Calcium Uptake Pattern and Its Transport Pathway in ‘Shixia’ Longan Fruit
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Sample Processing Method
2.3. Determination of the Total Calcium Content
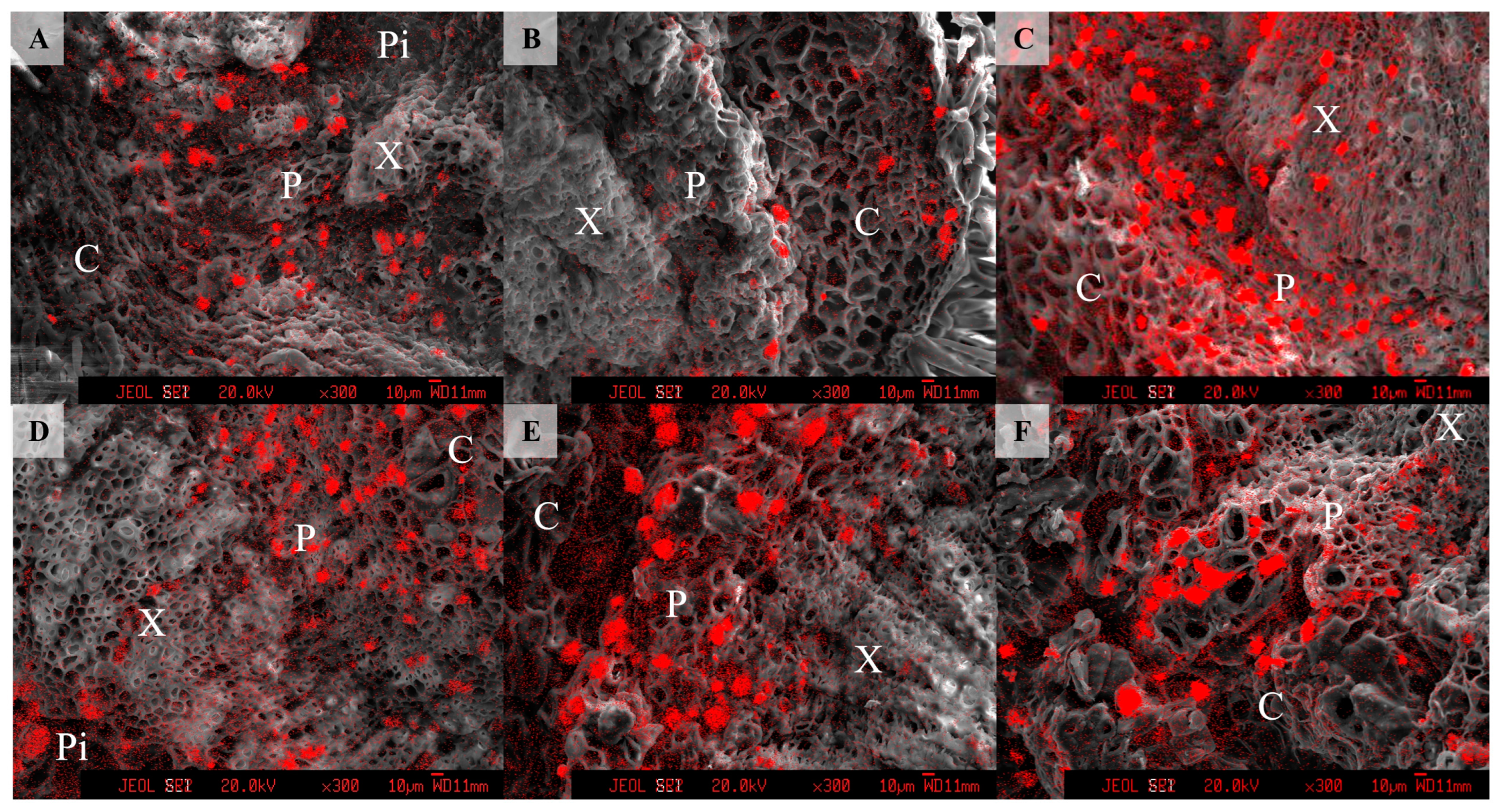
2.4. Calcium Mapping in Longan Tissues Using Electron Probe Microanalyzer (EPMA)
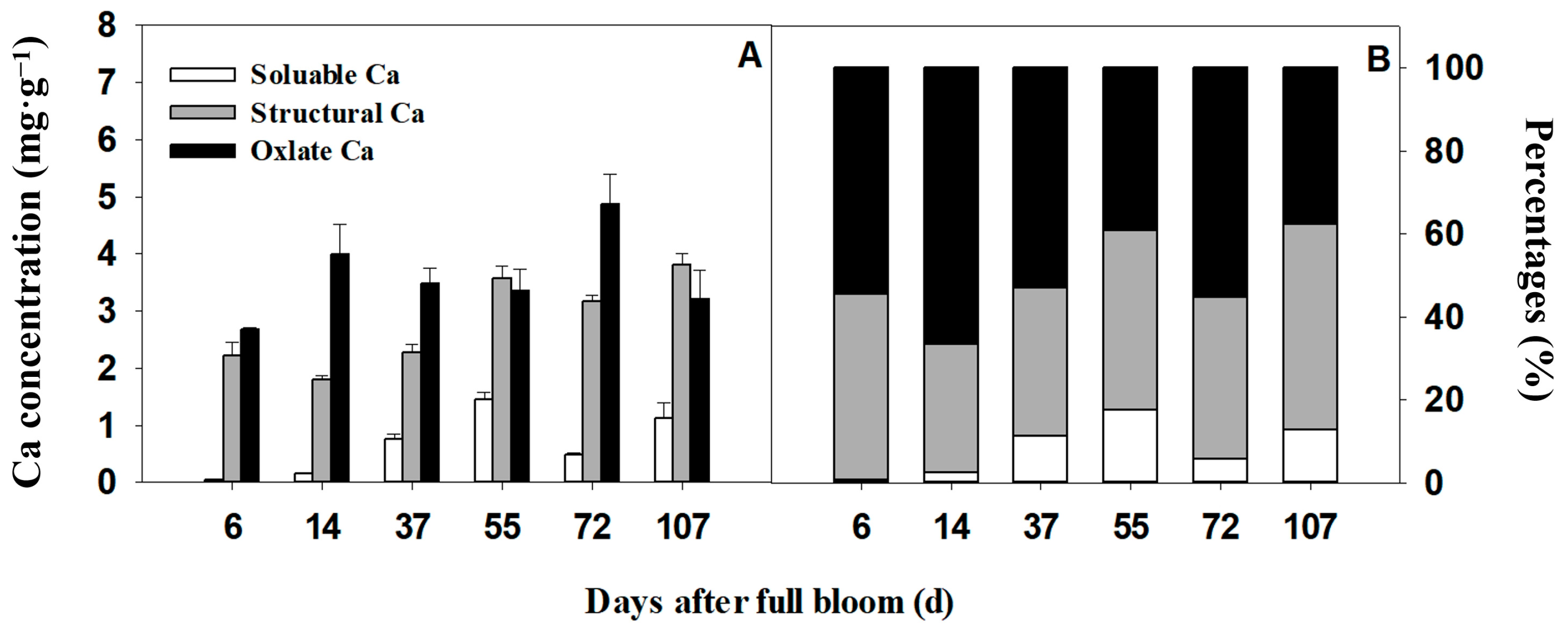
2.5. Extraction of Different Forms of Calcium from Tissues
2.6. Effects of Girdling on Calcium Uptake in Fruit
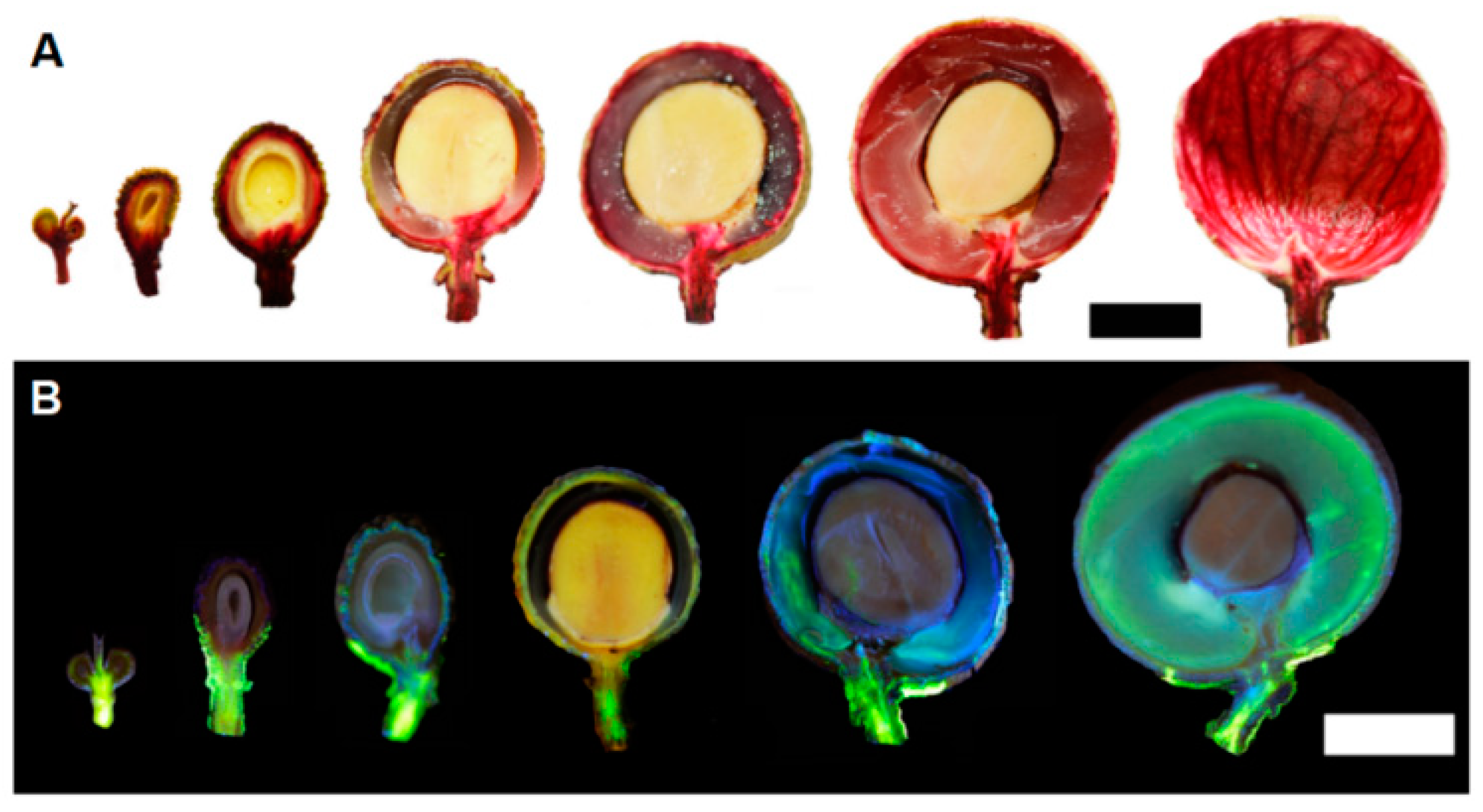
2.7. Tracer Observation of Ductal and Cytoplasmic Pathway Using Flux Dyes
2.8. Determination of IAA Content
2.9. Growth Hormone Analog (2,4-D) Treatment
2.10. Correlation Analysis of Seed Size and Fruit Calcium Uptake Capacity
2.11. Calculation of Fruit-Related Parameters
2.12. Statistical Analysis
3. Results
3.1. Dynamics of Calcium Accumulation in ‘Shixia’
3.2. Calcium Uptake Capacity of ‘Shixia’ Fruit at Various Stages
3.3. Variation Patterns and Correlation of Calcium Concentration in the Pedicel and the Fruit
3.4. Calcium Distribution in the Pedicels at Different Developmental Stages
3.5. Changes in Fruit Calcium Content After Girdling Treatment in ‘Shixia’
3.6. Dye Tracing of the Xylem/Apoplastic and Phloem/Symplastic Pathways
3.7. Role of Endogenous Indole-3-Acetic Acid (IAA) and Effect of 2,4-D on Calcium Uptake in Fruit
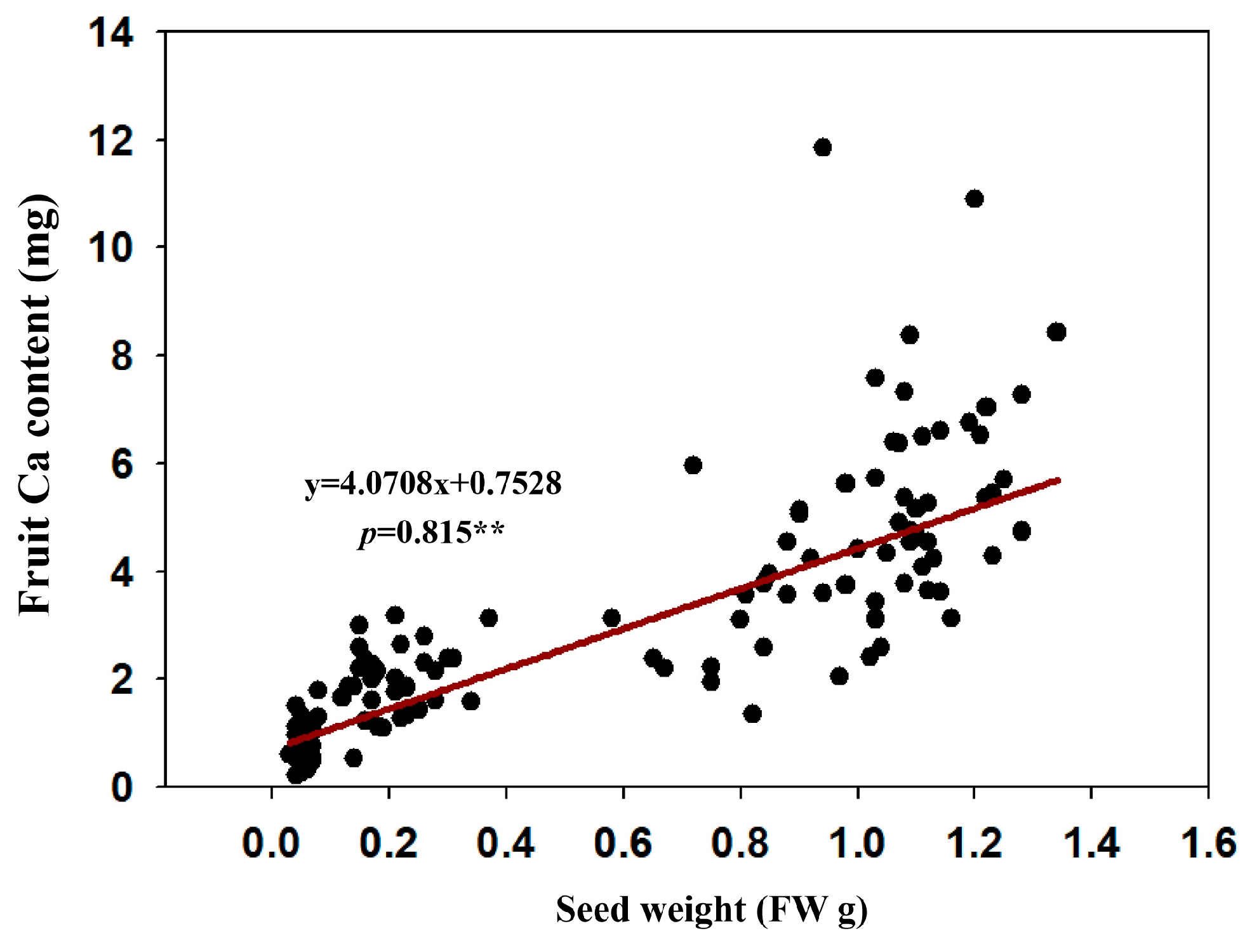
3.8. Seed Weight of ‘Shixia’ in Relation to Fruit Calcium Uptake
4. Discussion
5. Conclusions
- (1)
- Calcium distribution in the phloem: microscopic observations revealed an increased distribution of calcium in the phloem, particularly as calcium oxalate crystals.
- (2)
- Symplastic pathway continuity: the symplastic transport pathway remained functional throughout fruit development, with calcium concentrations in the pedicel being consistently higher than those in the fruit.
- (3)
- Impact of girdling: girdling treatments significantly affected both fruit enlargement and calcium uptake, highlighting the importance of the phloem in calcium transport.
