Nano-Food Farming: Toward Sustainable Applications of Proteins, Mushrooms, Nano-Nutrients, and Nanofibers
Abstract
1. Introduction
2. Toward Nano-Food Production
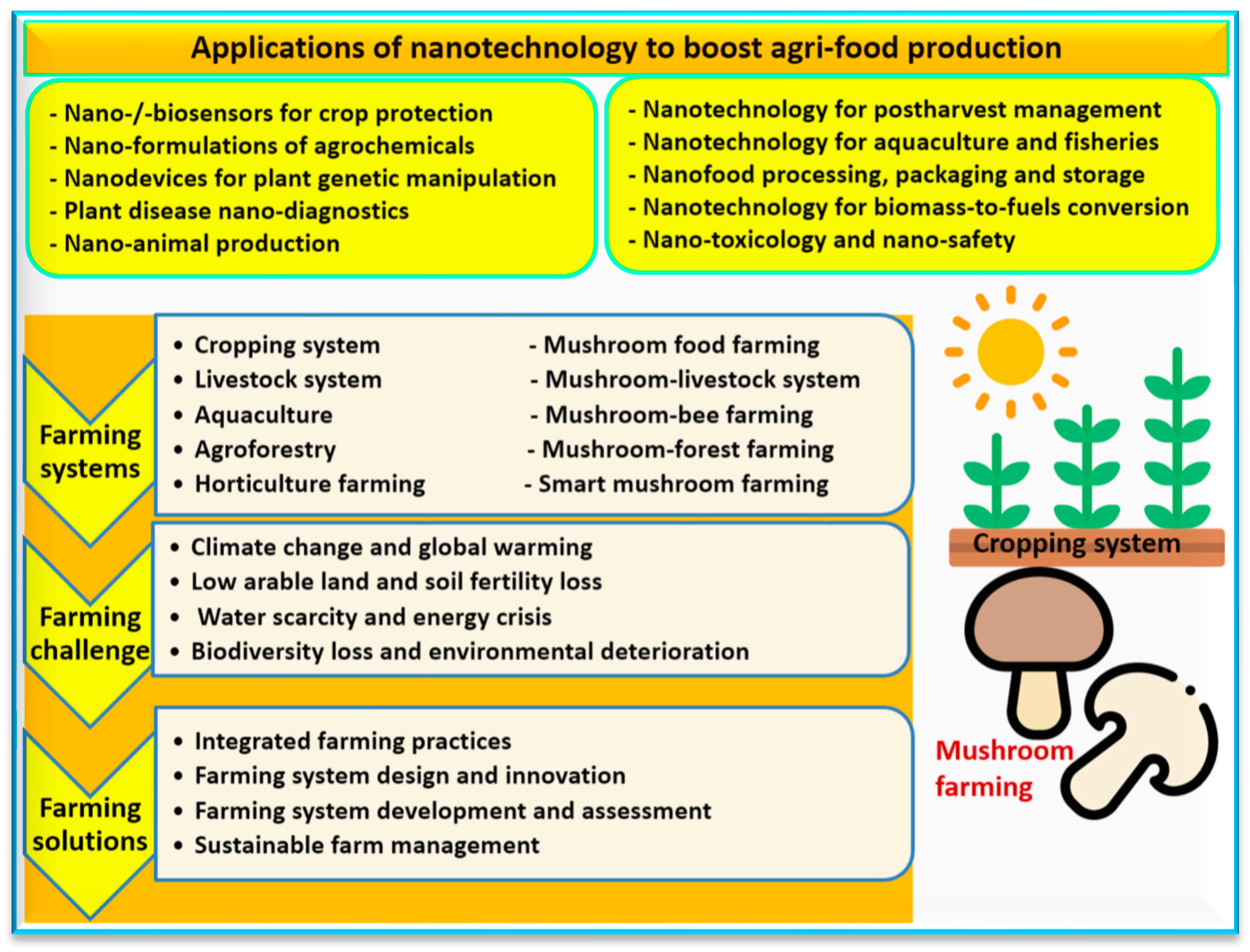
3. Global Food Crisis and Sustainable Agriculture
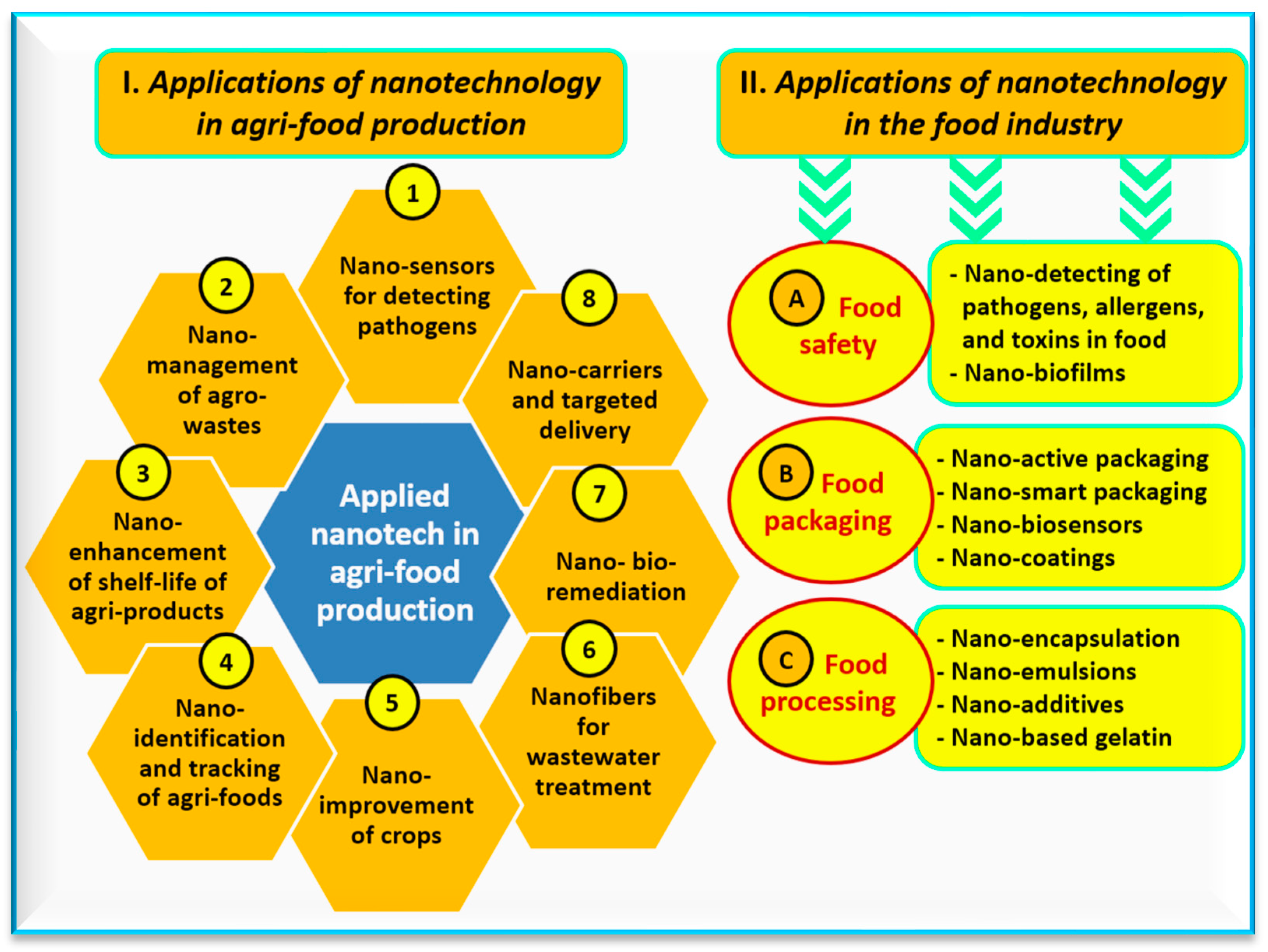
4. Nano-Food Farming: An Overview
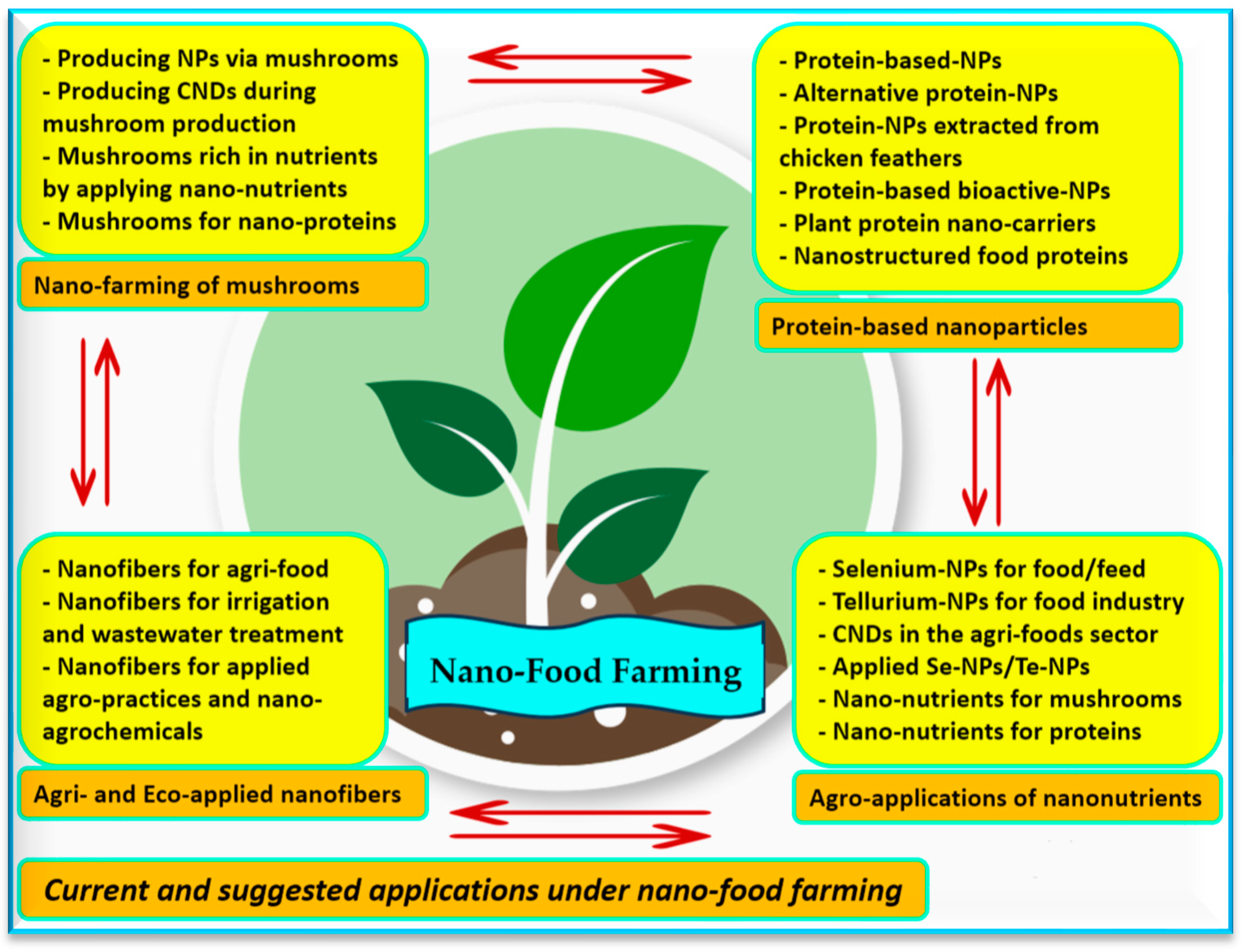
5. Protein-Based Nanoparticles
5.1. Protein-Based Nitrogen Nanoparticles
5.2. Alternative Protein Nanoparticles
6. Nano-Farming of Mushrooms
6.1. Mushroom-Based Nanoparticles
6.2. Nano-Applications in Food Packaging
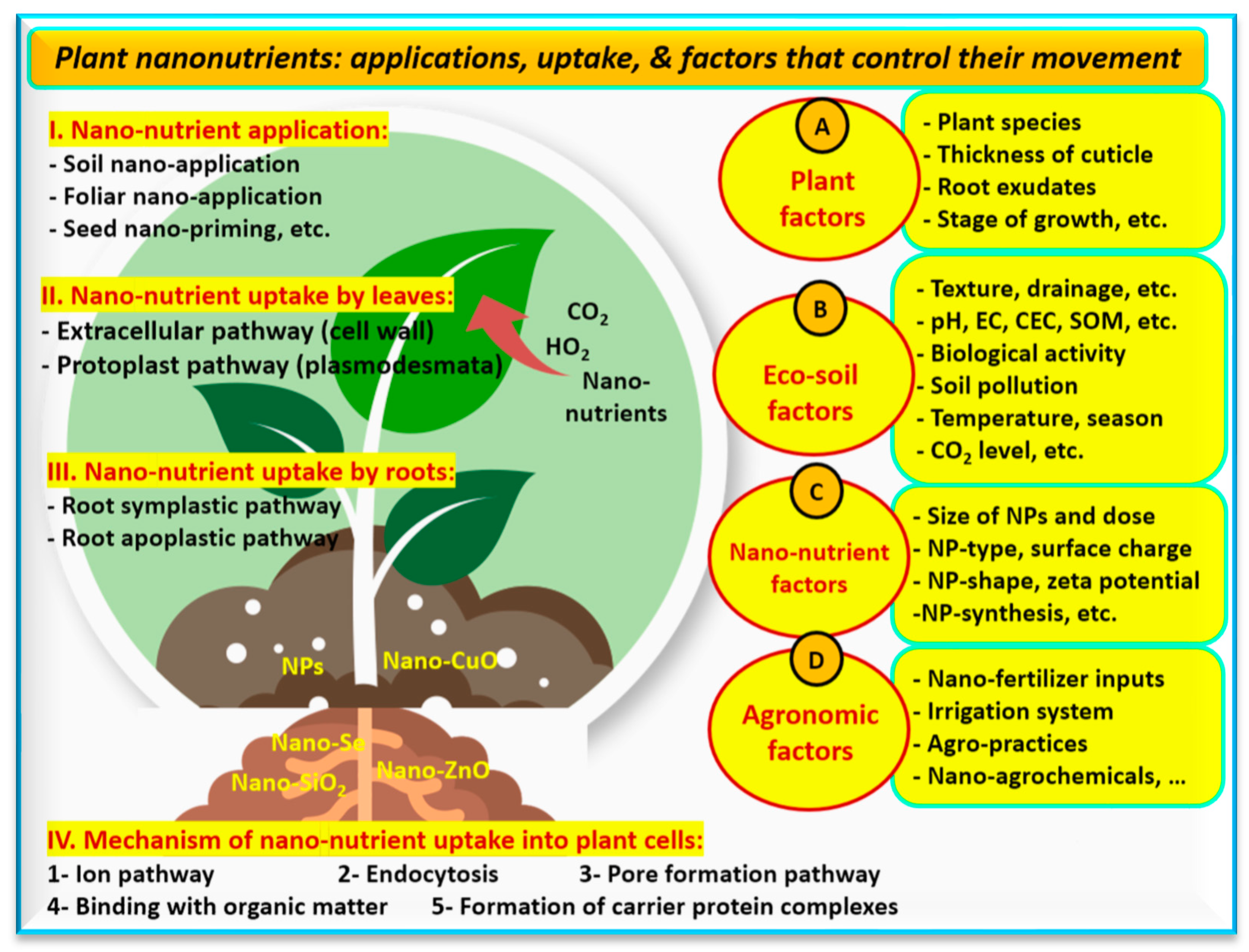
7. Nano-Food Farming: Role of Nano-Nutrients
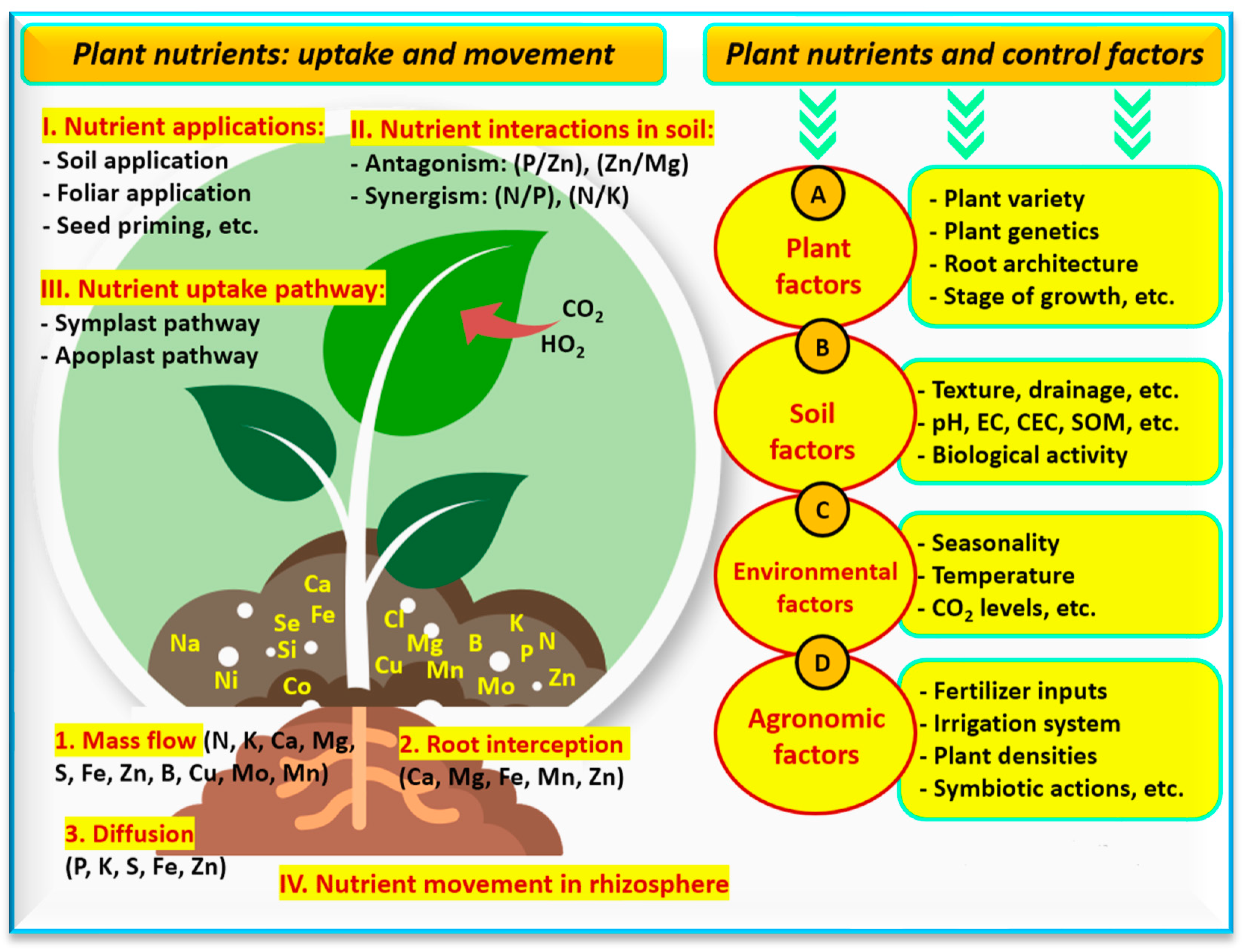
7.1. Nano-Selenium and Nano-Tellurium

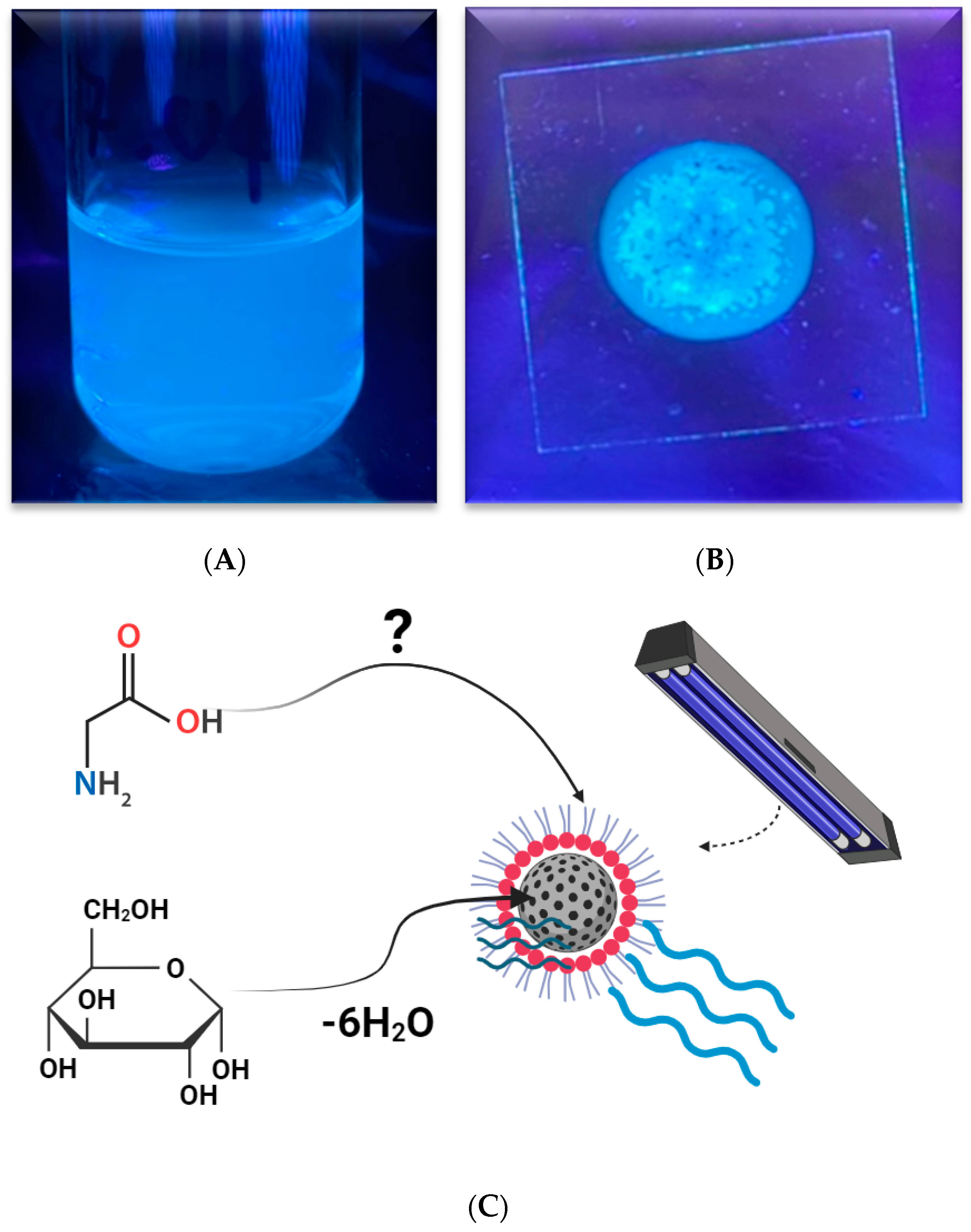
7.2. Carbon Nanodots
8. Nano-Farming: Applied Nanofibers
9. Nano-Food Farming Challenges
- -
- What are the main risks and benefits of nano-enabled agriculture?
- -
- What are the full life-cycle studies of the nanomaterials utilized in agriculture?
- -
- How do we effectively move our research from the laboratory to the field level?
- -
- To what extent does the field scale remain a critical knowledge gap?
- -
- To what extent is nano-enabled agriculture an emerging global issue for crop production?
- -
- What are the knowledge gaps that should be addressed concerning nano-safety?
- -
- To what extent can nanotechnology create safe and efficient delivery systems for food?
- -
- What are the required regulations, biosecurity measures, and public concern issues related to manufacturing, packing, and consuming nano-based food?
10. Conclusions and Further Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ebenso, B.; Otu, A.; Giusti, A.; Cousin, P.; Adetimirin, V.; Razafindralambo, H.; Effa, E.; Gkisakis, V.; Thiare, O.; Levavasseur, V.; et al. Nature-Based One Health Approaches to Urban Agriculture Can Deliver Food and Nutrition Security. Front. Nutr. 2022, 9, 773746. [Google Scholar] [CrossRef]
- van Dijk, M.; Morley, T.; Rau, M.L.; Saghai, Y. A Meta-Analysis of Projected Global Food Demand and Population at Risk of Hunger for the Period 2010–2050. Nat. Food 2021, 2, 494–501. Available online: https://www.nature.com/articles/s43016-021-00322-9 (accessed on 20 January 2024). [CrossRef]
- Spormann, S.; Nadais, P.; Sousa, F.; Pinto, M.; Martins, M.; Sousa, B.; Fidalgo, F.; Soares, C. Accumulation of Proline in Plants under Contaminated Soils—Are We on the Same Page? Antioxidants 2023, 12, 666. [Google Scholar] [CrossRef]
- Brevik, E.C. Agricultural Land Degradation in the United States of America. In Impact of Agriculture on Soil Degradation I; Pereira, P., Muñoz-Rojas, M., Bogunovic, I., Zhao, W., Eds.; The Handbook of Environmental Chemistry; Springer International Publishing: Cham, Switzerland, 2022; Volume 120, pp. 363–391. ISBN 978-3-031-32167-2. [Google Scholar]
- Avcı, B.C.; Kesgin, E.; Atam, M.; Tan, R.I.; Abdelkader, M. Short-Term Climate Change Influence on Surface Water Quality Impacts from Agricultural Activities. Environ. Sci. Pollut. Res. 2023, 30, 89581–89596. [Google Scholar] [CrossRef] [PubMed]
- Daszkiewicz, T. Food Production in the Context of Global Developmental Challenges. Agriculture 2022, 12, 832. [Google Scholar] [CrossRef]
- Haase, P.; Bowler, D.E.; Baker, N.J.; Bonada, N.; Domisch, S.; Garcia Marquez, J.R.; Heino, J.; Hering, D.; Jähnig, S.C.; Schmidt-Kloiber, A. The Recovery of European Freshwater Biodiversity Has Come to a Halt. Nature 2023, 620, 582–588. [Google Scholar] [CrossRef] [PubMed]
- Brevik, E.C. Soil, Food Security, and Human Health. In Soils, Plant Growth and Crop Production; Verheye, W.H., Ed.; EOLSS Publishers: Abu Dhabi, United Arab Emirates, 2010; Volume III, pp. 161–195. ISBN 978-1-84826-879-7. [Google Scholar]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in Sustainable Agriculture: Recent Developments, Challenges, and Perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed]
- Hafeez, A.; Ali, B.; Javed, M.A.; Saleem, A.; Fatima, M.; Fathi, A.; Afridi, M.S.; Aydin, V.; Oral, M.A.; Soudy, F.A. Plant Breeding for Harmony between Sustainable Agriculture, the Environment, and Global Food Security: An Era of Genomics-assisted Breeding. Planta 2023, 258, 97. [Google Scholar] [CrossRef] [PubMed]
- Aman Mohammadi, M.; Maximiano, M.R.; Hosseini, S.M.; Franco, O.L. CRISPR-Cas Engineering in Food Science and Sustainable Agriculture: Recent Advancements and Applications. Bioprocess Biosyst. Eng. 2023, 46, 483–497. [Google Scholar] [CrossRef] [PubMed]
- Goyal, V.; Rani, D.; Ritika; Mehrotra, S.; Deng, C.; Wang, Y. Unlocking the Potential of Nano-Enabled Precision Agriculture for Efficient and Sustainable Farming. Plants 2023, 12, 3744. [Google Scholar] [CrossRef] [PubMed]
- Karnwal, A.; Dohroo, A.; Malik, T. Unveiling the Potential of Bioinoculants and Nanoparticles in Sustainable Agriculture for Enhanced Plant Growth and Food Security. BioMed Res. Int. 2023, 2023, 6911851. [Google Scholar] [CrossRef]
- Haris, M.; Hussain, T.; Mohamed, H.I.; Khan, A.; Ansari, M.S.; Tauseef, A.; Khan, A.A.; Akhtar, N. Nanotechnology—A New Frontier of Nano-Farming in Agricultural and Food Production and Its Development. Sci. Total Environ. 2023, 857, 159639. [Google Scholar] [CrossRef] [PubMed]
- Sarker, P.K.; Paul, A.S.; Karmoker, D. Mitigating Climate Change and Pandemic Impacts on Global Food Security: Dual Sustainable Agriculture Approach (2S Approach). Planta 2023, 258, 104. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Tabla, R.; Anany, H.; Bastias, R.; Brøndsted, L.; Casado, S.; Cifuentes, P.; Deaton, J.; Denes, T.G.; Islam, M.A. ECOPHAGE: Combating Antimicrobial Resistance Using Bacteriophages for Eco-Sustainable Agriculture and Food Systems; MDPI: Basel, Switzerland, 2023; ISBN 1999-4915. [Google Scholar]
- Zain, M.; Ma, H.; Chaudhary, S.; Nuruzaman, M.; Azeem, I.; Mehmood, F.; Rahman, S.U.; Aiwang, D.; Sun, C. Nanotechnology in Precision Agriculture: Advancing towards Sustainable Crop Production. Plant Physiol. Biochem. 2023, 206, 108244. [Google Scholar] [CrossRef]
- Singh, A.; Rajput, V.D.; Pandey, D.; Sharma, R.; Ghazaryan, K.; Minkina, T. Nano Zinc-Enabled Strategies in Crops for Combatting Zinc Malnutrition in Human Health. Front. Biosci.-Landmark 2023, 28, 158. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, X.; Kang, Z.; Peralta-Videa, J.R.; Zhu, Y.-G. Nano-Enabled Seed Treatment: A New and Sustainable Approach to Engineering Climate-Resilient Crops. Sci. Total Environ. 2024, 910, 168640. [Google Scholar] [CrossRef] [PubMed]
- Daniel, A.I.; Keyster, M.; Klein, A. Biogenic Zinc Oxide Nanoparticles: A Viable Agricultural Tool to Control Plant Pathogenic Fungi and Its Potential Effects on Soil and Plants. Sci. Total Environ. 2023, 897, 165483. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Suman, K.; Karmakar, S.; Lakra, S.G.; Saurav, G.K.; Mahto, B.K. Regulation and Safety Measures for Nanotechnology-Based Agri-Products. Front. Genome Ed. 2023, 5, 1200987. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Rojas, C.; Pérez-de-Luque, A. Nanobiosensors and Nanoformulations in Agriculture: New Advances and Challenges for Sustainable Agriculture. Emerg. Top. Life Sci. 2023, 7, 229–238. [Google Scholar] [CrossRef]
- An, C.; Sun, C.; Li, N.; Huang, B.; Jiang, J.; Shen, Y.; Wang, C.; Zhao, X.; Cui, B.; Wang, C. Nanomaterials and Nanotechnology for the Delivery of Agrochemicals: Strategies towards Sustainable Agriculture. J. Nanobiotechnol. 2022, 20, 11. [Google Scholar] [CrossRef]
- Gangwar, J.; Kadanthottu Sebastian, J.; Puthukulangara Jaison, J.; Kurian, J.T. Nano-Technological Interventions in Crop Production—A Review. Physiol. Mol. Biol. Plants 2023, 29, 93–107. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Arora, K. Trends in Nano-Inspired Biosensors for Plants. Mater. Sci. Energy Technol. 2020, 3, 255–273. [Google Scholar] [CrossRef]
- Rahmani, M.K.I.; Ghanimi, H.M.A.; Jilani, S.F.; Aslam, M.; Alharbi, M.; Alroobaea, R.; Sengan, S. Early Pathogen Prediction in Crops Using Nano Biosensors and Neural Network-Based Feature Extraction and Classification. Big Data Res. 2023, 34, 100412. [Google Scholar] [CrossRef]
- Ijaz, M.; Khan, F.; Ahmed, T.; Noman, M.; Zulfiqar, F.; Rizwan, M.; Chen, J.; Siddique, K.H.; Li, B. Nanobiotechnology to Advance Stress Resilience in Plants: Current Opportunities and Challenges. Mater. Today Bio 2023, 22, 100759. [Google Scholar] [CrossRef] [PubMed]
- El-Bialy, S.M.; El-Mahrouk, M.E.; Elesawy, T.; Omara, A.E.-D.; Elbehiry, F.; El-Ramady, H.; Áron, B.; Prokisch, J.; Brevik, E.C.; Solberg, S.Ø. Biological Nanofertilizers to Enhance Growth Potential of Strawberry Seedlings by Boosting Photosynthetic Pigments, Plant Enzymatic Antioxidants, and Nutritional Status. Plants 2023, 12, 302. [Google Scholar] [CrossRef] [PubMed]
- Jha, A.; Pathania, D.; Damathia, B.; Raizada, P.; Rustagi, S.; Singh, P.; Rani, G.M.; Chaudhary, V. Panorama of Biogenic Nano-Fertilizers: A Road to Sustainable Agriculture. Environ. Res. 2023, 235, 116456. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, B.S. Nanotechnology in Agri-Food Production: An Overview. Nanotechnol. Sci. Appl. 2014, 7, 31–53. [Google Scholar] [CrossRef]
- Mohammad, Z.H.; Ahmad, F.; Ibrahim, S.A.; Zaidi, S. Application of Nanotechnology in Different Aspects of the Food Industry. Discov. Food 2022, 2, 12. [Google Scholar] [CrossRef]
- Mohammadi, S.; Jabbari, F.; Cidonio, G.; Babaeipour, V. Revolutionizing Agriculture: Harnessing Nano-Innovations for Sustainable Farming and Environmental Preservation. Pestic. Biochem. Physiol. 2024, 198, 105722. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Rajput, V.D.; Varshney, A.; Sharma, R.; Ghazaryan, K.; Minkina, T.; Alexiou, A.; El-Ramady, H. Revolutionizing Crop Production: Nanoscale Wonders—Current Applications, Advances, and Future Frontiers. Egypt. J. Soil Sci. 2024, 64, 221–258. [Google Scholar] [CrossRef]
- Sári, D.; Ferroudj, A.; Muthu, A.; Prokisch, J.; El-Ramady, H.; Elsakhawy, T.A.; Omara, A.E.-D.; Brevik, E. Nano-Enabled Agriculture Using Nano-Selenium for Crop Productivity: What Should Be Addressed More? Environ. Biodivers. Soil Secur. 2023, 7, 85–99. [Google Scholar] [CrossRef]
- Pretto, A.; Savio, G.; Gottardo, F.; Uccheddu, F.; Concheri, G. A Novel Low-Cost Visual Ear Tag Based Identification System for Precision Beef Cattle Livestock Farming. Inf. Process. Agric. 2022, 11, 117–126. [Google Scholar] [CrossRef]
