Looking for Fusarium Resistance in Oats: An Update
Abstract
1. Introduction
- I.
- Fusarium resistance in oats is a complex, quantitative trait. Five types of resistance mechanisms are known:
- -
- resistance to the initial infection (type I): is due to earliness that ensures the avoidance or to plant morphologies and chemical compounds that act as barriers against the fungus;
- -
- resistance to the spread of the infection (type II): plant morphologies, chemical compounds, and detoxification mechanisms that limit the spread of the fungus;
- -
- resistance to kernel infection (type III): kernel morphologies, chemical barriers, defense proteins, induced resistance mechanisms to limit kernel infection and late blight;
- -
- tolerance (type IV): antioxidant and detoxification strategies to compensate for infection;
- -
- toxin accumulation (type V): toxin modification and transportation to reduce the toxin content
- II.
- Inoculation methods for screening Fusarium-resistant plants.
- III.
- Phenotyping the FHB resistance.
- The available literature of the last 10 years was screened based on the keywords “Oats”, “Fusarium”, “FHB”, “mycotoxins”, “Fusarium resistance”, “Oats genomics”;
- The articles resulted from the search were examined carefully for their content and for their novelty in comparison with the already published reviews on the topic;
- In total, more than 100 articles were finally selected for this update.
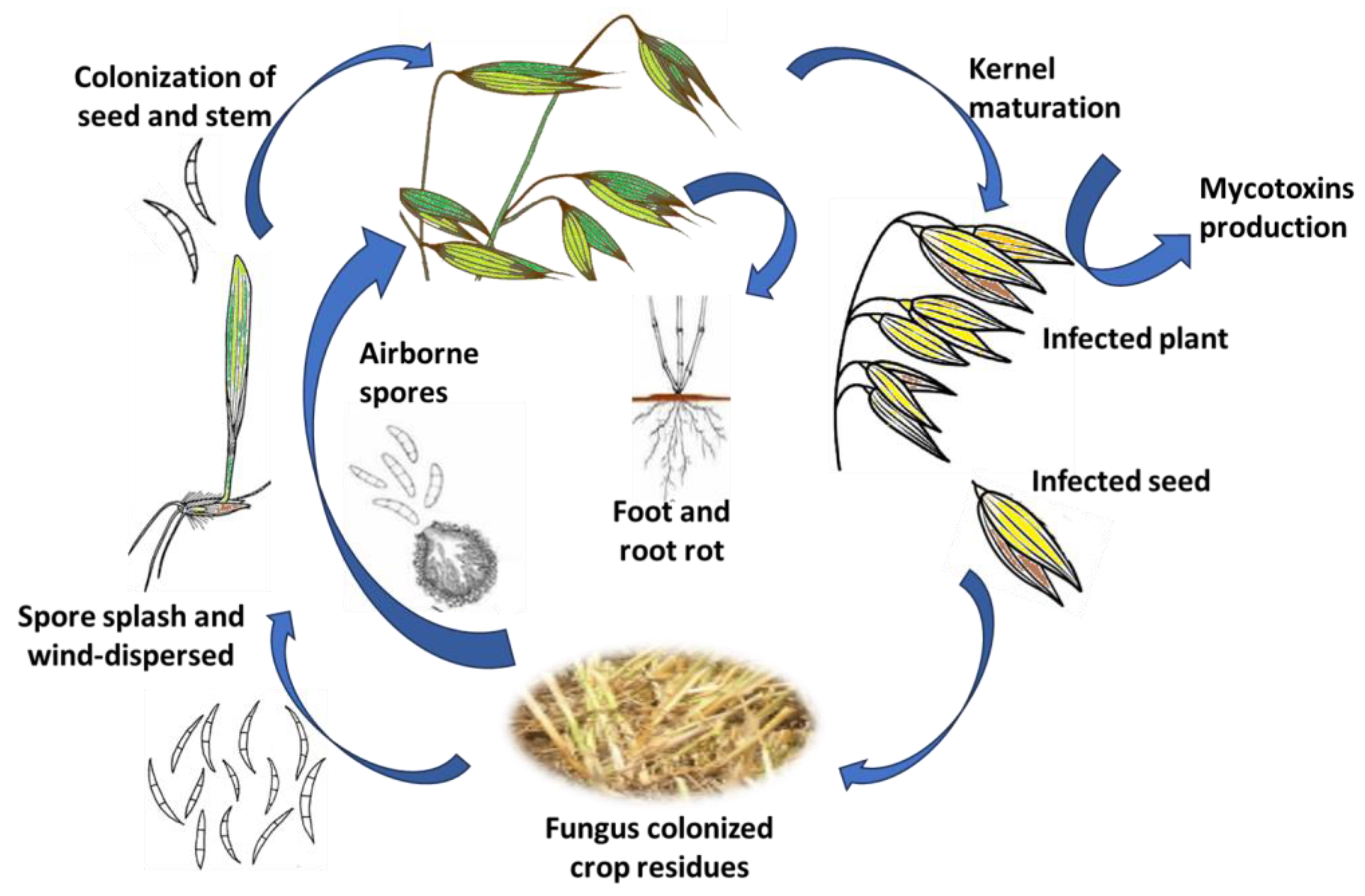
2. Epidemiology of Fusarium in Oats
| Region | Fungus | Mycotoxins | References |
|---|---|---|---|
| Northern and Central Europe | F. graminearum, F. culmorum | DON | [33] |
| Swedish | F. poae, F. langsethiae, F. graminearum | NIV, HT-2/T2, DON, ZEN | [34] |
| southeast Norway | F. langsethiae, F. graminearum, F. avenaceum | HT-2/T2, DON, ENNs, BEA | [26] |
| Spain | F. langsethiae, F. poae, F. tricinctum, F. cerealis | HT-2/T-2, B trichothecenes | [35] |
| Ireland | Fusarium spp. | T-2/HT-2 | [37] |
| Canada | F. poae, F.graminearum, F.sporotrichioides, F. avenaceum, F, culmorum | - | [39,40] |
| Urals and West Siberia | F. graminearum, F. langsethiae, F. sibiricum, F.sporotrichioides, F. avenaceum, F. poae, F. anguioides | DON, A-trichothecene | [41] |
| South-Western Siberian | F. sibiricum, F. globosum | fumonisin | [41] |
3. Genetic Resources as Valuable Sources of FHB Resistance
4. Phenotyping for Fusarium Resistance Using Artificial Infection and Field Trials
5. Phenotyping Strategies and Diagnostics Methods
6. Involvement of Peculiar Oat Compounds in FHB
7. Genotyping Tools in Oats
8. Mapping Fusarium Resistance
9. Progress in Understanding Plant-Pathogen Interactions, Genetic Engineering of R Protein, and Their Importance in the Future Development of FHB-Resistant Oats
10. Influence of Microbiological Environment on FHB Oat Resistance
11. Perspectives on Biotechnological Tools to Empower Oats against FBH
12. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 24 January 2024).
