Effects of Harvesting Method on Seed Yield and Seed Quality in Urochloa ruziziensis (cv. ‘OKI-1’ and cv. ‘Br-203’)
Abstract
1. Introduction
2. Materials and Methods
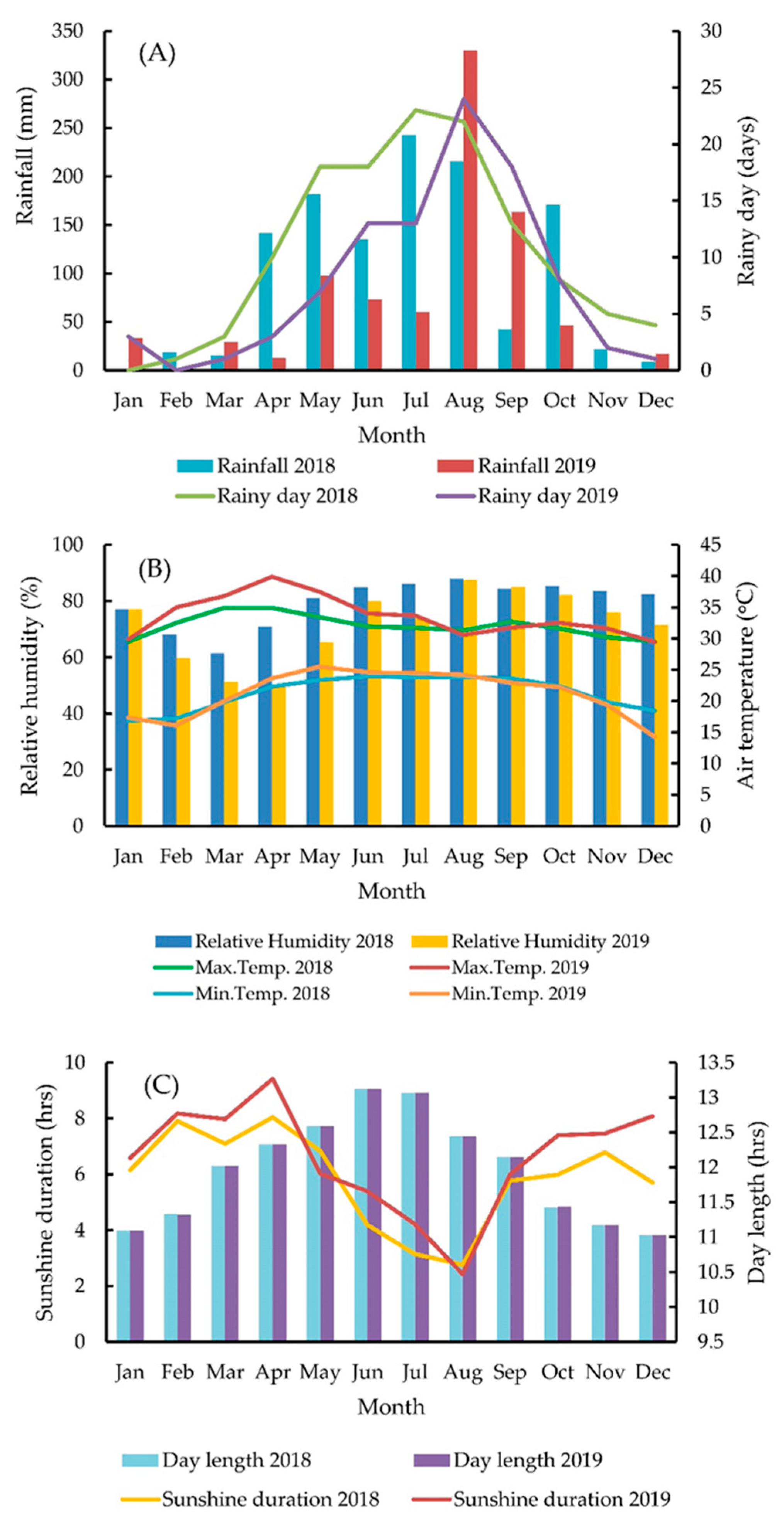
2.1. Location, Soil Characteristics, and Meteorological Data of the Experimental Sites
2.2. Trial 1—Effect of Harvesting Methods on Seed Yield and Seed Quality in OKI-1 Cultivar
2.2.1. Plant Cultivation
2.2.2. Experimental Design and Treatments
- T1—knocking the seeds from seedheads into a broad, shallow nylon net receptacle and collecting them once every day (Daily knocking);
- T2—knocking the seeds from seedheads into a broad, shallow nylon net receptacle and collecting them every three days (3-day knocking);
- T3—allowing ripe seeds to fall into a nylon net sheet stretched as a receptacle positioned beneath the seedheads and collecting them every five days (Under);
- T4—covering the tied seedheads with a light-weight nylon net bag, allowing the ripe seeds to fall into the nylon-net bag, and collecting them every five days (Cover).
