Application of Nitrate–Ammonium Nitrogen Fertilization Reduced Nitrogen Loss in Surface Runoff and Infiltration by Improving Root Morphology of Flue-Cured Tobacco
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site
2.2. Experimental Design
2.3. Data Collection
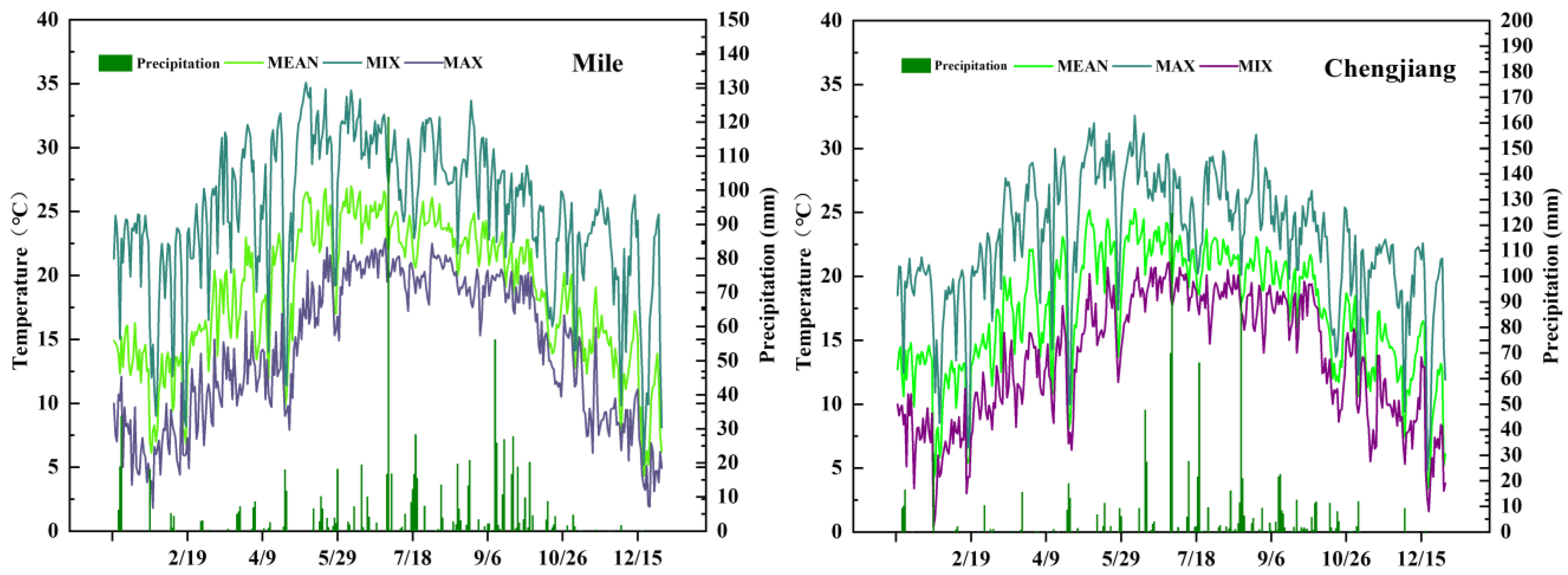
2.3.1. Meteorological Data Collection
2.3.2. Water Sample Collection and Measurement
2.3.3. Biomass and Root Index of Tobacco
2.4. Data Analysis
3. Results
3.1. Rainfall and Average Temperature in the Experiment Site
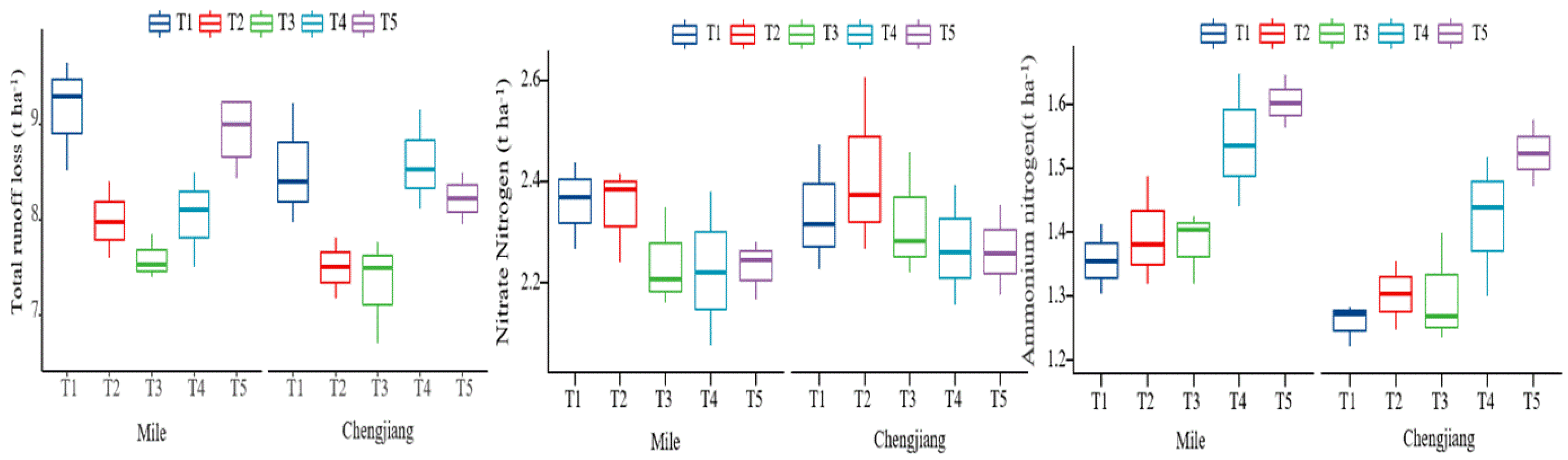
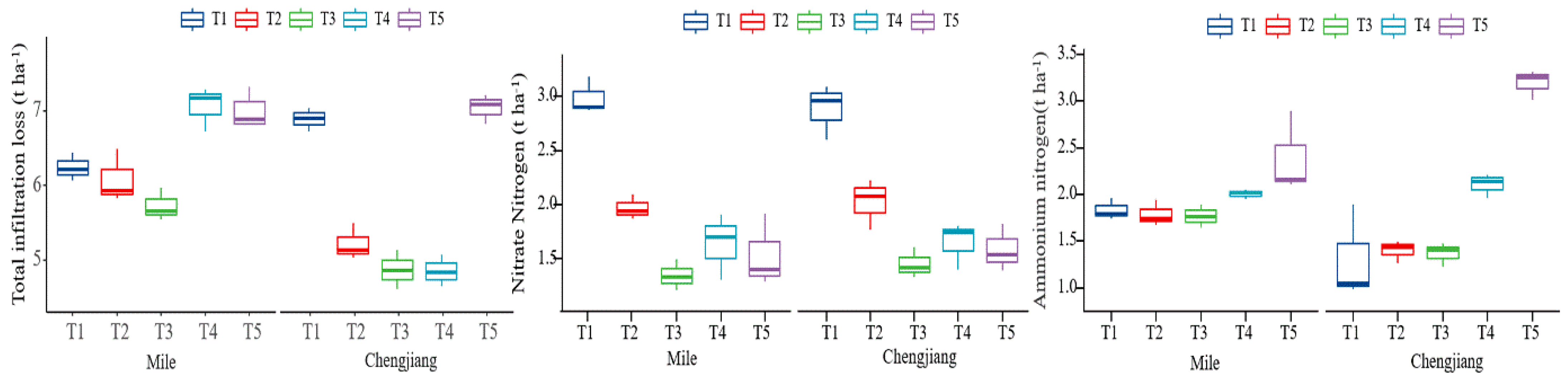
3.2. Nitrogen Loss from Tobacco Fields with Different Forms of Nitrogen Fertilizer
3.2.1. Nitrogen Loss in Surface Runoff
3.2.2. Nitrogen Loss in Infiltration Water
3.3. Root Spatial Distribution of Tobacco Roots with Different Forms of Nitrogen Fertilizer
3.3.1. Root Biomass of Nitrogen-Fertilized Tobacco
3.3.2. Root Surface Area of Nitrogen-Fertilized Tobacco
3.3.3. Root Distribution of Nitrogen-Fertilized Tobacco
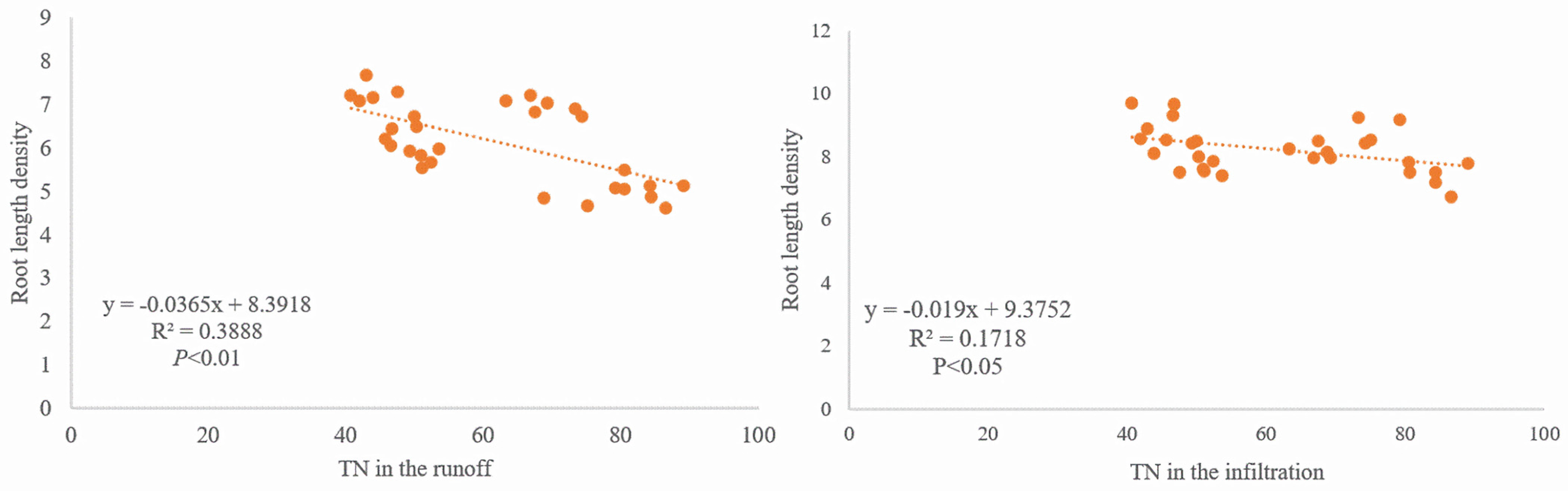
3.4. Relationship Between the Root System of Tobacco and Nitrogen Loss Under Different Forms of Nitrogen Fertilizer
3.5. Plant Biomass Under Different Forms of Nitrogen Fertilizer
4. Discussion
4.1. Nitrate–Ammonium Nitrogen Fertilizer Can Improve Root Morphology and Increase Flue-Cured Tobacco Biomass
4.2. Nitrate–Ammonium Nitrogen Fertilizer Can Reduce Total Nitrogen Runoff and Infiltration Loss
4.3. Nitrate–Ammonium Nitrogen Fertilizer Can Reduce Nitrogen Loss by Improving Root Morphology
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tan, C.; Ma, M.; Kuang, H. Spatial-temporal characteristics and climatic responses of water level fluctuations of global major lakes from 2002 to 2010. Remote Sens. 2017, 9, 150. [Google Scholar] [CrossRef]
