Genome-Wide Association Analysis of Seed Vigor-Related Traits in Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Standard Germination Test
day/number of test seeds) × 100%
day/number of test seeds) × 100%
2.3. Artificial Accelerated Aging Test
2.4. Electrical Conductivity Measurement
2.5. GWAS Analysis and Data Processing
2.6. Candidate Gene Analysis
3. Results
3.1. Variability Analysis of Wheat Seed Vigor-Related Traits under Different Experimental Conditions
3.1.1. Statistical Analysis of Wheat Seed Vigor-Related Traits
3.1.2. Analysis of Variance for Wheat Seed Vigor-Related Traits
3.1.3. Correlation Analysis of Wheat Seed Vigor-Related Traits
3.2. GWAS Analysis to Identify Seed Vigor-Related Genes
3.2.1. Genome-Wide Association Analysis of Traits under Standard Germination Test Conditions
3.2.2. Genome-Wide Association Analysis of Traits under Artificial Accelerated Aging Test Conditions
3.2.3. Genome-Wide Association Analysis of Electrical Conductivity
3.2.4. Candidate Genes for Seed Vigor-Related Traits
4. Discussion
4.1. Determination of Seed Vigor-Related Traits
4.2. QTL Mapping of Seed Vigor-Related Traits
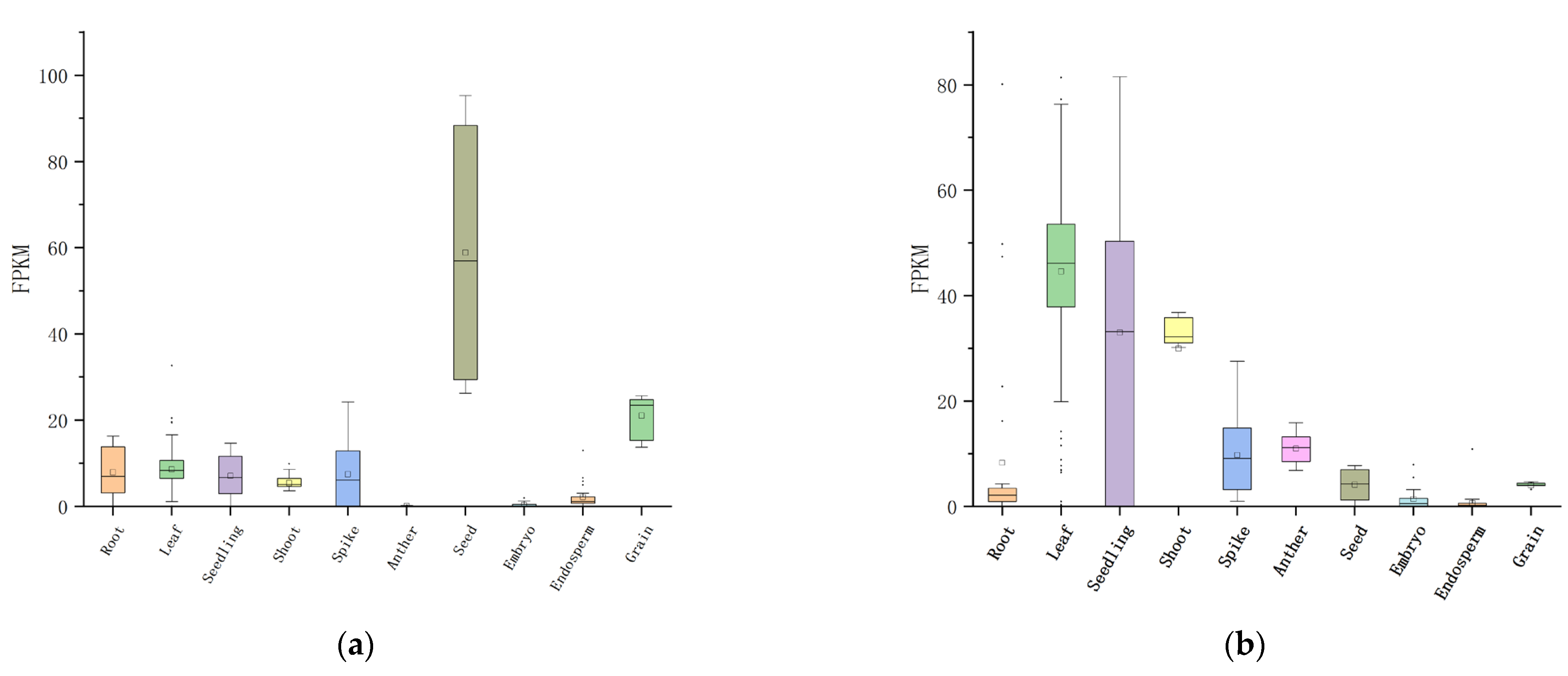
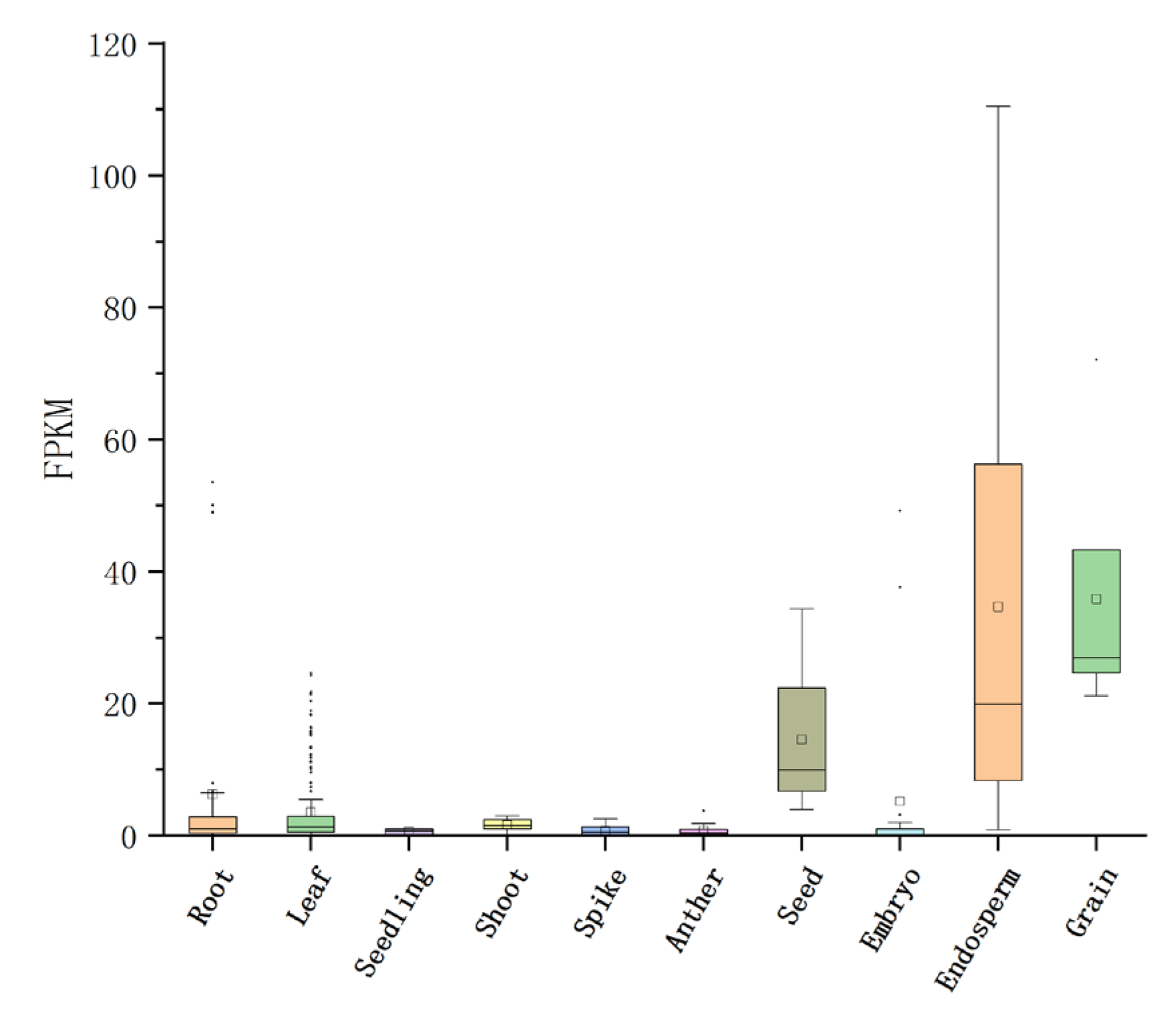
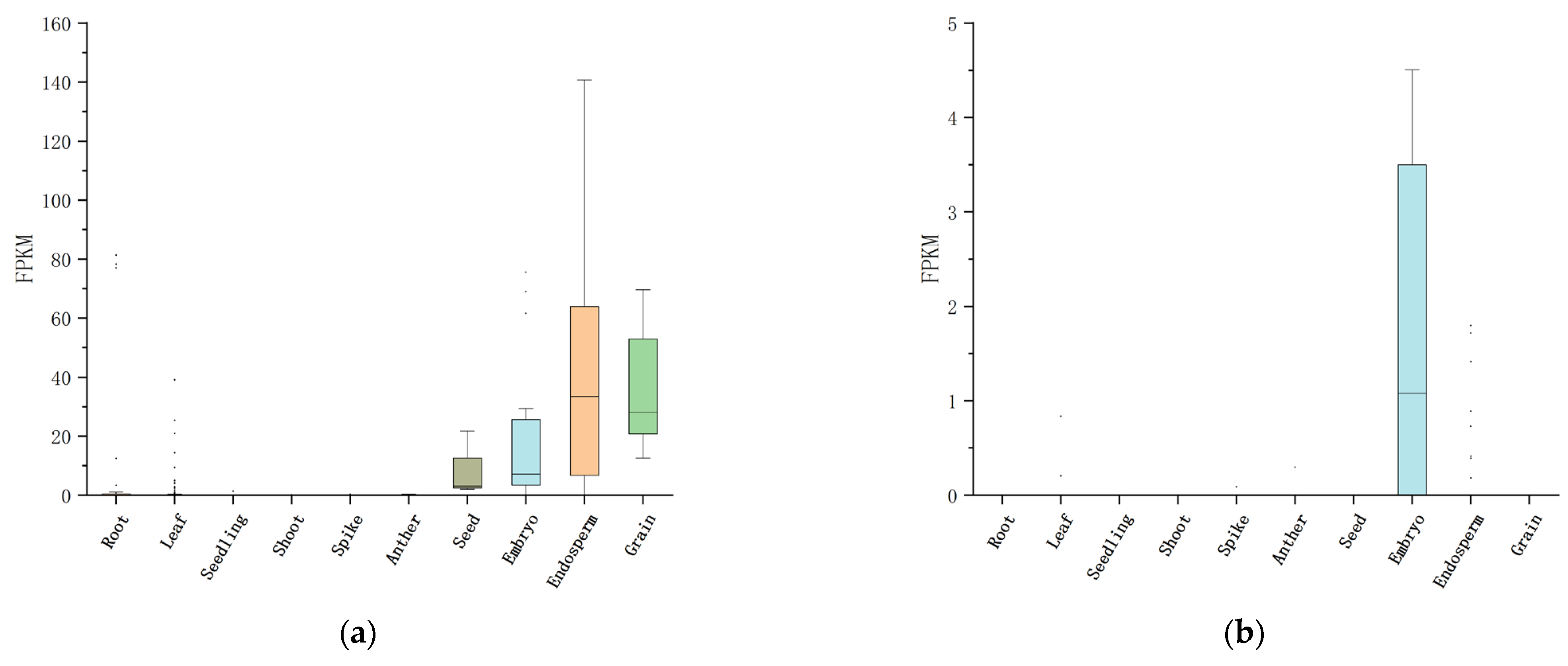
4.3. Functional Analysis of Candidate Genes for Seed Vigor
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, M.; Yang, Z.; Yang, W.; Yang, E. Genetic Diversity Assessment of the International Maize and Wheat Improvement Center and Chinese Wheat Core Germplasms by Non-Denaturing Fluorescence In Situ Hybridization. Plants 2022, 11, 1403. [Google Scholar] [CrossRef]
- Association of Official Seed Analysts. Seed Vigor Testing Handbook; Association of Official Seed Analysts: Lincon, OR, USA, 1983. [Google Scholar]
- Rajjou, L.; Duval, M.; Gallardo, K.; Catusse, J.; Bally, J.; Job, C.; Job, D. Seed germination and vigor. Annu. Rev. Plant Biol. 2012, 63, 507–533. [Google Scholar] [CrossRef]