2.2.3. Data Collection and Seed Harvesting
2.2.4. Seed Processing and Calculation of Secondary Attributes
2.2.5. Statistical Analysis
2.3. Trial 2—Effect of Harvesting Methods on Seed Yield and Seed Quality in Br-203 Cultivar
3. Results
3.1. Trial 1—Effect of Harvesting Methods on Seed Yield and Seed Quality in OKI-1 Cultivar
3.2. Trial 2—Effect of Harvesting Methods on Seed Yield and Seed Quality in Br-203 Cultivar
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ishigaki, G.; Gondo, T.; Suenaga, K.; Akashi, R. Induction of tetraploid ruzigrass (Brachiaria ruziziensis) plants by colchicine treatment of in vitro multiple-shoot clumps and seedlings. Grassl. Sci. 2009, 55, 164–170. [Google Scholar] [CrossRef]
- Kouki, K.; Ishigaki, G.; Akashi, R.; Shimabukuro, H. The Newly-Bred Cultivar Production in Brachiaria Grass. (1) Production of the High Seed Yield Strains Using Sexual Tetraploid Ruzigrass ‘Miyaokikoku-Ichigou’, Annual Report; Okinawa Prefectural Livestock and Grassland Research Center: Uruma, Japan, 2014; Volume 52, pp. 73–75. (In Japanese) [Google Scholar]
- Nakmanee, G.; Ebina, M.; Shimoda, K.; Thaikua, S.; Srisomporn, W.; Patipan, C.; Suenaga, K.; Ando, S.; Kouki, K.; Ishigaki, G.; et al. A new candidate cultivar of Brachiaria grass ‘Br-203’ developed with apomixis marker assisted selection, through a collaborative breeding activity of Thailand and Japan. In Proceedings of the 23rd International grassland congress, New Dehli, India, 20–24 November 2015. [Google Scholar]
- Thaikua, S.; Chanpeng, P.; Juntasin, W.; Donsawai, S.; Chuchuay, P.; Kouki, K.; Kawamoto, Y. Effect of ploidy on digestibility and fiber fraction of ruzigrass (Brachiaria ruziziensis). In Proceedings of the 18th Asian–Australasian Animal Production (AAAP) Congress, Sarawak, Malaysia, 1–5 August 2018; p. 434. [Google Scholar]
- Hare, M.D.; Pizarro, E.A.; Phengphet, S.; Songsiri, T.; Sutin, N. Evaluation of new hybrid Brachiaria lines in Thailand. 2. Seed production. Trop. Grassl.-Forrajes 2015, 3, 94–103. [Google Scholar] [CrossRef][Green Version]
- West, S.H.; Pitman, W.D. Seed production technology of tropical forages. In Tropical Forage Plants, Development and Use; Sotomayor-Ríos, A., Pitman, W.D., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 143–166. [Google Scholar]
- Benaseer, S.; Masilamani, P.; Alex Albert, V.; Govindaraj, M.; Selvaraju, P.; Bhaskaran, M. Impact of harvesting and threshing methods on seed quality—A review. Agric. Rev. 2018, 39, 183–192. [Google Scholar]
- Loch, D.S.; de Souza, F.H. Seed harvesting and drying: Grasses. In Forage Seed Production. Volume 2: Tropical and Subtropical Species; Loch, D.S., Ferguson, J.E., Eds.; CAB International Publishing: Wallingford, UK, 1999; pp. 191–212. [Google Scholar]
- Hare, M.D.; Tatsapong, S.; Saipraset, K. Seed production of two brachiaria hybrid cultivars in north-east Thailand. 2. Closing date defoliation. Trop. Grassl. 2007, 41, 35–42. [Google Scholar]
