Evaluation of Bioactive Compounds and Antioxidant Activity of Green and Red Kale (Brassica oleracea L. var. acephala) Microgreens Grown Under White, Red, and Blue LED Combinations
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Standards and Reagents
2.3. Extraction and Analysis of Photosynthetic Pigments
2.4. Extraction of Phenolic Compounds
2.5. Determination of Composition of Phenolic Compounds
2.6. Determination of Dietary Fiber Components
2.7. Determination of Antioxidant Activity
2.8. Statistical Analysis
3. Results
3.1. Chlorophyll (a, b, and Total) and Carotenoids
3.2. Total Phenolics, Flavonoids, and Anthocyanins
3.3. Profiles of Phenolic Compounds
3.4. Antioxidant Activity
3.5. Dietary Fiber
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dereje, B.; Jacquier, J.C.; Elliott-Kingston, C.; Harty, M.; Harbourne, N. Brassicaceae microgreens: Phytochemical compositions, influences of growing practices, postharvest technology, health, and food applications. ACS Food Sci. Technol. 2023, 3, 981–998. [Google Scholar] [CrossRef]
- Singh, A.; Singh, J.; Kaur, S.; Gunjal, M.; Kaur, J.; Nanda, V.; Ullah, R.; Ercisli, S.; Rasane, P. Emergence of microgreens as a valuable food, current understanding of their market and consumer perception: A review. Food Chem. X 2024, 23, 101527. [Google Scholar]
- Amil, M.A.; Saad, H.M.; Tan, C.H.; Sim, K.S. A Review of microgreens in Southeast Asia: Sustainable agriculture, phytochemicals, and biological activities. Int. J. Biotechnol. Biomed. 2024, 1, 19–71. [Google Scholar] [CrossRef]
- Bhaswant, M.; Shanmugam, D.K.; Miyazawa, T.; Abe, C.; Miyazawa, T. Microgreens—A comprehensive review of bioactive molecules and health benefits. Molecules 2023, 28, 867. [Google Scholar] [CrossRef] [PubMed]
- Tallei, T.E.; Kepel, B.J.; Wungouw, H.I.S.; Nurkolis, F.; Adam, A.A.; Fatimawali. A comprehensive review on the antioxidant activities and health benefits of microgreens: Current insights and future perspectives. Int. J. Food Sci. Technol. 2024, 59, 58–71. [Google Scholar] [CrossRef]
- Ebert, A.W. Sprouts and microgreens—Novel food sources for healthy diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef]
- Di Bella, M.C.; Toscano, S.; Arena, D.; Moreno, D.A.; Romano, D.; Branca, F. Effects of growing cycle and genotype on the morphometric properties and glucosinolates amount and profile of sprouts, microgreens and baby leaves of broccoli (Brassica oleracea L. var. italica Plenck) and Kale (B. oleracea L. var. acephala DC.). Agronomy 2021, 11, 1685. [Google Scholar] [CrossRef]
- Alloggia, F.P.; Bafumo, R.F.; Ramirez, D.A.; Maza, M.A.; Camargo, A.B. Brassicaceae microgreens: A novel and promissory source of sustainable bioactive compounds. Curr. Res. Food Sci. 2023, 6, 100480. [Google Scholar] [CrossRef]
