1. Introduction
Plants have been a natural source of medicinal substances for thousands of years. Most of these substances are secondary metabolites that do not affect plant growth or development [
1]. Secondary plant metabolites are compounds that are not directly necessary for the performance of basic life functions, but they play an important role in protection against unfavorable biotic factors by modifying biochemical and physiological processes, as well as the color and taste of flowers, fruits, and leaves [
2,
3]. The synthesis of secondary metabolites is, therefore, significantly and directly influenced by habitat conditions. The most important groups of secondary metabolites is represented by primary phenolic compounds, terpenes, and non-protein nitrogen compounds (
Table 1) [
2,
4]. These compounds play key roles in the defensive abilities of plants against pathogens, interacting with pollinators, and protecting against environmental stress [
4,
5,
6]. The presence of pathogens, pests, or symbionts (e.g., mycorrhizal fungi) can also affect the production of secondary metabolites. Mycorrhiza (a symbiosis of plant roots with fungi) and interactions with nitrogen-fixing bacteria can affect plant metabolism by increasing the availability of nutrients, which, in turn, can stimulate the production of specific compounds. Habitat conditions greatly impact the composition and concentration of biologically active compounds in medicinal plants [
5].
Plant individuals colonize disturbed habitats, for example, post-mining mineral sites, and then plants are exposed to unique environmental challenges [
7]. Plants subjected to harsher, more challenging environments often synthesize higher quantities of biologically active substances with medicinal properties. In general, the more stressful the habitat, the greater the abundance of active chemical compounds produced [
1,
6]. Therefore, in order to understand the therapeutic potential of a plant, knowledge about the environmental conditions in which a given species occurs is essential.
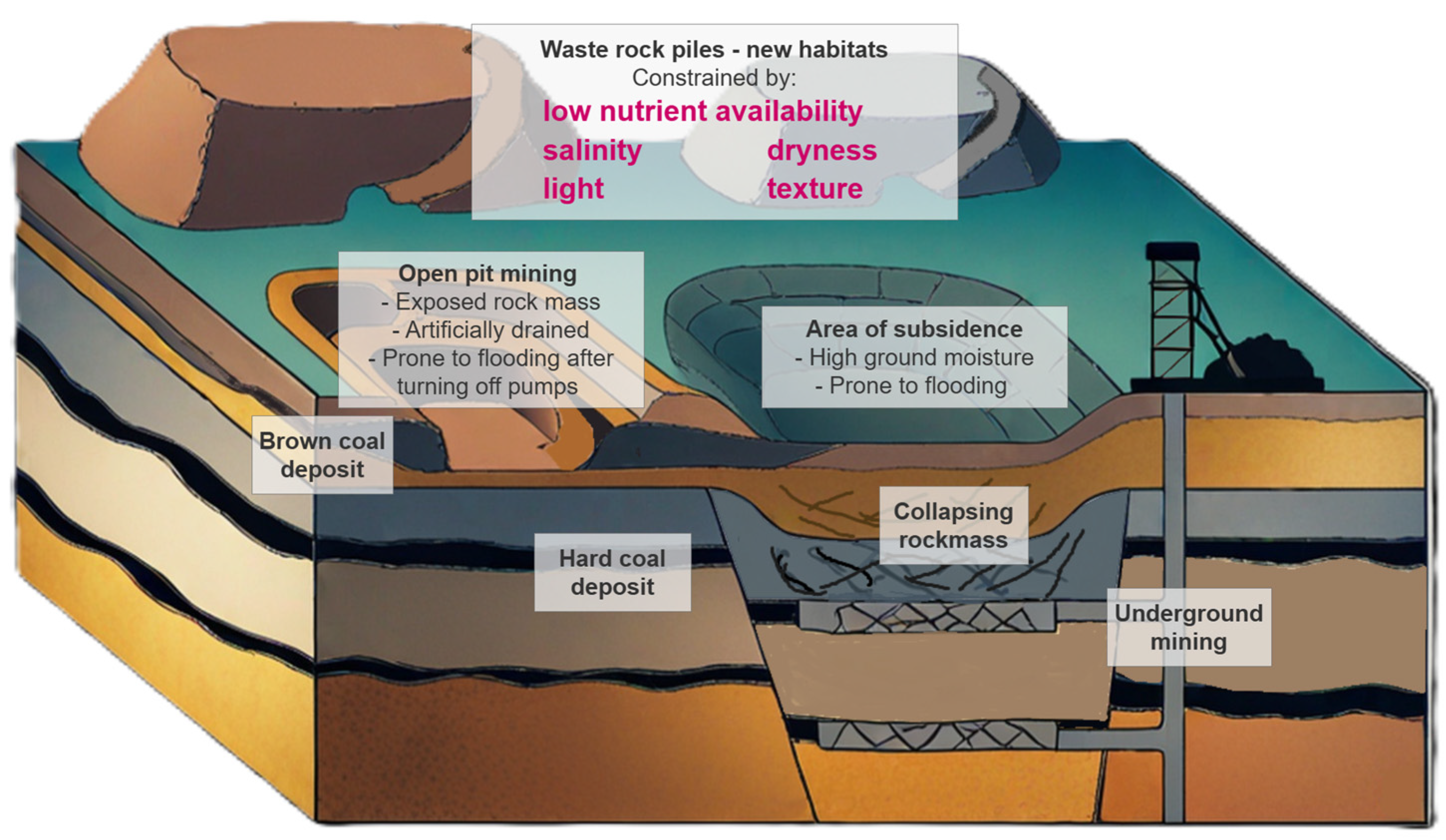
As a result of the authors’ own studies of the spontaneous vegetation on post-mining mineral sites, it was noted that several of the species recorded there belong to groups traditionally recognized as medicinal plants. The scientific question arises of how the challenging conditions (including salinity, extreme pH, drought, unfavorable texture of the mineral post-mining substrates, extreme temperature, and the occurrence of bacteria and fungi) of the habitats of these medicinal plants, influence the amount and quality of the synthesis of their secondary metabolites. The post-mining sites with medicinal plants growing spontaneously on such areas become a “Living Laboratory” and provide the opportunity to study the secondary metabolites from such plants [
8]. The “Living Laboratory” approach is “a new methodological approach—building on and adding distinctive features to the tradition of action research” [
9].
This article aims to review the current state of knowledge of the impact of the severe habitat conditions of post-mining mineral sites, such as salinity, extreme pH, drought, extreme light, temperature, and heavy metal presence, on the synthesis of secondary metabolites. In the future, this will allow for consideration of using post-mining habitats to enhance the physiological mechanisms of synthesizing secondary metabolites.
Table 1.
Basic division of secondary metabolites of vascular plants [
10].
Table 1.
Basic division of secondary metabolites of vascular plants [
10].
| Phenolics | Terpenes | N Containing Compounds | S Containing Compounds |
|---|
| flavonoids, e.g., quercitin | monoterpenes, e.g., menthol | alkaloids, e.g., vincristine | glutathione |
| isoflavonoids, e.g., genistein | diterpenes, e.g., rosmanol | cyanogenic glucosides, e.g., amigdalin | glucosinolates |
| coumarin, e.g., aesculin | triterpenes, e.g., betulin | non-protein amino acids, e.g., cycloserine | phytoalexine |
| furano-coumarin, e.g., psoralin | tetraterpenes, e.g., lycopene | | thionins |
| tannins, e.g., tannic acid | polyterpenes, e.g., guta-percha | | defensins |
| | sesquiterpenes, e.g., bisabolol | | allinin |
2. Major Types of Plant Secondary Metabolites Synthesized in Challenging Habitat Conditions
The chemical compounds of secondary metabolites are divided into three main groups (
Table 1).
Phenolic compounds are a group of secondary metabolites that protect plants against pathogens, attract pollinators, and enable adaptations to various stress conditions [
11]. The most important phenolic compounds are flavonoids, anthocyanins, and tannins [
12]. Flavonoids exhibit primarily antioxidant activity [
11]. Anthocyanins are pigments whose synthesis is stimulated by UVB radiation [
13]. Tannins taste bitter and protect plants from herbivores [
12].
Terpenes play many important roles in the physiological processes of plants. They protect plants from abiotic habitat conditions and stress [
12] and potentially play a chemoprotective role [
14,
15]. This group also includes pigments and antioxidants, such as carotenoids and xanthophylls [
13].
Non-protein nitrogen compounds are primarily stored in vacuoles. They are often toxic defensive substances to humans and animals [
16]. However, they are also valuable medicinal substances [
10,
12].
3. Stress Factors
The stress factors to which plants are subjected in their natural habitat are divided into (
Figure 1) [
6] the following categories:
Biotic—bacteria, viruses, fungi, herbivores, competing species causing allelopathy;
Abiotic—physical or chemical, which include the following:
- -
Mechanical damage, e.g., by wind, snow, ice;
- -
Salinity related to excess NaCl in the substrate causing disruption of osmotic pressure;
- -
Temperature—too low or too high;
- -
Exposure—too low or too high;
- -
UV—too intense (mutagenic factor);
- -
Heavy metals—too high concentration;
- -
pH—too low or too high.
Most stress factors disrupt homeostasis and thus disrupt physiological processes [
6].