- (4)
- Correlation with seed size: seed size exhibited a significant positive correlation with fruit calcium content, whereas IAA levels closely matched calcium accumulation patterns.
- (5)
- Effect of 2,4-D auxin: exogenous application of 2,4-D increased calcium concentration in the pedicel and enhanced the fruit’s calcium uptake rate within a week, indicating a synergistic effect between auxin and calcium.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hepler, P.K. Calcium: A central regulator of plant growth and development. Plant Cell 2005, 17, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Xu, X.; Fu, W.; Zhang, H.; Qu, S.; Wang, S. Difference in calcium accumulation in the fruit of two apple varieties and its relationship with vascular bundle development in the pedicel. Plant Physiol. Biochem. 2023, 201, 107833. [Google Scholar] [CrossRef] [PubMed]
- Dunemann, L.; Schwedt, G. Ionenaustausch- und gel-chromatographische Analysenverfahren zur Charakterisierung von Metall-Bindungsformen—Anwendung auf wäßrige Extrakte aus Soja- und Weizenmehl. Fresenius’ Z. Anal. Chem. 1986, 325, 121–125. [Google Scholar] [CrossRef]
- Jackson, L.S.; Lee, K. Chemical Forms of Iron, Calcium, Magnesium and Zinc in Black, Oolong, Green and Instant Black Tea. J. Food Sci. 1988, 53, 181–184. [Google Scholar] [CrossRef]
- Ilarslan, H.; Palmer, R.; Imsande, J.; Horner, H. Quantitative determination of calcium oxalate and oxalate in developing seeds of soybean (Leguminosae). Am. J. Bot. 1997, 84, 1042. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- Shear, C.B. Calcium-related Disorders of Fruits and Vegetables1. HortScience 1975, 10, 361–365. [Google Scholar] [CrossRef]
- de Freitas, S.T.; Mitcham, E.J. Factors Involved in Fruit Calcium Deficiency Disorders. In Horticultural Reviews; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 107–146. [Google Scholar]
- Storey, R.; Treeby, M.T.; Milne, J. Crease: Another Ca deficiency-related fruit disorder? J. Hortic. Sci. Biotechnol. 2002, 77, 565–571. [Google Scholar] [CrossRef]
