Abstract
Currently, the EU is focusing on less intensive agrotechnology and sustainable development. It is important to minimize the occurrence of mycotoxins (including Fusariotixins) in food, and to monitor mycotoxin concentration in the food chain. Therefore, this study evaluated Fusarium mycotoxin contamination, specifically type A and B trichothecenes and ergosterol concentration, in wheat grain from a three-year field experiment (2019–2021) conducted at IUNG-PIB in Osiny (Poland), along with its byproducts (bran, flour, bread). Four wheat cultivars were grown under different farming systems: organic (ORG), integrated (INT), and conventional (CONV). Ergosterol was analyzed using HPLC with an absorbance detector while Type A and B trichothecenes were analyzed using gas chromatography and mass spectrometry. Results showed that the farming system significantly influenced type B trichothecenes concentration in grain, with the highest concentration established in ORG-grown wheat. However, the grain concentration from the INT farming system was comparable to that from CONV. Type A trichothecenes concentrations were low and not significantly affected by the farming system. Bran exhibited the highest ergosterol and mycotoxin concentration, while flour and bread exhibited the lowest.
1. Introduction
Wheat is a cereal crop belonging to the grass family (Poaceae). It ranks second in the world in global production and, for the 2022–2023 growing season, this was estimated at approximately 808 million tons of grain [1]. Wheat grain is an essential resource in the global food system. It is crucial in ensuring food security, which has been starkly highlighted by the current military conflict in Ukraine [2,3].
The production of groats and flour uses wheat grain, making it a fundamental ingredient in many food products, such as bread, multi-ingredient breakfast cereals, pasta and cakes. Additionally, producers use it as a crucial raw material for animal feed production [4,5,6].
Wheat crops are susceptible to fungal diseases, notably fusarium head blight (FHB). The Fusarium genus, including in Poland, is widespread in wheat fields globally [6]. Under Polish climatic conditions, fungal species, such as Fusarium culmorum, F. graminearum, F. avenaceum, F. sporotrichoides, and F. poae, primarily cause FHB [7,8,9]. The detrimental effects of FHB include a reduction in grain yield—attributed to a lower thousand-kernel weight, decreased number of kernels per spike, reduced kernel weight per spike and a decline in the grain’s processing quality, including health safety. Certain strains of Fusarium fungi can biosynthesize harmful metabolites called mycotoxins, which accumulate in the grain and pose health risks to humans and animals [9,10,11,12,13]. Fungal species primarily produce these mycotoxins under field conditions, and their levels rarely increase post-harvest [14].
The main groups of toxins produced by Fusarium fungi belong to the trichothecenes, zearalenone, and fumonisins B1 and B2, from which trichothecenes are the most commonly found in wheat grain and grain byproducts. Trichothecenes include over 200 toxins grouped into four types (A, B, C, and D) based on their chemical structure [15]. The most frequently detected Fusarium mycotoxins contaminated wheat grain are trichothecenes type A, HT-2 toxin, T-2 Toxin, T-2 Tetraol, T-2 Triol, diacetoxyscirpenol and type B, deoxynivalenol and its acetylated derivatives (3-acetyldeoxynivalenol and 15-acetyldeoxynivalenol), nivalenol and fusarenon-X [16]. The occurrence of mycotoxins in cereal grains and their processed products, particularly trichothecenes, is associated with the potential existence of conjugated trichothecenes. Some plants can detoxify by conjugating toxic metabolites, such as glucose [17]. This phenomenon has been described and defined as the enzymatic detoxification of trichothecenes [18]. It should be emphasized here that conjugated mycotoxins “evade” the control of mycotoxin concentrations when using routine analysis methods.
Environmental conditions (soil, climate, and agronomic practices) along with the genotype resistance of the plants influence the infestation of crops by Fusarium fungi and the potential threat of grain contamination with their mycotoxins. Among these factors, meteorological conditions are considered the most critical. Crops are particularly susceptible to Fusarium mycotoxin contamination in humid and warm weather during the growing season [19]. Agronomic factors, such as soil cultivation, crop rotation, fertilization, harvest timing and conditions, and especially plant protection products (especially fungicides), also play a significant role [7,14,20,21,22].
The issue of mycotoxin presence is a critical concern in the context of food waste, food security, and consumer food safety [6]. These compounds accumulate in the body, leading to serious health consequences. Therefore, it is crucial to monitor their levels, especially in commonly consumed foods, such as grain products. To protect consumer health, international organizations, like the World Health Organization (WHO) and the Food and Agriculture Organization (FAO), have implemented regulations in the form of the Codex Alimentarius [23], which establishes international food safety standards. The European Union’s analog regulations have been implemented by the European Commission Regulation, which establishes maximum permitted levels of certain mycotoxins in cereal grains and their byproducts [24,25].
Therefore, this study assessed the impact of different farming systems (organic vs. integrated vs. conventional) on the grain contamination of selected spring wheat cultivars by Fusarium mycotoxins. Additionally, to demonstrate how various stages of grain processing affect mycotoxin concentration in the processed products, samples of flour, bran, and the final product, bread, were analyzed. Furthermore, ergosterol content, a biomarker for microscopic fungi, was determined in all tested samples. Ergosterol can be useful in monitoring grain quality and as an indicator of potential mycotoxin risk [26]. To our knowledge, this is the first comprehensive publication on this topic. Moreover, our work aligns with current EU Commission trends promoting less intensive agriculture, such as organic and integrated farming, and addressing food safety issues.
The experimental hypothesis assumed that differentiated agronomic factors, such as farming system and genotype (cultivar), affect ergosterol and mycotoxin concentration.
2. Materials and Methods
2.1. Research Material
The study material consisted of spring wheat grain (4 cultivars: Harenda, Kandela, Mandaryna, and Serenada) grown in three different farming systems: organic (ORG), integrated (INT), and conventional (CONV) at the Institute of Soil Science and Plant Cultivation State Research Institute in Osiny (51°27′ N; 22°2′ E), Poland, between 2019 and 2021. The tested wheat cultivars exhibited similar resistance to Fusarium, ranging from 8.0° (Mandaryna, Serenada) to 8.2° (Harenda, Kandela) on a 9-point scale [27]. In the INT and CONV farming systems, systemic fungicides were applied to protect wheat crops from Fusarium head blight. A comprehensive field research methodology, along with a description of the meteorological conditions during different growing periods, was presented in a previous publication [28].
For the research, 3 kg grain samples were collected from experimental plots with an area of 30 m2, where the sowing and harvesting area was 25 m2 [28]. To analyze the presence of Fusarium mycotoxins, 250 g samples were weighed from the collected grain samples, which were then ground in a laboratory mill (A11, IKA Works GmbH and Co., Staufen, Germany) to particle sizes below 1.0 mm.
Additionally, byproducts from wheat grain processing, including flour, bran, and bread baked from this flour, were analyzed for the presence of Fusariotoxins. The detailed methodology of the grain milling process and bread baking was presented in a previous publication [29]. Prior to analysis for Fusariotoxins, 250 g bread samples were dried and then ground in a laboratory mill (A11, IKA Works GmbH and Co., Staufen, Germany) to particle sizes below 1.0 mm.