Plants have evolved various stress-resistant mechanisms that enable adaptation to a spectrum of stressors and changed environmental conditions. A plant exposed to a stressor at low intensity activates “defence systems” that strengthen the plant’s resistance to more intense stress later on. This process is called conditioning [
6].
A common effect of abiotic stress is oxidative damage caused by reactive oxygen species (ROS). The formation of ROS accompanies metabolic processes occurring in cells under aerobic conditions. In plants, the main natural sources of ROS are thylakoid membranes and mitochondrial cristae, where electron transport occurs. To protect against oxidative stress, antioxidant systems function in cells to maintain the level of ROS at a level safe for the body [
6].
ROS are characterized by high chemical activity and, therefore, have a short lifetime [
17,
18]. Because of magnetic properties, ROS may be present in two molecular forms: reactive dia- and paramagnetic molecules [
19]. An example of diamagnetic ROS is singlet oxygen (
1O
2) produced in plants from chlorophyll exposure to light and hydrogen peroxide (H
2O
2). Paramagnetic properties include a hydroxyl radical (•OH) and a superoxide anion (O
2•). Both dia- and paramagnetic ROS participate in many chemical reactions in biological systems, leading to structural modifications. ROS radical reactions and reactions involving the products of these reactions occur in plants. ROS and their reactions are presented in detail in research works [
17,
18,
20,
21,
22].
4. Influence of Abiotic Factors on Plant Biosynthetic Activity
4.1. Drought
This stress factor quickly affects plant physiology, especially seed germination. One of the key mechanisms for dealing with water stress is osmotic adjustment, which aims to reduce the water potential in cells [
6]. Drought stress also increases the production of secondary metabolites.
Hypericum brasiliense increased the content of betulinic acid and phenolic compounds, including flavonoids, during drought [
23,
24]. In a state of moderate drought,
Scutellaria baicalensis accumulates larger amounts of baicaine [
25], and
Prunella vulgaris increases the content of rosmarinic and ursolic acid [
26]. The accumulation of substances such as phenols, alkaloids, and terpenoids by medicinal plants prevents the formation of ROS and protects plants against oxidative stress [
27].
4.2. Salinity
Most plants do not require the presence of sodium in the environment [
28], but excess sodium chloride in the substrate reduces the water potential of the soil solution, contributing to water stress in plants. Excessive accumulation of Na+ and Cl- ions causes a cytotoxic effect where metabolic processes slow down and plant organs age and die [
6]. Additionally, sodium ions can penetrate cells, competing with potassium ions, depriving the plant of an element essential for life [
28]. Excessive salinity significantly limits the growth and development of
Catharanthus roseus in crops. In this case, salinity tolerance is achieved through arbuscular mycorrhiza [
29]. In halophytes, one of the ways they cope with salt stress is to accumulate antioxidant phenolic compounds that eliminate ROS [
30].
Nigella sativa, when grown in conditions of increased salinity, had an increased content of, among others, medicinal apigenin and quercetin [
31]. In similar conditions, in
Matricaria chamomila, the amount of phenolic compounds was higher [
32], and in
Catharamtus rosaeus, the content of vincristine [
1], a cytostatic alkaloid used in anti-cancer therapies, was increased [
33]. In the shoots of
Ricinus communis, an increased content of ricin was found [
34]. Plants of
Plantago ovata growing in a saline medium had increased concentrations of flavonoids and saponins [
35].
4.3. Light
Light is a basic abiotic factor influencing plant growth, development, and metabolism. Wavelength, intensity of light exposure, and photoperiodism are important [
36]. Plant stress can be caused by too-low and too-high light intensity. Light stress primarily inhibits the photosynthesis process and the activity of antioxidant systems. It also affects the transpiration process.
To protect against excessive exposure to light, including excessive UV radiation, plants use the following strategies [
6,
37,
38]:
Changes in leaf orientation;
Changes in leaf reflectance by creating a protective layer on the leaf surface in the form of mechanical hairs;
Changes in chloroplast distribution within cells exposed to excessive light exposure;
Inactivation of PSII—photoinhibition, starting photorespiration; the resulting glutathione is an antioxidant;
Non-photochemical quenching;
Mehler reaction.
The most dangerous UV radiation naturally stimulates the synthesis of secondary metabolites with antioxidant activity [
5,
39,
40].
4.4. Temperature
Thermal stress is one of the key factors affecting the growth and development of plants in crops, and in natural and anthropogenically modified habitats [
1]. The response of plants to thermal stress is not uniform. In the case of
Astragalus compactus, an increase in temperature contributed to an increase in the content of secondary metabolites [
41].
Panax quinquefolium responded similarly, increasing the ginsenoside content in the radix root, a medicinal raw material [
42]. High temperature also stimulates the synthesis of terpenes [
43]. On the other hand, in cultivated
Chrysanthemum varieties imported from the Netherlands to Japan, high temperatures limited the synthesis of anthocyanins responsible for the color of the corolla petals [
44]. In turn, low-temperature stress increases the intensity of the production of
Arnica montana flavonoids used in medicine [
1]. Extreme temperatures caused by climate change often exceed the adaptive capacity of plants and lead to the production of ROS and oxidative stress.
4.5. Heavy and Trace Metals
The main reasons for their appearance in the environment are anthropogenic activities. They are not biodegradable and are characterized by significant mobility. They most often enter plants through the root system. Exposure to the toxic effects of metals stimulates the activity of antioxidant systems through increased production of, among others, glutathione and ascorbic acid [
6]. Plants with increased tolerance to the content of heavy metals in their body are called hyperaccumulators.
5. Influence of Biotic Factors on Plant Synthesizing Activity
5.1. Pathogens
A plant’s response to pathogens is always connected to the activation of several metabolic pathways under the influence of elicitors, responsible for, among others, the synthesis of stress proteins and secondary metabolites that have a toxic effect on the presence of the pathogen. The induced response mechanisms of the plant include intensive synthesis of compounds with antimicrobial properties, including phytoalexins. The content of these compounds in healthy cells is low. However, their concentration increases rapidly as a result of stress factors. The group of secreted compounds is usually characteristic of its botanical family, e.g.,
Fabaceae defensively produces mainly flavonoids, and
Solanaceae produces terpenoids [
6]. Plants rich in secondary metabolites are a rich source of substances that combat various pathogens [
45]. Many of these substances exhibit antimicrobial properties. Scientific studies have confirmed the antibacterial activity of, among others,
Sysygium aromaticum leaf extract, which proved effective in combating
Kliebsiella pneumoniae [
46]. In turn,
Artemisia absynthium leaf extract inhibits Enterobacter faecidum and
Streptopus aureus [
47]. Some secondary metabolites also exhibit antifungal activity. Extract of leaves and twigs of
Chamaecyparis pisifera showed activity against
Aspergillus oryzae [
48]. Substances contained in the celluli of
Allium sativum L. have demonstrated potent antiviral activity against many viruses [
49].
5.2. Arbuscular Mycorrhizal Fungi
Plant microorganismal interactions are frequent in vegetation patches in natural, seminatural, and disturbed ecosystems. Mycorrhizal fungi help plants adapt to difficult habitat conditions [
50]. Disturbed habitats with high abiotic stress potential are spaces for the appearance of new species of mycorrhizal fungi, such as
Rhizoglomus silesianum, discovered on a coal heap in Poland [
51]. According to Magurno [
50], “
Rhizoglomus silesianum will be useful for the production of inocula to replace synthetic fertilizers and pesticides. This feature makes it highly desirable for agriculture” [
50]. Moreover, medicinal plants colonized by arbuscular mycorrhizal fungi (AMF) show an increased content of various secondary metabolites constituting medicinal substances, which is a result of the activation of metabolic pathways of the defense response to stress factors [
52].
Plant-growth-promoting rhizobacteria enhance plant growth and promote the accumulation of various active substances in the roots [
53,
54,
55,
56]. AMF are identified as improving plant growth parameters and biomass and increasing secondary metabolite concentrations in many plant species [
57,
58]. Improvements in the absorption of mineral nutrients, increase in biomass establishment, and changes in the amount of polyphenols, flavonoids, and anthocyanins are the result of arbuscular mycorrhizal fungi inoculation [
59].
5.3. Pests
Pests are another factor that causes secondary metabolite synthesis. To defend against them, plants have developed antibiosis, which is a type of passive immunity based on the harmful effects of plant consumption through the accumulation of anti-nutritional substances such as enzyme inhibitors, toxins, or repellents (taste or odor compounds that repel pests) [
6,
16].
6. Post-Mining Site Habitat Conditions and Secondary Metabolite Synthesis
For a long time, natural habitat conditions have shaped the particular composition of primary and secondary metabolites. Individual plants responded to harsh and unfavorable habitat conditions with more complex and complex synthesis.
In primary metabolism, biosynthetic pathways are predominantly regulated at the genetic level. However, certain biosynthetic processes can also be stimulated by external environmental factors. These stressors initiate complex signaling cascades involving the production of signaling molecules [
4]. For example, the generation of reactive oxygen species (ROS) under stress conditions has been shown to induce the biosynthesis of apocarotenoids [
60]. Advances in our understanding of these biosynthetic pathways and the identification of key regulatory targets have opened new possibilities for the targeted engineering of secondary metabolism.