- Yang, C.-H.; Yang, P.; Geng, J.; Yin, H.-B.; Chen, K. Sediment internal nutrient loading in the most polluted area of a shallow eutrophic lake (Lake Chaohu; China) and its contribution to lake eutrophication. Environ. Pollut. 2020, 262, 114292. [Google Scholar] [CrossRef] [PubMed]
- Hou, P.; Jiang, Y.; Yan, L.; Petropoulos, E.; Chen, D.-L. Effect of fertilization on nitrogen losses through surface runoffs in Chinese farmlands: A meta-analysis. Sci. Total Environ. 2021, 793, 148554. [Google Scholar] [CrossRef] [PubMed]
- Husain, A.; Muneer, M.-A.; Fan, W.; Yin, G.-F.; Shen, S.-Z.; Wang, F.; Li, Y.; Zhang, K.-Q. Application of optimum n through different fertilizers alleviate NH4+-N, NO3−N and total nitrogen losses in the surface runoff and leached water and improve nitrogen use efficiency of rice crop in Erhai Lake Basin; China. Commun. Soil Sci. Plant Anal. 2019, 50, 716–738. [Google Scholar] [CrossRef]
- Barcellos, D.; Queiroz, H.-M.; Nóbrega, G.-N.; de Oliveira Filho, L.-R.; Santaella, S.-T.; Otero, X.-L.; Ferreira, T.-O. Phosphorus enriched effluents increase eutrophication risks for mangrove systems in northeastern Brazil. Mar. Pollut. Bull. 2019, 142, 58–63. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, G.; Sun, G.; Wu, Y.; Chen, Y. Assessment of lake water quality and eutrophication risk in an agricultural irrigation area: A case study of the Chagan Lake in northeast China. Water 2019, 11, 2380. [Google Scholar] [CrossRef]
- Tang, X.; Li, R.; Han, D.; Scholz, M. Response of eutrophication development to variations in nutrients and hydrological regime: A case study in the Changjiang River (Yangtze) Basin. Water 2020, 12, 1634. [Google Scholar] [CrossRef]
- Zhang, X.-C.; Razavi, B.; Liu, J.-X.; Wang, G.; Zhang, X.-C.; Li, Z.-Y.; Zhai, B.-N.; Wang, Z.-H.; Zamanian, K. Croplands conversion to cash crops in dry regions: Consequences of nitrogen losses and decreasing nitrogen use efficiency for the food chain system. Land Degrad. Dev. 2021, 32, 1103–1113. [Google Scholar] [CrossRef]
