Fusariotoxins Concentration in Common Wheat Grain Depending on the Farming System (Organic vs. Integrated vs. Conventional) and Changes During Grain Processing
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Material
2.2. Methods
2.2.1. Analysis of Ergosterol Concentration
2.2.2. Analysis of the Concentration of Type A and B Trichothecenes
2.3. Statistical Analysis
3. Results
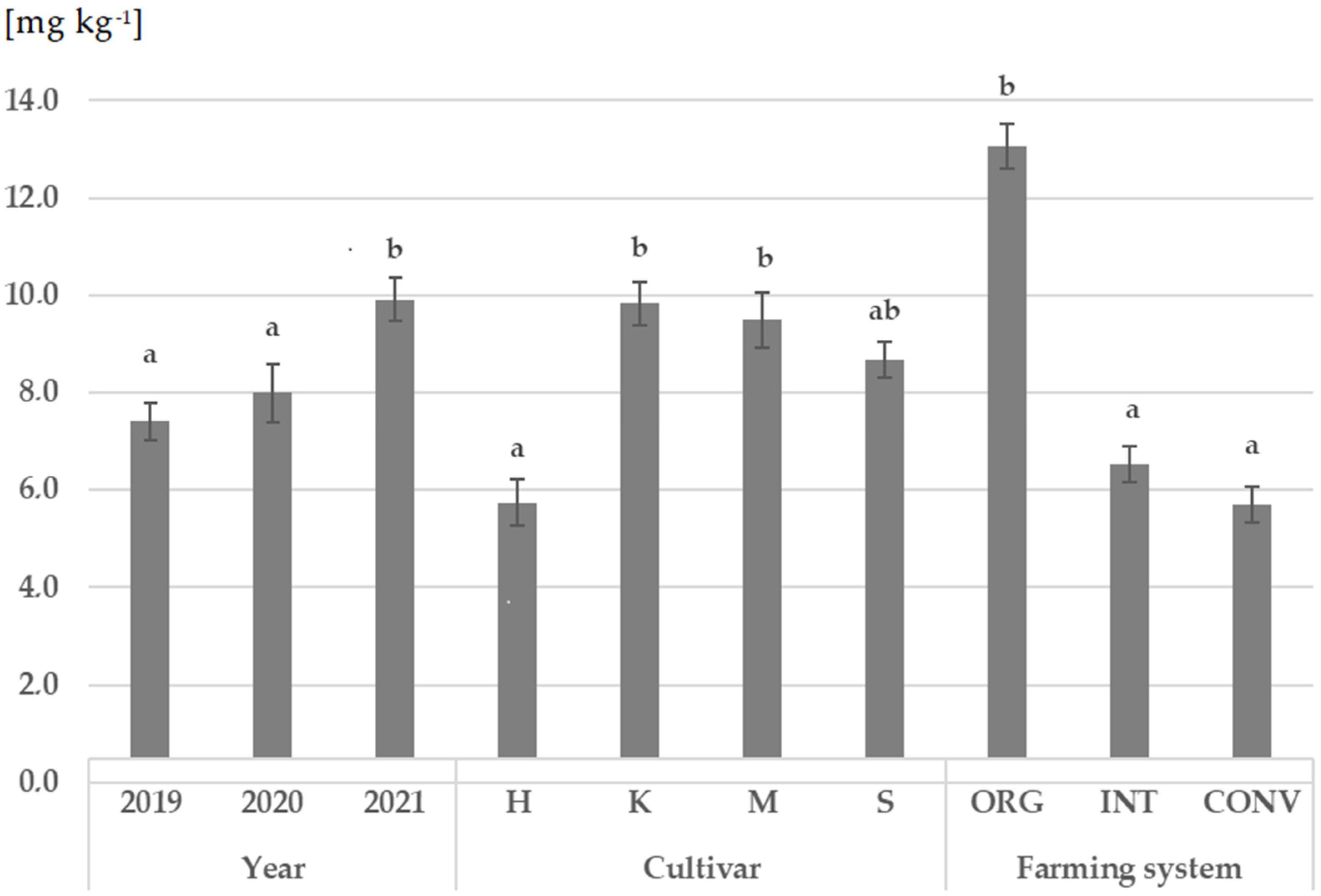
3.1. Ergosterol Concentration in Wheat Grain
3.2. Type A Trichothecenes Concentration in Wheat Grain
3.3. Type B Trichothecenes Concentration in Wheat Grain
3.4. Ergosterol Content in Wheat Grain and Grain Byproducts
3.5. Type A Trichothecenes Concentration in Wheat Grain and Grain Byproducts
3.6. Trichothecenes Type B Concentration in Wheat Grain and Grain Byproducts
3.7. PCA Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Available online: https://www.fao.org/ (accessed on 15 June 2024).