- Zhao, K.; Shen, X.; Zhou, P.; Wu, J. Effects of Nano-Cu2O on the Productivity in the Cu-Stripped Chinese Merino Sheep. Biol. Trace Elem. Res. 2023, 201, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Llanaj, X.; Törős, G.; Hajdu, P.; El-Ramady, H.; Peles, F.; Prokisch, J. Mushroom Cultivation Systems: Exploring Antimicrobial and Prebiotic Benefits. Environ. Biodivers. Soil Secur. 2023, 7, 101–120. [Google Scholar] [CrossRef]
- Zou, G.; Nielsen, J.B.; Wei, Y. Harnessing Synthetic Biology for Mushroom Farming. Trends Biotechnol. 2023, 41, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, Z.; El-Ramady, H.; Omara, A.E.-D.; Elsakhawy, T.; Bayoumi, Y.; Shalaby, T.; Prokisch, J. From Farm-to-Fork: A Pictorial Mini Review on Nano-Farming of Vegetables. Environ. Biodivers. Soil Secur. 2022, 6, 149–163. [Google Scholar] [CrossRef]
- Divya, S.; Rusyn, I.; Solorza-Feria, O.; Sathish-Kumar, K. Sustainable SMART Fertilizers in Agriculture Systems: A Review on Fundamentals to in-Field Applications. Sci. Total Environ. 2023, 904, 166729. [Google Scholar]
- Hamid, L.; Alsayari, A.; Tak, H.; Mir, S.A.; Almoyad, M.A.A.; Wahab, S.; Bader, G.N. An Insight into the Global Problem of Gastrointestinal Helminth Infections amongst Livestock: Does Nanotechnology Provide an Alternative? Agriculture 2023, 13, 1359. [Google Scholar] [CrossRef]
- Ahmed, J.; Vasagam, K.K.; Ramalingam, K. Nanoencapsulated Aquafeeds and Current Uses in Fisheries/Shrimps: A Review. Appl. Biochem. Biotechnol. 2023, 195, 7110–7131. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.R.; Nazir, F.; Maheshwari, C.; Chopra, P.; Chhillar, H.; Sreenivasulu, N. Mineral Nutrients in Plants under Changing Environments: A Road to Future Food and Nutrition Security. Plant Genome 2023, 16, e20362. [Google Scholar] [CrossRef] [PubMed]
- Brevik, E.C.; Slaughter, L.; Singh, B.R.; Steffan, J.J.; Collier, D.; Barnhart, P.; Pereira, P. Soil and Human Health: Current Status and Future Needs. Air Soil Water Res. 2020, 13, 117862212093444. [Google Scholar] [CrossRef]
- Bhaskar, M.; Kumar, A.; Rani, R. Application of Nano Formulations in Agriculture. Biocatal. Agric. Biotechnol. 2023, 54, 102934. [Google Scholar] [CrossRef]
- Sharma, R.; Kumar, V. Nano Enabled Agriculture for Sustainable Soil. Waste Manag. Bull. 2024, 2, 152–161. [Google Scholar] [CrossRef]
- Prokisch, J.; Széles, É.; Kovács, B.; Daróczy, L.; Zommara, M. Formation of Metal Selenium Nanospheres in Bacteria: Is It a Possible Detoxification Mechanism? Cereal Res. Commun. 2008, 36, 947–950. [Google Scholar]
- Prokisch, J.; Zommara, M.A. Process for Producing Elemental Selenium Nanospheres. U.S. Patent No. 8,003,071, 23 August 2011. [Google Scholar]
- El-Ramady, H.; Faizy, S.E.-D.; Abdalla, N.; Taha, H.; Domokos-Szabolcsy, É.; Fari, M.; Elsakhawy, T.; Omara, A.E.-D.; Shalaby, T.; Bayoumi, Y. Selenium and Nano-Selenium Biofortification for Human Health: Opportunities and Challenges. Soil Syst. 2020, 4, 57. [Google Scholar] [CrossRef]
- El-Ramady, H.; Taha, N.; Shalaby, T.; Elsakhawy, T.A.; Omara, A.E.-D.; Prokisch, J.; Bayoumi, Y. Nano-Selenium and Its Interaction with Other Nano-Nutrients in Soil under Stressful Plants: A Mini-Review. Environ. Biodivers. Soil Secur. 2021, 5, 205–220. [Google Scholar] [CrossRef]
- Saffan, M.M.; Koriem, M.A.; El-Henawy, A.; El-Mahdy, S.; El-Ramady, H.; Elbehiry, F.; Omara, A.E.-D.; Bayoumi, Y.; Badgar, K.; Prokisch, J. Sustainable Production of Tomato Plants (Solanum Lycopersicum L.) under Low-Quality Irrigation Water as Affected by Bio-Nanofertilizers of Selenium and Copper. Sustainability 2022, 14, 3236. [Google Scholar] [CrossRef]
- El-Ramady, H.; Shedeed, S.; Abdalla, Z.F.; El-Bassiony, A.E.-M.; El-Sawy, S.; Mahmoud, S.; Prokisch, J. Biofortification of Vegetables under Stress Conditions Using Biological Nano-Selenium: A Mini-Review. Environ. Biodivers. Soil Secur. 2023, 7, 23–35. [Google Scholar] [CrossRef]
- Taha, N.A.; Hamden, S.; Bayoumi, Y.A.; Elsakhawy, T.; El-Ramady, H.; Solberg, S.Ø. Nanofungicides with Selenium and Silicon Can Boost the Growth and Yield of Common Bean (Phaseolus Vulgaris L.) and Control Alternaria Leaf Spot Disease. Microorganisms 2023, 11, 728. [Google Scholar] [CrossRef] [PubMed]
- Törős, G.; El-Ramady, H.; Prokisch, J.; Velasco, F.; Llanaj, X.; Nguyen, D.H.; Peles, F. Modulation of the Gut Microbiota with Prebiotics and Antimicrobial Agents from Pleurotus Ostreatus Mushroom. Foods 2023, 12, 2010. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; Abdalla, N.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Eid, Y.; Prokisch, J. Edible Mushrooms for Sustainable and Healthy Human Food: Nutritional and Medicinal Attributes. Sustainability 2022, 14, 4941. [Google Scholar] [CrossRef]
- Elsakhawy, T.; Omara, A.E.-D.; Abowaly, M.; El-Ramady, H.; Badgar, K.; Llanaj, X.; Törős, G.; Hajdú, P.; Prokisch, J. Green Synthesis of Nanoparticles by Mushrooms: A Crucial Dimension for Sustainable Soil Management. Sustainability 2022, 14, 4328. [Google Scholar] [CrossRef]
- Nguyen, D.H.; El-Ramady, H.; Llanaj, X.; Törős, G.; Hajdú, P.; Prokisch, J. Chemical Composition and Health Attributes of Agri-Foods: A Scientific Overview on Black Foods. Sustainability 2023, 15, 3852. [Google Scholar] [CrossRef]
- Badgar, K.; Abdalla, N.; El-Ramady, H.; Prokisch, J. Sustainable Applications of Nanofibers in Agriculture and Water Treatment: A Review. Sustainability 2022, 14, 464. [Google Scholar] [CrossRef]
- Prokisch, J.; Sári, D.; Muthu, A.; Nagy, A.; El-Ramady, H.; Abdalla, N.; Dobránszki, J. Biotechnology of Nanofiber in Water, Energy, and Food Sectors. Agronomy 2023, 13, 2734. [Google Scholar] [CrossRef]
- Muthu, A.; Sári, D.; Ferroudj, A.; El-Ramady, H.; Béni, Á.; Badgar, K.; Prokisch, J. Microbial-Based Biotechnology: Production and Evaluation of Selenium-Tellurium Nanoalloys. Appl. Sci. 2023, 13, 11733. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Dang, Y.P.; Li, L. Farming System: A Systemic Solution to Sustainable Agricultural Development. Farming Syst. 2023, 1, 100007. [Google Scholar] [CrossRef]
- Brevik, E.C.; Pereg, L.; Pereira, P.; Steffan, J.J.; Burgess, L.C.; Gedeon, C.I. Shelter, Clothing, and Fuel: Often Overlooked Links between Soils, Ecosystem Services, and Human Health. Sci. Total Environ. 2019, 651, 134–142. [Google Scholar] [CrossRef]
- FAO. The State of Food Security and Nutrition in the World 2022: Repurposing Food and Agricultural Policies to Make Healthy Diets More Affordable; Food & Agriculture Organization: Rome, Italy, 2022; Volume 2022, ISBN 92-5-136499-0. [Google Scholar]
- Dehghani, M.H.; Ahmadi, S.; Ghosh, S.; Khan, M.S.; Othmani, A.; Khanday, W.A.; Gökkuş, Ö.; Osagie, C.; Ahmaruzzaman, M.; Mishra, S.R. Sustainable Remediation Technologies for Removal of Pesticides as Organic Micro-Pollutants from Water Environments: A Review. Appl. Surf. Sci. Adv. 2024, 19, 100558. [Google Scholar] [CrossRef]
- FAO Statistical Yearbook—World Food and Agriculture; FAO: Rome, Italy, 2023. [CrossRef]
- Adalibieke, W.; Cui, X.; Cai, H.; You, L.; Zhou, F. Global Crop-Specific Nitrogen Fertilization Dataset in 1961–2020. Sci. Data 2023, 10, 617. [Google Scholar] [CrossRef]
- Ale, A.; Andrade, V.S.; Gutierrez, M.F.; Bacchetta, C.; Rossi, A.S.; Orihuela, P.S.; Desimone, M.F.; Cazenave, J. Nanotechnology-Based Pesticides: Environmental Fate and Ecotoxicity. Toxicol. Appl. Pharmacol. 2023, 471, 116560. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Mao, Y.; Zhao, F.; Idris, A.L.; Liu, Q.; Zou, S.; Guan, X.; Huang, T. Towards Sustainable Green Adjuvants for Microbial Pesticides: Recent Progress, Upcoming Challenges, and Future Perspectives. Microorganisms 2023, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Daraban, G.M.; Hlihor, R.-M.; Suteu, D. Pesticides vs. Biopesticides: From Pest Management to Toxicity and Impacts on the Environment and Human Health. Toxics 2023, 11, 983. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Mukhopadhyay, A.; Paul, S.; Sarkar, S.; Mukhopadhyay, R. Nanocomposite-Based Smart Fertilizers: A Boon to Agricultural and Environmental Sustainability. Sci. Total Environ. 2023, 863, 160859. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Tiwari, S.; Kumawat, K.C.; Cardinale, M. Nano-Biofertilizers as Bio-Emerging Strategies for Sustainable Agriculture Development: Potentiality and Their Limitations. Sci. Total Environ. 2023, 860, 160476. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ying, D.; Li, J.; Deng, J.; Li, C.; Tian, S.; Zhao, G.; Wu, C.; Jiao, J.; Jiang, M.; et al. Global-Scale No-Tillage Impacts on Soil Aggregates and Associated Carbon and Nitrogen Concentrations in Croplands: A Meta-Analysis. Sci. Total Environ. 2023, 881, 163570. [Google Scholar] [CrossRef] [PubMed]
- Ghani, M.I.; Ali, A.; Atif, M.J.; Ali, M.; Ahanger, M.A.; Chen, X.; Cheng, Z. Different Leafy Vegetable Cropping Systems Regulate Growth, Photosynthesis, and PSII Functioning in Mono-Cropped Eggplant by Altering Chemical Properties and Upregulating the Antioxidant System. Front. Plant Sci. 2023, 14, 1132861. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Liu, J.; Liu, Q.; Sun, J.; Zhao, Y.; Liu, J.; Gao, W.; Chen, Y.; Sui, P. Knowledge Domain and Research Progress in the Field of Crop Rotation from 2000 to 2020: A Scientometric Review. Environ. Sci. Pollut. Res. 2023, 30, 86598–86617. [Google Scholar] [CrossRef] [PubMed]
- Ghahramani, A.; Kingwell, R.S.; Maraseni, T.N. Land Use Change in Australian Mixed Crop-Livestock Systems as a Transformative Climate Change Adaptation. Agric. Syst. 2020, 180, 102791. [Google Scholar] [CrossRef]
- Bhati, P.; Saikia, A.R.; Chaudhary, S.; Bahadur, R.; Nengparmoi, T.; Talukdar, N. Sanjay Hazarika Integrated Farming Systems for Environment Sustainability: A Comprehensive Review. J. Sci. Res. Rep. 2024, 30, 143–155. [Google Scholar] [CrossRef]
- Sooriyaarachchi, P.; Jayawardena, R. Impact of the Economic Crisis on Food Consumption of Sri Lankans: An Online Cross-Sectional Survey. Diabetes Metab. Syndr. Clin. Res. Rev. 2023, 17, 102786. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.A. The Growing Crisis of Food and Water Insecurity, and Homelessness, Afflicting the United States; Elsevier Science Inc.: New York, NY, USA, 2023; Volume 19, pp. 167–169. ISBN 1550-8307. [Google Scholar]
