The Combined Use of Soil Conditioner and Foliar Sulfur Spray Successfully Prevents Dark Pericarp Disease Induced by Manganese Toxicity in Litchi
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Litchi Orchard and Tree Used
2.2. Soil Conditioner, Ascorbic Acid, Sulfur, S Adjuvant and Standard Materials
2.3. Field Experiment Scheme
2.4. Fruit Harvest, Sample Collection and Preparation
2.5. Quantification of Pericarp Element
2.6. Measurement of Antioxidase Activity in Pericarp
2.7. Detection of Phenolic Compounds in Pericarp
2.8. Analysis of Sugar and Organic Acid Components in Pericarp and Flesh
2.9. Determination of Soil Property
2.10. Data Analysis and Statistics
3. Results
3.1. The Morbidity of DPD in Litchi Fruit
3.2. Pericarp Mn, Fe, Al, S, Ca, and Mg
3.3. Phenolic Compounds in Litchi Pericarp
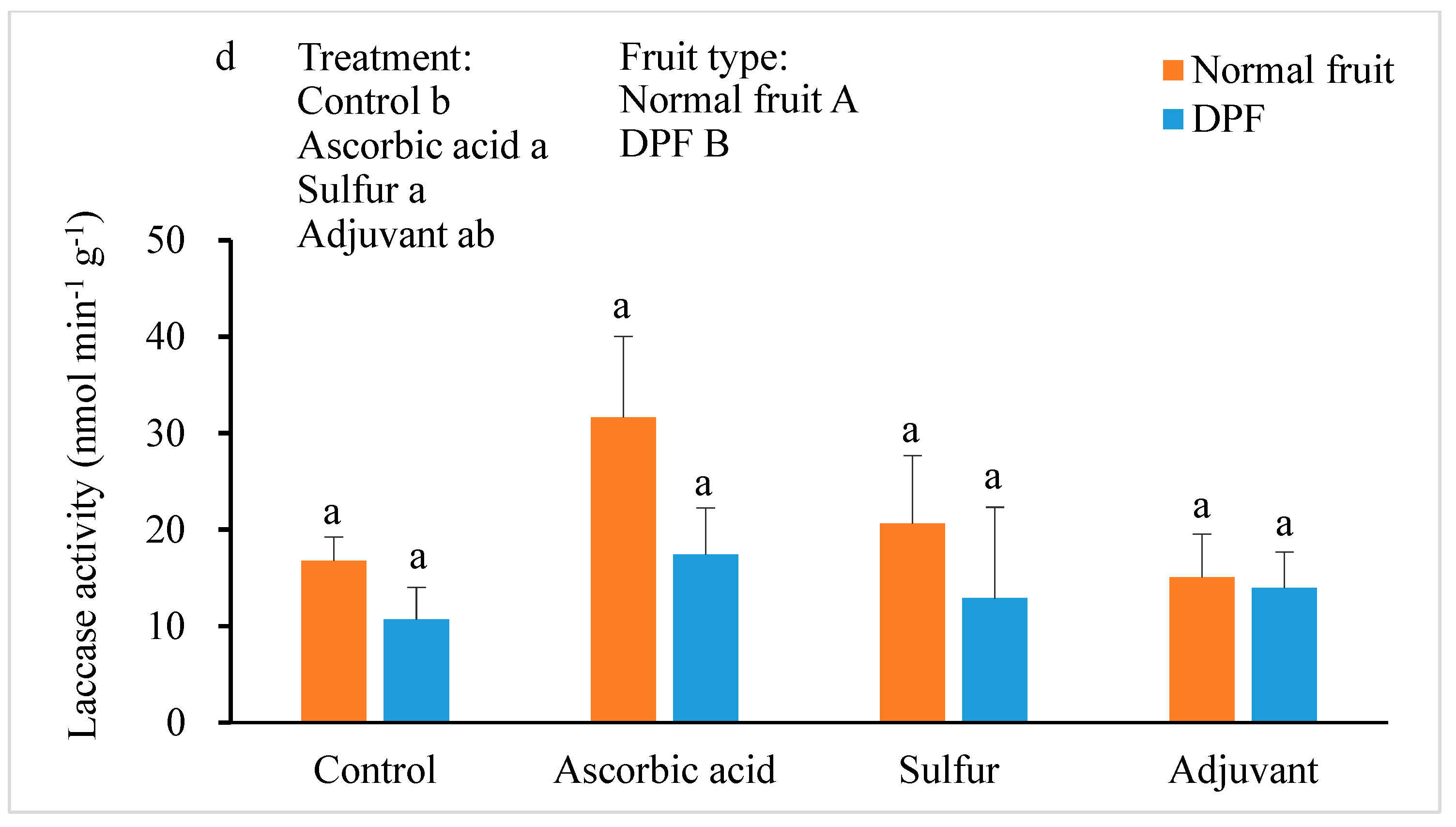
3.4. Activities of Antioxidant Enzymes
3.5. Sugar Components in Litchi Pericarp
3.6. Sugar and Organic Acid Components in Litchi Pulp
3.7. Soil Properties
4. Discussion
4.1. The Role of Soil Conditioner
4.2. The Effect of Sulfur Spray
4.3. The Influence of Ascorbic Acid Spray
4.4. The Impact of Adjuvant Spray
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Chen, L.S.; Jiang, H.X.; Tang, N.; Yang, L.T.; Lin, Z.H.; Li, Y.; Yang, G.H. Effects of manganese-excess on CO2 assimilation, ribulose-1,5-bisphosphate carboxylase/oxygenase, carbohydrates and photosynthetic electron transport of leaves, and antioxidant systems of leaves and roots in Citrus grandis seedlings. Bmc Plant Biol. 2010, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.E.; Cheng, H.B.; Luo, T.; Song, F.X. Development status, trend and suggestion of litchi production in mainland China. Guangdong Agric. Sci. 2019, 46, 132–139. (In Chinese) [Google Scholar]
- Xie, Z.N.; Zhuang, Y.M.; Wang, R.J.; Xu, W.B. Correlation between soil pH and the contents of available nutrients in selected soils from three kinds of orchards at subtropical zone in Fujian. Acta Hortic. Sin. 1997, 24, 209–214. (In Chinese) [Google Scholar]
- Guo, H.R.; Chen, Q.X.; Peng, Z.P.; Lin, C.L. Yield increasing effect of modifier application on litch and soil improvement. J. Yangtze Univ. (Nat. Sci. Ed.) 2005, 8, 16–19. (In Chinese) [Google Scholar]
- Su, X.X.; Liu, Q.H.; Bai, C.H.; Zhou, C.M.; Yao, L.X. Investigation and analysis on soil properties of litchi orchards in South China. Chin. J. Trop. Crops 2021, 42, 3165–3172. (In Chinese) [Google Scholar] [CrossRef]
- Su, X.X.; Zhu, Y.C.; Bai, C.H.; Liu, H.L.; Wei, Z.H.; Yao, L.X. Dark pericarp disease in litchi is induced by manganese stress. Plant Soil 2022, 481, 563–579. [Google Scholar] [CrossRef]
