Potential Momilactones in Rice Stress Tolerance and Health Advantages
Abstract
1. Introduction
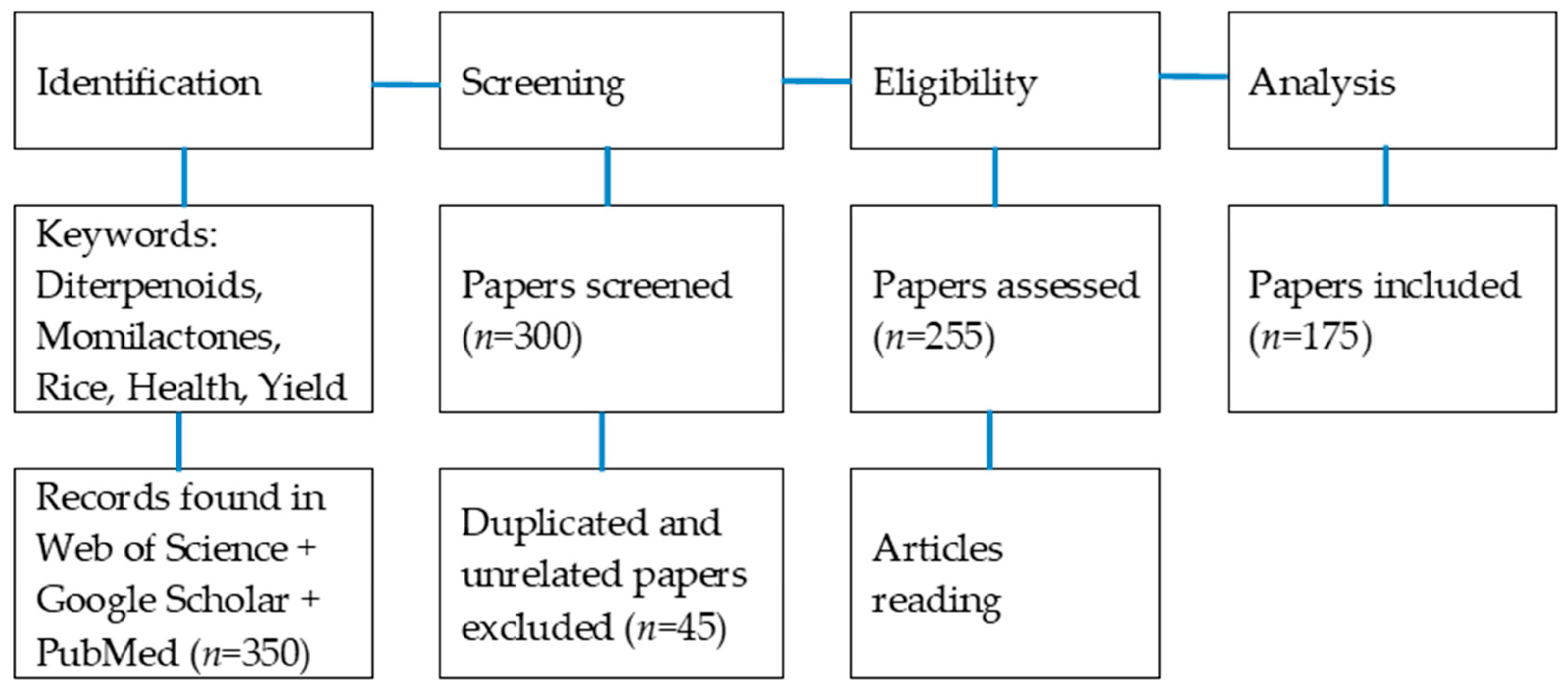
2. Literature Source and Search Methodology
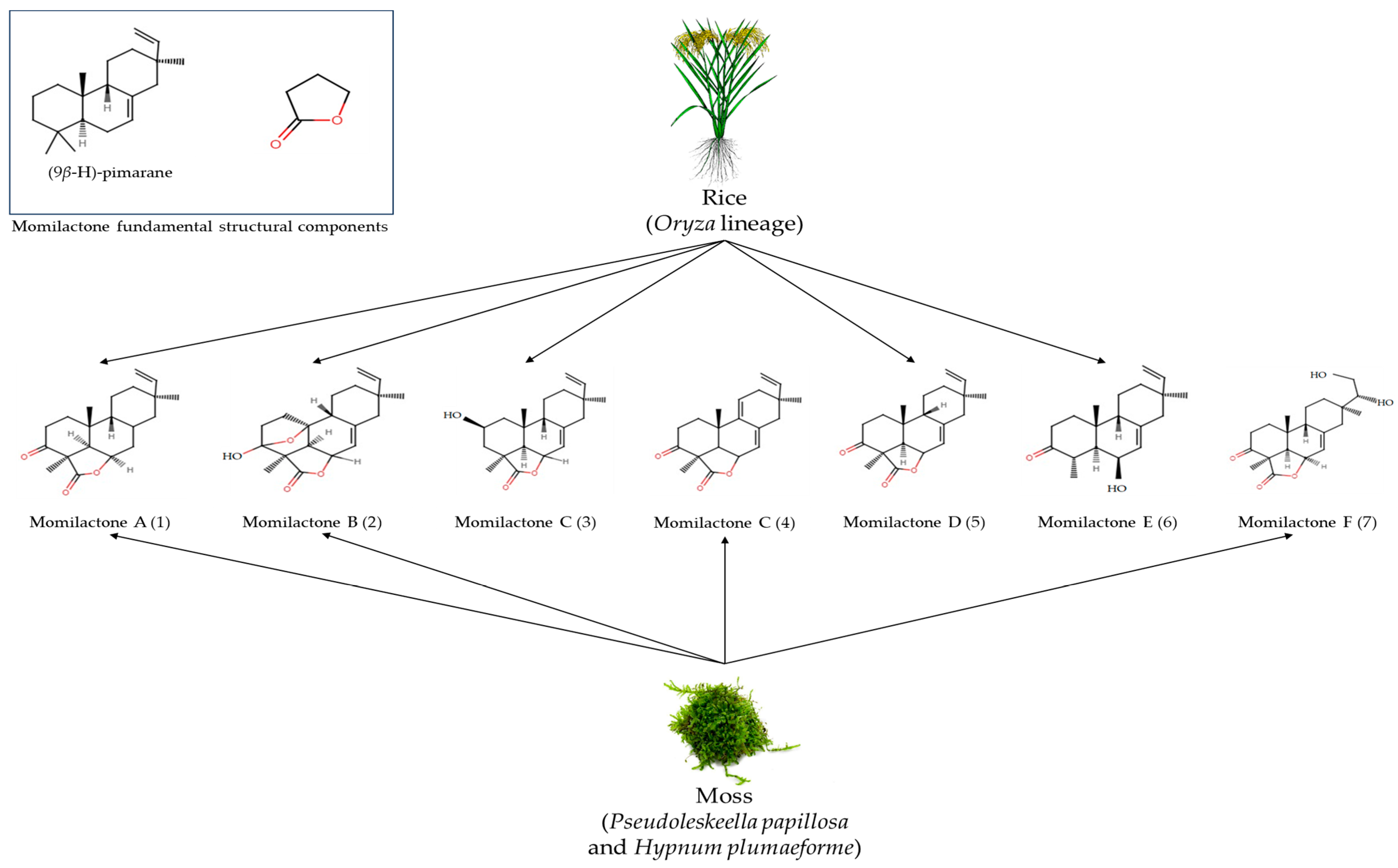
3. Rice Diterpenoids and Momilactones
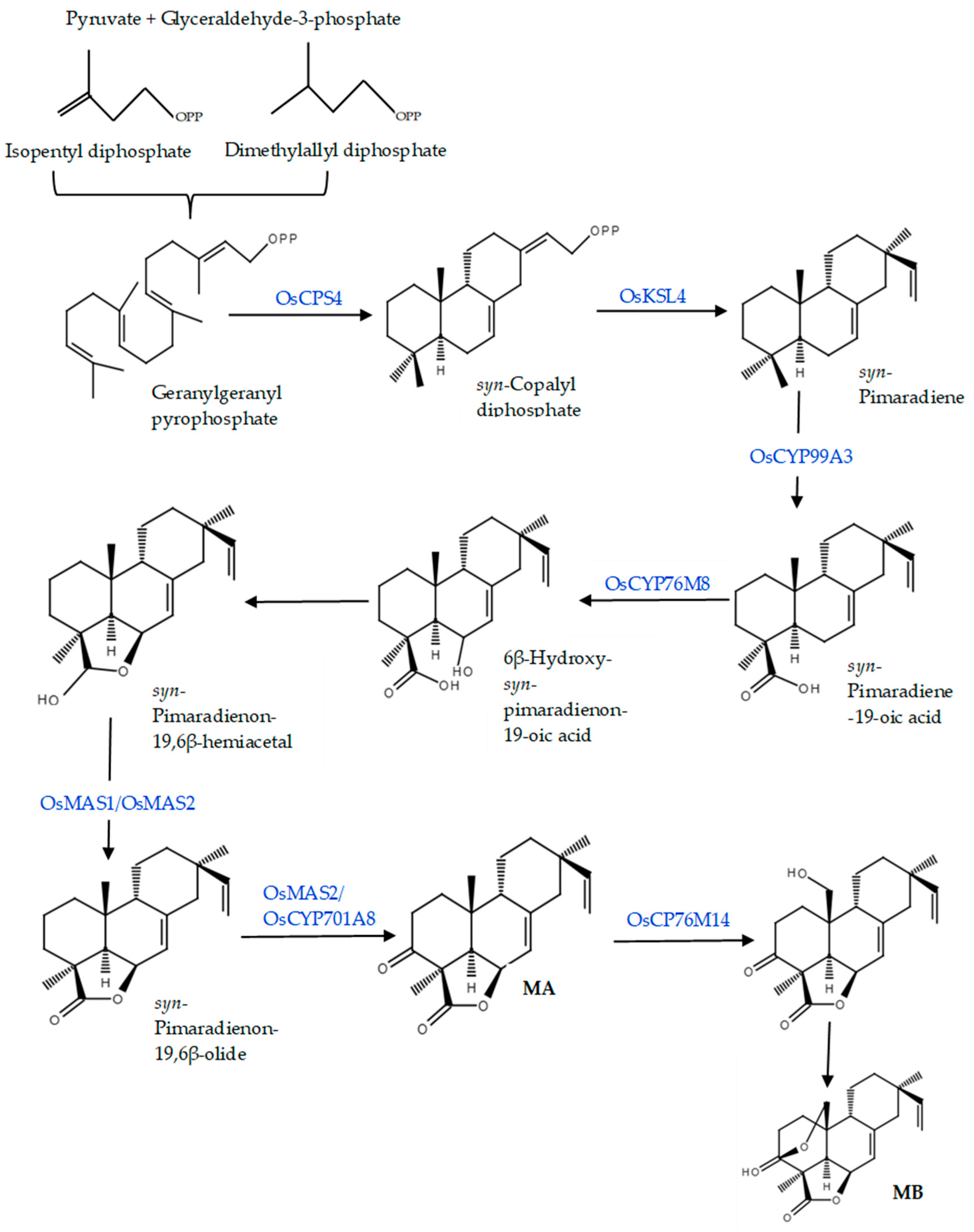
3.1. Biosynthesis Pathway of Rice Diterpenoids
3.2. Biosynthesis of Momilactones and Related Genes
4. Distribution of Momilactones in Rice and Their Correlation with Rice Growth and Quality
4.1. Distribution of Momilactones in Rice Plant Organs
4.2. Momilactones and Rice Quality
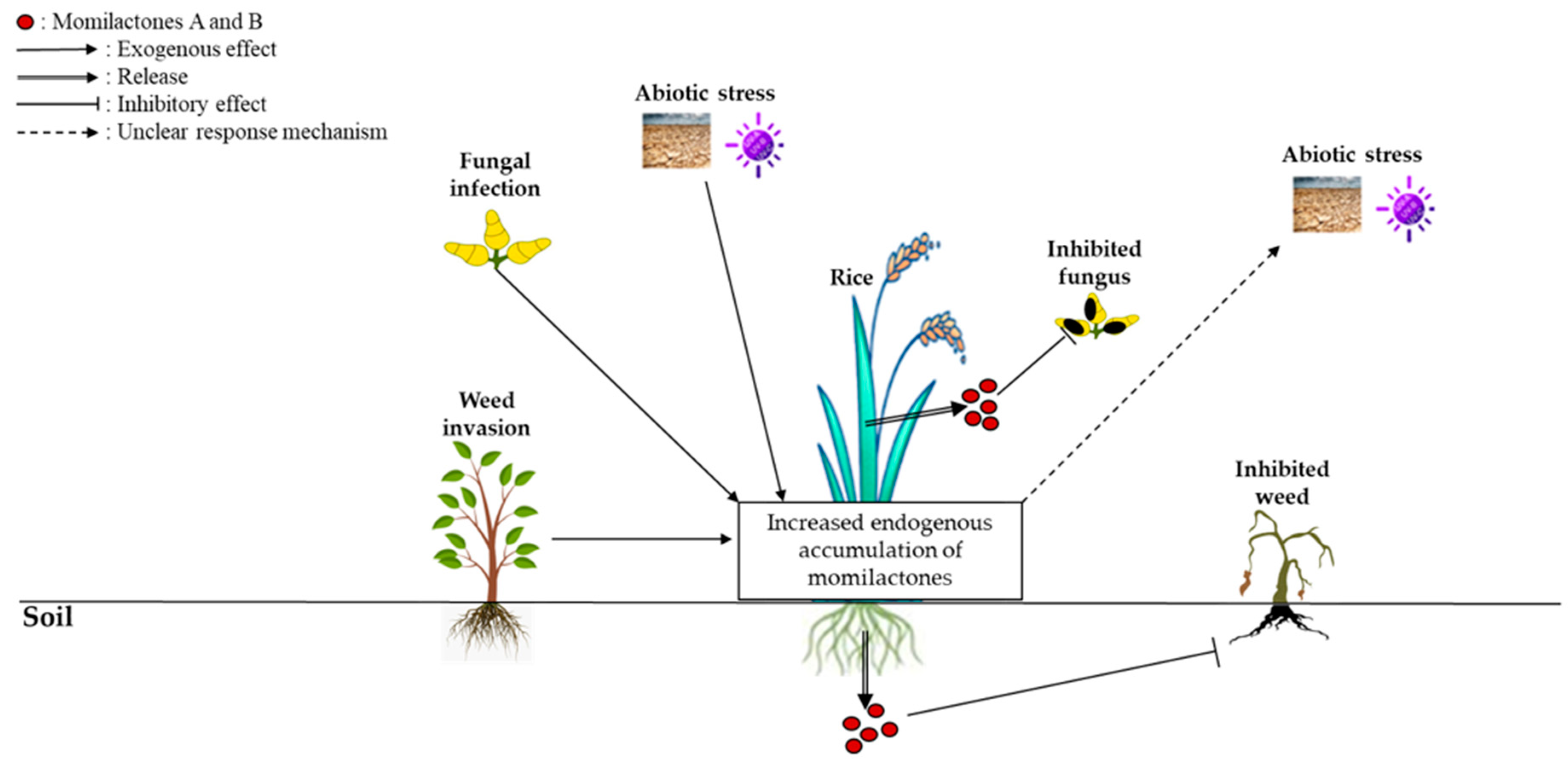
5. Involvement of Momilactones in Rice Response to Stresses
5.1. Involvement of Momilactones in Rice Resistance against Abiotic Stress
| Rice Type | Stress | MA Content (% Increase/Decrease over Control) | MB Content (% Increase/Decrease over Control) | Relevant Gene Expression | References |
|---|---|---|---|---|---|
| Japonica and Indica | Drought (4–7 days) | 68% | 30% | nd | [60] |
| Japonica | Salinity (5–12 dSm−1 NaCl) | 15.4% | 90% | nd | [51,60] |
| Japonica (Koshikari) | Chilling (6 °C) | −78% | −44% | Decreased expression of OsCPS4, OsKSL4, CYP99A3, OsMAS, and OsMAS2 | [61] |
| Japonica (Koshihikari) | UV (2–8 h) | 75% | 80% | Increased expression of CYP99A3, OsMAS, OsMAS2, OsKSL4, OsCPS4 | [61,63,64] |
| Japonica (Koshihikari) | Heavy metals (FeCl2, CuCl2) | nd | 50–72% | nd | [62] |
5.2. Involvement of Momilactones in Rice Resistance against Biotic Stress
5.2.1. Antifungal Activity
5.2.2. Allelopathic Activity
6. Contribution of Momilactones to Human Health Benefits
6.1. Antioxidant Activity
6.2. Anticancer Potential
6.3. Antidiabetic Potential
6.4. Anti-Skin Aging Potential
6.5. Anti-Inflammatory Potential
6.6. Antibacterial Activity
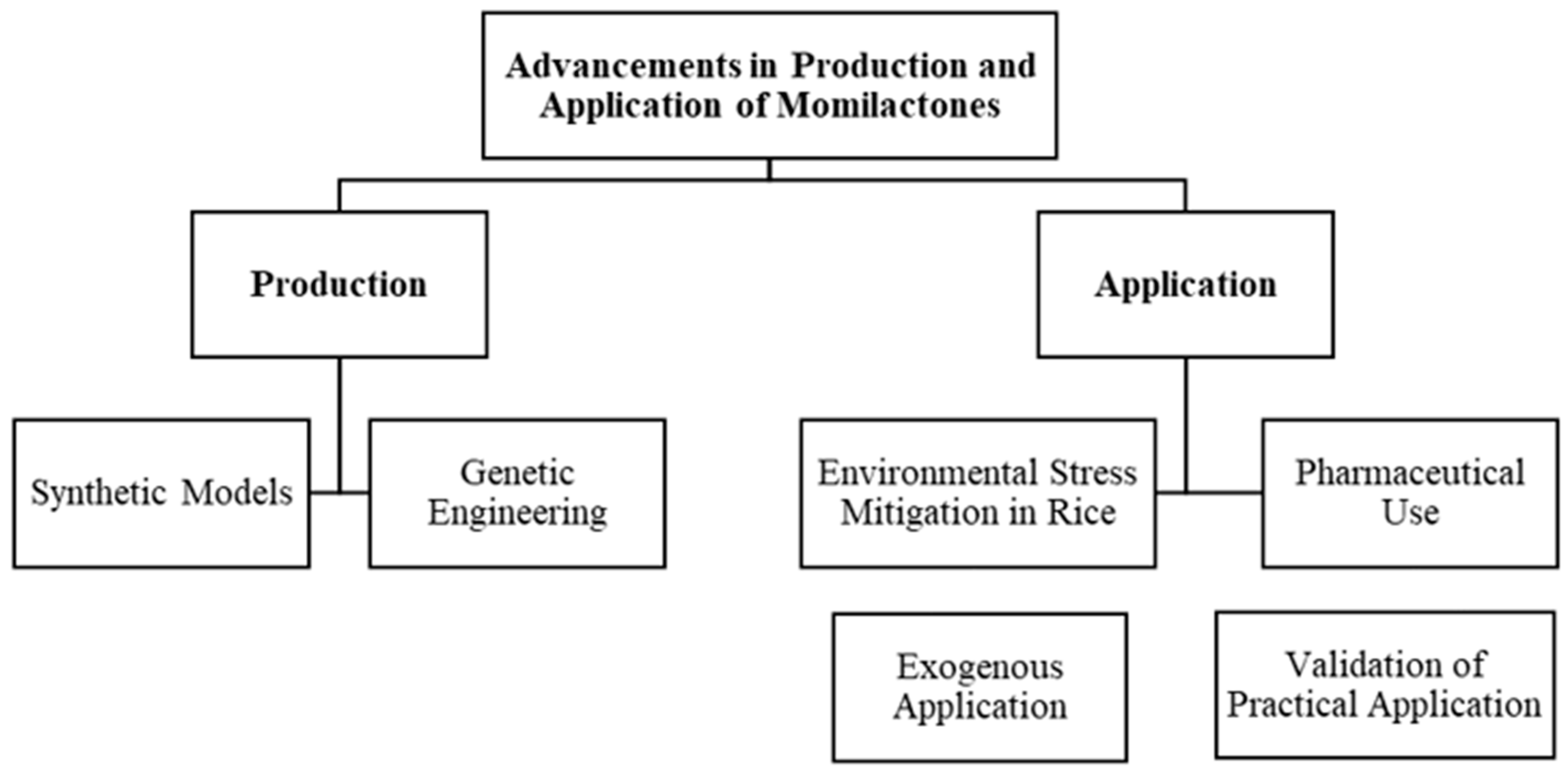
7. Future Research
7.1. Momilactones Distribution in Rice
7.2. Potential Contribution of Momilactones in Rice to Stress Tolerance
7.3. Improving Momilactone-Based Products for Pharmaceutical Purposes
7.4. Promising Approaches in Exploiting Beneficial Properties of Momilactones
7.4.1. Synthetic Models
7.4.2. Genetic Engineering
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peanparkdee, M.; Iwamoto, S. Bioactive compounds from by-products of rice cultivation and rice processing: Extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2019, 86, 109–117. [Google Scholar] [CrossRef]
- Rayee, R.; Tran, H.D.; Xuan, T.D.; Khanh, T.D. Imposed water deficit after anthesis for the improvement of macronutrients, quality, phytochemicals, and antioxidants in rice grain. Sustainability 2018, 10, 4843. [Google Scholar] [CrossRef]
- Panda, D.; Mishra, S.S.; Behera, P.K. Drought tolerance in rice: Focus on recent mechanisms and approaches. Rice Sci. 2021, 28, 119–132. [Google Scholar] [CrossRef]
- Rollins, J.A.; Habte, E.; Templer, S.E.; Colby, T.; Schmidt, J.; Von Korff, M. Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). J. Exp. Bot. 2013, 64, 3201–3212. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Ismail, A.M. Responses of photosynthesis, chlorophyll fluorescence and ROS-scavenging systems to salt stress during seedling and reproductive stages in rice. Ann. Bot. 2007, 6, 1161–1173. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Roche, D.; Monaco, T.A.; Hole, D. Stomatal conductance is a key parameter to assess limitations to photosynthesis and growth potential in barley genotypes. Plant Biol. 2006, 8, 515–521. [Google Scholar] [CrossRef]
- Hidema, J.; Kumagai, T. Sensitivity of rice to ultraviolet-B radiation. Ann. Bot. 2006, 97, 933–942. [Google Scholar] [CrossRef]
- Singh, R.; Jwa, N.S. Understanding the responses of rice to environmental stress using proteomics. J. Proteome Res. 2013, 12, 4652–4669. [Google Scholar] [CrossRef]
- Khan, M.I.R.; Fatma, M.; Per, T.S.; Anjum, N.A.; Khan, N.A. Salicylic acid-induced abiotic stress tolerance and underlying mechanisms in plants. Front. Plant Sci. 2015, 6, 462. [Google Scholar] [CrossRef]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef]
