1. Introduction
Kiwifruit (
Actinidia chinensis) is a popular and highly nutritious fruit with a unique flavor and aroma compounds [
1]. It is known for its high vitamin C content and a range of other health benefits [
2]. The main kiwifruit cultivar marketed in the world is ‘Hayward’ (
Actinidia chinensis var.
deliciosa). The introduction of new cultivars in the kiwifruit industry is confronted with significant challenges pertaining to successful commercialization, ensuring that the product meets consumer preferences in terms of good taste and being “ready to eat”, as well as revealing an attractive appearance to the consumer [
3]. In Chile, kiwifruit exports are primarily concentrated in the ‘Hayward’ cultivar, which constitutes 98% of total exports. The remaining 2% are comprised of yellow-fleshed cultivars, including ‘Dori’, ‘Jintao’ (Jing Gold), ‘Soreli’, A19 (‘Enza Gold’) and ‘Kiss’ [
4]. Despite the strong dominance of the ‘Hayward’ cultivar in Chilean plantations, there is an increasing intertest in growing new cultivars, particularly those with yellow and red flesh [
5].
From the point of view of the consumer, one of the main characteristics of the commercial cultivars is the vibrant color of their flesh due to the accumulation of pigments, including chlorophylls (green flesh), carotenoids (yellow flesh) and anthocyanins (red flesh). Adequate color development is therefore of commercial relevance, particularly for
Actinidia chinensis var.
chinensis cultivars, which require the breakdown of chlorophyll pigments (green) present in the flesh during fruit development to reveal the pigments associated with yellow or red flesh color, a process known as flesh degreening [
6]. In Hayward Kiwifruit (
A. chinensis var.
deliciosa), flesh color remains green throughout fruit development and ripening. However, in the yellow-fleshed cultivars (
A. chinensis var.
chinensis), the color changes from green to yellow due to activation of the chlorophyll breakdown pathway and the transformation of plastid structure from chloroplast to chromoplast, along with the unmasking the carotenoid pigments already present [
7]. The main increase in carotenoids, once chlorophyll degradation has been initiated, is attributed to beta-carotene concentration, under the control of
LCY-β (lycopene beta cyclase) genes [
8]. Carotenoid pigment concentrations in yellow-fleshed kiwifruit (
A. chinensis var.
chinensis) and green-fleshed kiwifruit (
A. chinensis var.
deliciosa) can be similar even in ripe fruit [
9].
Changes in flesh color can occur on or off the vine [
10]. To achieve the commercially required color, it is necessary to harvest the fruit already degreened, or at least with a sufficient level of degreening to allow the process during postharvest [
11]. Initial studies performed in the yellow-fleshed kiwifruit ‘Hort16A’ showed an influence of harvest maturity and temperature regimens on the flesh degreening potential [
12,
13]. These studies showed that ‘ Hort16A’, when picked at the usual harvest maturity, achieved complete degreening postharvest if stored at storage temperatures between 10 °C and 15 °C. Flesh degreening was complete if fruit were harvested earlier than commercial harvest with an initial color of 107.9° hue and stored for 42 days at 15 °C. Subsequent studies on ‘Zesy003’ (Gold9) indicated complete degreening after 42 days storage at between 10 °C and 15 °C and with the same sigmoidal decrease in hue angle [
14]. Similar results were found with an early cultivar, ‘Dori’, harvested seven days before commercial maturity [
15]. Conversely, the late cultivar ‘Kiss’ (initially described as Y-374) exhibited a more complex color evolution during its development on the plant, indicating a slower progression in color change compared to other maturity parameters [
16]. Therefore, temperature and exposure time are key factors for achieving complete flesh degreening off the vine. While high degreening temperatures can lead to increased softening in yellow-fleshed cultivars, they also demonstrated a beneficial effect by reducing symptoms of chilling injury [
15,
17].
Studies of degreening in species other than kiwifruit showed complete inhibition of degreening at high temperatures. Peel degreening in Cavendish bananas was reduced at temperatures between 30 and 34 °C, compared to at 20 °C when degreening was complete [
18]. In a later study with bananas (
Musa acuminata cv. Cavendish,
AAA group), peel degreening was analyzed for treatments in which temperatures of 20 or 30 °C were alternated or continuous [
19]. Peel degreening was inhibited when the fruit was treated with continuous temperatures at 30 °C, although the changes triggered by the maturation process continued normally and degreening was completed at 20 °C. The optimal temperature range for banana degreening lies between 18 and 24 °C [
19]. The inhibitory effect of 30 °C on degreening flesh has not been previously tested in kiwifruit, and it may serve as a means of elucidating the role of enzymes in the degreening process, particularly with consideration of the specific functions of chlorophyllase and magnesium dechelatase enzymes, closely associated with early chlorophyll degradation.
The genes involved in the enzymes of the biosynthesis and degradation pathway of chlorophyll are expressed in the green and yellow-fleshed cultivars of kiwifruit [
20]. Chlorophyll degradation involves a series of enzymatic reactions that lead to the degradation of the chlorophyll molecule and the formation of colorless breakdown products. This process of chlorophyll breakdown can be divided into an early phase occurring within the thylakoid membrane with the degradation of chlorophylls to simpler products, known as the PAO/Phyllobilin pathway, then a later phase in which these colorless chlorophyll catabolites are further modified and translocated from the chloroplast to the vacuole [
21]. Views differ on the specific enzymes responsible for triggering chlorophyll breakdown and on the role played by chlorophyllase in removing the phytol chain of the chlorophyll molecule through the formation of chlorophyllide as an intermediate compound [
21]. Additionally, studies indicate that magnesium dechelatase plays a pivotal role in initiating chlorophyll degradation by catalyzing the removal of the central magnesium atom. This step converts chlorophyll
a to pheophytin
a. leading to the formation of pheophytin, which is considered a fundamental part of the breakdown process. In the PAO/Phyllobilin pathway, Chlorophyll
a is converted to Pheophytin
a and the production of the magnesium-free porphyrin ring, colorless Pheophorbide
a, by Pheophorbide oxidase [
22,
23]. On the other hand, the PAO enzyme (Pheophorbide
a oxygenase) participates in the conversion of pheophorbide
a to red chlorophyll colorless catabolite (RCCs), which are primarily associated with the cellular processes of senescing leaves [
23,
24]. Additionally, the STAY GREEN (SGR) protein is involved in regulating the chlorophyll degradation process. Although it is not an enzyme itself, SGR plays a crucial role in modulating the activity of chlorophyll degradation enzymes and determining the timing of chlorophyll breakdown [
22].