7. Environmental Influence on the Composition of Primary and Secondary Metabolites in Plants
Plants have had to adapt and develop complex biochemical systems to enable them to grow in a wide range of environmental conditions. Natural habitat conditions, including nutrient availability, drought, extreme pH, high salinity, and complex texture composition, have had a crucial role in shaping the evolution of both primary and secondary metabolic pathways. Sugars, amino acids, and lipids are listed among the primary metabolites and these are essential for basic growth and plant development. Secondary metabolites, e.g., alkaloids, flavonoids, and terpenoids, function primarily in effective adaptive responses to environmental challenges. There is growing evidence to suggest that harsher and more challenging habitat conditions cause more complex and intensified synthesis of secondary metabolites to enhance both protective and ecological functions [
61].
7.1. Natural Habitats as Selective Filters of Metabolic Profiles
Natural environmental gradients, such as soil fertility, water availability, temperature extremes, UV radiation, and biotic interactions, exert strong selective pressures on the biochemical composition of plant individuals. These habitat constraints influence not only the diversity of species present but also intraspecific variation in their metabolic traits. As plants adapt, their primary metabolic pathways are adjusted to meet basic survival needs under stress, and these shifts often serve as precursors for regulation of secondary metabolite production [
62].
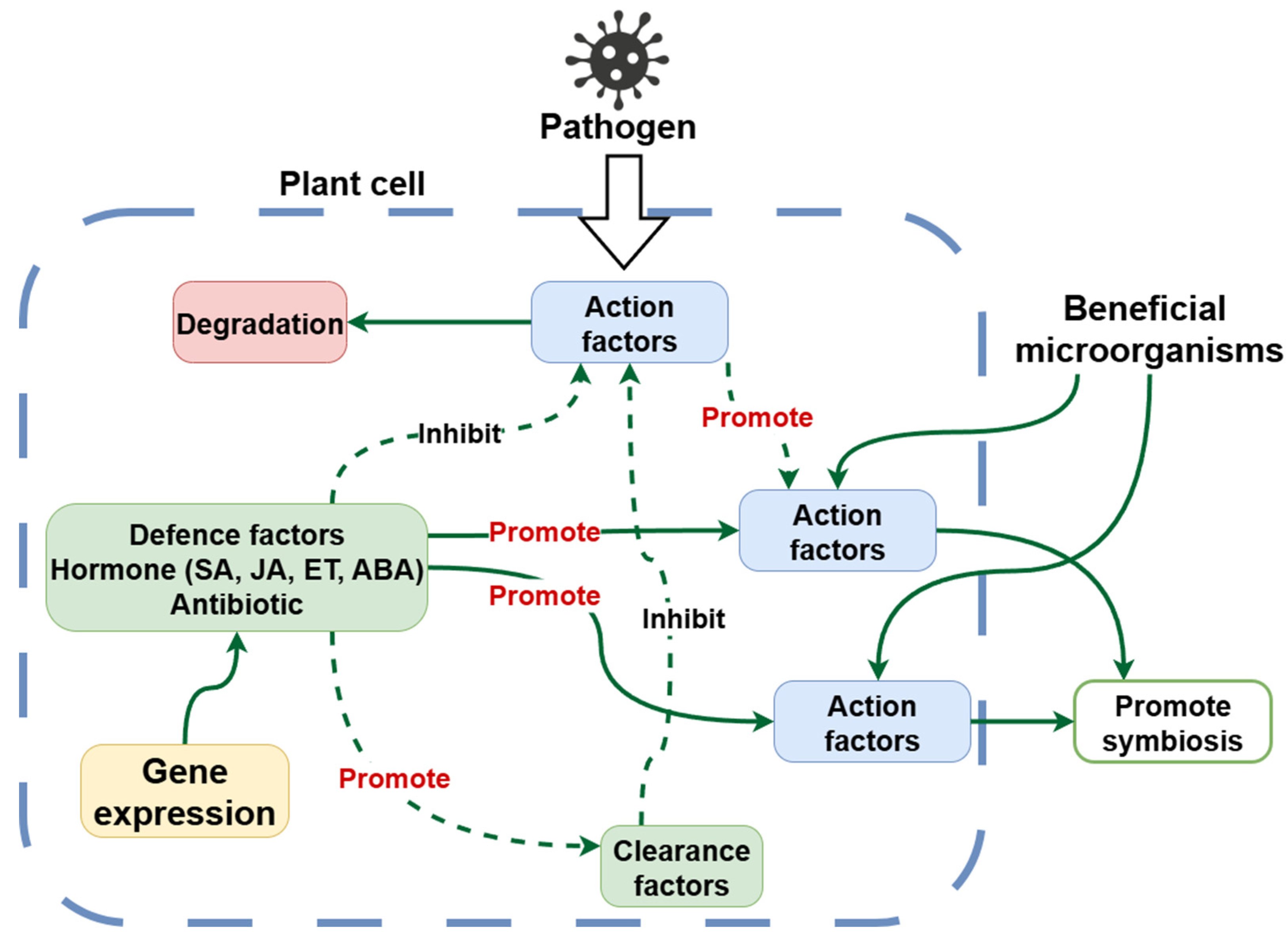
7.2. Harsh Conditions Stimulate Secondary Metabolite Synthesis
Under stressful conditions, plants often redirect resources toward the synthesis of defensive and protective compounds. These secondary metabolites function in UV protection, antioxidant activity, allelopathy, herbivore and pathogen resistance, and symbiotic interactions [
63]. For example, flavonoids increase under UVB radiation to protect against DNA damage [
64]. Phenolic compounds and terpenes often accumulate in response to pathogen attack or wounding [
65]. Plants growing in metal-contaminated soils tend to produce higher levels of polyphenols and alkaloids, which act as metal chelators or ROS scavengers (
Figure 2) [
66].
7.3. The Role of Phytohormones in Stress Responses
Plants are challenged by diverse biotic and abiotic stress factors. To survive and adapt, they rely on complex signaling networks that integrate external cues with endogenous regulatory mechanisms.
Plants have developed various mechanisms for responding to stress and rapidly regulating metabolism [
68]. Abiotic stress factors lead to the accumulation of salicylates in cells. Phytohormones participate in this regulation [
68]. Infection of plants with endophytes results in inhibition of the salicylic acid (SA) pathway, which, in turn, leads to stimulation of the JA pathway [
67]. Jasmonates are phytohormones that support the fight against necrotrophic pathogens and biting insects. Salicylates, in turn, participate in defense against biting animals and biotrophic and hemibiotrophic pathogens [
69]. Increased ethylene production accompanies plant responses to thermal stress, drought, and pest feeding [
69]. A key phytohormone in response to biotic and abiotic stress is abscisic acid (ABA) [
70]. Regulating stomatal function, it plays a key role in plant responses to drought, high temperatures, and osmotic stress, among other factors. It also protects the plant body against pathogen entry through stomata [
71]. Increased ethylene production accompanies the plant’s response to thermal stress, drought, and pest feeding.
8. Adaptive Significance of Metabolic Shifts
Plant individuals synthesize biological compounds, secondary metabolites, in response to challenging environmental factors. These synthesized metabolites are not only the by-products of plant metabolism but they enable the ability for strategic energy investment in long-term survival and reproductive success. These synthesized compounds are specifically adjusted to enhance fitness by mitigating the effects of abiotic stress and thus increasing competitive advantage. For instance, research on alpine plants has shown that individuals growing at higher altitudes, where UV exposure is greater and temperatures are lower, accumulate higher levels of anthocyanins and flavonoids [
72]. Moreover, intraspecific variation in metabolite profiles promotes phenotypic plasticity, allowing individual plants to adjust their metabolic strategies in response to local environmental conditions [
73].
Fikriah et al. [
74] performed a qualitative phytochemical analysis of individual plants growing in a post-coal mining and a non-mining soil substrate area, and the results showed the presence of flavonoids and polyphenols with antioxidant activity. Additionally, the presence of terpenoids without antioxidant potential was recorded, the level of which was slightly elevated.
9. Implications for Biodiversity and Bioprospecting
Understanding how natural habitats shape plant metabolic profiles has direct implications for biodiversity conservation, ecosystem functioning, and natural product discovery. Extreme habitats, such as post-mining sites, arid regions, and alpine environments, often harbor plants with unique secondary metabolite compositions, which makes them valuable for pharmaceutical and biotechnological exploration [
75].
Plants have developed mechanisms of protection against ROS particles. Plants control the level of ROS through a complex system of antioxidants, which includes antioxidant enzymes, as well as antioxidant substances such as the following:
Ascorbic acid (ASC);
Glutathione (GSH);
Tocopherol;
Flavonoids;
Anthocyanins;
Carotenoids.
Interaction of enzymatic and non-enzymatic antioxidants effectively prevents oxidative damage and enables adaptations to unfavorable environmental conditions [
6]. Antioxidants neutralize free radicals [
18,
76]. The interactions of plant antioxidants with free radicals can be examined with the use of electron paramagnetic resonance (EPR) spectroscopy and ultraviolet–visible spectrophotometry (UV-Vis) [
77,
78,
79,
80,
81,
82,
83,
84]. Both methods use the model free radical DPPH (2,2-diphenyl-1-picrylhydrazyl) [
18,
85]. A measure of antioxidant properties is a decrease in the amplitude of EPR spectra of DPPH free radicals due to interactions with antioxidants. The UV-Vis method determines a decrease in the absorbance of the wavelength of 515 nm after the addition of the antioxidant substance to the solution of DPPH [
79,
84].