- Tai, G.; Ba, R. A simple method to determine amino acid leakage and germination capabilities from single radish (Raphanus sativus L.) and Chinese cabbage (Brassica rapa ssp. pekinensis) seeds. Hortic. Environ. Biotechnol. 2013, 54, 249–256. [Google Scholar]
- Finch-Savage, W.; Bassel, G. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef]
- Ding, J.; Su, S.; Zhang, Y.; Li, C.; Zhu, X.; Guo, W. Seedling Growth and Recovery in Response to Waterlogging of Wheat Cultivars Grown in the Yangtze River Basin of China from Three Different Decades. J. Agric. Sci. 2017, 9, 128. [Google Scholar] [CrossRef][Green Version]
- Akhtar, N.; Ilyas, N.; Arshad, M.; Meraj, T.A.; Hefft, D.I.; Jan, B.L.; Ahmad, P. The Impact of Calcium, Potassium, and Boron Application on the Growth and Yield Characteristics of Durum Wheat under Drought Conditions. Agronomy 2022, 12, 1917. [Google Scholar] [CrossRef]
- Fu, Y.; Li, P.; Mounkaila, H.; Wan, S.; Gao, Y.; Wang, X. Effects of Single and Combined Drought and Salinity Stress on the Root Morphological Characteristics and Root Hydraulic Conductivity of Different Winter Wheat Varieties. Plants 2023, 12, 2694. [Google Scholar] [CrossRef]
- Zuo, J.; Liu, J.; Gao, F.; Yin, G.; Wang, Z.; Chen, F.; Li, X.; Xu, J.; Chen, T.; Li, L.; et al. Genome-wide linkage mapping reveals qtls for seed vigor-related traits under artificial aging in common wheat (Triticum aestivum). Front. Plant Sci. 2018, 9, 1101. [Google Scholar] [CrossRef]
- Shi, H.; Guan, W.; Shi, Y.; Wang, S.; Fan, H.; Yang, J.; Chen, W.; Zhang, W.; Sun, D.; Jing, R. QTL mapping and candidate gene analysis of seed vigor-related traits during artificial aging in wheat (Triticum aestivum). Sci. Rep. 2020, 10, 22060. [Google Scholar] [CrossRef]
- Beyer, S.; Iwgsc; Daba, S.; Tyagi, P.; Bockelman, H.; Brown-Guedira, G.; Mohammadi, M. Loci and candidate genes controlling root traits in wheat seedlings—A wheat root GWAS. Funct. Integr. Genom. 2019, 19, 91–107. [Google Scholar] [CrossRef]