2.2. Methods
2.2.1. Analysis of Ergosterol Concentration
Ergosterol was determined using a microwave-assisted basic hydrolysis procedure. Samples of 100 mg ground wheat grain were placed into 17 mL culture tubes, suspended in 1 mL of methanol, and treated with 0.1 mL of 2 M aqueous sodium hydroxide before being tightly sealed. The culture tubes were placed within 250 mL plastic bottles, sealed, and subjected to microwave irradiation at 2450 MHz and 900 W maximum output. The samples were irradiated for 20 s, allowed to rest for approximately 5 min, and then irradiated for an additional 20 s. The samples were subsequently extracted with pentane within the culture tubes. The combined pentane extracts were evaporated to dryness using a RapidVap Evaporator and a gentle stream of high-purity nitrogen. The dried extracts were stored at −25 °C until analysis. Before analysis, the samples were dissolved in 1 mL of methanol and filtered through 13 mm syringe filters with 0.22 mm pore size. Ergosterol concentrations were analyzed using an Acquity UPLC H-class system equipped with a Waters Acquity PDA detector. Chromatographic separation was achieved on an Acquity UPLC® BEH C18 (100 mm × 2.1 mm i.d., 1.7 μm) column. The mobile phase composition used for isocratic elution was as follows: A, 10% acetonitrile; B, 85% methanol; C, 5% water, at a flow rate of 0.5 mL min−1. Ergosterol concentrations were quantified using an external standard, with detection performed at a wavelength of λ = 282 nm. Compound identification was based on a comparison of the examined peak’s retention time with that of the standard, as well as by spiking the sample with a known amount of the standard and reanalyzing. The limit of detection was 0.1 mg kg−1. All chemical reagents used to determine ergosterol concentration in wheat grain and grain byproducts were purchased from Merck (Darmstadt, Germany).
2.2.2. Analysis of the Concentration of Type A and B Trichothecenes
Plant material (grain, flour, bran and bread) were analyzed for the presence of type A and B trichothecenes, according to Perkowski et al. [30].
Briefly, subsamples were extracted with a mixture of acetonitrile and water (82:18 v/v), and then purified using a charcoal column (Celite 545/charcoal Draco G/60/activated alumina neutral 4:3:4 (w/w/w). The type A trichothecenes were analysed as TFAA (trifluoroacetic acid anhydride) derivatives. The dried sample was treated with 100 μL of trifluoroacetic acid anhydride and, after 20 min, the reacting substance was evaporated to dryness under nitrogen. The residue was dissolved in isooctane, and 1 μL was injected into a gas chromatograph-mass spectrometer. The type B trichothecenes were analysed as TMS (trimethylsilyl) derivatives. The dried extract was treated with 100 μL of a TMSI/TMCS (trimethylsilyl imidazole/trimethylchlorosilane v/v 100/1) mixture and, after 10 min, 500 μL of isooctane was added, and the reaction was quenched with 1 mL of water. The isooctane layer was used for the analysis, and 1 μL of the sample was injected into a GC/MS system. The analyses were conducted using a gas chromatograph (Varian 450-GC, Varian Inc., Palo Alto, CA, USA) coupled to a mass spectrometer (Varian 320-MS, Walnut Creek, CA, USA), with an HP-5MS, 0.25 mm × 30 m capillary column. The injection port temperature was adjusted to 280 °C, and the transfer line temperature was concurrently kept at 280 °C. The analyses were performed with programmed temperatures, separately for type A and B trichothecenes. The type A trichothecenes were analyzed using an initial temperature of 80 °C, held for 1 min, followed by a ramp from 80 °C to 280 °C at 10 °C min−1, with the final temperature maintained for 4 min. For the type B trichothecenes, the initial temperature was 80 °C, held for 1 min, then ramped from 80 °C to 200 °C at 15 °C min−1, held for 6 min, and finally ramped from 200 °C to 280 °C at 10 °C min−1, with the final temperature maintained for 3 min. The helium flow rate was held constant at 0.7 mL min. Quantitative analysis was conducted using a single ion monitoring mode, with the following ions detected: 456 and 555 for STO; 455 and 568 for T-2 Tetraol; 455 and 569 for T-2 Triol; 402 and 374 for DAS; 455 and 327 for HT-2; 327 and 401 for T-2. For DON, the ions 103 and 512 were detected; for 3-AcDON, 117 and 482; for 15-AcDON, 193 and 482; for FUS X, 103 and 570; and for NIV, 191 and 600. Qualitative analysis was performed in SCAN mode. The recovery rates for the analysed toxins were as follows: STO, unknown; T-2 Triol, unknown; T-2, 86 ± 3.8%; T-2 Tetraol, 88 ± 4.0%; HT-2, 91 ± 3.3%; DAS, 84 ± 4.6%; DON, 84 ± 3.8%; 3-AcDON, 78 ± 4.8%; 15-AcDON, 74 ± 2.2%; FUS X, 87% ± 5.9%; and NIV, 81 ± 3.8%. The limit of detection was 0.1 μg kg−1. All chemical reagents used to determine Type A and B Trichothecenes concentration in wheat grain and grain byproducts were purchased from Merck (Darmstadt, Germany).
2.3. Statistical Analysis
To evaluate the impact of the harvest year, cultivars, and farming systems on the concentration of specific type A and B trichothecenes, as well as ergosterol, a three-way ANOVA was performed separately for wheat grain and their byproducts (bran, flour, bread). The differences in the concentration of individual trichothecene type A and B and ergosterol for wheat grain and its byproducts were assessed using a one-way ANOVA. Tukey’s test was applied as the post hoc test for these two types of variance analysis. The assumptions for applying ANOVA, including normality of distribution and homogeneity of variances, were verified and found to be satisfactory. Multivariate relationships (for all determined trichothecenes and ergosterol) between the studied factors were examined using Principal Components Analysis (PCA).
3. Results
3.1. Ergosterol Concentration in Wheat Grain
Ergosterol (ERG) was present in all grain samples, with concentrations ranging from 1.3 to 15.8 mg kg−1 (Table S1). The year, cultivar (genotype), and the farming system significantly influenced the concentration of ergosterol (ERG), as shown in Figure 1. The highest significant concentration of ERG was observed in the grain from 2021 (mean 9.9 mg kg−1). Grain samples obtained from 2019 and 2020 did not differ significantly in ERG concentration (mean: 7.4 and 7.9 mg kg−1, respectively). The wheat cultivars Kandela and Mandaryna (mean 9.5 mg kg−1 and 9.8 mg kg−1, respectively) had the highest concentrations of ERG, which were significantly higher than the concentration in the Harenda cultivar (mean 5.7 mg kg−1). The highest ERG concentration was confirmed in the grain obtained from the organic (ORG) system (mean 13.1 mg kg−1), which was significantly higher than that of the integrated (INT) and conventional (CONV) systems (means 6.5 and 5.7 mg kg−1, respectively).