- Liu, S.L.; Xiao, Y.P.; Bai, C.H.; Liu, H.L.; Su, X.X.; Jin, P.; Xu, H.T.; Cao, L.X.; Yao, L.X. The physiological and biochemical responses to dark pericarp disease induced by excess manganese in litchi. Plant Physiol. Biochem. 2024, 206, 108269. [Google Scholar] [CrossRef]
- Wei, J.B.; Zhang, X.; Zhong, R.H.; Liu, B.; Zhang, X.L.; Fang, F.; Zhang, Z.Q.; Pang, X.Q. Laccase-Mediated Flavonoid Polymerization Leads to the Pericarp Browning of Litchi Fruit. J. Agric. Food Chem. 2021, 69, 15218–15230. [Google Scholar] [CrossRef]
- Kula, E.; Wildova, E.; Hrdlicka, P. Accumulation and dynamics of manganese content in bilberry (Vaccinium myrtillus L.). Environ. Monit. Assess. 2018, 190, 224. [Google Scholar] [CrossRef]
- Wildová, E.; Elznicová, J.; Kula, E. Seasonal dynamics of manganese accumulation in European larch (Larix decidua Mill.), silver birch (Betula pendula Roth), and bilberry (Vaccinium myrtillus L.) over 10 years of monitoring. Environ. Monit. Assess. 2021, 193, 612. [Google Scholar] [CrossRef]
- Wollmer, A.C.; Pitann, B.; Mühling, K.H. Timing of waterlogging is crucial for the development of micronutrient deficiencies or toxicities in winter wheat and rapeseed. J. Plant Growth Regul. 2019, 38, 824–830. [Google Scholar] [CrossRef]
- Fernando, D.R.; Moroni, S.J.; Scott, B.J.; Conyers, M.K.; Lynch, J.P.; Marshall, A.T. Temperature and light drive manganese accumulation and stress in crops across three major plant families. Environ. Exp. Bot. 2016, 132, 66–79. [Google Scholar] [CrossRef]
- NCC. Blue Book on Climate Change in China 2021; Science Press: Beijing, China, 2022. (In Chinese)
- Xie, Q.X.; Gu, X.P.; Li, G.; Tang, T.R.; Li, Z.Y. Variation characteristics of rainstorms and floods in southwest China and their relationships with atmospheric circulation in the summer half-year. Atmosphere 2022, 13, 2103. [Google Scholar] [CrossRef]
- Park, B.J.; Min, S.K.; Weller, E. Lengthening of summer season over the Northern Hemisphere under 1.5 °C and 2.0 °C global warming. Environ. Res. Lett. 2021, 17, 014012. [Google Scholar] [CrossRef]
- Lin, W.; Wang, C.Z. Longer summers in the Northern Hemisphere under global warming. Clim. Dyn. 2022, 58, 2293–2307. [Google Scholar] [CrossRef]
- Chatzistathis, T.; Alifragis, D.; Papaioannou, A. The influence of liming on soil chemical properties and on the alleviation of manganese and copper toxicity in Juglans regia, Robinia pseudoacacia, Eucalyptus sp. and Populus sp. plantations. J. Environ. Manag. 2015, 150, 149–156. [Google Scholar] [CrossRef]
- Hue, N.V.; Mai, Y. Manganese toxicity in watermelon as affected by lime and compost amended to a Hawaiian acid oxisol. Hortscience 2002, 37, 656–661. [Google Scholar] [CrossRef]