- Dong, Z. Molecular Characterization of Tamyb10-1 Genes and QTL Mapping and Genome-Wide Association Analysis of Seed Vigor Related Traits in Wheat; Henan Agricultural University: Zhengzhou, China, 2018. (In Chinese) [Google Scholar]
- Zheng, Y. Association Mapping and Analysis of Combining Ability of Seed Vigor Traits in Wheat; Northwest A&F University: Xianyang, China, 2016. (In Chinese) [Google Scholar]
- Hu, J.; Zhang, H. Seed Science; Science Press: Beijing, China, 2016. [Google Scholar]
- Zhang, H. 152 Synthetic Wheat Yield-Related Traits Genome-Wide Association Study; Shanxi University: Taiyuan, China, 2019. (In Chinese) [Google Scholar]
- Danakumara, T.; Kumari, J.; Singh, A.K.; Sinha, S.K.; Pradhan, A.K.; Sharma, S.; Jha, S.K.; Bansal, R.; Kumar, S.; Jha, G.K.; et al. Genetic dissection of seedling root system architectural traits in a diverse panel of hexaploid wheat through multi-locus genome-wide association mapping for improving drought tolerance. Int. J. Mol. Sci. 2021, 22, 7188. [Google Scholar] [CrossRef]
- Turhan, K. Genome-Wide Correlation and Linkage Analysis of Salt Tolerance in Wheat Seed Germination; Xinjiang Agricultural University: Urumqi, China, 2020. (In Chinese) [Google Scholar]
- Khodaee, S.M.M.; Hashemi, M.; Mirlohi, A.; Majidi, M.M.; Sukumaran, S.; Moghaddam, M.E.; Abdollahi, M. Root characteristics of an elite spring wheat panel under contrasting water treatments and their genome-wide association study. Rhizosphere 2021, 19, 100413. [Google Scholar] [CrossRef]
- Tavares, L.C.; Rufino, C.A.; Tunes, L.M.; Trzeciak, M.B.; Pino, M.; Barros, A.C.S.A. Initial Growth of Soybean Plants from Seeds of High and Low Vigor Subjected to Water Stress. J. Agric. Sci. 2012, 4, 246. [Google Scholar] [CrossRef]
- Chen, L.T.; Sun, A.Q.; Yang, M.; Chen, L.L.; Ma, X.L.; Li, M.L.; Yin, Y.P. Seed Vigor Evaluation Based on Adversity Resistance Index of Wheat Seed Germination under Stress Conditions. Chin. J. Appl. Ecol. 2016, 27, 2968–2974. (In Chinese) [Google Scholar]