- Phaikaew, C.; Pholsen, P. Ruzigrass (Brachiaria ruziziensis) seed production and research in Thailand. In Proceedings of the Third Meeting of Regional Working Group on Grazing and Feed Resources of Southeast Asia, Khon Kaen, Thailand, 31 January–6 February 1993; pp. 165–173. [Google Scholar]
- Phaikaew, C.; Pholsen, P.; Chinosang, W. Effect of Harvesting Methods on Seed Yield and Quality of Purple Guinea Grass (Panicum maximum TD.58) Produced by Small Farmers in Khon Kaen, Annual Report; Bureau of Animal Nutrition Development, Department of Livestock Development: Bangkok, Thailand, 1995; pp. 103–108. [Google Scholar]
- Phaikaew, C.; Poonpipat, W.; Nakmanee, G.; Chinosang, W.; Sooksaran, W. Effect of Harvesting Methods on Seed Yield and Seed Quality of Panicum maximum cv. Mombaza, Annual Report; Bureau of Animal Nutrition Development, Department of Livestock Development: Bangkok, Thailand, 2010; pp. 194–204. [Google Scholar]
- Kamphayae, S.; Lertrattanapong, B.; Suksaket, A.; Pholsen, P. Effects of Plant Spacing and Harvesting Method on Seed Yield and Seed Quality of Umaku Guinea Grass (Panicum maximum cv. Umaku); Annual Report; Bureau of Animal Nutrition Development, Department of Livestock Development: Bangkok, Thailand, 2013; pp. 309–319. [Google Scholar]
- LDD (Land Development Department). 2009. Available online: http://oss101.ldd.go.th/osr_data&service/OSR_PDF/TB_SSK_Distribute/P_SSK529.pdf (accessed on 26 December 2023).
- LDD (Land Development Department). 2010. Available online: https://www.ldd.go.th/PMQA/2553/Manual/OSD-03.pdf (accessed on 26 December 2023).
- ISTA. International Rules for Seed Testing Edition 2011; The International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2011. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Rao, I.M.; Kerridge, P.C.; Macedo, M. Nutritional requirements of Brachiaria and adaptation to acid soils. In Brachiaria: Biology, Agronomy, and Improvement; Miles, J.W., Maass, B.L., do Valle, C.B., Eds.; Centro International de Agricultura Tropical (CIAT), International Center for Tropical Agriculture: Cali, Colombia, 1996; pp. 53–71. [Google Scholar]
- Boonman, J.G. Determinants of seed yield. In On the Seed Production of Tropical Grasses in Kenya; Leniger, H.A., Ed.; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1973; pp. 2–9. [Google Scholar]
- Stür, W.W.; Hopkinson, J.M.; Chen, C.P. Regional experience with Brachiaria: Asia, the South Pacific, and Australia. In Brachiaria: Biology, Agronomy, and Improvement; Miles, J.W., Maass, B.L., do Valle, C.B., Eds.; Centro International de Agricultura Tropical (CIAT), International Center for Tropical Agriculture: Cali, Colombia, 1996; pp. 258–271. [Google Scholar]
- Kendrick, C. Brachiaria hybrid. In Pastures Australia. 2008. Available online: https://keys.lucidcentral.org/keys/v3/pastures/Html/Brachiaria_hybrid.htm (accessed on 26 December 2023).