- de la Fuente, B.; López-García, G.; Máñez, V.; Alegría, A.; Barberá, R.; Cilla, A. Antiproliferative effect of bioaccessible fractions of four Brassicaceae microgreens on human colon cancer cells linked to their phytochemical composition. Antioxidants 2020, 9, 368. [Google Scholar] [CrossRef]
- Lee, S.; Park, C.H.; Kim, J.K.; Ahn, K.; Kwon, H.; Kim, J.K.; Park, S.U.; Yeo, H.J. LED lights influenced phytochemical contents and biological activities in kale (Brassica oleracea L. var. acephala) microgreens. Antioxidants 2023, 12, 1686. [Google Scholar] [CrossRef]
- Vaštakaitė-Kairienė, V.; Jurkonienė, S.; Rasiukevičiūtė, N.; Karklelienė, R.; Samuolienė, G. The influence of pre-harvest leds on phytochemical constituents and antioxidant activity of microgreens during short-term storage. Agronomy 2023, 13, 2188. [Google Scholar] [CrossRef]
- Wojciechowska, R.; Dąbrowa, A.; Kołton, A. How monochromatic and composed light affect the kale ‘Scarlet’ in its initial growth stage. Acta Sci. Pol. Hortorum Cultus 2023, 22, 93–100. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Sprouts vs. microgreens as novel functional foods: Variation of nutritional and phytochemical profiles and their in vitro bioactive properties. Molecules 2020, 25, 4648. [Google Scholar] [CrossRef]
- Artés-Hernández, F.; Castillejo, N.; Martínez-Zamora, L. UV and Visible Spectrum LED lighting as abiotic elicitors of bioactive compounds in sprouts, microgreens, and baby leaves—A comprehensive review including their mode of action. Foods 2022, 11, 265. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.; Marcone, M.F.; Tsao, R. Current review of the modulatory effects of LED lights on photosynthesis of secondary metabolites and future perspectives of microgreen vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Yuan, X.; Chen, X.; Lu, C. A review on the effects of light-emitting diode (LED) light on the nutrients of sprouts and microgreens. Trends Food Sci. Technol. 2020, 99, 203–216. [Google Scholar] [CrossRef]
- Frąszczak, B.; Kula-Maximenko, M.; Podsędek, A.; Sosnowska, D.; Unegbu, K.C.; Spiżewski, T. Morphological and photosynthetic parameters of green and red kale microgreens cultivated under different light spectra. Plants 2023, 12, 3800. [Google Scholar] [CrossRef] [PubMed]
- Frąszczak, B.; Kula-Maximenko, M. The biometric parameters of microgreen crops grown under various light conditions. Agriculture 2022, 12, 576. [Google Scholar] [CrossRef]
- Tantharapornrerk, N.; Vichitsoonthonkul, T.; Techavuthiporn, C.; Photchanachai, S. Growth and antioxidant system of Chinese kale microgreens in response to different illumination of light sources. N. Z. J. Crop Hortic. Sci. 2023, 51, 108–122. [Google Scholar] [CrossRef]
