Impact of Drought Stress on Yield-Related Agronomic Traits of Different Genotypes in Spring Wheat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Growth Conditions and Experiment Design
2.3. Agronomic Data
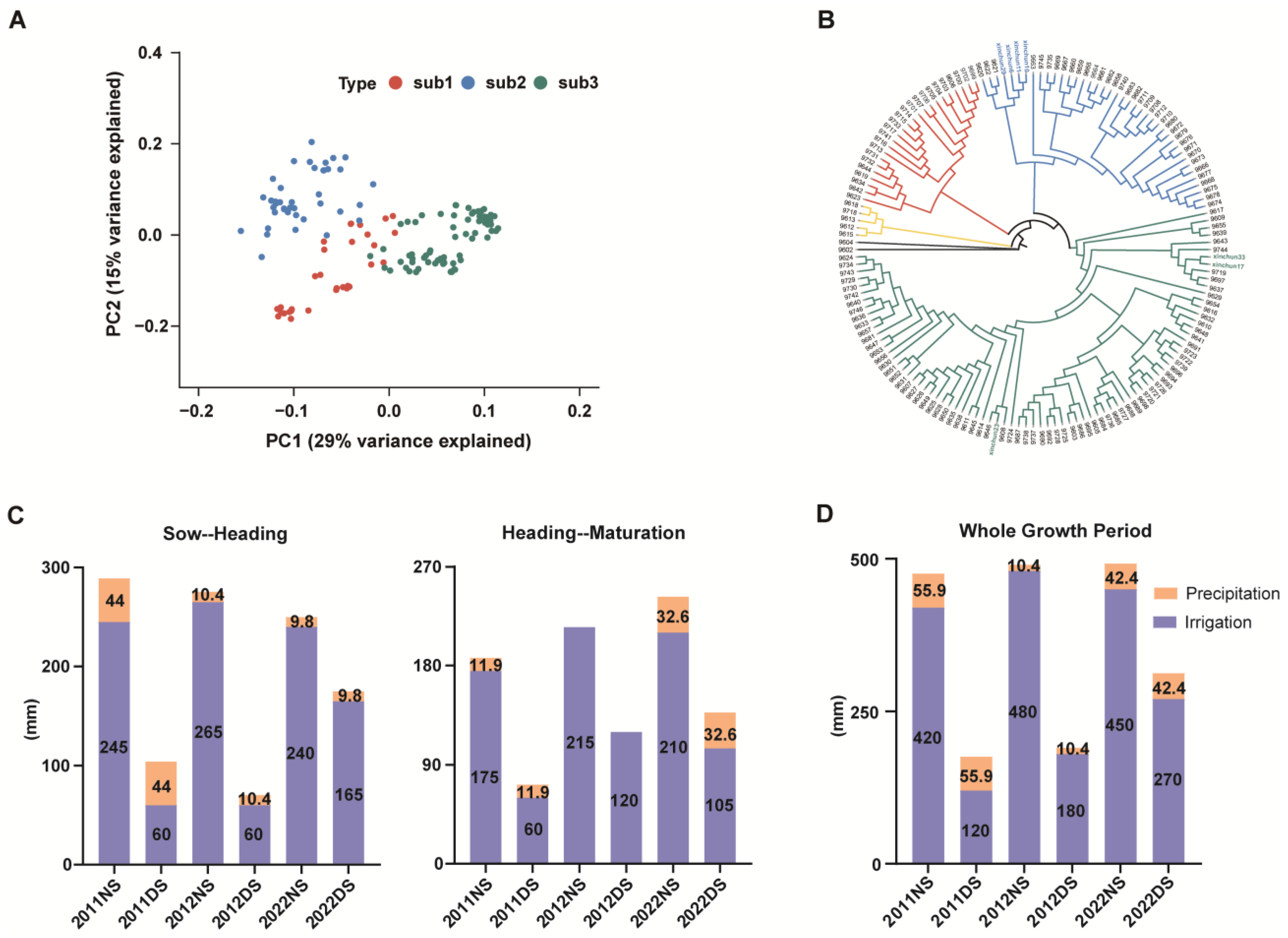
2.4. Principal Component Analysis (PCA) by Genotyping
2.5. Statistical and Data Analysis
2.6. Multiple Linear Regression Analysis
3. Results
3.1. Analysis of Population and Drought Stress Test
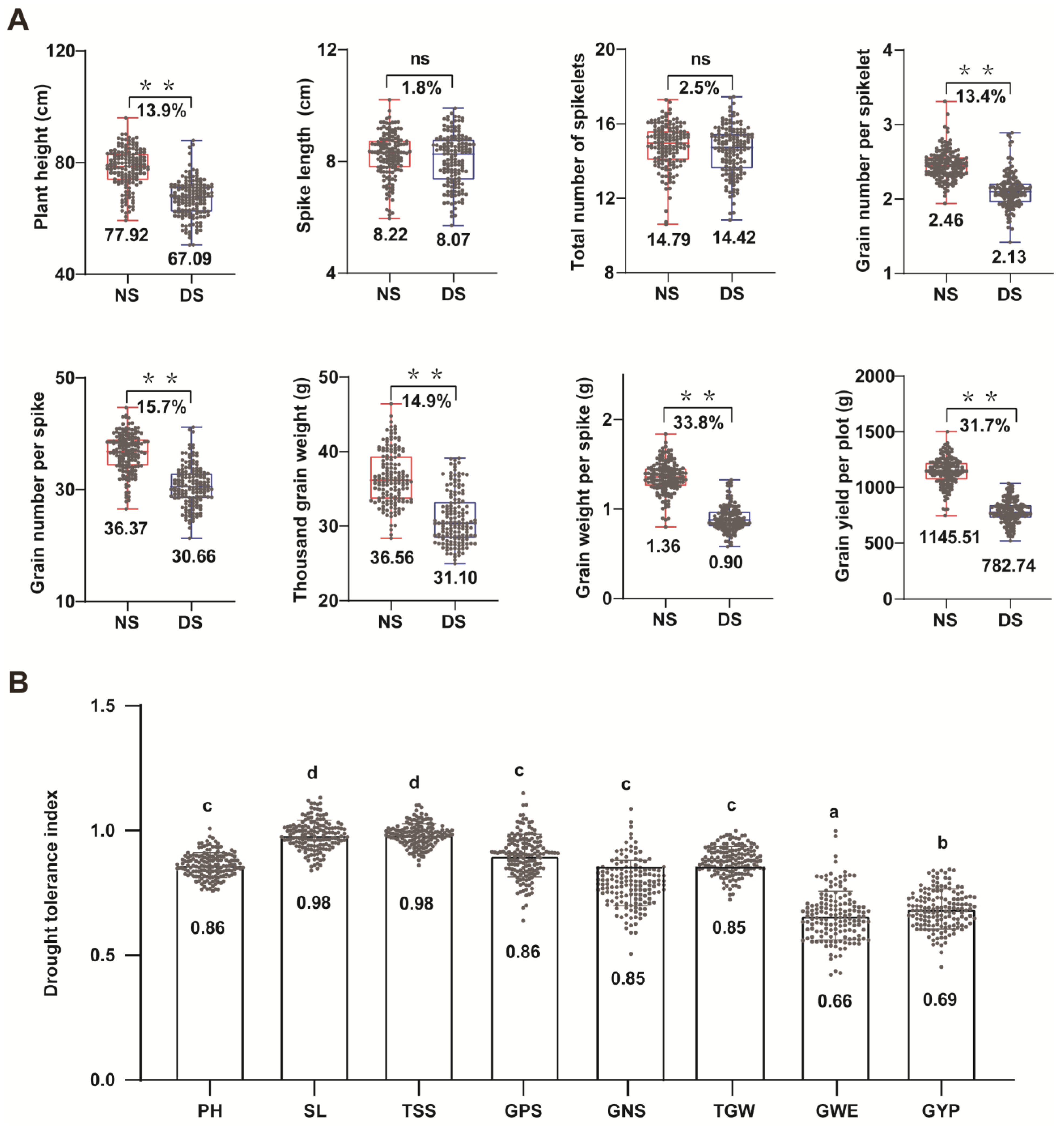
3.2. Effects of Drought Stress on Wheat Yield
3.3. Effects of Drought Stress on Wheat Physiology
3.4. Drought-Tolerant Germplasm Resources
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Godfray, H.C.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef]
- Langridge, P.; Reynolds, M. Breeding for drought and heat tolerance in wheat. Theor. Appl. Genet. 2021, 134, 1753–1769. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Chen, B.; Du, L.; Li, S.; Zhu, D.; Chen, N.; Zhang, Y.; Li, F.; Wang, Z.; Cheng, X. A gain-of-function allele of a DREB transcription factor gene ameliorates drought tolerance in wheat. Plant Cell 2022, 34, 4472–4494. [Google Scholar] [CrossRef] [PubMed]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Change 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Nevo, E.; Chen, G. Drought and salt tolerances in wild relatives for wheat and barley improvement. Plant Cell Environ. 2010, 33, 670–685. [Google Scholar] [CrossRef] [PubMed]
- Poggi, G.M.; Corneti, S.; Aloisi, I.; Ventura, F. Environment-oriented selection criteria to overcome controversies in breeding for drought resistance in wheat. J. Plant Physiol. 2023, 280, 153895. [Google Scholar] [CrossRef]
- Tricker, P.J.; ElHabti, A.; Schmidt, J.; Fleury, D. The physiological and genetic basis of combined drought and heat tolerance in wheat. J. Exp. Bot. 2018, 69, 3195–3210. [Google Scholar] [CrossRef]
