34,35] |
| Light sensing (C-DAX) | SSH | Moderate | Yes | Non-destructive | Provides fast, accurate estimates and relatively low cost among other advanced methods, no cloud cover challenges | Require different seasonal calibrations specific to the region | [36,37] |
| LiDAR | SSH | Moderate | Yes | Non-destructive | Less time and multiple measurements can be obtained from the same place | Relatively expensive, and poor ability to measure in windy conditions | [38] |
| Ultrasonic sensor aid | SSH | - | Yes | Non-destructive | Quick response, small instrument, low power consumption and automation | Low accuracy and not suitable for high levels of biomass and sward height | [24,39] |
| Hyperspectral sensing | SA | - | Yes | Non-destructive | Rapid, reliable approach for near real-time quantitative assessment and accurate | Expensive and needs more studies | [40,41] |
| Multispectral sensing | SA | - | Yes | Non-destructive | Reasonable accuracy and affordable | Lack of long-term studies | [40,42] |
| Satellite multispectral | SA | - | Yes | Non-destructive | Large aerial coverage, less time and remote sensing | Cloud cover challenges and needs more studies | [40,43] |
| Country | Pastures | Regression Equation | Season/Month | I | R2 | cv | Error | S.E. | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Japan | Bahia grass pasture (Paspalum notatum) | y = 294 x − 2205 | May | 10 | 0.92 | 0.237 | 481 ** | - | [49] |
| y = 192 x − 1091 | June | 50 | 0.92 | 0.179 | 306 ** | - | |||
| y = 176 x − 1378 | July | 50 | 0.97 | 0.120 | 166 ** | - | |||
| y = 232 x − 1460 | August | 10 | 0.93 | 0.184 | 270 ** | - | |||
| y = 164 x − 286 | September | 50 | 0.91 | 0.168 | 361 ** | - | |||
| y = 366 x − 2378 | October | 10 | 0.89 | 0.256 | 842 ** | - | |||
| Centipede grass pasture (Eremochloa ophiuroides) | y = 269 x − 893 | June | 50 | 0.88 | 0.200 | 338 ** | - | ||
| y = 359 x − 1657 | July | 10 | 0.98 | 0.106 | 318 ** | - | |||
| y = 422 x − 1451 | August | 50 | 0.84 | 0.264 | 1172 ** | - | |||
| y = 283 x − 424 | September | 50 | 0.97 | 0.093 | 341 ** | - | |||
| y = 510 x − 1450 | October | 10 | 0.97 | 0.109 | 443 ** | - | |||
| y = 314 x + 540 | November | 10 | 0.57 | 0.354 | 1275 ** | - | |||
| New Zealand | Plantain (Plantago lanceolata) mix | y = 124.4 x + 1647.8 | Early spring | 168 | 0.66 | - | 21 * | 6.93 | [20] |
| y = 152.6 x + 1609.5 | Late spring | 192 | 0.49 | - | 26 * | 11.26 | |||
| y = 161.1 x + 1188.1 | Summer | 72 | 0.74 | - | 23 * | 11.54 | |||
| y = 109.9 x + 843.3 | Autumn | 144 | 0.59 | - | 20 * | 7.75 | |||
| y = 129.4 x + 1418.5 | Mean | 576 | 0.50 | - | 29 * | 5.44 | |||
| Chicory (Cichorium intybus) mix | y = 118.5 x + 1553.3 | Early spring | 168 | 0.54 | - | 25 * | 8.53 | ||
| y = 142.6 x + 1465.8 | Late spring | 192 | 0.54 | - | 25 * | 9.48 | |||
| y = 119.1 x + 1534.8 | Summer | 72 | 0.56 | - | 26 * | 12.5 | |||
| y = 104.6 x + 814.2 | Autumn | 144 | 0.62 | - | 27 * | 6.89 | |||
| y = 112.5 x + 1453.8 | Mean | 576 | 0.46 | - | 30 * | 5.05 | |||
| Combined plantain and chicory | y = 121.1 x + 1603.9 | Early spring | 168 | 0.59 | - | 27 * | 5.48 | ||
| y = 144.0 x + 1569.8 | Late spring | 192 | 0.51 | - | 26 * | 7.29 | |||
| y = 135.1 x + 1396.7 | Summer | 72 | 0.64 | - | 25 * | 8.56 | |||
| y = 104.7 x + 854.0 | Autumn | 144 | 0.61 | - | 24 * | 4.94 | |||
| y = 118.4 x + 1460.1 | Mean | 576 | 0.47 | - | 30 * | 3.69 |
| Country | Pastures | Regression Equation | Season/Month | i | R2 | cv | Error | S.E. | Reference |
|---|---|---|---|---|---|---|---|---|---|