- Friend, J. Phenolic substances and plant disease. In Recent Advances in Phytochemistry, 1st ed.; Mark, A.B., Reinhard, J., Eds.; Phytochemical Society of North America: Boston, MA, USA, 1979; Volume 2, pp. 557–588. [Google Scholar]
- Mathivanan, N.; Prabavathy, V.R.; Vijayanandraj, V.R. The effect of fungal secondary metabolites on bacterial and fungal pathogens. Soil Biol. 2008, 14, 129–140. [Google Scholar]
- Yanping, T.; Shiqi, D.; Yonghua, Q.; Xin, X.; You, Y.; Liu, C.; Chuntai, W.; Changjie, J.; Xinqiong, L. Evaluation of medicinal plant extracts for rice blast disease control. Plant Sci. 2023, 30, 6–10. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Dang, P.; Zhao, S.; Lai, D.; Zhou, L. Rice secondary metabolites: Structures, roles, biosynthesis, and metabolic regulation. Molecules 2018, 23, 3098. [Google Scholar] [CrossRef] [PubMed]
- Hediye Sekmen, A.; Türkan, I.; Takio, S. Differential responses of antioxidative enzymes and lipid peroxidation to salt stress in salt-tolerant Plantago maritima and salt-sensitive Plantago media. Physiol. Plant 2007, 3, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Demiral, T.; Türkan, I. Comparative lipid peroxidation, antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance. Environ. Exp. Bot. 2005, 3, 247–257. [Google Scholar] [CrossRef]
- Kariya, K.; Ube, N.; Ueno, M.; Teraishi, M.; Okumoto, Y.; Mori, N.; Ueno, K.; Ishihara, A. Natural variation of diterpenoid phytoalexins in cultivated and wild rice species. Phytochemistry 2020, 18, 112518. [Google Scholar] [CrossRef]
- Dixon, R.A.; Dey, P.M.; Lamb, C.J. Phytoalexins: Molecular biology and enzymology. Adv. Enzymol. Relat. Areas Mol. Biol. 1983, 55, 69. [Google Scholar]
- Grayer, R.J.; Kokubun, T. Plant–fungal interactions: The search for phytoalexins and other antifungal compounds from higher plants. Phytochemistry 2001, 56, 253–263. [Google Scholar] [CrossRef]
- Hain, R.; Reif, H.J.; Krause, E.; Langebartels, R.; Kindl, H.; Vornam, B.; Stenzel, K. Disease resistance results from foreign phytoalexin expression in a novel plant. Nature 1993, 361, 153–156. [Google Scholar] [CrossRef]
- Jeandet, P. Phytoalexins: Current progress and prospects. Molecules 2015, 20, 2770–2774. [Google Scholar] [CrossRef]
- Mansfield, J.W. The role of phytoalexins in disease resistance. In Phytoalexins; Blackie: Glasgow, UK, 1982; pp. 253–288. [Google Scholar]
- Paxton, J. Soybean phytoalexins: Elicitation, nature, mode of action, and role. In Handbook of Phytoalexin Metabolism and Action; Routledge: London, UK, 1995; pp. 69–83. [Google Scholar]
- Kato, T.; Kabuto, C.; Sasaki, N.; Tsunagawa, M.; Aizawa, H.; Fujita, K.; Takahashi, N. Momilactones, growth inhibitors from rice, Oryza sativa L. Tetrahedron Lett. 1973, 39, 3861–3864. [Google Scholar] [CrossRef]
- Anh, L.H.; Dang Khanh, T.; Xuan, T.D. Biological roles of momilactones: Achievements, challenges, and promising approaches to exploit their beneficial properties. Front. Nat. Prod. 2023, 2, 1245869. [Google Scholar] [CrossRef]
- Cartwright, D.W.; Langcake, P.; Ride, J.P. Phytoalexin production in rice and its enhancement by a dichlorocyclopropane fungicide. Physiol. Plant Pathol. 1980, 17, 259. [Google Scholar] [CrossRef]
- Tsunakawa, M.; Ohba, A.; Sasaki, N.; Kabuto, C.; Kato, T.; Kitahara, Y.; Takahashi, N. Momilactone C, a minor constituent of growth inhibitors in rice husk. Chem. Lett. 1976, 5, 1157–1158. [Google Scholar] [CrossRef]
- Cho, J.G.; Cha, B.J.; Min Lee, S.; Shrestha, S.; Jeong, R.H.; Sung Lee, D.; Baek, N.I. Diterpenes from the roots of Oryza sativa L. and their inhibition activity on NO production in LPS-Stimulated RAW264. 7 macrophages. Chem. Biodivers. 2015, 12, 1356–1364. [Google Scholar] [CrossRef]
- Li, J.L.; Wie, L.L.; Chen, C.; Liu, D.; Gu, Y.Q.; Duan-Mu, J.X.; Song, Y. Bioactive constituents from the bryophyta Hypnum plumaeforme. Chem. Biodivers. 2020, 17, e2000552. [Google Scholar] [CrossRef]
