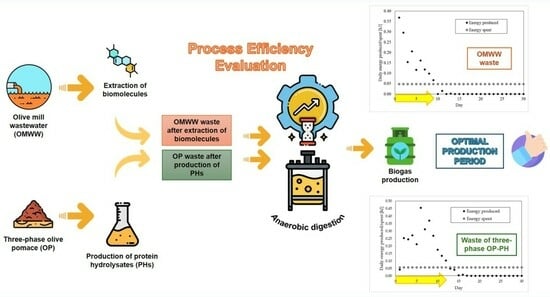
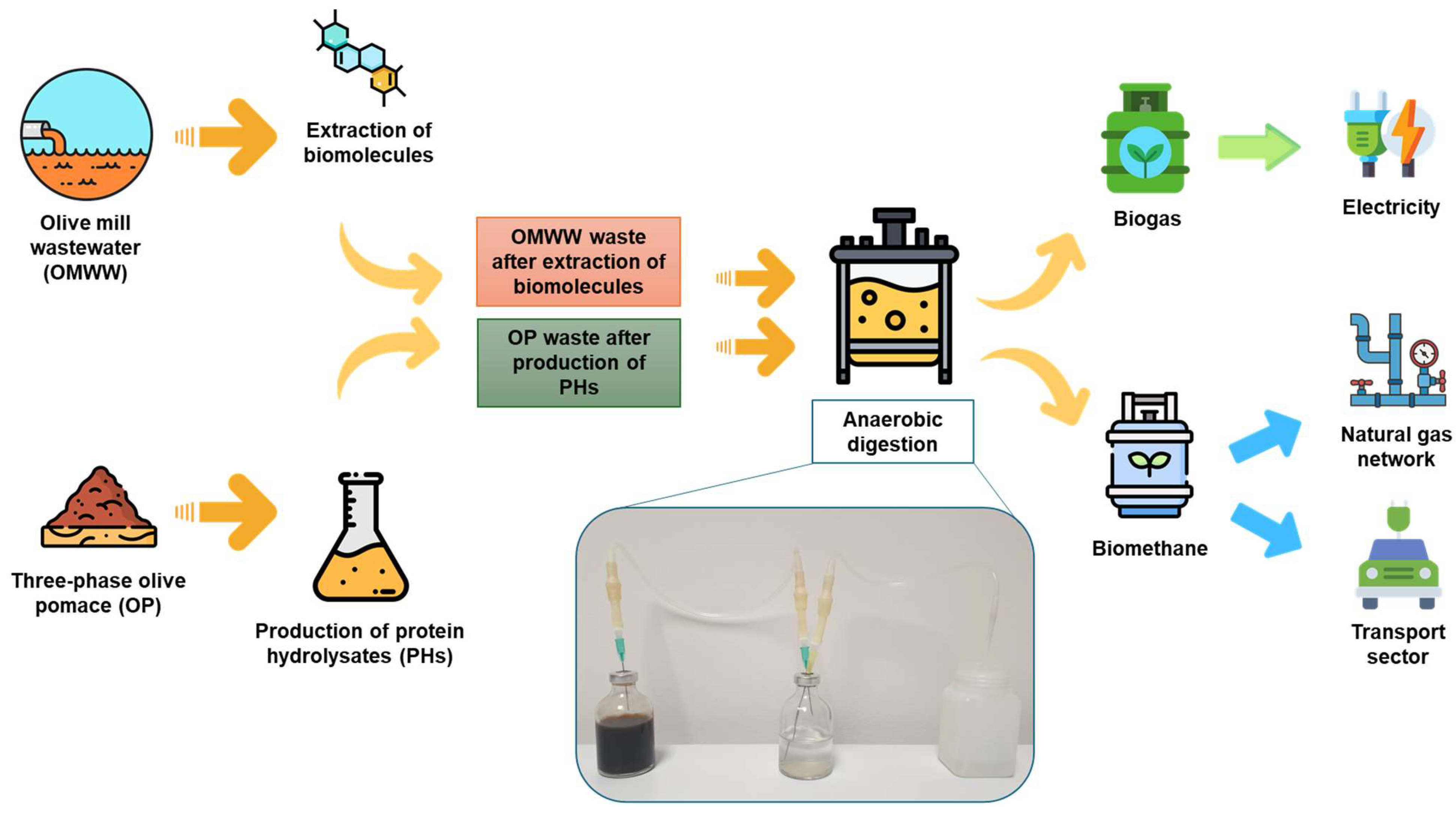
Biogas Production with Residuals Deriving from Olive Mill Wastewater and Olive Pomace Wastes: Quantification of Produced Energy, Spent Energy, and Process Efficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Determination of Moisture Content
2.3. Determination of Volatile Solids
2.4. Anaerobic Bioreactors
2.5. Statistical Analysis
3. Results and Discussion
- Sample 1 (sole inoculation): 47.52%;
- Sample 2 (inoculation + ¼ swine slurry): 53.23%;
- Sample 3 (inoculation + ¼ untreated three-phase OP): 66.30%;
- Sample 4 (inoculation + ¼ three-phase OP-PH waste): 58.44%;
- Sample 5 (inoculation + ¼ OMWW waste): 56.99%.