- Marshall, A.H.; Cowan, A.A.; Edwards, S.; Griffiths, I.M.; Howarth, C.; Langdon, T.; White, E. Crops that feed the world 9. Oats-a cereal crop for human and livestock feed with industrial applications. Food Secur. 2013, 5, 13–33. [Google Scholar] [CrossRef]
- Menon, R.; Gonzalez, T.; Ferruzzi, M.; Jackson, E.; Winderl, D.; Watson, J. Oats-from farm to fork. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2016; Volume 77, pp. 1–55. ISBN 978-0-12-804772-9. [Google Scholar]
- Morcia, C.; Finocchiaro, F.; Delbono, S.; Ghizzoni, R.; Reggiani, F.; Carnevali, P.; Tumino, G.; Carrara, I.; Terzi, V. Oats: Nutritional uniqueness and breeding of a healthy superfood. In Compendium of Crop Genome Designing for Nutraceuticals; Kole, C., Ed.; Springer Nature: Singapore, 2023; pp. 153–192. ISBN 978-981-19-4169-6. [Google Scholar]
- Dimberg, L.H.; Theander, O.; Lingnert, H. Avenanthramides-a group of phenolic antioxidants in oats. Cereal Chem. 1993, 70, 637–641. [Google Scholar]
- JECFA, Joint FAO/WHO Expert Committee on Food Additives. Safety Evaluation of Certain Mycotoxins in Food; Prepared by the Fifty-Sixth Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO: Geneva, Switzerland, 2001. [Google Scholar]
- Bottalico, A.; Perrone, G. Toxigenic Fusarium species and mycotoxins associated with head blight in small-grain cereals in Europe. Eur. J. Plant Pathol. 2002, 108, 611–624. [Google Scholar] [CrossRef]
- Henriksen, B.; Elen, O. Natural Fusarium grain infection level in wheat, barley and oat after early application of fungicides and herbicides. J. Phytopathol. 2005, 153, 214–220. [Google Scholar] [CrossRef]
- Münzing, K.; Hampshire, J.; Bruer, A. Deoxinivalenol (DON). Kontamination und Dekontamination Bei Hafer-Deoxynivalenol (DON)—Contamination and Decontamination of Oats; Jahresbericht Forschung; Institut für Getreide-, Kartoffel- und Stärketechnologie: Detmold, Germany, 2002; pp. 32–35. Available online: https://www.deutsche-digitale-bibliothek.de/item/ULYNDY33WWWOK3BHWRF3XN5XQ32S3AUU?lang=en (accessed on 10 January 2024).
- Tekauz, A.; McCallum, B.; Ames, N.; Mitchell Fetch, J. Fusarium Head Blight of oat—Current status in western Canada. Can. J. Plant Pathol. 2004, 26, 473–479. [Google Scholar] [CrossRef]
- Joint FAO/WHO Food Standards Programme Codex Committee on Contaminant in Food. Draft guidelines for risk analysis of instances of contaminants in food where there is no regulatory level or risk management framework established. In Proceedings of the 13th Session of the Codex Committee on Contaminants in Foods, Yogyakarta, Indonesia, 29 April–3 May 2019. CX/CF 19/13/8. [Google Scholar]
- Available online: https://www.who.int/news-room/fact-sheets/detail/mycotoxins (accessed on 24 January 2024).
- Commission Regulation (EU). Commission Regulation (EU) 2023/915 of 25 April 2023 on Maximum Levels for Certain Contaminants in Food and Repealing Regulation (EC) No 1881/2006 (Text with EEA Relevance). Off. J. Eur. Union 2023, L119, 103–157. [Google Scholar]
- EU Commission Recommendation of 27 March 2013 on the Presence of T-2 and HT-2 Toxin in Cereals and Cereal Products (2013/165/EU) GU L 91, 3.4.2013. pp. 12–15. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32013H0165 (accessed on 24 January 2024).
- Knutsen, H.K.; Alexander, J.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Grasl-Kraupp, B.; Hogstrand, C.; et al. Scientific opinion on the risks to human and animal health related to the presence of deoxynivalenol and its acetylated and modified forms in food and feed. EFSA J. 2017, 15, e04718. [Google Scholar] [CrossRef] [PubMed]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific opinion on the risks for public health related to the presence of zearalenone in food. EFSA J. 2011, 9, 2197. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain). Scientific opinion on the appropriateness to set a group health-based guidance value for zearalenone and its modified forms. EFSA J. 2016, 14, 4425. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.-K.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Scientific opinion on the appropriateness to set a group health based guidance value for T2 and HT2 toxin and its modified forms. EFSA J. 2017, 15, 4655. [Google Scholar] [CrossRef]
- EFSA (European Food Safety Authority); Arcella, D.; Gergelova, P.; Innocenti, M.L.; Steinkellner, H. Scientific report on human and animal dietary exposure to T-2 and HT-2 toxin. EFSA J. 2017, 15, 4972. [Google Scholar] [CrossRef]
- EFSA Panel on Contaminants in the Food Chain; Knutsen, H.K.; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Scientific opinion on the appropriateness to set a group health-based guidance value for fumonisins and their modified forms. EFSA J. 2018, 16, 5172. [Google Scholar] [CrossRef]
- EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain); Knutsen, H.-K.; Barregård, L.L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; et al. Scientific opinion on the appropriateness to set a group health based guidance value for nivalenol and its modified forms. EFSA J. 2017, 15, 4751. [Google Scholar] [CrossRef]