- Liu, N.; Wang, S.; Lou, H. A new pimarane-type diterpenoid from moss Pseudoleskeella papillosa (Lindb.) Kindb. Acta Pharm. Sin. B. 2012, 3, 256–259. [Google Scholar] [CrossRef][Green Version]
- Zhao, M.; Cheng, J.; Guo, B.; Duan, J.; Che, C.T. Momilactone and related diterpenoids as potential agricultural chemicals. J. Agric. Food Chem. 2018, 30, 7859–7872. [Google Scholar] [CrossRef]
- Miyamoto, K.; Shimizu, T.; Okada, K. Transcriptional regulation of the biosynthesis of phytoalexin: A lesson from specialized metabolites in rice. Plant Biotechnol. 2014, 31, 377–388. [Google Scholar] [CrossRef]
- Akatsuka, T.; Kodama, O.; Kato, H.; Kono, Y.; Takeuchi, S. 3-hydroxy-7-oxo-sandaracopimaradiene (oryzalexin A), a new phytoalexin isolated from rice blast leaves. Agric. Biol. Chem. 1983, 47, 445–447. [Google Scholar] [CrossRef]
- Horie, K.; Inoue, Y.; Sakai, M.; Yao, Q.; Tanimoto, Y.; Koga, J.; Toshima, H.; Hasegawa, M. Identification of UV-induced diterpenes including a new diterpene phytoalexin, phytocassane F, from rice leaves by complementary GC/MS and LC/MS approaches. J. Agric. Food Chem. 2015, 63, 4050–4059. [Google Scholar] [CrossRef]
- Inoue, Y.; Sakai, M.; Yao, Q.; Tanimoto, Y.; Toshima, H.; Hasegawa, M. Identification of a novel casbane-type diterpene phytoalexin, ent-10-oxodepressin, from rice leaves. Biosci. Biotechnol. Biochem. 2013, 77, 760–765. [Google Scholar] [CrossRef]
- Jin, X.; Baysal, C.; Gao, L.; Medina, V.; Drapal, M.; Ni, X.; Sheng, Y.; Shi, L.; Capell, T.; Fraser, P.D. The subcellular localization of two isopentenyl diphosphate isomerases in rice suggests a role for the endoplasmic reticulum in isoprenoid biosynthesis. Plant Cell Rep. 2020, 39, 119–133. [Google Scholar] [CrossRef]
- Okada, A.; Shimizu, T.; Okada, K.; Kuzuyama, T.; Koga, J.; Shibuya, N.; Nojiri, H.; Yamane, H. Elicitor induced activation of the methylerythritol phosphate pathway toward phytoalexins biosynthesis in rice. Plant Mol. Biol. 2007, 65, 177–187. [Google Scholar] [CrossRef]
- Yamane, H. Biosynthesis of phytoalexins and regulatory mechanisms of it in rice. Biosci. Biotechnol. Biochem. 2013, 77, 1141–1148. [Google Scholar] [CrossRef]
- Hemmerlin, A.; Hoeffler, J.F.; Meyer, O.; Tritsch, D.; Kagan, I.A.C.; Rohmer, M.; Bach, T.J. Cross-talk between the cytosolic mevalonate and the plastidial methyl erythritol phosphate pathways in tobacco bright yellow-2 cells. J. Biol. Chem. 2003, 278, 26666–26676. [Google Scholar] [CrossRef]
- Laule, O.; Fürholz, A.; Chang, H.S.; Zhu, T.; Wang, X.; Heifetz, P.B.; Gruissem, W.; Lange, M. Crosstalk between cytosolic and plastidial pathways of isoprenoid biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2003, 100, 6866–6871. [Google Scholar] [CrossRef]
- Vranová, E.; Coman, D.; Gruissem, W. Network analysis of the MVA and MEP pathways for isoprenoid synthesis. Annu. Rev. Plant Biol. 2013, 64, 665–700. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H. Defensive molecules momilactones A and B: Function, biosynthesis, induction and occurrence. Toxins 2023, 4, 241. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, T.; Miyura, K.; Itoh, H.; Tatsumi, T.; Ueguchi-Tanaka, M.; Ishiyama, K.; Kobayashi, M.; Agrawal, G.K.; Takeda, S.; Abe, K.; et al. Erratum: An overview of gibberellin metabolism enzyme genes and their related mutants in rice. Plant Physiol. 2004, 134, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
- Wilderman, P.R.; Xu, M.; Jin, Y.; Coates, R.M.; Peters, R.J. Identification of syn-pimara-7,15-diene synthase reveals functional clustering of terpene synthases involved in rice phytoalexin/allelochemical biosynthesis. Plant Physiol. 2004, 135, 2098–2105. [Google Scholar] [CrossRef]
- Kitaoka, N.; Zhang, J.; Oyagbenro, R.K.; Brown, B.; Wu, Y.; Yang, B.; Li, Z.; Peters, R.J. Interdependent evolution of biosynthetic gene clusters for momilactone production in rice. Plant Cell 2021, 2, 290–305. [Google Scholar] [CrossRef]
