Effects of Film Mulching on Soil Microbial Diversity and Community Structure in the Maize Root Zone under Drip Irrigation in Northwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Location
2.2. Materials
2.3. Experimental Design
2.4. Analysis of Soil Properties
2.5. High-Throughput Sequencing of Soil Microorganisms
2.6. Analysis of the Yield of Maize
2.7. Statistical and Bioinformatics Analysis
3. Results
3.1. Effect of Film Mulching Treatments on Soil Characteristics
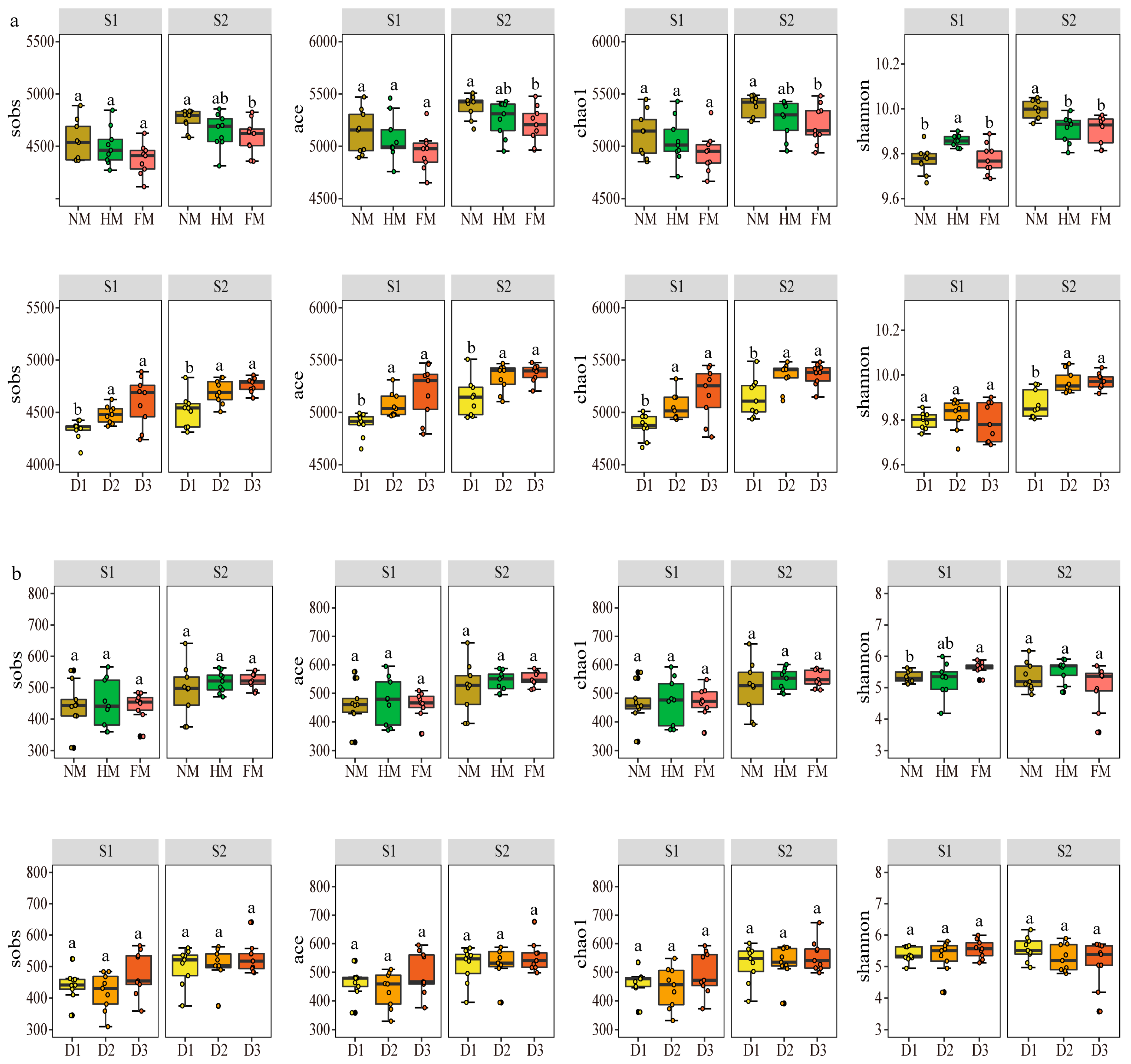
3.2. Response of Different Film Mulching Methods to Microbial α- and β-Diversity
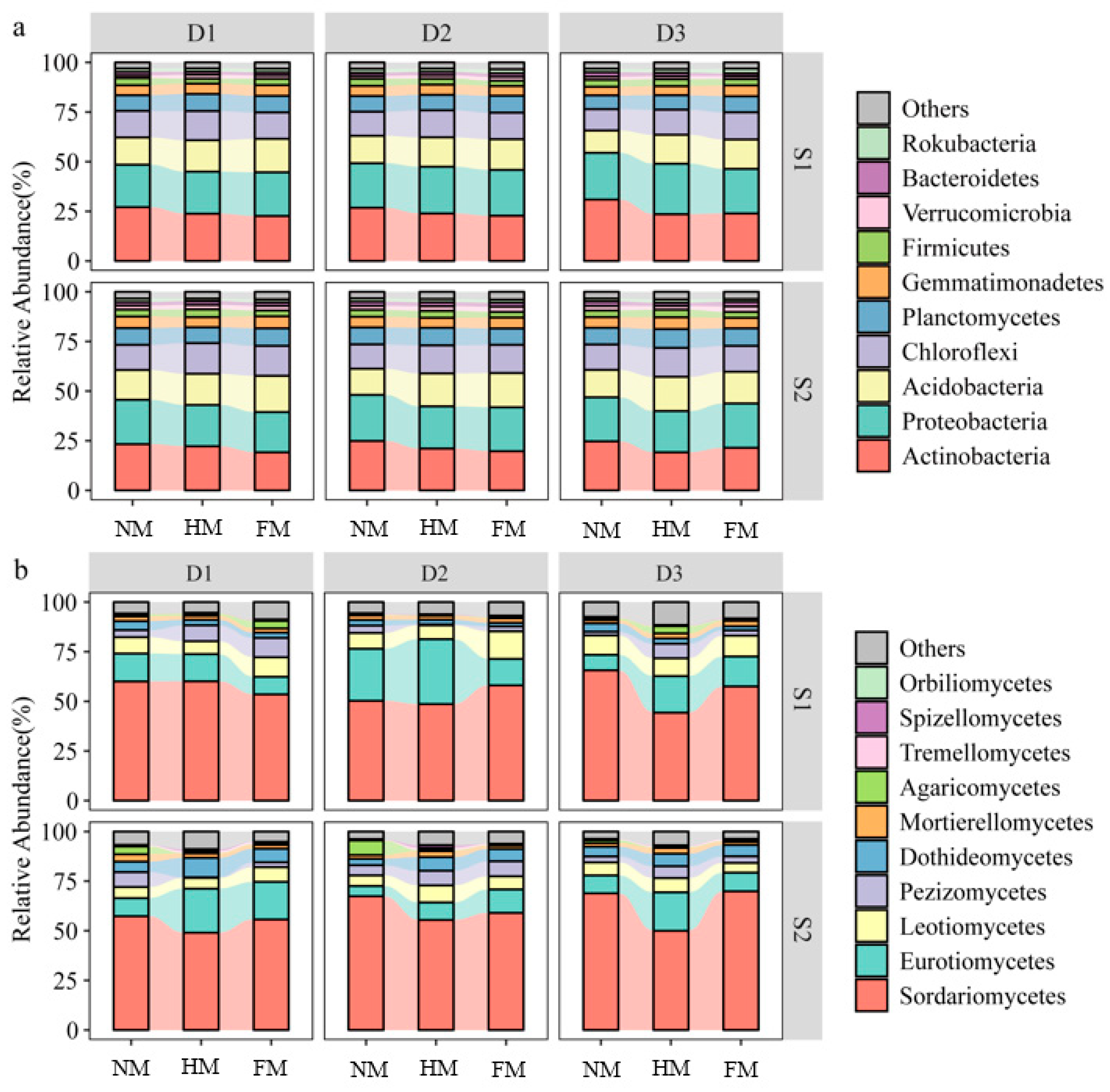
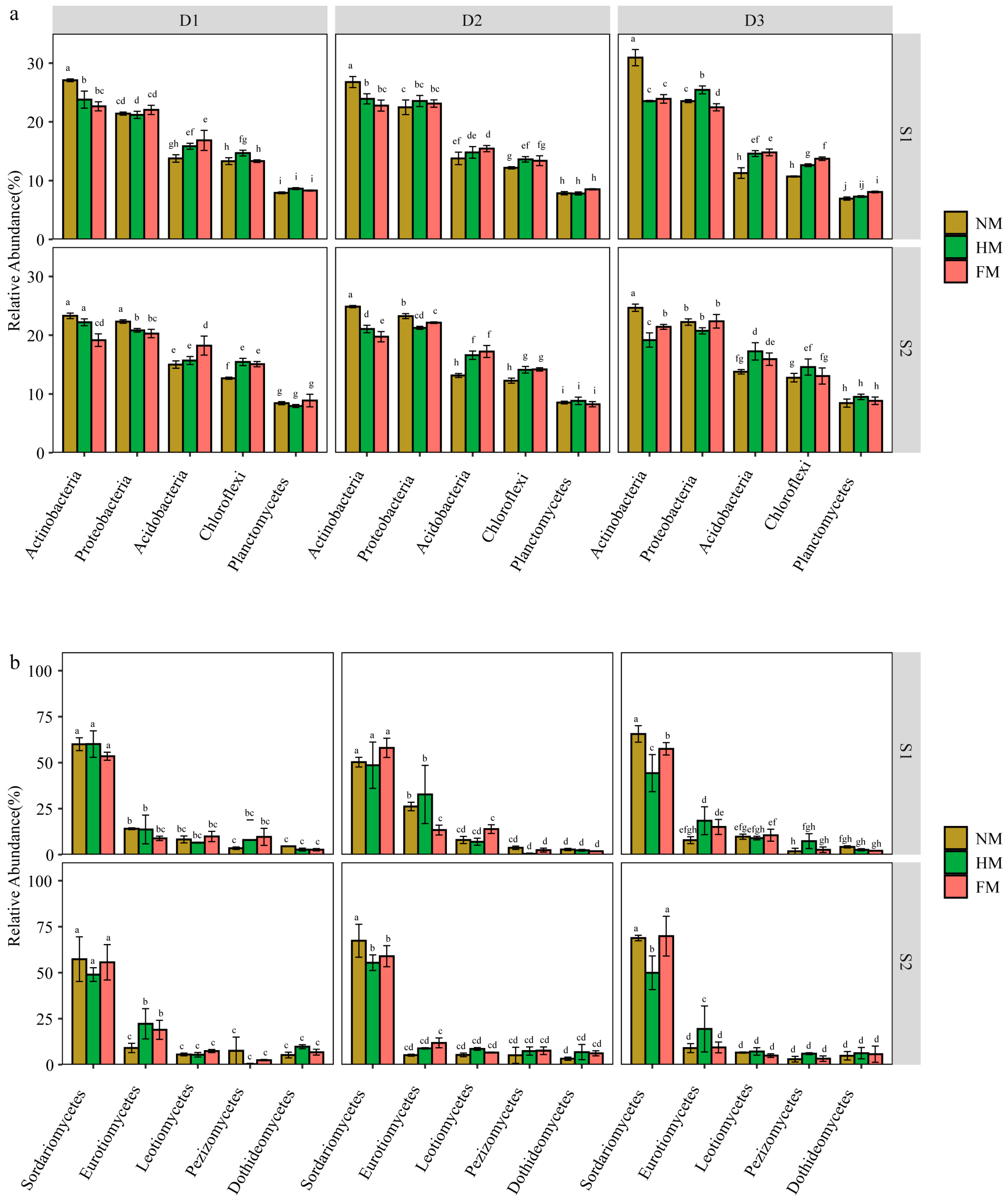
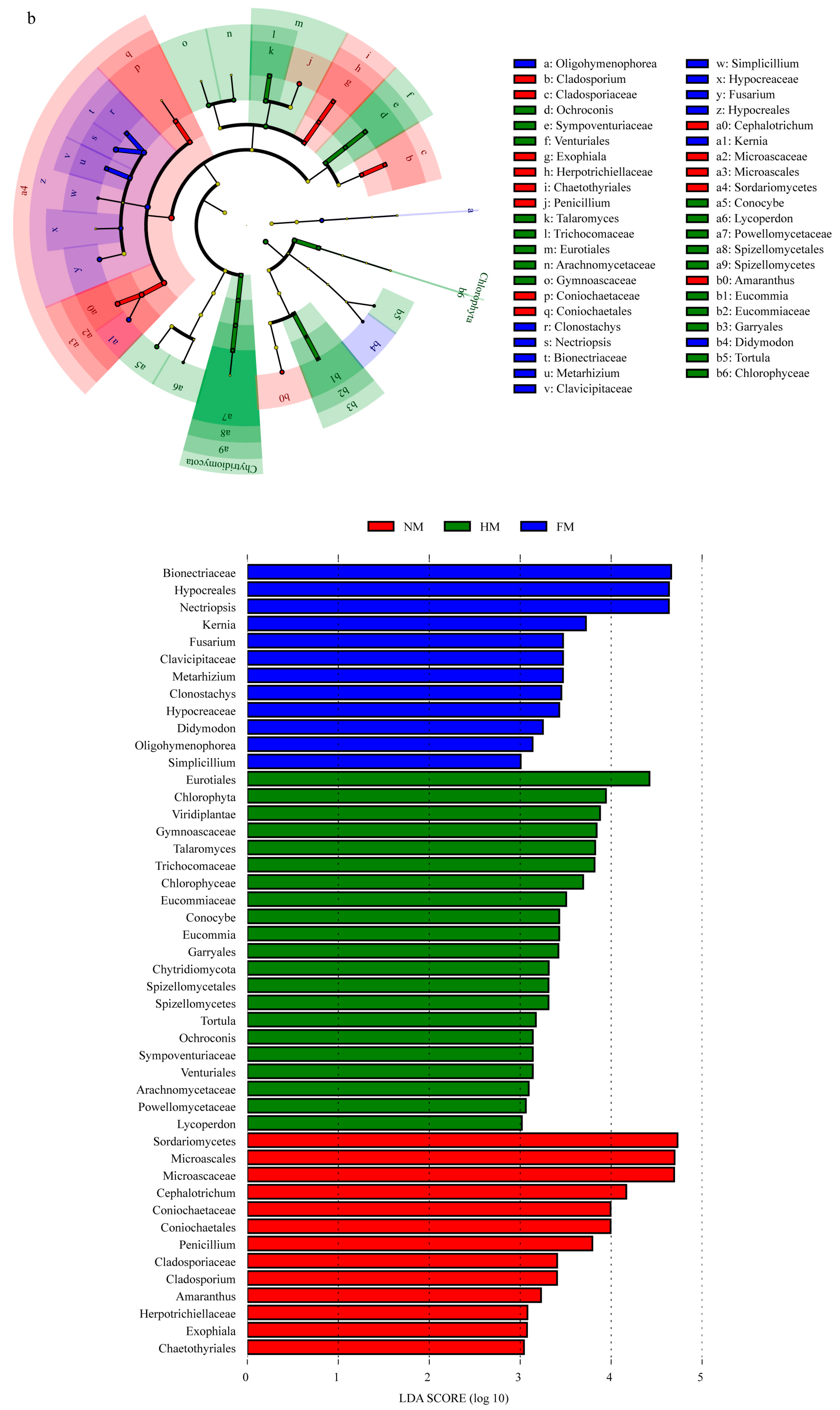
3.3. Changes in Microbial Community Composition under Different Film Mulching Methods
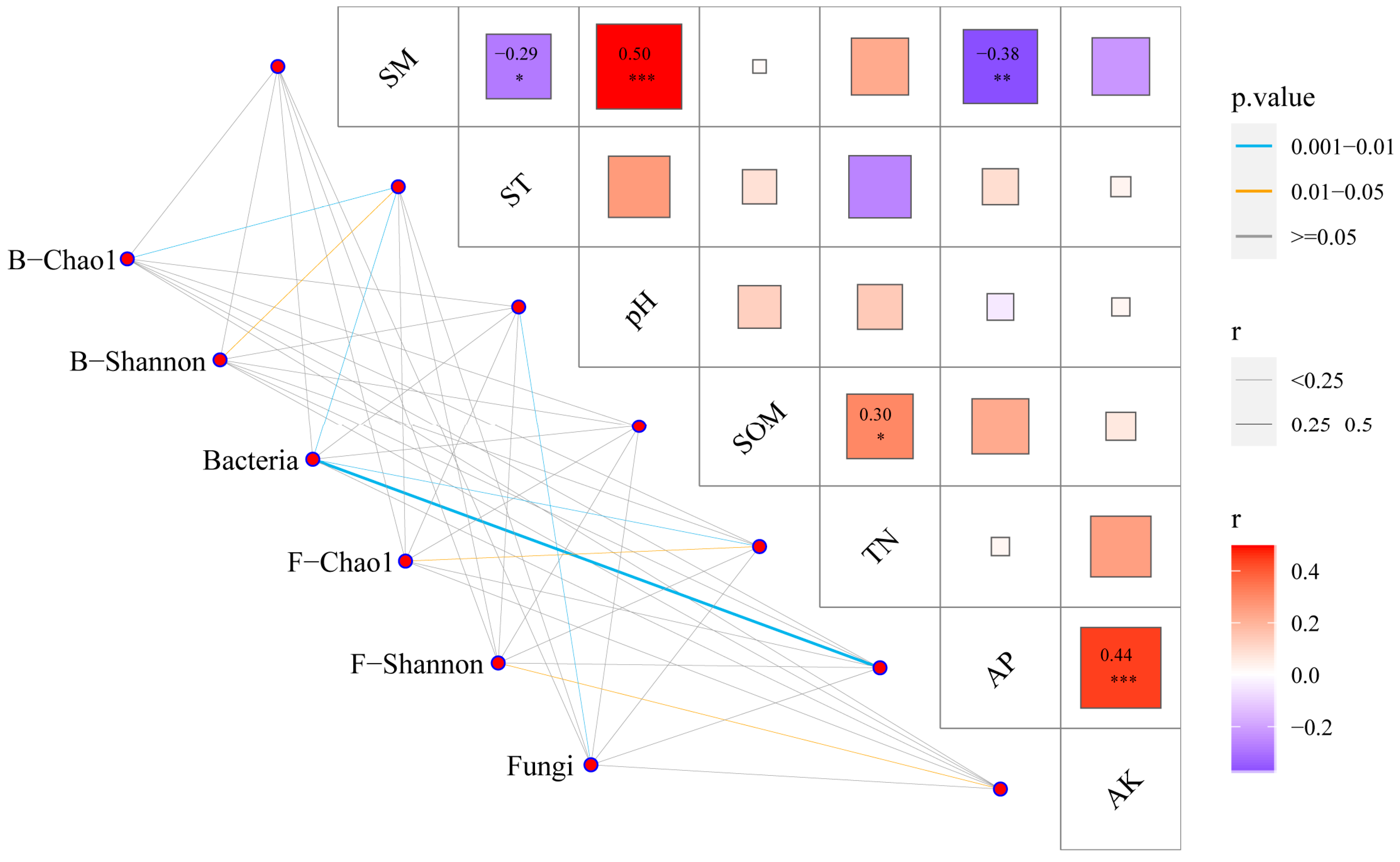
3.4. Correlation Analysis between Microbial Community Structure and Soil Properties
3.5. Analysis of the Soil Microbial Symbiotic Network by Different Film Mulching Methods
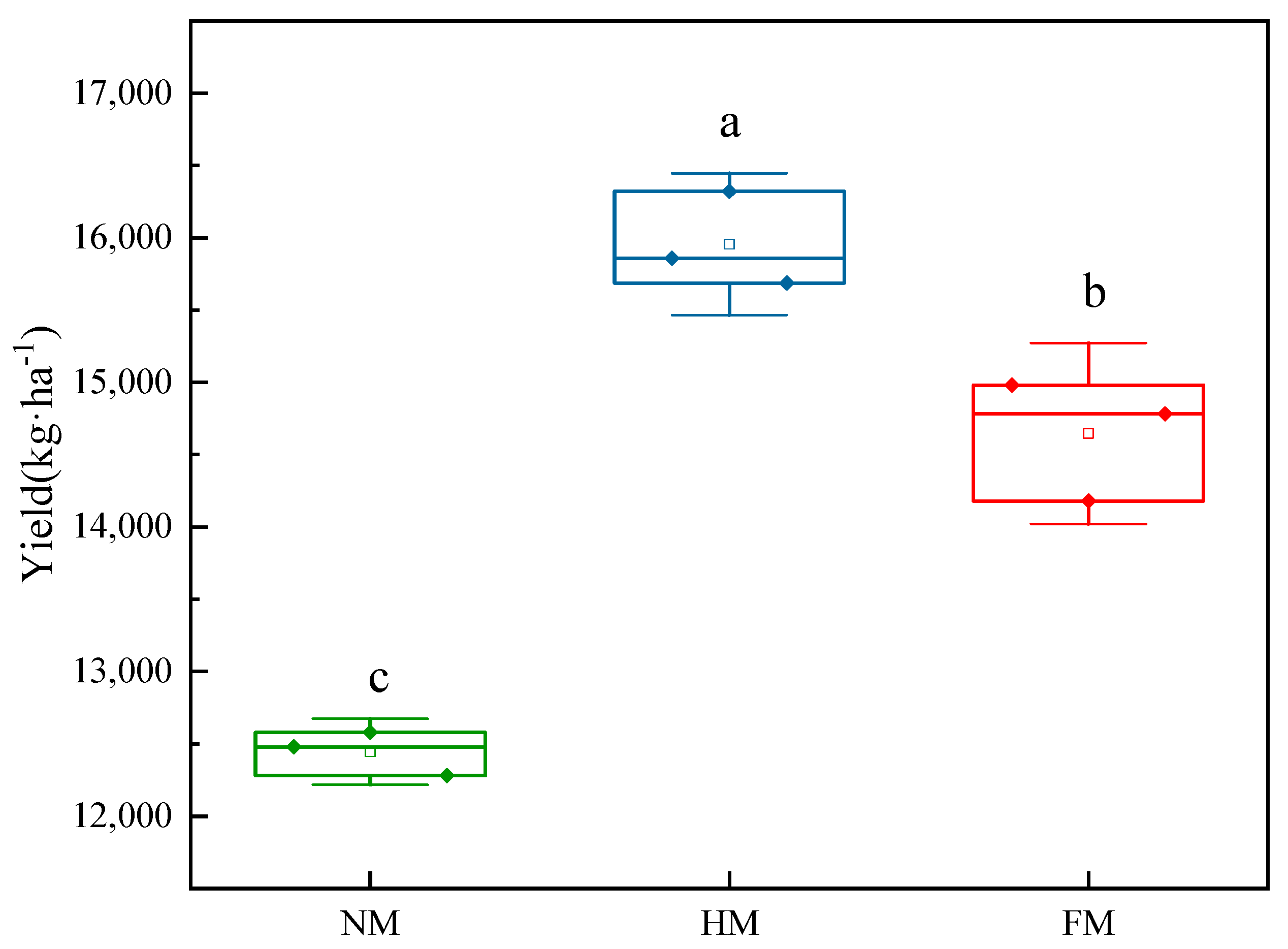
3.6. Effect of Mulching Treatments on the Yield of Drip-Irrigated Maize
4. Discussion
4.1. Effects of Different Film Mulching Methods on Soil Microbial Diversity
4.2. Effects of Different Film Mulching Methods on Soil Microbial Community Structure
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Q.; Chen, L.; Zhang, Q. Effects of Mulching on Soil Water Content and Maize Growth in Semiarid Regions. Comput. Electron. Agric. 2024, 241, 108677. [Google Scholar] [CrossRef]
- Demo, A.H.; Asefa Bogale, G. Enhancing crop yield and conserving soil moisture through mulching practices in dryland agriculture. Front. Agron. 2024, 6, 1361697. [Google Scholar] [CrossRef]
- Wang, J.; Liu, G.; Li, L.; Zhang, X.; He, D. Effects of Water Stress on Maize Yield and Soil Water in Different Mulching Methods. Water 2023, 16, 354. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Sun, Z.; Zheng, J.; Liu, E.; Feng, L.; Feng, C.; Si, P.; Bai, W.; Cai, Q.; et al. Plastic film covers during the fallow season preceding sowing increases yield and water use efficiency of rain-fed spring maize in a semi-arid climate. Agric. Water Manag. 2019, 212, 203–210. [Google Scholar] [CrossRef]