- Pang, X.Q.; Duan, X.W.; Zhang, Z.Q.; Xu, F.C.; Ji, Z.L. Purification and some properties of peroxidase from pericarp of Litchi (Litchi chinensis Sonn.). J. Trop. Subtrop. Bot. 2004, 12, 5. [Google Scholar]
- Liu, C.L. Characterization of peroxidase from pulp of litchi. Southwest China J. Agric. Sci. 2012, 25, 424–428. (In Chinese) [Google Scholar]
- Liu, L.; Cao, S.Q.; Xu, Y.J.; Zhang, M.W.; Xiao, G.S.; Deng, Q.C.; Xie, B.J. Oxidation of (-)-epicatechin is a precursor of litchi pericarp enzymatic browning. Food Chem. 2010, 118, 508–511. [Google Scholar] [CrossRef]
- Gacche, R.N.; Warangkar, S.C.; Ghole, V.S. Glutathione and cinnamic acid: Natural dietary components used in preventing the process of browning by inhibition of polyphenol oxidase in apple juice. J. Enzym. Inhib. Med. Chem. 2004, 19, 175–179. [Google Scholar] [CrossRef]
- Liang, Y.S.; Chen, N.L.; Ke, L.S. Influence of dipping in sodium metabisulfite on pericarp browning of litchi cv. Yu Her Pau (Feizixiao). Postharvest Biol. Technol. 2012, 68, 72–77. [Google Scholar] [CrossRef]
- Na, G.; Salt, D.E. The role of sulfur assimilation and sulfur-containing compounds in trace element homeostasis in plants. Environ. Exp. Bot. 2011, 72, 18–25. [Google Scholar] [CrossRef]
- Ragab, G.; Saad-Allah, K. Seed priming with greenly synthesized sulfur nanoparticles enhances antioxidative defense machinery and restricts oxidativeiInjury under manganese stress in Helianthus annuus (L.) seedlings. J. Plant Growth Regul. 2020, 40, 1894–1902. [Google Scholar] [CrossRef]
- Saad-Allah, K.M.; Ragab, G.A. Sulfur nanoparticles mediated improvement of salt tolerance in wheat relates to decreasing oxidative stress and regulating metabolic activity. Physiol. Mol. Biol. Plants 2020, 26, 2209–2223. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.Y.; Liu, Q.Q.; Guo, Z.; Fu, J.H.; Sun, Y.M.; Gu, C.S.; Xing, B.S.; Dhankher, O.P. Sulfur nanoparticles improved plant growth and reduced mercury toxicity via mitigating the oxidative stress in Brassica napus L. J. Clean. Prod. 2021, 318, 128589. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef]
- Lu, R.K. Methods for Soil and Agrochemical Analysis; China Agricultural Science & Technology Press: Beijing, China, 2000. (In Chinese) [Google Scholar]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.D.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Klisurova, D.; Petrova, I.; Ognyanov, M.; Georgiev, Y.; Kratchanova, M.; Denev, P. Co-pigmentation of black chokeberry (Aronia melanocarpa) anthocyanins with phenolic co-pigments and herbal extracts. Food Chem. 2019, 279, 162–170. [Google Scholar] [CrossRef]