- De La Peña, R.; Sattely, E.S. Rerouting plant terpene biosynthesis enables momilactone pathway elucidation. Nat. Chem. Biol. 2021, 17, 205–212. [Google Scholar] [CrossRef]
- Zhang, J.; Peters, R.J. Why are momilactones always associated with biosynthetic gene clusters in plants? Proc. Natl. Acad. Sci. USA 2020, 117, 13867–13869. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhang, J.; Li, Z.; Peters, R.J.; Yang, B. Dissecting the labdane-related diterpenoid biosynthetic gene clusters in rice reveals directional cross-cluster phytotoxicity. New Phytol. 2022, 233, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Kato-Noguchi, H.; Ino, T. Rice seedlings release momilactone B into the environment. Phytochemistry 2003, 5, 551–554. [Google Scholar] [CrossRef]
- Kato-Noguchi, H. Allelopathic substance in rice root exudates: Rediscovery of momilactone B as an allelochemical. J. Plant Physiol. 2004, 3, 271–276. [Google Scholar] [CrossRef]
- Huong, C.T.; Anh, T.T.T.; Dat, T.D.; Khanh, T.D.; Xuan, T.D. Uniparental inheritance of salinity tolerance and beneficial phytochemicals in rice. Agronomy 2020, 10, 1032. [Google Scholar] [CrossRef]
- Ahmad, A.; Xuan, T.D.; Minh, T.N.; Siddiqui, N.A.; Van Quan, N. Comparative extraction and simple isolation improvement techniques of active constituents’ momilactone A and B from rice husks of Oryza sativa L. by HPLC analysis and column chromatography. Saudi Pharm. J. 2019, 27, 17–24. [Google Scholar] [CrossRef]
- Quan, N.V.; Xuan, T.D.; Tran, H.D.; Ahmad, A.; Khanh, T.D.; Dat, T.D. Contribution of momilactones A and B to diabetes inhibitory potential of rice bran: Evidence from in vitro assays. Saudi Pharm. J. 2019, 27, 643–649. [Google Scholar] [CrossRef]
- Quan, N.V.; Thien, D.D.; Khanh, T.D.; Tran, H.D.; Xuan, T.D. Momilactones A, B, and tricin in rice grain and by-products are potential skin aging inhibitors. Food 2019, 12, 602. [Google Scholar] [CrossRef] [PubMed]
- Zu, X.; Lu, Y.; Wang, Q.; Chu, P.; Miao, W.; Wang, H.; La, H. A new method for evaluating the drought tolerance of upland rice cultivars. Crop J. 2017, 5, 488–498. [Google Scholar] [CrossRef]
- Xiong, Q.; Sun, C.; Wang, R.; Wang, R.; Wang, X.; Zhang, Y.; Zhu, J. The Key metabolites in rice quality formation of conventional japonica varieties. Curr. Issues Mol. Biol. 2023, 45, 990–1001. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Q.; Zhang, J.; Shi, Q.; Zhang, Y.; Sun, C.; Li, A.; Lu, W.; Hu, J.; Zhou, N.; Wei, H.; et al. The key metabolites associated with nutritional components in purple glutinous rice. Food Res. Int. 2022, 160, 111686. [Google Scholar] [CrossRef]
- Quan, N.V.; Tran, H.D.; Xuan, T.D.; Ahmad, A.; Dat, T.D.; Khanh, T.D.; Teschke, R. Momilactones A and B are α-amylase and α-glucosidase inhibitors. Molecules 2019, 3, 482. [Google Scholar] [CrossRef] [PubMed]
- Kakar, K.; Xuan, T.D.; Quan, N.V.; Wafa, I.K.; Tran, H.D.; Khanh, T.D.; Dat, T.D. Efficacy of N-methyl-N-nitrosourea mutation on physicochemical properties, phytochemicals, and momilactones A and B in rice. Sustainability 2019, 23, 6862. [Google Scholar] [CrossRef]
- Xuan, T.D.; Minh, T.N.; Anh, L.H.; Khanh, T.D. Allelopathic momilactones A and B are implied in rice drought and salinity tolerance, not weed resistance. Agron. Sustain. Dev. 2016, 36, 52. [Google Scholar] [CrossRef]
- Anh, L.H.; Van Quan, N.; Quang Lam, V.; Takami, A.; Dang Khanh, T.; Xuan, T.D. Rice momilactones and phenolics: Expression of relevant biosynthetic genes in response to UV and chilling stresses. Agronomy 2022, 12, 1731. [Google Scholar]
- Kato-Noguchi, H. Stress-induced allelopathic activity and momilactone B in rice. Plant Growth Regul. 2009, 59, 153–158. [Google Scholar] [CrossRef]
- Cartwright, D.; Langcake, P.; Pryce, R.J.; Leworthy, D.P.; Ride, J.P. Chemical activation of host defense mechanisms as a basis for crop protection. Nature 1977, 5611, 511–513. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kujime, H.; Ino, T. UV-induced momilactone B accumulation in rice rhizosphere. J. Plant Physiol. 2007, 164, 1548–1551. [Google Scholar] [CrossRef]