- Billen, G.; Lassaletta, L.; Garnier, J. A biogeochemical view of the global agro-food system: Nitrogen flows associated with protein production, consumption and trade. Glob. Food Secur. 2014, 3, 209–219. [Google Scholar] [CrossRef]
- Ben Hassen, T.; El Bilali, H. Impacts of the Russia-Ukraine War on Global Food Security: Towards More Sustainable and Resilient Food Systems? Foods 2022, 11, 2301. [Google Scholar] [CrossRef] [PubMed]
- Ficco, D.B.M.; Borrelli, G.M. Nutritional Components of Wheat Based Food: Composition, Properties, and Uses. Foods 2023, 12, 4010. [Google Scholar] [CrossRef]
- Wrigley, C.W.; Corke, H.; Seetharaman, K.; Faubion, J. (Eds.) Encyclopedia of Food Grains; Academic Press: New York, NY, USA, 2015. [Google Scholar]
- Łaba, S.; Cacak-Pietrzak, G.; Łaba, R.; Sułek, A.; Szczepański, K. Food Losses in Consumer Cereal Production in Poland in the Context of Food Security and Environmental Impact. Agriculture 2022, 12, 665. [Google Scholar] [CrossRef]
- Różewicz, M.; Wyzińska, M.; Grabiński, J. The Most Important Fungal Diseases of Cereals—Problems and Possible Solutions. Agronomy 2021, 11, 714. [Google Scholar] [CrossRef]
- Bilska, K.; Jurczak, S.; Kulik, T.; Ropelewska, E.; Olszewski, J.; Żelechowski, M.; Zapotoczny, P. Species Composition and Trichothecene Genotype Profiling of Fusarium Field Isolates Recovered from Wheat in Poland. Toxins 2018, 10, 325. [Google Scholar] [CrossRef]
- Suchorzyńska, M.; Misiewicz, A. Phytopathogenic Mycotoxin-Producing Fungi of the Genus Fusarium and Their Detection Using PCR Techniques. Post. Mikrobiol 2009, 48, 221–230. (In Polish) [Google Scholar]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The Impact of Fusarium Mycotoxins on Human and Animal Host Susceptibility to Infectious Diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef]
- Zhou, H.; Guog, T.; Dai, H.; Yu, Y.; Zhang, Y.; Ma, L. Deoxynivalenol: Toxicological profiles and perspective views for future research. World Mycotoxin J. 2020, 13, 179–188. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Nwozo, S.; Odongo, G.A.; Eseoghene, I.J.; Twinomuhwezi, H.; Ogbonna, C.U.; Upadhyay, A.K.; Adeleye, A.O.; Okpala, C.O.R. Mycotoxins’ Toxicological Mechanisms Involving Humans, Livestock and Their Associated Health Concerns: A Review. Toxins 2022, 14, 167. [Google Scholar] [CrossRef]