- Storey, R.; Treeby, M.T. Cryo-SEM study of the early symptoms of peteca in ‘Lisbon’ lemons. J. Hortic. Sci. Biotechnol. 2002, 77, 551–556. [Google Scholar] [CrossRef]
- Taylor, M.D.; Locascio, S.J. Blossom-End Rot: A Calcium Deficiency. J. Plant Nutr. 2004, 27, 123–139. [Google Scholar] [CrossRef]
- Volz, R.K.; Alspach, P.A.; Fletcher, D.J.; Ferguson, I.B. Genetic variation in bitter pit and fruit calcium concentrations within a diverse apple germplasm collection. Euphytica 2006, 149, 1–10. [Google Scholar] [CrossRef]
- Huang, X.M.; Yuan, W.Q.; Wang, H.C.; Li, J.G.; Huang, H.B. Early calcium accumulation may play a role in spongy tissue formation in litchi pericarp. J. Hortic. Sci. Biotechnol. 2004, 79, 947–952. [Google Scholar] [CrossRef]
- Huang, X.M.; Wang, H.C.; Zhong, W.L.; Yuan, W.Q.; Lu, J.M.; Li, J.G. Spraying calcium is not an effective way to increase structural calcium in litchi pericarp. Sci. Hortic. 2008, 117, 39–44. [Google Scholar] [CrossRef]
- Wilkinson, B.G. Mineral composition of apples IX.—Uptake of calcium by the fruit. J. Sci. Food Agric. 1968, 19, 646–647. [Google Scholar] [CrossRef]
- Bemadac, A.; Jean-Baptiste, I.; Bertoni, G.; Morard, P. Changes in calcium contents during melon (Cucumis melo L.) fruit development. Sci. Hortic. 1996, 66, 181–189. [Google Scholar] [CrossRef]
- Tagliavini, M.; Zavalloni, C.; RombolÃ, A.D.; Quartieri, M.; Malaguti, D.; Mazzanti, F.; Millard, P.; Marangoni, B. Mineral Nutrient Partitioning to Fruits of Deciduous Trees. Acta Hortic. 2000, 512, 131–140. [Google Scholar] [CrossRef]
- Quinlan, J.D. Chemical composition of developing and shed fruits of Laxton’s Fortune apple. J. Hortic. Sci. 1969, 44, 97–106. [Google Scholar] [CrossRef]
- Zindler-Frank, E. On the Formation of the Pattern of Crystal Idioblasts in Canavalia ensiformis DC.VII. Calcium and Oxalate Content of the Leaves in Dependence of Calcium Nutrition. Z. Pflanzenphysiol. 1975, 77, 80–85. [Google Scholar] [CrossRef]