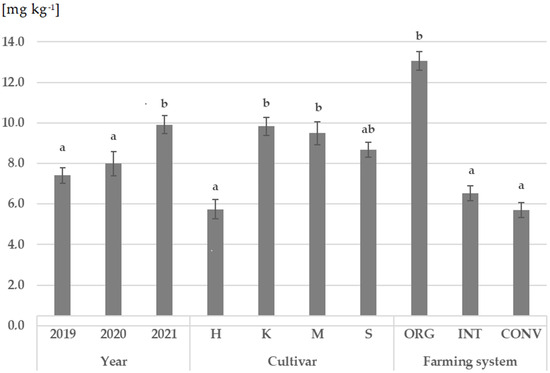
Figure 1.
The effect of year, cultivar, and farming system on wheat grainergosterol concentration (ERG). Abbreviations: different letters (a,b) on the top of the bar correspond to significant differences (α = 0.05) between means; according to Tukey’s test, error bars indicate the standard error of the mean. H—Harenda, K—Kandela, M—Mandaryna, S—Serenada, ORG—organic, INT—integrated, CONV—conventional.
The grain of the Serenada cultivar from the organic (ORG) system harvested in 2019 had the highest ergosterol (ERG) concentration, while the grain of the Kandela cultivar cultivated in the integrated (INT) system and harvested in 2019 had the lowest (Table S1).
3.2. Type A Trichothecenes Concentration in Wheat Grain
We found no significant effect of any of the studied factors (year, cultivar, farming system) on the concentration of type A trichothecenes, such as scirpentriol (STO), T-2 Tetraol, T-2 Triol, diacetoxyscirpenol (DAS), and HT-2, in wheat grain (Table 1). These mycotoxins were either in trace amounts or undetected in the analyzed grain samples (Table 1 and Table S2). In total, out of 36 samples tested, none of these mycotoxins was detected in 23 samples (Table S2).

Table 1.
Type A trichothecenes concentration in wheat grain depends on the year, cultivar, and farming system [μg kg−1].
Scirpentriol (STO) was detected only in the grain of the Mandaryna wheat cultivar from the CONV system harvested in 2021, with a concentration of 1.0 μg kg−1.
Only three wheat grain samples contained T-2 Tetraol, with 2 samples originating from the INT system and one from the CONV system (Table S2). T-2 Tetraol was not detected in any grain samples from the ORG system.
T-2 triol was detected in four wheat grain samples, of which three were from the INT system (cultivars Kandela and Serenada) and one from the CONV system (cultivar Serenada) (Table S2). No presence of T-2 Triol was found in grain from the ORG system, as well as in the Mandaryna and Harenda cultivars. DAS was detected in five grain samples from all three farming systems (Table 2 and Table S2).

Table 2.
Type A trichothecenes concentration in wheat grain [μg kg−1].
HT-2 was the most frequently occurring type A trichothecene in the wheat grain samples analyzed. We detected its presence in six grain samples: four from the CONV system and two from the INT system. We found the presence of HT-2 in grain from the ORG system. We observed the highest concentration of HT-2 in grain from the Harenda cultivar, cultivated in the INT system in 2020, with a concentration of 5 μg kg−1. This was the highest concentration among all the type A trichothecenes detected. HT-2 was negatively correlated with DON (r = −0.41), as shown in Figure 2.
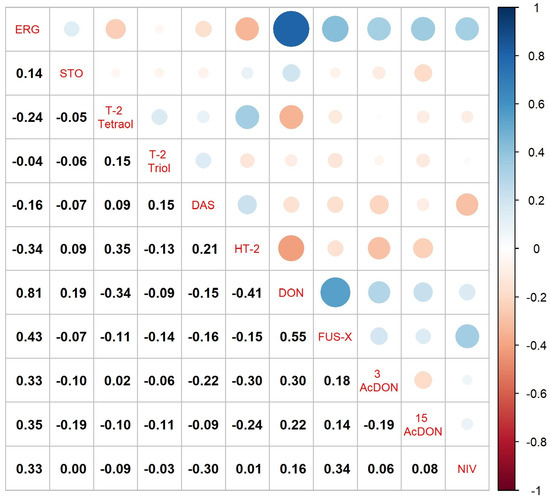
Figure 2.
Correlation between all study traits in wheat grain. Abbreviations: ERG—ergosterol, STO—scirpentriol, DAS—diacetoxyscirpenol, DON—deoxynivalenol, FUS-X—fusarenon X, 3AcDON—3-acetyldeoxynivalenol, 15AcDON—15-acetyldeoxynivalenol, NIV—nivalenol.
3.3. Type B Trichothecenes Concentration in Wheat Grain
The year, cultivar (genotype), and farming system significantly affected the concentration of individual type B trichothecenes. However, the deoxynivalenol derivative 3AcDON concentration was significantly influenced only by the farming system (Table 3).

Table 3.
Type B trichothecenes concentration in wheat grain depends on the year, cultivar, and farming system [μg kg−1].
Among all detected Fusarium mycotoxins, the highest concentration in wheat grain was that of DON (ranging from 15.0 to 157.0 μg kg−1), which was present in all analyzed grain samples (Table 4, Table S3). The highest DON concentration was observed in grain from 2020 (mean 92.0 μg kg−1), significantly higher than from the 2019 harvest (mean 72.9 μg kg−1). The Mandaryna and Kandela cultivars had significantly higher DON concentrations (mean 93.8 and 90.3 μg kg−1, respectively) than the Harenda and Serenada cultivars (mean 67.9 and 78.8 μg kg−1, respectively). We found the highest significant DON concentration in grain from the ORG system, with a mean of 122.1 μg kg−1. The grain from the INT and CONV systems did not differ significantly regarding DON concentration (mean 56.6 and 69.4 μg kg−1, respectively).

Table 4.
Type B trichothecenes concentration in the wheat grain [μg kg−1].
Only the farming system significantly influenced the concentration of 3AcDON in the grain (Table 3). We found the highest significant concentrations of 3AcDON in grain from the INT and CONV systems, with means of 28.7 and 32.7 μg kg−1, respectively. The concentration in grain from the ORG system was lower, with a mean of 19.8 μg kg−1. We confirmed the presence of 3AcDON in all analyzed grain samples except for the Serenada cultivar grown in the CONV system in 2019 (Table S3).
All experimental factors had a significant impact on the concentration of 15AcDON. The highest concentration of 15AcDON was observed in grain from 2019 (mean 11.2 μg kg−1), and was significantly higher than in 2021 (mean 7.8 μg kg−1). The highest concentration of 15AcDON was found in the Kandela cultivar (mean 12.3 μg kg−1), which was significantly higher than in the Harenda and Serenada (mean, respectively, 6.2 μg kg−1, 8.3 μg kg−1) cultivars, but not significantly different from the Mandaryna cultivar (mean 10.4 μg kg−1). The lowest concentration of 15AcDON was found in grain from the CONV system (mean 6.8 μg kg−1), and it was significantly lower than in grain from the ORG system (mean 12.8 μg kg−1). 15AcDON was detected in 28 out of 36 evaluated wheat grain samples (Table 4), with concentrations ranging from 1.0 to 30.0 μg kg−1 (Table S3).