- De Oliveira, I.C.; Rego, C.H.Q.; Cardoso, F.B.; Zuffo, A.M.; Cândido, A.C.d.S.; Alves, C.Z. Root Protrusion in Quality Evaluation of Chia Seeds. Rev. Caatinga 2019, 32, 282–287. [Google Scholar] [CrossRef]
- Hong, M.; Kim, J.; Seo, Y.; Kim, D. F-Box Genes in the Wheat Genome and Expression Profiling in Wheat at Different Developmental Stages. Genes 2020, 11, 1154. [Google Scholar] [CrossRef]
- Stone, S. The role of ubiquitin and the 26S proteasome in plant abiotic stress signaling. Front. Plant Sci. 2014, 5, 135. [Google Scholar] [CrossRef]
- Yu, Q.; Liu, Y.-L.; Sun, G.-Z.; Liu, Y.-X.; Chen, J.; Zhou, Y.-B.; Chen, M.; Ma, Y.-Z.; Xu, Z.-S.; Lan, J.-H. Genome-Wide Analysis of the Soybean Calmodulin-Binding Protein 60 Family and Identification of GmCBP60A-1 Responses to Drought and Salt Stresses. Int. J. Mol. Sci. 2021, 22, 13501. [Google Scholar] [CrossRef]
- Ali, E.; Raza, M.A.; Cai, M.; Hussain, N.; Shahzad, A.N.; Hussain, M.; Ali, M.; Bukhari, S.A.H.; Sun, P. Calmodulin-binding transcription activator (CAMTA) genes family: Genome-wide survey and phylogenetic analysis in flax (Linum usitatissimum). PLoS ONE 2020, 15, e0236454. [Google Scholar] [CrossRef]
- Li, M.; Zhu, X.; Yu, Q.; Yu, A.; Chen, L.; Kang, J.; Wang, X.; Yang, T.; Yang, Q.; Long, R. FtsH proteases confer protection against salt and oxidative stress in Medicago sativa L. Plant Sci. 2023, 338, 111915. [Google Scholar] [CrossRef]
- Cao, Q.; Lv, W.; Jiang, H.; Chen, X.; Wang, X.; Wang, Y. Genome-wide identification of glutathione S-transferase gene family members in tea plant (Camellia sinensis) and their response to environmental stress. Int. J. Biol. Macromol. 2022, 205, 749–760. [Google Scholar] [CrossRef]
| Env | Trait | Mean ± SD | Skewness | Kurtosis | CV (%) |
|---|---|---|---|---|---|
| 2020 Wenjiang (E1) | GE (%) | 73.94 ± 19.91 | −1.00 | 0.52 | 26.92 |
| GP (%) | 85.82 ± 15.94 | −2.07 | 4.64 | 18.58 | |
| GI | 58.85 ± 14.95 | −1.00 | 0.66 | 25.40 | |
| VI | 571.58 ± 191.12 | −0.02 | −0.17 | 33.44 | |