- Nakat, Z.; Tayoun, V.; Merhi, S.; Bou-Mitri, C.; Karam, L. Food Safety Culture in Food Companies amid the Lebanese Economic Crisis and the COVID-19 Pandemic. Heliyon 2023, 9, e19885. [Google Scholar] [CrossRef] [PubMed]
- Roubík, H.; Lošťák, M.; Ketuama, C.T.; Soukupová, J.; Procházka, P.; Hruška, A.; Hakl, J.; Pacek, L.; Karlík, P.; Menšíková, L.K. COVID-19 Crisis Interlinkage with Past Pandemics and Their Effects on Food Security. Glob. Health 2023, 19, 52. [Google Scholar] [CrossRef] [PubMed]
- Gorzycka-Sikora, A.; Mock, N.; Lacey, M. Multivariate Analysis of Food Consumption Profiles in Crisis Settings. PLoS ONE 2023, 18, e0283627. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Park, J. Quantification of Food Loss and Waste and Its Percentage Estimation along the Food Supply Chain in Korea. Waste Manag. Res. 2023, 41, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Valenzuela-Amaro, H.M.; Aguayo-Acosta, A.; Meléndez-Sánchez, E.R.; de la Rosa, O.; Vázquez-Ortega, P.G.; Oyervides-Muñoz, M.A.; Sosa-Hernández, J.E.; Parra-Saldívar, R. Emerging Applications of Nanobiosensors in Pathogen Detection in Water and Food. Biosensors 2023, 13, 922. [Google Scholar] [CrossRef] [PubMed]
- El-Ramady, H.; El-Henawy, A.; Amer, M.; Omara, A.E.-D.; Elsakhawy, T.; Elbasiouny, H.; Elbehiry, F.; Abou Elyazid, D.; El-Mahrouk, M. Agricultural Waste and Its Nano-Management: Mini Review. Egypt. J. Soil Sci. 2020, 60, 349–364. [Google Scholar] [CrossRef]
- Kumar, R.S.; Sasikumar, R.; Dhilipkumar, T. Exploiting Agro-Waste for Cleaner Production: A Review Focusing on Biofuel Generation, Bio-Composite Production, and Environmental Considerations. J. Clean. Prod. 2024, 435, 140536. [Google Scholar] [CrossRef]
- Sharma, A.; Hazarika, M.; Heisnam, P.; Pandey, H.; Devadas, V.S.; Singh, D.; Wangsu, M.; Kartha, B.D. Influence of Storage Conditions, Packaging, Post-Harvest Technology, Nanotechnology and Molecular Approaches on Shelf Life of Microgreens. J. Agric. Food Res. 2023, 14, 100835. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K.; Ahmad, R.; Abd-Elsalam, K.A. Emerging Frontiers in Nanotechnology for Precision Agriculture: Advancements, Hurdles and Prospects. Agrochemicals 2023, 2, 220–256. [Google Scholar] [CrossRef]
- Ahmed, T.; Noman, M.; Gardea-Torresdey, J.L.; White, J.C.; Li, B. Dynamic Interplay between Nano-Enabled Agrochemicals and the Plant-Associated Microbiome. Trends Plant Sci. 2023. [Google Scholar] [CrossRef] [PubMed]
- Zamel, D.; Khan, A.U.; Waris, A.; Ebrahim, A.; Abd El-Sattar, N.E. Nanomaterials Advancements for Enhanced Contaminant Removal in Wastewater Treatment: Nanoparticles, Nanofibers, and Metal-Organic Frameworks (MOFs). Results Chem. 2023, 6, 101092. [Google Scholar] [CrossRef]
- Chauhan, P.; Imam, A.; Kanaujia, P.K.; Suman, S.K. Nano-Bioremediation: An Eco-Friendly and Effective Step towards Petroleum Hydrocarbon Removal from Environment. Environ. Res. 2023, 231, 116224. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, Q.; Zhong, C.; Zhang, Z. Multifunctional Cell Membranes-Based Nano-Carriers for Targeted Therapies: A Review of Recent Trends and Future Perspective. Drug Deliv. 2023, 30, 2288797. [Google Scholar] [CrossRef] [PubMed]
- Abedi-Firoozjah, R.; Ebdali, H.; Soltani, M.; Abdolahi-Fard, P.; Heydari, M.; Assadpour, E.; Azizi-Lalabadi, M.; Zhang, F.; Jafari, S.M. Nanomaterial-Based Sensors for the Detection of Pathogens and Microbial Toxins in the Food Industry; a Review on Recent Progress. Coord. Chem. Rev. 2024, 500, 215545. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Miguel-Rojas, C.; Montanha, G.S.; Carmona, F.J.; Dal Sasso, G.; Sillero, J.C.; Skov Pedersen, J.; Masciocchi, N.; Guagliardi, A.; Pérez-de-Luque, A. Reducing Nitrogen Dosage in Triticum Durum Plants with Urea-Doped Nanofertilizers. Nanomaterials 2020, 10, 1043. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G. A New Era of Sustainability: Plant Biostimulants. Int. J. Mol. Sci. 2023, 24, 16329. [Google Scholar] [CrossRef]
- Agrahari, S.; Dubey, A. Nanoparticles in Plant Growth and Development. In Biogenic Nano-Particles and Their Use in Agro-Ecosystems; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 9–37. [Google Scholar] [CrossRef]
- Abhiram, G. Contributions of Nano-Nitrogen Fertilizers to Sustainable Development Goals: A Comprehensive Review. Nitrogen 2023, 4, 397–415. [Google Scholar] [CrossRef]
- Abou El-Enin, M.M.; Sheha, A.M.; El-Serafy, R.S.; Ali, O.A.; Saudy, H.S.; Shaaban, A. Foliage-Sprayed Nano-Chitosan-Loaded Nitrogen Boosts Yield Potentials, Competitive Ability, and Profitability of Intercropped Maize-Soybean. Int. J. Plant Prod. 2023, 17, 517–542. [Google Scholar] [CrossRef]
- Mejias, J.H.; Salazar, F.; Pérez Amaro, L.; Hube, S.; Rodriguez, M.; Alfaro, M. Nanofertilizers: A Cutting-Edge Approach to Increase Nitrogen Use Efficiency in Grasslands. Front. Environ. Sci. 2021, 9, 52. [Google Scholar] [CrossRef]
- Yan, X.; Xia, L.; Ti, C. Temporal and Spatial Variations in Nitrogen Use Efficiency of Crop Production in China. Environ. Pollut. 2022, 293, 118496. [Google Scholar] [CrossRef]
- Rop, K.; Mbui, D.; Karuku, G.N.; Michira, I.; Njomo, N. Characterization of Water Hyacinth Cellulose-g-Poly (Ammonium Acrylate-Co-Acrylic Acid)/Nano-Hydroxyapatite Polymer Hydrogel Composite for Potential Agricultural Application. Results Chem. 2020, 2, 100020. [Google Scholar] [CrossRef]
- Wang, Y.; Shaghaleh, H.; Hamoud, Y.A.; Zhang, S.; Li, P.; Xu, X.; Liu, H. Synthesis of a pH-Responsive Nano-Cellulose/Sodium Alginate/MOFs Hydrogel and Its Application in the Regulation of Water and N-Fertilizer. Int. J. Biol. Macromol. 2021, 187, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.; Meena, N.D.; Meena, R.N.; Choudhary, M.; Meena, S.; Kumar, S. Role of Nanotechnology in Organic Agriculture. In Advances in Resting-State Functional MRI; Woodhead Publishing: Sawston, UK, 2023; pp. 343–364. [Google Scholar] [CrossRef]
- Saad, A.M.; Alabdali, A.Y.M.; Ebaid, M.; Salama, E.; El-Saadony, M.T.; Selim, S.; Safhi, F.A.; ALshamrani, S.M.; Abdalla, H.; Mahdi, A.H. Impact of Green Chitosan Nanoparticles Fabricated from Shrimp Processing Waste as a Source of Nano Nitrogen Fertilizers on the Yield Quantity and Quality of Wheat (Triticum Aestivum L.) Cultivars. Molecules 2022, 27, 5640. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.M.; Soliman, M.I.; Abo Al-Saoud, A.M.; El-Sherbeny, G.A. Waste-Derived NPK Nanofertilizer Enhances Growth and Productivity of Capsicum Annuum L. Plants 2021, 10, 1144. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Gao, F.; Su, H.; Zheng, P.; Li, Y.; Yao, H. Nitrogen Distribution and Soil Microbial Community Characteristics in a Legume–Cereal Intercropping System: A Review. Agronomy 2022, 12, 1900. [Google Scholar] [CrossRef]
- El-Ghobashy, Y.E.; Elmehy, A.A.; El-Douby, K.A. Influence of Intercropping Cowpea with Some Maize Hybrids and N Nano-Mineral Fertilization on Productivity in Salinity Soil. Egypt. J. Agron. 2020, 42, 63–78. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, K.; Verma, P.; Singh, O.; Panwar, A.; Singh, T.; Kumar, Y.; Raliya, R. Effect of Nitrogen and Zinc Nanofertilizer with the Organic Farming Practices on Cereal and Oil Seed Crops. Sci. Rep. 2022, 12, 6938. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, L.; Li, Y.; Shakoor, N.; Sun, Y.; Jiang, Y.; Zhu, G.; Wang, F.; Shen, Y.; Rui, Y. Nano-Agriculture and Nitrogen Cycling: Opportunities and Challenges for Sustainable Farming. J. Clean. Prod. 2023, 421, 138489. [Google Scholar] [CrossRef]
- Gong, H.; Fu, H.; Zhang, J.; Zhang, Q.; Wang, Y.; Wang, D.; Cai, L.; Chen, J.; Yu, H.; Lyu, B. Preparation of Soybean Protein-Based Nanoparticles and Its Application as Encapsulation Carriers of Bioactive Substances. LWT 2023, 191, 115680. [Google Scholar] [CrossRef]
- Mohammadian, M.; Waly, M.I.; Moghadam, M.; Emam-Djomeh, Z.; Salami, M.; Moosavi-Movahedi, A.A. Nanostructured Food Proteins as Efficient Systems for the Encapsulation of Bioactive Compounds. Food Sci. Hum. Wellness 2020, 9, 199–213. [Google Scholar] [CrossRef]
- Bayraktar, O.; Oder, G.; Erdem, C.; Kose, M.D.; Cheaburu-Yilmaz, C.N. Selective Encapsulation of the Polyphenols on Silk Fibroin Nanoparticles: Optimization Approaches. Int. J. Mol. Sci. 2023, 24, 9327. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, S.M.; Barbinta-Patrascu, M.-E. Organic and Biogenic Nanocarriers as Bio-Friendly Systems for Bioactive Compounds’ Delivery: State-of-the Art and Challenges. Materials 2023, 16, 7550. [Google Scholar] [CrossRef]
- Zhang, T.; Li, L.; Chunta, S.; Wu, W.; Chen, Z.; Lu, Y. Enhanced Oral Bioavailability from Food Protein Nanoparticles: A Mini Review. J. Control. Release 2023, 354, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Liu, K.; Liu, X.; Rashid, M.T.; Zhang, H.; Wang, M. Research Progress of Protein-Based Bioactive Substance Nanoparticles. Foods 2023, 12, 2999. [Google Scholar] [CrossRef] [PubMed]
- Saif, A.; Anjum, L.; Faisal, Z.; Akram, N.; Shah, Y.A.; Irfan, R.; Saeed, F.; Afzaal, M.; Asif Shah, M. Recent Advances in Protein-Based Nanoparticles and Their Applications in the Delivery of Bioactive Compounds. Int. J. Food Prop. 2023, 26, 2866–2880. [Google Scholar] [CrossRef]
- Chen, S.; Wu, Q.; Ma, M.; Huang, Z.; Vriesekoop, F.; Liang, H. Designing Biocompatible Protein Nanoparticles for Improving the Cellular Uptake and Antioxidation Activity of Tetrahydrocurcumin. J. Drug Deliv. Sci. Technol. 2021, 63, 102404. [Google Scholar] [CrossRef]
- Kaltbeitzel, J.; Wich, P.R. Protein-based Nanoparticles: From Drug Delivery to Imaging, Nanocatalysis and Protein Therapy. Angew. Chem. Int. Ed. 2023, 62, e202216097. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Chen, L.; McClements, D.J.; Peng, X.; Xu, Z.; Meng, M.; Ji, H.; Qiu, C.; Long, J.; Jin, Z. Encapsulation of Polyphenols in Protein-Based Nanoparticles: Preparation, Properties, and Applications. Crit. Rev. Food Sci. Nutr. 2023, 1–15. [Google Scholar] [CrossRef]
- Eweje, F.; Walsh, M.L.; Ahmad, K.; Ibrahim, V.; Alrefai, A.; Chen, J.; Chaikof, E.L. Protein-Based Nanoparticles for Therapeutic Nucleic Acid Delivery. Biomaterials 2024, 305, 122464. [Google Scholar] [CrossRef]
- Liu, F.; Li, M.; Wang, Q.; Yan, J.; Han, S.; Ma, C.; Ma, P.; Liu, X.; McClements, D.J. Future Foods: Alternative Proteins, Food Architecture, Sustainable Packaging, and Precision Nutrition. Crit. Rev. Food Sci. Nutr. 2023, 63, 6423–6444. [Google Scholar] [CrossRef] [PubMed]