- Borchert, R. Calcium acetate induces calcium uptake and formation of calcium-oxalate crystals in isolated leaflets of Gleditsia triacanthos L. Planta 1986, 168, 571–578. [Google Scholar] [CrossRef]
- Franceschi, V.R. Calcium oxalate formation is a rapid and reversible process in Lemna minor L. Protoplasma 1989, 148, 130–137. [Google Scholar] [CrossRef]
- Tuason, M.M.; Arocena, J.M. Calcium oxalate biomineralization by Piloderma fallax in response to various levels of calcium and phosphorus. Appl. Environ. Microbiol. J. 2009, 75, 7079–7085. [Google Scholar] [CrossRef] [PubMed]
- Monje, P.; Baran, E. Characterization of Calcium Oxalate Biominerals in Some (Non-Cactaceae) Succulent Plant Species. Z. Naturforschung C 2014, 65, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Osuji, J.; Ndukwu, B.C. Probable functions and remobilisation of calcium oxalates in Musa L. Afr. J. Biotechnol. 2005, 4, 1139–1141. [Google Scholar]
- Chen, Y.; Zhang, S.; Lin, H.; Lu, W.; Wang, H.; Chen, Y.; Lin, Y.; Fan, Z. The role of cell wall polysaccharides disassembly in Lasiodiplodia theobromae-induced disease occurrence and softening of fresh longan fruit. Food Chem. 2021, 351, 129294. [Google Scholar] [CrossRef]
- Lin, Y.; Lin, L.; Lin, Y.; Lin, M.; Ritenour, M.A.; Lin, H. Comparison between two cultivars of longan fruit cv. ‘Dongbi’ and ‘Fuyan’ in the metabolisms of lipid and energy and its relation to pulp breakdown. Food Chem. 2023, 398, 133885. [Google Scholar] [CrossRef]
- Park, S.J.; Park, D.H.; Kim, D.H.; Lee, S.; Yoon, B.H.; Jung, W.Y.; Lee, K.T.; Cheong, J.H.; Ryu, J.H. The memory-enhancing effects of Euphoria longan fruit extract in mice. J. Ethnopharmacol. 2010, 128, 160–165. [Google Scholar] [CrossRef]
- Liu, L.; Lan, H.; Wang, Y.; Zhao, L.; Liu, X.; Hu, Z.; Wang, K. Acetylation at the O-6 position of t-Glc improved immunoactivity of α-1,6-glucan from longan by additionally activating Dectin-1 and CD14 receptors. Carbohydr. Polym. 2023, 320, 121199. [Google Scholar] [CrossRef]