NIV was detected in 30 grain samples, accounting for 83% of the analysed samples (Table 4). The concentration of NIV in samples where it was detected ranged from 1.0 to 26.0 μg kg−1 (Table S3). The concentration of NIV was significantly influenced by the year, cultivar (genotype), and farming system. The highest significant concentration of NIV was found in the grain from 2019 (mean 9.7 μg kg−1), which was approximately twice as high as in grain from 2020 (mean 4.6 μg kg−1). The Mandaryna cultivar had the highest significant NIV concentration (mean 10.4 μg kg−1). The Harenda and Serenada cultivars had significantly lower NIV concentrations (mean 4.2 and 5.0 μg kg−1, respectively) than the Mandaryna and Kandela cultivars (mean 10.4 and 8.8 μg kg−1, respectively). The highest significant NIV concentration was found in grain from the ORG system (mean 11.0 μg kg−1). In contrast, the NIV concentrations in grain from the INT and CONV systems did not differ significantly, with mean values of 5.9 and 4.4 μg kg−1, respectively.
FUS-X was the least frequently occurring type B trichothecene. Its presence was detected in 5 samples originating from the ORG system (Table 3 and Table S3). In addition to the farming system, the concentration of this mycotoxin was significantly influenced by the year and cultivar (genotype). The highest significant FUS-X concentrations were found in grain from 2020 and 2021 (mean 2.0 and 1.8 μg kg−1, respectively), while the concentration in grain from 2019 was 0.8 μg kg−1. The Mandaryna cultivar exhibited the highest significant FUS-X concentration (mean 3.9 μg kg−1), whereas no FUS-X was undetected in all samples of the Serenada cultivar. Our results show positive correlations of FUS-X with DON (r = 0.55), as ilustrated in Figure 2.
3.4. Ergosterol Content in Wheat Grain and Grain Byproducts
The concentration of ergosterol (ERG) significantly depends on the type of research material. The highest significant concentration of ERG was found in bran (BN) (mean 33.8 mg kg−1), whereas the significantly lowest concentration was observed in bread (BD) (mean 0.1 mg kg−1), as illustrated in Figure 3.

Figure 3.
The ergosterol concentration (ERG) in the wheat grain and grain byproducts. Abbreviations: different letters (a–d) on the top of the bar correspond to significant differences (α = 0.05) between means according to Tukey’s test; error bars indicate the standard error of the mean. GR—grain, BN—bran, FR—flour, BD—bread.
ERG was present in all BN samples, with concentrations ranging from 11.6 to 68.1 mg kg−1 (Table S1). Significantly lower ERG concentrations than BN were detected in grain (GR), ranging from 1.4 to 15.8 mg kg−1. Similar to BN, ERG was present in all GR-tested samples. We found that ERG concentration in flour (FR) was significantly lower than in BN and GR, ranging from 0.1 to 1.9 mg kg−1, and we detected ERG in all FR samples. We also found the lowest ERG concentration in the final product, BD, where we detected the compound in only 28% of the bread samples, ranging from 0.1 to 0.9 mg kg−1.
3.5. Type A Trichothecenes Concentration in Wheat Grain and Grain Byproducts
Similar to ERG concentrations, trichothecenes type A varied significantly depending on the research material (Table 5). The highest significant concentrations of individual type A trichothecenes, were found in BN, followed by lower concentrations in GR, and generally no presence in FR and BD (Table 5). DAS was an exception, detected in trace amounts in FR (mean 0.1 μg kg−1).

Table 5.
Type A trichothecenes concentration in wheat grain and grain byproducts [μg kg−1].
3.6. Trichothecenes Type B Concentration in Wheat Grain and Grain Byproducts
Similar to type A trichothecenes, concentration of trichothecenes type B significantly depended on the type of research material (grain, bran, flour, bread), as presented in Table 6. The highest concentrations of trichothecenes type B were found in BN, followed by GR, with either no presence or only trace amounts detected in FR and BD (Table 6).

Table 6.
Type B trichothecenes concentration in wheat grain and grain byproducts [μg kg−1].
The most common mycotoxin was DON, detected in GR and its byproducts, including the final product (BD). BN had the highest concentrations of all analyzed mycotoxins. The content of DON and FUS-X in BN averaged 82.7 and 3.5 μg kg−1, respectively, significantly higher than the concentrations in GR, FR, and BD. However, for DON derivatives (3AcDON, 15AcDON) and NIV, there was no significant difference in their concentrations between BN and GR. Nonetheless, the concentrations of these toxins were significantly higher than those in FR and BD.
3.7. PCA Analysis
Figure 4 presents the PCA results, showing that the first principal component explained approximately 72% of the variance, and the first two principal components together explained 93% of the total variance. The ergosterol (ERG) concentration in wheat GR strongly correlated with type B trichothecenes (FUS-X, NIV, DON, etc.). High concentrations of type B trichothecenes (FUS-X, NIV, DON, etc.) and ergosterol (ERG) were observed in BN and wheat GR cultivated in the ORG system. In contrast, for trichothecene type A (T-2 Triol, DAS, etc.), higher concentrations were found in BN from wheat GR grown in CONV and INT systems.
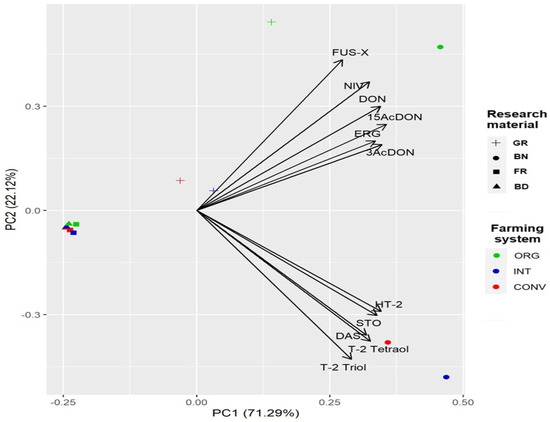
Figure 4.
Biplot of PCA for ergosterol and type A and B trichothecenes in wheat grain. Abbreviations: GR—grain, BN—bran, FR—flour, BD—bread, ORG—organic, INT—integrated, CONV—conventional, STO—scirpentriol, DAS—diacetoxyscirpenol, DON—deoxynivalenol, 3AcDON—3 acetyldeoxynivalenol, 15AcDON—15-acetyldeoxynivalenol, NIV—nivalenol, FUS-X—fusarenon X.
4. Discussion
The three-year field experiment conducted from 2019 to 2021 aimed to assess the impact of the harvest year (meteorological conditions), cultivar, and farming system (organic, integrated, conventional) on the concentration of ergosterol (ERG) and Fusarium mycotoxins belong to the type A and B trichothecenes in the grain of four cultivars of spring wheat. To demonstrate how milling and baking processes affect the concentration of ERG and mycotoxins in the grain, along with additional byproducts, bran, flour, and bread were analysed.