- Hefferon, K.L.; De Steur, H.; Perez-Cueto, F.J.; Herring, R. Alternative Protein Innovations and Challenges for Industry and Consumer: An Initial Overview. Front. Sustain. Food Syst. 2023, 7, 148. [Google Scholar] [CrossRef]
- Moura, M.A.F.E.; Martins, B.d.A.; Oliveira, G.P.d.; Takahashi, J.A. Alternative Protein Sources of Plant, Algal, Fungal and Insect Origins for Dietary Diversification in Search of Nutrition and Health. Crit. Rev. Food Sci. Nutr. 2023, 63, 10691–10708. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Chausali, N.; Saxena, J.; Prasad, R. Nanotechnology as a Sustainable Approach for Combating the Environmental Effects of Climate Change. J. Agric. Food Res. 2023, 12, 100541. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Demokritou, P.; Dokoozlian, N.; Hendren, C.O.; Karn, B.; Mauter, M.S.; Sadik, O.A.; Safarpour, M.; Unrine, J.M.; Viers, J.; et al. Nanotechnology for Sustainable Food Production: Promising Opportunities and Scientific Challenges. Environ. Sci. Nano 2017, 4, 767–781. [Google Scholar] [CrossRef]
- Reddy, N.; Rapisarda, M. Properties and Applications of Nanoparticles from Plant Proteins. Materials 2021, 14, 3607. [Google Scholar] [CrossRef] [PubMed]
- Aswathi, V.P.; Meera, S.; Maria, C.A.; Nidhin, M. Green Synthesis of Nanoparticles from Biodegradable Waste Extracts and Their Applications: A Critical Review. Nanotechnol. Environ. Eng. 2023, 8, 377–397. [Google Scholar] [CrossRef]
- Peng, H.; Gan, Z.; Xiong, H.; Luo, M.; Yu, N.; Wen, T.; Wang, R.; Li, Y. Self-Assembly of Protein Nanoparticles from Rice Bran Waste and Their Use as Delivery System for Curcumin. ACS Sustain. Chem. Eng. 2017, 5, 6605–6614. [Google Scholar] [CrossRef]
- Okagu, O.D.; Verma, O.; McClements, D.J.; Udenigwe, C.C. Utilization of Insect Proteins to Formulate Nutraceutical Delivery Systems: Encapsulation and Release of Curcumin Using Mealworm Protein-Chitosan Nano-Complexes. Int. J. Biol. Macromol. 2020, 151, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Akbar, I.; Jaswir, I.; Jamal, P.; Octavianti, F. Fish Gelatin Nanoparticles and Their Food Applications: A Review. Int. Food Res. J. 2017, 24, S255–S264. [Google Scholar]
- Salaeh, S.; Ahmed, F.; Ahmed, O.; Sayed, M. Preparation, Characterization and Properties of Protein Nanoparticles from Feather Waste. Egypt. J. Chem. 2020, 63, 993–999. [Google Scholar] [CrossRef]
- Campalani, C.; Causin, V.; Selva, M.; Perosa, A. Fish-Waste-Derived Gelatin and Carbon Dots for Biobased UV-Blocking Films. ACS Appl. Mater. Interfaces 2022, 14, 35148–35156. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Li, Y.; Long, H.; Lin, Y.; Zhang, Z.; Zhan, F.; Li, M.; Wu, C.; Liu, Z. Cell Membrane-Camouflaged DOX-Loaded β-Glucan Nanoparticles for Highly Efficient Cancer Immunochemotherapy. Int. J. Biol. Macromol. 2023, 225, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Basumallick, S. Green Synthesis of Chitosan Nano Particles at Different Temperature for Bio-Medical Applications. Asian J. Appl. Sci. Technol. 2023, 07, 52–59. [Google Scholar] [CrossRef]
- Sowmyya, T. Potential Forensic Applications of Carbon Nanodots. J. Phys. Conf. Ser. 2023, 2603, 012057. [Google Scholar] [CrossRef]
- Balakumaran, M.D.; Ramachandran, R.; Balashanmugam, P.; Mukeshkumar, D.J.; Kalaichelvan, P.T. Mycosynthesis of Silver and Gold Nanoparticles: Optimization, Characterization and Antimicrobial Activity against Human Pathogens. Microbiol. Res. 2016, 182, 8–20. [Google Scholar] [CrossRef]
- Atta-Allah, A.A.; Ahmed, R.F.; Shahin, A.A.; Hassan, E.A.; El-Bialy, H.A.-A.; El-Fouly, M.Z. Optimizing the Synthesis of Yeast Beta-Glucan via Response Surface Methodology for Nanotechnology Application. BMC Microbiol. 2023, 23, 110. [Google Scholar] [CrossRef]
- Tripathi, V.; Yadav, P.; Singh, M.P. Beta Glucan as an Immune Stimulant in Tumor Microenvironment—Insight into Lessons and Promises from Past Decade. Int. J. Biol. Macromol. 2023, 234, 123617. [Google Scholar]
- Shaheen, T.I.; Hussien, G.M.; Mekawey, A.A.; Ghalia, H.H.; El Mokadem, M.T. Facile Extraction of Nanosized β-Glucans from Edible Mushrooms and Their Antitumor Activities. J. Food Compos. Anal. 2022, 111, 104607. [Google Scholar] [CrossRef]
- Terkula Iber, B.; Azman Kasan, N.; Torsabo, D.; Wese Omuwa, J. A Review of Various Sources of Chitin and Chitosan in Nature. J. Renew. Mater. 2022, 10, 1097–1123. [Google Scholar] [CrossRef]
- Khalaf, E.M.; Abood, N.A.; Atta, R.Z.; Ramírez-Coronel, A.A.; Alazragi, R.; Parra, R.M.R.; Abed, O.H.; Abosaooda, M.; Jalil, A.T.; Mustafa, Y.F. Recent Progressions in Biomedical and Pharmaceutical Applications of Chitosan Nanoparticles: A Comprehensive Review. Int. J. Biol. Macromol. 2023, 231, 123354. [Google Scholar] [CrossRef]
- Park, H.-G.; Shim, Y.Y.; Choi, S.-O.; Park, W.-M. New Method Development for Nanoparticle Extraction of Water-Soluble β-(1→3)- d -Glucan from Edible Mushrooms, Sparassis Crispa and Phellinus Linteus. J. Agric. Food Chem. 2009, 57, 2147–2154. [Google Scholar] [CrossRef] [PubMed]
- Acay, H.; Yildirim, A.; Erdem Güzel, E.; Kaya, N.; Baran, M.F. Evaluation and Characterization of Pleurotus Eryngii Extract-Loaded Chitosan Nanoparticles as Antimicrobial Agents against Some Human Pathogens. Prep. Biochem. Biotechnol. 2020, 50, 897–906. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, Y.; Wang, W.; Yang, Y.; Shi, X.; Sun, M.; Hao, Y.; Li, Y. Comparison of Physicochemical and Rheology Properties of Shiitake Stipes-Derived Chitin Nanocrystals and Nanofibers. Carbohydr. Polym. 2020, 244, 116468. [Google Scholar] [CrossRef] [PubMed]
- Boobalan, T.; Sethupathi, M.; Sengottuvelan, N.; Kumar, P.; Balaji, P.; Gulyás, B.; Padmanabhan, P.; Selvan, S.T.; Arun, A. Mushroom-Derived Carbon Dots for Toxic Metal Ion Detection and as Antibacterial and Anticancer Agents. ACS Appl. Nano Mater. 2020, 3, 5910–5919. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Viswanath, B.; Reddy, A.S.; Yoon, M. Fungus-Derived Photoluminescent Carbon Nanodots for Ultrasensitive Detection of Hg2+ Ions and Photoinduced Bactericidal Activity. Sens. Actuators B Chem. 2018, 258, 172–183. [Google Scholar] [CrossRef]
- Manimaran, K.; Yanto, D.H.Y.; Kamaraj, C.; Selvaraj, K.; Pandiaraj, S.; Elgorban, A.M.; Vignesh, S.; Kim, H. Eco-Friendly Approaches of Mycosynthesized Copper Oxide Nanoparticles (CuONPs) Using Pleurotus Citrinopileatus Mushroom Extracts and Their Biological Applications. Environ. Res. 2023, 232, 116319. [Google Scholar] [CrossRef] [PubMed]
- Syed, I.A.; Ahmad, J.; Butt, S.; Ullah, A.; Ahmed, I.; Niaz, Z.; Hayat, S.; Ashique, S.; Zengin, G.; Farid, A. Synthesis of Silver Nanoparticles from Ganoderma Species and Their Activity against Multi Drug Resistant Pathogens. Chem. Biodivers. 2023, e202301304. [Google Scholar] [CrossRef]
- Dias, C.; Ayyanar, M.; Amalraj, S.; Khanal, P.; Subramaniyan, V.; Das, S.; Gandhale, P.; Biswa, V.; Ali, R.; Gurav, N. Biogenic Synthesis of Zinc Oxide Nanoparticles Using Mushroom Fungus Cordyceps Militaris: Characterization and Mechanistic Insights of Therapeutic Investigation. J. Drug Deliv. Sci. Technol. 2022, 73, 103444. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan Nanoparticles Preparation and Applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Perera, U.M.S.P.; Rajapakse, N. Chitosan Nanoparticles: Preparation, Characterization, and Applications. In Seafood Processing By-Products: Trends and Applications; Kim, S.-K., Ed.; Springer: New York, NY, USA, 2014; pp. 371–387. ISBN 978-1-4614-9590-1. [Google Scholar]
- Han, J.-F.; Lou, Q.; Ding, Z.-Z.; Zheng, G.-S.; Ni, Q.-C.; Song, R.-W.; Liu, K.-K.; Zang, J.-H.; Dong, L.; Shen, C.-L. Chemiluminescent Carbon Nanodots for Dynamic and Guided Antibacteria. Light Sci. Appl. 2023, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Bartolomei, B.; Prato, M. The Importance of the Purification Step and the Characterization of the Products in the Synthesis of Carbon Nanodots. Small 2023, 19, 2206714. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.-C.; Panda, P.K.; Li, C.-H.; Ting, Y.-X.; Ashraf Gandomi, Y.; Hsieh, C.-T. Hydrothermal Synthesis of Functionalized Carbon Nanodots and Their Clusters as Ionic Probe for High Sensitivity and Selectivity for Sulfate Anions with Excellent Detection Level. Polymers 2023, 15, 2655. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.; Shahadat, M.; ul Islam, S.; Adnan, R.; Mohamad Ibrahim, M.N.; Ullah, Q. Synthesis, Characterization, and Properties of Green Carbon Nanodots. In Green Carbon Materials for Environmental Analysis: Emerging Research and Future Opportunities; ACS Publications: Washington, DC, USA, 2023; pp. 25–39. ISBN 1947-5918. [Google Scholar]
- Nazibudin, N.A.; Zainuddin, M.F.; Abdullah, C.A.C. Hydrothermal Synthesis of Carbon Quantum Dots: An Updated Review. J. Adv. Res. Fluid Mech. Therm. Sci. 2023, 101, 192–206. [Google Scholar] [CrossRef]
- Amr, M.; Abu-Hussien, S.H.; Ismail, R.; Aboubakr, A.; Wael, R.; Yasser, M.; Hemdan, B.; El-Sayed, S.M.; Bakry, A.; Ebeed, N.M. Utilization of Biosynthesized Silver Nanoparticles from Agaricus Bisporus Extract for Food Safety Application: Synthesis, Characterization, Antimicrobial Efficacy, and Toxicological Assessment. Sci. Rep. 2023, 13, 15048. [Google Scholar] [CrossRef] [PubMed]
- Santhosh Kumar, D.S.R.; Senthilkumar, P.; Surendran, L.; Sudhagar, B. Ganoderma lucidum-oriental mushroom mediated synthesis of gold nanoparticles conjugated with doxorubicin and evaluation of its anticancer potential on human breast cancer MCF-7/DOX cells. Int. J. Pharm. Pharm. Sci. 2017, 9, 267. [Google Scholar] [CrossRef]
- Salem, S.S.; Fouda, A. Green Synthesis of Metallic Nanoparticles and Their Prospective Biotechnological Applications: An Overview. Biol. Trace Elem. Res. 2021, 199, 344–370. [Google Scholar] [CrossRef] [PubMed]
- Primožic, M.; Knez, Ž.; Leitgeb, M. Nanotechnology in Food Science—Food Packaging. Nanomaterials 2021, 11, 292. [Google Scholar] [CrossRef]
- Wani, N.R.; Dar, A.H.; Dash, K.K.; Pandey, V.K.; Srivastava, S.; Jan, S.Y.; Deka, P.; Sabahi, N. Recent Advances in the Production of Bionanomaterials for Development of Sustainable Food Packaging: A Comprehensive Review. Environ. Res. 2023, 237, 116948. [Google Scholar] [CrossRef]
- Yu, Z.; Boyarkina, V.; Liao, Z.; Lin, M.; Zeng, W.; Lu, X. Boosting Food System Sustainability through Intelligent Packaging: Application of Biodegradable Freshness Indicators. ACS Food Sci. Technol. 2023, 3, 199–212. [Google Scholar] [CrossRef]