- Khan, S.; Anwar, S.; Yu, S.; Sun, M.; Yang, Z.; Gao, Z.Q. Development of Drought-tolerant transgenic wheat: Achievements and limitations. Int. J. Mol. Sci. 2019, 20, 3350. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Onyemaobi, I.; Liu, H.; Siddique, K.H.; Yan, G. Both male and female malfunction contributes to yield reduction under water stress during meiosis in bread wheat. Front. Plant Sci. 2016, 7, 2071. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Shiran, B.; Wan, J.; Lewis, D.C.; Jenkins, C.L.; Condon, A.G.; Richards, R.A.; Dolferus, R. Importance of pre-anthesis anther sink strength for maintenance of grain number during reproductive stage water stress in wheat. Plant Cell Environ. 2010, 33, 926–942. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Zheng, X.; Liu, H.; Able, J.A.; Yang, H.; Zhao, H.; Zhang, M.; Qiao, Y.; Wang, Y.; Liu, M. Effects of drought stress on pollen sterility, grain yield, abscisic acid and protective enzymes in two winter wheat cultivars. Front. Plant Sci. 2017, 8, 1008. [Google Scholar] [CrossRef]
- Wang, X.; Vignjevic, M.; Jiang, D.; Jacobsen, S.; Wollenweber, B. Improved tolerance to drought stress after anthesis due to priming before anthesis in wheat (Triticum aestivum L.) var. Vinjett. J. Exp. Bot. 2014, 65, 6441–6456. [Google Scholar] [CrossRef] [PubMed]
- Senapati, N.; Halford, N.G.; Semenov, M.A. Vulnerability of European wheat to extreme heat and drought around flowering under future climate. Environ. Res. Lett. 2021, 16, 024052. [Google Scholar] [CrossRef]
- Webber, H.; Ewert, F.; Olesen, J.E.; Müller, C.; Fronzek, S.; Ruane, A.C.; Bourgault, M.; Martre, P.; Ababaei, B.; Bindi, M. Diverging importance of drought stress for maize and winter wheat in Europe. Nat. Commun. 2018, 9, 4249. [Google Scholar] [CrossRef]
- Luo, L.; Xia, H.; Lu, B.R. Editorial: Crop breeding for drought resistance. Front. Plant Sci. 2019, 10, 314. [Google Scholar] [CrossRef]
- Rabieyan, E.; Bihamta, M.R.; Moghaddam, M.E.; Alipour, H.; Mohammadi, V.; Azizyan, K.; Javid, S. Analysis of genetic diversity and genome-wide association study for drought tolerance related traits in Iranian bread wheat. BMC Plant Biol. 2023, 23, 431. [Google Scholar] [CrossRef]