- Liu, E.; He, W.; Yan, C. ‘White revolution’ to ‘white pollution’—Agricultural plastic film mulch in China. Environ. Res. Lett. 2014, 9, 091001. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Q.; Hu, W.; Qin, J.; Zheng, Y.; Wang, J.; Wang, Q.; Xu, Y.; Guo, G.; Hu, S.; et al. Effects of plastic mulch film residues on soil-microbe-plant systems under different soil pH conditions. Chemosphere 2020, 267, 128901. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.; Jing, B.; Chen, M.; Zheng, X.; Wu, W. Comprehensive environmental impact assessment of plastic film mulching with emphasis on waste disposal of discarded plastic film in sunflower production. J. Clean. Prod. 2023, 404, 136979. [Google Scholar] [CrossRef]
- Hu, Q.; Li, X.; Gonçalves, J.M.; Shi, H.; Tian, T.; Chen, N. Effects of residual plastic-film mulch on field corn growth and productivity. Sci. Total Environ. 2020, 729, 138901. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Dai, J.; Liu, Z.; Deng, X.; Yang, Y.; Zeng, Q. Film mulching alters soil properties and increases Cd uptake in Sedum alfredii Hance-oil crop rotation systems. Environ. Pollut. 2023, 318, 120948. [Google Scholar] [CrossRef] [PubMed]
- Streletskii, R.A.; Astaykina, A.A.; Belov, A.A.; Cheptsov, V.S.; Vetrova, A.A. Beneficial soil microorganisms and their role in sustainable agriculture. In Sustainable Agricultural Practices; Academic Press: Cambridge, MA, USA, 2024; pp. 293–333. [Google Scholar] [CrossRef]
- Zhang, S.; Li, X.; Chen, K.; Shi, J.; Wang, Y.; Luo, P.; Yang, J.; Wang, Y.; Han, X. Long-term fertilization altered microbial community structure in an aeolian sandy soil in northeast China. Front. Microbiol. 2022, 13, 979759. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xie, H.; Ren, Z.; Ding, Y.; Long, M.; Zhang, G.; Qin, X.; Siddique, K.H.; Liao, Y. Response of soil microbial community parameters to plastic film mulch: A meta-analysis. Geoderma 2022, 418, 115851. [Google Scholar] [CrossRef]
- Cai, F.; Luo, P.; Yang, J.; Irfan, M.; Zhang, S.; An, N.; Dai, J.; Han, X. Effect of long-term fertilization on ammonia-oxidizing microorganisms and nitrification in brown soil of Northeast China. Front. Microbiol. 2021, 11, 622454. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Guo, A.; Kang, Y.; Yang, X.; Zhang, W.; Liu, Y.; Zhang, R.; Wang, Y.; Qin, S. Effects of plastic film mulching and legume rotation on soil nutrients and microbial communities in the Loess Plateau of China. Chem. Biol. Technol. Agric. 2023, 10, 38. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, H.; Jin, T.; Liu, H.; Men, J.; Cai, G.; Cernava, T.; Duan, G.; Jin, D. Biodegradable mulch films significantly affected rhizosphere microbial communities and increased peanut yield. Sci. Total Environ. 2023, 871, 162034. [Google Scholar] [CrossRef]