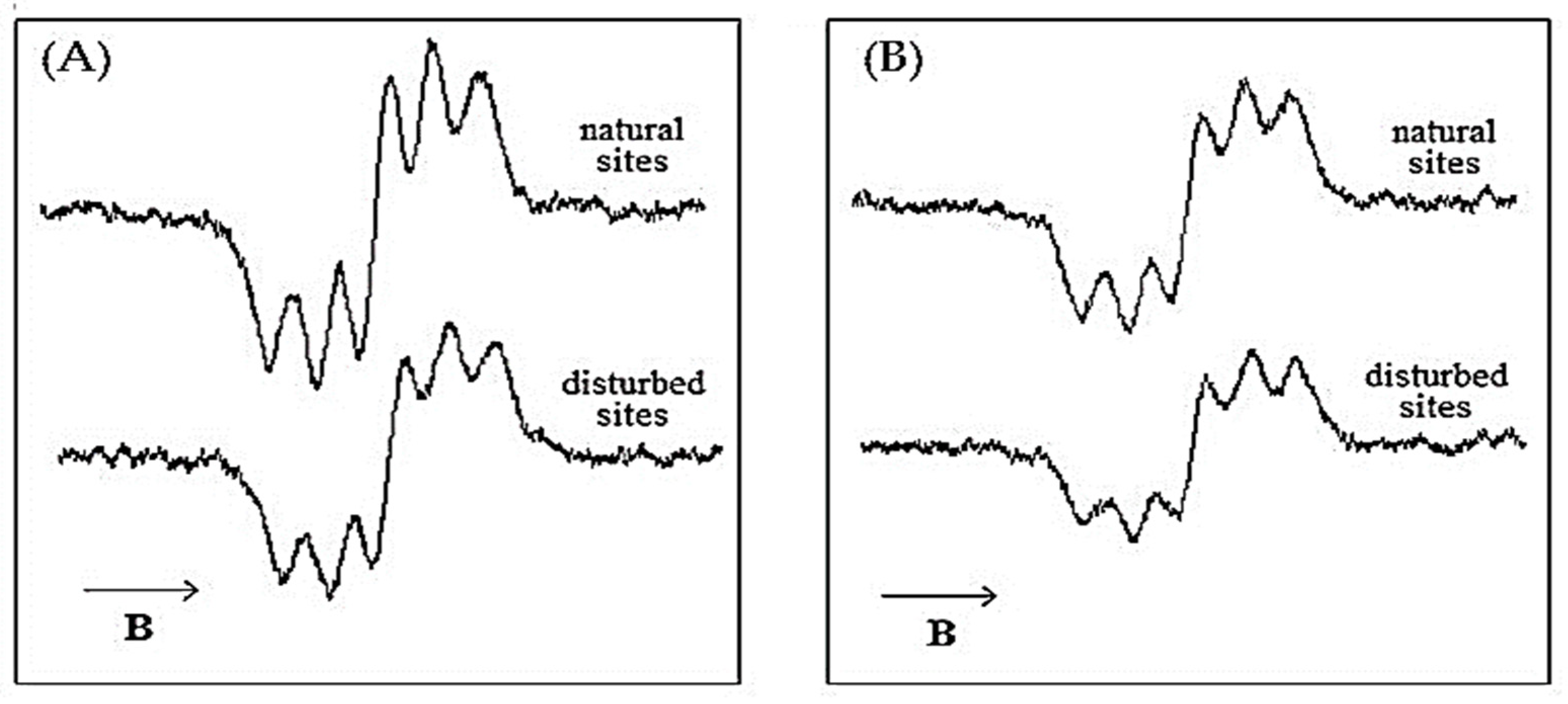
Our observations of different extracts of
Vaccinium myrtillus have indicated differences between the antioxidant properties of infusions obtained from
V. myrtillus individuals growing on natural and disturbed sites.
Figure 3 presents a comparison of the neutralization of DPPH free radicals by the exemplary infusions of
V. myrtillus individuals obtained from natural and disturbed sites. In the figure, the neutralization of DPPH free radicals was taken into account by studying the neutralization of DPPH free radicals after 10 min (
Figure 3A) and after 60 min (
Figure 3B).
Figure 3 reveals that the interactions of the infusions with DPPH free radicals are different for individuals growing in disturbed and natural sites. The quenching of DPPH free radicals is visible for the infusion obtained from
V. myrtillus from disturbed areas and is stronger than that from natural habitat conditions after both 10 min (
Figure 3A) and 60 min (
Figure 3B) of interactions. Detailed studies of this phenomenon were presented in the study of Bacler-Żbikowska et al. [
5].
An exemplary study of the antioxidant properties of plant extracts with the use of EPR and UV-Vis methods was performed for infusions of leaves of the bear berry
Arctostaphylos uva-ursi, lingonberries
Vaccinium vitis-idea, and bilberries
Vaccinium myrtillus [
5]. It was noted that stronger antioxidant properties were revealed in water extracts obtained from raw plant materials occurring in dry areas with increased UV radiation intensity compared with habitats with moderate water and radiation conditions.
10. Discussion
The challenging conditions of habitats, including salinity, extreme pH, drought, unfavorable texture of the mineral post-mining sites, high or low temperature, and the occurrence of bacteria and fungi, influence the synthesis of secondary metabolites in terms of amount and quality.
The biological functions of metabolites were unrecognized due to the frequently low concentrations of metabolites in plants, and earlier, they were recognized as metabolic waste or as bioproducts of detoxification. The current knowledge about secondary metabolites, which are known to have toxic effects on animal cells, is insufficient.
This review has highlighted the crucial role of severe habitat conditions found in mineral habitats in enhancing the synthesis of plant secondary metabolites. Today, it is estimated that more than 30% of medicinal compounds are derived, either directly or indirectly, from natural wild-growing plant sources [
86,
87,
88]. The synthesis of secondary metabolites is dependent on the ecophysiological process, which reflects the ecosystem functions’ adjustment to environmental challenges [
89]. Over the last century, rapid advancements in high-throughput and sophisticated analytical technologies have greatly accelerated the exploration and characterization of plant metabolites. This has resulted in the significant expansion of knowledge in this field. Moreover, growing recognition of the ecological importance and diverse benefits of secondary metabolites has further fueled interest and research in this area over the past four decades. One example of the underestimated role of secondary metabolites is their impact on the biomass biochemical [
90,
91].
The use of plant-derived metabolites dates back to around 2600 BC, and for the next 4000 years, secondary metabolites from plants were primarily utilized for medicinal, toxicological, and nutritional purposes. A major milestone in this field was the isolation of morphine from the opium poppy (
Papaver somniferum) in 1806, which marked the beginning of modern research into secondary metabolites [
92]. This discovery demonstrated that the biological activity of plant extracts could be attributed to distinct organic compounds with defined chemical identities that could be isolated and purified [
93].
Post-mining sites are often colonized by spontaneous plants and other organisms [
94,
95,
96,
97]. There are studies showing that among the spontaneous plants that colonize post-mining sites, there is a group of plant species of pharmacological importance [
5,
98]. Fikriah et al. [
74] studied pioneer plants of pharmacological importance colonizing mining sites in Indonesia. They studied the quantity and quality of the secondary metabolites of
Macaranga sp. They found that synthesis of the secondary metabolites of
Macaranga sp. is highly influenced by soil pH and nutrient content, which, in turn, impact their biological activities. Therefore, evaluating the secondary metabolite profile and antioxidant activity of
Macaranga spp., such as
M. tanarius growing on post-mining land, along with several soil parameters such as pH and nutrients, may help to evaluate the likely success of land recovery at post-mining sites [
74].
Fikriah et al. [
74] performed a qualitative phytochemical analysis of plants from post-coal mining areas and non-mining areas, which showed the presence of flavonoids and polyphenols with antioxidant activity, and the presence of terpenoids without antioxidant potential. Previous studies have revealed that individuals of selected species of the
Macaranga genus (
M. hosei) contain flavonoids [
99], and that
M. bancana synthesizes polyphenols and terpenoids [
100]. The quantitative analysis presented showed that plants from post-coal mining areas had significantly higher total phenolic compounds compared to non-mining areas.
The vast chemical diversity of plant metabolites includes many complex and difficult structures, which, in some cases, are considered impossible to replicate through industrial synthesis [
92,
101].
10.1. Oxidative Damage Induced by Reactive Oxygen Species (ROS) as a Common Effect of Abiotic Stress in Plants
One of the most common responses of plant individuals to stress from habitat conditions is the synthesis of secondary metabolites that neutralize reactive oxygen compounds. Plants, as sessile organisms, are exposed to various abiotic natural and human-induced stressors such as drought, salinity, extreme temperatures, heavy metal presence, and high light intensity, many of which occur on post-mining mineral sites. These stress conditions can disrupt cellular elements and metabolic functions, causing the overproduction of ROS. ROS, e.g., O
2−, H
2O
2, OH,
1O
2, occur in stressed organisms as partially reduced forms of molecular oxygen that can oxidize various cellular components [
102]. These reactive molecules are synthesized as a byproduct of aerobic metabolism in different parts of the cell. ROS are actively synthesized by specialized enzymes [
103]. In plants, ROS synthesis takes part mainly in the chloroplast during photosynthesis, the mitochondrion during respiration, and in the peroxisome and apoplast via enzymes such as NADPH oxidases (RBOHs) [
104]. ROS are crucial in cell signaling at particular physiological levels; their excessive accumulation under stress conditions results in oxidative damage, affecting plant growth, development, and survival [
103]. ROS are primarily produced in organelles associated with high metabolic activity, such as chloroplasts, mitochondria, and peroxisomes. Under optimal conditions, the generation and scavenging of ROS are tightly regulated. However, abiotic stress conditions often disrupt this balance, resulting in oxidative stress [
105].