- Diao, Y.; Li, H.; Jiang, H.; Li, H. Effects of changing fertilization since the 1980s on nitrogen runoff and leaching in rice–wheat rotation systems; Taihu Lake Basin. Water 2020, 12, 886. [Google Scholar] [CrossRef]
- Cai, A.; Xu, M.; Wang, B.; Zhang, W.; Liang, G.; Hou, E.; Luo, Y. Manure acts as a better fertilizer for increasing crop yields than synthetic fertilizer does by improving soil fertility. Soil Till. Res. 2019, 189, 168–175. [Google Scholar] [CrossRef]
- Lu, Y.-X.; Li, C.-J.; Zhang, F.-S. Transpiration, potassium uptake and flow in tobacco as affected by nitrogen forms and nutrient levels. Ann. Bot. 2005, 95, 991–998. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-H.; Cheng, X.-J.; Yu, Z.; Cheng, G.-L.; Zhao, L.-W. Response of non-point source pollution to landscape pattern: A case study in mountain-rural region, China. Environ. Sci. Pollut. Res. 2021, 28, 16602–16615. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.-S.; Hou, R.; Wu, F.-Q.; Keesstra, S. Effect of soil surface roughness on infiltration water; ponding and runoff on tilled soils under rainfall simulation experiments. Soil Till. Res. 2018, 179, 47–53. [Google Scholar] [CrossRef]
- Xue, L.; Hou, P.; Zhang, Z.; Shen, M.; Yang, L. Application of systematic strategy for agricultural non-point source pollution control in Yangtze River basin; China. Agric. Ecosyst. Environ. 2020, 304, 107148. [Google Scholar] [CrossRef]
- Monchamp, M.-E.; Pick, F.-R.; Beisner, B.-E.; Maranger, R. Nitrogen forms influence microcystin concentration and composition via changes in cyanobacterial community structure. PLoS ONE 2014, 9, e85573. [Google Scholar] [CrossRef]
- Ying, J.; Li, X.; Wang, N.; Lan, Z.; He, J.; Bai, Y. Contrasting effects of nitrogen forms and soil pH on ammonia oxidizing microorganisms and their responses to long-term nitrogen fertilization in a typical steppe ecosystem. Soil Biol. Biochem. 2017, 107, 10–18. [Google Scholar] [CrossRef]