- (1)
- Energy crop cultivation and feedstock pre-treatment;
- (2)
- Feedstock collection and transportation;
- (3)
- Biogas plant operation processes;
- (4)
- Biogas treatment and storage;
- (5)
- Digestate processing and handling.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cesaretti, A.; Montegiove, N.; Calzoni, E.; Leonardi, L.; Emiliani, C. Protein Hydrolysates: From Agricultural Waste Biomasses to High Added-Value Products (minireview). AgroLife Sci. J. 2020, 9, 79–87. [Google Scholar]
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions Closing the Loop—An EU Action Plan for the Circular Economy; European Commission: Brussels, Belgium, 2015. [Google Scholar]
- FAO. The State of Food and Agriculture: Moving Forward on Food Loss and Waste Reduction, the State of Food and Agriculture (SOFA); FAO: Rome, Italy, 2019. [Google Scholar] [CrossRef]
- Stahel, W.R. The circular economy. Nature 2016, 531, 435–438. [Google Scholar] [CrossRef]
- Tuck, C.O.; Pérez, E.; Horváth, I.T.; Sheldon, R.A.; Poliakoff, M. Valorization of Biomass: Deriving More Value from Waste. Science 2012, 337, 695–699. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Tacchi, S.; Caponi, S.; Pellegrino, R.M.; Luzi, F.; Cottone, F.; Fioretto, D.; Emiliani, C.; Di Michele, A. Covalent Immobilization of Proteases on Polylactic Acid for Proteins Hydrolysis and Waste Biomass Protein Content Valorization. Catalysts 2021, 11, 167. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- Martínez-Alvarez, O.; Chamorro, S.; Brenes, A. Protein hydrolysates from animal processing by-products as a source of bioactive molecules with interest in animal feeding: A review. Food Res. Int. 2015, 73, 204–212. [Google Scholar] [CrossRef]
- Puglia, D.; Pezzolla, D.; Gigliotti, G.; Torre, L.; Bartucca, M.L.; Del Buono, D. The Opportunity of Valorizing Agricultural Waste, Through Its Conversion into Biostimulants, Biofertilizers, and Biopolymers. Sustainability 2021, 13, 2710. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Montegiove, N.; Pellegrino, R.M.; Leonardi, L.; Emiliani, C. Protein and Amino Acid Profile Analysis of Agri-Food Waste Biomasses. Sci. Bull. Ser. F Biotechnol. 2021, 25, 19–25. [Google Scholar]
- Etemadian, Y.; Ghaemi, V.; Shaviklo, A.R.; Pourashouri, P.; Sadeghi Mahoonak, A.R.; Rafipour, F. Development of animal/ plant-based protein hydrolysate and its application in food, feed and nutraceutical industries: State of the art. J. Clean. Prod. 2021, 278, 123219. [Google Scholar] [CrossRef]
- Kiewiet, M.B.G.; Faas, M.M.; De Vos, P. Immunomodulatory Protein Hydrolysates and Their Application. Nutrients 2018, 10, 904. [Google Scholar] [CrossRef] [PubMed]
- Norzagaray-Valenzuela, C.D.; Valdez-Ortiz, A.; Shelton, L.M.; Jiménez-Edeza, M.; Rivera-López, J.; Valdez-Flores, M.A.; Germán-Báez, L.J. Residual biomasses and protein hydrolysates of three green microalgae species exhibit antioxidant and anti-aging activity. J. Appl. Phycol. 2017, 29, 189–198. [Google Scholar] [CrossRef]