- Blamey, F.P.C.; McKenna, B.A.; Li, C.; Cheng, M.; Tang, C.; Jiang, H.; Howard, D.L.; Paterson, D.J.; Kappen, P.; Wang, P.; et al. Manganese distribution and speciation help to explain the effects of silicate and phosphate on manganese toxicity in four crop species. New Phytol. 2018, 217, 1146–1160. [Google Scholar] [CrossRef]
- He, L.J.; Su, R.K.; Chen, Y.H.; Zeng, P.; Du, L.; Cai, B.; Zhang, A.S.; Zhu, H.H. Integration of manganese accumulation, subcellular distribution, chemical forms, and physiological responses to understand manganese tolerance in Macleaya cordata. Environ. Sci. Pollut. Res. 2022, 29, 39017–39026. [Google Scholar] [CrossRef]
- Baldisserotto, C.; Ferroni, L.; Anfuso, E.; Pagnoni, A.; Fasulo, M.P.; Pancaldi, S. Responses of Trapa natans L. floating laminae to high concentrations of manganese. Protoplasma 2007, 231, 65–82. [Google Scholar] [CrossRef]
- Wissemeier, A.H.; Horst, W.J. Effect of light intensity on manganese toxicity symptoms and callose formation in cowpea (Vigna unguiculata (L.) Walp.). Plant Soil 1992, 143, 299–309. [Google Scholar] [CrossRef]
- Fecht-Christoffers, M.M.; Maier, P.; Iwasaki, K.; Braun, H.P.; Horst, W.J. The role of the leaf apoplast in manganese toxicity and tolerance in cowpea (Vigna unguiculata L. Walp). In The Apoplast of Higher Plants: Compartment of Storage, Transport and Reactions; Sattelmacher, B., Horst, W.J., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 307–321. [Google Scholar]
- Ribera-Fonseca, A.; Inostroza-Blancheteau, C.; Cartes, P.; Rengel, Z.; Mora, M.L. Early induction of Fe-SOD gene expression is involved in tolerance to Mn toxicity in perennial ryegrass. Plant Physiol. Biochem. 2013, 73, 77–82. [Google Scholar] [CrossRef]
- Fecht-Christoffers, M.M.; Maier, P.; Horst, W.J. Apoplastic peroxidases and ascorbate are involved in manganese toxicity and tolerance of Vigna unguiculata. Physiol. Plant. 2003, 117, 237–244. [Google Scholar] [CrossRef]
- Fernando, D.R.; Lynch, J.P. Manganese phytotoxicity: New light on an old problem. Ann. Bot. 2015, 116, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Zhang, X.L.; Luo, H.H.; Zhou, J.J.; Gong, Y.H.; Li, W.J.; Shi, Z.W.; He, Q.; Wu, Q.; Li, L.; et al. An Intracellular Laccase Is Responsible for Epicatechin-Mediated Anthocyanin Degradation in Litchi Fruit Pericarp. Plant Physiol. 2015, 169, 2391–2408. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Cui, Z.X.; Xu, H.; Wei, Z.H.; Zhang, J.W.; Yao, L.X. Comparison of fruit quality and flavor characteristics of new and fine litchi cultivars. J. Chin. Inst. Food Sci. Technol. 2023, 23, 327–338. (In Chinese) [Google Scholar] [CrossRef]