The study of genes involved in kiwifruit flesh degreening in ‘Zesy003’ (yellow-fleshed) and Hayward (green-fleshed) varieties suggest that the expression of SGR2 and PAO1 genes account for the main differences in degreening among the yellow and green-fleshed kiwifruit cultivars [
14]. Both genes are described as components of the chlorophyll degradation pathway;
SGR, in particular, is proposed to encode the magnesium dechelatase enzyme, responsible for the conversion of chlorophyll
a to pheophytin
a by removing the central magnesium atom [
25]. In particular, SGR2 was found to be slightly upregulated in yellow-fleshed kiwifruit [
20]. However, the information regarding these genes has yet to be fully elucidated at the protein level.
Our research aims to further clarify the degreening behaviors and to assess the enzymatic response of chlorophyllase and magnesium dechelatase considering different storage temperature regimes, including high temperature, in two contrasting kiwifruit cultivars: the yellow-fleshed cv. ‘Kiss’ and the green-fleshed cv. ‘Hayward’.
2. Materials and Methods
2.1. Plant Material
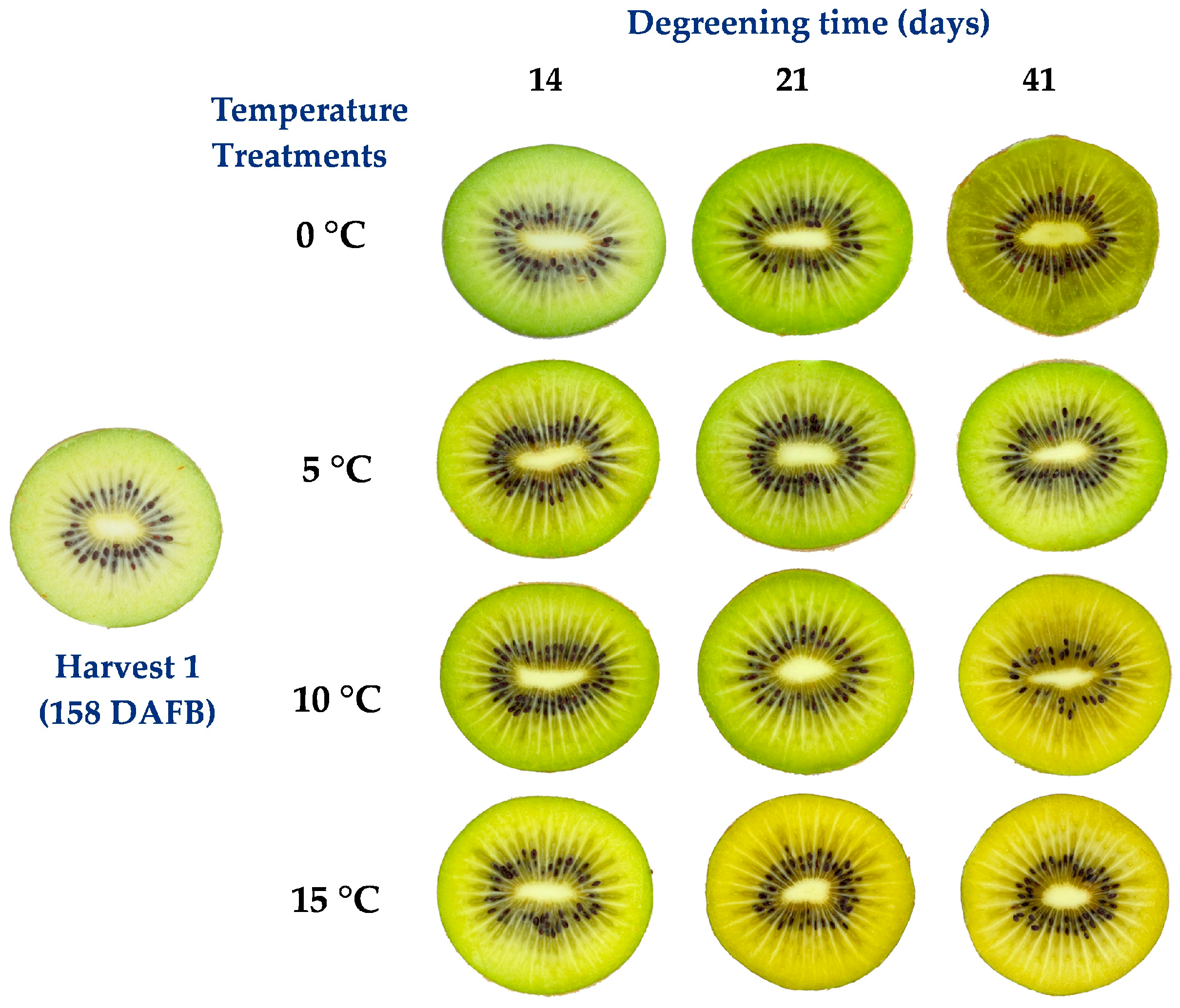
Two kiwifruit cultivars were chosen based on their distinct flesh degreening behaviors. The fruit were sourced from orchards in Chile’s sixth and seventh regions. The first cultivar was the yellow-fleshed
A. chinensis var.
chinensis cv. ‘Y-374’ (marketed as kiwifruit ‘Kiss’), a late cultivar with a reference harvest date similar to Hayward and Jintao [
26], harvested at 158 (initial of color brake) or at 166 days after full bloom (DAFB), corresponding to 21 and 14 days before commercial harvest, respectively. The second cultivar was the green-fleshed
A. chinensis var.
deliciosa cv. ‘Hayward’). which was harvested 161 DAFB, corresponding to three days before commercial harvest. In the case of ‘Hayward’, a single harvest time was selected when the temperature treatments were concentrated. The harvested fruit was transported to the Postharvest Physiology and Technology Laboratory of the Pontificia Universidad Católica de Chile.
2.2. Assay and Temperature Degreening Treatments
The degreening-enzyme study was conducted on two kiwifruit cultivars, yellow- and green-fleshed, under various temperature treatments. With cv. ‘Kiss’, different assays were carried out at two harvest stages. The first assay with ‘Kiss’ (harvested at 158 DAFB) aimed to determine the degreening behavior of flesh in fruit harvested 21 days before commercial harvest. This harvest involved 440 fruit; 40 fruit were chosen for maturity characterization, the others (400 fruit) were degreened for 41 days (d) at different temperatures: 0, 5, 10 or 15 °C with 100 fruit each, following the methodology described by Gambi et al. [
14]. The 0 °C storage temperature reflected the commercially-used handling temperature. For each storage temperature, four replications of five groups of fruit (20) were evaluated at 7, 14, 21, 30 and 41 d.