The results showed that the tested wheat grain had a lower type A trichothecenes concentration than type B. Literature data [16,18] show that type A trichothecenes are synthesized mainly by F. poae, F. langsethiae, F. sporotrichoides. Among the 36 grain samples tested, the presence of STO was found in one sample (2.8%), T-2 Tetraol in three samples (8.3%), T-2 Triol in four samples (11.1%), DAS in five samples (13.9%), and HT-2 in six samples (16.7%). The low concentration of type A trichothecenes in wheat grain from Europe is also indicated by Góral et al. [31]. The presented results on the concentration of trichothecenes in some cases indicate their non-detection. Specifically, T-2 toxin was not detected in any of the analysed samples. This may be due to the occurrence of these toxins at very low concentrations, the lack of biosynthesis of these mycotoxins by fungal strains due to genetic factors, as well as environmental conditions. It is also likely that a certain pool of trichothecenes is present in the plant material in a conjugated form, which is also not detected by the methods used in this study [17]. Significantly higher wheat grain was contaminated by type B trichothecenes. This could be explained by the fact that type B trichothecenes are synthesized by the most common Fusarium species in Europe, such as: F. culmorum and F. graminearum [20,31]. We found the presence of DON in all grain samples, 3AcDON in 35 samples (97.2%), 15AcDON in 28 samples (77.8%), NIV in 30 samples (83.3%), and FUS-X in 5 samples (13.9%).
Over the three-year study period, the concentration of type A and type B trichothecenes remained low. In none of the tested grain samples did the concentration of DON exceed the legislative limit of 1000 µg kg−1 set as the maximum safe level for wheat grain intended for processing for consumption purposes [24]. Similarly, the concentration of T-2 and HT-2 remained below the maximum levels set at 50 µg kg−1 of grain [25]. Literature data indicate that Fusarium mycotoxins are among the most frequently occurring contaminants in wheat grain under Polish climatic conditions [8,19,32,33,34,35] and in the Northern European area [18,36]. According to Rodrigues and Naehrer [37], this problem is less common in Southern Europe. The most commonly detected Fusarium mycotoxins in the world include DON, its derivatives, NIV, T-2 Tetraol, T-2 Triol, and HT-2. T-2 and HT-2 toxins have proven toxic effects on humans and animals, including negative impacts on the digestive and immune systems and carcinogenic properties [11,38,39,40]. In our studies, among the Fusarium mycotoxins analyzed, DON was detected at the highest concentrations in all grain samples. According to the literature, DON is one of the most common trichothecenes in wheat grain infected by F. graminearum and F. culmorum [23,40]. Acetylated derivatives of DON (3AcDON, 15AcDON) and NIV were detected in low amounts in the grain, consistent with the findings of Ochodzki et al. [41].
Literature data [33,34] indicate the problem of mycotoxin co-occurrence, which frequently involves DON and its derivatives (3AcDON, 15AcDON). This occurs because they are metabolites produced by the same species of Fusarium. This is corroborated by our studies, in which co-occurrence of DON and its acetylated derivatives was found in 30 out of 36 grain samples (83.3%). However, such a relationship was not observed for type A trichothecenes. Within this group, the simultaneous presence of three mycotoxins was confirmed in only one grain sample, two mycotoxins in four samples and, in the remaining samples, they either occurred singly or were not detected at all.
Numerous factors, primarily weather conditions, genotype, and agricultural practices used in cultivation, influence the contamination of wheat grain by Fusarium mycotoxins [7,14,20,21,22,40]. Fusarium contamination typically occurs pre-harvest [42]. The trichothecenes production depends on specific environmental conditions. Weather plays a particularly significant role in the contamination of grain with Fusarium mycotoxins [20,40,41,42,43,44]. High air humidity combined with high daily temperatures from the flowering stage until the grain harvest especially favors the infection of wheat spikes by Fusarium fungi [22,44]. Our studies partially confirm this. In 2020 and 2021, slightly higher concentrations of trichothecenes type B were observed compared to 2019. The high air humidity, high daily temperatures in May 2020, and approximately three times higher rainfall in August 2021, just before the grain harvest, compared to the long-term average (1951–2021), could explain this [28]. Perochon and Doohan [42] report that low temperatures and lack of nutrients are key factors favoring the production of trichothecenes. According to Erazo et al. [45], water activity may also play a role in the production of trichothecenes. Adverse meteorological conditions, such as damage caused by insects, hail, wind, or mechanical injuries during agricultural procedures, further facilitate the infection of wheat spikes by Fusarium fungi [46].
Results from multiple studies [20,21,22,33,34,35,47] indicate that wheat cultivars exhibit varying resistance to fungal diseases, including those caused by Fusarium species, which translates to differences in the contamination levels of grain with mycotoxins produced by these fungi. Our studies found no statistically significant cultivar differences in the type A trichothecenes concentration. However, the grain of the studied wheat cultivars was significantly differentiated in terms of the concentration of type B trichothecenes, except 3AcDON.
Numerous studies [19,31,48,49,50,51,52,53] indicate that the intensity of agronomic practices used during cultivation influences the presence of Fusarium mycotoxins in wheat grain. Our studies found no significant differences in the concentration of type A trichothecenes in wheat grain from different farming systems. The findings of other authors regarding the influence of the farming system on mycotoxin content in wheat grain are not always consistent, with most authors [19,48,49,50,51,52,53] suggesting that the mycotoxin concentration may be lower in grain from the ORG system compared to the CONV system. Bernhoft [50] explains these relationships, among other factors, by the high organic matter content in the soil and the prohibition of mineral nitrogen fertilizers in ORG systems, which limits the growth of mold fungi.
Research conducted by Góral et al. [31] and Mazurkiewicz et al. [53] indicates lower concentrations of Fusarium mycotoxins in grain from the CONV system compared to the ORG system, which these authors attribute to the chemical protection of crops used in the CONV system. In our studies, grain from the CONV and INT systems, where fungicides were used [28], had significantly lower concentrations of DON, 15AcDON, and NIV than grain from the ORG system and did not contain FUS-X. Notably, the DON concentration in grain from the INT and CONV systems was about twice as low as in grain from the ORG system.