- Hooks, T.; Sun, L.; Kong, Y.; Masabni, J.; Niu, G. Adding UVA and far-red light to white LED affects growth, morphology, and phytochemicals of indoor-grown microgreens. Sustainability 2022, 14, 8552. [Google Scholar] [CrossRef]
- Ying, Q.; Jones-Baumgardt, C.; Zheng, Y.; Bozzo, G. The proportion of blue light from light-emitting diodes (RB light environment) alters microgreen phytochemical profiles in a species-specific manner. HortScience 2021, 56, 13–20. [Google Scholar] [CrossRef]
- Kamal, K.Y.; Khodaeiaminjan, M.; El-Tantawy, A.A.; Moneim, D.A.; Salam, A.A.; Ash-Shormillesy, S.M.A.I.; Attia, A.; Ali, M.A.S.; Herranz, R.; El-Esawi, M.A.; et al. Evaluation of growth and nutritional value of Brassica microgreens grown under red, blue and green LEDs combinations. Physiol. Plant. 2020, 169, 625–638. [Google Scholar] [CrossRef] [PubMed]
- Kopsell, D.A.; Sams, C.E.; Morrow, R.C. Interaction of light quality and fertility on biomass, shoot pigmentation and xanthophyll cycle flux in Chinese kale. J. Sci. Food Agric. 2017, 97, 911–917. [Google Scholar] [CrossRef]
- Majdoub, N.; El-Guendouz, S.; Rezgui, M.; Carlier, J.; Costa, C.; Kaab, L.B.B.; Miguel, M.G. Growth, photosynthetic pigments, phenolic content and biological activities of Foeniculum vulgare Mill., Anethum graveolens L. and Pimpinella anisum L. (Apiaceae) in response to zinc. Ind. Crops Prod. 2017, 109, 627–636. [Google Scholar] [CrossRef]
- Sosnowska, D.; Kajszczak, D.; Podsędek, A. The effect of different growth stages of black chokeberry fruits on phytonutrients, anti-lipase activity, and antioxidant capacity. Molecules 2022, 27, 8031. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, S.; Tachibana, Y.; Okada, Y. Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha). Food Chem. 2005, 89, 569–575. [Google Scholar] [CrossRef]
- Podsędek, A.; Frąszczak, B.; Sosnowska, D.; Kajszczak, D.; Szymczak, K.; Bonikowski, R. LED light quality affected bioactive compounds, antioxidant potential, and nutritional value of red and white cabbage microgreens. Appl. Sci. 2023, 13, 5435. [Google Scholar] [CrossRef]
- Gouw, V.P.; Jung, J.; Zhao, Y. Functional properties, bioactive compounds, and in vitro gastrointestinal digestion study of dried fruit pomace powders as functional food ingredients. LWT—Food Sci. Technol. 2017, 80, 136–144. [Google Scholar] [CrossRef]
- Polka, D.; Podsędek, A.; Koziołkiewicz, M. Comparison of chemical composition and antioxidant capacity of fruit, flower and bark of Viburnum opulus. Plant Foods Hum. Nutr. 2019, 74, 436–442. [Google Scholar]
- Kim, D.O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Identification of the phenolic components of collard greens, kale, and Chinese broccoli. J. Agric. Food Chem. 2009, 57, 7401–7408. [Google Scholar] [CrossRef]