- Ranjan, R.; Yadav, R. Genetics of root traits influencing nitrogen use efficiency under varied nitrogen level in spring wheat (Triticum aestivum L.). Cereal Res. Commun. 2022, 50, 755–765. [Google Scholar] [CrossRef]
- Martínez-Dalmau, J.; Berbel, J.; Ordóñez-Fernández, R. Nitrogen fertilization. A review of the risks associated with the inefficiency of its use and policy responses. Sustainability 2021, 13, 5625. [Google Scholar] [CrossRef]
- Elsalam, H.-E.-A.; Sharnouby, M.-E.-E.; Mohamed, A.-E.; Raafat, B.-M.; El-Gamal, E.-H. Effect of sewage sludge compost usage on corn and faba bean growth; carbon and nitrogen forms in plants and soil. Agronomy 2021, 11, 628. [Google Scholar] [CrossRef]
- Pan, S.-G.; Liu, H.-D.; Mo, Z.-W.; Bob, P.; Duan, M.-Y.; Tian, H.; Hu, S.-J.; Tang, X.-R. Corrigendum: Effects of nitrogen and shading on root morphologies; nutrient accumulation; and photosynthetic parameters in different rice genotypes. Sci. Rep. 2017, 7, 45611. [Google Scholar] [CrossRef]
- Chen, J.-T.; Li, J.-H.; Li, W.-F.; Li, P.; Zhu, R.; Zhong, Y.-X.; Zhang, W.-F.; Li, T.-Y. The optimal ammonium-nitrate ratio for various crops: A Meta-analysis. Field Crops Res. 2024, 307, 109240. [Google Scholar] [CrossRef]
- Kurt, D.; Kinay, A. Effects of irrigation; nitrogen forms and topping on sun cured tobacco. Ind. Crops Prod. 2021, 162, 113276. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Dresbøll, D.-B.; Kristensen, H.-L. Crop yield; root growth; and nutrient dynamics in a conventional and three organic cropping systems with different levels of external inputs and N recycling through fertility building crops. Eur. J. Agron. 2012, 37, 66–82. [Google Scholar] [CrossRef]
- Kakar, K.-U.; Nawaz, Z.; Cui, Z.-Q.; Ahemd, N.; Ren, X.-L. Molecular breeding approaches for production of disease-resilient commercially important tobacco. Brief. Funct. Genom. 2020, 19, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Berg, L.D.; Riaz, M.; Arif, M.; Ahsmore, M. Nitrogen induced DOC and heavy metals leaching: Effects of nitrogen forms; deposition loads and liming. Environ. Pollut. 2020, 265 Pt B, 114981. [Google Scholar] [CrossRef]