10.2. Abiotic Stress and ROS Overproduction
Abiotic stress leads to changes in cellular redox status by enhancing ROS generation. The main abiotic stressors that induce ROS generation include the following:
Drought: reduced water availability limits CO
2 intake, causing an over-reduction in the photosynthetic electron transport chain and the leakage of electrons to form O
2− [
106].
Salinity: high salt concentrations cause osmotic and ionic imbalances, disrupting mitochondrial respiration and promoting the formation of ROS [
107].
High light and UV radiation: excess photons overwhelm the photosynthetic mechanism, generating ROS in chloroplasts [
108].
Heavy metals: metals such as cadmium (Cd), lead (Pb), and arsenic (As) enhance ROS presence by redox cycling or by impairing the antioxidant defense system [
109].
10.3. Consequences of Oxidative Damage
Higher levels of ROS surpass a plant’s detoxification ability. As a consequence, damage occurs to vital cellular components, including lipids, proteins, and DNA. The higher ROS presence also causes inhibition to photosynthesis, enzyme activity, nutrient transport, and cellular signaling. All these effects impact the growth of cells, frequently resulting in cell death [
110].
10.4. Plant Antioxidant Defense Mechanisms
Mechanisms to avoid oxidative stress are based on the antioxidant defense system, including enzymatic and antioxidants synthesis. The ROS function is the activation of the positive signaling molecules’ activity [
111].
The antioxidant activity of phenolic compounds in plants involves inhibiting the formation of free radicals and/or their removal and/or stimulating internal cellular defense mechanisms. Plant secondary metabolites may act through one of these methods or combine several synergistically. The antioxidant activity of polyphenols increases with the number of hydroxyl groups. An example of a phenolic compound with antioxidant properties is quercetin, present in many plant species rich in antioxidants, found on post-mining habitats. Examples of medicinal plants with strong antioxidant properties include quercetin-rich flowers of
Sambucus nigra (
Sambuci flos), fruits of
Vaccinium myrtillus containing anthocyanin (
Myrtylli fructus), or flowers of
Centurea cyanus (
Cyani Flos) [
112,
113,
114].
10.5. Root Colonization by Arbuscular Mycorrhizal Fungi
Colonization of the root by arbuscular mycorrhizal fungi has been shown to reduce the impact of herbivorous feeding by the aphid
Aphis gossypii by increasing the amount of flavonoids and phenolics [
115] and causing an increase in proline and phenol content in the plant body. These have been shown to improve plant growth under unfavorable conditions and to modify oxidative systems, hormones, and ionic homeostasis to improve plant salt tolerance [
116,
117]. The above studies results show that microorganisms support the protection mechanisms of plants from biotic and abiotic stresses. The protection mechanisms are based on the improvement in plant nutrient uptake, enhancing growth and metabolite accumulation while supporting plant resilience.
10.6. Implications for Agriculture and Stress Tolerance Breeding
Most of the studies related to the impact of the presence of ROS on plant growth are focused on plants used in agriculture. The study of the ROS mechanism and understanding their detoxification are crucial for successful breeding of crop stress tolerance. Studies in agronomy mainly focus on developing antioxidant capacity and resilience in plant varieties by using genetic engineering [
118].
Use of the extreme conditions of post-mining sites for enhancing the ability of individual plants to synthesize particular secondary metabolites has been considered by Fikriah et al. [
74]. Fikriah et al. [
74] studied the pioneer plant
Macaranga tanarius growing on post-mining sites. They aimed to assess and compare the secondary metabolites, and antioxidant activity of the ethanol extracts of the leaf obtained from plants growing on post-mining and non-mining areas. Both habitat types were characterized, along with several soil parameters such as pH and nutrients, and soil toxicity was assessed. The study considered the benefits of this medicinal plant growing on post-mining land for use in the pharmaceutical industry [
74]. The toxic effects of
M. tanarius leaves have shown that this species of plant might be a suitable anti-cancer candidate. Previous research reported that various members of the
Macaranga genus, including
M. hosei,
M. tanarius, and
M. gigantea, have revealed cytotoxic effects against cancer cell lines [
99,
119].
11. Conclusions
This review suggests the need to perform a detailed study on the content and quality of secondary metabolites synthesis in individuals of medical plant species which grow spontaneously in challenging habitat conditions such as post-mining mineral sites.
Mechanisms of the synthesis of secondary metabolites have developed through evolutionary processes, which generally last for a long time frame. However, harsh unfavorable environmental conditions cause fast adaptive responses, resulting in the synthesis of complex secondary metabolites. These chemical traits, deeply rooted in environmental stressors, underscore the dynamic relationship between ecological stress and metabolic expression. Apart from the practical, application aspect, the continued research in this field will deepen our understanding of plant adaptations and provide valuable insights into novel bioactive compounds.
In the future, this will allow for the possibility of using post-mining habitats to explore the physiological mechanisms of synthesizing secondary metabolites. Owing to the fact that mineral substrates do not contain dangerous pollutants, this will allow for the potential use of the stressful habitat conditions of mineral sites of post-mining areas as a source of raw medicinal plant materials with important secondary metabolites. This will allow them to be sourced naturally instead of deriving them from cultivation.
In particular, this review paper focused on (i.) summarizing the current stage of knowledge about the plant response to some habitat constraints (drought, salinity, texture, lack of soil profile) with respect to the intensity of secondary metabolites’ synthesis; (ii.) highlighting the relation between the harsh habitat conditions and the intensity of the secondary metabolite synthesis; (iii.) highlighting the potential role of harsh post-mining mineral habitats in enhancing the synthesis of secondary metabolites in medical plant individuals that spontaneously colonize the post-mining sites; and (iv.) underlining the need for future study on the potential use of mineral post-mining sites for enhancement in the secondary metabolites’ synthesis in the individuals of selected plant species for pharmaceutical application purposes.
Author Contributions
Conceptualization, B.B.-Ż., A.H., B.P. and E.C.; methodology, A.H., M.Z. and A.P.-S.; software, D.F. and L.M.; validation, A.H. and G.W.; formal analysis, A.H., A.P.-S. and G.W.; investigation, B.B.-Ż., A.H., B.P., E.C. and A.P.-S.; resources, B.B.-Ż., A.H., B.P. and E.C.; data curation, A.P.-S., D.F. and L.M.; writing—original draft preparation, B.B.-Ż., B.P., E.C. and M.Z.; writing—review and editing, A.H., D.F., L.M. and G.W.; visualization, D.F. and L.M.; supervision, G.W.; project administration, A.H. and G.W. All authors have read and agreed to the published version of the manuscript.
