Methanotrophic Inoculation Reduces Methane Emissions from Rice Cultivation Supplied with Pig-Livestock Biogas Digestive Effluent
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Preparation
2.3. Biogas Digestive Effluent
2.4. Methanotroph
2.5. Rice Cultivar
2.6. Experimental Design
2.7. Measurements
2.7.1. Weather Data
2.7.2. Physiochemical Characteristics of BDE, Soil and Soil water solution
2.7.3. CH4 and N2O Analysis
2.7.4. Plant Height, SPAD, Yield Components
2.7.5. RNA Extraction and qPCR of pmoA Transcript Copy Number
2.8. Data Processing
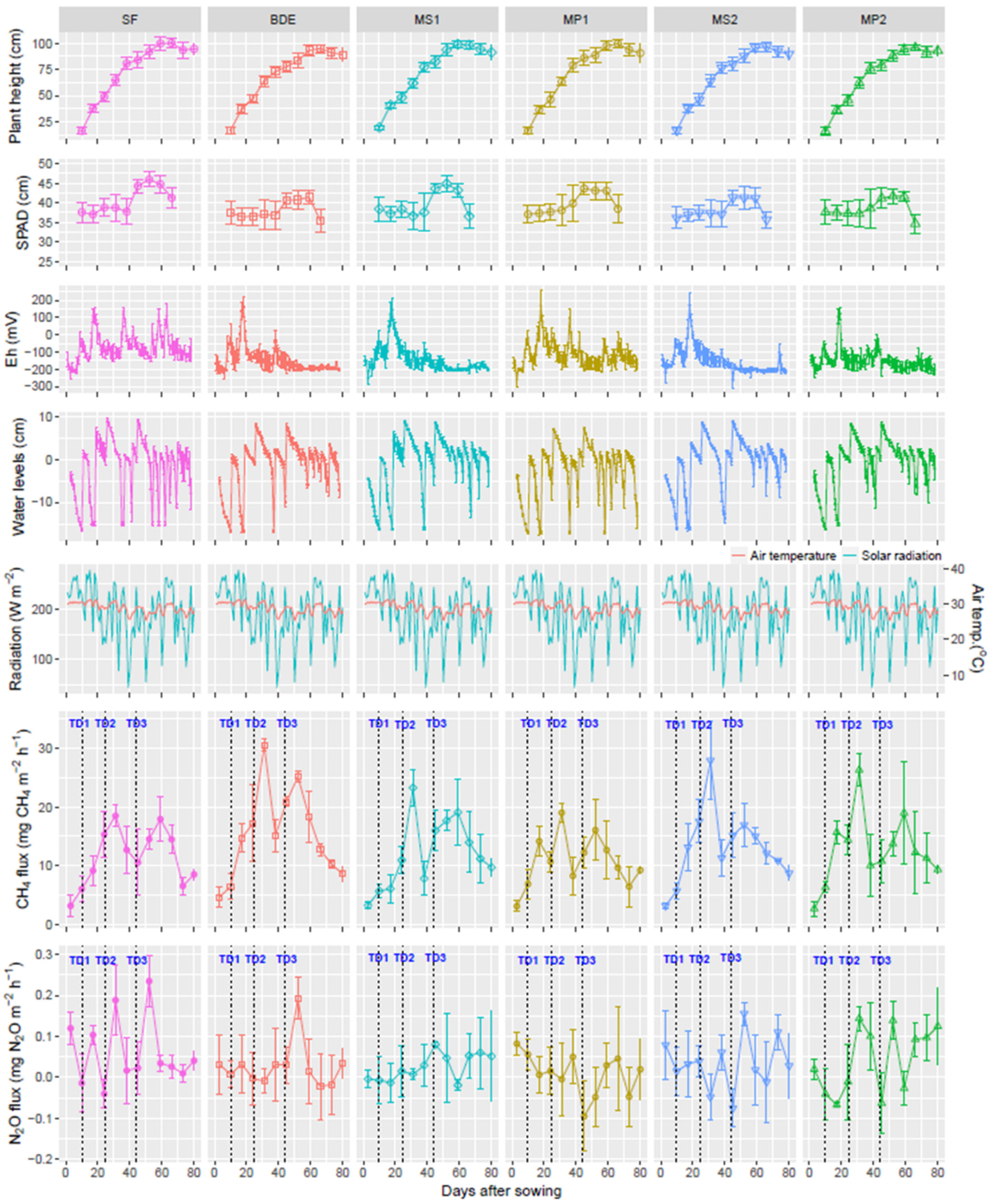
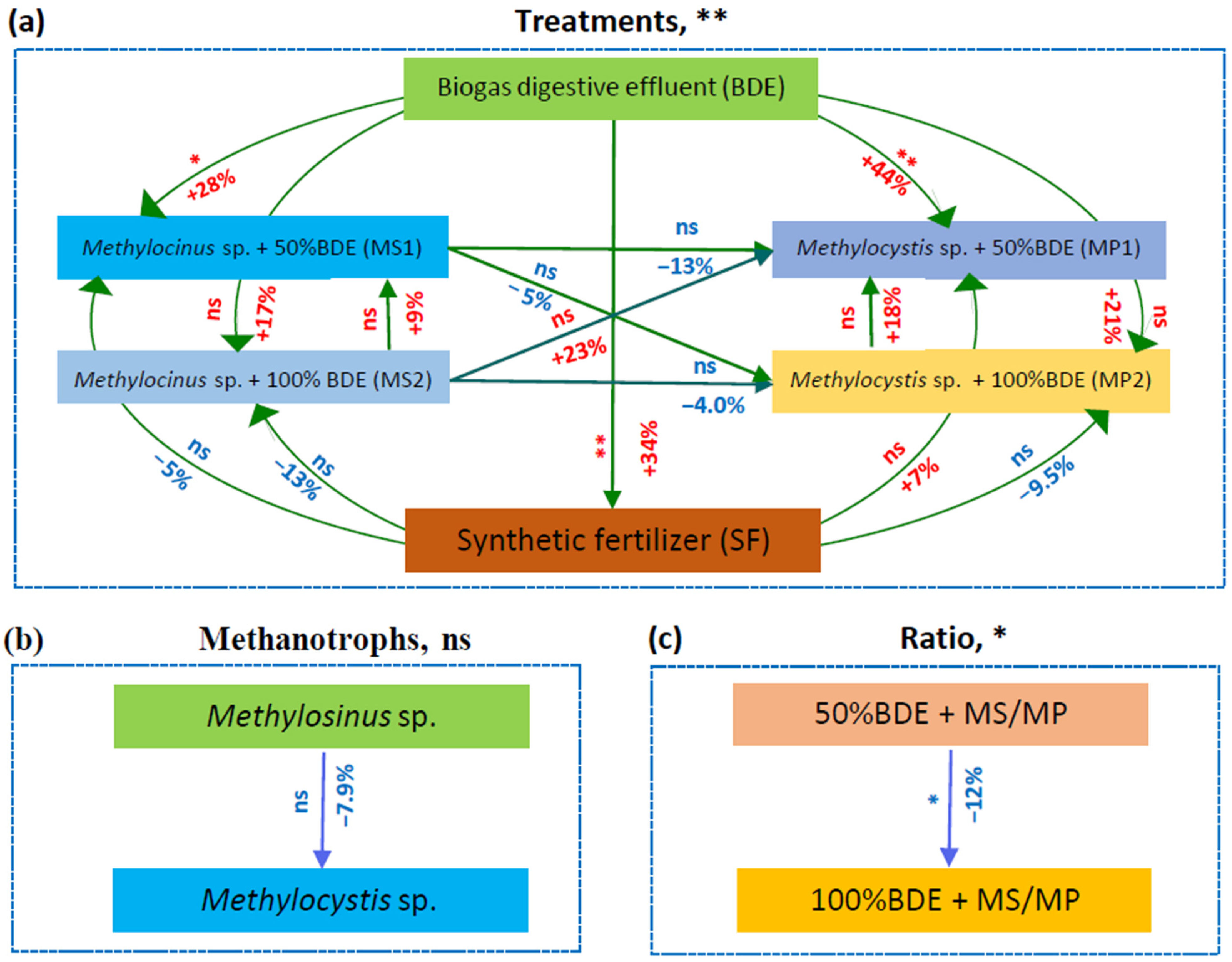
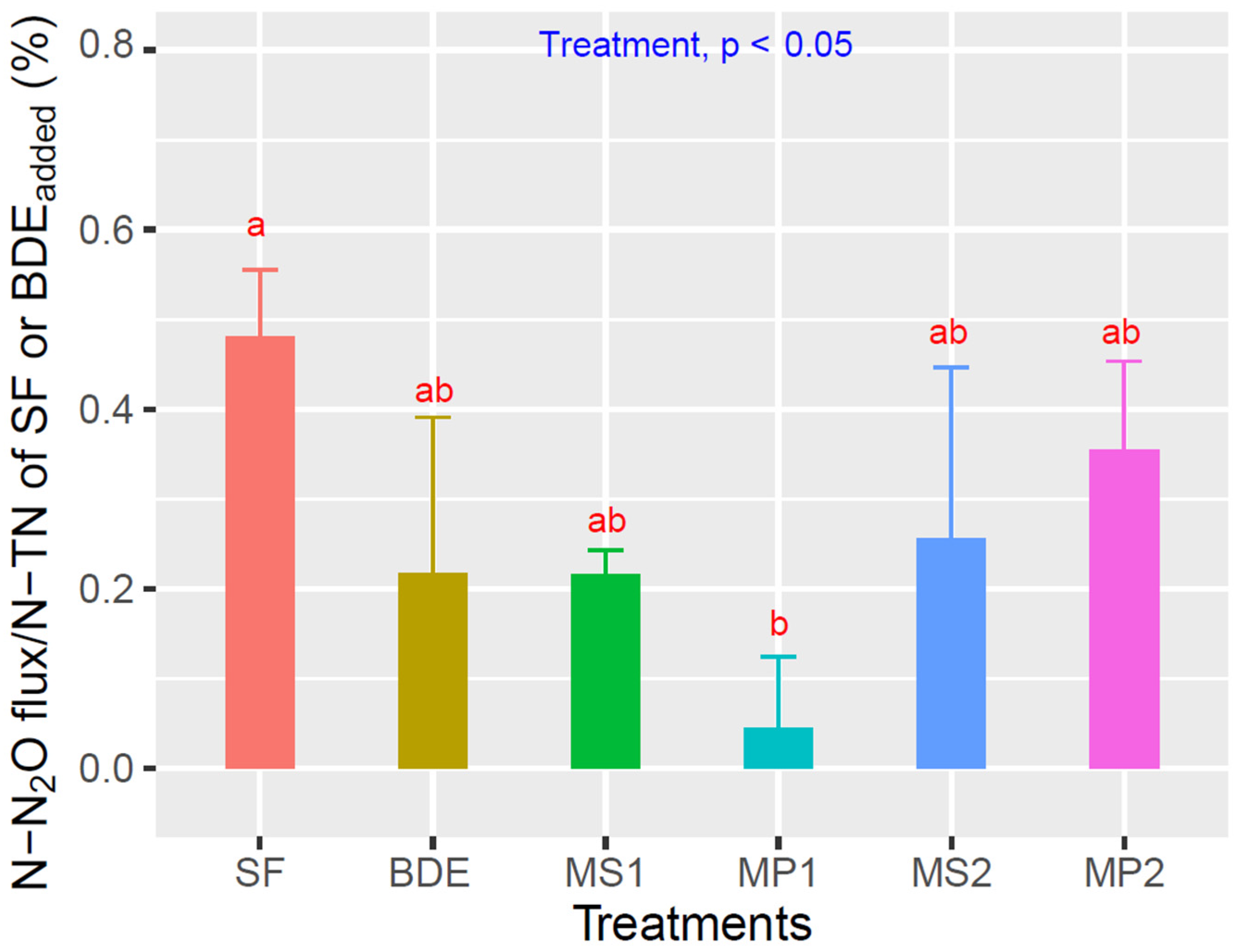
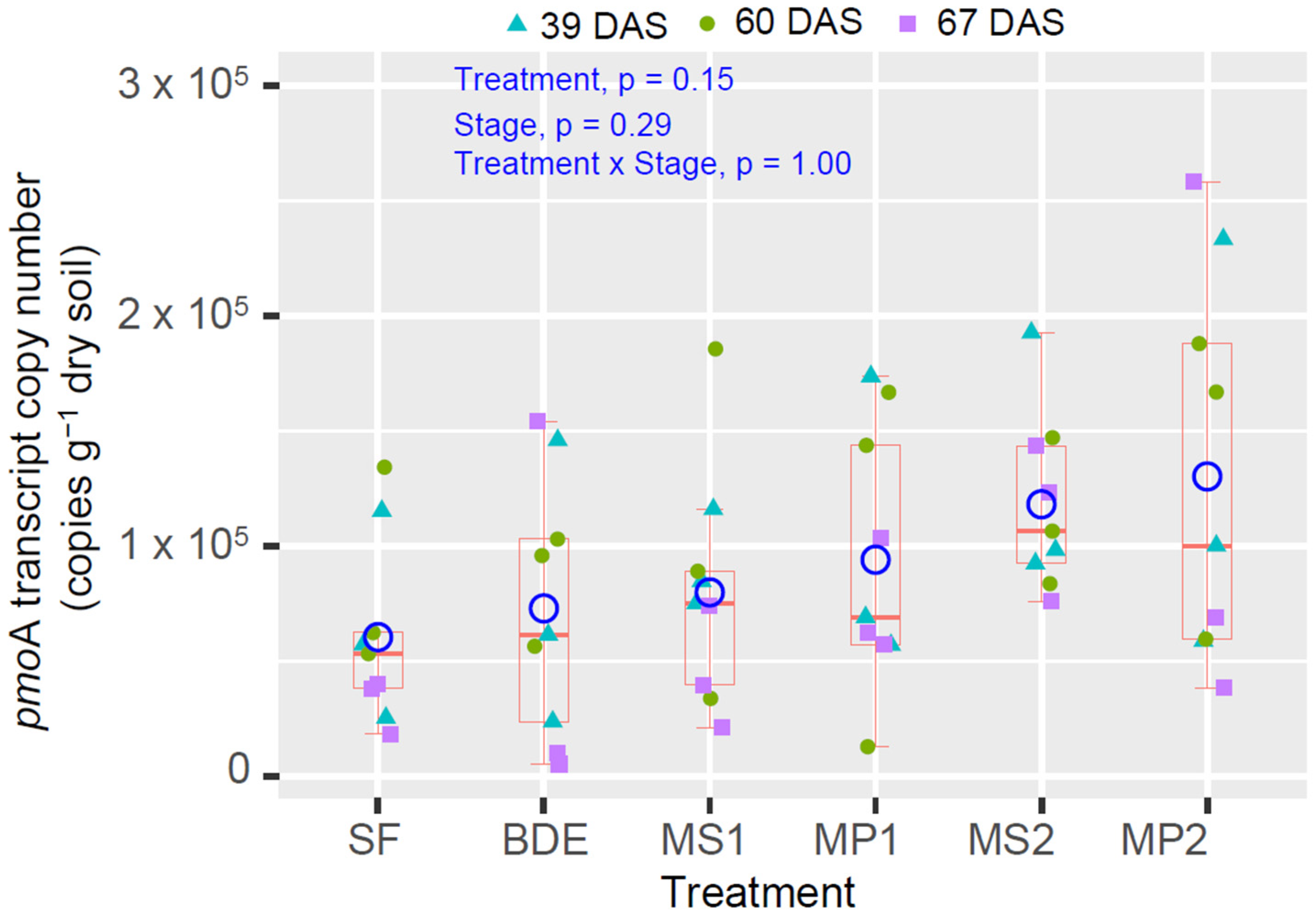
3. Results
3.1. Physiochemical Characteristics of Soil and Soil Water Solution
3.2. Eh, Water Levels, CH4, and N2O Emissions
3.3. Rice Growth, SPAD, and Yield Components
3.4. pmoA Transcript Copy Number
3.5. Factors Affecting Greenhouse Gas Emissions
4. Discussion
4.1. Soil and Soil Water Changes under Methanotroph-Inoculated BDE Application
4.2. Effects of Methanotroph-Inoculated BDE Application on Rice Growth and Yield Components
4.3. Effects of Methanotroph-Inoculated BDE on Methane and Nitrous Oxide Emissions
| Digestate | Experimental Condition | Water Management | N Applied (g N m−2) | CH4 Emission (g CH4 m−2) | N2O Emission (mg N2O m−2) | Reference |
|---|---|---|---|---|---|---|
| Cattle BDE | Applying 100% BDE based N-TKN, direct seeding, field experiment in VMD | AWD, MiDi, CF | 15 | 37 | 44 | [20] |