Wheat is one of the primary cereals processed for consumption, mainly used for flour production [29]. In our studies, the mycotoxin concentration in byproducts varied by wheat grain processing (milling and baking). Generally, the highest concentrations of both type A and type B trichothecenes, except NIV, were found in bran (BN), a byproduct mainly used for feed. The higher mycotoxin concentration in BN may be explained by the higher mycotoxin concentration in this fraction consisting of the outer part of the grain, namely the pericarp and seed coat, which serves a protective function and is most colonized by mold fungi [54]. In flour (FR), we only detected DAS among the type A trichothecenes. The concentration of type B trichothecenes (DON and its derivatives, NIV) in FR was significantly lower than in the GR, and FUS-X was undetected. There were no samples of FR in which the DON concentration exceeded the maximum level of 600 µg·kg−1 [24], and the sum toxins T-2 and HT-2, whose maximum level was set at 20 µg·kg−1, were also undetected [25]. In bread (BD), no type A trichothecenes were found, and only trace amounts of DON and NIV were present among type B trichothecenes. According to Twarużek et al. [19] and Tibola et al. [55], the bread-making process reduced the concentration of Fusarium mycotoxins. Tibola et al. [55] also claim that the baking process may be an additional strategy to decrease dietary intake of DON. In our studies, the maximum detected concentration of DON in BD was 17 µg·kg−1, which was more than 23 times lower than the maximum concentration set for bakery wares at 400 µg·kg−1 [24], indicating no health risk for consumers. According to the European Commission’s recommendations, T-2 and HT-2 toxins were not detected in BD, where the maximum level should not exceed 20 µg·kg−1 of product [25].
For the results presented in the study from 2019 to 2021, correlations were calculated between the concentration of ERG, an organic compound from the sterol group found primarily in fungal cells [56], and the detected type A and B trichothecenes. Stuper Szablewska et al. [57] state that ERG in wheat grain can be a biomarker for Fusarium mycotoxins. Our results partially confirmed this, showing a strong positive correlation between ERG and DON (r = 0.81). The correlation coefficients between ERG and other type B trichothecenes ranged from 0.33 to 0.43, while no positive correlations were found between ERG and type A trichothecenes. Among the individual mycotoxins detected, the highest positive correlation was between DON and FUS-X (r = 0.55). Góral et al. [31] did not find a relationship between ERG and DON but, similarly to our study, there was a higher concentration of ERG in grain from the ORG system compared to the CONV system. Contrary to our findings, these authors obtained positive correlations between ERG and type A trichothecenes and negative correlations between ERG and type B trichothecenes. These differences may be because, as Stanisz et al. [58] indicated, not all fungal strains synthesize mycotoxins. Furthermore, the death of fungi leads to a gradual decrease in ERG concentration in samples, while the concentration of mycotoxins usually remains constant. This suggests that ERG may not always be a reliable biomarker for the presence of Fusarium mycotoxins in wheat grain.
5. Conclusions
In summary, our research aimed to demonstrate which farming system used, in conjunction with the selection of wheat cultivar, could reduce the risk of grain contamination caused by Fusarium mycotoxins (type A and B trichothecenes). Additionally, considering the high toxicity of mycotoxins to humans and animals, our research was extended to assess the concentration of these toxins in wheat BN, primarily used as animal feed, in FR (as a raw material in the baking industry), and in BD. The study showed that wheat GR from the ORG system exhibited higher concentrations of type B trichothecenes than GR from the INT and CONV systems. In wheat GR from the INT system, the concentration of type B trichothecenes, which were the main detected mycotoxin, was comparable to that in wheat GR from the high-input CONV system. This suggests that using sustainable agricultural practices combined with cultivating wheat cultivars highly resistant to fungal diseases, such as Harenda and Serenada, can significantly reduce the risk of GR contamination by Fusarium mycotoxins.
Our research demonstrated that GR processing results in products with highly varied mycotoxin concentrations compared to the raw material. We found that the highest concentration of these toxins was present in BN, followed by the GR, FR, and the lowest in BD. This finding is crucial from a consumer safety and food security perspective. Among the Fusarium mycotoxins assessed in our study, DON, T-2, and HT-2 are considered serious health threats for humans and animals in Central and Northern Europe. It is important to note that, throughout our three-year study, none of the tested samples of BN, GR, FR, and BD exceeded the permissible limits for DON as specified in Commission Regulation (EU) 2024/1022, and T-2 and HT-2 were not detected. Nevertheless, monitoring the levels of Fusarium mycotoxins in GR and GR byproducts is essential to ensure food quality and public health protection.
In conclusion, considering food safety and environmental factors, our research suggests that the INT system can be recommended for broader application in agriculture.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agronomy14112535/s1, Table S1: Ergosterol concentration in wheat grain and grain byproducts [mg kg−1]; Table S2: Type A trichothecenes concentration in the wheat grain [μg kg−1]; Table S3: Type B trichothecenes concentration in the wheat grain [μg kg−1].
Author Contributions
Conceptualization, K.W. and G.C.-P.; methodology, K.W., G.C.-P. and M.B.; software, K.W. and M.S.; validation, K.W., G.C.-P.; formal analysis, K.W. and M.B.; investigation, K.W. and G.C.-P.; resources, K.W.; data curation, K.W.; writing—original draft preparation, K.W. and G.C.-P.; writing—review and editing, K.W. and G.C.-P.; visualization, K.W. and M.S.; supervision, G.C.-P.; project administration, G.C.-P.; funding acquisition, G.C.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author/s.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- FAOSTAT. Available online: https://www.fao.org/ (accessed on 15 June 2024).