- Sallam, A.; Alqudah, A.M.; Dawood, M.F.A.; Baenziger, P.S.; Börner, A. Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 2019, 20, 3137. [Google Scholar] [CrossRef]
- Du, L.; Huang, X.; Ding, L.; Wang, Z.; Tang, D.; Chen, B.; Ao, L.; Liu, Y.; Kang, Z.; Mao, H. TaERF87 and TaAKS1 synergistically regulate TaP5CS1/TaP5CR1-mediated proline biosynthesis to enhance drought tolerance in wheat. New Phytol. 2023, 237, 232–250. [Google Scholar] [CrossRef]
- Makino, A. Photosynthesis, grain yield, and nitrogen utilization in rice and wheat. Plant Physiol. 2011, 155, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Mao, X.; Wang, J.; Chang, X.; Reynolds, M.; Jing, R. Genetic dissection of drought and heat-responsive agronomic traits in wheat. Plant Cell Environ. 2019, 42, 2540–2553. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, S.; Cheng, M.; Jiang, H.; Zhang, X.; Peng, C.; Lu, X.; Zhang, M.; Jin, J. Effect of drought on agronomic traits of rice and wheat: A meta-analysis. Int. J. Env. Res. Pub. Health 2018, 15, 839. [Google Scholar] [CrossRef] [PubMed]
- Tsenov, N. Effects of drought on grain productivity and quality in winter bread wheat. Bulg. J. Agric. Sci. 2015, 21, 589–595. [Google Scholar]
- Nezhadahmadi, A.; Prodhan, Z.H.; Faruq, G. Drought tolerance in wheat. Sci. World J. 2013, 2013, 610721. [Google Scholar] [CrossRef] [PubMed]
- Amalova, A.; Abugalieva, S.; Babkenov, A.; Babkenova, S.; Turuspekov, Y. Genome-wide association study of yield components in spring wheat collection harvested under two water regimes in Northern Kazakhstan. PEER J. 2021, 9, e11857. [Google Scholar] [CrossRef]
- Tian, G.; Wang, S.; Wu, J.; Wang, Y.; Wang, X.; Liu, S.; Han, D.; Xia, G.; Wang, M. Allelic variation of TaWD40-4B.1 contributes to drought tolerance by modulating catalase activity in wheat. Nat. Commun. 2023, 14, 1200. [Google Scholar] [CrossRef]
- Eltaher, S.; Hashem, M.; Ahmed, A.A.M.; Baenziger, P.S.; Börner, A.; Sallam, A. Effectiveness of TaDreb-B1 and 1-FEH w3 KASP markers in spring and winter wheat populations for marker-assisted selection to improve drought tolerance. Int. J. Mol. Sci. 2023, 24, 8986. [Google Scholar] [CrossRef]