- Mielniczuk, E.; Skwaryło-Bednarz, B. Fusarium Head Blight, mycotoxins and strategies for their reduction. Agronomy 2020, 10, 509. [Google Scholar] [CrossRef]
- Hautsalo, J.; Jalli, M.; Manninen, O.; Veteläinen, M. Evaluation of resistance to Fusarium graminearum in oats. Euphytica 2018, 214, 139. [Google Scholar] [CrossRef]
- Hautsalo, J.; Jauhiainen, L.; Hannukkala, A.; Manninen, O.; Vetelainen, M.; Pietila, L.; Peltoniemi, K.; Jalli, M. Resistance to Fusarium head blight in oats based on analyses of multiple field and greenhouse studies. Eur. J. Plant Pathol. 2020, 158, 15–33. [Google Scholar] [CrossRef]
- Tekle, S.; Dill-Macky, R.; Skinnes, H.; Tronsmo, A.M.; Bjørnstad, Å. Infection Process of Fusarium Graminearum in Oats (Avena Sativa L.). Eur. J. Plant Pathol. 2012, 132, 431–442. [Google Scholar] [CrossRef]
- Hofgaard, I.S.; Aamot, H.U.; Seehusen, T.; Holen, B.M.; Riley, H.; Dill-Macky, R.; Edwards, S.G.; Brodal, G. Reduced risk of oat grain contamination with Fusarium langsethiae and HT-2 and T-2 toxins with increasing tillage intensity. Pathogens 2022, 11, 1288. [Google Scholar] [CrossRef]
- Bernhoft, A.; Wang, J.; Leifert, C. Effect of organic and conventional cereal production methods on Fusarium Head Blight and mycotoxin contamination levels. Agronomy 2022, 12, 797. [Google Scholar] [CrossRef]
- Julian Maywald, N.; Francioli, D.; Mang, M.; Ludewig, U. Role of mineral nitrogen nutrition in fungal plant diseases of cereal crops. Crit. Rev. Plant Sci. 2023, 42, 93–123. [Google Scholar] [CrossRef]
- Stępień, A.; Wojtkowiak, K.; Cwalina-Ambroziak, B.; Waśkiewicz, A. Mycotoxin level in winter wheat grain as impacted by nitrogen and manganese fertilisation. Appl. Sci. 2023, 13, 10086. [Google Scholar] [CrossRef]
- Nazari, L.; Pattori, E.; Terzi, V.; Morcia, C.; Rossi, V. Influence of temperature on infection, growth, and mycotoxin production by Fusarium langsethiae and F. sporotrichioides in durum wheat. Food Microbiol. 2014, 39, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Pascale, M.; Logrieco, A.F. Mycotoxin risks under a climate change scenario in Europe. Trends Food Sci. Technol. 2019, 84, 38–40. [Google Scholar] [CrossRef]
- Kos, J.; Anić, M.; Radić, B.; Zadravec, M.; Hajnal, E.J.; Pleadin, J. Climate change—A global threat resulting in increasing mycotoxin occurrence. Foods 2023, 12, 2704. [Google Scholar] [CrossRef] [PubMed]
- Sohlberg, E.; Virkajärvi, V.; Parikka, P.; Rämö, S.; Laitila, A.; Sarlin, T. Taqman qPCR quantification and Fusarium community analysis to evaluate toxigenic fungi in cereals. Toxins 2022, 14, 45. [Google Scholar] [CrossRef]
- Karlsson, I.; Mellqvist, E.; Persson, P. Temporal and spatial dynamics of Fusarium spp. and mycotoxins in Swedish cereals during 16 years. Mycotoxin Res. 2023, 39, 3–18. [Google Scholar] [CrossRef]
- Gil-Serna, J.; Patiño, B.; Verheecke-Vaessen, C.; Vázquez, C.; Medina, Á. Searching for the Fusarium spp. which are responsible for trichothecene contamination in oats. Using metataxonomy to compare the distribution of toxigenic species in fields from Spain and the UK. Toxins 2022, 14, 592. [Google Scholar] [CrossRef]
- Morcia, C.; Tumino, G.; Ghizzoni, R.; Badeck, F.; Lattanzio, V.; Pascale, M.; Terzi, V. Occurrence of Fusarium langsethiae and T-2 and HT-2 toxins in italian malting barley. Toxins 2016, 8, 247. [Google Scholar] [CrossRef]
- De Colli, L.; De Ruyck, K.; Abdallah, M.F.; Finnan, J.; Mullins, E.; Kildea, S.; Spink, J.; Elliott, C.; Danaher, M. Natural co-occurrence of multiple mycotoxins in unprocessed oats grown in Ireland with various production systems. Toxins 2021, 13, 188. [Google Scholar] [CrossRef]
- Strychar, R. World oat production, trade, and usage. In Oats: Chemistry and Technology, 2nd ed.; Webster, F.H., Wood, P.J., Eds.; American Association of Cereal Chemists, Inc. (AACC): St. Paul, MN, USA, 2016; pp. 1–10. ISBN 978-1-891127-64-9. [Google Scholar] [CrossRef]
- Xue, A.G.; Chen, Y.H.; Seifert, K.; Guo, W.; Blackwell, B.A.; Harris, L.J.; Overy, D.P. Prevalence of Fusarium species causing head blight of spring wheat, barley and oat in Ontario during 2001–2017. Can. J. Plant Pathol. 2019, 41, 392–402. [Google Scholar] [CrossRef]
- Islam, M.N.; Tabassum, M.; Banik, M.; Daayf, F.; Fernando, W.G.D.; Harris, L.J.; Sura, S.; Wang, X. Naturally occurring Fusarium species and mycotoxins in oat grains from Manitoba, Canada. Toxins 2021, 13, 670. [Google Scholar] [CrossRef]
- Gavrilova, O.P.; Gagkaeva, T.Y.; Orina, A.S.; Gogina, N.N. Diversity of Fusarium species and their mycotoxins in cereal crops from the Asian territory of Russia. Dokl. Biol. Sci. 2023, 508, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Wambugu, P.W.; Ndjiondjop, M.N.; Henry, R.J. Role of genomics in promoting the utilization of plant genetic resources in GeneBanks. Brief. Funct. Genom. 2018, 17, 198–206. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, M.A.A.P.; Bebeli, P.J.; Bettencourt, E.; Costa, G.; Dias, S.; Dos Santos, T.M.M.; Slaski, J.J. Cereal landraces genetic resources in worldwide GeneBanks. A review. Agron. Sustain. Dev. 2013, 33, 177–203. [Google Scholar] [CrossRef]