- Bouathong, C.; Hare, M.D.; Losirikul, M.; Wongpichet, K. Effect of nitrogen-rates on plant growth, seed yield and seed quality of three lines of Brachiaria hybrid grass. Khon Kaen Agric. J. 2011, 39, 295–306. [Google Scholar]
- Nadew, B.B. Effects of climatic and agronomic factors on yield and quality of bread wheat (Triticum aestivum L.) Seed: A review on selected factors. Adv. Crop. Sci. Tech. 2018, 6, 356. [Google Scholar]
- Wongsuwan, N. Seed Production Studies in Ruzi Grass (Brachiaria ruziziensis Germain and Everard). Master’s Thesis, Massey University, Palmerston North, New Zealand, 1994. [Google Scholar]
- Ison, R.L.; Hopkinson, J.M. Pasture legumes and grasses of warm climate regions. In CRC Handbook of Flowering; Halevy, A.H., Ed.; CRC Press: Boca Raton, FL, USA, 1985; Volume 1, pp. 203–251. [Google Scholar]
- Hopkinson, J.M.; de Souza, F.H.D.; Diulgheroff, S.; Ortiz, A.; Sánchez, M. Reproductive physiology, seed production and seed quality of Brachiaria. In Brachiaria: Biology, Agronomy, and Improvement; Miles, J.W., Maass, B.L., do Valle, C.B., Eds.; Centro International de Agricultura Tropical (CIAT), International Center for Tropical Agriculture: Cali, Colombia, 1996; pp. 124–140. [Google Scholar]
- Delouche, J.C. Environmental effects on seed development and seed quality. Hortic. Sci. 1980, 15, 13–18. [Google Scholar] [CrossRef]
- Chastain, T.G. Pollination and Seed Yield in Grass Seed Crops; Oregon State University: Corvallis, OR, USA, 2013; Available online: https://osu-wams-blogs-uploads.s3.amazonaws.com/blogs.dir/873/files/2013/06/Pollination-and-Seed-Yield.pdf (accessed on 26 December 2023).
- Phunphiphat, R.; Phaikaew, C.; Phunphiphat, W.; Nakmanee, G. Brachiaria Hybrids Regional Adaptability Test. 2. Yield and Chemical Composition of Herbage, Seed Yield and Seed Quality of Mulato, Mulato II and Ruzi Grass at Lampang, Annual Report; Bureau of Animal Nutrition Development, Department of Livestock Development: Bangkok, Thailand, 2007; pp. 234–244. [Google Scholar]
- Nakmanee, G.; Phaikaew, C.; Thinnakorn, S.; Kruemangkorn, P. Brachiaria Hybrids Regional Ability Test 1. Yield and Chemical Composition of Herbage, Seed Yield and Seed Quality of Mulato I, Mulato II and Ruzi grass at Pakchong Nakhonratchasima, Annual Report; Bureau of Animal Nutrition Development, Department of Livestock Development: Bangkok, Thailand, 2007; pp. 211–221. [Google Scholar]
- Phaikaew, C.; Pholsen, P.; Tudsri, S.; Tsuzuki, E.; Numaguchi, H.; Ishii, Y. Maximising seed yield and seed quality of Paspalum atratum through choice of harvest method. Trop. Grassl. 2001, 35, 11–18. [Google Scholar]
- Kowithayakorn, L.; Phaikaew, C. Harvesting and processing techniques of tropical grass and legume seeds for small farmers. In Proceedings of the XVII International Grassland Congress, Palmerston North, New Zealand, 8–21 February 1993; pp. 1809–1813. [Google Scholar]
- Hare, M.D.; Wongpichet, K.; Tatsapong, S.; Narksombat, S.; Saengkhum, M. Method of seed harvest, closing date and height of closing cut affect seed yield and seed yield components in Paspalum atratum in Thailand. Trop. Grassl. 1999, 33, 82–90. [Google Scholar]
- Gelderman, R.; Carson, P.; Gerwing, J. Fertilizing for grass seed production. Agric. Exp. Stn. Circ. 1987, 272. Available online: https://openprairie.sdstate.edu/cgi/viewcontent.cgi?article=1271&context=agexperimentsta_circ (accessed on 26 December 2023).