| SVI | 829.25 ± 221.28 | −0.35 | 0.44 | 26.68 | |
| AGE (%) | 21.27 ± 19.15 | 1.41 | 1.93 | 90.06 | |
| AGP (%) | 32.59 ± 23.61 | 0.79 | −0.14 | 72.45 | |
| AGI | 17.60 ± 14.66 | 1.26 | 1.40 | 83.29 | |
| AVI | 162.57 ± 148.19 | 1.49 | 2.34 | 91.16 | |
| ASVI | 295.67 ± 233.53 | 1.04 | 0.65 | 78.98 | |
| EC (µs/cm) | 22.71 ± 6.91 | 0.76 | 0.99 | 30.44 | |
| 2021 Wenjiang (E2) | GE (%) | 66.56 ± 19.12 | −0.83 | 0.20 | 28.73 |
| GP (%) | 73.21 ± 18.41 | −1.05 | 0.63 | 25.15 | |
| GI | 52.50 ± 15.09 | −0.81 | 0.12 | 28.74 | |
| VI | 555.93 ± 192.24 | −0.02 | −0.16 | 34.58 | |
| SVI | 773.28 ± 238.46 | −0.11 | −0.00 | 30.84 | |
| AGE (%) | 51.08 ± 23.45 | −0.29 | −0.80 | 45.90 | |
| AGP (%) | 62.02 ± 22.88 | −0.61 | −0.49 | 36.89 | |
| AGI | 41.29 ± 18.15 | −0.33 | −0.80 | 43.97 | |
| AVI | 437.00 ± 212.48 | −0.01 | −0.57 | 48.62 | |
| ASVI | 654.20 ± 276.31 | −0.19 | −0.45 | 42.24 | |
| EC (µs/cm) | 26.19 ± 7.99 | 0.78 | 0.34 | 30.53 | |
| 2021 Xi’an (E3) | GE (%) | 86.20 ± 14.17 | −1.87 | 3.47 | 16.44 |
| GP (%) | 93.74 ± 9.31 | −3.45 | 16.38 | 9.93 | |
| GI | 66.10 ± 10.44 | −1.53 | 2.81 | 15.79 | |
| VI | 711.21 ± 161.84 | 0.10 | 0.55 | 22.76 | |
| SVI | 1007.82 ± 184.67 | 0.00 | 1.10 | 18.32 | |
| AGE (%) | 34.20 ± 21.68 | 0.58 | −0.52 | 63.39 | |
| AGP (%) | 47.66 ± 22.72 | 0.12 | −0.88 | 47.67 | |
| AGI | 28.04 ± 16.45 | 0.51 | −0.55 | 58.68 | |
| AVI | 273.16 ± 181.41 | 0.84 | 0.45 | 66.41 | |
| ASVI | 458.29 ± 253.94 | 0.53 | −0.15 | 55.41 | |
| EC (µs/cm) | 19.93 ± 4.23 | 0.86 | 0.64 | 21.20 |
| Test | Trait | F Value (E) | F Value (V) | F Value (E&V) |
|---|---|---|---|---|
| Standard germination test (SGT) | GE | 286.93 ** | 3.85 ** | 93.17 ** |
| GP | 426.88 ** | 3.27 ** | 100.76 ** | |
| GI | 254.16 ** | 4.06 ** | 70.58 ** | |
| VI | 188.40 ** | 4.44 ** | 49.82 ** | |
| SVI | 246.34 ** | 4.12 ** | 49.49 ** | |
| Accelerated aging test (AAT) | AGE | 409.74 ** | 3.05 ** | 139.39 ** |
| AGP | 338.43 ** | 3.38 ** | 110.00 ** | |