In the second assay with ‘Kiss’ (harvested at 166 DAFB), the objective was to corroborate the degreening behavior observed in the first assay by testing the effect of very high temperature over a short period of time, in contrast to the conventional storage temperature of 0 or 15 °C. The selection of a very high temperature (30 °C) was supported by literature that indicates different degreening responses in other fruit [
18,
19,
27]. In this assay, 280 fruit were harvested, 40 fruit were used for maturity characterization and the others (240 fruit) were held for 30 d at 0 or 15 °C and a third treatment of initial high temperature degreening of 7 d at 30 °C, followed by storage at 15 °C for 23 d. For each of these three treatments, four replications of five groups of fruit (20) were evaluated at 7, 14, 21, and 30 d.
The experiment with ‘Hayward’ (harvested at 161 DAFB) included the same temperature treatments used in the ‘Kiss’ assays, with storage degreening temperatures of 0, 5, 10 or 15 °C or 7 d at 30 °C, with subsequent storage at 15 °C, until 41 d. The total number of fruits harvested was 440; as well as the first harvest of ‘Kiss’, 40 fruit were evaluated at harvest and the others were divided into different temperature treatments following the same distribution described earlier but, in the case of cv. ‘Hayward’, the fruit was assessed only after 7, 14, 21 and 41 d. The quality assessment of the fruit at each time and at different temperature treatments is described above.
Ethylene traps based on potassium permanganate (KMnO4) were adapted to ensure ethylene gas concentrations were consistently <0.05 µL L−1. Trap performance was checked by quantifying ethylene in a storage chamber using a GC-8340 Gas Chromatograph (Fisons Instruments Inc., MA, USA), equipped with a Chromosorb 102 glass-filled column (Thermo Electron Corporation, Milan, Italy).
2.3. Quality Assessments
The characterization of maturity parameters was carried out according to the methodology described by Zoffoli et al. [
28]. Flesh color was measured using a CR-400 Chroma meter (Konica Minolta Sensing Inc., Osaka, Japan) after making a cross-sectional cut of the fruit. The measurement unit used was hue angle (° h) (L × C × Hue color space). Flesh firmness was measured with an FT327 penetrometer (Effegi, Alfonsine, Italy) fitted with a 7.9 mm plunger and expressed as newtons (N). Soluble solids’ concentration (SSC) was obtained individually (for each fruit) with a thermo-compensated optical refractometer (ATAGO, Master Alpha, Tokyo, Japan), and titratable acidity (TA) was obtained by titration of 5 mL of juice per repetition with 0.1 N NaOH to pH 8.2 using a pH-211 pH meter (Hanna Instruments, Romania) and expressed as percentage of citric acid equivalents. Additionally, at harvest, the dry matter (DM) was calculated from the fresh and dry weight obtained from a 3 mm thick sheet dried in the oven at 65 °C until a constant weight was achieved. After the destructive analysis of the maturity parameters described above, a flesh sample was taken from each fruit and stored separately at −20 °C for each repetition for further analysis (pigment concentration and enzyme activity).
2.4. Pigment Analyses
The carotenoids and chlorophylls were analyzed from flesh samples (without columella or seeds) frozen at −20 °C, which were ground in the presence of liquid nitrogen until complete pulverization was obtained. After this, the amounts required for each methodology were used.
2.4.1. Total Carotenoids
The Total Carotenoids were extracted according to the method described by Xia et al. [
29] with modifications. A 0.5 g frozen pulverized sample was weighed and 5 mL of 100%
v/
v acetone solution containing 0.1% butylated hydroxytoluene (BHT) was added immediately and vortexed for 30 s. Ultrasonic extraction was performed for 60 min in a pre-cooled distilled water bath at 0 °C. The samples were centrifuged at 9000×
g for 10 min at 4 °C (Sorvall Super T21, Dupont, MA, USA). The supernatant was collected with a 0.45 µm filter integrated into a 5 mL syringe. The calculation of the TC concentration was obtained from the following equation:
The total carotenoids is expressed in mg per kilogram of fresh weight (mg kg−1 FW−1), A corresponds to the absorbance measured at 450 nm and is an absorption coefficient, which in this case corresponds to 2332 (acetone solvent).
2.4.2. Chlorophylls and Pheophytin a
The concentrations of chlorophyll
a (Chl
a), chlorophyll
b (Chl
b) and pheophytin
a (
Phe a) were obtained using the method of Costa et al. [
30] with modifications. Briefly, 0.5 g of sample was weighed, and 5 mL of 100%
v/
v acetone pre-cooled at 0 °C was added. Ultrasonic extraction was performed for 60 min at 4 °C. Then, the samples were centrifuged at 9000×
g for 10 min at 4 °C. The supernatant was collected and filtered with a 0.45 µm filter integrated into a 5 mL syringe. The absorbance was measured in a spectrophotometer UV–VIS (Optizen Pop, K Lab Co., Ltd., Daejeon, Republic of Korea) at the wavelengths indicated by the following equations [
31]:
After measuring the absorbance for chlorophylls, the samples were returned to the tubes, and a drop of 25%
v/
v HCl was added, which generated the start of the conversion reaction from chlorophyll
a to pheophytin
a. The concentration of pheophytin
a was calculated as the absorbance difference according to the following equation [
31]:
The pigment concentrations obtained by entering the absorbance values for each specific wavelength are expressed in milligrams per kilogram of fresh weight (mg kg−1 FW−1).
2.5. Chlorophyllase and Magnesium Dechelatase Activity
The procedure was standardized for analyses of kiwifruit samples from the methodology proposed by Costa et al. [
30] with modifications. Two extraction buffers were prepared. Extraction buffer 1 was prepared with 0.1 mol L
−1 Na
2HPO
4, 0.1 mol L
−1 NaH
2PO
4, 2 mL L
−1 Triton X–100, 20 g L
−1 PVPP (poly vinyl-poly-pyrrolidone), 5 mmol L
−1 cysteine pH 6.0 and 1 mmol L
−1 PMSF (phenyl-methyl-sulfonyl fluoride). Due to the instability of the PMSF reagent when dissolved in water (susceptibility to fluoride hydrolysis), it was first dissolved in 3 mL of 100% (
v/
v) ethanol and then added to the final solution containing all the reagents from extraction buffer 1. The second mixture of extraction buffer 2 consisted of 0.1 mol L
−1 sodium phosphate buffer pH 7.0, 1.5 mL L
−1 Triton X–100, 10 µmol L
−1 chlorophyll extract and 16% (
v/
v) acetone.