- Hautsalo, J.; Latvala, S.; Manninen, O.; Haapalainen, M.; Hannukkala, A.; Jalli, M. Two oat genotypes with different field resistance to Fusarium head blight respond similarly to the infection at spikelet level. J. Plant Pathol. 2021, 103, 299–304. [Google Scholar] [CrossRef]
- Šliková, S.; Gregová, E.; Pastirčák, M. Toxin accumulation in avena species after different spray inoculation by Fusarium graminearum and F. culmorum. Cereal Res. Commun. 2019, 47, 656–668. [Google Scholar] [CrossRef]
- Tekle, S.; Lillemo, M.; Skinnes, H.; Reitan, L.; Buraas, T.; Bjørnstad, Å. Screening of oat accessions for Fusarium head blight resistance using spawn-inoculated field experiments. Crop Sci. 2018, 58, 143–151. [Google Scholar] [CrossRef]
- Bentivenga, G.; Spina, A.; Ammar, K.; Allegra, M.; Cacciola, S.O. Screening of durum wheat (Triticum turgidum L. subsp. durum (Desf.) Husn.) Italian cultivars for susceptibility to Fusarium Head Blight incited by Fusarium graminearum. Plants 2021, 10, 68. [Google Scholar] [CrossRef]
- Schöneberg, T.; Kibler, K.; Wettstein, F.E.; Bucheli, T.D.; Forrer, H.R.; Musa, T.; Mascher, F.; Bertossa, M.; Keller, B.; Vogelgsang, S. Influence of temperature, humidity duration and growth stage on the infection and mycotoxin production by Fusarium langsethiae and Fusarium poae in oats. Plant Pathol. 2019, 68, 173–184. [Google Scholar] [CrossRef]
- Aamot, H.U.; Mousavi, H.; Razzaghian, J.; Brodal, G.; Sulyok, M.; Krska, R.; Edwards, S.G.; Hofgaard, I.S. Fusarium langsethiae and mycotoxin contamination in oat grain differed with growth stage at inoculation. Eur. J. Plant Pathol. 2022, 164, 59–78. [Google Scholar] [CrossRef]
- Xu, X.-M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Brennan, J.; Monaghan, S.; Moretti, A.; Mule, G.; et al. Relationship between the fungal complex causing Fusarium head blight of wheat and environmental conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef]
- Parikka, P.; Hakala, K.; Tiilikkala, K. Expected shifts in Fusarium species’ composition on cereal grain in Northern Europe due to climatic change. Food Addit. Contam. Part A 2012, 29, 1543–1555. [Google Scholar] [CrossRef]
- Czaban, J.; Wróblewska, B.; Sułek, A.; Mikos, M.; Boguszewska, E.; Podolska, G.; Nieróbca, A. Colonisation of winter wheat grain by Fusarium spp. and mycotoxin content as dependent on a wheat variety, crop rotation, a crop management system and weather conditions. Food Addit. Contam. Part A 2015, 32, 874–910. [Google Scholar] [CrossRef]
- Medina, A.; Magan, N. Comparisons of water activity and temperature impacts on growth of Fusarium langsethiae strains from northern Europe on oat-based media. Int. J. Food Microbiol. 2010, 142, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Madden, L.V.; Edwards, S. Modelling the effects of environmental conditions on HT2 andT2 toxin accumulation in field oat grains. Phytopathology 2014, 104, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Divon, H.H.; Razzaghian, J.; Aamot, H.U.; Klemsdal, S.S. Fusarium langsethiae (Torp and Nirenberg), investigation of alternative infection routes in oats. Eur. J. Plant Pathol. 2012, 132, 147–161. [Google Scholar] [CrossRef]
- Gilbert, J.; Woods, S.M.; Kromer, U. Germination of ascospores of Gibberella zeae after exposure to various levels of relative humidity and temperature. Phytopathology 2008, 98, 504–508. [Google Scholar] [CrossRef]
- Kaukoranta, T.; Hietaniemi, V.; Ramo, S.; Koivisto, T.; Parikka, P. Contrasting responses of T-2, HT-2 and DON mycotoxins and Fusarium species in oat to climate, weather, tillage and cereal intensity. Eur. J. Plant Pathol. 2019, 155, 93–110. [Google Scholar] [CrossRef]
- Kolawole, O.; De Ruyck, K.; Greer, B.; Meneely, J.; Doohan, F.; Danaher, M.; Elliott, C. Agronomic factors influencing the scale of Fusarium mycotoxin contamination of oats. J. Fungi 2021, 7, 965. [Google Scholar] [CrossRef]
- Mielniczuk, E.; Wit, M.; Patkowska, E.; Cegiełko, M.; Wakuliński, W. Reaction of oat genotypes to Fusarium equiseti (Corda) Sacc. infection and mycotoxin concentrations in grain. Agronomy 2022, 12, 295. [Google Scholar] [CrossRef]
- Morcia, C.; Ghizzoni, R.; Delogu, C.; Andreani, L.; Carnevali, P.; Terzi, V. Digital PCR: What relevance to plant studies? Biology 2020, 9, 433. [Google Scholar] [CrossRef]
- Morcia, C.; Tumino, G.; Gasparo, G.; Ceresoli, C.; Fattorini, C.; Ghizzoni, R.; Carnevali, P.; Terzi, V. Moving from qPCR to chip digital PCR assays for tracking of some Fusarium species causing Fusarium Head Blight in cereals. Microorganisms 2020, 8, 1307. [Google Scholar] [CrossRef] [PubMed]
- Belyakov, M.V.; Moskovskiy, M.N.; Litvinov, M.A.; Lavrov, A.V.; Khamuev, V.G.; Efremenkov, I.Y.; Gerasimenko, S.A. Method of optical diagnostics of grain seeds infected with Fusarium. Appl. Sci. 2022, 12, 4824. [Google Scholar] [CrossRef]
- Moskovskiy, M.N.; Belyakov, M.V.; Dorokhov, A.S.; Boyko, A.A.; Belousov, S.V.; Noy, O.V.; Gulyaev, A.A.; Akulov, S.I.; Povolotskaya, A.; Efremenkov, I.Y. design of device for optical luminescent diagnostic of the seeds infected by Fusarium. Agriculture 2023, 13, 619. [Google Scholar] [CrossRef]