- Forster-Carneiro, T.; Berni, M.D.; Dorileo, I.L.; Rostagno, M.A. Biorefinery study of availability of agriculture residues and wastes for integrated biorefineries in Brazil. Resour. Conserv. Recycl. 2013, 77, 78–88. [Google Scholar] [CrossRef]
- Salisu, J.; Gao, N.; Quan, C. Techno-economic Assessment of Co-gasification of Rice Husk and Plastic Waste as an Off-grid Power Source for Small Scale Rice Milling—An Aspen Plus Model. J. Anal. Appl. Pyrolysis 2021, 158, 105157. [Google Scholar] [CrossRef]
- Dermeche, S.; Nadour, M.; Larroche, C.; Moulti-Mati, F.; Michaud, P. Olive mill wastes: Biochemical characterizations and valorization strategies. Process Biochem. 2013, 48, 1532–1552. [Google Scholar] [CrossRef]
- Espadas-Aldana, G.; Vialle, C.; Belaud, J.-P.; Vaca-Garcia, C.; Sablayrolles, C. Analysis and trends for Life Cycle Assessment of olive oil production. Sustain. Prod. Consum. 2019, 19, 216–230. [Google Scholar] [CrossRef]
- Behera, B.; Venkata Supraja, K.; Paramasivan, B. Integrated microalgal biorefinery for the production and application of biostimulants in circular bioeconomy. Bioresour. Technol. 2021, 339, 125588. [Google Scholar] [CrossRef]
- Gigliotti, G.; Proietti, P.; Said-Pullicino, D.; Nasini, L.; Pezzolla, D.; Rosati, L.; Porceddu, P.R. Co-composting of olive husks with high moisture contents: Organic matter dynamics and compost quality. Int. Biodeterior. Biodegrad. 2012, 67, 8–14. [Google Scholar] [CrossRef]
- Otero, P.; Garcia-Oliveira, P.; Carpena, M.; Barral-Martinez, M.; Chamorro, F.; Echave, J.; Garcia-Perez, P.; Cao, H.; Xiao, J.; Simal-Gandara, J.; et al. Applications of by-products from the olive oil processing: Revalorization strategies based on target molecules and green extraction technologies. Trends Food Sci. Technol. 2021, 116, 1084–1104. [Google Scholar] [CrossRef]
- Çelekli, A.; Gün, D.; Bozkurt, H. Bleaching of olive pomace oil with Spirulina platensis as an eco-friendly process. Algal Res. 2021, 54, 102210. [Google Scholar] [CrossRef]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of by-products from olive oil industry and added-value applications for innovative functional foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Laca, A.; Laca, A.; Díaz, M. Value-Added Products from Fruit and Vegetable Wastes: A Review. CLEAN—Soil Air Water 2021, 49, 2000376. [Google Scholar] [CrossRef]
- Torrecilla, J.S. Chapter 40-Phenolic Compounds in Olive Oil Mill Wastewater. In Olives and Olive Oil in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Eds.; Academic Press: San Diego, CA, USA, 2010; pp. 357–365. [Google Scholar] [CrossRef]
- Torrecilla, J.S. The Olive: Its Processing and Waste Management; Nova Science Publishers: Hauppauge, NY, USA, 2010. [Google Scholar]
- Galanakis, C.M.; Kotsiou, K. Chapter 10-Recovery of bioactive compounds from olive mill waste. In Olive Mill Waste; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 205–229. [Google Scholar] [CrossRef]