- Fukuta, M.; Xuan, T.D.; Deba, F.; Tawata, S.; Khanh, T.D.; Chung, I.M. Comparative efficacies in vitro of antibacterial, fungicidal, antioxidant, and herbicidal activities of momilactones A and B. J. Plant Interact. 2007, 4, 245–251. [Google Scholar] [CrossRef]
- Adou, E.; Williams, R.B.; Schilling, J.K.; Malone, S.; Meyer, J.; Wisse, J.H.; Kingston, D.G. Cytotoxic diterpenoids from two lianas from the Suriname rainforest. Bioorg. Med. Chem. 2005, 13, 6009–6014. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Hasegawa, M.; Ino, T.; Ota, K.; Kujime, H. Contribution of momilactone A and B to rice allelopathy. J. Plant Physio. 2010, 167, 787–791. [Google Scholar] [CrossRef]
- Chung, I.M.; Hahn, S.J.; Ahmad, A. Confirmation of potential herbicidal agents in hulls of rice, Oryza sativa. J. Chem. Ecol. 2005, 31, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Quan, N.V.; Xuan, T.D.; Tran, H.D.; Dieu Thuy, N.T. Inhibitory activities of momilactones A, B, E, and 7-Ketostigmasterol isolated from rice husk on paddy and invasive weeds. Plants 2019, 8, 159. [Google Scholar] [CrossRef] [PubMed]
- Mennan, H.; Ngouajio, M.; Sahin, M.; Isik, D.; Kaya Altop, E. Quantification of momilactone B in rice hulls and the phytotoxic potential of rice extracts on the seed germination of Alisma plantago-aquatica. Weed Biol. Manag. 2012, 12, 29–39. [Google Scholar] [CrossRef]
- Horie, K.; Sakai, K.; Okugi, M.; Toshima, H.; Hasegawa, M. Ultraviolet-induced amides and casbene diterpenoids from rice leaves. Phytochem. Lett. 2016, 15, 57–62. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kitajima, S. Momilactone sensitive proteins in Arabidopsis thaliana. Nat. Prod. Commun. 2015, 10, 1934578X1501000508. [Google Scholar] [CrossRef]
- Valletta, A.; Iozia, L.M.; Fattorini, L.; Leonelli, F. Rice Phytoalexins: Half a Century of Amazing Discoveries; Part I: Distribution, Biosynthesis, Chemical Synthesis, and Biological Activities. Plants 2023, 12, 260. [Google Scholar] [CrossRef]
- Anh, L.H.; Lam, V.Q.; Takami, A.; Khanh, T.D.; Quan, N.V.; Xuan, T.D. Cytotoxic mechanism of momilactones A and B against acute promyelocytic leukemia and multiple myeloma cell Lines. Cancers 2022, 14, 4848. [Google Scholar] [CrossRef]
- Park, C.; Jeong, N.Y.; Kim, G.Y.; Han, M.H.; Chung, I.M.; Kim, W.J.; Choi, Y.H. Momilactone B induces apoptosis and G1 arrest of the cell cycle in human monocytic leukemia U937 cells through downregulation of pRB phosphorylation and induction of the cyclin-dependent kinase inhibitor. Oncol. Rep. 2014, 31, 1653–1660. [Google Scholar] [CrossRef]
- Lee, S.C.; Chung, I.M.; Jin, Y.J.; Song, Y.S.; Seo, S.Y.; Park, B.S.; Yoo, Y.H. Momilactone B, an allelochemical of rice hulls, induces apoptosis on human lymphoma cells (Jurkat) in a micromolar concentration. Nut. Cancer 2008, 60, 542–551. [Google Scholar] [CrossRef]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Elbadry, M.I.; Taniwaki, M.; Harada, K.; Trung, L.Q.; Nakagawa, N.; Nakao, S. The simultaneous inhibition of the mTOR and MAPK pathways with Gnetin-C induces apoptosis in acute myeloid leukemia. Cancer Lett. 2017, 400, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Anh, L.H.; Xuan, T.D. Momilactones and phenolics in brown rice: Enrichment, optimized extraction, and potential for antioxidant and anti-diabetic activities. Separations 2023, 11, 6. [Google Scholar] [CrossRef]
- Lemus, C.; Angelis, A.; Halabalaki, M.; Skaltsounis, A.L. γ-Oryzanol: An attractive bioactive component from rice bran. In Wheat and Rice in Disease Prevention and Health, 1st ed.; Ronald, R.W., Sherma, Z., Victor, R.P., Eds.; Academic Press: Cambridege, MA, USA, 2014; pp. 409–430. [Google Scholar]