- Wongsuwan, N.; Chu, A.C.P.; Watkin, B.R. Effects of water stress on seed production in Ruzi grass (Brachiaria ruziziensis Germain and Everard). In Proceedings of the XIX International Grassland Congress, São Paulo, Brazil, 11–21 February 2001. [Google Scholar]
- Sehgal, A.; Sita, K.; Siddique, K.H.M.; Kumar, R.; Bhogireddy, S.; Varshney, R.K.; HanumanthaRao, B.; Nair, R.M.; Prasad, P.V.V.; Nayyar, H. Drought or/and heat-stress effects on seed filling in food crops: Impacts on functional biochemistry, seed yields, and nutritional quality. Front. Plant Sci. 2018, 9, 1705. [Google Scholar] [CrossRef] [PubMed]



| Trial | pH | OM (%) | Total N (%) | Extractable (mg/kg) | EC (dS/m) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | K | Ca | Mg | Na | S | Mn | Fe | Cu | Zn | |||||
| 1 | 5.22 | 0.71 | 0.04 | 2.72 | 51.71 | 74.44 | 11.45 | 3.38 | 2.45 | 19.12 | 54.48 | 0.19 | 0.02 | 0.41 |
| 2 | 5.33 | 0.49 | 0.02 | 0.64 | 81.13 | 278.42 | 43.53 | 2.44 | 2.75 | 10.78 | 46.91 | 0.15 | 0.06 | 0.30 |
| Event | Year | |
|---|---|---|
| 2018 | 2019 | |
| Start of flowering | 16 October | 13 October |
| Period of harvesting | 20 November–12 December | 3 December–25 December |
| Post-harvest cut of herbage | 16 December | 30 December |
| Year | Harvesting Method | TSY (kg/ha) | TN (No./m2) | FTN (No./m2) | IN (No./m2) | FTP (%) | IN/T (No.) | RN/I (No.) | SN/R (No.) |
|---|---|---|---|---|---|---|---|---|---|
| 2018 | T1 ‘Daily knocking’ | 579.96 | 84.17 | 55.50 | 238.18 a | 65.72 | 4.32 a | 2.86 | - |
| T2 ‘3-day knocking’ | 660.42 | 88.58 | 58.33 | 182.26 ab | 65.40 | 3.12 b | 3.26 | - | |
| T3 ‘Under’ | 764.83 | 72.83 | 45.83 | 118.38 b | 62.32 | 2.57 b | 3.34 | - | |
| T4 ‘Cover’ | 669.60 | 75.75 | 50.17 | 144.30 b | 66.14 | 2.90 b | 3.35 | - | |
| 2019 | T1 ‘Daily knocking’ | 253.26 | 80.25 | 78.00 | 115.83 | 97.31 | 1.48 | 4.17 | 34.40 |
| T2 ‘3-day knocking’ | 362.18 | 78.50 | 75.42 | 119.98 | 96.08 | 1.60 | 4.19 | 36.62 | |
| T3 ‘Under’ | 307.68 | 69.83 | 68.50 | 105.73 | 97.83 | 1.48 | 4.34 | 33.66 | |
| T4 ‘Cover’ | 311.23 | 74.42 | 73.08 | 101.23 | 98.15 | 1.35 | 4.24 | 32.54 | |
| 2-y avg | T1 ‘Daily knocking’ | 416.61 | 82.21 | 66.75 | 177.00 a | 81.51 | 2.90 a | 3.52 | - |
| T2 ‘3-day knocking’ | 511.30 | 83.54 | 66.88 | 151.12 ab | 80.74 | 2.36 b | 3.72 | - | |