Enzyme substrates were prepared as a reference for the case of chlorophyll and chlorophyllin from fresh spinach leaves [
30]. Chlorophyllin is an Mg-chlorin derived from chlorophyll and was extracted following the procedure described by Vicentini et al. [
32] with some modifications. Briefly, 6 g of spinach was weighed and mixed with 60 mL of 80% (
v/
v) acetone. After achieving complete homogenization, the extract was centrifuged at 9000×
g for 10 min at 4 °C (Sorvall Super T21, Dupont, MA, USA). The supernatant was separated, and 40 mL of petroleum ether was added. To evaporate away the petroleum ether, gaseous nitrogen was added under the extraction hood and a green precipitate was obtained. For the chlorophyll extract, the precipitate was reconstituted with 5 mL of 100% (
v/
v) acetone. To obtain the chlorophyllin extract, the precipitate was washed three times with 40 mL of distilled water and 1 mL of 30% (
w/
v) KOH solution in methanol was added for each milligram of chlorophyll present. The chlorophyllin extract was centrifuged at 5500×
g for 15 min at 4 °C. The precipitate was dissolved in 10 mL of distilled water and brought to pH 9.0 by adding tricin (1 mol L
−1) to generate a stock solution.
For initial enzyme extraction, 8 g of kiwifruit frozen sample was mixed with 25 mL of extraction buffer 1. This was vortexed for 60 s and then centrifuged at 9000× g for 20 min at 4 °C. The separated supernatant was considered as the crude enzyme extract.
2.5.1. Chlorophyllase Activity
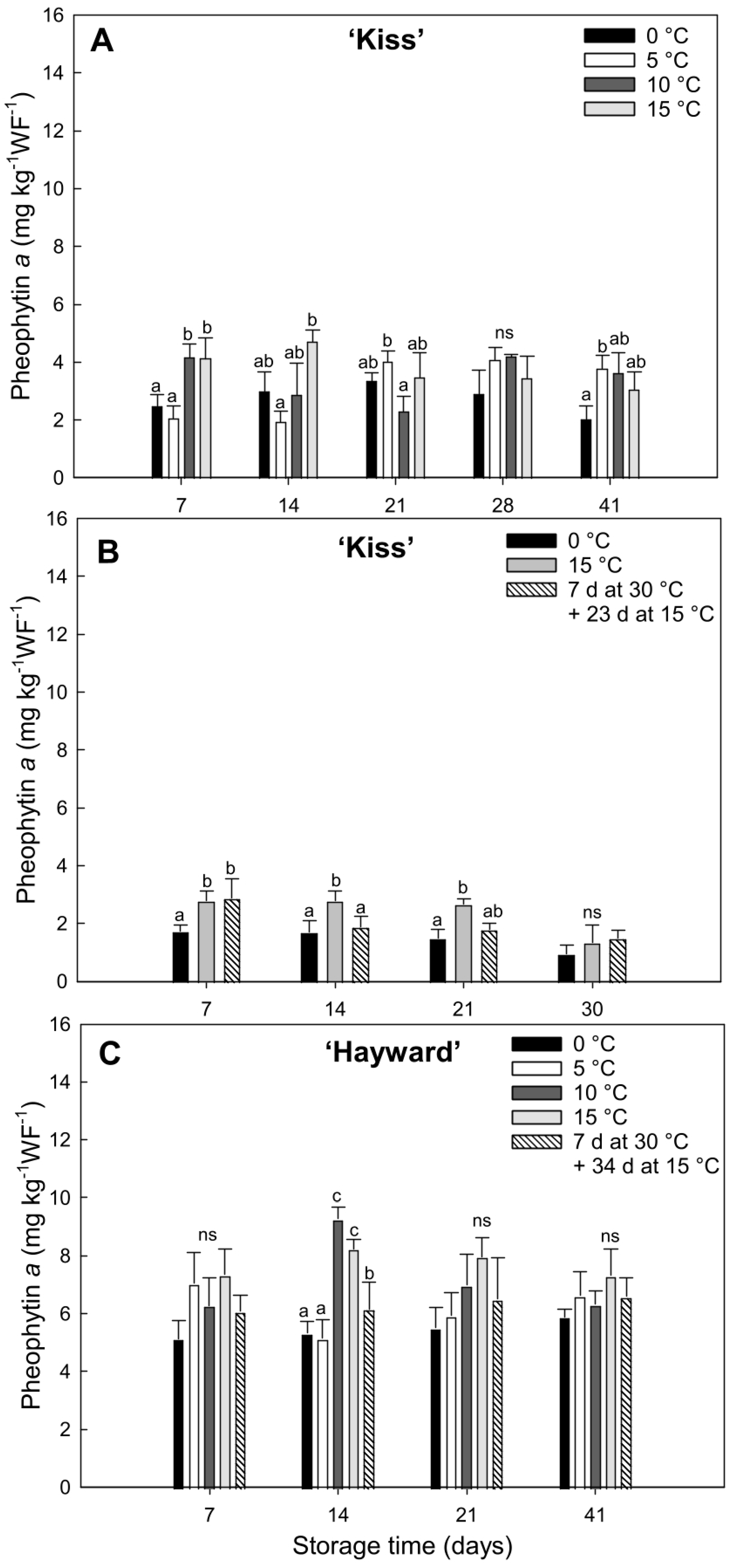
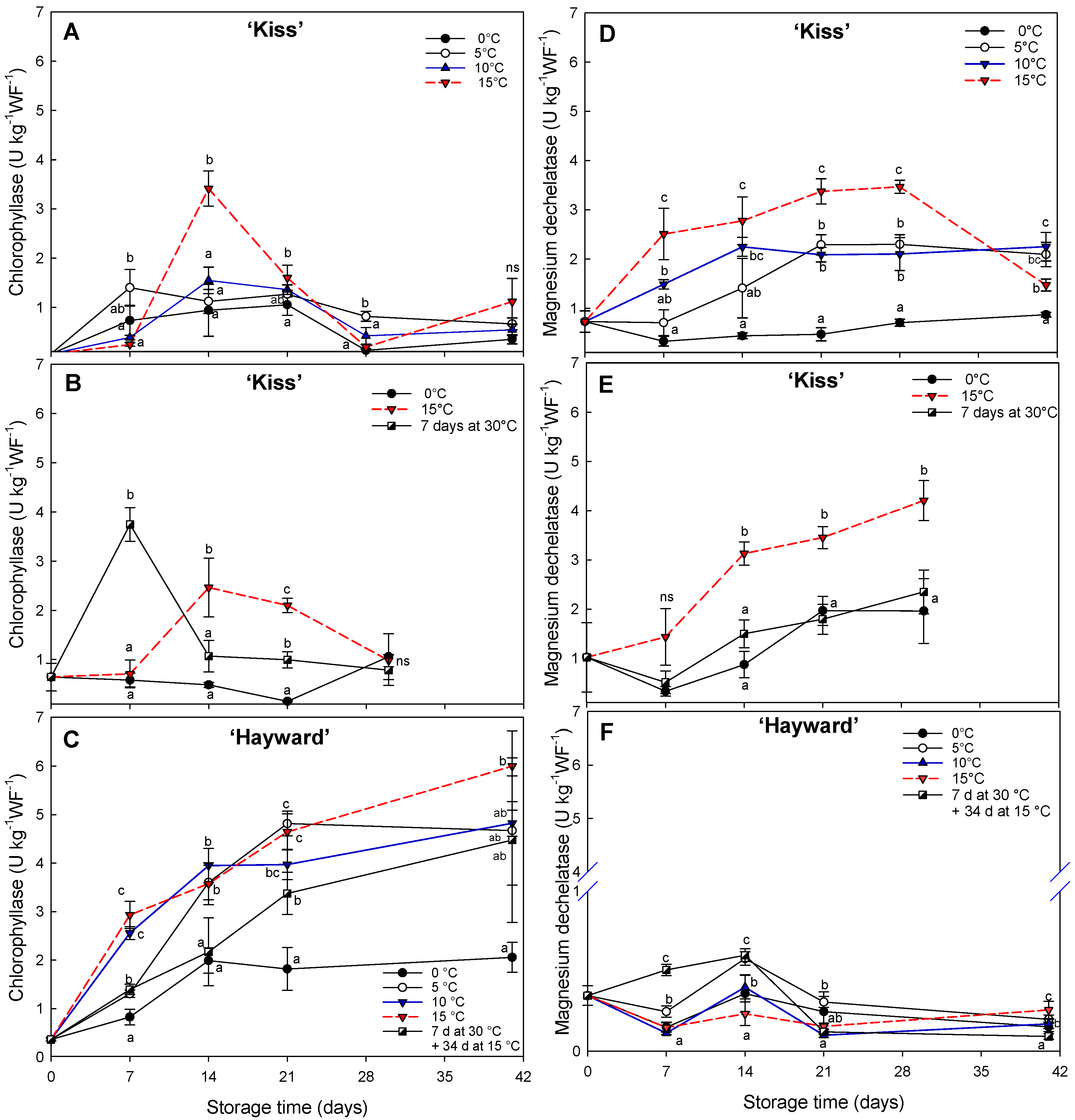
A sample of 2 mL of the crude enzyme extract was separated and 11 mL of extraction buffer 2 was added. The samples were incubated at 40 °C and taken in duplicates of 2 mL each from 0 to 60 min from the start of the reaction. Then, 5 mL of hexane–acetone mixture (7:3) pre-cooled in ice water was added. It was stirred vigorously