- Ezati, P.; Khan, A.; Priyadarshi, R.; Bhattacharya, T.; Tammina, S.K.; Rhim, J.-W. Biopolymer-Based UV Protection Functional Films for Food Packaging. Food Hydrocoll. 2023, 142, 108771. [Google Scholar] [CrossRef]
- Huang, F.; Zhang, Q.; Wang, L.; Zhang, C.; Zhang, Y. Are Biodegradable Mulch Films a Sustainable Solution to Microplastic Mulch Film Pollution? A Biogeochemical Perspective. J. Hazard. Mater. 2023, 459, 132024. [Google Scholar] [CrossRef]
- Bhatt, G.S.; Aarthi, S. Biopolymer Sustainable Films for Food Industries: Properties and Application Based on Chitosan. In Tailored Functional Materials for Clean and Sustainable Development; Apple Academic Press: Palm Bay, FL, USA, 2024; ISBN 978-1-00-339476-1. [Google Scholar]
- Sultanbawa, F.; Sultanbawa, Y. Mineral Nutrient-Rich Plants—Do They Occur? Appl. Food Res. 2023, 3, 100347. [Google Scholar] [CrossRef]
- Santa-María, G.E.; Lavres, J.; Rubio, G. The Concept of Mineral Plant Nutrient in the Light of Evolution. Plant Sci. 2023, 334, 111747. [Google Scholar] [CrossRef] [PubMed]
- Supriatna, J.; Setiawati, M.R.; Sudirja, R.; Suherman, C.; Bonneau, X. Migration from Inorganic to Organic Fertilization for a More Sustainable Oil Palm Agro-Industry. Heliyon 2023, 9, e22868. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Lambers, H.; Preece, C.; Alrefaei, A.F.; Penuelas, J. Role of Mycorrhizas and Root Exudates in Plant Uptake of Soil Nutrients (Calcium, Iron, Magnesium, and Potassium): Has the Puzzle Been Completely Solved? Plant J. 2023, 114, 1227–1242. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Liu, S.; Jiang, C.; Zhang, T.; Chen, W. Recent Advances in Stimuli-Response Mechanisms of Nano-Enabled Controlled-Release Fertilizers and Pesticides. Eco-Environ. Health 2023, 2, 161–175. [Google Scholar] [CrossRef]
- Khan, Z.; Thounaojam, T.C.; Chowdhury, D.; Upadhyaya, H. The Role of Selenium and Nano Selenium on Physiological Responses in Plant: A Review. Plant Growth Regul. 2023, 100, 409–433. [Google Scholar] [CrossRef]
- Flores-Balderas, X.; Peña-Peña, M.; Rada, K.M.; Alvarez-Alvarez, Y.Q.; Guzmán-Martín, C.A.; Sánchez-Gloria, J.L.; Huang, F.; Ruiz-Ojeda, D.; Morán-Ramos, S.; Springall, R. Beneficial Effects of Plant-Based Diets on Skin Health and Inflammatory Skin Diseases. Nutrients 2023, 15, 2842. [Google Scholar] [CrossRef]
- Mehta, P.; Tawfeeq, S.; Padte, S.; Sunasra, R.; Desai, H.; Surani, S.; Kashyap, R. Plant-Based Diet and Its Effect on Coronary Artery Disease: A Narrative Review. World J. Clin. Cases 2023, 11, 4752. [Google Scholar] [CrossRef] [PubMed]
- Salehin, S.; Rasmussen, P.; Mai, S.; Mushtaq, M.; Agarwal, M.; Hasan, S.M.; Salehin, S.; Raja, M.; Gilani, S.; Khalife, W.I. Plant Based Diet and Its Effect on Cardiovascular Disease. Int. J. Environ. Res. Public. Health 2023, 20, 3337. [Google Scholar] [CrossRef]
- Thompson, A.S.; Tresserra-Rimbau, A.; Karavasiloglou, N.; Jennings, A.; Cantwell, M.; Hill, C.; Perez-Cornago, A.; Bondonno, N.P.; Murphy, N.; Rohrmann, S. Association of Healthful Plant-Based Diet Adherence with Risk of Mortality and Major Chronic Diseases among Adults in the UK. JAMA Netw. Open 2023, 6, e234714. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Gu, Y.; Meng, G.; Wu, H.; Zhang, S.; Wang, X.; Zhang, J.; Huang, T.; Niu, K. Quality of Plant-Based Diet and the Risk of Dementia and Depression among Middle-Aged and Older Population. Age Ageing 2023, 52, afad070. [Google Scholar] [CrossRef]
- Chiba, M.; Morita, N. Incorporation of Plant-Based Diet Surpasses Current Standards in Therapeutic Outcomes in Inflammatory Bowel Disease. Metabolites 2023, 13, 332. [Google Scholar] [CrossRef]
- Wang, Y.B.; Page, A.J.; Gill, T.K.; Melaku, Y.A. The Association between Diet Quality, Plant-Based Diets, Systemic Inflammation, and Mortality Risk: Findings from NHANES. Eur. J. Nutr. 2023, 62, 2723–2737. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Kaimori, J.-Y.; Isaka, Y. Plant-Dominant Low Protein Diet: A Potential Alternative Dietary Practice for Patients with Chronic Kidney Disease. Nutrients 2023, 15, 1002. [Google Scholar] [CrossRef]
- Rosenfeld, R.M.; Juszczak, H.M.; Wong, M.A. Scoping Review of the Association of Plant-Based Diet Quality with Health Outcomes. Front. Nutr. 2023, 10, 1211535. [Google Scholar] [CrossRef]
- Chen, N.; Yao, P.; Zhang, W.; Zhang, Y.; Xin, N.; Wei, H.; Zhang, T.; Zhao, C. Selenium Nanoparticles: Enhanced Nutrition and Beyond. Crit. Rev. Food Sci. Nutr. 2023, 63, 12360–12371. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, R.; Li, F.; Yu, S.; Nie, Y.; Li, J.-Q.; Pan, C.; Zhu, W.; Zhou, Z.; Diao, J. Nano-Selenium Repaired the Damage Caused by Fungicides on Strawberry Flavor Quality and Antioxidant Capacity by Regulating ABA Biosynthesis and Ripening-Related Transcription Factors. Pestic. Biochem. Physiol. 2024, 198, 105753. [Google Scholar] [CrossRef] [PubMed]
- Moulick, D.; Mukherjee, A.; Das, A.; Roy, A.; Majumdar, A.; Dhar, A.; Pattanaik, B.K.; Chowardhara, B.; Ghosh, D.; Upadhyay, M.K.; et al. Selenium—An Environmentally Friendly Micronutrient in Agroecosystem in the Modern Era: An Overview of 50-Year Findings. Ecotoxicol. Environ. Saf. 2024, 270, 115832. [Google Scholar] [CrossRef] [PubMed]
- Kulhánek, M.; Asrade, D.A.; Suran, P.; Sedlář, O.; Černý, J.; Balík, J. Plant Nutrition—New Methods Based on the Lessons of History: A Review. Plants 2023, 12, 4150. [Google Scholar] [CrossRef]
- Wu, H.; Li, Z. Nano-Enabled Agriculture: How Do Nanoparticles Cross Barriers in Plants? Plant Commun. 2022, 3, 100346. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef] [PubMed]
- Sembada, A.A.; Lenggoro, I.W. Transport of Nanoparticles into Plants and Their Detection Methods. Nanomaterials 2024, 14, 131. [Google Scholar] [CrossRef]
- Geoffrion, L.D.; Guisbiers, G. Physico-Chemical Properties of Selenium–Tellurium Alloys across the Scales. Nanoscale Adv. 2021, 3, 4254–4270. [Google Scholar] [CrossRef]
- Zambonino, M.C.; Quizhpe, E.M.; Jaramillo, F.E.; Rahman, A.; Santiago Vispo, N.; Jeffryes, C.; Dahoumane, S.A. Green Synthesis of Selenium and Tellurium Nanoparticles: Current Trends, Biological Properties and Biomedical Applications. Int. J. Mol. Sci. 2021, 22, 989. [Google Scholar] [CrossRef]
- Chang, Y.; Huang, J.; Shi, S.; Xu, L.; Lin, H.; Chen, T. Precise-engineering of Se/Te Nanochaperone for Reinvigorating Cancer Radio-immunotherapy. Adv. Mater. 2023, 35, 2212178. [Google Scholar] [CrossRef]
- Liu, K.; Niu, J.; Liu, L.; Tian, F.; Nie, H.; Liu, X.; Chen, K.; Zhao, R.; Sun, S.; Jiao, M.; et al. LUMO-Mediated Se and HOMO-Mediated Te Nanozymes for Selective Redox Biocatalysis. Nano Lett. 2023, 23, 5131–5140. [Google Scholar] [CrossRef]
- Beleneva, I.A.; Kharchenko, U.V.; Kukhlevsky, A.D.; Boroda, A.V.; Izotov, N.V.; Gnedenkov, A.S.; Egorkin, V.S. Biogenic Synthesis of Selenium and Tellurium Nanoparticles by Marine Bacteria and Their Biological Activity. World J. Microbiol. Biotechnol. 2022, 38, 188. [Google Scholar] [CrossRef]
- Nwoko, K.C.; Liang, X.; Perez, M.A.; Krupp, E.; Gadd, G.M.; Feldmann, J. Characterisation of Selenium and Tellurium Nanoparticles Produced by Aureobasidium Pullulans Using a Multi-Method Approach. J. Chromatogr. A 2021, 1642, 462022. [Google Scholar] [CrossRef] [PubMed]
- Shah, V.; Medina-Cruz, D.; Vernet-Crua, A.; Truong, L.B.; Sotelo, E.; Mostafavi, E.; González, M.U.; García-Martín, J.M.; Cholula-Díaz, J.L.; Webster, T.J. Pepper-Mediated Green Synthesis of Selenium and Tellurium Nanoparticles with Antibacterial and Anticancer Potential. J. Funct. Biomater. 2022, 14, 24. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fan, L.; Wang, K.; Liu, H.; Zhang, J.; Yan, S. Progress in the Synthesis and Application of Tellurium Nanomaterials. Nanomaterials 2023, 13, 2057. [Google Scholar] [CrossRef] [PubMed]
- Abo Elsoud, M.M.; Al-Hagar, O.E.A.; Abdelkhalek, E.S.; Sidkey, N.M. Synthesis and Investigations on Tellurium Myconanoparticles. Biotechnol. Rep. 2018, 18, e00247. [Google Scholar] [CrossRef] [PubMed]
- Reddy, G.K.K.; Pathak, S.; Nancharaiah, Y.V. Aerobic Reduction of Selenite and Tellurite to Elemental Selenium and Tellurium Nanostructures by Alteromonas Sp. under Saline Conditions. Int. Biodeterior. Biodegrad. 2023, 179, 105571. [Google Scholar] [CrossRef]
- Saikia, S.; Sinharoy, A.; Lens, P.N.L. Adsorptive Removal of Gallium from Aqueous Solution onto Biogenic Elemental Tellurium Nanoparticles. Sep. Purif. Technol. 2022, 286, 120462. [Google Scholar] [CrossRef]
- Sathiyaseelan, A.; Zhang, X.; Wang, M.-H. Biosynthesis of Gallic Acid Fabricated Tellurium Nanoparticles (GA-Te NPs) for Enhanced Antibacterial, Antioxidant, and Cytotoxicity Applications. Environ. Res. 2024, 240, 117461. [Google Scholar] [CrossRef]
- Hosseini, F.; Hadian, M.; Lashani, E.; Moghimi, H. Simultaneous Bioreduction of Tellurite and Selenite by Yarrowia Lipolytica, Trichosporon Cutaneum, and Their Co-Culture along with Characterization of Biosynthesized Te–Se Nanoparticles. Microb. Cell Factories 2023, 22, 193. [Google Scholar] [CrossRef] [PubMed]
- Nikam, P.B.; Salunkhe, J.D.; Minkina, T.; Rajput, V.D.; Kim, B.S.; Patil, S.V. A Review on Green Synthesis and Recent Applications of Red Nano Selenium. Results Chem. 2022, 4, 100581. [Google Scholar] [CrossRef]
- Shi, M.-T.; Zhang, T.-J.; Fang, Y.; Pan, C.-P.; Fu, H.-Y.; Gao, S.-J. Nano-Selenium Enhances Sugarcane Resistance to Xanthomonas Albilineans Infection and Improvement of Juice Quality. Ecotoxicol. Environ. Saf. 2023, 254, 114759. [Google Scholar] [CrossRef]
- Ghanbari, F.; Bag-Nazari, M.; Azizi, A. Exogenous Application of Selenium and Nano-Selenium Alleviates Salt Stress and Improves Secondary Metabolites in Lemon Verbena under Salinity Stress. Sci. Rep. 2023, 13, 5352. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Miao, P.; Li, D.; Wu, Y.; Zhou, C.; Pan, C. Improving Red Pitaya Fruit Quality by Nano-Selenium Biofortification to Enhance Phenylpropanoid and Betalain Biosynthesis. Ecotoxicol. Environ. Saf. 2023, 267, 115653. [Google Scholar] [CrossRef] [PubMed]
- Sindireva, A.; Golubkina, N.; Bezuglova, H.; Fedotov, M.; Alpatov, A.; Erdenotsogt, E.; Sękara, A.; Murariu, O.C.; Caruso, G. Effects of High Doses of Selenate, Selenite and Nano-Selenium on Biometrical Characteristics, Yield and Biofortification Levels of Vicia Faba L. Cultivars. Plants 2023, 12, 2847. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Mashwani, Z.-R.; Raja, N.I.; Mohammad, S.; Luna-Arias, J.P.; Ahmad, A.; Kaushik, P. Phytomediated Selenium Nanoparticles and Light Regimes Elicited in Vitro Callus Cultures for Biomass Accumulation and Secondary Metabolite Production in Caralluma Tuberculata. Front. Plant Sci. 2023, 14, 1253193. [Google Scholar] [CrossRef] [PubMed]