- Billen, G.; Lassaletta, L.; Garnier, J. A biogeochemical view of the global agro-food system: Nitrogen flows associated with protein production, consumption and trade. Glob. Food Secur. 2014, 3, 209–219. [Google Scholar] [CrossRef]
- Ben Hassen, T.; El Bilali, H. Impacts of the Russia-Ukraine War on Global Food Security: Towards More Sustainable and Resilient Food Systems? Foods 2022, 11, 2301. [Google Scholar] [CrossRef] [PubMed]
- Ficco, D.B.M.; Borrelli, G.M. Nutritional Components of Wheat Based Food: Composition, Properties, and Uses. Foods 2023, 12, 4010. [Google Scholar] [CrossRef]
- Wrigley, C.W.; Corke, H.; Seetharaman, K.; Faubion, J. (Eds.) Encyclopedia of Food Grains; Academic Press: New York, NY, USA, 2015. [Google Scholar]
- Łaba, S.; Cacak-Pietrzak, G.; Łaba, R.; Sułek, A.; Szczepański, K. Food Losses in Consumer Cereal Production in Poland in the Context of Food Security and Environmental Impact. Agriculture 2022, 12, 665. [Google Scholar] [CrossRef]
- Różewicz, M.; Wyzińska, M.; Grabiński, J. The Most Important Fungal Diseases of Cereals—Problems and Possible Solutions. Agronomy 2021, 11, 714. [Google Scholar] [CrossRef]
- Bilska, K.; Jurczak, S.; Kulik, T.; Ropelewska, E.; Olszewski, J.; Żelechowski, M.; Zapotoczny, P. Species Composition and Trichothecene Genotype Profiling of Fusarium Field Isolates Recovered from Wheat in Poland. Toxins 2018, 10, 325. [Google Scholar] [CrossRef]
- Suchorzyńska, M.; Misiewicz, A. Phytopathogenic Mycotoxin-Producing Fungi of the Genus Fusarium and Their Detection Using PCR Techniques. Post. Mikrobiol 2009, 48, 221–230. (In Polish) [Google Scholar]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The Impact of Fusarium Mycotoxins on Human and Animal Host Susceptibility to Infectious Diseases. Toxins 2014, 6, 430–452. [Google Scholar] [CrossRef]
- Zhou, H.; Guog, T.; Dai, H.; Yu, Y.; Zhang, Y.; Ma, L. Deoxynivalenol: Toxicological profiles and perspective views for future research. World Mycotoxin J. 2020, 13, 179–188. [Google Scholar] [CrossRef]
- Awuchi, C.G.; Ondari, E.N.; Nwozo, S.; Odongo, G.A.; Eseoghene, I.J.; Twinomuhwezi, H.; Ogbonna, C.U.; Upadhyay, A.K.; Adeleye, A.O.; Okpala, C.O.R. Mycotoxins’ Toxicological Mechanisms Involving Humans, Livestock and Their Associated Health Concerns: A Review. Toxins 2022, 14, 167. [Google Scholar] [CrossRef]
- Cighir, A.; Mare, A.D.; Vultur, F.; Cighir, T.; Pop, S.D.; Horvath, K.; Man, A. Fusarium spp. in Human Disease: Exploring the Boundaries between Commensalism and Pathogenesis. Life 2023, 13, 1440. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, L. Mycotoxigenic Fusarium species from agricultural crops in Malaysia. JSM Mycotoxins 2017, 67, 67–75. [Google Scholar] [CrossRef][Green Version]
- McCormick, S.P.; Stanley, A.M.; Stover, N.A.; Alexander, N.J. Trichothecenes: From Simple to Complex Mycotoxins. Toxins 2011, 3, 802–814. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Yang, J.; Yang, X.; Zhang, J.; Zhao, Z. Type A Trichothecene Metabolic Profile Differentiation, Mechanisms, Biosynthetic Pathways, and Evolution in Fusarium Species—A Mini Review. Toxins 2023, 15, 446. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Ksieniewicz-Woźniak, E.; Szymczyk, K.; Jędrzejczak, R. Modified Fusarium Mycotoxins in Cereals and Their Products—Metabolism, Occurrence, and Toxicity: An Updated Review. Molecules 2018, 23, 963. [Google Scholar] [CrossRef] [PubMed]
- Foroud, N.A.; Baines, D.; Gagkaeva, T.Y.; Thakor, N.; Badea, A.; Steiner, B.; Bürstmayr, M.; Bürstmayr, H. Trichothecenes in Cereal Grains—An Update. Toxins 2019, 11, 634. [Google Scholar] [CrossRef]
- Twarużek, M.; Grajewska-Wanat, N.; Błajet-Kosicka, A.; Grajewski, J. Occurrence of Fusarium and major mycotoxins in cereal grains harvested in 2011–2012. Prog. Plant Prot. 2013, 53, 801–806. (In Polish) [Google Scholar] [CrossRef][Green Version]
- Aleksandrowicz, E. Factors influencing the occurrence of Fusarium mycotoxins in the grain of winter wheat. Pol. J. Agron. 2020, 43, 102–113. [Google Scholar]
- Birr, T.; Hasler, M.; Verreet, J.-A.; Klink, H. Composition and Predominance of Fusarium Species Causing Fusarium Head Blight in Winter Wheat Grain Depending on Cultivar Susceptibility and Meteorological Factors. Microorganisms 2020, 8, 617. [Google Scholar] [CrossRef]
- Wegulo, S.N. Factors Influencing Deoxynivalenol Accumulation in Small Grain Cereals. Toxins 2012, 4, 1157–1180. [Google Scholar] [CrossRef]
- Chen, C.; Frank, K.; Wang, T.; Wu, F. Global wheat trade and Codex Alimentarius guidelines for deoxynivalenol: A mycotoxin common in wheat. Glob. Food Sec. 2021, 29, 100538. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2024/1022 of 8 April 2024 Amending Regulation (EU) 2023/915 as Regards Maximum Levels of Deoxynivalenol in Food. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32024R1022 (accessed on 10 July 2024).
- Commission Regulation (EU) 2024/1038 of 9 April 2024 Amending Regulation (EU) 2023/915 as Regards Maximum Levels of T-2 and HT-2 Toxins in Food. Available online: https://eur-lex.europa.eu/eli/reg/2024/1038/oj (accessed on 10 July 2024).
- Perkowski, J.; Buśko, M.; Stuper, K.; Kostecki, M.; Matysiak, A.; Szwajkowska-Michałek, L. Concentration of ergosterol in small-grained naturally contaminated and inoculated cereals. Biologia 2008, 63, 542–547. [Google Scholar] [CrossRef]
- Available online: https://www.iung.pl/ (accessed on 20 October 2024).
- Mitura, K.; Cacak-Pietrzak, G.; Feledyn-Szewczyk, B.; Szablewski, T.; Studnicki, M. Yield and Grain Quality of Common Wheat (Triticum aestivum L.) Depending on the Different Farming Systems (Organic vs. Integrated vs. Conventional). Plants 2023, 12, 1022. [Google Scholar] [CrossRef]
- Wysocka, K.; Cacak-Pietrzak, G.; Feledyn-Szewczyk, B.; Studnicki, M. The Baking Quality of Wheat Flour (Triticum aestivum L.) Obtained from Wheat Grains Cultivated in Various Farming Systems (Organic vs. Integrated vs. Conventional). Appl. Sci. 2024, 14, 1886. [Google Scholar] [CrossRef]
- Perkowski, J.; Wiwart, M.; Buśko, M.; Laskowska, M.; Berthiller, A.; Kandler, S.; Krska, R. Fusarium toxins and total fungal biomass indicators in naturally contaminated wheat samples from north-eastern Poland in 2003. Food Additiv. Contam. 2007, 24, 1292–1298. [Google Scholar] [CrossRef]
- Góral, T.; Ochodzki, P.; Nielsen, L.K.; Walentyn-Góral, D. Species of the genus Fusarium and Fusarium toxins in the grain of winter and spring wheat in Poland. Biul. IHAR 2021, 296, 25–42. [Google Scholar] [CrossRef]