- Suyala, Q.; Liguo, J.; Qin, Y.-L.; Chen, Y.; Fan, M.-S. Effects of different nitrogen forms on potato growth and development. J. Plant Nutr. 2017, 40, 1651–1659. [Google Scholar]
- Xu, Y.; Huang, G. A Risk-Based interval two-stage programming model for agricultural system management under uncertainty. Math. Probl. Eng. 2016, 7438913, 1–13. [Google Scholar] [CrossRef]
- Chen, X.; Mao, A.; Alice, Z.; Zhang, Y.; Chang, L.; Gao, J.; Thompson, H.-J.; Michael, L. Carbon and nitrogen forms in soil organic matter influenced by incorporated wheat and corn residues. Soil Sci. Plant Nutr. 2017, 63, 377–387. [Google Scholar] [CrossRef]
- Hachiya, T.; Sakakibara, H. Interactions between nitrate and ammonium in their uptake, allocation, assimilation, and signaling in plants. J. Exp. Bot. 2017, 68, 2501–2512. [Google Scholar] [CrossRef]
- Bisseling, T.; Scheres, B. Nutrient computation for root architecture. Science 2014, 346, 300–301. [Google Scholar] [CrossRef]
- Li, K.; Guo, Y.; Liu, C.; Lu, X.; Liao, H. Effects of different NH4+/NO3 ratios on soybean growth, nodulation and biological N fixation. Chinese. J. Oil Crop Sci. 2014, 36, 349–356. [Google Scholar]
- Cesco, S.; Mimmo, T.; Tonon, G.; Tomasi, N.; Pinton, R.; Terzano, R.; Neumann, G.; Weisskopf, L.; Renella, G.; Landi, L.; et al. Plant-borne flavonoids released into the rhizosphere: Impact on soil bio-activities related to plant nutrition. A review. Biol. Fertil. Soils. 2012, 48, 123–149. [Google Scholar] [CrossRef]
- Ashraf, M.; Shahzad, S.M.; Imtiaz, M.; Rizwan, M.-S. Salinity effects on nitrogen metabolism in plants—Focusing on the activities of nitrogen metabolizing enzymes: A review. J. Plant Nutr. 2018, 41, 1065–1081. [Google Scholar] [CrossRef]