- Chung, I.M.; Ali, M.; Hahn, S.J.; Siddiqui, N.A.; Lim, Y.H.; Ahmad, A. Chemical constituents from the hulls of Oryza sativa L. with cytotoxic activity. Chem. Nat. Compd. 2005, 41, 182–189. [Google Scholar] [CrossRef]
- Hanai, H.; Ishida, S.; Saito, C.; Maita, T.; Kusano, M.; Tamogami, S.; Noma, M. Stimulation of mycelia growth in several mushroom species by rice husks. Biosci. Biotechnol. Biochem. 2005, 69, 123–127. [Google Scholar] [CrossRef]
- Cliver, D.O.; Riemann, H.P. Foodborne Infections and Intoxications, 5th ed.; Glenn Morris, J., Vugia, D.J., Eds.; Academic Press: London, UK, 2011. [Google Scholar]
- Tena, D.; Martínez-Torres, J.Á.; Pérez-Pomata, M.T.; Sáez-Nieto, J.A.; Rubio, V.; Bisquert, J. Cutaneous infection due to Bacillus pumilus: Report of 3 cases. Clin. Infect. Dis. 2007, 44, 40–42. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Ino, T. Concentration and release level of momilactone B in the seedlings of eight rice cultivars. J. Plant Physiol. 2005, 162, 965–969. [Google Scholar] [CrossRef]
- Miyamoto, K.; Fujita, M.; Shenton, M.R.; Akashi, S.; Sugawara, C.; Sakai, A. Evolutionary trajectory of phytoalexin biosynthetic gene clusters in rice. Plant J. 2016, 87, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Colebrook, E.H.; Thomas, S.G.; Phillips, A.L.; Hedden, P. The role of gibberellin signaling in plant responses to abiotic stress. J. Exp. Biol. 2014, 217, 67–75. [Google Scholar] [CrossRef]
- Xuan, T.D.; Huong, C.T.; Quan, N.V.; Anh, L.H.; Khanh, T.D.; Rayee, R. Improvement of salinity tolerance in rice seedlings by exogenous magnesium sulfate application. Soil Syst. 2022, 6, 69. [Google Scholar] [CrossRef]
- Jesmin, A.; Anh, L.H.; Mai, N.P.; Khanh, T.D.; Xuan, T.D. Fulvic acid improves salinity tolerance of rice seedlings: Evidence from phenotypic performance, relevant phenolic acids, and momilactones. Plants 2023, 12, 2359. [Google Scholar] [CrossRef]
- Kato-Noguchi, H.; Kobayashi, K. Jasmonic acid, protein phosphatase inhibitor, metals and UV-irradiation increased momilactone A and B concentrations in the moss Hypnum plumaeforme. J. Plant Physiol. 2009, 166, 1118–1122. [Google Scholar] [CrossRef]
- Rakwal, R.; Tamogami, S.; Kodama, O. Role of jasmonic acid as a signaling molecule in copper chloride-elicited rice phytoalexin production. Biosci. Biotechnol. Biochem. 1996, 60, 1046–1048. [Google Scholar] [CrossRef]
- Daw, B.D.; Zhang, L.H.; Wang, Z.Z. Salicylic acid enhances antifungal resistance to Magnaporthe grisea in rice plants. Aust. Plant Pathol. 2008, 37, 637–644. [Google Scholar] [CrossRef]
- Bajsa-Hirschel, J.; Pan, Z.; Duke, S.O. Rice momilactone gene cluster: Transcriptional response to barnyard grass (Echinochloa crus-galli). Mol. Biol. Rep. 2020, 47, 1507–1512. [Google Scholar] [CrossRef]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenet. Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef]
- Libby, G.; Donnelly, L.A.; Donnan, P.T.; Alessi, D.R.; Morris, A.D.; Evans, J.M.M. New users of metformin are at low risk of incident cancer. Diabetes Care 2009, 32, 1620–1625. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the interdependence between oxidative stress and inflammation explain the antioxidant paradox? Oxid. Med. Cell. Longev. 2016, 2016, 1–9. [Google Scholar] [CrossRef]
- Teschke, R.; Xuan, T.D. Active nature-based ingredients for drug discovery with pivotal role of clinical efficacy: Review and prospective. J. Mod. Med. Chem. 2020, 8, 4–18. [Google Scholar] [CrossRef]
- Quan, N.V.; Xuan, T.D.; Teschke, R. Potential hepatotoxins found in herbal medicinal products: A systematic review. Int. J. Mol. Sci. 2020, 21, 5011. [Google Scholar] [CrossRef]
- Sanz, M.A.; Fenaux, P.; Tallman, M.S.; Estey, E.H.; Löwenberg, B.; Naoe, T. Management of acute promyelocytic leukemia: Updated recommendations from an expert panel of the European leukemianet. Blood 2019, 133, 1630–1643. [Google Scholar] [CrossRef]