- Jiao, L.; Cao, X.; Wang, C.; Chen, F.; Zou, H.; Yue, L.; Wang, Z. Crosstalk between in Situ Root Exudates and Rhizobacteria to Promote Rice Growth by Selenium Nanomaterials. Sci. Total Environ. 2023, 878, 163175. [Google Scholar] [CrossRef] [PubMed]
- Ran, M.; Wu, T.; Jiao, Y.; Wu, J.; Li, J. Selenium Bio-Nanocomposite Based on Extracellular Polymeric Substances (EPS): Synthesis, Characterization and Application in Alleviating Cadmium Toxicity in Rice (Oryza Sativa L.). Int. J. Biol. Macromol. 2024, 258, 129089. [Google Scholar] [CrossRef] [PubMed]
- Shang, H.; Ma, C.; Li, C.; Cai, Z.; Shen, Y.; Han, L.; Wang, C.; Tran, J.; Elmer, W.H.; White, J.C. Aloe Vera Extract Gel-Biosynthesized Selenium Nanoparticles Enhance Disease Resistance in Lettuce by Modulating the Metabolite Profile and Bacterial Endophytes Composition. ACS Nano 2023, 17, 13672–13684. [Google Scholar] [CrossRef] [PubMed]
- Kang, L.; Wu, Y.; Jia, Y.; Chen, Z.; Kang, D.; Zhang, L.; Pan, C. Nano-Selenium Enhances Melon Resistance to Podosphaera Xanthii by Enhancing the Antioxidant Capacity and Promoting Alterations in the Polyamine, Phenylpropanoid and Hormone Signaling Pathways. J. Nanobiotechnol. 2023, 21, 377. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Kang, L.; Wu, Y.; Zhou, C.; Cai, R.; Zhang, H.; Li, J.; Chen, Z.; Kang, D.; Zhang, L. Nano-selenium Foliar Intervention-induced Resistance of Cucumber to Botrytis Cinerea by Activating Jasmonic Acid Biosynthesis and Regulating Phenolic Acid and Cucurbitacin. Pest Manag. Sci. 2023, 80, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Akram, A.; Mehak, A.; ul Haq, E.; Fatima, N.; Wareen, G.; Fitriatin, B.N.; Sayyed, R.Z.; Ilyas, N.; Sabullah, M.K. Evaluation of Selenium Nanoparticles in Inducing Disease Resistance against Spot Blotch Disease and Promoting Growth in Wheat under Biotic Stress. Plants 2023, 12, 761. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Le, X.C.; Tran, T.N.M.; Nguyen, N.T.T.; Pham, B.N.; Vu, D. Nano Selenium–Alginate Edible Coating Extends Hydroponic Strawberry Shelf Life and Provides Selenium Fortification as a Micro-Nutrient. Food Biosci. 2023, 53, 102597. [Google Scholar] [CrossRef]
- Samynathan, R.; Venkidasamy, B.; Ramya, K.; Muthuramalingam, P.; Shin, H.; Kumari, P.S.; Thangavel, S.; Sivanesan, I. A Recent Update on the Impact of Nano-Selenium on Plant Growth, Metabolism, and Stress Tolerance. Plants 2023, 12, 853. [Google Scholar] [CrossRef]
- El-Batal, A.I.; Ismail, M.A.; Amin, M.A.; El-Sayyad, G.S.; Osman, M.S. Selenium Nanoparticles Induce Growth and Physiological Tolerance of Wastewater-stressed Carrot Plants. Biologia 2023, 78, 2339–2355. [Google Scholar] [CrossRef]
- Wang, Y.; Rao, C.; Huang, L.; Wu, J.; Sun, P.; Zhan, J.; Wu, J.; Liu, S.; Zhou, C.; Hu, L. Effects of Organic Selenium and Nanoselenium on Drought Stress of Pak Choi (Brassica Chinensis Var. Pekinensis. Cv.‘Suzhouqing’) and Its Transcriptomic Analysis. Agronomy 2023, 14, 78. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Quan, J.; Sun, J.; Lu, J.; Zhao, G. Dietary Nano-Selenium Alleviates Heat Stress-Induced Intestinal Damage through Affecting Intestinal Antioxidant Capacity and Microbiota in Rainbow Trout (Oncorhynchus Mykiss). Fish Shellfish Immunol. 2023, 133, 108537. [Google Scholar] [CrossRef]
- Rathore, S.S.; Hanumappa, S.M.; Yusufzai, S.I.; Suyani, N.K.; Abdullah-Al-Mamun, M.; Nasren, S.; Sidiq, M.J.; Hanumanthappa, S.K.; Kalyani, R. Dietary Administration of Engineered Nano-Selenium and Vitamin C Ameliorates Immune Response, Nutritional Physiology, Oxidative Stress, and Resistance Against Aeromonas Hydrophila in Nile Tilapia (Oreochromis Niloticus). Biol. Trace Elem. Res. 2023, 201, 4079–4092. [Google Scholar] [CrossRef] [PubMed]
- Mansuriya, B.D.; Altintas, Z. Carbon Dots: Classification, Properties, Synthesis, Characterization, and Applications in Health Care—An Updated Review (2018–2021). Nanomaterials 2021, 11, 2525. [Google Scholar] [CrossRef] [PubMed]
- Chandel, M.; Kaur, K.; Sahu, B.K.; Sharma, S.; Panneerselvam, R.; Shanmugam, V. Promise of Nano-Carbon to the next Generation Sustainable Agriculture. Carbon 2022, 188, 461–481. [Google Scholar] [CrossRef]
- Bhattacharya, N.; Cahill, D.M.; Yang, W.; Kochar, M. Graphene as a Nano-Delivery Vehicle in Agriculture–Current Knowledge and Future Prospects. Crit. Rev. Biotechnol. 2023, 43, 851–869. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Majumdar, S.; Servin, A.D.; Pagano, L.; Dhankher, O.P.; White, J.C. Carbon Nanomaterials in Agriculture: A Critical Review. Front. Plant Sci. 2016, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Mocci, F.; de Villiers Engelbrecht, L.; Olla, C.; Cappai, A.; Casula, M.F.; Melis, C.; Stagi, L.; Laaksonen, A.; Carbonaro, C.M. Carbon Nanodots from an in Silico Perspective. Chem. Rev. 2022, 122, 13709–13799. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Chen, L.; Gu, J.; Ma, H.; Wu, H. Carbon-Based Nanomaterials for Sustainable Agriculture: Their Application as Light Converters, Nanosensors, and Delivery Tools. Plants 2022, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Rezaei Cherati, S.; Anas, M.; Liu, S.; Shanmugam, S.; Pandey, K.; Angtuaco, S.; Shelton, R.; Khalfaoui, A.N.; Alena, S.V.; Porter, E. Comprehensive Risk Assessment of Carbon Nanotubes Used for Agricultural Applications. ACS Nano 2022, 16, 12061–12072. [Google Scholar] [CrossRef]
- Safdar, M.; Kim, W.; Park, S.; Gwon, Y.; Kim, Y.-O.; Kim, J. Engineering Plants with Carbon Nanotubes: A Sustainable Agriculture Approach. J. Nanobiotechnol. 2022, 20, 275. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, D.K.; Dasgupta-Schubert, N.; Villaseñor Cendejas, L.M.; Villegas, J.; Carreto Montoya, L.; Borjas García, S.E. Interfacing Carbon Nanotubes (CNT) with Plants: Enhancement of Growth, Water and Ionic Nutrient Uptake in Maize (Zea Mays) and Implications for Nanoagriculture. Appl. Nanosci. 2014, 4, 577–591. [Google Scholar] [CrossRef]
- Lahiani, M.H.; Nima, Z.A.; Villagarcia, H.; Biris, A.S.; Khodakovskaya, M.V. Assessment of Effects of the Long-Term Exposure of Agricultural Crops to Carbon Nanotubes. J. Agric. Food Chem. 2017, 66, 6654–6662. [Google Scholar] [CrossRef] [PubMed]
- Nepal, J.; Xin, X.; Maltais-Landry, G.; Ahmad, W.; Pereira, J.; Santra, S.; Wright, A.L.; Ogram, A.; Stofella, P.J.; He, Z. Carbon Nanomaterials Are a Superior Soil Amendment for Sandy Soils than Biochar Based on Impacts on Lettuce Growth, Physiology and Soil Biochemical Quality. NanoImpact 2023, 31, 100480. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C. Cytotoxicity of Carbon Nanotubes, Graphene, Fullerenes, and Dots. Nanomaterials 2023, 13, 1458. [Google Scholar] [CrossRef] [PubMed]
- Krumova, S.; Petrova, A.; Petrova, N.; Stoichev, S.; Ilkov, D.; Tsonev, T.; Petrov, P.; Koleva, D.; Velikova, V. Seed Priming with Single-Walled Carbon Nanotubes Grafted with Pluronic P85 Preserves the Functional and Structural Characteristics of Pea Plants. Nanomaterials 2023, 13, 1332. [Google Scholar] [CrossRef]
- Fu, T.; Zhang, B.; Gao, X.; Cui, S.; Guan, C.-Y.; Zhang, Y.; Zhang, B.; Peng, Y. Recent Progresses, Challenges, and Opportunities of Carbon-Based Materials Applied in Heavy Metal Polluted Soil Remediation. Sci. Total Environ. 2023, 856, 158810. [Google Scholar] [CrossRef]
- Sk, M.P.; Jaiswal, A.; Paul, A.; Ghosh, S.S.; Chattopadhyay, A. Presence of Amorphous Carbon Nanoparticles in Food Caramels. Sci. Rep. 2012, 2, 383. [Google Scholar] [CrossRef] [PubMed]
- Husen, A. Carbon-Based Nanomaterials and Their Interactions with Agricultural Crops. In Nanomaterials for Agriculture and Forestry Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 199–218. [Google Scholar]
- Samadi, S.; Asgari Lajayer, B.; Moghiseh, E.; Rodríguez-Couto, S. Effect of Carbon Nanomaterials on Cell Toxicity, Biomass Production, Nutritional and Active Compound Accumulation in Plants. Environ. Technol. Innov. 2021, 21, 101323. [Google Scholar] [CrossRef]
- Shojaei, T.R.; Salleh, M.A.M.; Tabatabaei, M.; Mobli, H.; Aghbashlo, M.; Rashid, S.A.; Tan, T. Applications of Nanotechnology and Carbon Nanoparticles in Agriculture. In Synthesis, Technology and Applications of Carbon Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 247–277. [Google Scholar]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S.A. Nanoparticle-Based Sustainable Agriculture and Food Science: Recent Advances and Future Outlook. Front. Nanotechnol. 2020, 2, 579954. [Google Scholar] [CrossRef]
- Hao, Y.; Yu, Y.; Sun, G.; Gong, X.; Jiang, Y.; Lv, G.; Zhang, Y.; Li, L.; Zhao, Y.; Sun, D. Effects of Multi-Walled Carbon Nanotubes and Nano-Silica on Root Development, Leaf Photosynthesis, Active Oxygen and Nitrogen Metabolism in Maize. Plants 2023, 12, 1604. [Google Scholar] [CrossRef] [PubMed]
- Nurdin, M.; Sari, I.D.W.; Mardhatillah, M.; Herdianto, N.; Wibowo, D.; Maulidiyah, M.; Mappasomba, M.; Ansharullah, A.; Bijang, C. Highly Ecofriendly Inorganic Pesticide Based on TiO2 Incorporated with Nano-Carbon Composites for Phytophthora Palmivora Fungus Disinfection. Indian J. Microbiol. 2023, 63, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Xin, X.; Cao, Y.; Su, D.; Ji, P.; Zhu, Z.; He, Z. Use of Carbon Nanoparticles to Improve Soil Fertility, Crop Growth and Nutrient Uptake by Corn (Zea Mays L.). Nanomaterials 2021, 11, 2717. [Google Scholar] [CrossRef] [PubMed]
- Behpour, M.; Shadi, M.; Nojavan, S. Preparation of an Efficient Magnetic Nano-Sorbent Based on Modified Cellulose and Carboxylated Carbon Nano-Tubes for Extraction of Pesticides from Food and Agricultural Water Samples before GC-FID Analysis. Food Chem. 2023, 407, 135067. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Wong, S.C.; Lisak, G. Effects of Plastic-Derived Carbon Dots on Germination and Growth of Pea (Pisum Sativum) via Seed Nano-Priming. Chemosphere 2023, 316, 137868. [Google Scholar] [CrossRef]
- Jing, X.; Liu, Y.; Liu, X.; Wang, X.-F.; You, C.; Chang, D.; Zhang, S. Nitrogen-Doped Carbon Dots Enhanced Seedling Growth and Salt Tolerance with Distinct Requirements of Excitation Light. RSC Adv. 2023, 13, 12114–12122. [Google Scholar] [CrossRef]