- Ge, S.F.; Hao, W.Q.; Jiang, H.; Wei, S.C.; Jiang, Y.M. Distribution characteristics of soil organic matter and pH and the correlation to soil nutrients in apple orchards of Yantai. Chin. Agric. Sci. Bull. 2014, 30, 274–278. (In Chinese) [Google Scholar] [CrossRef]
- Li, G.L.; Yao, L.X.; He, Z.H.; Zhang, Y.C.; Zhou, C.M.; Tu, S.H. Evaluation on soil nutrient fertility status in litchi plantations of Guangdong. Chin. J. Soil Sci. 2009, 40, 800–804. (In Chinese) [Google Scholar]
- Sparrow, L.A.; Uren, N.C. Manganese oxidation and reduction in soils: Effects of temperature, water potential, pH and their interactions. Soil Res. 2014, 52, 483–494. [Google Scholar] [CrossRef]
- Ragab, G.A.; Saad-Allah, K.M. Green synthesis of sulfur nanoparticles using Ocimum basilicum leaves and its prospective effect on manganese-stressed Helianthus annuus (L.) seedlings. Ecotoxicol. Environ. Saf. 2020, 191, 110242. [Google Scholar] [CrossRef]
- Sheng, H.J.; Zeng, J.; Liu, Y.; Wang, X.L.; Wang, Y.; Kang, H.Y.; Fan, X.; Sha, L.N.; Zhang, H.Q.; Zhou, Y.H. Sulfur mediated alleviation of Mn toxicity in Polish wheat relates to regulating Mn allocation and improving antioxidant system. Front. Plant Sci. 2016, 7, 1382. [Google Scholar] [CrossRef] [PubMed]
- Zakari, S.; Jiang, X.; Zhu, X.; Liu, W.; Allakonon, M.G.B.; Singh, A.K.; Chen, C.; Zou, X.; Akponikpe, P.B.I.; Dossa, G.G.O.; et al. Influence of sulfur amendments on heavy metals phytoextraction from agricultural contaminated soils: A meta-analysis. Environ. Pollut. 2021, 288, 117820. [Google Scholar] [CrossRef] [PubMed]
- Buchner, P.; Stuiver, C.E.; Westerman, S.; Wirtz, M.; Hell, R.; Hawkesford, M.J.; De Kok, L.J. Regulation of sulfate uptake and expression of sulfate transporter genes in Brassica oleracea as affected by atmospheric H2S and pedospheric sulfate nutrition. Plant Physiol. 2004, 136, 3396–3408. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H. Sulfate transport systems in plants: Functional diversity and molecular mechanisms underlying regulatory coordination. J. Exp. Bot. 2019, 70, 4075–4087. [Google Scholar] [CrossRef] [PubMed]
- Su, X.X.; Zhang, X.T.; Bai, C.H.; Liu, H.L.; Cao, X.Y.; Yao, L.X. Asymmetric distribution of mineral nutrients aggregates uneven fruit pigmentation driven by sunlight exposure in litchi. Planta 2023, 258, 96. [Google Scholar] [CrossRef] [PubMed]
- Rausch, T.; Wachter, A. Sulfur metabolism: A versatile platform for launching defence operations. Trends Plant Sci. 2005, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Cobbett, C.S. Phytochelatins and their roles in heavy metal detoxification. Plant Physiol. 2000, 123, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Cadmium stress tolerance in crop plants: Probing the role of sulfur. Plant Signal. Behav. 2011, 6, 215–222. [Google Scholar] [CrossRef]