- Cighir, A.; Mare, A.D.; Vultur, F.; Cighir, T.; Pop, S.D.; Horvath, K.; Man, A. Fusarium spp. in Human Disease: Exploring the Boundaries between Commensalism and Pathogenesis. Life 2023, 13, 1440. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, L. Mycotoxigenic Fusarium species from agricultural crops in Malaysia. JSM Mycotoxins 2017, 67, 67–75. [Google Scholar] [CrossRef][Green Version]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From Simple to Complex Mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Yang, J.; Yang, X.; Zhang, J.; Zhao, Z. Type A Trichothecene Metabolic Profile Differentiation, Mechanisms, Biosynthetic Pathways, and Evolution in Fusarium Species—A Mini Review. Toxins 2023, 15, 446. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Ksieniewicz-Woźniak, E.; Szymczyk, K.; Jędrzejczak, R. Modified Fusarium Mycotoxins in Cereals and Their Products—Metabolism, Occurrence, and Toxicity: An Updated Review. Molecules 2018, 23, 963. [Google Scholar] [CrossRef] [PubMed]
- Foroud, N.A.; Baines, D.; Gagkaeva, T.Y.; Thakor, N.; Badea, A.; Steiner, B.; Bürstmayr, M.; Bürstmayr, H. Trichothecenes in Cereal Grains—An Update. Toxins 2019, 11, 634. [Google Scholar] [CrossRef]
- Twarużek, M.; Grajewska-Wanat, N.; Błajet-Kosicka, A.; Grajewski, J. Occurrence of Fusarium and major mycotoxins in cereal grains harvested in 2011–2012. Prog. Plant Prot. 2013, 53, 801–806. (In Polish) [Google Scholar] [CrossRef][Green Version]
- Aleksandrowicz, E. Factors influencing the occurrence of Fusarium mycotoxins in the grain of winter wheat. Pol. J. Agron. 2020, 43, 102–113. [Google Scholar]
- Birr, T.; Hasler, M.; Verreet, J.-A.; Klink, H. Composition and Predominance of Fusarium Species Causing Fusarium Head Blight in Winter Wheat Grain Depending on Cultivar Susceptibility and Meteorological Factors. Microorganisms 2020, 8, 617. [Google Scholar] [CrossRef]
- Wegulo, S.N. Factors Influencing Deoxynivalenol Accumulation in Small Grain Cereals. Toxins 2012, 4, 1157–1180. [Google Scholar] [CrossRef]
- Chen, C.; Frank, K.; Wang, T.; Wu, F. Global wheat trade and Codex Alimentarius guidelines for deoxynivalenol: A mycotoxin common in wheat. Glob. Food Sec. 2021, 29, 100538. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2024/1022 of 8 April 2024 Amending Regulation (EU) 2023/915 as Regards Maximum Levels of Deoxynivalenol in Food. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32024R1022 (accessed on 10 July 2024).
- Commission Regulation (EU) 2024/1038 of 9 April 2024 Amending Regulation (EU) 2023/915 as Regards Maximum Levels of T-2 and HT-2 Toxins in Food. Available online: https://eur-lex.europa.eu/eli/reg/2024/1038/oj (accessed on 10 July 2024).
- Perkowski, J.; Buśko, M.; Stuper, K.; Kostecki, M.; Matysiak, A.; Szwajkowska-Michałek, L. Concentration of ergosterol in small-grained naturally contaminated and inoculated cereals. Biologia 2008, 63, 542–547. [Google Scholar] [CrossRef]
- Available online: https://www.iung.pl/ (accessed on 20 October 2024).