- Urban, A.; Rogowski, P.; Wasilewska-Dębowska, W.; Romanowska, E. Understanding maize response to nitrogen limitation in different light conditions for the improvement of photosynthesis. Plants 2021, 10, 1932. [Google Scholar] [CrossRef]
- Bhambri, A.; Karn, S.-K. Biotechnique for nitrogen and phosphorus removal: A possible insight. Chem. Ecol. 2020, 36, 785–809. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, M.; Tsang, D.C.W.; Geng, N.; Lu, D.; Zhu, L.; Igalavithana, A.D.; Dissanayake, P.D.; Rinklebe, J.; Yang, X.; et al. Recent advances in control technologies for non-point source pollution with nitrogen and phosphorous from agricultural runoff: Current practices and future prospects. Appl. Biol. Chem. 2020, 63, 8. [Google Scholar] [CrossRef]
- Xu, G.; Jiang, M.; Lu, D.; Wang, H.; Chen, M. Nitrogen forms affect the root characteristic; photosynthesis; grain yield; and nitrogen use efficiency of rice under different irrigation regimes. Crop Sci. 2020, 60, 2594–2610. [Google Scholar] [CrossRef]
- Beeckman, F.; Motte, H.; Beeckman, T. Nitrification in agricultural soils: Impact, actors and mitigation. Curr. Opin. Biotech. 2018, 50, 166–173. [Google Scholar] [CrossRef]
- Schortemeyer, M.; Feil, B.; Stamp, P. Root morphology and nitrogen uptake of maize simultaneously supplied with ammonium and nitrate in a split-root system. Ann. Bot. 1993, 72, 107–115. [Google Scholar] [CrossRef]
- Wang, J.-F.; Zhu, C.-Y.; Weng, B.-S.; Mo, P.-W.; Xu, Z.-J.; Ping, T.; Cui, B.-S.; Bai, J.-H. Regulation of heavy metals accumulated by Acorus calamus L. in constructed wetland through different nitrogen forms. Chemosphere 2021, 281, 130773. [Google Scholar] [CrossRef]
- Wang, J.-L.; Fu, Z.-S.; Chen, G.-F.; Zou, G.-Y.; Song, X.-F.; Liu, F.-X. Runoff nitrogen (N) losses and related metabolism enzyme activities in paddy field under different nitrogen fertilizer levels. Environ. Sci. Rollut. Res. 2018, 25, 27583–27593. [Google Scholar] [CrossRef] [PubMed]