- Panahirad, S.; Dadpour, M.; Gohari, G.; Akbari, A.; Mahdavinia, G.; Jafari, H.; Kulak, M.; Alcázar, R.; Fotopoulos, V. Putrescine-Functionalized Carbon Quantum Dot (Put-CQD) Nanoparticle: A Promising Stress-Protecting Agent against Cadmium Stress in Grapevine (Vitis Vinifera Cv. Sultana). Plant Physiol. Biochem. 2023, 197, 107653. [Google Scholar] [CrossRef]
- Zhao, W.-B.; Wang, Y.; Li, F.-K.; Guo, R.; Jiao, F.-H.; Song, S.-Y.; Chang, S.-L.; Dong, L.; Liu, K.-K.; Shan, C.-X. Highly Antibacterial and Antioxidative Carbon Nanodots/Silk Fibroin Films for Fruit Preservation. Nano Lett. 2023, 23, 11755–11762. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, H.; Liu, L.; Jia, M.; Li, X.; Li, J. Nano-Biosensor Based on Manganese Dioxide Nanosheets and Carbon Dots for Dual-Mode Determination of Staphylococcus Aureus. Food Chem. 2024, 432, 137144. [Google Scholar] [CrossRef]
- Zaim, N.S.H.B.H.; Tan, H.L.; Rahman, S.M.A.; Abu Bakar, N.F.; Osman, M.S.; Thakur, V.K.; Radacsi, N. Recent Advances in Seed Coating Treatment Using Nanoparticles and Nanofibers for Enhanced Seed Germination and Protection. J. Plant Growth Regul. 2023, 42, 7374–7402. [Google Scholar] [CrossRef]
- Fincheira, P.; Hoffmann, N.; Tortella, G.; Ruiz, A.; Cornejo, P.; Diez, M.C.; Seabra, A.B.; Benavides-Mendoza, A.; Rubilar, O. Eco-Efficient Systems Based on Nanocarriers for the Controlled Release of Fertilizers and Pesticides: Toward Smart Agriculture. Nanomaterials 2023, 13, 1978. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, N.; Rajput, V.D.; Mandzhieva, S.; Minkina, T.; Saharan, B.S.; Kumar, D.; Sadh, P.K.; Duhan, J.S. Advances in Biopolymeric Nanopesticides: A New Eco-Friendly/Eco-Protective Perspective in Precision Agriculture. Nanomaterials 2022, 12, 3964. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, J.; Wang, X.; Zhang, T.; Zhang, F.; Zhang, S.; Li, Y.; Gao, W.; You, C.; Wang, X. Cellulose Nanofibers Extracted from Natural Wood Improve the Postharvest Appearance Quality of Apples. Front. Nutr. 2022, 9, 881783. [Google Scholar] [CrossRef]
- Bao, J.; Hu, Y.; Farag, M.A.; Huan, W.; Wu, J.; Yang, D.; Song, L. Carbon Dots, Cellulose Nanofiber, and Essential Oil from Torreya Grandis Aril Added to Fish Scale Gelatin Film for Tomato Preservation. Int. J. Biol. Macromol. 2023, 245, 125482. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Fang, D.; Huang, C.; Lyu, L.; Wu, W.; Li, W. Electrospun Biopolymer Material for Antimicrobial Function of Fresh Fruit and Vegetables: Application Perspective and Challenges. LWT 2023, 174, 114374. [Google Scholar] [CrossRef]
- Sharma, N.; Allardyce, B.J.; Rajkhowa, R.; Agrawal, R. Biodegradable Cellulose and Cellulose Nanofibres-Based Coating Materials as a Postharvest Preservative for Horticultural Products. J. Polym. Environ. 2024, 32, 1500–1512. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P.; Tkaczewska, J.; Dordevic, D.; Jancikova, S.; Kulawik, P.; Milosavljevic, V.; Dolezelikova, K.; Smerkova, K.; Svec, P.; et al. Nanocomposite Furcellaran Films—The Influence of Nanofillers on Functional Properties of Furcellaran Films and Effect on Linseed Oil Preservation. Polymers 2019, 11, 2046. [Google Scholar] [CrossRef] [PubMed]
- Shaghaleh, H.; Hamoud, Y.A.; Sun, Q. Functionalized Nanocellulose Nanocomposite Hydrogels for Soil and Water Pollution Prevention, Remediation, and Monitoring: A Critical Review on Fabrication, Application Properties, and Potential Mechanisms. J. Environ. Chem. Eng. 2024, 12, 111892. [Google Scholar] [CrossRef]
- Cota-Leal, M.; García-Valenzuela, J.A.; Borbón-Nuñez, H.A.; Cota, L.; Olivas, A. CuS/Cellulose Acetate Nanofiber Composite: A Study on Adsorption and Photocatalytic Activity for Water Remediation. Polymer 2023, 293, 126627. [Google Scholar] [CrossRef]
- Pratim Das, P.; Kalyani, P.; Kumar, R.; Khandelwal, M. Cellulose-Based Natural Nanofibers for Fresh Produce Packaging: Current Status, Sustainability and Future Outlook. Sustain. Food Technol. 2023, 1, 528–544. [Google Scholar] [CrossRef]
- Jiang, L.; Huang, X.; Tian, C.; Zhong, Y.; Yan, M.; Miao, C.; Wu, T.; Zhou, X. Preparation and Characterization of Porous Cellulose Acetate Nanofiber Hydrogels. Gels 2023, 9, 484. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, C.; He, D.; Zhao, H.; Zhao, M.; Xu, E.; Jin, Z.; Yuan, C.; Guo, L.; Wu, Z.; et al. Intelligent Food Tag: A Starch-Anthocyanin-Based pH-Sensitive Electrospun Nanofiber Mat for Real-Time Food Freshness Monitoring. Int. J. Biol. Macromol. 2024, 256, 128384. [Google Scholar] [CrossRef] [PubMed]
- Brandão, R.M.; Batista, L.R.; de Oliveira, J.E.; Barbosa, R.B.; Nelson, D.L.; Cardoso, M.G. In Vitro and in Vivo Efficacy of Poly (Lactic Acid) Nanofiber Packaging Containing Essential Oils from Ocimum Basilicum L. and Ocimum Gratissimum L. against Aspergillus Carbonarius and Aspergillus Niger in Table Grapes. Food Chem. 2023, 400, 134087. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lv, H.; Wang, C.; He, D.; Xu, E.; Jin, Z.; Yuan, C.; Guo, L.; Wu, Z.; Liu, P. Organic Solvent-Free Starch-Based Green Electrospun Nanofiber Mats for Curcumin Encapsulation and Delivery. Int. J. Biol. Macromol. 2023, 232, 123497. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.H.; Yun, J.S. Natural Cellulose-Based Multifunctional Nanofibers for the Effective Removal of Particulate Matter and Volatile Organic Compounds. Nanomaterials 2023, 13, 1720. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.C.; Soares, J.C.; Dos Santos, D.M.; Migliorini, F.L.; Popolin-Neto, M.; dos Santos Cinelli Pinto, D.; Carvalho, W.A.; Brandão, H.M.; Paulovich, F.V.; Correa, D.S. Nanoarchitectonic E-Tongue of Electrospun Zein/Curcumin Carbon Dots for Detecting Staphylococcus Aureus in Milk. ACS Omega 2023, 8, 13721–13732. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, C.; Hu, J.; Huang, M.; Zhao, L.; He, J.; Zhang, S.; Shen, F.; Tian, D. Cascade utilization of crop straw through a FeCl3-mediated deep eutectic solvent biorefinery: Lignin-containing cellulose nanofibers flocculant fabrication followed by fertilizer production. Chem. Eng. J. 2023, 472, 144823. [Google Scholar] [CrossRef]
- Priya, E.; Jha, A.; Sarkar, S.; Maji, P.K. A Urea-Loaded Hydrogel Comprising of Cellulose Nanofibers and Carboxymethyl Cellulose: An Effective Slow-Release Fertilizer. J. Clean. Prod. 2023, 434, 140215. [Google Scholar]
- Wagh, R.V.; Khan, A.; Priyadarshi, R.; Ezati, P.; Rhim, J.-W. Cellulose Nanofiber-Based Multifunctional Films Integrated with Carbon Dots and Anthocyanins from Brassica Oleracea for Active and Intelligent Food Packaging Applications. Int. J. Biol. Macromol. 2023, 233, 123567. [Google Scholar] [CrossRef] [PubMed]
- Stie, M.B.; Öblom, H.; Hansen, A.C.N.; Jacobsen, J.; Chronakis, I.S.; Rantanen, J.; Nielsen, H.M.; Genina, N. Mucoadhesive Chitosan- and Cellulose Derivative-Based Nanofiber-on-Foam-on-Film System for Non-Invasive Peptide Delivery. Carbohydr. Polym. 2023, 303, 120429. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.A.; Adeel, M.; Shakoor, N.; Ali, I.; Ishfaq, M.; Haider, F.U.; Deng, X. Unraveling the Roles of Modified Nanomaterials in Nano Enabled Agriculture. Plant Physiol. Biochem. 2023, 202, 107944. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Dutt, S.; Sharma, P.; Sundramoorthy, A.K.; Dubey, A.; Singh, A.; Arya, S. Future of Nanotechnology in Food Industry: Challenges in Processing, Packaging, and Food Safety. Glob. Chall. 2023, 7, 2200209. [Google Scholar] [CrossRef] [PubMed]
- Shelar, A.; Nile, S.H.; Singh, A.V.; Rothenstein, D.; Bill, J.; Xiao, J.; Chaskar, M.; Kai, G.; Patil, R. Recent Advances in Nano-Enabled Seed Treatment Strategies for Sustainable Agriculture: Challenges, Risk Assessment, and Future Perspectives. Nano-Micro Lett. 2023, 15, 54. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Duan, L.; Li, X.; Jiang, C.; Zhang, T.; Chen, W. Citrate-Promoted Dissolution of Nanostructured Manganese Oxides: Implications for Nano-Enabled Sustainable Agriculture. J. Environ. Sci. 2023, 125, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Pagano, L.; Rossi, R.; White, J.C.; Marmiroli, N.; Marmiroli, M. Nanomaterials Biotransformation: In Planta Mechanisms of Action. Environ. Pollut. 2023, 318, 120834. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, Y.; Zhang, X.; Liu, J.; Gong, P.; Su, Z.; Fan, L.; Li, G. Emerging Nanoparticles in Food: Sources, Application, and Safety. J. Agric. Food Chem. 2023, 71, 3564–3582. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Bielicka, M.; Klekotka, U.; Kalska-Szostko, B. Nanoparticle Applications in Food—A Review. Food Funct. 2023, 14, 2544–2567. [Google Scholar] [CrossRef] [PubMed]
- Jahani, R.; Behnamian, M.; Dezhsetan, S.; Karimirad, R.; Chamani, E. Chitosan Nano-Biopolymer/Citrus Paradisi Peel Oil Delivery System Enhanced Shelf-Life and Postharvest Quality of Cherry Tomato. Int. J. Biol. Macromol. 2023, 225, 1212–1223. [Google Scholar] [CrossRef]
- Cheng, J.; Wang, H. Construction and Application of Nano ZnO/Eugenol@yam Starch/Microcrystalline Cellulose Active Antibacterial Film. Int. J. Biol. Macromol. 2023, 239, 124215. [Google Scholar] [CrossRef]
- Liao, J.; Zhou, Y.; Hou, B.; Zhang, J.; Huang, H. Nano-Chitin: Preparation Strategies and Food Biopolymer Film Reinforcement and Applications. Carbohydr. Polym. 2023, 305, 120553. [Google Scholar] [CrossRef]
- Verstegen, J.; Günther, K. Ubiquitous Occurrence of Nano Selenium in Food Plants. Foods 2023, 12, 3203. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, N.A.; McCann, R.; Kakavas, D.; Rochfort, K.D.; Sreenilayam, S.P.; Alkan, G.; Stornetta, T.; McGivern, A.R.; Grintzalis, K.; Friedrich, B. Production of Silver Nano-Inks and Surface Coatings for Anti-Microbial Food Packaging and Its Ecological Impact. Int. J. Mol. Sci. 2023, 24, 5341. [Google Scholar] [CrossRef] [PubMed]