- Mitura, K.; Cacak-Pietrzak, G.; Feledyn-Szewczyk, B.; Szablewski, T.; Studnicki, M. Yield and Grain Quality of Common Wheat (Triticum aestivum L.) Depending on the Different Farming Systems (Organic vs. Integrated vs. Conventional). Plants 2023, 12, 1022. [Google Scholar] [CrossRef]
- Wysocka, K.; Cacak-Pietrzak, G.; Feledyn-Szewczyk, B.; Studnicki, M. The Baking Quality of Wheat Flour (Triticum aestivum L.) Obtained from Wheat Grains Cultivated in Various Farming Systems (Organic vs. Integrated vs. Conventional). Appl. Sci. 2024, 14, 1886. [Google Scholar] [CrossRef]
- Perkowski, J.; Wiwart, M.; Buśko, M.; Laskowska, M.; Berthiller, A.; Kandler, S.; Krska, R. Fusarium toxins and total fungal biomass indicators in naturally contaminated wheat samples from north-eastern Poland in 2003. Food Additiv. Contam. 2007, 24, 1292–1298. [Google Scholar] [CrossRef]
- Góral, T.; Ochodzki, P.; Nielsen, L.K.; Walentyn-Góral, D. Species of the genus Fusarium and Fusarium toxins in the grain of winter and spring wheat in Poland. Biul. IHAR 2021, 296, 25–42. [Google Scholar] [CrossRef]
- Okorski, A.; Milewska, A.; Pszczółkowska, A.; Karpiesiuk, K.; Kozera, W.; Dąbrowska, J.A.; Radwińska, J. Prevalence of Fusarium fungi and Deoxynivalenol Levels in Winter Wheat Grain in Different Climatic Regions of Poland. Toxins 2022, 14, 102. [Google Scholar] [CrossRef]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Waśkiewicz, A.; Szymczyk, K.; Jędrzejczak, R. Natural Occurrence of Nivalenol, Deoxynivalenol, and Deoxynivalenol-3-Glucoside in Polish Winter Wheat. Toxins 2018, 10, 81. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K.; Jędrzejczak, R.; Damaziak, K.; Sułek, A. Occurrence of 26 Mycotoxins in the Grain of Cereals Cultivated in Poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Cacak-Pietrzak, G.; Lenc, L.; Gromadzka, K.; Dziki, D. Milling and Baking Quality of Spring Wheat (Triticum aestivum L.) from Organic Farming. Agriculture 2021, 11, 765. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.; Naehrer, K. Three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Pierzgalski, A.; Bryła, M.; Kanabus, J.; Modrzewska, M.; Podolska, G. Updated Review of the Toxicity of Selected Fusarium Toxins and Their Modified Forms. Toxins 2021, 13, 768. [Google Scholar] [CrossRef]
- Meneely, J.; Greer, B.; Kolawole, O.; Elliott, C. T-2 and HT-2 Toxins: Toxicity, Occurrence and Analysis: A Review. Toxins 2023, 15, 481. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Gupta, A.; Pandhi, S.; Sharma, B.; Dhawan, K.; Vasundhara; Mishra, S.; Kumar, M.; Tripathi, A.D.; et al. Deoxynivalenol: An Overview on Occurrence, Chemistry, Biosynthesis, Health Effects and Its Detection, Management, and Control Strategies in Food and Feed. Microbiol. Res. 2022, 13, 292–314. [Google Scholar] [CrossRef]
- Ochodzki, P.; Twardawska, A.; Wiśniewska, H.; Góral, T. Resistance to Fusarium Head Blight, Kernel Damage, and Concentrations of Fusarium Mycotoxins in the Grain of Winter Wheat Lines. Agronomy 2021, 11, 1690. [Google Scholar] [CrossRef]