| Pig biogas slurry | Combination of chemical fertilizers and BDE (25–100%), transplanting, a field experiment in Jiangsu, China | MD | 24 | 16–54.4 | NA | [19] |
| Pig biogas slurry | Applying slurry based on N-NH4+, transplanting, a field experiment in Fuchu, Tokyo, Japan | NA | 10–30 | 32–43.7 | −0.16–0.26 | [21] |
| Pig biogas slurry | Applying slurry based on N-NH4+-effects of rice cultivars, transplanting, a field experiment in Fuchu Tokyo, Japan | NA | 10–30 | 52–80 | NA | [62] |
| Cattle slurry | Mixing slurry with wood vinegar, transplanting, Lysimeter experiment in Fuchu Tokyo, Japan | CF | 30 | 60–150 | 0.01–0.23 | [57] |
| Pig biogas slurry | Applying slurry based on N-NH4+-effects of rice cultivars and Eh control, transplanting, field experiment in Fuchu Tokyo, Japan | CF, WS | 30 | 8.4–23.8 | 0.11–0.14 | [24] |
| Cattle slurry | Mixing biogas slurry and urea, transplanting, a field experiment in New Delhi, India | CF | 12 | 2.21 | NA | [63] |
| Cattle slurry | Fertilizing slurry based on N-NH4+, transplanting, a field experiment in Osaka, Japan. | CF, MiDi | 12 | 17.1–31.4 | NA | [17] |
| Pig BDE inoculated with methanotrophs | Fertilizing effluent based on N-TN-methanotroph inoculation, Direct seeding, a pot experiment in the VMD | MD | 15 | 21.3–30.6 | 10.6–83.6 | Current study |
4.4. The Role of Methanotrophs in CH4 Emission Reduction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Qian, H.; Zhu, X.; Huang, S.; Linquist, B.; Kuzyakov, Y.; Wassmann, R.; Minamikawa, K.; Martinez-Eixarch, M.; Yan, X.; Zhou, F.; et al. Greenhouse Gas Emissions and Mitigation in Rice Agriculture. Nat. Rev. Earth Environ. 2023, 4, 716–732. [Google Scholar] [CrossRef]