- Rathee, S.; Ojha, A.; Upadhyay, A.; Xiao, J.; Bajpai, V.K.; Ali, S.; Shukla, S. Biogenic Engineered Nanomaterials for Enhancing Bioavailability via Developing Nano-Iron-Fortified Smart Foods: Advances, Insight, and Prospects of Nanobionics in Fortification of Food. Food Funct. 2023, 14, 9083–9099. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, J.; Zeng, Y.; Liu, X.; Chen, M.; Dai, J.; Li, S.; Qin, W.; Liu, Y. Nanofibrous Composite Membranes Based on Chitosan-Nano Zinc Oxide and Curcumin for Kyoho Grapes Preservation. Int. J. Biol. Macromol. 2023, 242, 124661. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Jia, X.; Zhao, Z.; Ran, Y.; Du, M.; Ji, H.; Pan, Y.; Li, Z.; Ma, X.; Liu, Y. Innovative Natural Antimicrobial Natamycin Incorporated Titanium Dioxide (Nano-TiO2)/Poly (Butylene Adipate-Co-Terephthalate)(PBAT)/Poly (Lactic Acid)(PLA) Biodegradable Active Film (NTP@PLA) and Application in Grape Preservation. Food Chem. 2023, 400, 134100. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Yang, J.; Miao, J.; Jia, R.; Qin, Z. Production of the Protein-Based Nitrogen-Doped Carbon Quantum Dots/TiO2 Nanoparticles with Rapid and Efficient Photocatalytic Degradation of Hexavalent Chromium. J. Photochem. Photobiol. Chem. 2023, 444, 114947. [Google Scholar] [CrossRef]
| Source/Produced Nanoparticles | Mushroom Species | Nano Size (nm) | Application | Ref. |
|---|---|---|---|---|
| Water-soluble β-(1→3)-d-glucan | Sparassis crispa; Phellinus linteus | 150–390 | Food, cosmetic, and pharmaceutical industries | [142] |
| β-glucans | Lentinula edodes | 10–25 | Cancer treatment and therapeutic interventions | [139] |
| β-glucans | Pleurotus ostreatus | 40–50 | Cancer treatment and therapeutic interventions | [139] |
| Chitosan nanoparticles | Pleurotus eryngii | 2.25 | Antimicrobial coatings, drug delivery systems, wound dressings, food additives | [143] |
| Chitin nanocrystals | Lentinula edodes | 142–182 | Biomedical applications, drug encapsulation, polymer composite, cosmetics, textile industry | [144] |
| Carbon nanodots | Pleurotus spp. | 4.7–8.8 | Cancer treatment and therapeutic interventions, antimicrobial coatings, drug delivery systems, wound dressings, food additives | [145] |
| Carbon nanodots | Pleurotus spp. | 2–10 | Ultrasensitive detection of Hg2+ ions and photoinduced bactericidal activity | [146] |
| Copper oxide nanoparticles | Pleurotus citrinopileatus | 20 | Biomedical applications (mainly antimicrobial and anticancer) | [147] |
| Silver nanoparticles | Ganoderma spp. | ------ | Antibacterial and therapeutic agents | [148] |
| Zinc oxide nanoparticles | Cordyceps militaris | 1.83 | Therapeutic investigations as antioxidant, antidiabetic, and antibacterial potential | [149] |
| Crop | Applied Dose of Nano-Se | Farming Practices | Main Impacts of Nano-Se | Refs. |
|---|---|---|---|---|
| Sugarcane (Saccharum spp. hybrids) | Foliar application of 5 and 10 mg L−1 | Growing seedlings under biotic stress | Nano-Se enhanced antioxidants and jasmonic acid content; reduced accumulation of ROS and H2O2 under biotic stress as an eco-fungicide. | [202] |
| Lemon verbena (Lippia citriodora Kunth) | Foliar application of 10 uM nano-Se | Before full flowering stage | Nano-Se alleviated salt stress by improving secondary metabolites (protein, proline, and soluble sugars) and antioxidants. | [203] |
| Red Pitaya (Hylocereus undatus) | Foliar at 5 mg L−1 and soil at 3 mg L−1 | Biofortification of fruits and post-harvest | Nano-Se enhanced antioxidant capacity and nutritional value by boosting biosynthesis of amino acids, phenylpropanoid, and betalain. | [204] |
| Faba bean (Vicia faba L.) | Foliar application at 100 mg L−1 | Biofortification of bean seeds | Nano-Se improved the seed weight, yield and quality, and biofortification level. | [205] |
| Common Bean (Phaseolus vulgaris L.) | Applied 50 and 100 ppm nano-Se and nano-Si | Control Alternaria leaf spot disease | Applied nano-Se and nano-Si was an effective alternative to fungicide against the studied phytopathogen. | [53] |
| Caralluma tuberculata | In vitro containing 100 µg L−1 nano-Se | Producing secondary metabolites | Se-NPs elicited the production of antidiabetic metabolites (gallic acid, cumarin, ferulic acid, caffeic acid, catechin, quercetin and rutin). | [206] |
| Rice (Oryza sativa L.) | Applied nano-Se to soil at 0.1 mg·kg−1 | Rice seedling growth | Nano-Se enhanced root exudates and rhizobacteria, which promoted rice growth by increasing malic and citric acid content. | [207] |
| Rice (Oryza sativa L.) | Se bio-nanocomposite | Alleviating cadmium toxicity | Applied nanocomposite alleviated the inhibition of plant growth and Cd-oxidative stress by reducing Cd accumulation in rice plants. | [208] |
| Lettuce (Lactuca sativa L.) | Applied Se-NPs to the soil at 50 mg kg−1 | Enhancing plant disease resistance | Se-NPs suppressed Fusarium-induced wilt disease in lettuce by modulating the shoot metabolite levels of citrate, succinate, malate and upregulated jasmonic acid. | [209] |
| Strawberry (Fragaria × ananassa) | Nano-Se at 25, 50, 75, and 100 mg L−1 | Seedling production | Se-NPs enhanced growth of seedlings by promoting nutritional status, photosynthetic pigments, and enzymatic antioxidants. | [28] |
| Type of C Nanodot | Farming Practices | Main Impacts | Refs. |
|---|---|---|---|
| Multi-walled carbon nano-tubes (MCNs) | Seedling development | An application of 800 mg·L−1 MCN promoted maize root length, height, seedling dry weight, and photosynthesis enzymes related to nitrogen metabolism in maize seedlings. | [238] |
| Nano-carbon composites | Nano-pesticide against fungus | Applied nanocomposite of C-TiO2 inhibited the fungus Phytophthora palmivora, serving as a disinfectant for agricultural plant pathogens. | [239] |
| Carbon nanomaterials (CNMs) | Amendment of sandy soils | CNMs applied at 200–400 mg kg−1 increased shoot biomass of lettuce, total chlorophyll content, photosynthesis activity, and bioavailability of soil nutrients. | [229] |
| Carbon nanoparticles (CNPs) | Soil fertility improvement | CNPs applied at 200 mg kg−1 enhanced maize growth by improving nutrient use efficiency, plant height, the uptake of nutrients, and biomass yield. | [240] |
| Carbon nano-tubes | Magnetic nano-sorption pesticides | Using magnetic nanocomposite cellulose for sorption agro-pesticide samples due to high porosity, high surface area and good reusability up to 15 times for the extraction of pesticides. | [241] |
| Carbon dots (CDs) | Seed nano-priming | CDs applied at 0.25–2 mg mL−1 accelerated germination of pea seeds, increased biomass accumulation and elongation of shoots and roots compared to the control. | [242] |
| Nitrogen -doped carbon dots (N-CDs) | Seedlings growth under salt stress (150 mM NaCl) | N-CDs enhanced Arabidopsis salt stress tolerance and induced plant growth, chlorophyll content, and reduced malondialdehyde content compared to the control. | [243] |
| Carbon quantum dots (CQDs) | Protecting agent against Cd-stress | Putrescine-functionalized-CQD-NPs increased the fresh and dry leaf weight of grapes and mediated Cd-stress by promoting enzymatic activity, anthocyanin. and phenolics. | [244] |
| Carbon nanodots | Food preservation | Carbon nano-dot/silk fibroin films were antibacterial and antioxidative films that increased fruit preservation as a multifunctional and eco-friendly packaging system. | [245] |
| MnO2 nano-sheets and carbon dot (MnO2-CD) | Nano-biosensor for food safety | The MnO2-CD was an efficient nano-biosensor for Staphylococcus aureus having higher stability, good biocompatibility, and catalytic activity compared to natural enzymes. | [246] |
| Nanofibers (NFs) | Farming Practices | Main Impacts of NFs | Refs. |
|---|---|---|---|
| Natural multifunctional nanofibers from cellulose | Multi-functional air filtration | The NFs created an eco-friendly filter to remove indoor pollutants like volatile organic compounds and particulate matter. | [262] |
| Zein nanofibers coated by carbon dots | Dairy farming | NFs were able to detect the pathogen Staphylococcus aureus, which causes mammary infections in dairy cows. | [263] |
| Cellulose nanofibers based on lignin | Farmland drainage | This nanofiber can utilize crop straw through an FeCl3-mediated deep eutectic solvent biorefinery and lignin-containing cellulose nanofibers flocculant fabrication followed by P-fertilizer production. | [264] |
| Cellulose and carboxy-methyl cellulose nanofibers | Slow-release fertilization | Urea-loaded hydrogel applied via nanofibers enhanced seed germination and plant growth through effective and sustainable transport of fertilizer and water. | [265] |
| Cellulose nanofiber with anthocyanins and carbon nanodots | Applications in food packaging | NFs extended the shelf life of packaged perishable food. | [266] |
| Poly-lactic acid nanofibers | Active food packaging | This NF has strong antifungal activity that suppressed the proliferation of microbes in the preserved grapes and improved their quality. | [260] |
| Citrus insoluble nanofiber | Producing fat replacers | A composite NF gel showed potential as a fat replacer and for inhibiting lipid digestion. | [264] |
| Green nanofiber | Delivery of active foods | Curcumin-loaded starch-based fast-dissolving NF showed promise in the pharmaceutical and food fields. | [261] |
| Chitosan-based nanofibers | Delivery of drugs | Cellulose and chitosan derivative-based NFs showed promise as biocompatible drug delivery systems. | [267] |
| Starch-based nanofibers | Monitoring of food freshness | NFs can reflect food freshness as a degradable, non-toxic, and smart food label with pH-sensitive nanofiber mats. | [259] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prokisch, J.; Törős, G.; Nguyen, D.H.H.; Neji, C.; Ferroudj, A.; Sári, D.; Muthu, A.; Brevik, E.C.; El-Ramady, H. Nano-Food Farming: Toward Sustainable Applications of Proteins, Mushrooms, Nano-Nutrients, and Nanofibers. Agronomy 2024, 14, 606. https://doi.org/10.3390/agronomy14030606
Prokisch J, Törős G, Nguyen DHH, Neji C, Ferroudj A, Sári D, Muthu A, Brevik EC, El-Ramady H. Nano-Food Farming: Toward Sustainable Applications of Proteins, Mushrooms, Nano-Nutrients, and Nanofibers. Agronomy. 2024; 14(3):606. https://doi.org/10.3390/agronomy14030606
Chicago/Turabian StyleProkisch, József, Greta Törős, Duyen H. H. Nguyen, Chaima Neji, Aya Ferroudj, Daniella Sári, Arjun Muthu, Eric C. Brevik, and Hassan El-Ramady. 2024. "Nano-Food Farming: Toward Sustainable Applications of Proteins, Mushrooms, Nano-Nutrients, and Nanofibers" Agronomy 14, no. 3: 606. https://doi.org/10.3390/agronomy14030606
APA StyleProkisch, J., Törős, G., Nguyen, D. H. H., Neji, C., Ferroudj, A., Sári, D., Muthu, A., Brevik, E. C., & El-Ramady, H. (2024). Nano-Food Farming: Toward Sustainable Applications of Proteins, Mushrooms, Nano-Nutrients, and Nanofibers. Agronomy, 14(3), 606. https://doi.org/10.3390/agronomy14030606