- Fan, P.; Yin, J.; Zhong, G.D.; Wu, Z.H. Ascorbic acid alleviation of manganese-induced toxicity in Vallisneria natans (Lour.) Hara. Environ. Sci. Pollut. Res. 2020, 27, 32695–32706. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.J.; Ren, J.; Wang, X.H.; Liu, T.K.; Hou, X.L.; Li, Y. Ascorbic acid alleviates toxicity induced by excess copper in Brassica campestris ssp. Chinensis Makino. Commun. Soil Sci. Plant Anal. 2017, 48, 656–664. [Google Scholar] [CrossRef]
- Fecht-Christoffers, M.M.; Horst, W.J. Does apoplastic ascorbic acid enhance manganese tolerance of Vigna unguiculata and Phaseolus vulgaris? J. Plant Nutr. Soil Sci. 2005, 168, 590–599. [Google Scholar] [CrossRef]
- Ali, B.; Pantha, S.; Acharya, R.; Ueda, Y.; Wu, L.B.; Ashrafuzzaman, M.; Ishizaki, T.; Wissuwa, M.; Bulley, S.; Frei, M. Enhanced ascorbate level improves multi-stress tolerance in a widely grown indica rice variety without compromising its agronomic characteristics. J. Plant Physiol. 2019, 240, 152998. [Google Scholar] [CrossRef] [PubMed]



| Item | Mn (mg kg−1) | Fe (mg kg−1) | Al (mg kg−1) | S (g kg−1) | Ca (g kg−1) | Mg (g kg−1) |
|---|---|---|---|---|---|---|
| Treatment | ||||||
| Control | 226.2 ± 70.4 A | 34.1 ± 4.6 A | 40.9 ± 6.6 a | 0.83 ± 0.11 a | 4.30 ± 1.03 ab | 1.29 ± 0.27 B |
| Ascorbic acid | 204.6 ± 37.8 AB | 33.3 ± 6.7 A | 40.2 ± 4.3 a | 0.80 ± 0.06 a | 4.86 ± 0.48 ab | 1.42 ± 0.16 B |
| Sulfur | 190.2 ± 34.3 B | 28.2 ± 7.0 B | 40.0 ± 6.2 a | 0.80 ± 0.11 a | 4.98 ± 0.63 a | 1.63 ± 0.24 A |
| Adjuvant | 213.3 ± 21.6 AB | 34.0 ± 3.4 A | 38.4 ± 5.0 a | 0.81 ± 0.11 a | 4.04 ± 0.99 b | 1.34 ± 0.31 B |
| Fruit type | ||||||
| Normal fruit | 186.8 ± 31.4 B | 32.2 ± 5.1 a | 39.6 ± 5.4 a | 0.80 ± 0.09 a | 4.33 ± 0.99 b | 1.41 ± 0.27 a |
| DPF | 230.4 ± 48.0 A | 32.6 ± 7.0 a | 40.2 ± 5.9 a | 0.82 ± 0.10 a | 4.47 ± 0.96 a | 1.43 ± 0.29 a |
| Treatment × Fruit type | p < 0.05 | p < 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 |
| Item | Epicatechin (g kg−1) | Proanthocyanin A2 (g kg−1) | Proanthocyanin B2 (g kg−1) | Rutin (mg kg−1) | Ferulic Acid (mg kg−1) | Quercetin-3-Glucoside (mg kg−1) | Kaempferol-3-Glucoside (mg kg−1) | Cyanidin-3-Glucoside (mg kg−1) | Cyanidin-3-Rutinoside (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|
| Treatment | |||||||||
| Control | 6.5 ± 1.5 a | 11.8 ± 0.5 ab | 1.25 ± 0.24 a | 907 ± 158 ab | 76.9 ± 25.6 a | 93.3 ± 25.7 a | 235 ± 52 a | 20.8 ± 5.1 a | 343 ± 156 b |
| Ascorbic acid | 7.8 ± 1.4 a | 12.2 ± 0.6 a | 1.20 ± 0.30 a | 929 ± 89 ab | 77.9 ± 28.9 a | 86.2 ± 20.3 a | 217 ± 57 a | 21.4 ± 4.0 a | 431 ± 140 ab |