| New Zealand | High-sugar ryegrass and clover | y = 34.9 + 136.6 x | Summer Autumn Winter Spring | 50 | 0.84 | - | 4.16 * | - | [61] |
| Perennial ryegrass and clover | y = 150.4 + 132.5 x | 0.76 | - | 5.23 * | - | ||||
| Tall fescue and clover | y = 139.4 + 118.5 x | 0.81 | - | 3.95 * | - | ||||
| High-sugar ryegrass, clover, herbs | y = 450.5 + 105.3 x | 0.86 | - | 2.90 * | - | ||||
| Perennial ryegrass, prairie grass, clover, herbs | y = 381.4 + 99.1 x | 0.80 | - | 3.40 * | - | ||||
| Tall fescue, lucerne, prairie, grass, clover, herbs | y = 610.6 + 83.5 x | 0.80 | - | 2.90 * | - | ||||
| New Zealand | Chicory | y = 86 x + 235 | Summer | 244 | 0.73 | - | 664 ** | - | [31] |
| Plantain | y = 94 x + 455 | 135 | 0.70 | - | 711 ** | - | |||
| Ryegrass-based | y = 218 x + 48 | 135 | 0.73 | - | 772 ** | - | |||
| New Zealand | Plantain mix | y = 86.3 x + 1884.7 | Early spring | 168 | 0.63 | - | 22 * | 5.09 | [20] |
| y = 107.4 x + 1753.6 | Late spring | 192 | 0.54 | - | 25 * | 7.12 | |||
| y = 129.9 x + 1204.4 | Summer | 72 | 0.61 | - | 27 * | 12.3 | |||
| y = 100.3 x + 843.0 | Autumn | 144 | 0.68 | - | 18 * | 5.78 | |||
| y = 100.4 x + 1511.1 | Mean | 576 | 0.54 | - | 28 * | 3.86 | |||
| Chicory mix | y = 84.2 x + 1677.6 | Early spring | 168 | 0.52 | - | 21 * | 6.28 | ||
| y = 91.3 x + 1660.2 | Late spring | 192 | 0.55 | - | 25 * | 6.04 | |||
| y = 72.9 x + 1768.6 | Summer | 72 | 0.57 | - | 25 * | 7.50 | |||
| y = 76.3 x + 869.4 | Autumn | 144 | 0.66 | - | 26 * | 4.61 | |||
| y = 77.7 x + 1561.3 | Mean | 576 | 0.48 | - | 30 * | 3.36 | |||
| Combined plantain and chicory | y = 84.4 x + 1794.6 | Early spring | 168 | 0.57 | - | 24 * | 4.03 | ||
| y = 95 x + 1752.4 | Late spring | 192 | 0.52 | - | 26 * | 4.63 | |||
| y = 83.7 x + 1716.1 | Summer | 72 | 0.53 | - | 29 * | 6.59 | |||
| y = 75.5 x + 1019.8 | Autumn | 144 | 0.63 | - | 24 * | 3.41 | |||
| y = 83.8 x + 1596.7 | Mean | 576 | 0.49 | - | 27 * | 2.53 | |||
| Colombia | Ryegrass and kikuyu | y = 79.7 x + 319.7 | Summer | 825 | 0.85 | - | - | 0.53 | [62] |
| Corvallis | Grass-based | y = 87.7 x − 305.5 | Spring | 350 | 0.64 | - | - | - | [63] |
| Legume-based | y = 110.3 x − 405.7 | 350 | 0.81 | - | - | - | |||
| Grass-based | y = 65.1 x − 32.9 | 350 | 0.72 | - | - | - | |||
| Legume-based | y = 61.0 x − 79.2 | 350 | 0.81 | - | - | - | |||
| Herb-based | y = 79.1 x − 403.5 | 350 | 0.84 | - | - | - | |||
| Ireland | Perennial and hybrid ryegrass | y = −227.6 + 233.3 x −5.35 × 2 | Spring, Summer, and Autumn | 1640 | 0.59 | - | 19.8 * | - | [57] |
| y = −446.5 + t + m + 263.9 x − 6.6 × 2 | 1640 | 0.70 | - | 17.9 * | - | ||||
| y = 111.8 + t + m + 8.9 d + 118.7 x | 1640 | 0.68 | - | 19.2 * | - | ||||
| Austria | Grass-based | y = (x − 40) × 25 | Spring, Summer, and Autumn | 3796 | 0.73 | 33.7 | - | - | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susruthan, V.; Donaghy, D.J.; Kenyon, P.R.; Sneddon, N.W.; Cartmill, A.D. Measuring Herbage Mass: A Review. Agronomy 2025, 15, 2264. https://doi.org/10.3390/agronomy15102264
Susruthan V, Donaghy DJ, Kenyon PR, Sneddon NW, Cartmill AD. Measuring Herbage Mass: A Review. Agronomy. 2025; 15(10):2264. https://doi.org/10.3390/agronomy15102264
Chicago/Turabian StyleSusruthan, Varthani, Daniel J. Donaghy, Paul R. Kenyon, Nicholas W. Sneddon, and Andrew D. Cartmill. 2025. "Measuring Herbage Mass: A Review" Agronomy 15, no. 10: 2264. https://doi.org/10.3390/agronomy15102264
APA StyleSusruthan, V., Donaghy, D. J., Kenyon, P. R., Sneddon, N. W., & Cartmill, A. D. (2025). Measuring Herbage Mass: A Review. Agronomy, 15(10), 2264. https://doi.org/10.3390/agronomy15102264