- Olsen, H.; Aaby, K.; Borge, G.I.A. Characterization and quantification of flavonoids and hydroxycinnamic acids in curly kale (Brassica oleracea L. convar. acephala var. sabellica) by HPLC-DAD-ESI-MS. J. Agric. Food Chem. 2009, 57, 2816–2825. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Shi, J.; Wan, J.; Pham, Q.; Zhang, Z.; Sun, J.; Yu, L.; Wang, T.T.Y.; Chen, P. Profiling of polyphenols and glucosinolates in kale and broccoli microgreens grown under chamber and windowsill conditions by Ultrahigh-Performance Liquid Chromatography High-Resolution Mass Spectrometry. ACS Food Sci. Technol. 2022, 2, 101–113. [Google Scholar] [CrossRef]
- Bujor, O.C.; Le Bourvellec, C.; Volf, I.; Popa, V.I.; Dufour, C. Seasonal variations of the phenolic constituents in bilberry (Vaccinium myrtillus L.) leaves, stems and fruits, and their antioxidant activity. Food Chem. 2016, 213, 58–68. [Google Scholar] [PubMed]
- Zhu, P.; Tian, Z.; Pan, Z.; Feng, X. Identification and quantification of anthocyanins in different coloured cultivars of ornamental kale (Brassica oleracea L. var. acephala DC). J. Hortic. Sci. Biotechnol. 2018, 93, 466–473. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef]
- Brazaityte, A.; Miliauskiene, J.; Vaštakaite-Kairiene, V.; Sutuliene, R.; Laužike, K.; Duchovskis, P.; Małek, S. Effect of different ratios of blue and red led light on Brassicaceae microgreens under a controlled environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Sams, C.E.; Metallo, R.M.; Waterland, N.L.; Kopsell, D.E. Biomass, carbohydrates, pigments, and mineral elements in kale (Brassica oleracea var acephala) microgreens respond to LED blue-light wavelength. Sci. Hortic. 2024, 328, 112929. [Google Scholar] [CrossRef]
- Frede, K.; Winkelmann, S.; Busse, L.; Baldermann, S. The effect of LED light quality on the carotenoid metabolism and related gene expression in the genus Brassica. BMC Plant Biol. 2023, 23, 328. [Google Scholar] [CrossRef]
- Maru, R.N.; Wesonga, J.; Okazawa, H.; Kavoo, A.; Neondo, J.O.; Mazibuko, D.M.; Maskey, S.; Orsini, F. Evaluation of growth, yield and bioactive compounds of Ethiopian kale (Brassica carinata A. Braun) microgreens under different LED light spectra and substrates. Horticulturae 2024, 10, 436. [Google Scholar] [CrossRef]
- Flores, M.; Hernández-Adasme, C.; Guevara, M.J.; Escalona, V.H. Effect of different light intensities on agronomic characteristics and antioxidant compounds of Brassicaceae microgreens in a vertical farm system. Front. Sustain. Food Syst. 2024, 8, 1349423. [Google Scholar] [CrossRef]