- Rong, Y.; Yang, R.; Yang, Y.; Wen, Y.; Liu, S.; Li, C.; Hu, Z.; Cheng, X.; Li, W. Structural characterization of an active polysaccharide of longan and evaluation of immunological activity. Carbohydr. Polym. 2019, 213, 247–256. [Google Scholar] [CrossRef]
- Song, W.P.; Chen, W.; F. Kurniadinata, O.; Wang, H.; Huang, X.M. Application of electron probe to the observation of in situ calcium distribution in fruit tissues. J. Fruit Sci. 2014, 31, 730–732+752. [Google Scholar] [CrossRef]
- Okamoto, M.; Hanada, A.; Kamiya, Y.; Yamaguchi, S.; Nambara, E. Measurement of Abscisic Acid and Gibberellins by Gas Chromatography/Mass Spectrometry. In Plant Hormones: Methods and Protocols; Cutler, S., Bonetta, D., Eds.; Humana Press: Totowa, NJ, USA, 2009; pp. 53–60. [Google Scholar] [CrossRef]
- Montanaro, G.; Dichio, B.; Xiloyannis, C. Significance of fruit transpiration on calcium nutrition in developing apricot fruit. J. Plant Nutr. Soil Sci. 2010, 173, 618–622. [Google Scholar] [CrossRef]
- Miqueloto, A.; Amarante, C.V.T.d.; Steffens, C.A.; dos Santos, A.; Mitcham, E. Relationship between xylem functionality, calcium content and the incidence of bitter pit in apple fruit. Sci. Hortic. 2014, 165, 319–323. [Google Scholar] [CrossRef]
- Ferreira Torres, L.; López de Andrade, S.A.; Mazzafera, P. Split-root, grafting and girdling as experimental tools to study root-to shoot-to root signaling. Environ. Exp. Bot. 2021, 191, 104631. [Google Scholar] [CrossRef]
- Di Vaio, C.; Petito, A.; Buccheri, M. Effect of Girdling on Gas Exchanges and Leaf Mineral Content in the ‘INDEPENDENCE’ Nectarine. J. Plant Nutr. 2001, 24, 1047–1060. [Google Scholar] [CrossRef]
- Stebbins, R.L.; Dewey, D.H. Role of Transpiration and Phloem Transport in Accumulation of 45 Calcium in Leaves of Young Apple Trees1. J. Am. Soc. Hortic. Sci. 1972, 97, 471–474. [Google Scholar] [CrossRef]
- Vemmos, S.N. Effects of shoot girdling on bud abscission, carbohydrate and nutrient concentrations in pistachio (Pistacia vera L.). J. Hortic. Sci. Biotechnol. 2005, 80, 529–536. [Google Scholar] [CrossRef]