- Amini, A.; Majidi, M.M.; Mokhtari, N.; Ghanavati, M. Drought stress memory in a germplasm of synthetic and common wheat: Antioxidant system, physiological and morphological consequences. Sci. Rep. 2023, 13, 8569. [Google Scholar] [CrossRef]
- Bouslama, M.; Schapaugh, W.T. Stress tolerance in soybeans. I. Evaluation of three screening techniques for heat and drought tolerance. Crop Sci. 1950, 24, 933–937. [Google Scholar] [CrossRef]
- Qin, P.; Lin, Y.; Hu, Y.; Liu, K.; Mao, S.; Li, Z.; Wang, J.; Liu, Y.; Wei, Y.; Zheng, Y. Genome-wide association study of drought-related resistance traits in Aegilops tauschii. Gent. Mol. Biol. 2016, 39, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Boliko, M.C. FAO and the situation of food security and nutrition in the world. J. Nutr. Sci. Vitaminol. 2019, 65, S4–S8. [Google Scholar] [CrossRef] [PubMed]
- Cattivelli, L.; Rizza, F.; Badeck, F.W.; Mazzucotelli, E.; Mastrangelo, A.M.; Francia, E.; Marè, C.; Tondelli, A.; Stanca, A.M.J.F.C.R. Drought tolerance improvement in crop plants: An integrated view from breeding to genomics. Field Crop Res. 2008, 105, 1–14. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Abberton, M.; Batley, J.; Bentley, A.; Bryant, J.; Cai, H.; Cockram, J.; Oliveira, A.C.; Cseke, L.J.; Dempewolf, H.; De, P.C. Global agricultural intensification during climate change: A role for genomics. Plant Biotechnol. J. 2016, 14, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Vinocur, B.; Altman, A. Recent advances in engineering plant tolerance to abiotic stress: Achievements and limitations. Curr. Opin. Biotech. 2005, 16, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Gholami, Z.; Ehsanzadeh, P. Exogenous proline improves osmoregulation, physiological functions, essential oil, and seed yield of fennel. Ind. Crop. Prod. 2018, 111, 133–140. [Google Scholar] [CrossRef]