| T3 ‘Under’ | 536.25 | 71.33 | 57.17 | 112.06 b | 80.07 | 2.02 b | 3.84 | - | |
| T4 ‘Cover’ | 490.42 | 75.08 | 61.62 | 122.77 b | 82.14 | 2.12 b | 3.80 | - |
| Year | Harvesting Method | FSP (%) | TSW (g) | PP (%) | PSY (kg/ha) | GP (%) | PGSY (kg/ha) |
|---|---|---|---|---|---|---|---|
| 2018 | T1 ‘Daily knocking’ | 32.03 | 9.12 | 99.72 | 578.41 | 81.75 | 473.06 |
| T2 ‘3-day knocking’ | 40.12 | 9.60 | 99.82 | 659.23 | 77.25 | 528.97 | |
| T3 ‘Under’ | 40.65 | 9.53 | 99.78 | 763.14 | 88.00 | 683.61 | |
| T4 ‘Cover’ | 32.19 | 9.42 | 99.88 | 668.74 | 77.75 | 519.67 | |
| 2019 | T1 ‘Daily knocking’ | 32.67 | 8.21 | 97.78 b | 246.64 | 96.25 | 236.65 |
| T2 ‘3-day knocking’ | 33.28 | 8.76 | 98.62 ab | 357.49 | 95.00 | 339.95 | |
| T3 ‘Under’ | 38.14 | 8.91 | 99.50 a | 306.20 | 95.75 | 293.39 | |
| T4 ‘Cover’ | 35.67 | 8.85 | 99.78 a | 310.53 | 92.50 | 287.25 | |
| 2-y avg | T1 ‘Daily knocking’ | 32.35 | 8.66 | 98.75 b | 412.53 | 89.00 | 354.85 |
| T2 ‘3-day knocking’ | 38.76 | 9.18 | 99.22 ab | 508.36 | 86.12 | 434.46 | |
| T3 ‘Under’ | 39.40 | 9.22 | 99.64 a | 534.67 | 91.88 | 488.50 | |
| T4 ‘Cover’ | 33.35 | 9.13 | 99.82 a | 489.64 | 85.12 | 403.46 |
| Year | Harvesting Method | TSY (kg/ha) | TN (No./m2) | FTN (No./m2) | IN (No./m2) | FTP (%) | IN/T (No.) | RN/I (No.) | SN/R (No.) |
|---|---|---|---|---|---|---|---|---|---|
| 2018 | T1 ‘Daily knocking’ | 277.10 | 68.42 a | 52.00 a | 218.12 | 76.27 | 4.17 | 3.27 | - |
| T2 ‘3-day knocking’ | 257.28 | 54.00 b | 40.25 ab | 178.64 | 73.48 | 4.38 | 3.27 | - | |
| T3 ‘Under’ | 417.89 | 53.08 b | 38.92 b | 163.34 | 72.77 | 4.25 | 3.17 | - | |
| T4 ‘Cover’ | 379.15 | 62.75 ab | 45.83 ab | 172.69 | 73.06 | 3.75 | 2.79 | - | |
| 2019 | T1 ‘Daily knocking’ | 136.80 | 79.00 | 71.08 | 85.50 b | 88.53 | 1.22 c | 4.88 | 34.40 |
| T2 ‘3-day knocking’ | 160.67 | 94.92 | 82.42 | 143.88 ab | 86.80 | 1.70 b | 4.08 | 36.62 | |
| T3 ‘Under’ | 268.37 | 83.67 | 77.25 | 155.45 ab | 92.40 | 2.12 a | 4.33 | 33.66 | |
| T4 ‘Cover’ | 197.61 | 101.83 | 90.92 | 164.27 a | 89.67 | 1.80 ab | 4.05 | 32.54 | |
| 2-y avg | T1 ‘Daily knocking’ | 206.94 b | 73.71 | 61.54 | 151.81 | 83.08 | 2.69 | 4.07 | - |
| T2 ‘3-day knocking’ | 208.97 b | 74.46 | 61.33 | 161.26 | 81.96 | 3.04 | 3.63 | - | |
| T3 ‘Under’ | 343.13 a | 68.38 | 58.08 | 159.40 | 84.82 | 3.18 | 3.80 | - | |