- Kamel, H.; Khawla, I.; Selma, F.; Tarek, S.; Chedly, A.; Kadambot, S.; Cristina, C. Interactive effects of salinity and nitrogen forms on plant growth; photosynthesis and osmotic adjustment in maize. Plant Physiol. Biochem. 2019, 139, 171–178. [Google Scholar]
- Bergstrom, A.-K.; Jansson, M. Atmospheric nitrogen deposition has caused nitrogen enrichment and eutrophication of lakes in the northern hemisphere. Global Chang. Biol. 2006, 12, 635–643. [Google Scholar] [CrossRef]
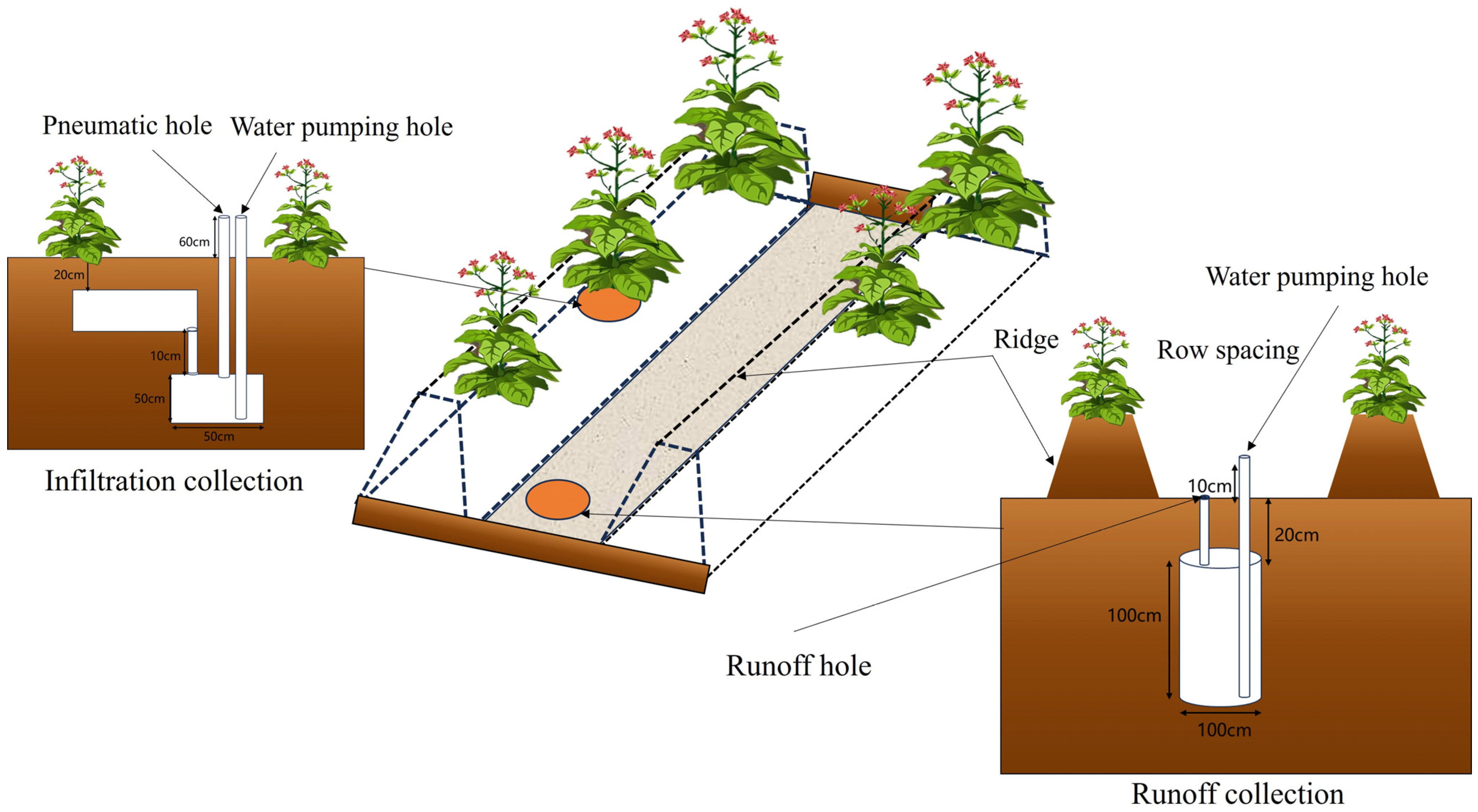
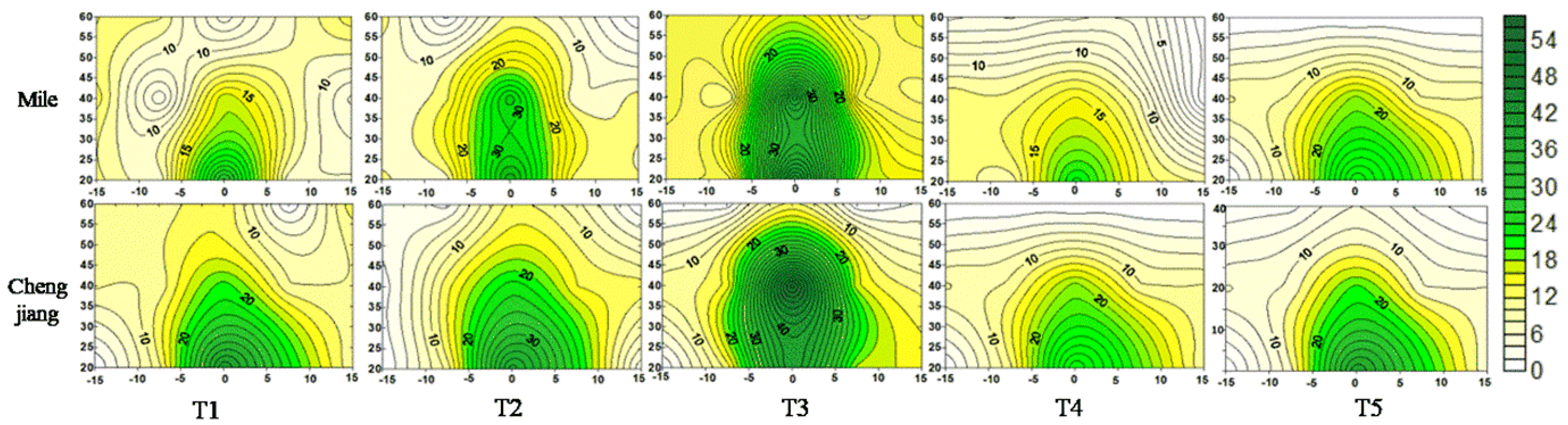
| Experimental Site | Treatments | Vertical Distance (cm) | Horizontal Distance (cm) | ||||
|---|---|---|---|---|---|---|---|
| 0−20 cm | 20−40 cm | 40−60 cm | 0−10 cm | 10−20 cm | 20−30 cm | ||
| Mile County | T1 | 601.79 ± 20.24 b | 309.27 ± 13.29 b | 381.93 ± 36.21 a | 293.38 ± 23.13 a | 708.5 ± 62.11 bc | 291.1 ± 19.20 a |
| T2 | 637.22 ± 11.89 a | 342.41 ± 20.76 a | 333.59 ± 20.28 b | 300.05 ± 31.11 a | 725.63 ± 27.66 b | 286.54 ± 23.28 a | |
| T3 | 642.76 ± 20.11 a | 356.06 ± 26.98 b | 315.33 ± 30.19 b | 247.61 ± 27.00 b | 764.95 ± 33.29 a | 301.59 ± 17.55 a | |
| T4 | 604.29 ± 10.59 b | 306.08 ± 18.36 c | 303.29 ± 29.10 b | 159.27 ± 16.14 c | 649.12 ± 49.01 c | 325.27 ± 18.02 a | |
| T5 | 569.41 ± 30.41 c | 254.87 ± 30.23 d | 301.29 ± 19.28 b | 185.32 ± 12.18 c | 641.09 ± 38.44 c | 299.06 ± 20.00 a | |
| Chengjiang County | T1 | 586.71 ± 18.20 b | 321.58 ± 21.48 a | 166.27 ± 10.15 b | 143.21 ± 17.77 a | 812.36 ± 12.09 b | 139.62 ± 12.98 b |
| T2 | 607.34 ± 32.17 ab | 333.17 ± 19.00 a | 217.87 ± 17.00 a | 156.44 ± 13.00 a | 837.25 ± 19.00 a | 165.23 ± 15.00 a | |
| T3 | 621.00 ± 28.29 a | 346.57 ± 23.33 a | 174.20 ± 27.19 b | 163.87 ± 10.38 a | 840.76 ± 16.44 a | 167.14 ± 12.87 a | |
| T4 | 589.91 ± 20.17 b | 308.33 ± 15.29 b | 220.73 ± 20.19 a | 149.64 ± 12.67 a | 822.90 ± 16.20 ab | 146.32 ± 14.23 b | |
| T5 | 663.14 ± 30.10 a | 299.31 ± 19.20 b | 111.31 ± 15.25 c | 137.41 ± 11.19 b | 795.43 ± 10.99 b | 141.10 ± 11.11 b | |