- Myhre, G.; Shindell, D.; Bréon, F.M.; Collins, W.; Fuglestvedt, J.; Huang, J.; Koch, D.; Lamarque, J.F.; Lee, D.; Mendoza, B.; et al. Anthropogenic and Natural Radiative Forcing. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M.M.B., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013; pp. 659–740. [Google Scholar]
- Carlson, K.M.; Gerber, J.S.; Mueller, N.D.; Herrero, M.; MacDonald, G.K.; Brauman, K.A.; Havlik, P.; O’Connell, C.S.; Johnson, J.A.; Saatchi, S.; et al. Greenhouse Gas Emissions Intensity of Global Croplands. Nat. Clim. Chang. 2017, 7, 63–68. [Google Scholar] [CrossRef]
- Yan, X.; Yagi, K.; Akiyama, H.; Akimoto, H. Statistical Analysis of the Major Variables Controlling Methane Emission from Rice Fields. Glob. Chang. Biol. 2005, 11, 1131–1141. [Google Scholar] [CrossRef]
- Nam, T.S.; Van Cong, N.; Van Thao, H. Enhancing Renewable Energy Production from Water Hyacinth (Eichhornia crassipes) by a Biogas-Aerating Recirculation System: A Case Study in the Vietnamese Mekong Delta. Case Stud. Chem. Environ. Eng. 2023, 7, 100340. [Google Scholar] [CrossRef]
- Rajendran, K.; Aslanzadeh, S.; Taherzadeh, M.J. Household Biogas Digesters—A Review. Energies 2012, 5, 2911–2942. [Google Scholar] [CrossRef]
- Thoa, L.T. Lack of Overall Strategy on the Expansion of Biogas Production. Biogas J. 2020, 1–3. Available online: https://gizenergy.org.vn/wp-content/uploads/Biogas_Journal_Aug-2020_ENG-1.pdf (accessed on 22 May 2024).