- Song, W.P.; Chen, W.; Yi, J.W.; Wang, H.C.; Huang, X.M. Ca Distribution Pattern in Litchi Fruit and Pedicel and Impact of Ca Channel Inhibitor, La3+. Front. Plant Sci. 2018, 8, 2228. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.H.; Xu, T.; Qi, M.F.; Li, S.S.; Li, J.H.; Li, H.; Gao, S.; Li, T.L. The Role of Calcium in Mediating SIIAAs Expression in Tomato Pedicel Explants. Agric. Sci. China 2010, 9, 980–984. [Google Scholar] [CrossRef]
- Banuelos, G.S.; Bangerth, F.; Marschner, H. Relationship between polar basipetal auxin transport and acropetal Ca2+ transport into tomato fruits. Physiol. Plant. 1987, 71, 321–327. [Google Scholar] [CrossRef]
- Brown, M.M.; Ho, L.C. Factors Affecting Calcium Transport and Basipetal IAA Movement in Tomato Fruit in Relation to Blossom-end Rot. J. Exp. Bot. 1993, 44, 1111–1117. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Zhong, X.M.; Wang, H.C.; Zhang, L.; Li, J.G.; Huang, X.M. Starving longan fruit sends weakened abscission-suppressing signal rather than enhanced abscission-triggering signal to the abscission zone. Sci. Hortic. 2022, 293, 110667. [Google Scholar] [CrossRef]
- Yu, J.; Niu, L.; Yu, J.; Liao, W.; Xie, J.; Lv, J.; Feng, Z.; Hu, L.; Dawuda, M.M. The Involvement of Ethylene in Calcium-Induced Adventitious Root Formation in Cucumber under Salt Stress. Int. J. Mol. Sci. 2019, 20, 1047. [Google Scholar] [CrossRef] [PubMed]
- Waidyarathne, P.; Samarasinghe, S. Boolean Calcium Signalling Model Predicts Calcium Role in Acceleration and Stability of Abscisic Acid-Mediated Stomatal Closure. Sci. Rep. 2018, 8, 17635. [Google Scholar] [CrossRef] [PubMed]
- Bangerth, F. Polar auxin transport as a signal in the regulation of tree and fruit development. Acta Hortic. 1993, 329, 10. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, R.R.; Tyagi, S.K. Pre-harvest foliar application of calcium and boron influences physiological disorders, fruit yield and quality of strawberry (Fragaria×ananassa Duch.). Sci. Hortic. 