- Mateos, R.; Sarria, B.; Bravo, L. Nutritional and other health properties of olive pomace oil. Crit. Rev. Food Sci. Nutr. 2020, 60, 3506–3521. [Google Scholar] [CrossRef]
- Rodrigues, F.; Pimentel, F.B.; Oliveira, M.B.P.P. Olive by-products: Challenge application in cosmetic industry. Ind. Crops Prod. 2015, 70, 116–124. [Google Scholar] [CrossRef]
- Ruiz, E.; Romero-García, J.M.; Romero, I.; Manzanares, P.; Negro, M.J.; Castro, E. Olive-derived biomass as a source of energy and chemicals. Biofuels Bioprod. Biorefin. 2017, 11, 1077–1094. [Google Scholar] [CrossRef]
- Di Lecce, G.; Cassano, A.; Bendini, A.; Conidi, C.; Giorno, L.; Toschi, T.G. Characterization of olive mill wastewater fractions treatment by integrated membrane process. J. Sci. Food Agric. 2014, 94, 2935–2942. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, A.; Gentili, A.; Isidori, M.; Monaco, P.; Nardelli, A.; Parrella, A.; Temussi, F. Environmental Effects Caused by Olive Mill Wastewaters: Toxicity Comparison of Low-Molecular-Weight Phenol Components. J. Agric. Food Chem. 2003, 51, 1005–1009. [Google Scholar] [CrossRef] [PubMed]
- Justino, C.I.L.; Pereira, R.; Freitas, A.C.; Rocha-Santos, T.A.P.; Panteleitchouk, T.S.L.; Duarte, A.C. Olive oil mill wastewaters before and after treatment: A critical review from the ecotoxicological point of view. Ecotoxicology 2012, 21, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Cucina, M.; Pezzolla, D.; Tacconi, C.; Gigliotti, G. Anaerobic co-digestion of a lignocellulosic residue with different organic wastes: Relationship between biomethane yield, soluble organic matter and process stability. Biomass Bioenergy 2021, 153, 106209. [Google Scholar] [CrossRef]
- Di Maria, F.; Gigliotti, G.; Sordi, A.; Micale, C.; Zadra, C.; Massaccesi, L. Hybrid solid anaerobic digestion batch: Biomethane production and mass recovery from the organic fraction of solid waste. Waste. Manag. Res. 2013, 31, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Yun, S.; Li, B.; Wang, K.; Chen, J.; Jia, B.; Ke, T.; An, J. Coconut-shell-derived bio-based carbon enhanced microbial electrolysis cells for upgrading anaerobic co-digestion of cow manure and aloe peel waste. Bioresour. Technol. 2021, 338, 125520. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Shah, G.; Sahota, S.; Singh, L.; Vijay, V.K. Chapter 7-Biogas production from waste: Technical overview, progress, and challenges. In Bioreactors; Singh, L., Yousuf, A., Mahapatra, D.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 89–104. [Google Scholar] [CrossRef]
- Nwokolo, N.; Mukumba, P.; Obileke, K.; Enebe, M. Waste to Energy: A Focus on the Impact of Substrate Type in Biogas Production. Processes 2020, 8, 1224. [Google Scholar] [CrossRef]
- Li, B.; Yun, S.; Xing, T.; Wang, K.; Ke, T.; An, J. A strategy for understanding the enhanced anaerobic co-digestion via dual-heteroatom doped bio-based carbon and its functional groups. Chem. Eng. J. 2021, 425, 130473. [Google Scholar] [CrossRef]