- Farmer, J.; Zhang, B.; Jin, X.; Zhang, P.; Wang, J. Long-term effect of plastic film mulching and fertilization on bacterial communities in a brown soil revealed by high through-put sequencing. Arch. Agronom. Soil Sci. 2017, 63, 230–241. [Google Scholar] [CrossRef]
- Yang, C.; Huang, Y.; Long, B.; Gao, X. Effects of biodegradable and polyethylene film mulches and their residues on soil bacterial communities. Environ. Sci. Pollut. Res. 2022, 29, 89698–89711. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Zhang, J.; Yao, Z.; Luo, S.; Tian, L.; Tian, C.; Sun, Y. Preliminary findings of polypropylene carbonate (PPC) plastic film mulching effects on the soil microbial community. Agriculture 2022, 12, 406. [Google Scholar] [CrossRef]
- Wang, J.; Huang, M.; Wang, Q.; Sun, Y.; Zhao, Y.; Huang, Y. LDPE microplastics significantly alter the temporal turnover of soil microbial communities. Sci. Total Environ. 2020, 726, 138682. [Google Scholar] [CrossRef]
- Liu, J.; Li, S.; Yue, S.; Tian, J.; Chen, H.; Jiang, H.; Siddique, K.H.; Zhan, A.; Fang, Q.; Yu, Q. Soil microbial community and network changes after long-term use of plastic mulch and nitrogen fertilization on semiarid farmland. Geoderma 2021, 396, 115086. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Song, D.; Liang, S.; Qin, X.; Siddique, K.H.M. The effects of straw incorporation with plastic film mulch on soil properties and bacterial community structure on the loess plateau. Eur. J. Soil Sci. 2021, 72, 979–994. [Google Scholar] [CrossRef]
- Luo, S.; Wang, S.; Zhang, H.; Zhang, J.; Tian, C. Plastic film mulching reduces microbial interactions in black soil of northeastern China. Appl. Soil Ecol. 2021, 169, 104187. [Google Scholar] [CrossRef]
- Miao, L.; Wang, C.; Adyel, T.M.; Wu, J.; Liu, Z.; You, G.; Meng, M.; Qu, H.; Huang, L.; Yu, Y.; et al. Microbial carbon metabolic functions of biofilms on plastic debris influenced by the substrate types and environmental factors. Environ. Int. 2020, 143, 106007. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Fan, J.; Fu, W.; Niu, X.; Yang, Q.; Hao, M. Changes in soil microbial community and co-occurrence network after long-term no-tillage and mulching in dryland farming. Plant Soil 2023, 495, 201–220. [Google Scholar] [CrossRef]