- Pankin, D.; Povolotckaia, A.; Kalinichev, A.; Povolotskiy, A.; Borisov, E.; Moskovskiy, M.; Gulyaev, A.; Lavrov, A.; Izmailov, A. Complex spectroscopic study for Fusarium genus fungi infection diagnostics of “Zalp” cultivar oat. Agronomy 2021, 11, 2402. [Google Scholar] [CrossRef]
- Janik, E.; Niemcewicz, M.; Podogrocki, M.; Ceremuga, M.; Gorniak, L.; Stela, M.; Bijak, M. The existing methods and novel approaches in mycotoxins’ detection. Molecules 2021, 26, 3981. [Google Scholar] [CrossRef]
- Majer-Baranyi, K.; Adányi, N.; Székács, A. Current trends in mycotoxin detection with various types of biosensors. Toxins 2023, 15, 645. [Google Scholar] [CrossRef]
- Meneely, J.; Greer, B.; Kolawole, O.; Elliott, C. T-2 and HT-2 toxins: Toxicity, occurrence and analysis: A review. Toxins 2023, 15, 481. [Google Scholar] [CrossRef]
- Salina, M.; Tagliabue, G.; Ghizzoni, R.; Terzi, V.; Morcia, C. A Point-of-Care assay based on Reflective Phantom Interface (RPI) technology for fast, multi-toxin screening in wheat. Agronomy 2022, 12, 493. [Google Scholar] [CrossRef]
- Lattanzio, V.M.; Ciasca, B.; Terzi, V.; Ghizzoni, R.; McCormick, S.P.; Pascale, M. Study of the natural occurrence of T-2 and HT-2 toxins and their glucosyl derivatives from field barley to malt by high-resolution Orbitrap mass spectrometry. Food Addit. Contam. Part A 2015, 32, 1647–1655. [Google Scholar] [CrossRef]
- Tarazona, A.; Gómez, J.V.; Mateo, F.; Jiménez, M.; Mateo, E.M. Potential health risk associated with mycotoxins in oat grains consumed in Spain. Toxins 2021, 13, 421. [Google Scholar] [CrossRef]
- D’Agnello, P.; Vita, V.; Franchino, C.; Urbano, L.; Curiale, A.; Debegnach, F.; Iammarino, M.; Marchesani, G.; Chiaravalle, A.E.; De Pace, R. ELISA and UPLC/FLD as screening and confirmatory techniques for T-2/HT-2 mycotoxin determination in cereals. Appl. Sci. 2021, 11, 1688. [Google Scholar] [CrossRef]
- Teixido-Orries, I.; Molino, F.; Gatius, F.; Sanchis, V.; Marín, S. Near-infrared hyperspectral imaging as a novel approach for T-2 and HT-2 toxins estimation in oat samples. Food Control 2023, 153, 109952. [Google Scholar] [CrossRef]
- Doehlert, D.C.; Rays-Duarte, P.; McMullen, M.S. Inhibition of Fusarium graminearum growth in flour gel cultures by hexane-soluble compounds from oat (Avena sativa L. ) flour. J. Food Prot. 2011, 74, 2188–2191. [Google Scholar] [CrossRef] [PubMed]
- Farrokhi, N.; Burton, R.A.; Brownfield, L.; Hrmova, M.; Wilson, S.M.; Bacic, A.; Fincher, G.B. Plant cell wall biosynthesis: Genetic, biochemical and functional genomics approaches to the identification of key genes. Plant Biotechnol. J. 2006, 4, 145–167. [Google Scholar] [CrossRef] [PubMed]
- Sella, L.; Gazzetti, K.; Castiglioni, C.; Schäfer, W.; Favaron, F. Fusarium graminearum possesses virulence factors common to Fusarium Head Blight of wheat and seedling rot of soybean but differing in their impact on disease severity. Phytopathology 2014, 104, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Tini, F.; Beccari, G.; Benfield, A.H.; Gardiner, D.M.; Covarelli, L. Role of the XylA gene, encoding a cell wall degrading en-zyme, during common wheat, durum wheat and barley colonization by Fusarium graminearum. Fungal Genet. Biol. 2020, 136, 103318. [Google Scholar] [CrossRef]
- Moscetti, I.; Tundo, S.; Janni, M.; Sella, L.; Gazzetti, K.; Tauzin, A.; Giardina, T.; Masci, S.; Favaron, F.; D’Ovidio, R. Constitutive expression of the xylanase inhibitor TAXI-III delays Fusarium head blight symptoms in durum wheat transgenic plants. Mol. Plant Microbe Interact. 2013, 26, 1464–1472. [Google Scholar] [CrossRef]
- Hoson, T. Physiological functions of plant cell coverings. J. Plant Res. 2002, 115, 277–282. [Google Scholar] [CrossRef]
- Luna, E.; Pastor, V.; Robert, J.; Flors, V.; Mauch-Mani, B.; Ton, J. Callose deposition: A multifaceted plant defense response. Mol. Plant Microbe Interact. 2011, 24, 183–193. [Google Scholar] [CrossRef]
- Piršelová, B.; Matušíková, I. Callose: The plant cell wall polysaccharide with multiple biological functions. Acta Physiol. Plant. 2013, 35, 635–644. [Google Scholar] [CrossRef]
- Buckeridge, M.S.; Rayon, C.; Urbanowicz, B.; Tine, M.A.S.; Carpita, N. Mixed linkage (1-3),(1-4)-β-D-glucans of Grasses. Cereal Chem. J. 2004, 81, 115–127. [Google Scholar] [CrossRef]
- Havrlentová, M.; Deáková, L.; Kraic, J.; Žofajová, A. Can β-D-glucan protect oat seeds against a heat stress? Nova Biotechnol. Chim. 2016, 15, 107–113. [Google Scholar] [CrossRef]
- Vega- Sanchez, M.E.; Verhertbruggen, Y.; Christensen, U.; Chen, X.; Sharma, V.; Varanasi, P.; Jobling, S.A.; Talbot, M.; White, R.G.; Joo, M.; et al. Loss of Cellulose synthase-like F6 function affects mixed-linkage glucan deposition, cell wall mechanical properties and defense responses in vegetative tissues of rice. Plant Physiol. 2012, 159, 56–69. [Google Scholar] [CrossRef]
- Havrlentová, M.; Gregusová, V.; Šliková, S.; Nemĕcek, P.; Hudcovicová, M.; Kuzmová, D. Relationship between the content of-D-Glucans and infection with Fusarium pathogens in oat (Avena sativa L.) plants. Plants 2020, 9, 1776. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.L.; Doehlert, D.C.; McMullen, M.S. Association of avenanthramide concentration in oat (Avena sativa L.) grain with crown rust incidence and genetic resistance. Cereal Chem. 2008, 85, 639–641. [Google Scholar] [CrossRef]