- Fleyfel, L.M.; Leitner, N.K.V.; Deborde, M.; Matta, J.; El Najjar, N.H. Olive oil liquid wastes–characteristics and treatments: A literature review. Process Saf. Environ. Protection. 2022, 168, 1031–1048. [Google Scholar] [CrossRef]
- Tekin, A.R.; Dalgıç, A.C. Biogas production from olive pomace. Resour. Conserv. Recycl. 2000, 30, 301–313. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Dunbabin, J.S.; Bowmer, K.H. Potential use of constructed wetlands for treatment of industrial wastewaters containing metals. Sci. Total Environ. 1992, 111, 151–168. [Google Scholar] [CrossRef]
- Paritosh, K.; Yadav, M.; Mathur, S.; Balan, V.; Liao, W.; Pareek, N.; Vivekanand, V. Organic fraction of municipal solid waste: Overview of treatment methodologies to enhance anaerobic biodegradability. Front. Energy Res. 2018, 6, 75. [Google Scholar] [CrossRef]
- Amani, T.; Nosrati, M.; Mousavi, S.M.; Kermanshahi, R.K. Study of syntrophic anaerobic digestion of volatile fatty acids using enriched cultures at mesophilic conditions. Int. J. Environ. Sci. Technol. 2011, 8, 83–96. [Google Scholar] [CrossRef]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Ullah Khan, I.; Hafiz Dzarfan Othman, M.; Hashim, H.; Matsuura, T.; Ismail, A.F.; Rezaei-DashtArzhandi, M.; Wan Azelee, I. Biogas as a renewable energy fuel—A review of biogas upgrading, utilisation and storage. Energy Convers. Manag. 2017, 150, 277–294. [Google Scholar] [CrossRef]
- Cao, X.; Bian, J. Supersonic separation technology for natural gas processing: A review. Chem. Eng. Process.-Process Intensif. 2019, 136, 138–151. [Google Scholar] [CrossRef]
- Fajrina, N.; Yusof, N.; Ismail, A.F.; Aziz, F.; Bilad, M.R.; Alkahtani, M. A crucial review on the challenges and recent gas membrane development for biogas upgrading. J. Environ. Chem. Eng. 2023, 11, 110235. [Google Scholar] [CrossRef]
- Pöschl, M.; Ward, S.; Owende, P. Evaluation of energy efficiency of various biogas production and utilization pathways. Appl. Energy 2010, 87, 3305–3321. [Google Scholar] [CrossRef]
- Berglund, M.; Börjesson, P. Assessment of energy performance in the life-cycle of biogas production. Biomass Bioenergy 2006, 30, 254–266. [Google Scholar] [CrossRef]
- Gkotsis, P.; Kougias, P.; Mitrakas, M.; Zouboulis, A. Biogas upgrading technologies–Recent advances in membrane-based processes. Int. J. Hydrogen Energy 2023, 48, 3965–3993. [Google Scholar] [CrossRef]
- Prade, T.; Svensson, S.-E.; Mattsson, J.E. Energy balances for biogas and solid biofuel production from industrial hemp. Biomass Bioenergy 2012, 40, 36–52. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Chapter 41-Sustainability of food industry wastes: A microbial approach. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 829–854. [Google Scholar] [CrossRef]
- Skaltsounis, A.-L.; Argyropoulou, A.; Aligiannis, N.; Xynos, N. 11-Recovery of High Added Value Compounds from Olive Tree Products and Olive Processing Byproducts. In Olive and Olive Oil Bioactive Constituents; Boskou, D., Ed.; AOCS Press: Urbana, IL, USA, 2015; pp. 333–356. [Google Scholar] [CrossRef]