- Yang, Y.; Nan, R.; Mi, T.; Song, Y.; Shi, F.; Liu, X.; Wang, Y.; Sun, F.; Xi, Y.; Zhang, C. Rapid and nondestructive evaluation of wheat chlorophyll under drought stress using hyperspectral imaging. Int. J. Mol. Sci. 2023, 24, 5825. [Google Scholar] [CrossRef]
- Ghaffar, A.; Hussain, N.; Ajaj, R.; Shahin, S.M.; Bano, H.; Javed, M.; Khalid, A.; Yasmin, M.; Shah, K.H.; Zaheer, M.; et al. Photosynthetic activity and metabolic profiling of bread wheat cultivars contrasting in drought tolerance. Front. Plant Sci. 2023, 14, 1123080. [Google Scholar] [CrossRef]
- Kiliç, H.; Yağbasanlar, T. The effect of drought stress on grain yield, yield components and some quality traits of durum wheat (Triticum turgidum ssp. durum) Cultivars. Not. Bot. Horti Agrobot. 2010, 38, 164–170. [Google Scholar]
- Yasir, T.A.; Wasaya, A.; Hussain, M.; Ijaz, M.; Farooq, M.; Farooq, O.; Nawaz, A.; Hu, Y.G. Evaluation of physiological markers for assessing drought tolerance and yield potential in bread wheat. Physiol. Mol. Biol. Plants 2019, 25, 1163–1174. [Google Scholar] [CrossRef] [PubMed]
- Itam, M.; Mega, R.; Tadano, S.; Abdelrahman, M.; Matsunaga, S.; Yamasaki, Y.; Akash, K.; Tsujimoto, H. Metabolic and physiological responses to progressive drought stress in bread wheat. Sci. Rep. 2020, 10, 17189. [Google Scholar] [CrossRef] [PubMed]
- Dawood, M.F.A.; Moursi, Y.S.; Amro, A.; Baenziger, P.S.; Sallam, A.J.A. Investigation of heat-induced changes in the grain yield and grains metabolites, with molecular insights on the candidate genes in barley. Agronomy 2020, 10, 1730. [Google Scholar] [CrossRef]
- Mao, H.; Li, S.; Chen, B.; Jian, C.; Mei, F.; Zhang, Y.; Li, F.; Chen, N.; Li, T.; Du, L.; et al. Variation in cis-regulation of a NAC transcription factor contributes to drought tolerance in wheat. Mol. Plant 2022, 15, 276–292. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Li, T.; Ni, C.; Han, L.; Du, P.; Xiao, K. TaPYL4, an ABA receptor gene of wheat, positively regulates plant drought adaptation through modulating the osmotic stress-associated processes. BMC Plant Biol. 2022, 22, 423. [Google Scholar] [CrossRef]