- Okazaki, Y.; Isobe, T.; Iwata, Y.; Matsukawa, T.; Matsuda, F.; Miyagawa, H.; Ishihara, A.; Nishioka, T.; Iwamura, H. Metabolism of avenanthramide phytoalexins in oats. Plant J. 2004, 39, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.L. Effect of chemical systemic acquired resistance elicitors on avenanthramide biosynthesis in oat (Avena sativa). J. Agric. Food Chem. 2011, 59, 7028–7038. [Google Scholar] [CrossRef] [PubMed]
- Wise, M.L.; Vinje, M.A.; Conley, S.P. Field application of benzothiadiazole (BTH) to oats (Avena sativa): Effects on crown rust resistance and avenanthramide production. Crop Sci. 2016, 56, 1904–1913. [Google Scholar] [CrossRef]
- Loskutov, I.G.; Khlestkina, E.K. Wheat, barley, and oat breeding for health benefit components in grain. Plants 2021, 10, 86. [Google Scholar] [CrossRef]
- Mahmoud, M.; Zhou, Z.; Kaur, R.; Bekele, W.; Tinker, N.A.; Singh, J. Toward the development of Ac/Ds transposon-mediated gene tagging system for functional genomics in oat (Avena sativa L.). Funct. Integr. Genom. 2022, 22, 669–681. [Google Scholar] [CrossRef]
- Isidro-Sánchez, J.; D’Arcy, K.; Verheecke-Vaessen, C.; Kahla, A.; Bekele, W.; Doohan, F.; Magan, N.; Medina, A. Genome-wide association mapping of Fusarium langsethiae infection and mycotoxin accumulation in oat (Avena sativa L.). Plant Genome 2020, 13, e20023. [Google Scholar] [CrossRef]
- Blake, V.C.; Woodhouse, M.R.; Lazo, G.R.; Odell, S.G.; Wight, C.P.; Tinker, N.A.; Wang, Y.; Gu, Y.Q.; Birkett, C.L.; Jannink, J.L.; et al. GrainGenes: Centralized small grain resources and digital platform for geneticists and breeders. Database 2019, 2019, baz065. [Google Scholar] [CrossRef]
- Tinker, N.A.; Kilian, A.; Wight, C.P.; Heller-, K.; Wenzl, P.; Rines, H.W.; Bjørnstad, Å.; Howarth, C.J.; Jannink, J.; Anderson, J.M.; et al. New DArT markers for oat provide enhanced map coverage and global germplasm characterization. BMC Genom. 2009, 22, 39. [Google Scholar] [CrossRef]
- Yao, E.; Blake, V.C.; Cooper, L.; Wight, C.P.; Michel, S.; Cagirici, H.B.; Lazo, G.R.; Birkett, C.L.; Waring, D.J.; Jannink, J.-L.; et al. GrainGenes: A data-rich repository for small grains genetics and genomics. Database 2022, 2022, baac034. [Google Scholar] [CrossRef]
- Maughan, P.J.; Lee, R.; Walstead, R.; Vickerstaff, R.J.; Fogarty, M.C.; Brouwer, C.R.; Reid, R.R.; Jay, J.J.; Bekele, W.A.; Jackson, E.W.; et al. Genomic insights from the first chromosome-scale assemblies of oat (Avena spp.) diploid species. BMC Biol. 2019, 17, 92. [Google Scholar] [CrossRef]
- Kamal, N.; Tsardakas Renhuldt, N.; Bentzer, J.; Gundlach, H.; Haberer, G.; Juhász, A.; Lux, T.; Bose, U.; Tye-Din, J.A.; Lang, D.; et al. The mosaic oat genome gives insights into a uniquely healthy cereal crop. Nature 2022, 606, 113–119. [Google Scholar] [CrossRef]
- Mascher, M. Towards a pan-genome for hexaploidy OAT. OAT 2022. In Proceedings of the 11th International Oat Conference, Perth, Australia, 10–13 October 2022. [Google Scholar]
- Tumino, G.; Voorrips, R.E.; Rizza, F.; Badeck, F.W.; Morcia, C.; Ghizzoni, R.; Germeier, C.U.; Paulo, M.-J.; Terzi, V.; Smulders, M.J. Population structure and genome-wide association analysis for frost tolerance in oat using continuous SNP array signal intensity ratios. Theor. Appl. Genet. 2016, 129, 1711–1724. [Google Scholar] [CrossRef]
- Tumino, G.; Voorrips, R.E.; Morcia, C.; Ghizzoni, R.; Germeier, C.U.; Paulo, M.-J.; Terzi, V.; Smulders, M.J. Genome-wide association analysis for lodging tolerance and plant height in a diverse European hexaploid oat collection. Euphytica 2017, 213, 163. [Google Scholar] [CrossRef]
- Haikka, H.; Manninen, O.; Hautsalo, J.; Pietilä, L.; Jalli, M.; Veteläinen, M. Genome-wide association study and genomic prediction for Fusarium graminearum resistance traits in nordic oat (Avena sativa L.). Agronomy 2020, 10, 174. [Google Scholar] [CrossRef]
- Khairullina, A.; Tsardakas Renhuldt, N.; Wiesenberger, G.; Bentzer, J.; Collinge, D.B.; Adam, G.; Bülow, L. Identification and functional characterisation of two oat UDP-glucosyltransferases involved in deoxynivalenol detoxification. Toxins 2022, 14, 446. [Google Scholar] [CrossRef]
- Stancic, T. Identification of Fusarium Resistance Traits in UK Oat Varieties. Ph.D. Thesis, Harper Adams University, Newport, UK, 2016. Available online: https://hau.repository.guildhe.ac.uk/id/eprint/17313 (accessed on 10 January 2024).
- Chaffin, A.S.; Huang, Y.-F.; Smith, S.; Bekele, W.A.; Babiker, E.; Gnanesh, B.N.; Foresman, B.J.; Blanchard, S.G.; Jay, J.J.; Reid, R.W.; et al. A consensus map in cultivated hexaploid oat reveals conserved grass synteny with substantial subgenome rearrangement. Plant Genome 2016, 9, plantgenome2015-10. [Google Scholar] [CrossRef]
- Blackshaw-Crosby, J. Investigating Fusarium Resistance in UK Winter Oats. Ph.D. Thesis, Harper Adams University, Newport, UK, 2022. Available online: https://hau.repository.guildhe.ac.uk/id/eprint/17857 (accessed on 10 January 2024).