- Perochon, A.; Doohan, F.M. Trichothecenes and Fumonisins: Key Players in Fusarium–Cereal Ecosystem Interactions. Toxins 2024, 16, 90. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Athar, T.; Choudhary, S.; Deval, R.; Gezgin, S.; Hamurcu, M.; Topal, A.; Atmaca, E.; Santos, P.A.; et al. Fusarium head blight in wheat: Con-temporary status and molecular approaches. 3 Biotech 2020, 10, 172. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Cacak-Pietrzak, G.; Lenc, L.; Stalenga, J. Rating of Spring Wheat Varieties (Triticum aestivum L.) According to Their Suitability for Organic Agriculture. Agronomy 2020, 10, 1900. [Google Scholar] [CrossRef]
- Erazo, J.G.; Palacios, S.A.; Veliz, N.A.; Del Canto, A.; Plem, S.; Ramirez, M.L.; Torres, A.M. Effect of Temperature, Water Activity and Incubation Time on Trichothecene Production by Fusarium cereals Isolated from Durum Wheat Grains. Pathogens 2023, 12, 736. [Google Scholar] [CrossRef]
- Friskop, A.; Bergstrom, G.; Bradley, C.; Kleczewski, N.; Marshall, J.; Smith, D.; Tenuta, A.; Wise, K. An Overview of Fusarium Head Blight; Crop Protection Network: Washington, WA, USA, 2021. [Google Scholar] [CrossRef]
- Weber, R.; Plaskowska, E. Variability of the incidence of Fusarium species and mycotoxins in the grain of wheat, depending on soil tillage system and cultivar. Folia Pomeranae Univ. Technol. Stetin. Agric. Aliment. Piscaria Zootech. 2017, 334, 187–200. [Google Scholar] [CrossRef]
- Köpke, U.; Thiel, B.; Elmholt, S. Strategies to reduce mycotoxin and fungal alkaloid contamination in organic and conventional cereal production systems. In Handbook of Organic Food Safety and Quality; Cooper, J., Niggli, U., Leifert, C., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 353–391. [Google Scholar]
- Bernhoft, A.; Wang, J.; Leifert, C. Effect of Organic and Conventional Cereal Production Methods on Fusarium Head Blight and Mycotoxin Contamination Levels. Agronomy 2022, 12, 797. [Google Scholar] [CrossRef]
- Bernhoft, A.; Torp, M.; Clasen, P.E.; Løes, A.K.; Kristoffersen, A.B. Influence of Agronomic and Climatic Factors on Fusarium Infestation and Mycotoxin Contamination of Cereals in Norway. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1129–1140. [Google Scholar] [CrossRef]
- Mruczyk, K.; Mizgier, M.; Wójciak, R.W.; Cisek-Woźniak, A. Comparison of deoxynivalenol and zearaleone concentration in conventional and organic cereal products in western Poland. Ann. Agric. Environ. Med. 2021, 28, 44–48. [Google Scholar] [CrossRef]
- Váňová, M.; Klem, K.; Míša, P.; Matušinsky, P.; Hajšlová, J.; Lancová, K. The content of Fusarium mycotoxins grain yield and quality of winter wheat cultivars under organic and conventional cropping system. Plant Soil Environ. 2008, 54, 395–402. Available online: https://pse.agriculturejournals.cz/artkey/pse-200809-0005_the-content-of-fusarium-mycotoxins-grain-yield-and-quality-of-winter-wheat-cultivars-under-organic-and-convent.php (accessed on 15 July 2024). [CrossRef]