| Sulfur | 7.5 ± 1.1 a | 12.1 ± 0.4 a | 1.26 ± 0.22 a | 1002 ± 79 a | 87.9 ± 29.3 a | 92.0 ± 15.2 a | 210 ± 37 a | 22.6 ± 2.2 a | 511 ± 117 a |
| Adjuvant | 6.5 ± 1.6 a | 11.5 ± 0.9 b | 1.04 ± 0.31 a | 868 ± 80 b | 67.7 ± 19.3 a | 80.0 ± 13.0 a | 191 ± 56 a | 22.3 ± 4.8 a | 400 ± 175 ab |
| Fruit type | |||||||||
| Normal fruit | 6.5 ± 1.5 a | 11.7 ± 0.8 a | 1.1 ± 0.3 a | 915 ± 118 a | 68.7 ± 22.1 a | 78.1 ± 15.3 B | 197 ± 50 b | 22.8 ± 4.4 a | 466 ± 183 a |
| DPF | 7.2 ± 1.5 a | 12.1 ± 0.5 a | 1.2 ± 0.2 a | 939 ± 115 a | 86.1 ± 27.2 a | 97.5 ± 18.3 A | 229 ± 48 a | 20.8 ± 3.0 a | 384 ± 114 a |
| Treatment × Fruit type | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p < 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 |
| Item | Sucrose (g kg−1) | Glucose (g kg−1) | Galactose (g kg−1) | Fructose (g kg−1) |
|---|---|---|---|---|
| Treatment | ||||
| Control | 3.92 ± 1.52 b | 3.91 ± 1.00 ab | 8.76 ± 1.08 a | 4.22 ± 1.83 a |
| Ascorbic acid | 4.34 ± 0.88 b | 3.08 ± 1.07 b | 8.09 ± 0.87 a | 4.33 ± 1.40 a |
| Sulfur | 5.53 ± 0.97 a | 3.89 ± 0.81 ab | 8.15 ± 0.79 a | 5.38 ± 1.08 a |
| Adjuvant | 4.85 ± 1.50 ab | 4.22 ± 0.99 a | 8.78 ± 0.79 a | 4.93 ± 1.65 a |
| Fruit type | ||||
| Normal fruit | 4.88 ± 1.36 a | 3.86 ± 1.16 a | 8.35 ± 0.81 a | 5.02 ± 1.62 a |
| DPF | 4.43 ± 1.34 a | 3.69 ± 0.89 a | 8.54 ± 1.02 a | 4.41 ± 1.41 a |
| Treatment × Fruit type | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 |
| Item | Sucrose (g kg−1) | Glucose (g kg−1) | Galactose (g kg−1) | Fructose (g kg−1) |
|---|---|---|---|---|
| Treatment | ||||
| Control | 31.7 ± 7.5 a | 40.6 ± 4.0 a | 2.6 ± 0.4 b | 43.0 ± 4.2 a |
| Ascorbic acid | 29.7 ± 7.1 a | 37.8 ± 3.6 a | 3.5 ± 0.8 a | 40.0 ± 3.8 a |
| Sulfur | 29.7 ± 7.9 a | 37.2 ± 2.0 a | 3.0 ± 0.4 ab | 40.1 ± 2.0 a |
| Adjuvant | 31.3 ± 9.0 a | 40.5 ± 5.0 a | 2.6 ± 0.5 b | 43.0 ± 4.7 a |
| Fruit type | ||||
| Normal fruit | 29.3 ± 6.0 a | 39.4 ± 4.1 a | 2.8 ± 0.5 a | 42.1 ± 4.0 a |
| DPF | 31.9 ± 9.0 a | 38.7 ± 3.9 a | 3.0 ± 0.8 a | 41.0 ± 4.0 a |
| Treatment × Fruit type | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 |
| Item | Quinic Acid (g kg−1) | Malic Acid (g kg−1) | Tartaric Acid (g kg−1) | Oxalic Acid (mg kg−1) | Shikimic Acid (mg kg−1) | Ketoglutaric Acid (mg kg−1) | Citric Acid (mg kg−1) | Fumaric Acid (mg kg−1) | Ascorbic Acid (mg kg−1) | Acetic Acid (mg kg−1) |
|---|---|---|---|---|---|---|---|---|---|---|
| Treatment | ||||||||||
| Control | 2.80 ± 0.47 a | 3.57 ± 0.92 b | 3.81 ± 0.93 a | 286.9 ± 47.7 B | 155.6 ± 37.1 a | 96.0 ± 14.1 B | 293.9 ± 37.1 a | 13.3 ± 3.6 a | 556.1 ± 213.6 a | 729.4 ± 265.8 b |
| Ascorbic acid | 2.83 ± 0.54 a | 2.73 ± 0.53 b | 3.27 ± 0.52 a | 362.4 ± 49.6 A | 145.1 ± 40.5 ab | 88.7 ± 14.0 B | 277.0 ± 28.3 ab | 11.5 ± 4.5 ab | 608.2 ± 92.8 a | 892.0 ± 187.2 ab |