| AGI | 436.21 ** | 3.10 ** | 131.88 ** | |
| AVI | 492.12 ** | 3.03 ** | 126.46 ** | |
| ASVI | 422.38 ** | 3.32 ** | 106.05 ** | |
| Electrical conductivity measurement | EC (µs/cm) | 194.73 ** | 5.55 ** | 41.08 ** |
| Env | Trait | GE (%) | GP (%) | GI | VI | SVI |
|---|---|---|---|---|---|---|
| 2020 Wenjiang (E1) | GP (%) | 0.886 ** | ||||
| GI | 0.985 ** | 0.913 ** | ||||
| VI | 0.844 ** | 0.750 ** | 0.862 ** | |||
| SVI | 0.739 ** | 0.770 ** | 0.766 ** | 0.952 ** | ||
| 2021 Wenjiang (E2) | GP (%) | 0.965 ** | ||||
| GI | 0.989 ** | 0.966 ** | ||||
| VI | 0.854 ** | 0.826 ** | 0.876 ** | |||
| SVI | 0.822 ** | 0.840 ** | 0.840 ** | 0.979 ** | ||
| 2021 Xi’an (E3) | GP (%) | 0.852 ** | ||||
| GI | 0.926 ** | 0.834 ** | ||||
| VI | 0.651 ** | 0.610 ** | 0.746 ** | |||
| SVI | 0.453 ** | 0.569 ** | 0.506 ** | 0.915 ** |
| Env | Trait | AGE (%) | AGP (%) | AGI | AVI | ASVI |
|---|---|---|---|---|---|---|
| 2020 Wenjiang (E1) | AGP (%) | 0.931 ** | ||||
| AGI | 0.985 ** | 0.965 ** | ||||
| AVI | 0.954 ** | 0.902 ** | 0.962 ** | |||
| ASVI | 0.929 ** | 0.948 ** | 0.950 ** | 0.974 ** | ||
| 2021 Wenjiang (E2) | AGP (%) | 0.948 ** | ||||
| AGI | 0.988 ** | 0.969 ** | ||||
| AVI | 0.920 ** | 0.901 ** | 0.932 ** | |||
| ASVI | 0.871 ** | 0.913 ** | 0.892 ** | 0.975 ** | ||
| 2021 Xi’an (E3) | AGP (%) | 0.935 ** | ||||
| AGI | 0.981 ** | 0.961 ** | ||||
| AVI | 0.912 ** | 0.871 ** | 0.926 ** | |||
| ASVI | 0.875 ** | 0.901 ** | 0.894 ** | 0.972 ** |
| Env | Trait | GE (%) | GP (%) | GI | VI | SVI |
|---|---|---|---|---|---|---|
| 2020 Wenjiang (E1) | EC | −0.508 ** | −0.494 ** | −0.524 ** | −0.504 ** | −0.481 ** |
| 2021 Wenjiang (E2) | EC | −0.433 ** | −0.431 ** | −0.452 ** | −0.498 ** | −0.491 ** |
| 2021 Xi’an (E3) | EC | −0.300 ** | −0.308 ** | −0.295 ** | −0.365 ** | −0.365 ** |
| Index | Chr | Env | SNPs | SNP Position | −log10 P | R2 (%) |
|---|---|---|---|---|---|---|
| GE | 7A | 21X, 20W | 14 | 733.665462–734.156444 | 3.27–3.51 | 3.42–3.77 |