- Mazurkiewicz, J.; Solarska, E.; Kuzdraliński, A.; Muszyńska, M. The occurrence of fusarium toxins in winter wheat depending on fertilization. J. Res. Appl. Agric. Eng. 2008, 53, 15–17. (In Polish) [Google Scholar]
- Zhang, C.; Qu, Z.; Hou, J.; Yao, Y. Contamination and Control of Mycotoxins in Grain and Oil Crops. Microorganisms 2024, 12, 567. [Google Scholar] [CrossRef]
- Tibola, C.S.; Zavariz de Miranda, M.; Paiva, F.F.; Cunha Fernandes, J.M.; Guarienti, E.M.; Nicolau, M. Effect of breadmaking process on mycotoxin content in white and whole wheat breads. Cereal Chem. 2018, 95, 660–665. [Google Scholar] [CrossRef]
- Rodrigues, M.L. The Multifunctional Fungal Ergosterol. mBio 2018, 18, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stuper-Szablewska, K.; Przybylska-Balcerek, A.; Kurasiak-Popowska, D.; Ryńska, B.; Bilska, A. Determination of Influence of Contamination of Wheat Grain with Microscopic Fungi and their Metabolites on the Quality of Products of Grain Processing. Sci. Nat. Technol. 2019, 13, 5. [Google Scholar] [CrossRef]
- Stanisz, E.; Zgoła-Grześkowiak, A.; Waskiewicz, A.; Stępień, Ł. Can Ergosterol be an Indicator of Fusarium Fungi and Mycotoxins in Cereal Products? J. Braz. Chem. Soc. 2015, 26, 705–712. [Google Scholar] [CrossRef]



| Source of Variation | STO | T-2 Tetraol | T-2 Triol | DAS | HT-2 |
|---|---|---|---|---|---|
| Year | n.s. | n.s. | n.s. | n.s. | n.s. |
| 2019 | n.d. | n.d. | 0.1 a ± 0.3 | 0.1 a ± 0.3 | 0.4 a ± 1.0 |
| 2020 | n.d. | 0.1 a ± 0.3 | 0.2 a ± 0.6 | 0.2 a ± 0.4 | 0.5 a± 1.4 |
| 2021 | 0.1 ± 0.3 | 0.3 a ± 0.6 | 0.3 a ± 0.8 | 0.2 a ± 0.4 | 0.3 a ± 0.9 |
| Cultivar | n.s. | n.s. | n.s. | n.s. | n.s. |
| Harenda | n.d. | 0.2 a ± 4.2 | n.d. | 0.1 a ± 4.2 | 0.8 a ± 4.3 |
| Kandela | n.d. | n.d. | 0.3 a ± 4.2 | 0.1 a ± 0.3 | 0.3 a ± 1.0 |
| Mandaryna | 0.1 ± 4.3 | 0.1 a ± 4.3 | n.d. | 0.1 a ± 4.3 | 0.2 a ± 4.3 |
| Serenada | n.d. | 0.1 a ± 0.3 | 0.4 a ± 0.9 | 0.2 a ± 0.4 | 0.2 a ± 0.7 |
| Farming system | n.s. | n.s. | n.s. | n.s. | n.s. |
| ORG | n.d. | n.d. | n.d. | 0.2 a ± 0.4 | n.d. |
| INT | n.d. | 0.3 a ± 0.6 | 0.4 a ± 0.8 | 0.2 a ± 0.4 | 0.7 a ± 1.6 |
| CONV | 0.1 ± 0.3 | 0.1 a ± 0.3 | 0.2 a ± 0.6 | 0.1 a ± 0.3 | 0.6 a ± 1.0 |
| Mycotoxins | The Positive Samples | Min Concentration | Max Concentration | Mean | |
|---|---|---|---|---|---|
| Per Total (36) | Percentage [%] | ||||
| STO | 1/36 | 2.8 | 1.0 | 1.0 | 1.0 |
| T-2 Tetraol | 3/36 | 8.3 | 1.0 | 2.0 | 1.3 |
| T-2 Triol | 4/36 | 11.1 | 1.0 | 2.0 | 1.7 |
| DAS | 5/36 | 13.9 | 1.0 | 1.0 | 1.0 |
| HT-2 | 6/36 | 16.7 | 1.0 | 5.0 | 2.5 |
| Source of Variation | DON | 3AcDON | 15AcDON | NIV | FUS-X |
|---|---|---|---|---|---|
| Year | ** | n.s. | ** | ** | ** |
| 2019 | 72.9 a ± 37.5 | 25.2 a ± 18.1 | 11.2 b ± 8.5 | 9.7 c ± 7.9 | 0.8 a ± 2.9 |
| 2020 | 92.0 b ± 50.9 | 28.1 a ± 15.2 | 9.1 ab ± 8.7 | 4.6 a ± 5.6 | 2.0 b ± 4.7 |
| 2021 | 83.2 ab ± 32.6 | 27.9 a ± 12.4 | 7.8 a ± 8.6 | 7.1 b ± 8.4 | 1.8 b ± 4.3 |
| Cultivar | ** | n.s. | ** | ** | ** |
| Harenda | 67.9 a ± 52.2 | 28.8 a ± 17.6 | 6.2 a ± 6.7 | 4.2 a ± 4.0 | 1.1 b ± 4.2 |