- Dinh, T.X. An Overview of Agricultural Pollution in Vietnam: The Livestock Sector; World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Xu, M.; Xian, Y.; Wu, J.; Gu, Y.; Yang, G.; Zhang, X.; Peng, H.; Yu, X.; Xiao, Y.; Li, L. Effect of Biogas Slurry Addition on Soil Properties, Yields, and Bacterial Composition in the Rice-Rape Rotation Ecosystem over 3 Years. J. Soils Sediments 2019, 19, 2534–2542. [Google Scholar] [CrossRef]
- Nguyen Vo Chau, N.; Huynh Van, T.; Nguyen Cong, T.; Kim, L.; Pham, D.V. Water Lettuce ( Pistia stratiotes L.) Increases Biogas Effluent Pollutant Removal Efficacy and Proves a Positive Substrate for Renewable Energy Production. PeerJ 2023, 11, e15879. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Liu, X.; Sheng, L.; Zhang, D.; Li, L.; Wang, A. Study on the Pollution Status and Control Measures for the Livestock and Poultry Breeding Industry in Northeastern China. Environ. Sci. Pollut. Res. 2018, 25, 4435–4445. [Google Scholar] [CrossRef] [PubMed]
- Biagini, D.; Lazzaroni, C. Eutrophication Risk Arising from Intensive Dairy Cattle Rearing Systems and Assessment of the Potential Effect of Mitigation Strategies. Agric. Ecosyst. Environ. 2018, 266, 76–83. [Google Scholar] [CrossRef]
- Chen, S.; Yu, W.; Zhang, Z.; Luo, S. Soil Properties and Enzyme Activities as Affected by Biogas Slurry Irrigation in the Three Gorges Reservoir Areas of China. J. Environ. Biol. 2015, 36, 513–520. [Google Scholar] [PubMed]
- Ding, W.; Niu, H.; Chen, J.; Du, J.; Wu, Y. Influence of Household Biogas Digester Use on Household Energy Consumption in a Semi-Arid Rural Region of Northwest China. Appl. Energy 2012, 97, 16–23. [Google Scholar] [CrossRef]
- Yang, W.-J.; Shao, D.-D.; Zhou, Z.; Xia, Q.-C.; Chen, J.; Cao, X.-L.; Zheng, T.; Sun, S.-P. Carbon Quantum Dots (CQDs) Nanofiltration Membranes towards Efficient Biogas Slurry Valorization. Chem. Eng. J. 2020, 385, 123993. [Google Scholar] [CrossRef]
- Minamikawa, K.; Khanh, H.C.; Hosen, Y.; Nam, T.S.; Chiem, N.H. Variable-Timing, Fixed-Rate Application of Cattle Biogas Effluent to Rice Using a Leaf Color Chart: Microcosm Experiments in Vietnam. Soil Sci. Plant Nutr. 2020, 66, 225–234. [Google Scholar] [CrossRef]
- Tanaka, T.S.T.; Nitta, Y.; Kido, K.; Nishikawa, T.; Matoh, T.; Inamura, T. Effect of the Long-Term Application of Anaerobically Digested Residual Slurry on Methane Emissions in a Rice Paddy Field. Soil Sci. Plant Nutr. 2017, 63, 300–305. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, Z.; Zhao, J.; Ma, J.; Yu, Q.; Zou, P.; Lin, H.; Ma, J. Fate of Heavy Metals and Bacterial Community Composition Following Biogas Slurry Application in a Single Rice Cropping System. J. Soils Sediments 2022, 22, 968–981. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Cao, J.-L.; Wu, H.-S.; Ye, X.-M.; Ma, Y.; Yu, J.-G.; Shen, Q.-R.; Chang, Z.-Z. Elevated Methane Emissions from a Paddy Field in Southeast China Occur after Applying Anaerobic Digestion Slurry. GCB Bioenergy 2014, 6, 465–472. [Google Scholar] [CrossRef]
- Minamikawa, K.; Huynh, K.C.; Uno, K.; Tran, N.S.; Nguyen, C.H. Cattle Biogas Effluent Application with Multiple Drainage Mitigates Methane and Nitrous Oxide Emissions from a Lowland Rice Paddy in the Mekong Delta, Vietnam. Agric. Ecosyst. Environ. 2021, 319, 107568. [Google Scholar] [CrossRef]
- Win, A.T.; Toyota, K.; Win, K.T.; Motobayashi, T.; Ookawa, T.; Hirasawa, T.; Chen, D.; Lu, J. Effect of Biogas Slurry Application on CH4 and N2O Emissions, Cu and Zn Uptakes by Whole Crop Rice in a Paddy Field in Japan. Soil Sci. Plant Nutr. 2014, 60, 411–422. [Google Scholar] [CrossRef]
- Lu, Y.; Wassmann, R.; Neue, H.U.; Huang, C.; Bueno, C.S. Methanogenic Responses to Exogenous Substrates in Anaerobic Rice Soils. Soil Biol. Biochem. 2000, 32, 1683–1690. [Google Scholar] [CrossRef]
- Wassmann, R.; Tölg, M.; Papen, H.; Rennenberg, H.; Seiler, W.; Cheng, D.X.; Wang, M.X. Spatial and Seasonal Distribution of Organic Amendments Affecting Methane Emission from Chinese Rice Fields. Biol. Fert. Soils 1996, 22, 191–195. [Google Scholar] [CrossRef]