Funding
The research carried out as part of the Institute’s statutory research of Institute of Biology, Biotechnology and Environmental Protection, Faculty of Natural Sciences, Institute of Biology, University of Silesia in Katowice.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to thank Franco Magurno for the primary reading of this article and his useful comments that contributed to the shape of the work. Moreover, we would like to thank Lynn Besenyei for her professional language correction.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ABA | abscisic acid |
| AMF | arbuscular mycorrhizal fungi |
| ASC | ascorbic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EPR | electron paramagnetic resonance |
| ET | ethylene |
| GSH | glutathione |
| JA | jasmonic acid |
| NADPH | reducing Nicotinamide adenine dinucleotide phosphate |
| PS II | photosystem II |
| ROS | reactive oxygen species |
| SA | salicylic acid |
| UV | ultraviolet radiation |
| UVB | ultraviolet radiation B |
| UV-Vis | ultraviolet–visible spectrophotometry |
References
- Punetha, A.; Kumar, D.; Suryavanshi, P.; Padalia, R.C.; Venkatesha, K.T. Environmental abiotic stress and secondary metabolites production in medicinal plants: A review. J. Agric. Sci. 2022, 28, 351–362. [Google Scholar] [CrossRef]
- Nawrot-Chorabik, K.; Sułkowska, M.; Gumulak, N. Secondary metabolites produced by trees and fungi: Achievements so far and challenges remaining. Forest 2022, 13, 1338. [Google Scholar] [CrossRef]
- Salam, U.; Ullah, S.; Tang, Z.H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant metabolomics: An overview of the role of primary and secondary metabolites against different environmental stress factors. Life 2023, 13, 706. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental stress and secondary metabolites in plants: An Overview. In Plant Metabolites and Regulation under Environmental Stress; Ahmad, P., Ahanger, A.M., Singh, V.J., Tripathi, D.K., Alam, P., Alyemeni, M.N., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 153–167. [Google Scholar]
- Bacler-Żbikowska, B.; Zdybel, M.; Pilawa, B.; Chodurek, E.; Woźniak, G.; Gaj, R. Porównanie właściwości antyoksydacyjnych ekstraktów roślinnych z surowców leczniczych pozyskanych z siedlisk zaburzonych na przykładzie gatunków z rodziny Ericaceae. Fides Ratio Patria. Stud. Toruńskie 2023, 19, 12–29. (In Polish) [Google Scholar] [CrossRef]
- Szmidt-Jaworska, A.; Tyburski, J. Fizjologia stresu. In Fizjologia roślin; Kopcewicz, J., Szmidt-Jaworska, A., Eds.; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2020; pp. 589–633. (In Polish) [Google Scholar]
- Pandey, V.; Bhatt, I.D.; Nandi, S.K. Environmental stresses in Himalayan medicinal plants: Research needs and future priorities. Biodivers. Conserv. 2019, 28, 2431–2455. [Google Scholar] [CrossRef]
- Lupp, G.; Zingraff-Hamed, A.; Huang, J.J.; Oen, A.; Pauleit, S. Living labs—A concept for co-designing nature-based solutions. Sustainability 2021, 13, 188. [Google Scholar] [CrossRef]
- Higgins, A.; Klein, S. Introduction to the living lab approach. In Accelerating Global Supply Chains with IT-Innovation; Tan, Y.-H., Björn-Andersen, N., Klein, S., Rukanova, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Chapter 2; pp. 31–36. [Google Scholar]
- Twaij, B.M.; Hasan, M.N. Bioactive secondary metabolites from plant sources: Types, synthesis, and their therapeutic uses. Int. J. Plant Biol. 2022, 13, 4–14. [Google Scholar] [CrossRef]
- Brunetti, C.; Guidi, L.; Sebastiani, F.; Tattini, M. Isoprenoids and phenylpropanoids are key components of the antioxidant defense system of plants facing severe excess light stress. Environ. Exp. Bot. 2015, 119, 54–62. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Martel, A.B.; Strugnell, C.A. Environmental factors regulate plant secondary metabolites. Plants 2023, 12, 447. [Google Scholar] [CrossRef]
- Wahid, A. Physiological implications of metabolite biosynthesis for net assimilation and heat-stress tolerance of sugarcane (Saccharum officinarum) sprouts. J. Plant. Res. 2007, 120, 219–228. [Google Scholar] [CrossRef]
- Singsaas, E.L. Terpenes and the thermotolerance of photosynthesis. New Phytol. 2000, 146, 1–4. [Google Scholar] [CrossRef]
- Goh, H.H.; Khairudin, K.; Sukiran, N.A.; Normah, M.N.; Baharum, S.N. Metabolite profiling reveals temperature effects on the VOCs and flavonoids of different plant populations. Plant Biol. 2016, 18, 130–139. [Google Scholar] [CrossRef]
- Ahmed, S.; Jamil, S.; Siddiqui, M.U.A. Secondary metabolites—God gifted arsenal for plants. J. Pharmacogn. Phytochem. 2024, 13, 38–43. [Google Scholar] [CrossRef]
- Jóźwiak, Z.; Bartosz, G. (Eds.) Biofizyka. Wybrane Zagadnienia Wraz z Ćwiczeniami; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2013; pp. 1–556. (In Polish) [Google Scholar]
- Bartosz, G. Druga Twarz Tlenu. Wolne Rodniki w Przyrodzie; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2021; pp. 1–448. (In Polish) [Google Scholar]
- Morrish, A.H. Fizyczne Podstawy Magnetyzmu; Państwowe Wydawnictwo Naukowe PWN: Warszawa, Poland, 1970; pp. 1–599. (In Polish) [Google Scholar]
- Rozancew, E.G.; Szolle, W.D. Chemia Organiczna Wolnych Rodników; Wydawnictwo Naukowe PWN: Warszawa, Poland, 1985; pp. 1–316. (In Polish) [Google Scholar]
- Jaroszyk, F. Biofizyka; Wydawnictwo Lekarskie PZWL: Warszawa, Poland, 2009; pp. 1–912. (In Polish) [Google Scholar]
- Karbarz, M. Źródła powstawania i oddziaływanie środowiskowe wolnych rodników. Zesz. Nauk. SGSP 2010, 40, 59–67. (In Polish) [Google Scholar]
- de Abreu, I.N.; Mazzafera, P. Effect of water and temperature stress on the content of active constituents of Hypericum brasiliense Choisy. Plant Physiol. Biochem. 2005, 43, 241–248. [Google Scholar] [CrossRef]
- Ramakrishna, A.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef]
- Cheng, L.; Han, M.; Yang, L.M.; Yang, L.; Sun, Z.; Zhang, T. Changes in the physiological characteristics and baicalin biosynthesis metabolism of Scutellaria baicalensis Georgi under drought stress. Ind. Crops Prod. 2018, 122, 473–482. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, Q.; Liu, L.; Liao, L.; Zhu, Z. Influence of fertilization and drought stress on the growth and production of secondary metabolites in Prunella vulgaris L. J. Med. Plants Res. 2011, 5, 1749–1755. [Google Scholar]
- Radácsi, P.; Inotai, K.; Sárosi, S.; Czövek, P.; Bernáth, J.; Németh, É. Effect of water supply on the physiological characteristics and production of basil (Ocimum basilicum L.). Eur. J. Hortic. Sci. 2010, 75, 193–197. [Google Scholar] [CrossRef]
- Raven, P.H.; Eichhorn, S.E.; Evert, R.F. Biologia Roślin; Wydawnictwo Naukowe PWN S.A.: Warszawa, Poland, 2023; pp. 731–732. (In Polish) [Google Scholar]
- Sharma, P.; Kaushal, S.; Baishaya, R. Biofertilizers alleviate salinity stress in medicinally important plant—Catharanthus roseus (L.) G. Don by enhancing morphological and photosynthetic attributes. Int. J. Environ. Sci. 2023, 49, 387–397. [Google Scholar] [CrossRef]
- Ksouri, R.; Megdiche, W.; Debez, A.; Falleh, H.; Grignon, C.; Abdelly, C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol. Biochem. 2007, 45, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Bourgou, S.; Kchouk, M.E.; Bellila, A.; Marzouk, B. Effect of salinity on phenolic composition and biological activity of Nigella sativa. Acta Hortic. 2010, 853, 57–60. [Google Scholar] [CrossRef]
- Cik, J.K.; Klejdus, B.; Hedbavny, J.; Bačkor, M. Salicylic acid alleviates NaCl-induced changes in the metabolism of Matricaria chamomilla plants. Ecotoxicology 2009, 18, 544–554. [Google Scholar]
- Chmielewska, S.; Zambrzycka, A.; Czyżewska, U.; Czerniecka, M.; Tylicki, A. Nowoczesne terapie celowane w leczeniu nowotworów jako alternatywa dla konwencjonalnej chemioterapii. Postępy Biol. Komórki 2020, 47, 365–390. (In Polish) [Google Scholar]
- Ali, R.M.; Elfeky, S.S.; Abbas, H. Response of salt stressed Ricinus communis L. to exogenous application of glycerol and/or aspartic acid. J. Biol. Sci. 2008, 8, 171–175. [Google Scholar]
- Haghighi, Z.; Modarresi, M.; Mollayi, S. Enhancement of compatible solute and secondary metabolites production in Plantago ovate Forsk. by salinity stress. J. Med. Plants Res. 2012, 6, 3495–3500. [Google Scholar]
- Casal, J.J.; Yanovsky, M.J. Regulation of gene expression by light. Int. J. Dev. Biol. 2005, 49, 501–511. [Google Scholar] [CrossRef]
- Mehler, A. Studies on reactions of illuminated chloroplasts: I. Mechanism of the reduction of oxygen and other hill reagents. Arch. Biochem. Biophys. 1951, 33, 65–77. [Google Scholar] [CrossRef]