| Kandela | 90.3 c ± 41.1 | 27.3 a ± 13.4 | 12.3 c ± 8.2 | 8.8 b ± 9.9 | 1.2 b ± 3.7 |
| Mandaryna | 93.8 c ± 58.6 | 25.4 a ± 19.6 | 10.4 bc ± 7.0 | 10.4 c ± 8.2 | 3.9 c ± 5.9 |
| Serenada | 78.8 b ± 22.5 | 26.7 a ± 26.7 | 8.3 b ± 9.8 | 5.0 a ± 6.5 | n.d. |
| Farming system | ** | ** | ** | ** | ** |
| ORG | 122.1 b ± 22.4 | 32.7 b ± 14.3 | 12.8 b ± 9.0 | 11.0 b ± 8.4 | 4.7 b ± 5.8 |
| INT | 56.6 a ± 26.2 | 28.7 b ± 15.6 | 8.3 a ± 8.6 | 5.9 a ± 7.0 | n.d. |
| CONV | 69.4 a ± 38.0 | 19.8 a ± 13.3 | 6.8 a ± 7.1 | 4.4 a ± 5.7 | n.d. |
| Mycotoxins | Number of Positive Samples | Minimum Concentration | Maximum Concentration | Mean | |
|---|---|---|---|---|---|
| Per Total (36) | Percentage [%] | ||||
| DON | 36/36 | 100.0 | 15.0 | 157.0 | 82.7 |
| 3AcDON | 35/36 | 97.2 | 10.0 | 53.0 | 27.0 |
| 15AcDON | 28/36 | 77.8 | 1.0 | 30.0 | 9.3 |
| NIV | 30/36 | 83.0 | 1.0 | 26.0 | 7.1 |
| FUS-X | 5/36 | 13.9 | 10.0 | 13.0 | 1.5 |
| Research Material | STO | T-2 Tetraol | T-2 Triol | DAS | HT-2 |
|---|---|---|---|---|---|
| ** | ** | ** | ** | ** | |
| GR | 0.1 a ± 0.17 | 0.1 a ± 0.40 | 0.2 a ± 0.58 | 0.1 a ± 0.35 | 0.4 a ± 1.10 |
| BN | 1.3 b ± 1.56 | 1.3 b ± 1.58 | 0.8 b ± 1.42 | 1.0 b ± 1.66 | 1.8 b ± 2.14 |
| FR | n.d. | n.d. | n.d. | 0.1 a ± 0.32 | n.d. |
| BD | n.d. | n.d. | n.d. | n.d. | n.d. |
| Research Material | DON | 3AcDON | 15AcDON | NIV | FUS-X |
|---|---|---|---|---|---|
| ** | ** | ** | ** | ** | |
| GR | 82.7 b ± 40.66 | 27.1 b ± 15.02 | 9.3 b ± 8.44 | 7.1 b ± 7.48 | 1.6 a ± 3.95 |
| BN | 104.1 c ± 45.75 | 29.4 b ± 14.71 | 11.7 b ± 9.25 | 7.9 b ± 11.18 | 3.5 b ± 5.58 |
| FR | 2.9 a ± 4.05 | 0.6 a ± 1.44 | 0.4 a ± 1.30 | 0.8 a ± 1.64 | n.d. |
| BD | 2.5 a ± 4.40 | n.d. | n.d. | 0.8 a ± 1.64 | n.d. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysocka, K.; Cacak-Pietrzak, G.; Buśko, M.; Studnicki, M. Fusariotoxins Concentration in Common Wheat Grain Depending on the Farming System (Organic vs. Integrated vs. Conventional) and Changes During Grain Processing. Agronomy 2024, 14, 2535. https://doi.org/10.3390/agronomy14112535
Wysocka K, Cacak-Pietrzak G, Buśko M, Studnicki M. Fusariotoxins Concentration in Common Wheat Grain Depending on the Farming System (Organic vs. Integrated vs. Conventional) and Changes During Grain Processing. Agronomy. 2024; 14(11):2535. https://doi.org/10.3390/agronomy14112535
Chicago/Turabian StyleWysocka, Katarzyna, Grażyna Cacak-Pietrzak, Maciej Buśko, and Marcin Studnicki. 2024. "Fusariotoxins Concentration in Common Wheat Grain Depending on the Farming System (Organic vs. Integrated vs. Conventional) and Changes During Grain Processing" Agronomy 14, no. 11: 2535. https://doi.org/10.3390/agronomy14112535
APA StyleWysocka, K., Cacak-Pietrzak, G., Buśko, M., & Studnicki, M. (2024). Fusariotoxins Concentration in Common Wheat Grain Depending on the Farming System (Organic vs. Integrated vs. Conventional) and Changes During Grain Processing. Agronomy, 14(11), 2535. https://doi.org/10.3390/agronomy14112535