- Win, K.T.; Nonaka, R.; Win, A.T.; Sasada, Y.; Toyota, K.; Motobayashi, T. Effects of Water Saving Irrigation and Rice Variety on Greenhouse Gas Emissions and Water Use Efficiency in a Paddy Field Fertilized with Anaerobically Digested Pig Slurry. Paddy Water Environ. 2015, 13, 51–60. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, Y.; Jiang, P.; Zhang, C.; Smith, T.J.; Murrell, J.C.; Xing, X.-H. Methanotrophs: Multifunctional Bacteria with Promising Applications in Environmental Bioengineering. Biochem. Eng. J. 2010, 49, 277–288. [Google Scholar] [CrossRef]
- Davamani, V.; Parameswari, E.; Arulmani, S. Mitigation of Methane Gas Emissions in Flooded Paddy Soil through the Utilization of Methanotrophs. Sci. Total Environ. 2020, 726, 138570. [Google Scholar] [CrossRef]
- Singh, J.S.; Pandey, V.C.; Singh, D.P.; Singh, R.P. Influence of Pyrite and Farmyard Manure on Population Dynamics of Soil Methanotroph and Rice Yield in Saline Rain-Fed Paddy Field. Agric. Ecosyst. Environ. 2010, 139, 74–79. [Google Scholar] [CrossRef]
- Fan, L.; Schneider, D.; Dippold, M.A.; Poehlein, A.; Wu, W.; Gui, H.; Ge, T.; Wu, J.; Thiel, V.; Kuzyakov, Y.; et al. Active Metabolic Pathways of Anaerobic Methane Oxidation in Paddy Soils. Soil Biol. Biochem. 2021, 156, 108215. [Google Scholar] [CrossRef]
- Thauer, R.K. Functionalization of Methane in Anaerobic Microorganisms. Angew. Chem. Int. Ed. 2010, 49, 6712–6713. [Google Scholar] [CrossRef]
- Rani, V.; Bhatia, A.; Kaushik, R. Inoculation of Plant Growth Promoting-Methane Utilizing Bacteria in Different N-Fertilizer Regime Influences Methane Emission and Crop Growth of Flooded Paddy. Sci. Total Environ. 2021, 775, 145826. [Google Scholar] [CrossRef]
- Auman, A.J.; Speake, C.C.; Lidstrom, M.E. nifH Sequences and Nitrogen Fixation in Type I and Type II Methanotrophs. Appl. Environ. Microbiol. 2001, 67, 4009–4016. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, M.; Chen, L.; Zhang, S.; Luo, Y.; Cao, W.; Zhao, J.; Wang, L.; Jia, Z.; Bao, Z. Methanotrophs Contribute to Nitrogen Fixation in Emergent Macrophytes. Front. Microbiol. 2022, 13, 851424. [Google Scholar] [CrossRef]
- Statistical Yearbook of Vietnam. General Statistics Office, Statistical Publishing House. 2022. Available online: https://www.gso.gov.vn/wp-content/uploads/2023/06/Sach-Nien-giam-TK-2022-update-21.7_file-nen-Water.pdf (accessed on 25 February 2024). (In Vietnamese)
- Uno, K.; Ishido, K.; Nguyen Xuan, L.; Nguyen Huu, C.; Minamikawa, K. Multiple Drainage Can Deliver Higher Rice Yield and Lower Methane Emission in Paddy Fields in An Giang Province, Vietnam. Paddy Water Environ. 2021, 19, 623–634. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis; CRC Press: Boca Raton, FL, USA, 2008; p. 1240. [Google Scholar] [CrossRef]
- Walkley, A.J.; Black, I.A. Estimation of soil organic carbon by the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association, American Water Works Association and Water Environmental Federation: Washington, DC, USA, 1998. [Google Scholar]
- Houba, J.; van der Lee, J.; Novozamsky, I.; Walinga, I. Soil analysis procedures. In Soil and Plants Analysis; Novozamski, I., van der Lee, J.J., Houba, V.J.G., Walinga, I., van Vark, W., Temminghoff, E., Eds.; Wageningen University: Wageningen, The Netherlands, 1988; pp. 25–27. [Google Scholar]
- Bray, R.A.; Kurtz, L.T. Determination of Total Organic and Available Forms of Phosphorus in Soils. Soil Sci. 1945, 59, 39–45. [Google Scholar] [CrossRef]
- Minamikawa, K.; Tokida, T.; Sudo, S.; Padre, A.; Yagi, K. Guidelines for Measuring CH4 and N2O Emissions from Rice Paddies by a Manually Operated Closed Chamber Method; National Institute for Agro-Environmental Sciences: Tsukuba, Japan, 2015. [Google Scholar]
- Sakoda, M.; Tokida, T.; Sakai, Y.; Senoo, K.; Nishizawa, T. Mitigation of Paddy Field Soil Methane Emissions by Betaproteobacterium Azoarcus Inoculation of Rice Seeds. Microb. Environ. 2022, 37, ME22052. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, J.; Wu, H.; Yan, S.; Guo, D.; Wang, G.; Ma, Y. Soil Chemical and Microbial Responses to Biogas Slurry Amendment and Its Effect on Fusarium Wilt Suppression. Appl. Soil Ecol. 2016, 107, 116–123. [Google Scholar] [CrossRef]