- Ruban, A.; Wilson, S. The Mechanism of Non-Photochemical Quenching in Plants: Localization and Driving Forces. Plant Cell Physiol. 2021, 62, 1063–1072. [Google Scholar] [CrossRef]
- Alyas, J.; Khalid, N.; Ishaque, S.; Fatima, H.; Hashim, M.; Hassan, S.; Bukhari, S.; Hano, C.; Abbasi, B.H.; Anjum, S. Light (High Light/UV Radiation) Modulates Adaptation Mechanisms and Secondary Metabolite Production in Medicinal Plants. In Medicinal Plants; Husen, A., Iqbal, M., Eds.; Springer: Singapore, 2023; pp. 363–390. [Google Scholar]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B irradiation changes specifically the secondary metabolite profile in broccoli sprouts: Induced signaling overlaps with defense response to biotic stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef] [PubMed]
- Naghiloo, S.; Movafeghi, A.; Delazar, A.; Nazemiyeh, H.; Asnaashari, S.; Dadpour, M.R. Ontogenetic variation of total phenolics and antioxidant activity in roots: Leaves and flowers of Astragalus compactus Lam. (Fabaceae). BioImpacts 2012, 2, 105–109. [Google Scholar]
- Jochum, G.M.; Mudge, K.W.; Thomas, R.B. Elevated temperatures increase leaf senescence and root secondary metabolite concentration in the understory herb Panax quinquefolius (Araliaceae). Am. J. Bot. 2007, 94, 819–826. [Google Scholar] [PubMed]
- Loreto, F.; Schnitzler, J.P. Abiotic stress and induced BVOCs. Trends Plant Sci. 2010, 15, 154–166. [Google Scholar] [CrossRef]
- Shibata, M.; Amano, M.; Kawata, J.; Uda, M. Breeding process and characteristics of ‘Summer Queen’, a spray-type chrysanthemum. Bull. Natl. Res. Inst. Veg. Ornam. Plants Tea Ser. A 1988, A, 245–255. [Google Scholar]
- Keita, K.; Darkoh, C.; Okafor, F. Secondary plant metabolites as potent drug candidates against antimicrobial-resistant pathogens. SN Appl. Sci. 2022, 4, 209. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Islam, B.; Akram, M.; Shakil, S.; Ahmad, A.; Ali, S.M.; Siddiqui, M.; Khan, A.U. Antimicrobial activity of five herbalextracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules 2009, 14, 586–597. [Google Scholar]
- Khan, M.F.; Tang, H.; Lyles, J.T.; Pineau, R.; Mashwani, Z.U.; Quave, C.L. Antibacterial properties of medicinal plants from Pakistan against multidrug-resistant ESKAPE pathogens. Front. Pharmacol. 2018, 9, 815. [Google Scholar] [CrossRef]
- Kobayashi, K.; Nishino, C.; Tomita, H.; Fukushima, M. Anti-fungal activity of pisiferic acid derivatives against the rice blast fungus. Phytochemistry 1987, 26, 3175–3179. [Google Scholar] [CrossRef]
- Rouf, R.; Uddin, S.J.; Sarker, D.K.; Islam, M.T.; Ali, E.S.; Shilpi, J.A.; Nahar, L.; Tiralongo, E.; Sarker, S.D. Antiviral potential of garlic (Allium Sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 2020, 104, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Sztuka, M.; Magurno, F. Fungi in the Revitalization of Post-industrial Areas. No Limits 2021, 2, 22–23. [Google Scholar] [CrossRef]
- Błaszkowski, J.; Niezgoda, P.; Piątek, M.; Magurno, F.; Malicka, M.; Zubek, S.; Mleczko, P.; Yorou, N.S.; Jobim, K.; Vista, X.M.; et al. Rhizoglomus dalpeae, R. maiae, and R. silesianum, new species. Mycologia 2019, 111, 965–980. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Cartabia, A.; Lalaym, I.; Declerck, S. Arbuscular mycorrhizal fungi and production of secondary metabolites in medicinal plants. Mycorrhiza 2022, 32, 221–256. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Patil, D.; Rajamohanan, P.R.; Ahmad, A. Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS ONE 2013, 8, e71805. [Google Scholar] [CrossRef]
- Kumari, S.; Vaishnav, A.; Jain, S.; Varma, A.; Choudhary, D.K. Bacterial-Mediated Induction of Systemic Tolerance to Salinity with Expression of Stress Alleviating Enzymes in Soybean (Glycine max L. Merrill). J. Plant Growth Regul. 2015, 34, 558–573. [Google Scholar] [CrossRef]
- He, X.; Xu, M.; Wei, Q.; Tang, M.; Guan, L.; Lou, L.; Xu, X.; Hu, Z.; Chen, Y.; Shen, Z.; et al. Promotion of growth and phytoextraction of cadmium and lead in Solanum nigrum L. mediated by plant-growth-promoting rhizobacteria. Ecotox. Environ. Safe 2020, 205, 111333. [Google Scholar] [CrossRef]
- Khoso, M.A.; Wagan, S.; Alam, I.; Hussain, A.; Ali, Q.; Saha, S.; Poudel, T.R.; Manghwar, H.; Liu, F. Impact of plant growth-promoting rhizobacteria (PGPR) on plant nutrition and root characteristics: Current perspective. Plant Stress. 2024, 11, 100341. [Google Scholar] [CrossRef]
- Yang, Y.; He, C.; Huang, L.; Ban, Y.; Tang, M. The effects of arbuscular mycorrhizal fungi on glomalin-related soil protein distribution, aggregate stability and their relationships with soil properties at different soil depths in lead-zinc contaminated area. PLoS ONE 2017, 12, e0182264. [Google Scholar] [CrossRef]
- Yang, Y.; Ou, X.; Yang, G.; Xia, Y.; Chen, M.; Guo, L.; Liu, D. Arbuscular mycorrhizal fungi regulate the growth and phyto-active compound of Salvia miltiorrhiza seedlings. Appl. Sci. 2017, 7, 68. [Google Scholar] [CrossRef]
- Lu, F.C.; Lee, C.Y.; Wang, C.L. The influence of arbuscular mycorrhizal fungi inoculation on yam (Dioscorea spp.) tuber weights and secondary metabolite content. PeerJ 2015, 3, e1266. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.I.; Quamar Abba, S.; Reigosa, M.J. Activities and novel applications of secondary metabolite coumarins. Planta daninha 2018, 36, e018174040. [Google Scholar] [CrossRef]
- Khare, S.; Singh, N.B.; Singh, A.; Hussin, I.; Niharika, K.; Tadav, V.; Bano, C.; Yadav, R.K.; Amist, N. Plant secondary metabolites synthesis and their regulations under biotic and abiotic constraints. J. Plant Biol. 2020, 63, 203–216. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2023, 30, 239–264. [Google Scholar] [CrossRef]
- Kessler, A.; Kalske, A. Plant secondary metabolite diversity and species interactions. Annu. Rev. Ecol. Evol. Syst. 2018, 49, 115–138. [Google Scholar] [CrossRef]
- Jansen, M.A.K.; Gaba, V.; Greenberg, B.M. Higher plants and UV-B radiation: Balancing damage, repair and acclimation. Trends Plant Sci. 1998, 3, 131–135. [Google Scholar] [CrossRef]
- Dixon, R.A. Natural products and plant disease resistance. Nature 2001, 411, 843–847. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef]
- Li, Z.; Wen, W.; Qin, M.; He, Y.; Xu, D.; Li, L. Biosynthetic mechanisms of secondary metabolites promoted by the interaction between endophytes and plant hosts. Front. Microbiol. 2022, 13, 928967. [Google Scholar] [CrossRef]
- Panozzo, A.; Bolla, P.K.; Barion, G.; Botton, A.; Vamerali, T. Phytohormonal regulation of abiotic stress tolerance, leaf senescence and yield response in field corps: A comprehensive review. BioTech 2025, 14, 14. [Google Scholar] [CrossRef]
- Kopcewicz, J.; Lewak, S. Podstawy procesów życiowych. In Fizjologia Roślin; Kopcewicz, J., Szmidt-Jaworska, A., Eds.; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2020; pp. 91–119. (In Polish) [Google Scholar]
- Zhu, J.K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002, 53, 247–273. [Google Scholar] [CrossRef]
- Eyidogan, F.; Oz, M.T.; Yucel, M.; Oktem, H.K. Signal transduction of phytohormones under abiotic stresses. In Phytohormones and Abiotic Stress Tolerance in Plants; Khan, N., Nazar, R., Iqbal, N., Anjum, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–48. [Google Scholar]
- Zobayed, S.M.A.; Afreen, F.; Kozai, T. Temperature stress can alter the photosynthetic efficiency and secondary metabolite concentrations in St. John’s Wort. Pl. Physiol. Biochem. 2005, 43, 977–984. [Google Scholar] [CrossRef]
- Moore, B.D.; Andrew, R.L.; Külheim, C.; Foley, W.J. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014, 201, 733–750. [Google Scholar] [CrossRef]
- Fikriah, I.; Masruhin, M.A.; Paramita, S.; Marliana, E.; Panggabean, A.S.; Ismail, S.; Kusuma, I.W.; Kim, Y.U.; Kim, S.Y. Acute toxicity, secondary metabolites, and antioxidant activity of Macaranga tanarius from post-coal mining and non-mining areas in East Kalimantan, Indonesia. Narra J. 2024, 4, 791. [Google Scholar] [CrossRef]
- Sahu, S.C.; Gangopadhyay, M.; Dewanjee, S. Elicitor-induced rosmarinic acid accumulation and secondarymetabolism enzyme activities in Solenostemon scutellarioides. Acta Physiol. Plant 2013, 35, 1473–1481. [Google Scholar] [CrossRef]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA; Singapore, 2005; pp. 1–1024. [Google Scholar]
- Symons, M. Spektroskopia EPR w Chemii i Biochemii; Państwowe Wydawnictwo Naukowe: Warszawa, Poland, 1987; pp. 1–272. (In Polish) [Google Scholar]