| Sulfur | 2.70 ± 0.50 a | 3.54 ± 1.21 b | 3.46 ± 0.60 a | 340.6 ± 45.5 A | 135.2 ± 21.7 ab | 88.1 ± 20.1 B | 269.0 ± 30.4 ab | 13.3 ± 3.2 a | 531.5 ± 99.8 a | 747.6 ± 99.5 b |
| Adjuvant | 2.66 ± 0.26 a | 4.74 ± 0.90 a | 3.59 ± 0.55 a | 349.1 ± 19.7 A | 121.9 ± 16.9 b | 131.8 ± 15.4 A | 260.4 ± 22.6 b | 9.6 ± 2.6 b | 555.0 ± 119.4 a | 977.4 ± 176.9 a |
| Fruit type | ||||||||||
| Normal fruit | 2.76 ± 0.41 a | 3.49 ± 1.31 a | 3.55 ± 0.72 a | 338.9 ± 52.9 a | 142.3 ± 33.6 a | 100.7 ± 22.9 a | 272.0 ± 31.7 a | 11.9 ± 3.8 a | 566.0 ± 146.1 a | 826.7 ± 219.4 a |
| DPF | 2.73 ± 0.48 a | 3.85 ± 0.95 a | 3.51 ± 0.64 a | 330.6 ± 48.0 a | 136.6 ± 31.1 a | 101.6 ± 25.4 a | 277.1 ± 31.2 a | 11.8 ± 3.7 a | 559.3 ± 132.2 a | 846.5 ± 208.6 a |
| Treatment × Fruit type | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 | p > 0.05 |
| Soil Attribute | Before the Use of Soil Conditioner | After the Use of Soil Conditioner (at Fruit Harvest) |
|---|---|---|
| pH | 4.54 ± 0.02 B | 5.37 ± 0.10 A |
| Organic matter (g kg−1) | 18.4 ± 0.1 B | 28.5 ± 0.4 A |
| Alkali-hydrolyzed N (mg kg−1) | 207 ± 1 A | 156 ± 7 B |
| Available P (mg kg−1) | 17.3 ± 0.4 a | 17.9 ± 0.7 a |
| Available K (mg kg−1) | 622 ± 23 a | 585 ± 53 a |
| Available Fe (mg kg−1) | 15.0 ± 1.0 a | 13.7 ± 3.7 a |
| Available Mn (mg kg−1) | 657 ± 26 A | 150 ± 24 B |
| Available Ca (mg kg−1) | 1583 ± 100 B | 2665 ± 79 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Bai, C.; Guo, Y.; Yang, Z.; Luo, X.; Liu, S.; Huang, Y.; Yao, L. The Combined Use of Soil Conditioner and Foliar Sulfur Spray Successfully Prevents Dark Pericarp Disease Induced by Manganese Toxicity in Litchi. Agronomy 2024, 14, 449. https://doi.org/10.3390/agronomy14030449
Liu H, Bai C, Guo Y, Yang Z, Luo X, Liu S, Huang Y, Yao L. The Combined Use of Soil Conditioner and Foliar Sulfur Spray Successfully Prevents Dark Pericarp Disease Induced by Manganese Toxicity in Litchi. Agronomy. 2024; 14(3):449. https://doi.org/10.3390/agronomy14030449
Chicago/Turabian StyleLiu, Huilin, Cuihua Bai, Yongjun Guo, Zhuo Yang, Xinping Luo, Silin Liu, Yinghui Huang, and Lixian Yao. 2024. "The Combined Use of Soil Conditioner and Foliar Sulfur Spray Successfully Prevents Dark Pericarp Disease Induced by Manganese Toxicity in Litchi" Agronomy 14, no. 3: 449. https://doi.org/10.3390/agronomy14030449
APA StyleLiu, H., Bai, C., Guo, Y., Yang, Z., Luo, X., Liu, S., Huang, Y., & Yao, L. (2024). The Combined Use of Soil Conditioner and Foliar Sulfur Spray Successfully Prevents Dark Pericarp Disease Induced by Manganese Toxicity in Litchi. Agronomy, 14(3), 449. https://doi.org/10.3390/agronomy14030449