- Willforss, J.; Leonova, S.; Tillander, J.; Andreasson, E.; Marttila, S.; Olsson, O.; Chawade, A.; Levander, F. Interactive proteogenomic exploration of response to Fusarium head blight in oat varieties with different resistance. J. Proteom. 2020, 218, 103688. [Google Scholar] [CrossRef]
- Yuan, M.; Jiang, Z.; Bi, G.; Nomura, K.; Liu, M.; Wang, Y.; Cai, B.; Zhou, J.-M.; He, S.Y.; Xin, X.-F. Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature 2021, 592, 105–109. [Google Scholar] [CrossRef]
- Ngou, B.P.M.; Ahn, H.K.; Ding, P.; Jones, J.D.G. Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature 2021, 592, 110–115. [Google Scholar] [CrossRef]
- Pruitt, R.N.; Gust, A.A.; Nurnberger, T. Plant immunity unified. Nat. Plants 2021, 7, 382–383. [Google Scholar] [CrossRef]
- Chen, R.; Gajendiran, K.; Wulff, B.B. R we there yet? Advances in cloning resistance genes for engineering immunity in crop plants. Cur. Opin. Plant Biol. 2024, 77, 102489. [Google Scholar] [CrossRef]
- Kourelis, J.; van der Hoor, R.A.L. Defended to the nines: 25 years of resistance gene cloning identifies nine mechanisms for R protein function. Plant Cell 2018, 30, 285–299. [Google Scholar] [CrossRef]
- Förderer, A.; Li, E.; Lawson, A.W.; Deng, Y.N.; Sun, Y.; Logemann, E.; Zhang, X.; Wen, J.; Han, Z.; Chang, J.; et al. A wheat resistosome defines common principles of immune receptor channels. Nature 2022, 610, 532–539. [Google Scholar] [CrossRef]
- Maidment, J.H.R.; Shimizu, M.; Bentham, A.R.; Vera, S.; Franceschetti, M.; Longya, A.; Stevenson, C.E.M.; De la Concepcion, J.C.; Białas, A.; Kamoun, S.; et al. Effector target-guided engineering of an integrated domain expands the disease resistance profile of a rice NLR immune receptor. eLife 2023, 12, e81123. [Google Scholar] [CrossRef]
- Kourelis, J.; Marchal, C.; Posbeyikian, A.; Harant, A.; Kamoun, S. NLR immune receptor-nanobody fusions confer plant disease resistance. Science 2023, 379, 934–939. [Google Scholar] [CrossRef]
- Li, S.; Lin, D.; Zhang, Y.; Deng, M.; Chen, Y.; Lv, B.; Li, B.; Lei, Y.; Wang, Y.; Zhao, L.; et al. Genome-edited powdery mildew resistance in wheat without growth penalties. Nature 2022, 602, 455–460. [Google Scholar] [CrossRef]
- Gawehns, F.; Cornelissen, B.J.C.; Takken, F.L.W. The potential of effector-target genes in breeding for plant innate immunity. J. Microbial. Biotechnol. 2013, 6, 223–229. [Google Scholar] [CrossRef]
- Moonjely, S.; Ebert, M.; Paton-Glassbrook, D.; ANoel, Z.A.; Roze, L.; Shay, R.; Watkins, T.; Trail, F. Update on the state of research to manage Fusarium head blight. Fungal Gen. Biol. 2023, 169, 103829. [Google Scholar] [CrossRef]
- Mentges, M.; Glasenapp, A.; Boenisch, M.; Malz, S.; Henrissat, B.; Frandsen, R.J.; Güldener, U.; Münsterkötter, M.; Bormann, J.; Lebrun, M.H.; et al. Infection cushions of Fusarium graminearum are fungal arsenals for wheat infection. Mol. Plant Pathol. 2020, 21, 1070–1087. [Google Scholar] [CrossRef]
- Xu, M.; Huang, Z.; Zhu, W.; Liu, Y.; Bai, X.; Zhang, H. Fusarium-Derived secondary metabolites with antimicrobial effects. Molecules 2023, 28, 3424. [Google Scholar] [CrossRef]
- Snelders, N.C.; Rovenich, H.; Petti, G.C.; Rocafort, M.; van den Berg, G.C.M.; Vorholt, J.A.; Mesters, J.R.; Seidl, M.F.; Nijland, R.; Thomma, B.P.H.J. Microbiome manipulation by a soil-borne fungal plant pathogen using effector proteins. Nat. Plants 2020, 6, 1365–1374. [Google Scholar] [CrossRef]
- Gunupuru, L.R.; Perochon, A.; Doohan, F.M. Deoxynivalenol resistance as a component of FHB resistance. Trop. Plant Pathol. 2017, 42, 175–183. [Google Scholar] [CrossRef]
- Collemare, J.; O’Connell, R.; Lebrun, M. Nonproteinaceous effectors: The terra incognita of plant-fungal interactions. New Phytol. 2019, 223, 590–596. [Google Scholar] [CrossRef]
- Abid, M.; Fayolle, L.; Edel-Hermann, V.; Gautheron, N.; Héraud, C.; Leplat, J.; Stein-berg, C. Fate of deoxynivalenol (DON) and impact on the soil microflora and soil fauna. Appl. Soil Ecol. 2021, 162, 103898. [Google Scholar] [CrossRef]
- Gao, X.; Mu, P.; Wen, J.; Sun, Y.; Chen, Q.; Deng, Y. Detoxification of trichothecene mycotoxins by a novel bacterium, Eggerthella sp. DII-9. Food Chem. Toxicol. 2018, 112, 310–319. [Google Scholar] [CrossRef]
- Hassan, Z.U.; Al Thani, R.; Alsafran, M.; Migheli, Q.; Jaoua, S. Selection of Bacillus spp. with decontamination potential on multiple Fusarium mycotoxins. Food Control 2021, 127, 108119. [Google Scholar] [CrossRef]
- Liu, M.; Zhao, L.; Gong, G.; Zhang, L.; Shi, L.; Dai, J.; Han, Y.; Wu, Y.; Khalil, M.M.; Sun, L. Invited review: Remediation strategies for mycotoxin control in feed. J. Anim. Sci. Biotechnol. 2022, 13, 19. [Google Scholar] [CrossRef]
- Noel, Z.A.; Roze, L.V.; Breunig, M.; Trail, F. Endophytic fungi as a promising biocontrol agent to protect wheat from Fusarium graminearum Head Blight. Plant Dis. 2022, 106, 595–602. [Google Scholar] [CrossRef]
- Karlsson, I.; Persson, P.; Friberg, H. Fusarium Head Blight from a microbiome perspective. Front. Microbiol. 2021, 12, 371. [Google Scholar] [CrossRef]
- Elnahal, A.S.M.; El-Saadony, M.T.; Saad, A.M.; Desoky, E.S.M.; El-Tahan, A.M.; Rady, M.M.; AbuQamar, S.F.; El-Tarabily, K.A. The use of microbial inoculants for biological control, plant growth promotion, and sustainable agriculture: A review. Eur. J. Plant Pathol. 2022, 162, 759–792. [Google Scholar] [CrossRef]
- Available online: https://scanoats.se/controlling-fusarium-head-blight-in-oat/ (accessed on 10 January 2024).