- Toscano, S.; Cavallaro, V.; Ferrante, A.; Romano, D.; Patané, C. Effects of different light spectra on final biomass production and nutritional quality of two microgreens. Plants 2021, 10, 1584. [Google Scholar] [CrossRef] [PubMed]
- Marchiosi, R.; dos Santos, W.D.; Constantin, R.P.; de Lima, R.B.; Soares, A.R.; Finger-Teixeira, A.; Mota, T.R.; de Oliveira, D.M.; de Paiva Foletto-Felipe, M.; Abrahao, J.; et al. Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev. 2020, 19, 865–906. [Google Scholar]
- Guo, N.; Han, S.; Zong, M.; Wang, G.; Zheng, S.; Liu, F. Identification and differential expression analysis of anthocyanin biosynthetic genes in leaf color variants of ornamental kale. BMC Genom. 2019, 20, 564. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, H.; Huang, X.; Xia, S.; Chen, C.; Nie, Q.; Nie, S. Efficient enrichment of total flavonoids from kale (Brassica oleracea L. var. acephala L.) extracts by NKA-9 resin and antioxidant activities of flavonoids extract in vitro. Food Chem. 2022, 374, 131508. [Google Scholar] [CrossRef]
- Yang, I.; Jayaprakasha, G.K.; Patil, B. In vitro digestion with bile acids enhances the bioaccessibility of kale polyphenols. Food Funct. 2018, 9, 1235–1244. [Google Scholar] [CrossRef]
- Maciejak, A.; Kosmala, M. Kale (Brassica oleracea L. var. acephala) as a source of dietary fibre. Biotechnol. Food Sci. 2019, 83, 135–141. [Google Scholar]
- Kowalska, H.; Kowalska, J.; Ignaczak, A.; Masiarz, E.; Domian, E.; Galus, S.; Ciurzyńska, A.; Salamon, A.; Zając, A.; Marzec, A. Development of a high-fibre multigrain bar technology with the addition of curly kale. Molecules 2021, 26, 3939. [Google Scholar] [CrossRef]
- Ghoora, M.D.; Babu, D.R.; Srividya, N. Nutrient composition, oxalate content and nutritional ranking of ten culinary microgreens. J. Food Compost. Anal. 2020, 91, 103495. [Google Scholar] [CrossRef]
- Waddell, I.S.; Orfila, C. Dietary fiber in the prevention of obesity and obesity-related chronic diseases: From epidemiological evidence to potential molecular mechanisms. Crit. Rev. Food Sci. Nutr. 2023, 63, 8752–8767. [Google Scholar] [CrossRef]
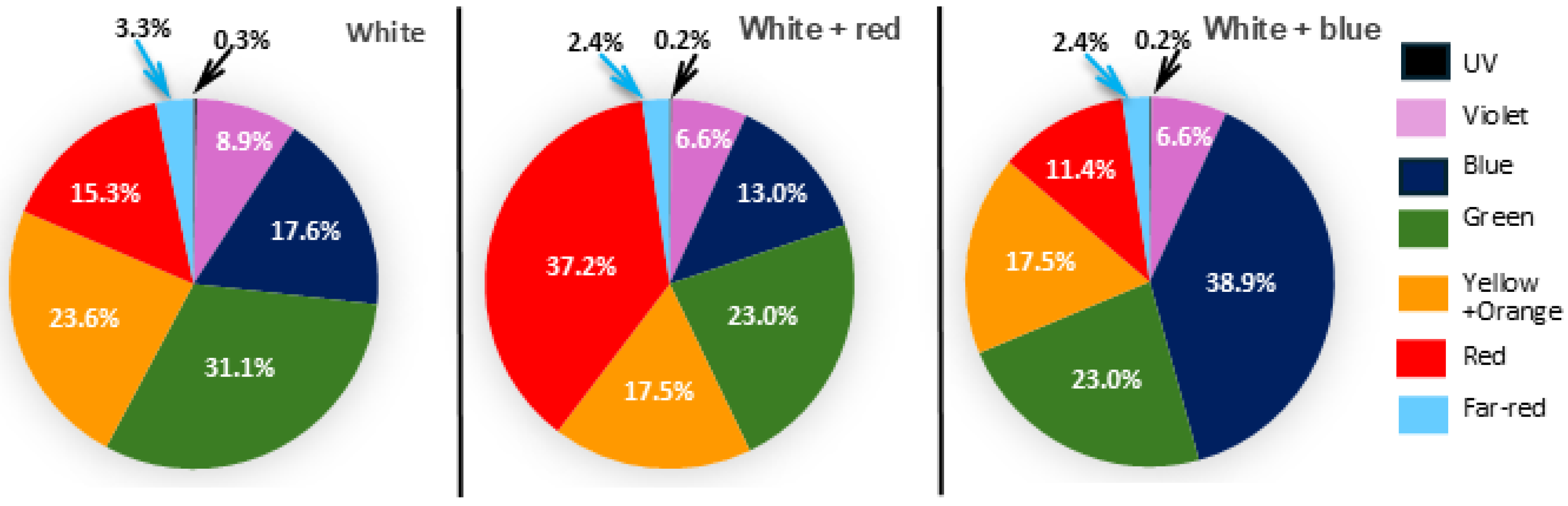
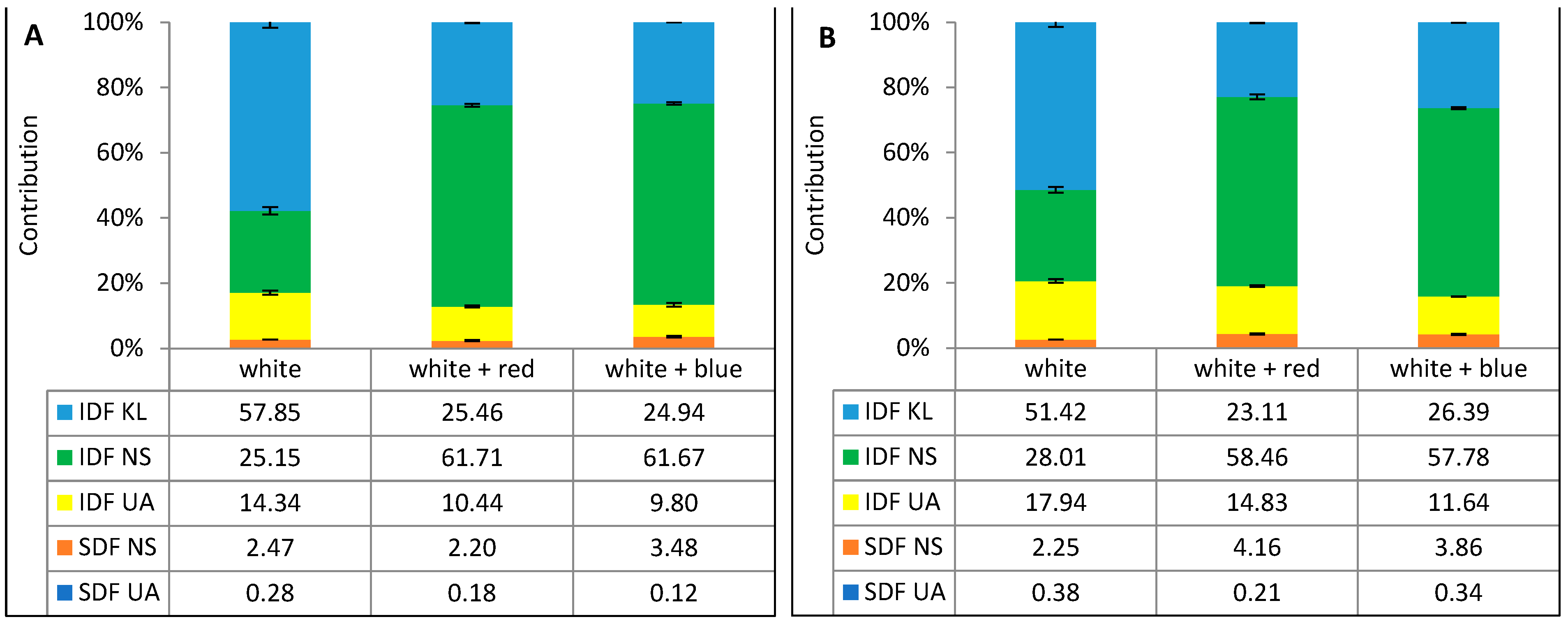
| Factor | Green Kale Microgreens | Red Kale Microgreens | ||||
|---|---|---|---|---|---|---|
| White | White + Red | White + Blue | White | White + Red | White + Blue | |
| Total carotenoids | 0.58 ± 0.03 b | 0.32 ± 0.02 a | 0.29 ± 0.02 a | 0.55 ± 0.04 b | 0.23 ± 0.06 a | 0.21 ± 0.03 a |
| Total chlorophylls | 8.57 ± 0.36 b | 3.67 ± 0.08 a | 3.42 ± 0.09 a | 6.63 ± 0.09 c | 3.61 ± 0.08 a | 4.56 ± 0.13 b |
| Chlorophyll a | 2.78 ± 0.19 b | 1.13 ± 0.04 a | 1.17 ± 0.07 a | 2.14 ± 0.08 c | 1.10 ± 0.09 a | 1.51 ± 0.06 b |
| Chlorophyll b | 3.39 ± 0.05 c | 1.49 ± 0.02 b | 1.31 ± 0.01 a | 2.63 ± 0.02 c | 1.47 ± 0.04 a | 1.77 ± 0.03 b |