| 7B | 21W, 20W | 9 | 680.066797–680.280913 | 3.13–3.50 | 3.34–3.64 | |
| GP | 3A | 21X, 21W | 6 | 11.350165–12.781485 | 3.00–3.26 | 2.92–3.26 |
| 7A | 21X, 21W, 20W | 4 | 724.086225–724.137239 | 3.11–3.93 | 3.09–4.38 | |
| 7B | 21W, 20W | 10 | 680.279781–680.280913 | 3.17–3.81 | 3.40–4.01 | |
| GI | 2B | 21X, 21W | 9 | 28.893387–30.465929 | 3.06–3.33 | 2.87–3.43 |
| 7B | 21W, 20W | 9 | 680.066797–680.280913 | 3.06–3.25 | 3.10–3.49 | |
| SVI | 4A | 21X, 20W | 16 | 12.377158–14.025321 | 3.12–3.79 | 3.10–3.71 |
| 5B | 21W, 20W | 14 | 482.999332–484.735571 | 3.02–3.74 | 3.10–3.91 | |
| GE, GP, GI | 7B | 21W, 20W | 11 | 680.066797–680.280913 | 3.06–3.81 | 3.10–4.01 |
| GE | 7A | 21X, 20W | 14 | 733.665462–734.156444 | 3.27–3.51 | 3.42–3.77 |
| Index | Chr | Env | SNPs | SNP Position | −log10 P | R2 (%) |
|---|---|---|---|---|---|---|
| AGE | 1A | 21X, 21W | 16 | 504.245992–504.615933 | 3.03–3.71 | 3.01–3.73 |
| AGP | 2A | 21X, 21W | 2 | 2.656936–4.169395 | 3.03–3.78 | 3.07–3.76 |
| AVI | 1B | 21X, 20W | 4 | 588.489918–589.070711 | 3.03–3.39 | 2.88–3.29 |
| 3D | 21W, 20W | 3 | 2.141767–3.549827 | 3.17–3.32 | 3.22–3.45 | |
| 6A | 21W, 20W | 4 | 55.032771–56.250082 | 3.07–3.09 | 3.10–3.17 | |
| 7B | 21W, 20W | 8 | 46.349379–47.744438 | 3.08–3.31 | 3.13–3.44 | |
| ASVI | 1B | 21W, 20W | 3 | 113.520204–115.276513 | 3.02–3.53 | 3.11–3.64 |
| 2B | 21W, 20W | 7 | 17.437505–17.514520 | 3.02–3.71 | 3.02–3.97 | |
| 2B | 21X, 21W | 3 | 790.228468–791.480914 | 3.08–4.44 | 3.09–4.50 | |
| 5A | 21X, 21W | 2 | 0.665300–2.409084 | 3.13–3.20 | 3.06–3.16 | |
| 7B | 21W, 20W | 9 | 46.349379–48.188686 | 3.01–3.65 | 3.10–3.79 | |
| AVI, ASVI | 7B | 21W, 20W | 11 | 46.349379–48.188686 | 3.01–3.65 | 3.10–3.79 |
| Index | Chr | Env | SNPs | SNP Position | −log10 P | R2 (%) |
|---|---|---|---|---|---|---|
| EC | 1B | 21X, 21W | 4 | 13.245681–14.149556 | 3.04–3.11 | 2.90–3.14 |
| 1B | 21X, 20W | 3 | 262.065376–263.572005 | 3.00–3.15 | 2.85–3.20 | |
| 4A | 21X, 20W | 3 | 732.513787–733.432588 | 3.18–3.24 | 3.12–3.24 | |