| Parameter | Inoculum |
|---|---|
| Moisture (%) | 93.3 |
| pH | 8.76 |
| TOC (% on DM) | 56.2 |
| TKN (% on DM) | 5.8 |
| Total P (g/kg of DM) | 3.30 |
| Total K (g/kg of DM) | 80.11 |
| WEOC (g/kg of DM) | 117.06 |
| WEN (g/kg of DM) | 71.89 |
| Day n° | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 |
|---|---|---|---|---|---|
| 1 | 0 | 2.5 | 7.2 | 2.2 | 19.3 |
| 2 | 0.2 | 6.8 | 8.2 | 15.4 | 34.8 |
| 3 | 1.3 | 9.5 | 14.6 | 28.3 | 42.9 |
| 4 | 11.8 | 14.0 | 22.1 | 42.4 | 53.7 |
| 5 | 11.9 | 16.5 | 26.4 | 53.5 | 59.8 |
| 6 | 12.9 | 18.3 | 37.2 | 77.4 | 68.3 |
| 7 | 14.7 | 21.8 | 46.0 | 93.7 | 74.5 |
| 8 | 14.7 | 23.6 | 56.8 | 113.0 | 79.7 |
| 9 | 17.2 | 25.2 | 65.2 | 127.1 | 83.2 |
| 10 | 21.5 | 31.5 | 71.3 | 136.2 | 83.2 |
| 11 | 27.0 | 36.0 | 76.8 | 143.2 | 84.0 |
| 12 | 33.9 | 39.2 | 80.0 | 148.6 | 84.0 |
| 13 | 38.6 | 43.7 | 80.0 | 151.1 | 84.1 |
| 14 | 43.1 | 46.2 | 80.0 | 153.2 | 84.1 |
| 15 | 47.6 | 51.0 | 80.0 | 153.4 | 84.1 |
| 16 | 48.8 | 54.2 | 80.0 | 153.8 | 84.1 |
| 17 | 51.7 | 58.0 | 80.0 | 153.9 | 84.1 |
| 18 | 53.5 | 60.1 | 80.0 | 153.9 | 84.1 |
| 19 | 53.9 | 60.6 | 80.0 | 153.9 | 84.1 |
| 20 | 55.1 | 62.1 | 80.0 | 153.9 | 84.1 |
| 21 | 55.1 | 62.5 | 80.0 | 153.9 | 84.1 |
| 22 | 55.8 | 62.5 | 80.0 | 153.9 | 84.1 |
| 23 | 56.1 | 62.5 | 80.0 | 153.9 | 84.1 |
| 24 | 56.5 | 62.5 | 80.0 | 153.9 | 84.1 |
| 25 | 56.5 | 62.5 | 80.0 | 153.9 | 84.1 |
| 26 | 56.5 | 62.5 | 80.0 | 153.9 | 84.1 |
| 27 | 56.5 | 62.5 | 80.0 | 153.9 | 84.1 |
| 28 | 56.5 | 62.5 | 80.0 | 153.9 | 84.1 |
| 29 | 56.5 | 62.5 | 80.0 | 153.9 | 84.1 |
| 30 | 56.5 | 62.5 | 80.0 | 153.9 | 84.1 |
| Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day Num° | Biogas [kg] | Biomethane [kg] | Energy [kJ] | Biogas [kg] | Biomethane [kg] | Energy [kJ] | Biogas [kg] | Biomethane [kg] | Energy [kJ] | Biogas [kg] | Biomethane [kg] | Energy [kJ] | Biogas [kg] | Biomethane [kg] | Energy [kJ] |
| 1 | 0 | 0 | 0 | 9.62 × 105 | 1.54 × 10−6 | 8.19 × 10−7 | 4.52 × 10−6 | 3.00 × 10−6 | 1.56 × 10−1 | 1.36 × 10−6 | 7.96 × 10−7 | 4.14 × 10−2 | 1.21 × 10−5 | 7.09 × 10−6 | 3.69 × 10−1 |
| 2 | 9.43 × 10−8 | 4.48 × 10−8 | 2.33 × 10−3 | 1.72 × 10−4 | 2.75 × 10−6 | 1.46 × 10−6 | 6.28 × 10−7 | 4.17 × 10−7 | 2.17 × 10−2 | 8.32 × 10−6 | 4.86 × 10−6 | 2.53 × 10−1 | 9.74 × 10−6 | 5.69 × 10−6 | 2.96 × 10−1 |
| 3 | 7.23 × 10−7 | 3.43 × 10−7 | 1.79 × 10−2 | 1.06 × 10−4 | 1.69 × 10−6 | 9.00 × 10−7 | 4.02 × 10−6 | 2.67 × 10−6 | 1.39 × 10−1 | 8.06 × 10−6 | 4.71 × 10−6 | 2.45 × 10−1 | 5.09 × 10−6 | 2.97 × 10−6 | 1.55 × 10−1 |
| 4 | 6.60 × 10−6 | 3.14 × 10−6 | 1.63 × 10−1 | 1.77 × 10−4 | 2.83 × 10−6 | 1.51 × 10−6 | 4.71 × 10−6 | 3.12 × 10−6 | 1.62 × 10−1 | 8.91 × 10−6 | 5.21 × 10−6 | 2.71 × 10−1 | 6.79 × 10−6 | 3.97 × 10−6 | 2.06 × 10−1 |
| 5 | 7.86 × 10−8 | 3.73 × 10−8 | 1.94 × 10−3 | 9.82 × 10−5 | 1.57 × 10−6 | 8.36 × 10−7 | 2.70 × 10−6 | 1.79 × 10−6 | 9.32 × 10−2 | 6.97 × 10−6 | 4.08 × 10−6 | 2.12 × 10−1 | 3.83 × 10−6 | 2.24 × 10−6 | 1.16 × 10−1 |