| T4 ‘Cover’ | 288.38 ab | 82.29 | 68.38 | 168.48 | 83.39 | 2.78 | 3.42 | - |
| Year | Harvesting Method | FSP (%) | TSW (g) | PP (%) | PSY (kg/ha) | GP (%) | PGSY (kg/ha) |
|---|---|---|---|---|---|---|---|
| 2018 | T1 ‘Daily knocking’ | 28.93 | 6.94 | 98.52 | 273.46 | 34.50 | 90.17 |
| T2 ‘3-day knocking’ | 24.28 | 7.28 | 98.12 | 252.93 | 35.00 | 87.11 | |
| T3 ‘Under’ | 17.53 | 6.89 | 97.52 | 407.70 | 41.12 | 180.71 | |
| T4 ‘Cover’ | 14.62 | 6.96 | 98.10 | 373.79 | 39.19 | 140.27 | |
| 2019 | T1 ‘Daily knocking’ | 26.72 | 6.51 | 97.15 | 133.05 b | 94.25 | 125.44 |
| T2 ‘3-day knocking’ | 12.26 | 6.49 | 97.42 | 156.52 ab | 95.25 | 148.98 | |
| T3 ‘Under’ | 15.33 | 7.45 | 96.70 | 258.52 a | 84.25 | 219.55 | |
| T4 ‘Cover’ | 10.57 | 6.69 | 96.32 | 190.61 ab | 89.75 | 170.82 | |
| 2-y avg | T1 ‘Daily knocking’ | 27.83 | 6.72 | 97.84 | 203.26 b | 64.38 | 107.80 |
| T2 ‘3-day knocking’ | 18.27 | 6.88 | 97.78 | 204.72 b | 65.12 | 118.04 | |
| T3 ‘Under’ | 16.43 | 7.17 | 97.11 | 333.11 a | 62.69 | 200.13 | |
| T4 ‘Cover’ | 12.59 | 6.83 | 97.21 | 282.20 ab | 64.47 | 155.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imura, Y.; Nakamura, I.; Juntasin, W.; Hossain, M.A.; Thaikua, S.; Poungkaew, R.; Kawamoto, Y. Effects of Harvesting Method on Seed Yield and Seed Quality in Urochloa ruziziensis (cv. ‘OKI-1’ and cv. ‘Br-203’). Agronomy 2024, 14, 509. https://doi.org/10.3390/agronomy14030509
Imura Y, Nakamura I, Juntasin W, Hossain MA, Thaikua S, Poungkaew R, Kawamoto Y. Effects of Harvesting Method on Seed Yield and Seed Quality in Urochloa ruziziensis (cv. ‘OKI-1’ and cv. ‘Br-203’). Agronomy. 2024; 14(3):509. https://doi.org/10.3390/agronomy14030509
Chicago/Turabian StyleImura, Yoshimi, Ichiro Nakamura, Weenaporn Juntasin, Mohammad Amzad Hossain, Sarayut Thaikua, Rattikan Poungkaew, and Yasuhiro Kawamoto. 2024. "Effects of Harvesting Method on Seed Yield and Seed Quality in Urochloa ruziziensis (cv. ‘OKI-1’ and cv. ‘Br-203’)" Agronomy 14, no. 3: 509. https://doi.org/10.3390/agronomy14030509
APA StyleImura, Y., Nakamura, I., Juntasin, W., Hossain, M. A., Thaikua, S., Poungkaew, R., & Kawamoto, Y. (2024). Effects of Harvesting Method on Seed Yield and Seed Quality in Urochloa ruziziensis (cv. ‘OKI-1’ and cv. ‘Br-203’). Agronomy, 14(3), 509. https://doi.org/10.3390/agronomy14030509