- Gupta, P.K.; Balyan, H.S.; Sharma, S.; Kumar, R. Genetics of yield, abiotic stress tolerance and biofortification in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2020, 133, 1569–1602. [Google Scholar] [CrossRef]
- Mwadzingeni, L.; Shimelis, H.; Dube, E.; Laing, M.D.; Tsilo, T.J. Breeding wheat for drought tolerance: Progress and technologies. J. Integr. Agr. 2016, 15, 935–943. [Google Scholar] [CrossRef]
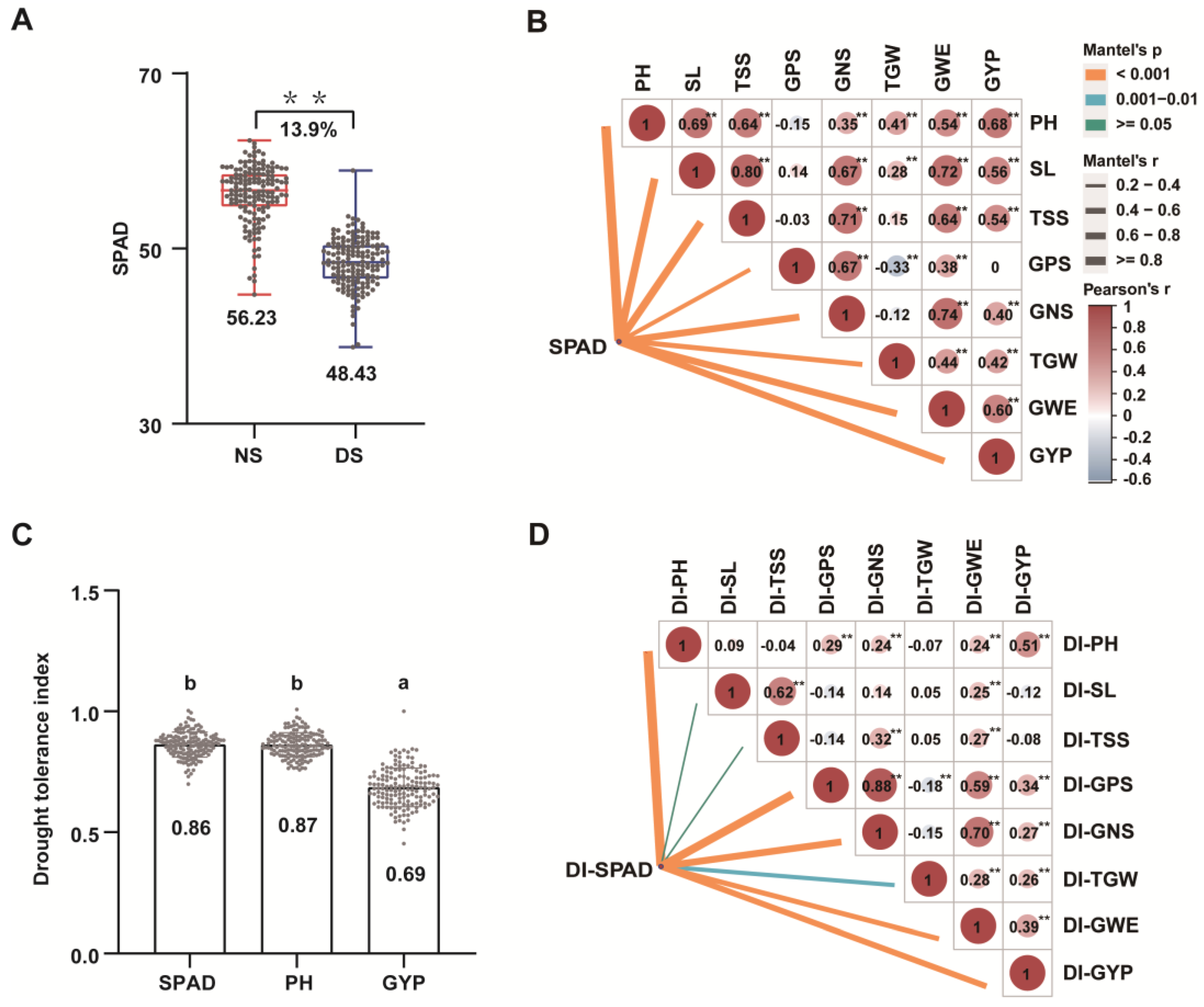

| Traits | Regression Equation | DI-PH (X1) | DI-GNS (X2) | DI-GPS (X3) | DI-TGW (X4) | Adjusted R2 | p-Value |
|---|---|---|---|---|---|---|---|
| DI-GWE | Y = −0.77+ 0.19X1 + 0.80X2 + 0.70X4 | 0.0094 | 0.0730 | -- | 0.0390 | 0.6421 | <2.2 × 10−16 |
| DI-GYP | Y = −0.59 + 0.72X1 + 0.26X3 + 0.50X4 | 0.0360 | -- | 0.0224 | 0.0277 | 0.3987 | <2.2 × 10−16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Lai, X.; Ren, Y.; Yang, H.; Wang, H.; Wang, C.; Xia, J.; Wang, Z.; Yang, Z.; Geng, H.; et al. Impact of Drought Stress on Yield-Related Agronomic Traits of Different Genotypes in Spring Wheat. Agronomy 2023, 13, 2968. https://doi.org/10.3390/agronomy13122968
Xu Z, Lai X, Ren Y, Yang H, Wang H, Wang C, Xia J, Wang Z, Yang Z, Geng H, et al. Impact of Drought Stress on Yield-Related Agronomic Traits of Different Genotypes in Spring Wheat. Agronomy. 2023; 13(12):2968. https://doi.org/10.3390/agronomy13122968
Chicago/Turabian StyleXu, Zihan, Xiangjun Lai, Yi Ren, Hongmei Yang, Haobo Wang, Chunsheng Wang, Jianqiang Xia, Zhenlong Wang, Zhenyu Yang, Hongwei Geng, and et al. 2023. "Impact of Drought Stress on Yield-Related Agronomic Traits of Different Genotypes in Spring Wheat" Agronomy 13, no. 12: 2968. https://doi.org/10.3390/agronomy13122968
APA StyleXu, Z., Lai, X., Ren, Y., Yang, H., Wang, H., Wang, C., Xia, J., Wang, Z., Yang, Z., Geng, H., Shi, X., & Zhang, Y. (2023). Impact of Drought Stress on Yield-Related Agronomic Traits of Different Genotypes in Spring Wheat. Agronomy, 13(12), 2968. https://doi.org/10.3390/agronomy13122968