| 6 | 6.28 × 10−7 | 2.99 × 10−7 | 1.55 × 10−2 | 7.07 × 10−5 | 1.13 × 10−6 | 6.02 × 10−7 | 6.79 × 10−6 | 4.50 × 10−6 | 2.34 × 10−1 | 1.50 × 10−5 | 8.75 × 10−6 | 4.55 × 10−1 | 5.34 × 10−6 | 3.12 × 10−6 | 1.62 × 10−1 |
| 7 | 1.13 × 10−6 | 5.38 × 10−7 | 2.79 × 10−2 | 1.37 × 10−4 | 2.20 × 10−6 | 1.17 × 10−6 | 5.53 × 10−6 | 3.67 × 10−6 | 1.91 × 10−1 | 1.03 × 10−5 | 6.00 × 10−6 | 3.12 × 10−1 | 3.90 × 10−6 | 2.28 × 10−6 | 1.18 × 10−1 |
| 8 | 0 | 0 | 0 | 7.07 × 10−5 | 1.13 × 10−6 | 6.02 × 10−7 | 6.79 × 10−6 | 4.50 × 10−6 | 2.34 × 10−1 | 1.21 × 10−5 | 7.10 × 10−6 | 3.69 × 10−1 | 3.27 × 10−6 | 1.91 × 10−6 | 9.93 × 10−2 |
| 9 | 1.57 × 10−6 | 7.47 × 10−7 | 3.88 × 10−2 | 6.28 × 10−5 | 1.01 × 10−6 | 5.35 × 10−7 | 5.28 × 10−6 | 3.50 × 10−6 | 1.82 × 10−1 | 8.81 × 10−6 | 5.15 × 10−6 | 2.68 × 10−1 | 2.20 × 10−6 | 1.29 × 10−6 | 6.68 × 10−2 |
| 10 | 2.67 × 10−6 | 1.30 × 10−6 | 6.60 × 10−2 | 2.45 × 10−4 | 3.93 × 10−6 | 2.09 × 10−6 | 3.83 × 10−6 | 2.54 × 10−6 | 1.32 × 10−1 | 5.76 × 10−6 | 3.37 × 10−6 | 1.75 × 10−1 | 0 | 0 | 0 |
| 11 | 3.46 × 10−6 | 1.64 × 10−6 | 8.54 × 10−2 | 1.77 × 10−4 | 2.83 × 10−6 | 1.51 × 10−6 | 3.46 × 10−6 | 2.29 × 10−6 | 1.19 × 10−1 | 4.40 × 10−6 | 2.57 × 10−6 | 1.34 × 10−1 | 5.03 × 10−7 | 2.94 × 10−7 | 1.53 × 10−2 |
| 12 | 4.34 × 10−6 | 2.0 × 10−6 | 1.07 × 10−1 | 1.28 × 10−4 | 2.04 × 10−6 | 1.09 × 10−6 | 0 | 0 | 0 | 3.35 × 10−6 | 1.96 × 10−6 | 1.02 × 10−1 | 0 | 0 | 0 |
| 13 | 2.99 × 10−6 | 1.42 × 10−6 | 7.38 × 10−2 | 1.77 × 10−4 | 2.83 × 10−6 | 1.51 × 10−6 | 0 | 0 | 0 | 1.61 × 10−6 | 9.43 × 10−7 | 4.90 × 10−2 | 6.28 × 10−8 | 3.67 × 10−8 | 1.91 × 10−3 |
| 14 | 2.83 × 10−6 | 1.34 × 10−6 | 6.99 × 10−2 | 9.82 × 10−5 | 1.57 × 10−6 | 8.36 × 10−7 | 0 | 0 | 0 | 1.33 × 10−6 | 7.77 × 10−7 | 4.04 × 10−2 | 0 | 0 | 0 |
| 15 | 2.83 × 10−6 | 1.34 × 10−6 | 6.99 × 10−2 | 1.87 × 10−4 | 2.98 × 10−6 | 1.59 × 10−6 | 0 | 0 | 0 | 1.26 × 10−7 | 7.34 × 10−8 | 3.82 × 10−3 | 0 | 0 | 0 |
| 16 | 7.23 × 10−7 | 3.43 × 10−7 | 1.79 × 10−2 | 1.28 × 10−4 | 2.04 × 10−6 | 1.09 × 10−6 | 0 | 0 | 0 | 2.51 × 10−7 | 1.47 × 10−7 | 7.64 × 10−3 | 0 | 0 | 0 |
| 17 | 1.84 × 10−6 | 8.76 × 10−7 | 4.56 × 10−2 | 1.47 × 10−4 | 2.36 × 10−6 | 1.25 × 10−6 | 0 | 0 | 0 | 2.62 × 10−8 | 1.53 × 10−8 | 7.96 × 10−4 | 0 | 0 | 0 |
| 18 | 1.13 × 10−6 | 5.38 × 10−7 | 2.79 × 10−2 | 8.44 × 10−5 | 1.35 × 10−6 | 7.19 × 10−7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 19 | 2.14 × 10−7 | 1.02 × 10−7 | 5.28 × 10−3 | 1.77 × 10−5 | 2.83 × 10−7 | 1.51 × 10−7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 20 | 7.85 × 10−7 | 3.73 × 10−7 | 1.94 × 10−2 | 5.89 × 10−5 | 9.43 × 10−7 | 5.02 × 10−7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 21 | 0 | 0 | 0 | 1.77 × 10−5 | 2.83 × 10−7 | 1.51 × 10−7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 22 | 4.40 × 10−7 | 2.09 × 10−7 | 1.09 × 10−2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 23 | 1.57 × 10−7 | 7.47 × 10−8 | 3.88 × 10−3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 24 | 2.51 × 10−7 | 1.19 × 10−7 | 6.21 × 10−3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 26 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 27 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 28 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 29 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