- Anh, L.H.; Xuan, T.D.; Thuy, N.T.D.; Quan, N.V.; Trang, L.T. Antioxidant and α-amylase inhibitory activities and phytocompounds of Clausena indica fruits. Medicines 2020, 7, 10. [Google Scholar]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Liu, Q.; Huang, J.; Chen, Y. A synthetic view on momilactones and related 9β-H pimarane skeleton diterpenoids. Front. Chem. 2022, 10, 882404. [Google Scholar] [CrossRef]
- Sicherer-Roetman, A.; Jansen, B.J.M.; de Groot, A. Investigations into the total synthesis of momilactones. Stereoselective preparation of (±)-4,4-dinor-9βH-pimara-7,19-diene. Recl. Trav. Chim. 2010, 104, 193–202. [Google Scholar] [CrossRef]
- Sichererroetman, A.; Jansen, B.J.M.; de Groot, A. Stereospecific preparation of (±)-4,4-dinor-9-beta-H-pimara-7,15-diene, a model for the total synthesis of momilactone type diterpenes. Tetrahedron Lett. 1984, 25, 2593–2596. [Google Scholar] [CrossRef]
- Jansen, B.J.M.; Schepers, G.C.; de Groot, A. The stereoselective synthesis of (±)-9βH-pimara-7,19-diene. Tetrahedron Lett. 1989, 45, 2773–2776. [Google Scholar] [CrossRef]
- Yajima, A.; Toda, K.; Okada, K.; Yamane, H.; Yamamoto, M.; Hasegawa, M. Stereocontrolled total synthesis of (±)-3β-hydroxy-9β-pimara-7,15-diene, a putative biosynthetic intermediate of momilactones. Tetrahedron Lett. 2011, 52, 3212–3215. [Google Scholar] [CrossRef]
- Macías, F.A.; Galindo, J.C.G.; Molinillo, J.M.G.; Castellano, D.; Velasco, R.F.; Chinchilla, D. Developing new herbicide models from allelochemicals. Pestic. Sci. 1999, 55, 662–665. [Google Scholar] [CrossRef]
- Macías, F.A.; Molinillo, J.M.G.; Varela, R.M.; Galindo, J.C.G. Allelopathy—A natural alternative for weed control. Pest Manag. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef]
- Bourgaud, F.; Gravot, A.; Milesi, S.; Gontier, E. Production of plant secondary metabolites: A historical perspective. Plant Sci. 2001, 161, 839–851. [Google Scholar] [CrossRef]
- Okada, K. The biosynthesis of isoprenoids and the mechanisms regulating it in plants. Biosci. Biotechnol. Biochem. 2011, 75, 1219–1225. [Google Scholar] [CrossRef]
- Kurusu, T.; Hamada, J.; Nokajima, H.; Kitagawa, Y.; Kiyoduka, M.; Takahashi, A. Regulation of microbe-associated molecular pattern-induced hypersensitive cell death, phytoalexin production, and defense gene expression by calcineurin B-like protein-interacting protein kinases, OsCIPK14/15, in rice cultured cells. Plant Physiol. 2010, 153, 678–692. [Google Scholar] [CrossRef]
- Kim, J.-A.; Cho, K.; Singh, R.; Jung, Y.-H.; Jeong, S.-H.; Kim, S.-H. Rice OsACDR1 (Oryza sativa accelerated cell death and resistance 1) is a potential positive regulator of fungal disease resistance. Mol. Cells 2009, 28, 431–439. [Google Scholar] [CrossRef]
- Ono, E.; Wong, H.L.; Kawasaki, T.; Hasegawa, M.; Kodama, O.; Shimamoto, K. Essential role of the small GTPase Rac in disease resistance of rice. Proc. Natl. Acad. Sci. USA 2001, 98, 759–764. [Google Scholar] [CrossRef]
- Sawada, K.; Hasegawa, M.; Tokuda, L.; Kameyama, J.; Kodama, O.; Kohchi, T. Enhanced resistance to blast fungus and bacterial blight in transgenic rice constitutively expressing OsSBP, a rice homologue of mammalian seleniumbinding proteins. Biosci. Biotechnol. Biochem. 2004, 68, 873–880. [Google Scholar] [CrossRef]
- Mori, M.; Tomita, C.; Sugimoto, K.; Hasegawa, M.; Hayashi, N.; Dubouzet, J.G. Isolation andmolecular characterization of a Spotted leaf 18 mutants by modified activation-tagging in rice. Plant Mol. Biol. 2007, 63, 847–860. [Google Scholar] [CrossRef]
- Shimura, K.; Okada, A.; Okada, K.; Jikumaru, Y.; Ko, K.-W.; Toyomasu, T. Identification of a biosynthetic gene cluster in rice for momilactones. J. Biol. Chem. 2007, 28, 34013–34018. [Google Scholar] [CrossRef]
- Wang, Q.; Hillwig, M.L.; Peters, R.J. CYP99A3: Functional identification of a diterpene oxidase from the momilactone biosynthetic gene cluster in rice. Plant J. 2011, 65, 87–95. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information (NCBI). Available online: https://www.ncbi.nlm.nih.gov (accessed on 20 December 2023).