- Chen, R.; Blagodatskaya, E.; Senbayram, M.; Blagodatsky, S.; Myachina, O.; Dittert, K.; Kuzyakov, Y. Decomposition of Biogas Residues in Soil and Their Effects on Microbial Growth Kinetics and Enzyme Activities. Biomass Bioenergy 2012, 45, 221–229. [Google Scholar] [CrossRef]
- Insam, H.; Gómez-Brandón, M.; Ascher, J. Manure-Based Biogas Fermentation Residues—Friend or Foe of Soil Fertility? Soil Biol. Biochem. 2015, 84, 1–14. [Google Scholar] [CrossRef]
- Yan, L.; Liu, C.; Zhang, Y.; Liu, S.; Zhang, Y. Effects of C/N Ratio Variation in Swine Biogas Slurry on Soil Dissolved Organic Matter: Content and Fluorescence Characteristics. Ecotoxicol. Environ. Saf. 2021, 209, 111804. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Zhang, J.; Zhong, C.; Zhu, L.; Cao, X.; Yu, S.; Allen Bohr, J.; Hu, J.; Jin, Q. Effects of Salt Stress on Rice Growth, Development Characteristics, and the Regulating Ways: A Review. J. Integr. Agric. 2017, 16, 2357–2374. [Google Scholar] [CrossRef]
- Wentzel, S.; Schmidt, R.; Piepho, H.-P.; Semmler-Busch, U.; Joergensen, R.G. Response of Soil Fertility Indices to Long-Term Application of Biogas and Raw Slurry under Organic Farming. Appl. Soil Ecol. 2015, 96, 99–107. [Google Scholar] [CrossRef]
- Chen, P.; Xu, J.; Zhang, Z.; Nie, T. “Preferential” Ammonium Uptake by Rice Does Not Always Turn into Higher N Recovery of Fertilizer Sources under Water-Saving Irrigation. Agric. Water Manag. 2022, 272, 107867. [Google Scholar] [CrossRef]
- Zhu, C.Q.; Zhang, J.H.; Zhu, L.F.; Abliz, B.; Zhong, C.; Bai, Z.G.; Hu, W.J.; Sajid, H.; James, A.B.; Cao, X.C.; et al. NH4+ Facilitates Iron Reutilization in the Cell Walls of Rice (Oryza sativa) Roots under Iron-Deficiency Conditions. Environ. Exp. Bot. 2018, 151, 21–31. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural Benefits and Environmental Risks of Soil Fertilization with Anaerobic Digestates: A Review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef]
- Abubaker, J.; Risberg, K.; Pell, M. Biogas Residues as Fertilisers—Effects on Wheat Growth and Soil Microbial Activities. Appl. Energy 2012, 99, 126–134. [Google Scholar] [CrossRef]
- Lu, Y.; Wassmann, R.; Neue, H.-U.; Huang, C. Dynamics of Dissolved Organic Carbon and Methane Emissions in a Flooded Rice Soil. Soil Sci. Soc. Amer J. 2000, 64, 2011–2017. [Google Scholar] [CrossRef]
- Huynh, K.C.; Minamikawa, K.; Nguyen, N.V.C.; Nguyen, C.H.; Nguyen, C.V. Effects of Cattle Biogas Effluent Application and Irrigation Regimes on Rice Growth and Yield: A Mesocosm Experiment. Jpn. Agric. Res. Q. JARQ 2022, 56, 341–348. [Google Scholar] [CrossRef]
- Tran Sy, N.; Huynh Van, T.; Nguyen Huu, C.; Nguyen Van, C.; Mitsunori, T. Rice Husk and Melaleuca Biochar Additions Reduce Soil CH4 and N2O Emissions and Increase Soil Organic Matter and Nutrient Availability. F1000Res 2021, 10, 1128. [Google Scholar] [CrossRef] [PubMed]
- Arai, H. Increased Rice Yield and Reduced Greenhouse Gas Emissions through Alternate Wetting and Drying in a Triple-Cropped Rice Field in the Mekong Delta. Sci. Total Environ. 2022, 842, 156958. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.B.T.; Wassmann, R.; Mai, V.T.; Vu, D.Q.; Bui, T.P.L.; Vu, T.H.; Dinh, Q.H.; Yen, B.T.; Asch, F.; Sander, B.O. Methane Emission Factors from Vietnamese Rice Production: Pooling Data of 36 Field Sites for Meta-Analysis. Climate 2020, 8, 74. [Google Scholar] [CrossRef]
- Win, K.T.; Nonaka, R.; Toyota, K.; Motobayashi, T.; Hosomi, M. Effects of Option Mitigating Ammonia Volatilization on CH4 and N2O Emissions from a Paddy Field Fertilized with Anaerobically Digested Cattle Slurry. Biol. Fertil. Soils 2010, 46, 589–595. [Google Scholar] [CrossRef]
- Kumar, A.; Verma, L.M.; Sharma, S.; Singh, N. Overview on Agricultural Potentials of Biogas Slurry (BGS): Applications, Challenges, and Solutions. Biomass Conv. Bioref. 2022, 13, 13729–13769. [Google Scholar] [CrossRef] [PubMed]