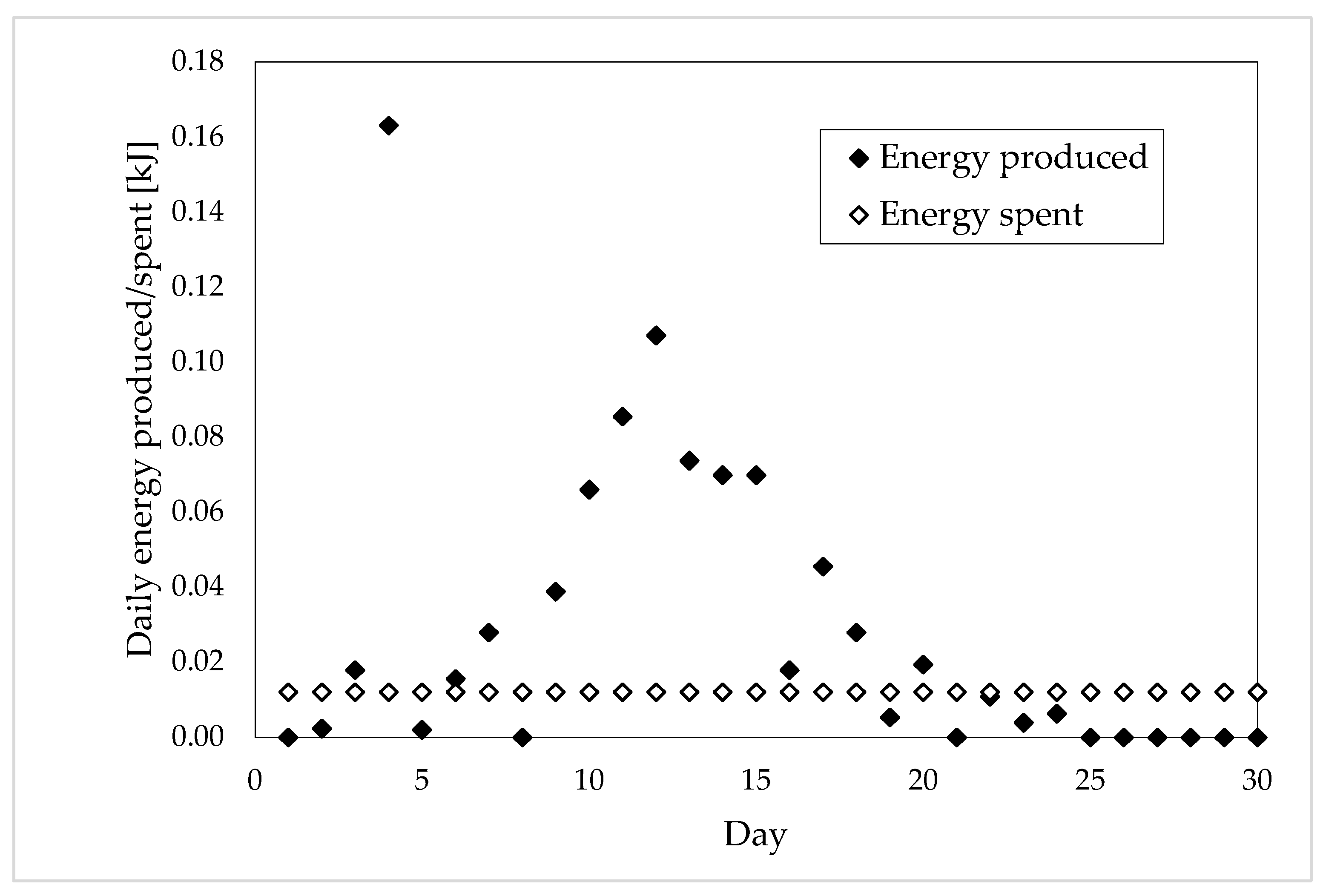
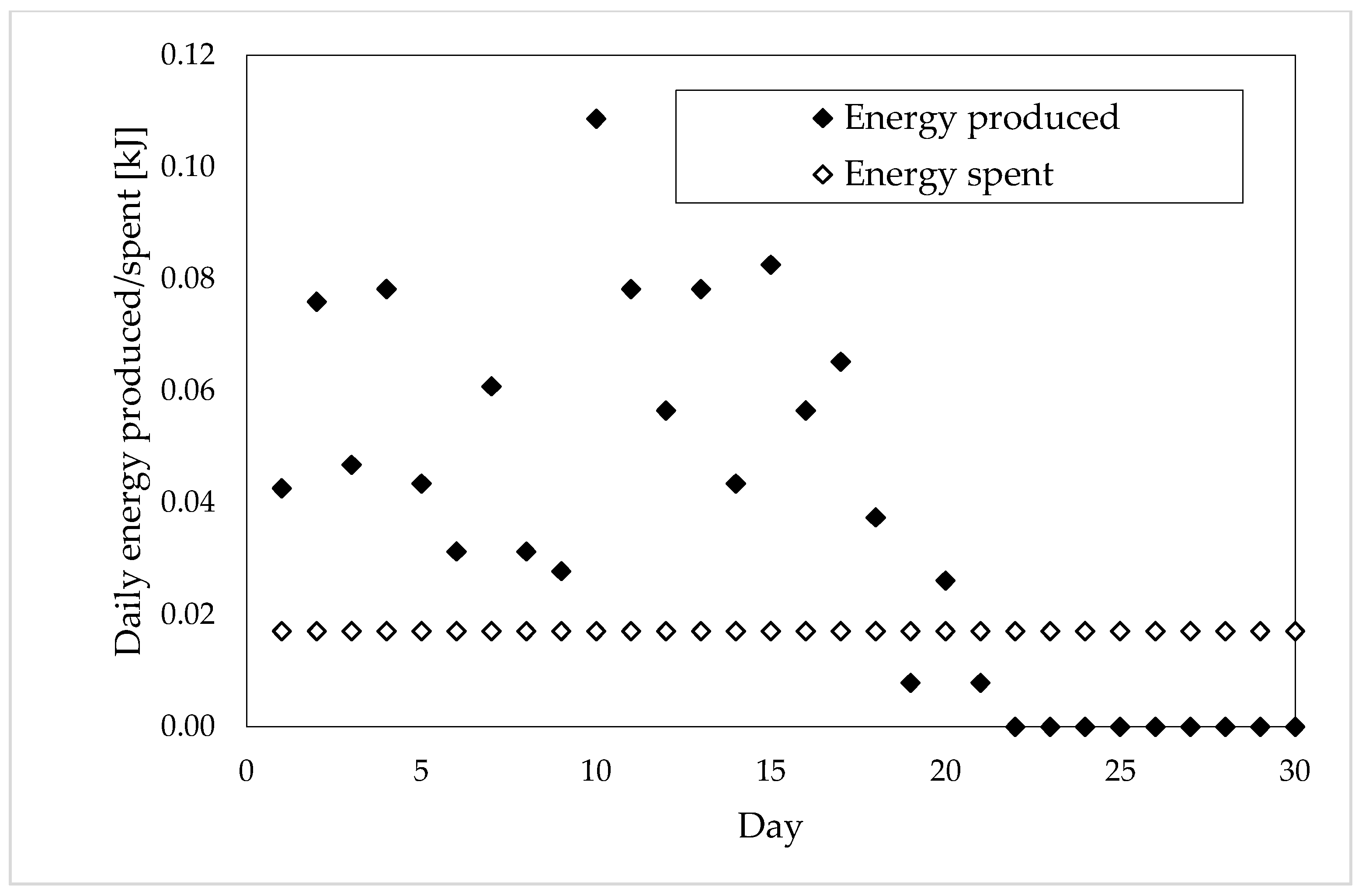
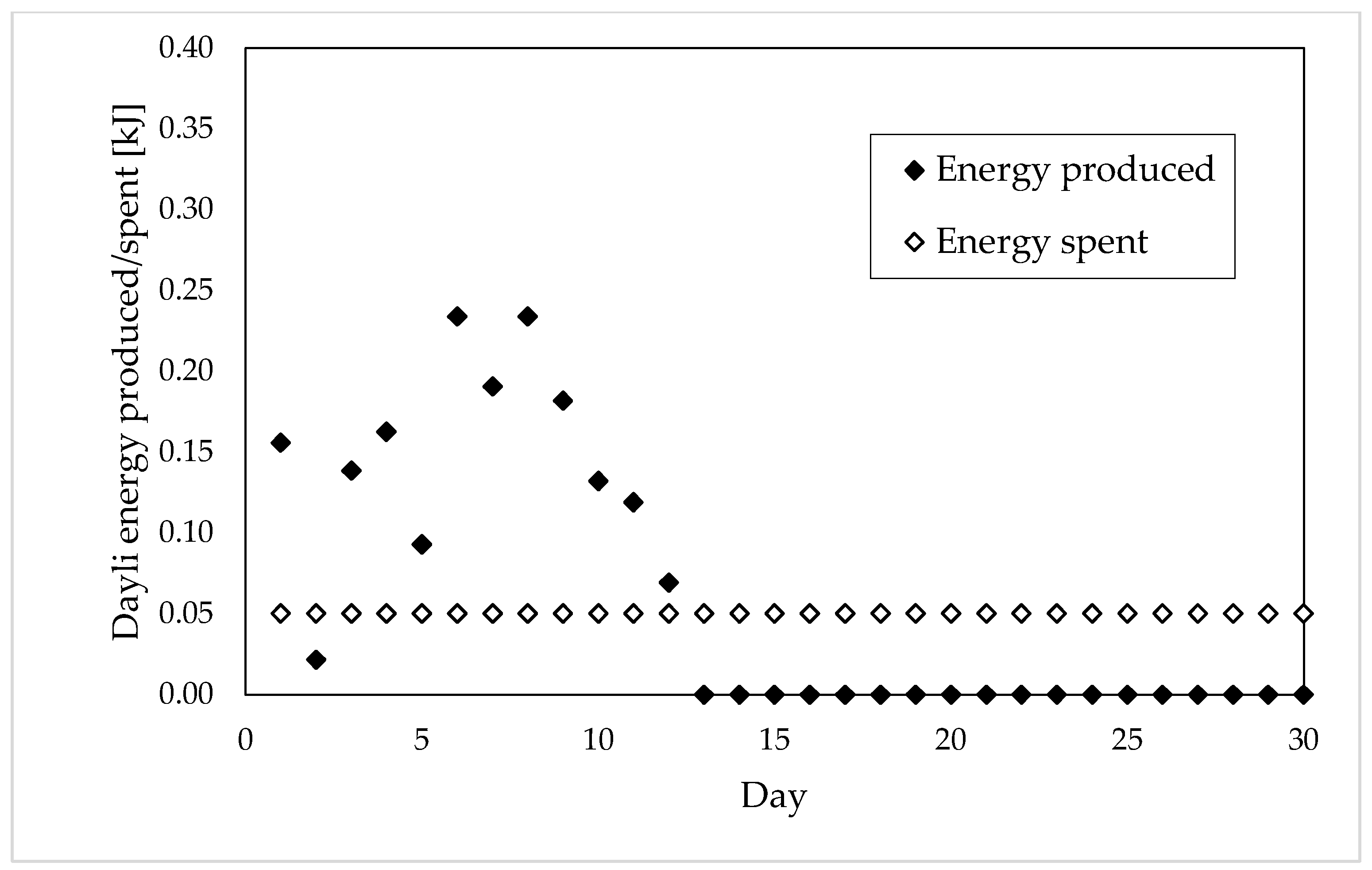
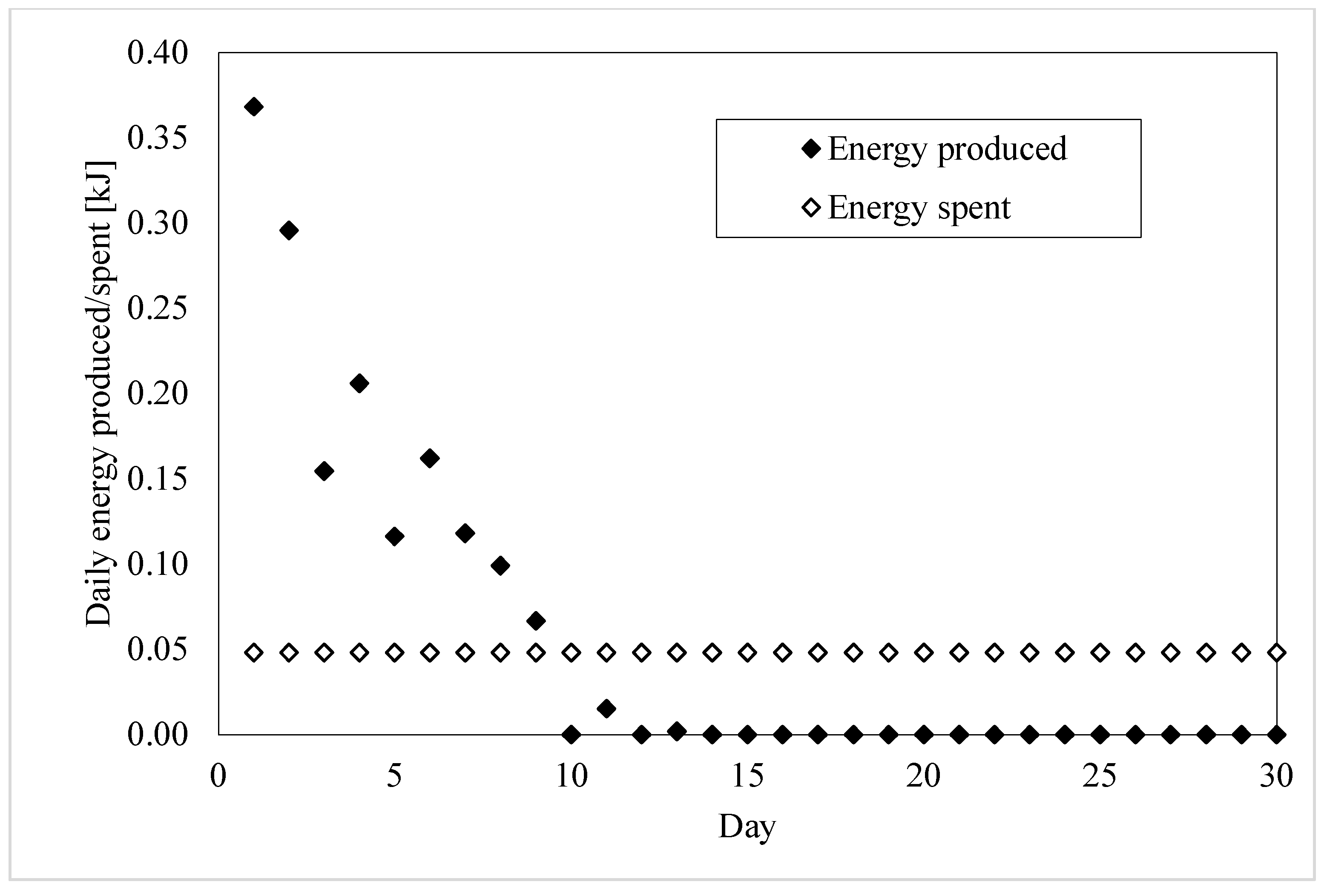
| Total Energy Consumed [kJ] | Days of Production | Daily Energy Consumed [kJ] | |
|---|---|---|---|
| Sample 1 | 0.29 ± 0.005 | 24 | 0.01 ± 0.005 |
| Sample 2 | 0.36 ± 0.005 | 21 | 0.02 ± 0.005 |
| Sample 3 | 0.52 ± 0.005 | 12 | 0.05 ± 0.005 |
| Sample 4 | 0.97 ± 0.005 | 17 | 0.06 ± 0.005 |
| Sample 5 | 0.52 ± 0.005 | 13 | 0.05 ± 0.005 |
| Whole Production of Energy [kJ] | Optimal Production Period [Day] | Energy Produced in the Optimal Period [kJ] | Portion of Energy Lost [%] | |
|---|---|---|---|---|
| Sample 1 | 0.88 | 18 | 0.80 | 9.09 |
| Sample 2 | 1.09 | 18 | 1.05 | 3.67 |
| Sample 3 | 1.73 | 12 | 1.73 | 0 |
| Sample 4 | 2.94 | 12 | 2.84 | 3.40 |
| Sample 5 | 1.60 | 9 | 1.58 | 1.25 |
| η [%] | ηOPT [%] | |
|---|---|---|
| Sample 1 | 67.0 | 74.8 |
| Sample 2 | 67.0 | 72.3 |
| Sample 3 | 67.0 | 81.7 |
| Sample 4 | 67.0 | 76.7 |
| Sample 5 | 67.0 | 75.9 |
| TS [kg] | ETS [kJ/kg] | VS [kg] | EVS [kJ/kg] | |
|---|---|---|---|---|
| Sample 1 (sole inoculation) | 2.43 × 10−3 | 361.1 | 1.65 × 10−3 | 530.3 |
| Sample 2 (with swine slurry) | 2.22 × 10−3 | 490.1 | 1.44 × 10−3 | 756.0 |
| Sample 4 (with OP-PH waste) | 3.10 × 10−3 | 946.5 | 1.70 ×10−3 | 1727.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montegiove, N.; Gambelli, A.M.; Calzoni, E.; Bertoldi, A.; Puglia, D.; Zadra, C.; Emiliani, C.; Gigliotti, G. Biogas Production with Residuals Deriving from Olive Mill Wastewater and Olive Pomace Wastes: Quantification of Produced Energy, Spent Energy, and Process Efficiency. Agronomy 2024, 14, 531. https://doi.org/10.3390/agronomy14030531
Montegiove N, Gambelli AM, Calzoni E, Bertoldi A, Puglia D, Zadra C, Emiliani C, Gigliotti G. Biogas Production with Residuals Deriving from Olive Mill Wastewater and Olive Pomace Wastes: Quantification of Produced Energy, Spent Energy, and Process Efficiency. Agronomy. 2024; 14(3):531. https://doi.org/10.3390/agronomy14030531
Chicago/Turabian StyleMontegiove, Nicolò, Alberto Maria Gambelli, Eleonora Calzoni, Agnese Bertoldi, Debora Puglia, Claudia Zadra, Carla Emiliani, and Giovanni Gigliotti. 2024. "Biogas Production with Residuals Deriving from Olive Mill Wastewater and Olive Pomace Wastes: Quantification of Produced Energy, Spent Energy, and Process Efficiency" Agronomy 14, no. 3: 531. https://doi.org/10.3390/agronomy14030531
APA StyleMontegiove, N., Gambelli, A. M., Calzoni, E., Bertoldi, A., Puglia, D., Zadra, C., Emiliani, C., & Gigliotti, G. (2024). Biogas Production with Residuals Deriving from Olive Mill Wastewater and Olive Pomace Wastes: Quantification of Produced Energy, Spent Energy, and Process Efficiency. Agronomy, 14(3), 531. https://doi.org/10.3390/agronomy14030531