- Cui, R.; An, X.; Luo, X.; Chen, C.; Liu, T.; Zou, L. Effects of Biodegradable Mulch on Soil Microorganisms. Mol. Plant Breed. 2024, 15, 34–41. [Google Scholar] [CrossRef]
- Chinese Soil Taxonomy Cooperative Research Group. Chinese Soil Taxonomy (Revised Proposal); Chinese Agricultural Science and Technology Press: Beijing, China, 1995. [Google Scholar]
- Shao, K.; Bai, C.; Cai, J.; Hu, Y.; Gong, Y.; Chao, J.; Dai, J.; Wang, Y.; Ba, T.; Tang, X.; et al. Illumina sequencing revealed soil microbial communities in a Chinese alpine grassland. Geomicrobiol. J. 2019, 36, 204–211. [Google Scholar] [CrossRef]
- Kõljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef] [PubMed]
- Abuzar, M.; Sadozai, G.; Baloch, M.; Baloch, A.; Shah, I.; Javaid, T.; Hussain, N. Effect of plant population densities on yield of maize. J. Anim. Plant Sci. 2011, 21, 692–695. [Google Scholar]
- Zhou, H.; Zhang, D.; Jiang, Z.; Sun, P.; Xiao, H.; Yuxin, W.; Chen, J. Changes in the soil microbial communities of alpine steppe at Qinghai-Tibetan Plateau under different degradation levels. Sci. Total Environ. 2018, 651, 2281–2291. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, J.; Shi, W.; Du, N.; Xu, X.; Zhang, Y.; Ji, P.; Zhang, F.; Jia, Z.; Wang, Y.; et al. Dysbiosis of maternal and neonatal microbiota associated with gestational diabetes mellitus. Gut 2018, 67, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, S. Nitrogen addition shapes soil enzyme activity patterns by changing pH rather than the composition of the plant and microbial communities in an alpine meadow soil. Plant Soil 2019, 440, 11–24. [Google Scholar] [CrossRef]
- Layeghifard, M.; Hwang, D.M.; Guttman, D.S. Disentangling interactions in the microbiome: A network perspective. Trends Microbiol. 2017, 25, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Zhang, Y.; Luo, W.; Wang, Y. Root colonization and effect of biocontrol fungus paecilomyces lilacinus on composition of ammonia-oxidizing bacteria, ammonia-oxidizing archaea and fungal populations of tomato rhizosphere. Biol. Fertil. Soils 2014, 51, 343–351. [Google Scholar] [CrossRef]