- Linquist, B.A.; Adviento-Borbe, M.A.; Pittelkow, C.M.; Van Kessel, C.; Van Groenigen, K.J. Fertilizer Management Practices and Greenhouse Gas Emissions from Rice Systems: A Quantitative Review and Analysis. Field Crops Res. 2012, 135, 10–21. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, R.; Zheng, X.; Mei, B.; Zhou, Z.; Xie, B.; Dong, H.; Liu, C.; Han, S.; Xu, Z.; et al. Elevated Atmospheric CO2 Reduces Yield-scaled N2O Fluxes from Subtropical Rice Systems: Six Site-years Field Experiments. Glob. Chang. Biol. 2021, 27, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Sun, Y.; Zhu, X.; Wang, H.; Wang, Y.; Chen, J.; Zhang, J.; Zeng, Y.; Zeng, Y.; Huang, S. Identifying Agronomic Practices with Higher Yield and Lower Global Warming Potential in Rice Paddies: A Global Meta-Analysis. Agric. Ecosyst. Environ. 2021, 322, 107663. [Google Scholar] [CrossRef]
- Win, A.T.; Toyota, K.; Ito, D.; Chikamatsu, S.; Motobayashi, T.; Takahashi, N.; Ookawa, T.; Hirasawa, T. Effect of Two Whole-Crop Rice (Oryza sativa L.) Cultivars on Methane Emission and Cu and Zn Uptake in a Paddy Field Fertilized with Biogas Slurry. Soil Sci. Plant Nutr. 2016, 62, 99–105. [Google Scholar] [CrossRef]
- Debnath, G.; Jain, M.C.; Kumar, S.; Sarkar, K.; Sinha, S.K. Methane Emissions from Rice Fields Amended with Biogas Slurry and Farm Yard Manure. Clim. Chang. 1996, 33, 97–109. [Google Scholar] [CrossRef]
- Wei, L.; Ge, T.; Zhu, Z.; Luo, Y.; Yang, Y.; Xiao, M.; Yan, Z.; Li, Y.; Wu, J.; Kuzyakov, Y. Comparing Carbon and Nitrogen Stocks in Paddy and Upland Soils: Accumulation, Stabilization Mechanisms, and Environmental Drivers. Geoderma 2021, 398, 115121. [Google Scholar] [CrossRef]
- Honda, S.; Ohkubo, S.; San, N.S.; Nakkasame, A.; Tomisawa, K.; Katsura, K.; Ookawa, T.; Nagano, A.J.; Adachi, S. Maintaining Higher Leaf Photosynthesis after Heading Stage Could Promote Biomass Accumulation in Rice. Sci. Rep. 2021, 11, 7579. [Google Scholar] [CrossRef]
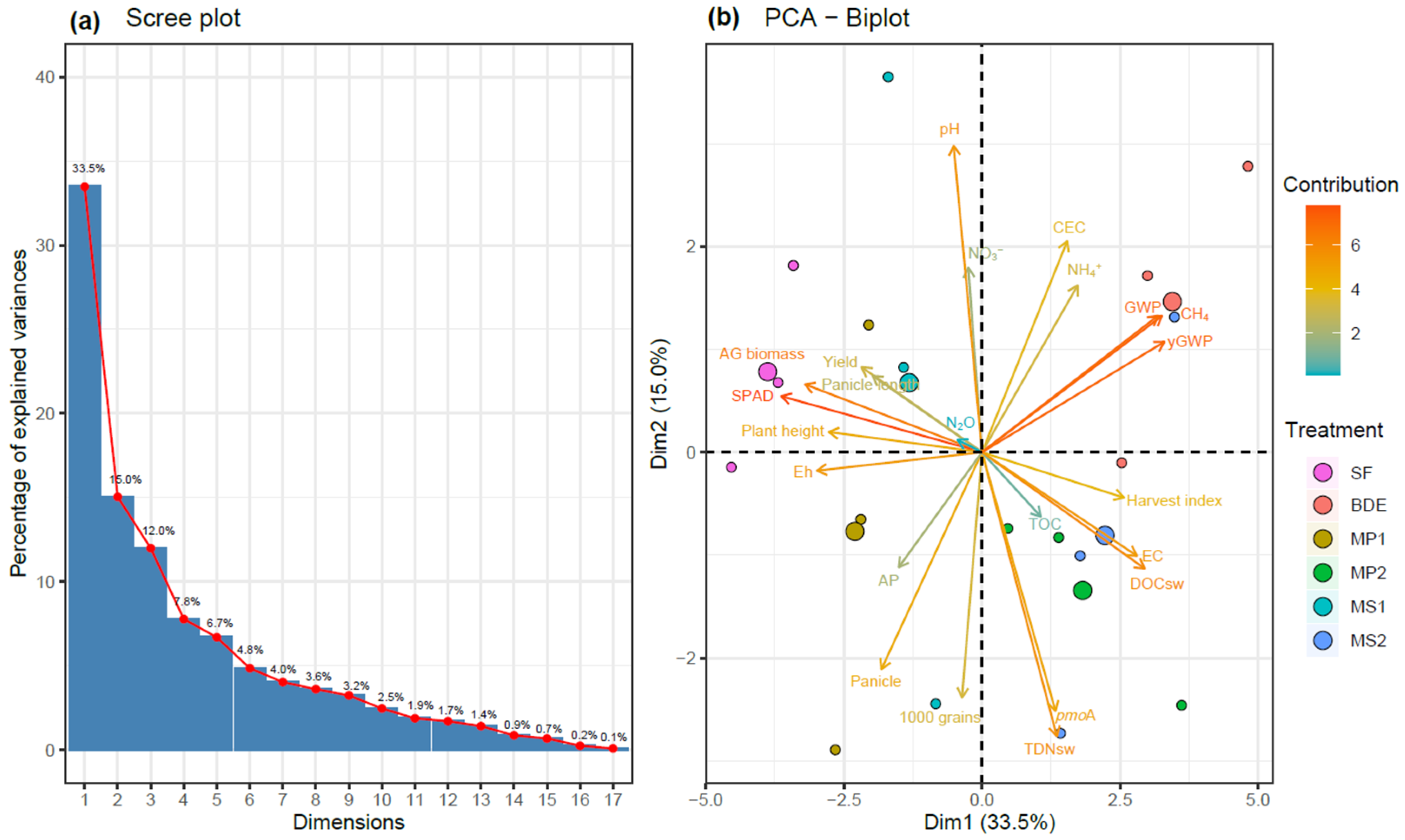
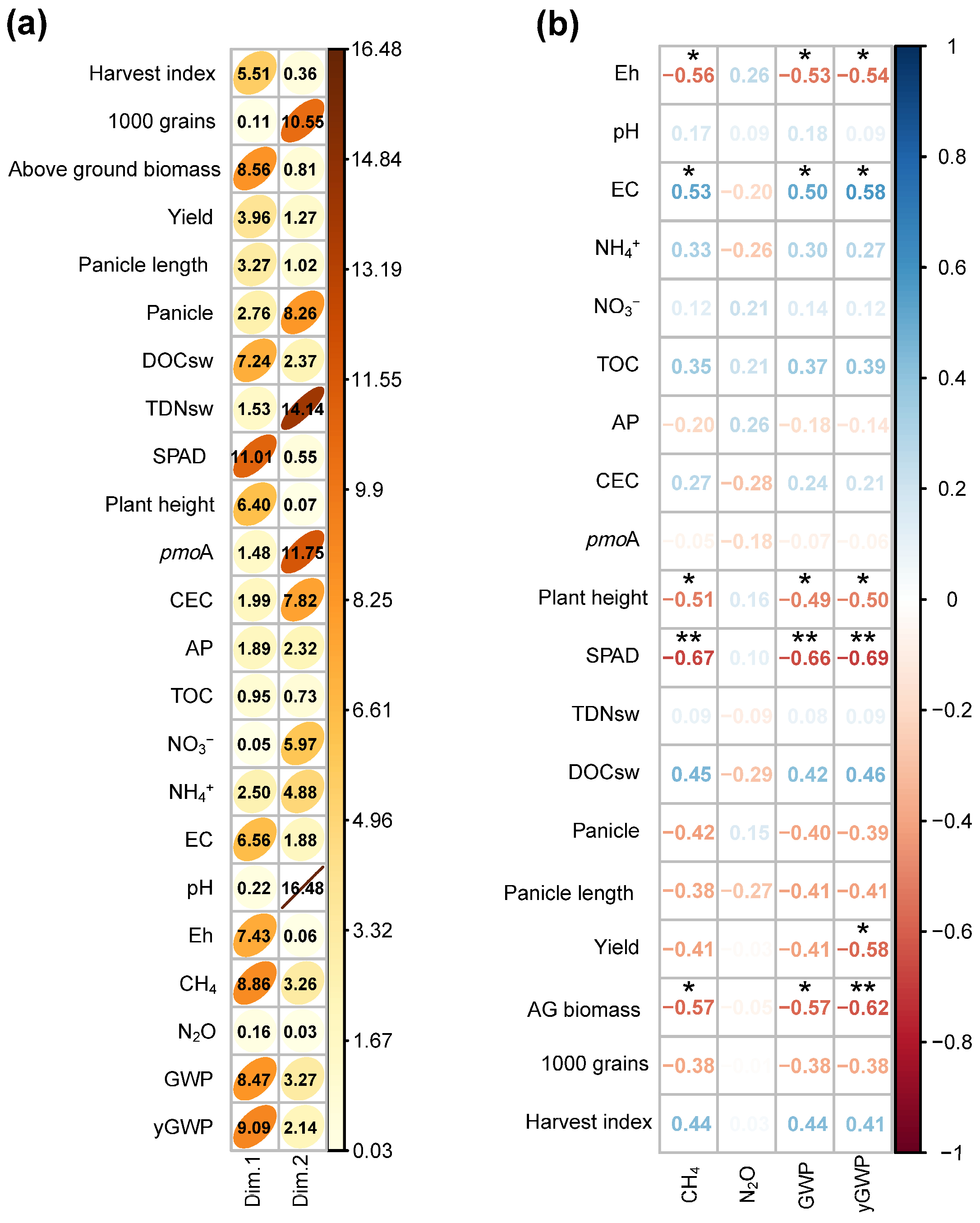
| Parameters | Unit | BDE Applied | ||
|---|---|---|---|---|
| First | Second | Third | ||
| pH | – | 6.54 ± 0.13 | 6.79 ± 0.16 | 6.82 ± 0.23 |
| EC | mS cm−1 | 23.0 ± 0.08 | 22.3 ± 0.19 | 21.8 ± 0.08 |
| TAN | mg N L−1 | 172 ± 25.3 | 165 ± 24.1 | 152 ± 18.3 |
| NO3− | mg N L−1 | 12.2 ± 2.21 | 13.5 ± 1.32 | 12.7 ± 1.17 |
| NO2− | mg N L−1 | 7.60 ± 1.23 | 8.86 ± 1.76 | 7.93 ± 2.14 |
| PO43− | mg P L−1 | 84.3 ± 6.56 | 78.2 ± 5.32 | 72.5 ± 6.38 |
| TOC | mg C L−1 | 333 ± 32.1 | 326 ± 36.3 | 316 ± 28.7 |
| TN | mg N L−1 | 334 ± 16.2 | 325 ± 22.4 | 320 ± 17.9 |
| Treatments | TD1 (10 DAS) | TD2 (25 DAS) | TD3 (44 DAS) | |||
|---|---|---|---|---|---|---|
| N-P2O-K2O (g m−2) | BDE (L m−2) | N-P2O-K2O (g m−2) | BDE (L m−2) | N-P2O-K2O (g m−2) | BDE (L m−2) | |
| SF | 3-1.3-0 | – | 7.0-1.3-3.0 | – | 5.0-1.3-3.0 | – |
| BDE | – | 9.0 | – | 21.5 | – | 15.6 |
| MS1 | 1.5-0.65-0 | 4.5 | 3.5-0.65-1.5 | 10.8 | 2.5-0.65-1.5 | 7.8 |
| MP1 | 1.5-0.65-0 | 4.5 | 3.5-0.65-1.5 | 10.8 | 2.5-0.65-1.5 | 7.8 |
| MS2 | – | 9.0 | – | 21.5 | – | 15.6 |
| MP2 | – | 9.0 | – | 21.5 | – | 15.6 |
| Items | pH (2) | EC (2) (mS cm−1) | NH4+ (2) (mg N kg−1) | NO3− (2) (mg N kg−1) | TOC (mg C kg−1) | AP (2) (mg P kg−1) | CEC (meq 100g−1) |
|---|---|---|---|---|---|---|---|
| Treatments (T) | |||||||
| SF | 5.17 ± 0.13 | 0.44 ± 0.21 b | 18.57 ± 5.13 | 0.76 ± 0.17 | 41.98 ± 0.89 | 30.14 ± 6.39 | 15.64 ± 0.63 |
| BDE | 5.18 ± 0.14 | 0.57 ± 0.23 a | 20.73 ± 8.57 | 0.73 ± 0.20 | 42.54 ± 1.36 | 27.74 ± 4.97 | 16.15 ± 0.87 |
| MS1 | 5.15 ± 0.11 | 0.45 ± 0.18 b | 20.47 ± 7.53 | 0.75 ± 0.21 | 41.79 ± 1.15 | 27.93 ± 7.89 | 16.11 ± 1.04 |
| MP1 | 5.13 ± 0.13 | 0.51 ± 0.21 ab | 20.90 ± 7.82 | 0.72 ± 0.12 | 41.81 ± 0.88 | 29.03 ± 6.70 | 15.83 ± 1.07 |
| MS2 | 5.14 ± 0.07 | 0.60 ± 0.23 ab | 21.60 ± 8.23 | 0.70 ± 0.29 | 42.33 ± 0.78 | 27.34 ± 7.13 | 16.09 ± 0.90 |
| MP2 | 5.09 ± 0.09 | 0.54 ± 0.23 ab | 21.56 ± 7.01 | 0.74 ± 0.20 | 42.34 ± 0.83 | 28.80 ± 8.14 | 16.09 ± 1.04 |
| Stages (S) | |||||||
| 0 DAS | 5.24 ± 0.13 a | 0.28 ± 0.05 d | 18.87 ± 3.42 b | 0.76 ± 0.15 ab | 41.55 ± 0.93 | 28.17 ± 6.19 ab | 15.85 ± 0.86 |
| 25 DAS | 5.08 ± 0.06 b | 0.35 ± 0.03 cd | 17.96 ± 3.21 b | 0.73 ± 0.08 ab | 42.21 ± 1.34 | 32.22 ± 7.19 a | 16.28 ± 1.02 |
| 39 DAS | 5.12 ± 0.07 b | 0.45 ± 0.11 c | 11.71 ± 1.92 c | 0.57 ± 0.21 b | 42.03 ± 0.81 | 27.31 ± 5.60 ab | 15.82 ± 0.77 |