2007, 112, 215–220. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, U.M.; Manohar, M.; Gaur, V.S. Calcium transport from source to sink: Understanding the mechanism(s) of acquisition, translocation, and accumulation for crop biofortification. Acta Physiol. Plant. 2014, 37, 1722. [Google Scholar] [CrossRef]
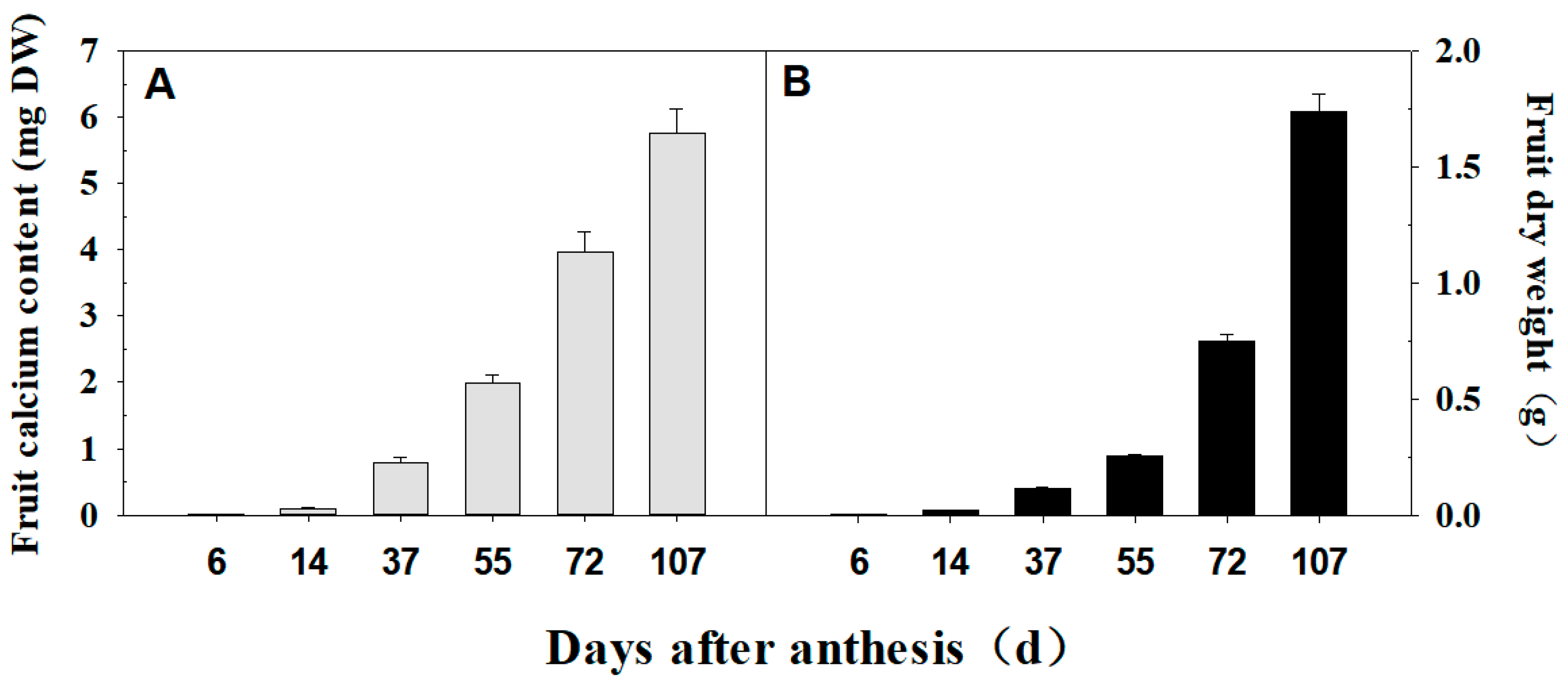

| Stage (Day After Anthesis) | Mass Growth (g) | Fruit Growth Rate (mg·d−1) | Ca Uptake (mg) | Fruit Ca Uptake Rate (μg·d−1) | Ca Uptake in Relative Growth (mg·g−1) | Fruit Ca Uptake Activity (μg·d−1·g−1) |
|---|---|---|---|---|---|---|
| 6–14 | 0.02 ± 0.00 | 1.96 ± 0.07 | 0.08 ± 0.01 | 9.83 ± 0.94 | 4.98 ± 0.36 | 792.29 ± 47.46 |
| 14–37 | 0.09 ± 0.00 | 3.91 ± 0.21 | 0.50 ± 0.10 | 21.87 ± 4.24 | 5.37 ± 0.82 | 345.02 ± 65.45 |
| 37–55 | 0.14 ± 0.01 | 7.65 ± 0.51 | 1.19 ± 0.12 | 66.31 ± 6.89 | 8.81 ± 0.88 | 360.74 ± 38.94 |
| 55–72 | 0.50 ± 0.03 | 29.15 ± 1.66 | 1.97 ± 0.31 | 115.90 ± 18.06 | 3.72 ± 0.58 | 225.70 ± 34.35 |
| 72–107 | 0.99 ± 0.07 | 28.29 ± 2.09 | 1.80 ± 0.43 | 51.29 ± 12.27 | 1.83 ± 0.46 | 42.50 ± 9.86 |
| Sample Date (DAA) | Relative Content of Calcium (%) | |||
|---|---|---|---|---|
| Pith | Xylem | Phloem | Cortex | |
| 6 | 7.52 ± 3.40 a | 2.73 ± 1.44 b | 0.41 ± 0.41 b | 1.31 ± 0.28 b |
| 14 | 0.79 ± 0.79 b | 0.90 ± 0.18 b | 2.79 ± 1.25 a | 1.62 ± 0.69 ab |
| 37 | 2.01 ± 1.09 b | 0.87 ± 0.36 b | 4.04 ± 0.32 a | 2.35 ± 0.53 b |
| 55 | 7.08 ± 2.29 a | 3.89 ± 0.61 b | 4.35 ± 0.42 b | 1.88 ± 0.09 b |
| 72 | 15.22 ± 9.28 a | 1.24 ± 0.16 b | 3.68 ± 0.44 b | 2.20 ± 0.33 b |
| 107 | 5.32 ± 0.88 a | 2.76 ± 0.33 c | 3.90 ± 0.23 b | 0.94 ± 0.24 d |
| Treatment | Fruit Weight (g) | Ca Uptake (mg) | Fruit Ca Uptake Rate (μg·d−1) | Ca Uptake in Relative Growth (mg·g−1) | Fruit Ca Uptake Activity (μg·d−1·g−1) |
|---|---|---|---|---|---|
| Control | 0.33 ± 0.01 | 1.03 ± 0.07 | 103.45 ± 6.76 | 6.11 ± 0.44 | 988.35 ± 62.88 |
| Girdling | 0.17 ± 0.01 * | 0.60 ± 0.12 | 60.11 ± 12.18 * | 8.25 ± 1.81 | 1063.01 ± 220.17 |