- Eaton, G.R.; Eaton, S.S.; Salikhov, K.M. Foundations of Modern EPR; World Scientific: Singapore, 1998; pp. 1–832. [Google Scholar]
- Kęcki, Z. Podstawy Spektroskopii Molekularnej; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2006; pp. 1–338. (In Polish) [Google Scholar]
- Stankowski, J.; Hilczer, W. Wstęp do Spektroskopii Rezonansów Magnetycznych; Państwowe Wydawnictwo Naukowe PWN: Warszawa, Poland, 2005; pp. 1–92. (In Polish) [Google Scholar]
- Weil, J.A.; Bolton, J.R. Electron Paramagnetic Resonance: Elementary Theory and Practical Applications, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2007; pp. 1–664. [Google Scholar]
- Dyrek, K. Elektronowy rezonans paramagnetyczny. In Fizyczne Metody Badań w Biologii, Medycynie i Ochronie Środowiska; Hrynkiewicz, A.Z., Rokita, E., Eds.; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2013; pp. 136–159. (In Polish) [Google Scholar]
- Pilawa, B.; Ramos, P. Spektroskopia EPR. Ćwiczenia dla Studentów Analityki Medycznej i Medycyny; Śląski Uniwersytet Medyczny w Katowicach: Katowice, Poland, 2017; pp. 1–125. (In Polish) [Google Scholar]
- Pilawa, B.; Chodurek, E.; Stec, M.; Zdybel, M. Zastosowanie Spektroskopii Elektronowego Rezonansu Paramagnetycznego do Badania Wolnych Rodników i Antyoksydantów w Produktach Spożywczych; Śląski Uniwersytet Medyczny w Katowicach: Katowice, Poland, 2024; pp. 1–71. (In Polish) [Google Scholar]
- Tirzitis, G.; Bartosz, G. Determination of antiradical and antioxidant activity: Basic principles and new insights. Acta Biochem. Pol. 2010, 57, 139–142. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Marine natural products and related compounds in clinical and advanced preclinical trials. J. Nat. Prod. 2004, 67, 1216–1238. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta BBA Gen. Subj. 2013, 1830, 3670–3695. [Google Scholar] [CrossRef] [PubMed]
- Kompała-Bąba, A.; Sierka, E.; Bierza, W.; Bąba, W.; Błońska, A.; Woźniak, G. Eco-physiological responses of Calamagrostis epigejos L (Roth) and Solidago gigantea Aition to complex environmental stresses in coal-mine spoil heaps. Land Degrad. Dev. 2021, 32, 5427–5442. [Google Scholar] [CrossRef]
- Ryś, K.; Chmura, D.; Prostański, D.; Woźniak, G. Biomass amounts of spontaneous vegetation on post-coal mine novel ecosystem in relation to biotic parameters. Energies 2023, 16, 7513. [Google Scholar] [CrossRef]
- Szuba, A.; Ratajczak, E.; Leski, T.; Tomaszewski, D.; Ratajczak, I.; Woźniak, G.; Jagodziński, A.M. The high adaptive potential of Abies alba Mill. seedlings—Biochemical and physiological studies of succession along the environmental gradient of a Cambrian quarry. BMC Plant Biol. 2025, 25, 820. [Google Scholar] [CrossRef]
- Sánchez, S.; Demain, A.L. Secondary Metabolites; American Society of Plant Physiologists: Derwood, MD, USA, 2000; pp. 1–13. [Google Scholar]
- Tsao, R.; Yang, R.; Xie, S.; Sockovie, E.; Khanizadeh, S. Which polyphenolic compounds contribute to the total antioxidant activities of apple? J. Agric. Food Chem. 2005, 53, 4989–4995. [Google Scholar] [CrossRef] [PubMed]
- Błońska, A.; Kompała-Bąba, A.; Sierka, E.; Besenyei, L.; Magurno, F.; Bierza, W.; Frydecka, K.; Woźniak, G. Impact of selected plant species on enzymatic activity of soil substratum on post-mining heaps. J. Ecol. Eng. 2019, 20, 138–144. [Google Scholar] [CrossRef]
- Woźniak, G.; Chmura, D.; Nowak, T.; Bacler-Żbikowska, B.; Besenyei, L.; Hutniczak, A. Post-extraction novel ecosystems support plant and vegetation diversity in urban-industrial landscapes. Sustainability 2022, 14, 7611. [Google Scholar] [CrossRef]
- Bierza, W.; Czarnecka, J.; Błońska, A.; Kompała-Bąba, A.; Hutniczak, A.; Jendrzejek, B.; Bakr, J.; Jagodziński, A.M.; Prostański, D.; Woźniak, G. Plant Diversity and Species Composition in Relation to Soil Enzymatic Activity in the Novel Ecosystems of Urban–Industrial Landscapes. Sustainability 2023, 15, 7284. [Google Scholar] [CrossRef]
- Bakr, J.; Kompalała-Bąba, A.; Bierza, W.; Hutniczak, A.; Błońska, A.; Chmura, D.; Magurno, F.; Jagodziński, A.M.; Besenyei, L.; Bacler-Żbikowska, B.; et al. Plant Species and Functional Diversity of Novel Forests Growing on Coal Mine Heaps Compared with Managed Coniferous and Deciduous Mixed Forests. Forests 2024, 15, 730. [Google Scholar] [CrossRef]
- Bacler-Żbikowska, B.; Hutniczak, A.; Bierza, W.; Bakr, J.; Błońska, A.; Piekarska-Stachowiak, A.; Olszewski, P.; Pieprzyca, A.; Kucharski, P.; Stebel, A.; et al. Railway Infrastructure as a Substitute Habitat for Valuable Medicinal Plant Species Using the Example of Bearberry Arctostaphylos uva-ursi. Agronomy 2024, 14, 2739. [Google Scholar] [CrossRef]
- Marliana, E.; Astuti, W.; Kosala, K.; Hairani, R.; Tjahjandarie, T.S.; Tanjung, M. Chemical composition and anticancer activity of Macaranga hosei leaves. Asian J. Chem. 2018, 30, 795–798. [Google Scholar] [CrossRef]
- Putri, R.; Sy, R.H.; Teruna, H.Y. Phytochemical screening and toxicity test from extracts of mahang (Macaranga bancana) leaves. Photon J. Nat. Sci. Technol. 2019, 9, 230–234. [Google Scholar] [CrossRef]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M.C. Free Radicals in Biology and Medicine, 5th ed.; Oxford University Press: New York, NY, USA, 2015; pp. 1–944. [Google Scholar]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Mignolet-Spruyt, L.; Xu, E.; Idänheimo, N.; Hoeberichts, F.A.; Mühlenbock, P.; Brosché, M.; Van Breusegem, F.; Kangasjärv, J. Spreading the news: Subcellular and organellar reactive oxygen species production and signalling. J. Exp. Bot. 2016, 67, 3831–3844. [Google Scholar] [CrossRef]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 28, 909–930. [Google Scholar] [CrossRef]
- Cruz de Carvalho, M.H. Drought stress and reactive oxygen species: Production, scavenging and signaling. Plant Signal. Behav. 2008, 3, 156–165. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftici-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Shahid, I.; Rizwan, M.; Baig, D.N.; Saleem, R.S.; Malik, K.A.; Mehnaz, S. Secondary Metabolites Production and Plant Growth Promotion by Pseudomonas chlororaphis and P. aurantiaca Strains Isolated from Cactus, Cotton, and Para Grass. J. Microbiol. Biotechnol. 2017, 27, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Foyer, C.H.; Noctor, G. Redox homeostasis and antioxidant signaling: A metabolic interface between stress perception and physiological responses. Plant Cell 2005, 17, 1866–1875. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef]
- Escher, G.B.; Santos, J.S.; Rosso, N.D.; Marques, M.B.; Azevedo, L.; do Camo, M.A.V.; Dauguer, H.; Molognoni, L.; Do Prado-Silva, L.; Sant’Ana, A.S.; et al. Chemical study, antioxidant, anti-hypertensive, and cytotoxic/cytoprotective activities of Centaurea cyanus L. petals aqueous extract. Food Chem. Toxicol. 2018, 118, 439–453. [Google Scholar] [CrossRef]
- Marčetič, M.; Arsenijević, J. Antioxidant activity of plant secondary metabolites. Arch. Pharm. 2023, 73, 264–277. [Google Scholar]
- Vollmannová, A.; Bojňanská, T.; Musilová, J.; Lidiková, J.; Cifrová, M. Quercetin as one of the most abundant represented biological valuable plant components with remarkable chemoprotective effects—A review. Heliyon 2024, 10, e33342. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.S.; Silva, F.S.; Hijri, M.; Kapoor, R. Arbuscular mycorrhiza-mediated augmentation of plant secondary metabolite production. Front. Plant Sci. 2023, 14, 1150900. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.A.; Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D. Bioremediation of adverse impact of cadmium toxicity on Cassia italica Mill by arbuscular mycorrhizal fungi. Saudi J. Biol. Sci. 2016, 23, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; Egamberdieva, D. Arbuscular Mycorrhizal Fungi and Plant Stress Tolerance. In Plant Microbiome: Stress Response; Egamberdieva, D., Ahmad, P., Eds.; Springer: Singapore, 2018; pp. 81–103. [Google Scholar]
- Badawi, G.H.; Kawano, N.; Yamauchi, Y.; Shimada, E.; Sasaki, R.; Kubo, A.; Tanaka, K. Over- expression of ascorbate peroxidase in tobacco chloroplasts enhances the tolerance to salt stress and water deficit. Physiol. Plant. 2004, 121, 231–238. [Google Scholar] [CrossRef]
- Arung, E.T.; Amirta, R.; Zhu, Q.; Amen, Y.; Shimizu, K. Effect of wood, bark and leaf extracts of Macaranga trees on cytotoxic activity in some cancer and normal cell lines. J. Indian. Acad. Wood Sci. 2018, 15, 115–119. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).