to generate an emulsion, achieving an instantaneous separation of two phases. The samples were kept in the dark for 15 min at 4 °C and finally centrifuged at 6000× g for 5 min at 4 °C. The upper phase contains chlorophyll and the lower phase chlorophyllide (an intermediate compound generated from the degradation of chlorophyll). Monitoring of the enzyme activity was carried out in the lower phase and expressed as one enzyme unit per kilogram of fresh weight (U kg−1WF−1). One enzyme unit (U) of chlorophyllase activity was defined as an increase in optical density (ΔOD) at 663 nm for the conversion of one micromole of substrate per minute.
2.5.2. Magnesium Dechelatase Activity
The following reaction mixture was prepared: 1800 μL Tris-Tricine buffer pH 8.8, 300 μL chlorophyllin extract and 900 μL crude enzyme extract. The mixture was incubated at 37 °C for 60 min, and samples were taken in duplicate of 2 mL each from 0 to 60 min after the onset of the reaction. Similarly, the activity of magnesium dechelatase enzymes was expressed as U kg−1. One enzyme unit (U) of magnesium dechelatase activity was defined as an increase in optical density (ΔOD) at 686 nm for the conversion of one micromole of substrate per minute. Due to the nature of the methodologies described (pigments and enzyme activity), the samples were kept at low temperatures throughout (4–8 °C).
2.6. Statistical Analyses
A completely randomized experimental design was used with four repetitions. The experimental unit considered five fruit each as a repetition. One-way analysis of variance was performed, and mean comparison analysis was carried out using the Fisher method of least significant differences (LSD) with a significance level of p ≤ 0.05 using the StatGraphics Centurion XVII software v. 17 2018 (Statpoint Technologies Inc., VA, USA). and curves were fitted using SigmaPlot v 15.0 2022 (Grafiti LLC., Waverley, BC, USA).