- De Curtis, F.; de Felice, D.; Ianiri, G.; De Cicco, V.; Castoria, R. Environmental factors affect the activity of biocontrol agents against ochratoxigenic Aspergillus carbonarius on wine grape. Int. J. Food Microbiol. 2012, 159, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Lin, T.; Sun, Z.; Yan, A.; Zhang, J.; Jiang, P.; Wu, F.; Zhang, H. Effects of mulching film on soil microbial diversity and community of cotton. AMB Express 2022, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Zhang, L.; Duan, Y.; Sun, L.; Zhao, P.; van der Werf, W.; Evers, J.B.; Wang, Q.; Wang, R.; Sun, Z. Ridge and furrow systems with film cover increase maize yields and mitigate climate risks of cold and drought stress in continental climates. Field Crop. Res. 2017, 207, 71–78. [Google Scholar] [CrossRef]
- Huang, F.; Liu, Z.; Mou, H.; Zhang, P.; Jia, Z. Effects of different long-term farmland mulching practices on the loessial soil fungal community in a semiarid region of China. Appl. Soil Ecol. 2019, 137, 111–119. [Google Scholar] [CrossRef]
- Smith, A.; Marín-Spiotta, E.; de Graaff, M.; Balser, T. Microbial community structure varies across soil organic matter aggregate pools during tropical land cover change. Soil Biol. Biochem. 2014, 77, 292–303. [Google Scholar] [CrossRef]
- Xue, Y.; Jin, T.; Gao, C.; Li, C.; Zhou, T.; Wan, D.; Yang, M. Effects of biodegradable film mulching on bacterial diversity in soils. Arch. Microbiol. 2022, 204, 195. [Google Scholar] [CrossRef] [PubMed]
- Tayyab, M.; Islam, W.; Lee, C.G.; Pang, Z.; Khalil, F.; Lin, S.; Lin, W.; Zhang, H. Short-term effects of different organic amendments on soil fungal composition. Sustainability 2019, 11, 198. [Google Scholar] [CrossRef]
- Liu, B.; Dai, Y.; Cheng, X.; He, X.; Bei, Q.; Wang, Y.; Zhou, Y.; Zhu, B.; Zhang, K.; Tian, X.; et al. Straw mulch improves soil carbon and nitrogen cycle by mediating microbial community structure and function in the maize field. Front. Microbiol. 2023, 14, 1217966. [Google Scholar] [CrossRef] [PubMed]
- Grum, B.; Assefa, D.; Hessel, R.; Woldearegay, K.; Ritsema, C.J.; Aregawi, B.; Geissen, V. Improving on-site water availability by combining in-situ water harvesting techniques in semi-arid Northern Ethiopia. Agric. Water Manag. 2017, 193, 153–162. [Google Scholar] [CrossRef]
- Zhalnina, K.; Dias, R.; De Quadros, P.D.; Davis-Richardson, A.; Camargo, F.A.O.; Clark, I.M.; McGrath, S.P.; Hirsch, P.R.; Triplett, E.W. Soil pH determines microbial diversity and composition in the park grass experiment. Microb. Ecol. 2014, 69, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Ren, B.; Hu, Y.; Chen, B.; Zhang, Y.; Thiele, J.; Shi, R.; Liu, M.; Bu, R. Soil pH and plant diversity shape soil bacterial community structure in the active layer across the latitudinal gradients in continuous permafrost region of Northeastern China. Sci. Rep. 2018, 8, 5619. [Google Scholar] [CrossRef] [PubMed]