- Nguyen, T.-D.; Dang, T.-T.T. Demystifying the momilactone pathway. Nat. Chem. Biol. 2021, 17, 126–128. [Google Scholar] [CrossRef] [PubMed]

| Compound | Plant Part | Concentration (μg/g) | Reference |
|---|---|---|---|
| MA | Root | 8.06 | [47,50] |
| Leaf | 4.28 | [17,44,51] | |
| Husk | 16.44 | [44,52] | |
| Bran | 6.65 | [53] | |
| Grain | 2.07 | [54] | |
| MB | Root | 5.69 | [48,50,55] |
| Leaf | 12.73 | [44,50,51] | |
| Husk | 9.24 | [44,52] | |
| Bran | 6.24 | [53] | |
| Grain | 1.06 | [54] |
| Compound | Rice Type/Variety | Plant Part Used for Isolation | Activity and Model Organism | Observations * | References |
|---|---|---|---|---|---|
| MA | Japonica, (Koshihikari, and Akitakomachi) | Coleoptiles and, Husk | Antifungal (P. oryzae, M. oryzae, B. cinerea, F. solani, C. gloeosporioide, and F. oxysporum) | IC50 (μg/mL): 78 to 198 | [17,31,63,65,66] |
| MB | Japonica (Koshihikari and, Nipponebare) | Seedlings | Allelopathic (E. galli, Lepidum sativum L. and Arabidopsis thaliana) | Inhibition (%): 2 to 9 | [49,67,68,69,70,71,72] |
| Japonica, (Koshihikari, and Akitakomachi) | Husk and, Bran | Antifungal (P. oryzae, M. oryzae, B. cinerea, F. solani, C. gloeospori-oide, and F. oxysporum) | IC50 (μg/mL): 53.4 to 137.4 | [17,31,63,65,67] | |
| Japonica (Koshihikari and, Nipponebare) | Seedlings | Allelopathic (E. galli, Lepidum sativum L. and Arabidopsis thaliana) | Inhibition (%): 14 to 40 | [49,66,68,69,70,71,72] | |
| MC | Koshihikari | Seeds | Allelopathic (L. sativa) | IC50 (mg/mL): 1.0 | [27,73] |
| Compound | Rice Variety | Plant Part Used for Isolation | Activity | Assay | Inhibitory Effects * | References |
|---|---|---|---|---|---|---|
| MA | Japonica/Koshihikari | Husk, grain | Antioxidant | ABTS, Reducing Power | IC50 (mg/mL): 2.8 EC50 (μg): 783 | [54,65] |
| Koshihikari | Husk, bran | Anti-leukemia Anti-lymphoma | HL-60 U-937 | - - | [74,75,76,77,78] | |
| Koshihikari | Husk, bran | Antidiabetic | α-Amylase α-Glucosidase | IC50 (μg/mL): 133 IC50 (μg/mL): 182 | [53,58,76] | |
| Koshihikari, Shinnosuke | Grain | Anti-skin aging | Elastase Tyrosinase | Inhibition (%): 30.9 Inhibition (%): 37.6 | [79] | |
| Japonica | Roots | Anti-inflammatory | LPS-stiumulated NO production | IC50 (μg/mL): 0.53 | [28] | |
| Japonica, (Koshihikari, and Akitakomachi) | Husk | Antibacterial | E. coli P. ovalis B. cereus B. pumilus M. aeruginosa | Inhibition (mm): 11 to 17 | [31,63,65,80,81] | |
| MB | Japonica/Koshihikari | Husk, grain | Antioxidant | DPPH ABTS | IC50 (mg/mL): 1.3 EC50 (μg): 790 | [54,65] |
| Koshihikari | Husk, Bran | Anti-leukemia Anti-lymphoma | HL-60 U-266 | IC50 (µM): 4.49 IC50 (µM): 5.09 | [74,78] | |
| Koshihikari | Husk, Bran | Antidiabetic | α-Amylase α-Glucosidase | IC50 (μg/mL): 129.02 to 146.85 IC50 (μg/mL): 612.03 | [53,58,76] | |
| Koshihikari, Shinnosuke | Grain | Anti-skin aging | Pancreatic elastase Tyrosinase | Inhibition (%): 18.5 Inhibition (%): 12.6 | [54,79] | |
| Japonica, (Koshihikari, and Akitakomachi) | Coleoptiles and, Husk | Antibacterial | E. coli P. ovalis B. cereus B. pumilus M. aeruginosa | Inhibition (mm): 14.2 to 17.2 | [31,63,65,80,81] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayee, R.; Anh, L.H.; Khanh, T.D.; Xuan, T.D. Potential Momilactones in Rice Stress Tolerance and Health Advantages. Agronomy 2024, 14, 405. https://doi.org/10.3390/agronomy14030405
Rayee R, Anh LH, Khanh TD, Xuan TD. Potential Momilactones in Rice Stress Tolerance and Health Advantages. Agronomy. 2024; 14(3):405. https://doi.org/10.3390/agronomy14030405
Chicago/Turabian StyleRayee, Ramin, La Hoang Anh, Tran Dang Khanh, and Tran Dang Xuan. 2024. "Potential Momilactones in Rice Stress Tolerance and Health Advantages" Agronomy 14, no. 3: 405. https://doi.org/10.3390/agronomy14030405
APA StyleRayee, R., Anh, L. H., Khanh, T. D., & Xuan, T. D. (2024). Potential Momilactones in Rice Stress Tolerance and Health Advantages. Agronomy, 14(3), 405. https://doi.org/10.3390/agronomy14030405