4. Discussion
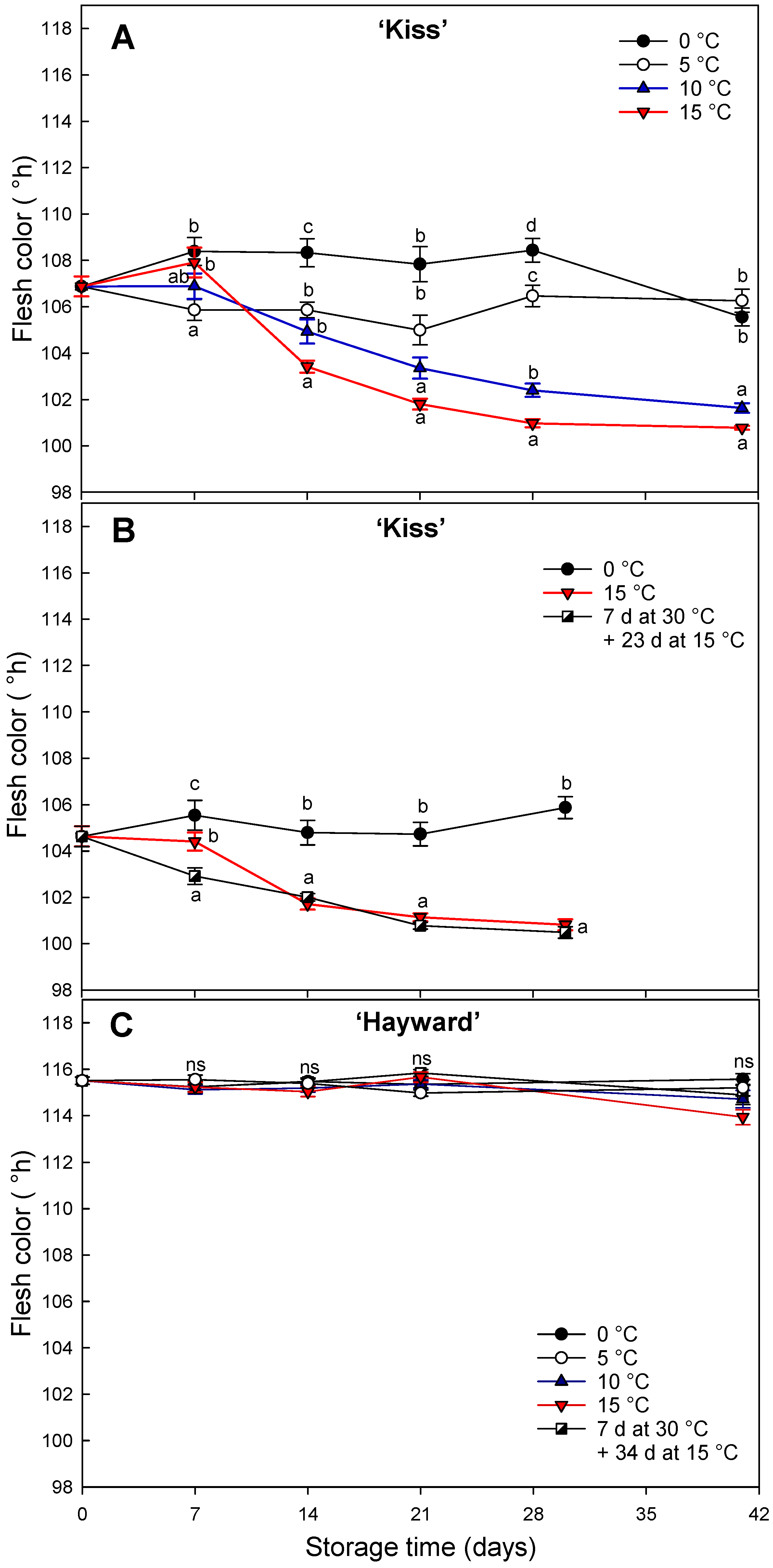
The kiwifruit cultivars ‘Kiss’ and ‘Hayward’ showed different degreening behaviors when exposed to 0, 5, 10 or 15 °C and a short exposure of seven days at 30 °C. Degreening of ‘Kiss’ kiwifruit was greater and faster at higher temperatures. The decrease in hue angle was sigmoidal, being most rapid in the first two weeks, a finding similar to that of Gambi et al. [
14] in cv. ’Zesy003’, which degreened almost completely after 30 d at 10 °C or 15 °C. The slightly later harvest resulted in a shortening of the sigmoidal pattern of hue angle decline, while the slightly earlier harvest accentuated it [
13]. This suggests a need to determine the optimal harvest time, to achieve complete degreening off-vine as discussed for the yellow-fleshed cv. ’Dori’ [
15]. Unlike other fruit types, fruit firmness in these two kiwifruit cultivars was not positively related to increases in storage temperature. This behavior has been noted previously [
33], suggesting that softening is regulated by low temperatures, independently of ethylene and where some chilling injury occurs at 0 °C, with fruit degreened at 15 °C softening more slowly than fruit degreened at 5 or 10 °C. Similar results have been reported for the kiwifruit cvs. ‘Hayward’ and ’Zesh004’, where fruit held at 16 °C softened less than fruit held at temperatures lower than 10 °C [
34].
The high-temperature degreening responses studied here (7 d at 30 °C) have previously been reported in other species, including in bananas and lemons that require degreening of the peel. In bananas, the use of high temperatures (above 25 °C) for more than 48 h is associated with inhibition of chlorophyll breakdown, resulting in a retention of their green color that persists even when fruit temperature is subsequently dropped to 20 °C [
18,
19,
27]. Similarly, with lemons, temperatures above 25 °C inhibit chlorophyll breakdown resulting in incomplete degreening [
35]. In our study, high temperatures (7 d at 30 °C) did not affect negatively the degreening of the flesh but instead favored an initial rapid decline in the hue angle compared to the high values maintained when the fruit was stored continuously at 0 °C. However, this rapid decrease in hue angle was not persistent, resulting in a similar result as when stored continuously at 15 °C. This is probably because it was not supported by the production of internal ethylene [
33,
36].