- Larkin, R.P. Characterization of soil microbial communities under different potato cropping systems by microbial population dynamics, substrate utilization, and fatty acid profiles. Soil Biol. Biochem. 2023, 35, 1451–1466. [Google Scholar] [CrossRef]
- Wan, P.; Zhang, F.; Zhang, K.; Li, Y.; Qin, R.; Yang, J.; Fang, C.; Kuzyakov, Y.; Li, S.; Li, F.-M. Soil warming decreases carbon availability and reduces metabolic functions of bacteria. Catena 2023, 223, 106913. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Sun, L.; Qiu, C.; Ding, Y.; Gu, H.; Wang, L.; Wang, Z.; Ding, Z. Organic mulching positively regulates the soil microbial communities and ecosystem functions in tea plantation. BMC Microbiol. 2020, 20, 103. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Lv, W.; Wang, X.; Xie, Z.; Wang, Y. Long-term effects of gravel mulching and straw mulching on soil physicochemical properties and bacterial and fungal community composition in the Loess Plateau of China. Eur. J. Soil Biol. 2020, 98, 103188. [Google Scholar] [CrossRef]
- Shange, R.S.; Ankumah, R.O.; Ibekwe, A.M.; Zabawa, R.; Dowd, S.E. Distinct soil bacterial communities revealed under a diversely managed agroecosystem. PLOS ONE 2012, 7, e40338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Deng, Y.; Luo, F.; He, Z.; Tu, Q.; Zhi, X. Functional molecular ecological networks. mBio 2010, 1, e00169-00110. [Google Scholar] [CrossRef] [PubMed]
- Yuanqiao, L.L.; Caixia, Z.Z.; Changrong, Y.Y.; Lili, M.M.; Qi, L.L.; Zhen, L.L.; Wenqing, H.H. Effects of agricultural plastic film residues on transportation and distribution of water and nitrate in soil. Chemosphere 2020, 242, 125131. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.-Y.; Wang, P.-Y.; Wang, Y.-B.; Zhou, R.; Koskei, K.; Munyasya, A.N.; Liu, S.-T.; Wang, W.; Su, Y.-Z.; Xiong, Y.-C. Fate of plastic film residues in agro-ecosystem and its effects on aggregate-associated soil carbon and nitrogen stocks. J. Hazard. Mater. 2021, 416, 125954. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Zhuang, S.; Gao, J.; Tang, L.; Harindintwali, J.D.; Wang, F. Aeration increases soil bacterial diversity and nutrient transformation under mulching-induced hypoxic conditions. Sci. Total Environ. 2022, 817, 153017. [Google Scholar] [CrossRef] [PubMed]