- Okorski, A.; Milewska, A.; Pszczółkowska, A.; Karpiesiuk, K.; Kozera, W.; Dąbrowska, J.A.; Radwińska, J. Prevalence of Fusarium fungi and Deoxynivalenol Levels in Winter Wheat Grain in Different Climatic Regions of Poland. Toxins 2022, 14, 102. [Google Scholar] [CrossRef]
- Bryła, M.; Ksieniewicz-Woźniak, E.; Waśkiewicz, A.; Szymczyk, K.; Jędrzejczak, R. Natural Occurrence of Nivalenol, Deoxynivalenol, and Deoxynivalenol-3-Glucoside in Polish Winter Wheat. Toxins 2018, 10, 81. [Google Scholar] [CrossRef]
- Bryła, M.; Waśkiewicz, A.; Podolska, G.; Szymczyk, K.; Jędrzejczak, R.; Damaziak, K.; Sułek, A. Occurrence of 26 Mycotoxins in the Grain of Cereals Cultivated in Poland. Toxins 2016, 8, 160. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Cacak-Pietrzak, G.; Lenc, L.; Gromadzka, K.; Dziki, D. Milling and Baking Quality of Spring Wheat (Triticum aestivum L.) from Organic Farming. Agriculture 2021, 11, 765. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, I.; Naehrer, K. Three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Pierzgalski, A.; Bryła, M.; Kanabus, J.; Modrzewska, M.; Podolska, G. Updated Review of the Toxicity of Selected Fusarium Toxins and Their Modified Forms. Toxins 2021, 13, 768. [Google Scholar] [CrossRef]
- Meneely, J.; Greer, B.; Kolawole, O.; Elliott, C. T-2 and HT-2 Toxins: Toxicity, Occurrence and Analysis: A Review. Toxins 2023, 15, 481. [Google Scholar] [CrossRef] [PubMed]
- Kamle, M.; Mahato, D.K.; Gupta, A.; Pandhi, S.; Sharma, B.; Dhawan, K.; Vasundhara; Mishra, S.; Kumar, M.; Tripathi, A.D.; et al. Deoxynivalenol: An Overview on Occurrence, Chemistry, Biosynthesis, Health Effects and Its Detection, Management, and Control Strategies in Food and Feed. Microbiol. Res. 2022, 13, 292–314. [Google Scholar] [CrossRef]
- Ochodzki, P.; Twardawska, A.; Wiśniewska, H.; Góral, T. Resistance to Fusarium Head Blight, Kernel Damage, and Concentrations of Fusarium Mycotoxins in the Grain of Winter Wheat Lines. Agronomy 2021, 11, 1690. [Google Scholar] [CrossRef]
- Perochon, A.; Doohan, F.M. Trichothecenes and Fumonisins: Key Players in Fusarium–Cereal Ecosystem Interactions. Toxins 2024, 16, 90. [Google Scholar] [CrossRef]
- Khan, M.K.; Pandey, A.; Athar, T.; Choudhary, S.; Deval, R.; Gezgin, S.; Hamurcu, M.; Topal, A.; Atmaca, E.; Santos, P.A.; et al. Fusarium head blight in wheat: Con-temporary status and molecular approaches. 3 Biotech 2020, 10, 172. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Cacak-Pietrzak, G.; Lenc, L.; Stalenga, J. Rating of Spring Wheat Varieties (Triticum aestivum L.) According to Their Suitability for Organic Agriculture. Agronomy 2020, 10, 1900. [Google Scholar] [CrossRef]
- Erazo, J.G.; Palacios, S.A.; Veliz, N.A.; Del Canto, A.; Plem, S.; Ramirez, M.L.; Torres, A.M. Effect of Temperature, Water Activity and Incubation Time on Trichothecene Production by Fusarium cereals Isolated from Durum Wheat Grains. Pathogens 2023, 12, 736. [Google Scholar] [CrossRef]
- Friskop, A.; Bergstrom, G.; Bradley, C.; Kleczewski, N.; Marshall, J.; Smith, D.; Tenuta, A.; Wise, K. An Overview of Fusarium Head Blight; Crop Protection Network: Washington, WA, USA, 2021. [Google Scholar] [CrossRef]
- Weber, R.; Plaskowska, E. Variability of the incidence of Fusarium species and mycotoxins in the grain of wheat, depending on soil tillage system and cultivar. Folia Pomeranae Univ. Technol. Stetin. Agric. Aliment. Piscaria Zootech. 2017, 334, 187–200. [Google Scholar] [CrossRef]
- Köpke, U.; Thiel, B.; Elmholt, S. Strategies to reduce mycotoxin and fungal alkaloid contamination in organic and conventional cereal production systems. In Handbook of Organic Food Safety and Quality; Cooper, J., Niggli, U., Leifert, C., Eds.; CRC Press: Boca Raton, FL, USA, 2007; pp. 353–391. [Google Scholar]
- Bernhoft, A.; Wang, J.; Leifert, C. Effect of Organic and Conventional Cereal Production Methods on Fusarium Head Blight and Mycotoxin Contamination Levels. Agronomy 2022, 12, 797. [Google Scholar] [CrossRef]
- Bernhoft, A.; Torp, M.; Clasen, P.E.; Løes, A.K.; Kristoffersen, A.B. Influence of Agronomic and Climatic Factors on Fusarium Infestation and Mycotoxin Contamination of Cereals in Norway. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2012, 29, 1129–1140. [Google Scholar] [CrossRef]
- Mruczyk, K.; Mizgier, M.; Wójciak, R.W.; Cisek-Woźniak, A. Comparison of deoxynivalenol and zearaleone concentration in conventional and organic cereal products in western Poland. Ann. Agric. Environ. Med. 2021, 28, 44–48. [Google Scholar] [CrossRef]
- Váňová, M.; Klem, K.; Míša, P.; Matušinsky, P.; Hajšlová, J.; Lancová, K. The content of Fusarium mycotoxins grain yield and quality of winter wheat cultivars under organic and conventional cropping system. Plant Soil Environ. 2008, 54, 395–402. Available online: https://pse.agriculturejournals.cz/artkey/pse-200809-0005_the-content-of-fusarium-mycotoxins-grain-yield-and-quality-of-winter-wheat-cultivars-under-organic-and-convent.php (accessed on 15 July 2024). [CrossRef]
- Mazurkiewicz, J.; Solarska, E.; Kuzdraliński, A.; Muszyńska, M. The occurrence of fusarium toxins in winter wheat depending on fertilization. J. Res. Appl. Agric. Eng. 2008, 53, 15–17. (In Polish) [Google Scholar]
- Zhang, C.; Qu, Z.; Hou, J.; Yao, Y. Contamination and Control of Mycotoxins in Grain and Oil Crops. Microorganisms 2024, 12, 567. [Google Scholar] [CrossRef]
- Tibola, C.S.; Zavariz de Miranda, M.; Paiva, F.F.; Cunha Fernandes, J.M.; Guarienti, E.M.; Nicolau, M. Effect of breadmaking process on mycotoxin content in white and whole wheat breads. Cereal Chem. 2018, 95, 660–665. [Google Scholar] [CrossRef]
- Rodrigues, M.L. The Multifunctional Fungal Ergosterol. mBio 2018, 18, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Stuper-Szablewska, K.; Przybylska-Balcerek, A.; Kurasiak-Popowska, D.; Ryńska, B.; Bilska, A. Determination of Influence of Contamination of Wheat Grain with Microscopic Fungi and their Metabolites on the Quality of Products of Grain Processing. Sci. Nat. Technol. 2019, 13, 5. [Google Scholar] [CrossRef]
- Stanisz, E.; Zgoła-Grześkowiak, A.; Waskiewicz, A.; Stępień, Ł. Can Ergosterol be an Indicator of Fusarium Fungi and Mycotoxins in Cereal Products? J. Braz. Chem. Soc. 2015, 26, 705–712. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).