| Treatments | Biomass (g plant−1) | |||||
|---|---|---|---|---|---|---|
| Roots | Stems | Leaves | ||||
| Mile County | Chengjiang County | Mile County | Chengjiang County | Mile County | Chengjiang County | |
| T1 | 93.15 ± 6.00 b | 99.90 ± 5.76 ab | 87.30 ± 5.89 b | 93.15 ± 5.21 ab | 204.00 ± 12.88 b | 205.50 ± 6.55 ab |
| T2 | 97.65 ± 6.02 b | 105.75 ± 7.77 a | 105.30 ± 7.00 a | 100.80 ± 7.00 a | 205.50 ± 9.21 b | 201.50 ± 7.08 ab |
| T3 | 106.65 ± 11.28 a | 107.10 ± 8.32 a | 97.20 ± 6.54 ab | 103.50 ± 5.30 a | 238.00 ± 10.09 a | 212.50 ± 6.32 a |
| T4 | 110.70 ± 9.02 a | 96.30 ± 4.20 ab | 94.05 ± 5.30 ab | 94.95 ± 3.02 ab | 184.00 ± 8.21 c | 200.50 ± 10.21 ab |
| T5 | 96.30 ± 5.98 b | 94.50 ± 5.29 b | 90.90 ± 6.42 b | 90.45 ± 4.98 b | 203.50 ± 14.90 b | 199.50 ± 5.08 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, C.; Yang, K.; Zhao, Z. Application of Nitrate–Ammonium Nitrogen Fertilization Reduced Nitrogen Loss in Surface Runoff and Infiltration by Improving Root Morphology of Flue-Cured Tobacco. Agronomy 2024, 14, 2532. https://doi.org/10.3390/agronomy14112532
Ouyang C, Yang K, Zhao Z. Application of Nitrate–Ammonium Nitrogen Fertilization Reduced Nitrogen Loss in Surface Runoff and Infiltration by Improving Root Morphology of Flue-Cured Tobacco. Agronomy. 2024; 14(11):2532. https://doi.org/10.3390/agronomy14112532
Chicago/Turabian StyleOuyang, Chengren, Kang Yang, and Zhengxiong Zhao. 2024. "Application of Nitrate–Ammonium Nitrogen Fertilization Reduced Nitrogen Loss in Surface Runoff and Infiltration by Improving Root Morphology of Flue-Cured Tobacco" Agronomy 14, no. 11: 2532. https://doi.org/10.3390/agronomy14112532
APA StyleOuyang, C., Yang, K., & Zhao, Z. (2024). Application of Nitrate–Ammonium Nitrogen Fertilization Reduced Nitrogen Loss in Surface Runoff and Infiltration by Improving Root Morphology of Flue-Cured Tobacco. Agronomy, 14(11), 2532. https://doi.org/10.3390/agronomy14112532