- Comby, M.; Gacoin, M.; Robineau, M.; Rabenoelina, F.; Ptas, S.; Dupont, J.; Profizi, C.; Baillieul, F. Screening of wheat endophytes as biological control agents against Fusarium head blight using two different in vitro tests. Microbiol. Res. 2017, 202, 11–20. [Google Scholar] [CrossRef]
- Rojas, E.C.; Jensen, B.; Jørgensen, H.J.L.; Latz, M.A.C.; Esteban, P.; Ding, Y.; Collinge, D.B. Selection of fungal endophytes with biocontrol potential against Fusarium head blight in wheat. Biol. Control 2020, 144, 104222. [Google Scholar] [CrossRef]
- Wang, L.S.; Zhang, Y.; Zhang, M.Q.; Gong, D.C.; Mei, Y.Z.; Dai, C.C. Engineered Phomopsis liquidambaris with Fhb1 and Fhb7 enhances resistance to Fusarium graminearum in wheat. J. Agric. Food Chem. 2023, 71, 1391–1404. [Google Scholar] [CrossRef]
- Petrucci, A.; Khairullina, A.; Sarrocco, S.; Jensen, D.F.; Jensen, B.; Jørgensen, H.J.L.; Collinge, D.B. Understanding the mechanisms underlying biological control of Fusarium diseases in cereals. Eur. J. Plant Pathol. 2023, 167, 453–476. [Google Scholar] [CrossRef]
- Khairullina, A. Controlling Fusarium Head Blight in Oat. Pure and Applied Biochemistry. Ph.D. Thesis, Lund University, Center for Chemistry and Chemical Engineering, Department of Pure and Applied Biochemistry, Lund, Sweden, 2023. [Google Scholar]
- Khairullina, A.; Micic, N.; Jørgensen, H.J.L.; Bjarnholt, N.; Bülow, L.; Collinge, D.B.; Jensen, B. Biocontrol effect of Clonostachys rosea on Fusarium graminearum infection and mycotoxin detoxification in oat (Avena sativa). Plants 2023, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Pathi, K.M.; Sprink, T. From petri dish to field: Plant tissue culture and genetic engineering of oats for improved agricultural outcomes. Plants 2023, 12, 3782. [Google Scholar] [CrossRef] [PubMed]
- Donoso, T. Standardizing the CRISPR-Cas9 System in Oat to Understand Beta-Glucan Regulation. Master’s Thesis, McGill University, Montréal, QC, Canada, 2021. Available online: https://escholarship.mcgill.ca/concern/theses/7p88cn79j (accessed on 10 January 2024).
- Bekalu, Z.E.; Krogh Madsen, C.; Dionisio, G.; Bæksted Holme, I.; Jørgensen, L.N.; Fomsgaard, I.S.; Brinch-Pedersen, H. Overexpression of nepenthesin HvNEP-1 in barley endosperm reduces Fusarium Head Blight and mycotoxin accumulation. Agronomy 2020, 10, 203. [Google Scholar] [CrossRef]
- Joshi, R.K.; Nayak, S. Gene pyramiding-A broad spectrum technique for developing durable stress resistance in crops. Biotechnol. Mol. Biol. Rev. 2010, 5, 51–60. [Google Scholar]

| Mycotoxin | TDI μg·kg−1 Bodyweight per Day | Food | Maximum Level (μg·kg−1) | Indicative Level (μg·kg−1) |
|---|---|---|---|---|
| DON | 1.0 (EFSA Journal 2017;15(9):4718) [15] | Unprocessed durum wheat grains oat grains | 1750 | |
| Cereals placed on the market for the final consumer, cereal flour, semolina, bran and germ as final product placed on the market for the final consumer except milling products of maize | 750 | |||
| Bread, pastries, biscuits, cereal snacks and breakfast cereals | 500 | |||
| Baby food and processed cereal-based food for infants and young children | 200 | |||
| ZEN | 0.25 (EFSA Journal 2011;9(6):2197 2016;14(4):4425) [16,17] | Unprocessed cereal grains except maize grains | 100 | |
| Cereals placed on the market for the final consumer, cereal flour, bran and germ as final product placed on the market for the final consumer except maize | 75 | |||
| Bread, pastries, biscuits, cereal snacks and breakfast cereals except maize | 50 | |||
| Baby food and processed cereal-based food for infants and young children | 20 | |||
| T-2, HT-2 | 0.02 (EFSA Journal 2017;15(1):4655 2017;15(8):4972) [18,19] | Unprocessed cereals: oats (with husk) | 1000 | |
| Cereal grains for direct human consumption: oats | 200 | |||
| Oat bran and flaked oats | 200 | |||
| Oat milling products other than oat bran and flaked oats | 100 | |||
| Breakfast cereals including formed cereal flakes | 75 | |||
| Bread (including small bakery wares), pastries, biscuits, cereal snacks, pasta | 25 | |||
| Cereal-based foods for infants and young children | 15 | |||
| Fumonisin B1 | 1.0 (EFSA Journal 2018;16(2):5172) [20] | Oat grains and oat products | ND * | ND * |
| NIV | 1.2 (EFSA Journal 2017;15(4):4751) [21] | Oat grains and oat products | ND * | ND * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morcia, C.; Terzi, V.; Ghizzoni, R.; Carrara, I.; Gazzetti, K. Looking for Fusarium Resistance in Oats: An Update. Agronomy 2024, 14, 505. https://doi.org/10.3390/agronomy14030505
Morcia C, Terzi V, Ghizzoni R, Carrara I, Gazzetti K. Looking for Fusarium Resistance in Oats: An Update. Agronomy. 2024; 14(3):505. https://doi.org/10.3390/agronomy14030505
Chicago/Turabian StyleMorcia, Caterina, Valeria Terzi, Roberta Ghizzoni, Ilaria Carrara, and Katia Gazzetti. 2024. "Looking for Fusarium Resistance in Oats: An Update" Agronomy 14, no. 3: 505. https://doi.org/10.3390/agronomy14030505
APA StyleMorcia, C., Terzi, V., Ghizzoni, R., Carrara, I., & Gazzetti, K. (2024). Looking for Fusarium Resistance in Oats: An Update. Agronomy, 14(3), 505. https://doi.org/10.3390/agronomy14030505