| 60 DAS | 5.19 ± 0.12 ab | 0.58 ± 0.15 b | 32.29 ± 4.66 a | 0.75 ± 0.07 ab | 42.15 ± 1.02 | 29.28 ± 6.11 ab | 16.10 ± 0.99 |
| 67 DAS | 5.15 ± 0.10 ab | 0.68 ± 0.19 ab | 21.05 ± 4.43 b | 0.78 ± 0.32 a | 42.22 ± 0.90 | 30.63 ± 6.93 a | 16.03 ± 1.15 |
| 80 DAS | 5.08 ± 0.12 b | 0.77 ± 0.20 a | 21.94 ± 5.76 b | 0.81 ± 0.18 a | 42.63 ± 0.87 | 23.36 ± 6.24 b | 15.84 ± 0.78 |
| p-value (1) | |||||||
| T | 0.20 | *** | 0.29 | 0.96 | 0.19 | 0.79 | 0.58 |
| S | *** | *** | *** | * | 0.10 | ** | 0.69 |
| T × S | 0.99 | 0.54 | 0.77 | 0.93 | 0.99 | 0.28 | 0.88 |
| Items | DNsw (mg N L−1) | DOCsw (mg C L−1) (2) |
|---|---|---|
| Treatments (T) | ||
| SF | 1.01 ± 0.88 | 9.26 ± 3.25 b |
| BDE | 1.42 ± 0.48 | 22.15 ± 7.64 a |
| MS1 | 1.53 ± 0.91 | 14.25 ± 5.36 ab |
| MP1 | 1.59 ± 0.87 | 17.69 ± 13.29 ab |
| MS2 | 1.81 ± 0.78 | 21.23 ± 12.27 a |
| MP2 | 1.71 ± 0.69 | 21.80 ± 8.46 a |
| Stages (S) | ||
| 39 DAS | 1.46 ± 0.87 | 11.45 ± 4.36 b |
| 60 DAS | 1.34 ± 0.52 | 18.88 ± 8.99 a |
| 67 DAS | 1.72 ± 0.91 | 22.86 ± 11.52 a |
| p-value (1) | ||
| T | 0.32 | ** |
| S | 0.32 | *** |
| T × S | 0.27 | 0.16 |
| Items | CH4 (3) (g CH4 m−2) | N2O (3) (mg N2O m−2) | GWP (3) (g CO2-eq m−2) | yGWP (3) (g CO2-eq g−1) |
|---|---|---|---|---|
| Treatments (T) | ||||
| SF | 22.8 ± 1.4 b | 113 ± 17.7 a | 808 ± 55.0 b | 1.09 ± 0.10 b |
| BDE | 30.6 ± 1.8 a | 51.2 ± 40.8 ab | 1055 ± 73.4 a | 1.48 ± 0.12 a |
| MS1 | 24.0 ± 0.73 b | 50.7 ± 6.40 ab | 831 ± 26.2 b | 1.11 ± 0.07 b |
| MP1 | 21.3 ± 1.39 b | 10.6 ± 18.7 b | 727 ± 41.7 b | 0.99 ± 0.04 b |
| MS2 | 26.2 ± 3.72 ab | 60.5 ± 44.8 ab | 908 ± 119 ab | 1.27 ± 0.19 ab |
| MP2 | 25.2 ± 2.0 ab | 83.6 ± 23.1 ab | 883 ± 70.7 ab | 1.25 ± 0.09 ab |
| p-value (1) | ** | * | ** | ** |
| Methanotrophs (M) | ||||
| MS | 25.09 ± 2.68 | 55.7 ± 29.2 | 870 ± 88.0 | 1.19 ± 15.2 |
| MP | 23.26 ± 2.65 | 47.1 ± 44.2 | 805 ± 100 | 1.12 ± 0.16 |
| Methanotroph-inoculated BDE rates (MR) | ||||
| 50% BDE | 22.64 ± 1.79 b | 30.7 ± 25.4 b | 779 ± 65.2 b | 1.05 ± 0.08 b |
| 100% BDE | 25.71 ± 2.72 a | 72.1 ± 34.3 a | 896 ± 88.6 a | 1.26 ± 0.13 a |
| p-value (2) | ||||
| M | 0.20 | 0.60 | 0.16 | 0.33 |
| MR | * | * | * | * |
| M × MR | 0.51 | 0.08 | 0.38 | 0.43 |
| Treatments | Panicle (panicle m−2) | Panicle Length (cm) | Grain Yield (g m−2) | AG Biomass (g m−2) | Weight of 1000 Grains (g m−2) | Harvest Index |
|---|---|---|---|---|---|---|
| SF | 573 ± 6.93 | 20.5 ± 0.30 | 740 ± 21.1 | 1718 ± 49.2 ab | 24.0 ± 0.26 | 0.43 ± 0.02 |
| BDE | 555 ± 13.4 | 19.4 ± 0.61 | 713 ± 24.8 | 1534 ± 114 b | 23.8 ± 0.33 | 0.47 ± 0.02 |
| MS1 | 565 ± 25.0 | 20.6 ± 1.04 | 749 ± 24.5 | 1657 ± 117 ab | 24.1 ± 0.30 | 0.47 ± 0.04 |
| MP1 | 570 ± 23.3 | 20.4 ± 1.00 | 733 ± 13.0 | 1750 ± 37.9 a | 24.2 ± 0.21 | 0.42 ± 0.01 |
| MS2 | 566 ± 18.9 | 20.0 ± 0.75 | 718 ± 26.8 | 1530 ± 45.2 b | 24.2 ± 0.01 | 0.47 ± 0.01 |
| MP2 | 560 ± 22.8 | 20.0 ± 0.61 | 704 ± 15.4 | 1525 ± 39.1 b | 24.3 ± 0.11 | 0.46 ± 0.01 |
| p-value | 0.87 | 0.528 | 0.171 | ** | 0.197 | † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thao, H.V.; Tarao, M.; Takada, H.; Nishizawa, T.; Nam, T.S.; Cong, N.V.; Xuan, D.T. Methanotrophic Inoculation Reduces Methane Emissions from Rice Cultivation Supplied with Pig-Livestock Biogas Digestive Effluent. Agronomy 2024, 14, 1140. https://doi.org/10.3390/agronomy14061140
Thao HV, Tarao M, Takada H, Nishizawa T, Nam TS, Cong NV, Xuan DT. Methanotrophic Inoculation Reduces Methane Emissions from Rice Cultivation Supplied with Pig-Livestock Biogas Digestive Effluent. Agronomy. 2024; 14(6):1140. https://doi.org/10.3390/agronomy14061140
Chicago/Turabian StyleThao, Huynh Van, Mitsunori Tarao, Hideshige Takada, Tomoyasu Nishizawa, Tran Sy Nam, Nguyen Van Cong, and Do Thi Xuan. 2024. "Methanotrophic Inoculation Reduces Methane Emissions from Rice Cultivation Supplied with Pig-Livestock Biogas Digestive Effluent" Agronomy 14, no. 6: 1140. https://doi.org/10.3390/agronomy14061140
APA StyleThao, H. V., Tarao, M., Takada, H., Nishizawa, T., Nam, T. S., Cong, N. V., & Xuan, D. T. (2024). Methanotrophic Inoculation Reduces Methane Emissions from Rice Cultivation Supplied with Pig-Livestock Biogas Digestive Effluent. Agronomy, 14(6), 1140. https://doi.org/10.3390/agronomy14061140