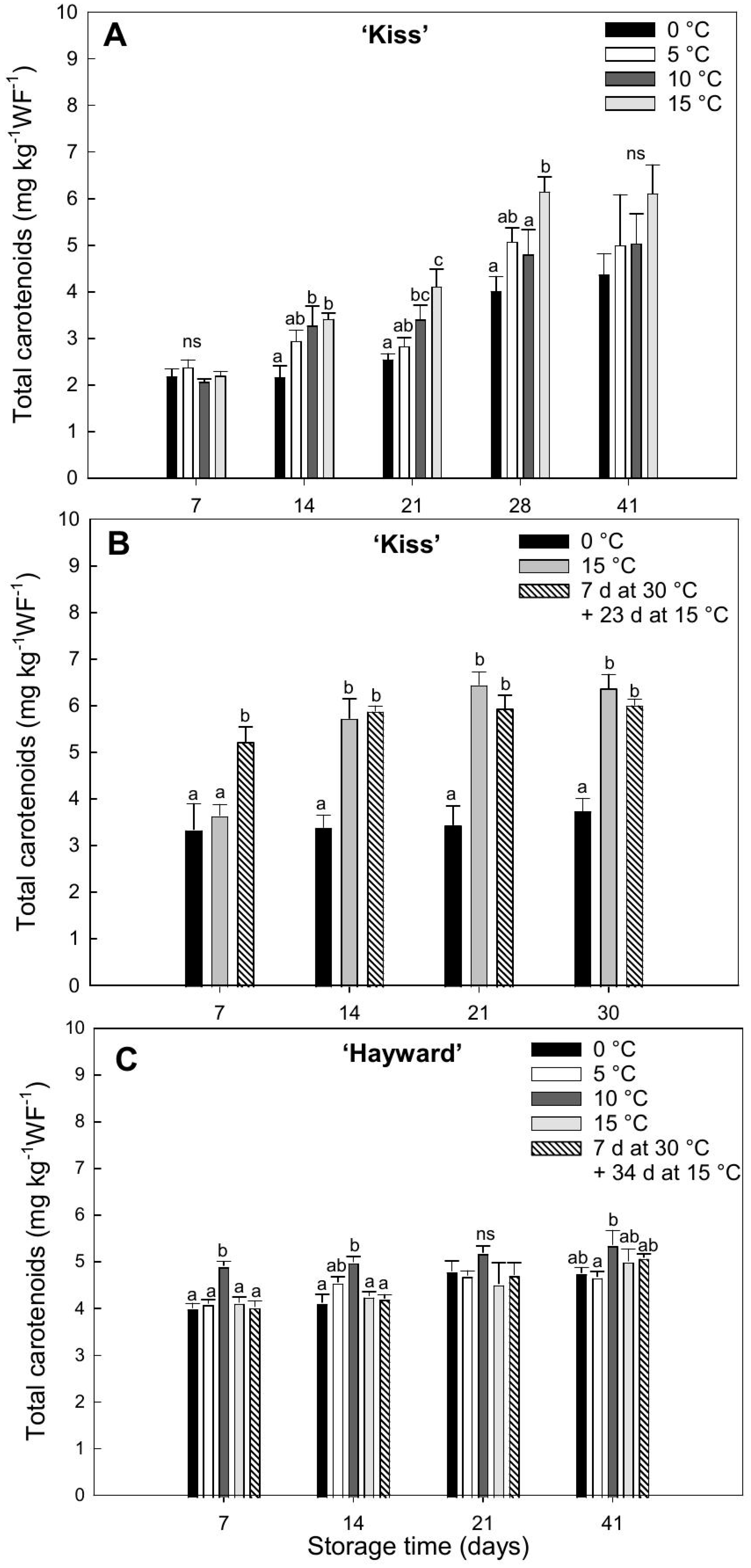
Most cultivars of kiwifruit have green flesh early in development. In
A. deliciosa, the green color persists to maturity. However, in
A. chinensis, the chlorophylls are degraded to reveal the underlying carotenoids and/or anthocyanins [
9,
37,
38]. The total carotenoid concentration in ‘Kiss’ was higher when fruit were degreened at higher temperature, with an increase during the first weeks of storage. According to Xia et al. [
29], in the yellow-fleshed cultivars ‘Jinshi 1’ and ‘Jinyan’, an increase in carotenoid concentration was detected after storage for 32 d at 20 °C with a rise in the expressions of genes associated with carotenoid metabolism, upregulating of the synthetic gene
PSY (Phytoene synthase) and downregulating of the genes
CCD1 (carotenoid cleavage dioxygenase) and
NCED1 (9-cisepoxycarotenoid dioxygenase). Increases in total carotenoids have also been reported in other species, such as in satsuma mandarins (
Citrus unshiu Marc.), which show increases after prolonged exposure to 20 °C under ethylene-free conditions [
39]. This suggests that the yellow flesh color in kiwifruit, in addition to being related to chlorophyll degradation under high temperature conditions, is also linked to increases in the synthesis of carotenoids [
29].
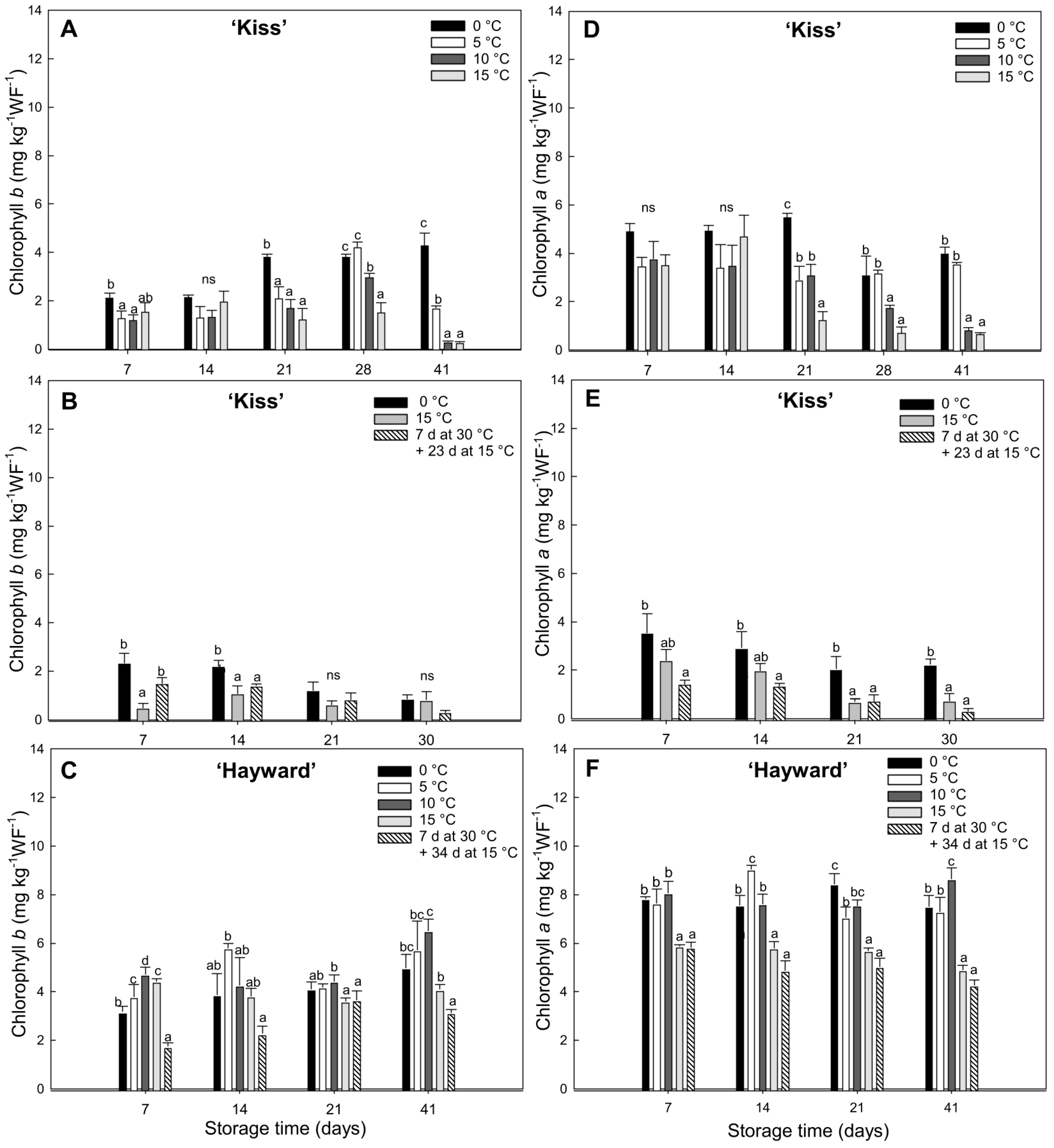
The degreening of ‘Kiss’ kiwifruit at high temperatures enhanced carotenoid synthesis and this was accompanied by decreases in chlorophyll. High temperature treatments at 15 °C (either continuously or with an initial 7 d at 30 °C) achieved the fastest decreases in chlorophyll, indicating an activation of the chlorophyll degradation pathway at these temperatures. At lower temperatures (0 and 5 °C), the high chlorophyll levels persisted until the end of the storage period. There are also reports of chlorophyll retention in other species, such as
Arabidopsis thaliana and Broccoli (
Brassica oleracea L. var.