| Growth Period | Seedling Period | Jointing Period | Small Bell Mouth Period | Big Bell-Mouth Period | Heading Period | Flowering Period | Silking Period | Grain Formation Period | Milk-Ripe Period | Total |
|---|---|---|---|---|---|---|---|---|---|---|
| Irrigation and Fertilization Date | 5/13 | 6/15 | 6/28 | 7/7 | 7/15 | 7/27 | 8/6 | 8/14 | 8/25 | |
| Irrigation amount (m3·ha−1) | 163.6 | 600 | 600 | 600 | 600 | 600 | 600 | 563.6 | 472.8 | 4800.0 |
| Urea (kg·ha−1) | 0 | 81.8 | 81.8 | 90.9 | 81.8 | 81.8 | 72.7 | 54.7 | 0 | 545.5 |
| Monoammonium phosphate (kg·ha−1) | 36.4 | 36.4 | 45.5 | 45.5 | 45.5 | 27.3 | 18.2 | 18.2 | 0 | 273.0 |
| Potassium sulphate (kg·ha−1) | 0 | 18.2 | 27.3 | 27.3 | 36.4 | 22.7 | 18.2 | 13.6 | 0 | 163.7 |
| Depth (cm) | Reproductive Period | Treatment | SM (%) | ST (°C) | SOM (g·kg−1) | TN (g·kg−1) | AP (mg·kg−1) | AK (mg·kg−1) | pH |
|---|---|---|---|---|---|---|---|---|---|
| 0–10 | Heading period | NM | 14.31 ± 0.16 c | 21.02 ± 0.19 b | 12.19 ± 0.29 c | 1.29 ± 0.07 b | 27.20 ± 0.50 a | 416.56 ± 0.43 a | 8.61 |
| HM | 15.72 ± 0.29 b | 22.27 ± 0.36 a | 17.79 ± 0.25 a | 1.41 ± 0.11 ab | 27.35 ± 0.64 a | 418.07 ± 0.69 a | 8.97 | ||
| FM | 18.03 ± 0.33 a | 18.03 ± 0.33 c | 16.73 ± 0.32 b | 1.76 ± 0.23 a | 26.84 ± 0.23 a | 417.79 ± 0.44 a | 8.99 | ||
| Maturity period | NM | 8.61 ± 0.48 c | 19.31 ± 0.26 c | 14.30 ± 0.11 c | 1.16 ± 0.22 b | 27.61 ± 0.65 a | 417.62 ± 0.77 a | 8.47 | |
| HM | 16.62 ± 0.32 b | 20.14 ± 0.10 b | 18.31 ± 0.27 a | 1.39 ± 0.03 a | 27.70 ± 0.62 a | 419.00 ± 0.59 a | 8.79 | ||
| FM | 21.93 ± 0.13 a | 21.43 ± 0.28 a | 17.24 ± 0.29 b | 1.34 ± 0.15 a | 27.70 ± 0.65 a | 418.64 ± 0.65 a | 8.41 | ||
| 10–20 | Heading period | NM | 22.33 ± 0.23 c | 21.07 ± 0.09 c | 13.17 ± 0.34 b | 1.43 ± 0.07 b | 27.49 ± 0.63 a | 417.26 ± 0.36 b | 8.37 |
| HM | 25.45 ± 0.30 b | 23.14 ± 0.36 b | 17.81 ± 0.39 a | 1.64 ± 0.12 a | 27.22 ± 0.39 a | 418.89 ± 0.58 a | 8.68 | ||
| FM | 26.59 ± 0.31 a | 24.39 ± 0.28 a | 16.88 ± 0.35 a | 1.51 ± 0.25 ab | 27.23 ± 0.42 a | 418.85 ± 0.56 a | 8.43 | ||
| Maturity period | NM | 8.72 ± 0.25 c | 19.44 ± 0.18 c | 14.34 ± 0.52 c | 1.51 ± 0.11 a | 27.50 ± 0.33 a | 417.79 ± 0.47 a | 8.06 | |
| HM | 18.63 ± 0.33 b | 23.65 ± 0.21 a | 19.56 ± 0.24 a | 1.55 ± 0.11 a | 27.68 ± 0.65 a | 420.82 ± 0.43 a | 8.28 | ||
| FM | 22.07 ± 0.28 a | 22.54 ± 0.35 b | 17.26 ± 0.34 b | 1.49 ± 0.03 a | 27.95 ± 0.62 a | 418.93 ± 0.64 a | 8.20 | ||