| 5A | 21X, 21W | 26 | 561.172374–562.864480 | 3.00–3.76 | 2.85–3.93 | |
| 5A | 21X, 21W | 7 | 563.755662–565.549837 | 3.07–3.66 | 3.08–3.61 | |
| 5B | 21W, 20W | 4 | 580.731933–581.146804 | 3.21–3.95 | 3.27–4.17 |
| Index | Chr | Env | SNPs | SNP Position | −log10 P | R2 (%) |
|---|---|---|---|---|---|---|
| GE | 7A | 21X, 20W | 13 | 733.665462–734.156444 | 3.27–3.51 | 3.42–3.77 |
| 7B | 21W, 20W | 10 | 680.066797–680.280913 | 3.13–3.40 | 3.27–3.64 | |
| GI | 7B | 21W, 20W | 10 | 680.066797–680.280913 | 3.09–3.25 | 3.10–3.49 |
| GP | 3A | 21X, 21W | 7 | 11.350165–12.781485 | 3.00–3.26 | 2.92–3.33 |
| 7A | 21X, 21W, 20W | 4 | 724.086225–724.137239 | 3.11–3.93 | 3.09–4.38 | |
| 7B | 21W, 20W | 11 | 680.066797–680.280913 | 3.17–3.81 | 3.40–4.01 | |
| SVI | 4A | 21X, 20W | 17 | 12.377158–14.025321 | 3.12–3.79 | 3.10–3.71 |
| 5B | 21W, 20W | 13 | 482.999332–484.735572 | 3.02–3.74 | 3.10–3.91 | |
| AVI | 7B | 21W, 20W | 9 | 46.349379–47.744438 | 3.08–3.31 | 3.13–3.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Q.; Shi, B.; Lai, Y.; Zhang, Y.; Wu, Y.; Li, Z.; Li, Y.; Zhu, X.; Pu, Z.; Liu, Z. Genome-Wide Association Analysis of Seed Vigor-Related Traits in Wheat. Agronomy 2024, 14, 410. https://doi.org/10.3390/agronomy14030410
Wu Q, Shi B, Lai Y, Zhang Y, Wu Y, Li Z, Li Y, Zhu X, Pu Z, Liu Z. Genome-Wide Association Analysis of Seed Vigor-Related Traits in Wheat. Agronomy. 2024; 14(3):410. https://doi.org/10.3390/agronomy14030410
Chicago/Turabian StyleWu, Qinxuan, Bingxin Shi, Yao Lai, Yuanyuan Zhang, Yu Wu, Zhi Li, Yang Li, Xiaofei Zhu, Zhien Pu, and Zihui Liu. 2024. "Genome-Wide Association Analysis of Seed Vigor-Related Traits in Wheat" Agronomy 14, no. 3: 410. https://doi.org/10.3390/agronomy14030410
APA StyleWu, Q., Shi, B., Lai, Y., Zhang, Y., Wu, Y., Li, Z., Li, Y., Zhu, X., Pu, Z., & Liu, Z. (2024). Genome-Wide Association Analysis of Seed Vigor-Related Traits in Wheat. Agronomy, 14(3), 410. https://doi.org/10.3390/agronomy14030410