italica) under specific conditions of light and temperature. The reaction of chlorophyll
b to chlorophyll
a is carried out by chlorophyllide reductase enzymes
a and
b; this is an early phase of chlorophyll degradation [
40].
The relationships between the concentrations of different pigments and the activities of the enzymes associated with chlorophyll breakdown provide information on the behavior of flesh degreening. Traditionally, the participation of chlorophyllase enzymes in the initial chlorophyll degradation process was described in the reaction of chlorophyll
a to pheophorbide
a through the generation of chlorophyllide intermediates [
21], in addition to enzymes with a magnesium dechelatase activity, apparently involved in the direct generation of the chlorophyllide intermediates [
21]. The kiwifruit cv. ‘Kiss’ showed a transient increase in chlorophyllase activity at 15 °C at both 14 and 21 d at 15 °C. In contrast, magnesium dechelatase activity remained high during the period at temperatures higher than 0 °C but was diminished at low temperatures (0 °C) or when degreening at 15 °C was preceded by seven days at 30 °C. Interestingly, during the initial period of 7 d at 30 °C, a storage condition that stimulates the degreening process of the yellow-fleshed kiwifruits, the change was associated mainly with chlorophyllase activity in conjunction with a low activity of magnesium dechelatase. This indicates that there is a differential activity of enzymes related to chlorophyll degradation according to exposure to different temperatures. For example, in tropical fruit, such as bananas (
Musa acuminata group AAA) and plantains (
Musa acuminata group ABB), the activity of enzymes associated with the degreening of the skin differ at different temperature ranges. In the case of bananas, the full peel degreening (hue less than 95°) was achieved with temperatures between 15 and 20 °C and characterized by low chlorophyll content and accompanied by greater activity of magnesium dechelatase enzymes and chlorophyllase.
The opposite occurs at 30 °C, where the green color persists and the concentration of chlorophylls and chlorophyllase activity are high but magnesium dechelatase remains low [
27]. In plantains, the situation is reversed, presenting greater chlorophyllase activity at 20 °C and high magnesium dechelatase activity at 30 °C with full degreening [
27]. This suggests that both enzymes work together to stimulate chlorophyll degradation, with chlorophyllase playing a more prominent role during this particular stage. In ‘Hayward’, chlorophyllase activity was very high, in contrast with the remarkably low activity of magnesium dechelatase enzyme, despite maintaining the green color with high levels of chlorophylls present, with a high chlorophyll
a/
b ratio. Similar results have been observed by Ghasemnezhad et al. [
41] in cv. ‘Hayward’ and cv. ’Tomua’, both green-fleshed kiwifruits.
Previous senescence studies in
A. thaliana suggest that the chlorophyllase enzymes are not the only ones involved in the initial degradation of the chlorophyll
a molecule [
42,
43], postulating the existence of enzymes with magnesium dechelatase activity. The expression of
SGR (
STAY GREEN) genes has been described in regulating enzymes with magnesium dechelatase activity. Gamby et al. [
14] demonstrated that
SGR gene expression was high in yellow-fleshed kiwifruit degreened at storage temperatures higher than conventional, and specifically with high expression at 15 °C and low expression in green-fleshed kiwifruit. Similarly, in our work at enzyme level, the comparison with cv. ‘Hayward’, which retains its green flesh, with high concentrations of chlorophylls, high chlorophyllase activity and practically no magnesium dechelatase activity, suggests a central role for magnesium dechelatase enzymes in the degreening of the yellow-fleshed kiwifruit cultivars.
5. Conclusions
Postharvest degreening at 15 °C of fruit harvested 14 or 21 d before the usual commercial harvest date improves the development of flesh color in ‘Kiss’ kiwifruit, a process dependent on exposure time and temperature. This is different in ‘Hayward’, where it is not possible to induce the same process. This suggests that the use of storage temperatures above 0 °C in yellow-fleshed kiwifruit offers the potential for enhanced degreening, with the possibility of complete degreening at continuous temperatures of 15 °C. The application of high temperatures (30 °C) over a brief period of time did not impede the flesh degreening process in ‘Kiss’ kiwifruit. Nevertheless, the decline in the hue angle was faster, with the activity of the chlorophyllase enzyme prevailing over that of the enzyme magnesium dechelatase (inhibited) for 7 d at 30 °C. The findings substantiate the pivotal role of temperature in the degreening process.
According to the enzyme study at different temperature treatments, in the yellow-fleshed kiwifruit, an increase in degreening temperature is associated with a higher activity of magnesium dechelatase, indicating its involvement in the breakdown of chlorophyll, mainly at 15 °C. In contrast, the chlorophyllase enzymes showed increased activities associated with exposure to very high temperatures (30 °C), thus triggering a much faster flesh degreening, and associated with a drop in firmness. The coexistence of a high chlorophyll concentration, high chlorophyllase activity and a low magnesium dechelatase activity observed in ‘Hayward’ in response to high storage temperatures supports the hypothesis that chlorophyllase enzymes are not the only enzymes involved in the process of chlorophyll breakdown and the development of the yellow flesh in the Actinidia chinensis cultivars.
Elucidating the enzymatic processes involved in chlorophyll degradation will improve our understanding of differences in degreening both on and off the vine among the different cultivars. This knowledge will contribute to the development of more effective degreening strategies to achieve the desired flesh color in a wide range of yellow-fleshed kiwifruit cultivars.