| Days After Full Bloom (Days) | IAA Content (ng·Fruit−1) | Fruit Ca Concentration (mg·g−1) | Fruit Total Ca (mg·Fruit−1) |
|---|---|---|---|
| 14 | 1.64 ± 0.23 a | 4.77 ± 0.26 b | 0.10 ± 0.01 c |
| 37 | 2.65 ± 0.35 a | 6.67 ± 0.45 ab | 0.80 ± 0.07 bc |
| 55 | 3.21 ± 0.45 a | 7.87 ± 0.45 a | 1.99 ± 0.12 a |
| Days After Treatment (Days) | Treatment | Pedicel Ca Concentration (mg·g−1) | Fruit Weight (g) | Fruit Ca Concentration (mg·g−1) | Fruit Total Ca (mg·Fruit−1) |
|---|---|---|---|---|---|
| 10 | Control | 4.85 ± 0.79 | 0.22 ± 0.01 | 3.85 ± 0.88 | 0.35 ± 0.09 |
| 2,4-D | 7.94 ± 1.08 * | 0.22 ± 0.03 | 5.21 ± 0.81 | 0.46 ± 0.10 | |
| 20 | Control | 7.83 ± 0.77 | 0.39 ± 0.03 | 4.99 ± 0.27 | 0.73 ± 0.03 |
| 2,4-D | 7.88 ± 1.61 | 0.48 ± 0.05 | 6.46 ± 1.33 | 1.07 ± 0.19 |
| Treatment | Fruit Dry Weight (g) | Fruit Ca Content (mg) | Fruit Ca Uptake Rate (μg·d−1) | Fruit Ca Uptake Activity (μg·d−1·g−1) |
|---|---|---|---|---|
| Control | 0.06 ± 0.01 | 0.36 ± 0.17 | 36.08 ± 16.65 | 313.32 ± 150.38 |
| 2,4-D | 0.09 ± 0.02 | 0.66 ± 0.31 | 66.45 ± 30.59 | 585.32 ± 288.83 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, W.; Huang, S.; Kurniadinata, O.F.; Yang, Z.; Huang, X. Calcium Uptake Pattern and Its Transport Pathway in ‘Shixia’ Longan Fruit. Agronomy 2024, 14, 2480. https://doi.org/10.3390/agronomy14112480
Song W, Huang S, Kurniadinata OF, Yang Z, Huang X. Calcium Uptake Pattern and Its Transport Pathway in ‘Shixia’ Longan Fruit. Agronomy. 2024; 14(11):2480. https://doi.org/10.3390/agronomy14112480
Chicago/Turabian StyleSong, Wenpei, Siqi Huang, Odit F. Kurniadinata, Ziqin Yang, and Xuming Huang. 2024. "Calcium Uptake Pattern and Its Transport Pathway in ‘Shixia’ Longan Fruit" Agronomy 14, no. 11: 2480. https://doi.org/10.3390/agronomy14112480
APA StyleSong, W., Huang, S., Kurniadinata, O. F., Yang, Z., & Huang, X. (2024). Calcium Uptake Pattern and Its Transport Pathway in ‘Shixia’ Longan Fruit. Agronomy, 14(11), 2480. https://doi.org/10.3390/agronomy14112480