| 20–40 | Heading period | NM | 32.51 ± 0.50 b | 21.43 ± 0.11 b | 13.23 ± 0.23 c | 1.20 ± 0.12 a | 24.79 ± 0.35 b | 296.88 ± 0.40 c | 8.18 |
| HM | 32.74 ± 0.38 ab | 23.56 ± 0.23 ab | 18.31 ± 0.39 a | 1.44 ± 0.02 a | 25.97 ± 0.29 ab | 308.31 ± 0.54 a | 8.56 | ||
| FM | 33.93 ± 0.70 a | 24.61 ± 0.27 a | 17.11 ± 0.27 b | 1.35 ± 0.14 a | 26.38 ± 0.38 a | 301.35 ± 0.61 b | 8.36 | ||
| Maturity period | NM | 11.33 ± 0.44 c | 20.15 ± 0.11 c | 14.85 ± 0.21 c | 1.35 ± 0.09 b | 24.77 ± 0.69 a | 300.89 ± 0.58 c | 7.88 | |
| HM | 20.62 ± 0.44 ab | 24.22 ± 0.24 b | 19.71 ± 0.17 a | 1.53 ± 0.07 a | 25.65 ± 0.70 a | 309.14 ± 0.36 a | 8.26 | ||
| FM | 22.12 ± 0.23 a | 25.53 ± 0.27 a | 17.28 ± 0.37 b | 1.28 ± 0.03 b | 25.26 ± 0.53 a | 305.16 ± 0.35 b | 8.07 |
| Network Properties | Treatment | Nodes | Edges | Average Degree | Clustering Coefficient | Modularity |
|---|---|---|---|---|---|---|
| Bacteria | NM | 418 | 689 | 0.297 | 0.249 | 0.840 |
| HM | 956 | 3106 | 6.498 | 0.298 | 0.685 | |
| FM | 236 | 246 | 2.085 | 0.336 | 0.609 | |
| Fungus | NM | 167 | 147 | 1.760 | 0.458 | 0.906 |
| HM | 174 | 141 | 1.621 | 0.703 | 0.909 | |
| FM | 178 | 242 | 2.719 | 0.806 | 0.970 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Zheng, J.; Li, Q.; Liang, F.; Mu, X.; Pei, D.; Jia, H.; Wang, Z. Effects of Film Mulching on Soil Microbial Diversity and Community Structure in the Maize Root Zone under Drip Irrigation in Northwest China. Agronomy 2024, 14, 1139. https://doi.org/10.3390/agronomy14061139
Liu M, Zheng J, Li Q, Liang F, Mu X, Pei D, Jia H, Wang Z. Effects of Film Mulching on Soil Microbial Diversity and Community Structure in the Maize Root Zone under Drip Irrigation in Northwest China. Agronomy. 2024; 14(6):1139. https://doi.org/10.3390/agronomy14061139
Chicago/Turabian StyleLiu, Mengjie, Jiliang Zheng, Quansheng Li, Fei Liang, Xiaoguo Mu, Dongjie Pei, Hongtao Jia, and Zhenhua Wang. 2024. "Effects of Film Mulching on Soil Microbial Diversity and Community Structure in the Maize Root Zone under Drip Irrigation in Northwest China" Agronomy 14, no. 6: 1139. https://doi.org/10.3390/agronomy14061139
APA StyleLiu, M., Zheng, J., Li, Q., Liang, F., Mu, X., Pei, D., Jia, H., & Wang, Z. (2024). Effects of Film Mulching on Soil Microbial Diversity and Community Structure in the Maize Root Zone under Drip Irrigation in Northwest China. Agronomy, 14(6), 1139. https://doi.org/10.3390/agronomy14061139