| Microgreens | Light Treatments | Total Phenolics | Total Flavonoids | Total Anthocyanins |
|---|---|---|---|---|
| White | 8.07 ± 0.52 a | 2.34 ± 0.20 a | 0 | |
| Green kale | White + red | 10.66 ± 0.47 b | 3.83 ± 0.21 b | 0 |
| White + blue | 11.03 ± 0.72 b | 5.62 ± 0.34 c | 0 | |
| White | 6.45 ± 0.29 a | 1.95 ± 0.17 a | 0.86 ± 0.03 a | |
| Red kale | White + red | 12.36 ± 0.74 b | 3.74 ± 0.23 b | 3.17 ± 0.20 b |
| White + blue | 12.67 ± 0.64 b | 4.01 ± 0.12 c | 3.94 ± 0.10 c |
| tR (min) | [MS-H]− [MS+H]+ (m/z) | MS/MS (m/z) | Tentative Identification | Light Treatments | ||
|---|---|---|---|---|---|---|
| White | White + red | White + blue | ||||
| 3.60 | 353 | 134/191 | Chlorogenic acid | 0.14 ± 0.00 | 0.15 ± 0.01 | 0.16 ± 0.01 |
| 4.01 | 337 | 119/160/191 | p-Coumaroylquinic acid [31] | 0.04 ± 0.01 | 0.04 ± 0.00 | - |
| 4.19 | 353 | 134/191/128/103 | Caffeoylquinic acid [32] | 0.02 ± 0.01 | - | - |
| 4.20 | 367 | 191/134 | Feruloylquinic acid [31] | 0.03 ± 0.00 | - | - |
| 4.26 | 355 | 193/132/161 | Feruloyl-glucoside [33] | 0.02 ± 0.00 | - | |
| 4.45 | 385 | 175/147/119/190 | Sinapoyl-glucoside [33] | 0.06 ± 0.00 | 0.10 ± 0.01 | 0.08 ± 0.01 |
| 7.48 | 753 | 223/205/190/164/149 | Disinapoyl-diglucoside [32] | 0.55 ± 0.01 | 0.45 ± 0.00 | 0.34 ± 0.03 |
| 7.71 | 723 | 179/160/193/134/149 | Sinapoyl- feruloyl-diglucoside [32] | 0.09 ± 0.00 | 0.31 ± 0.01 | 0.24 ± 0.02 |
| 8.85 | 959 | 205/223/190/164/247 | Trisinapoyl-diglucoside [32] | 0.46 ± 0.00 | 0.28 ± 0.00 | - |
| Total phenolic acids | 1.37 ± 0.01 b | 1.35 ± 0.04 b | 0.82 ± 0.06 a | |||
| 3.66 | 787 | 299/271/463/624 | Quercetin 3-diglucoside-7-glucoside [32] | - | 0.03 ± 0.00 | 0.04 ± 0.00 |
| 3.72 | 447 | 198/285/149/242/147 | Kaempferol 3-glucoside | 0.05 ± 0.00 | 0.04 ± 0.00 | 0.02 ± 0.00 |
| 3.77 | 771 | 285/161/255/415 | Kaempferol 3-diglucoside-7-glucoside [32] | - | 0.02 ± 0.00 | - |
| 3.80 | 1111 | 625/949/300/801/179 | Quercetin 3-triglucoside-7-diglucoside [32] | - | - | 0.08 ± 0.01 |
| 3.82 | 949 | 301/625/979 | Quercetin 3-diglucoside-7-diglucoside [32] | - | - | 0.03 ± 0.00 |
| 3.90 | 1125 | 949/625/300/801 | Quercetin 3-feruloyl-diglucoside-7-diglucoside [32] | 0.01 ± 0.00 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| 3.94 | 1317 | 947/771/815/753/300 | Quercetin 3-sinapoyl-diglucoside-7-diglucoside [33] | - | - | 0.05 ± 0.00 |
| 3.99 | 963 | 609/284/191/176 | Kaempferol 3-hydroxyferuloyl-diglucoside -7-glucoside [32] | - | - | 0.03 ± 0.00 |
| 4.00 | 1095 | 609/771/161/284 | Kaempferol 3-triglucoside-7-diglucoside [31] | - | 0.04 ± 0.00 | - |
| 4.03 | 1155 | 625/949/787/1111 | Quercetin 3-sinapoyl-diglucoside-7-diglucoside [32] | - | 0.03 ± 0.00 | 0.06 ± 0.00 |
| 4.04 | 1125 | 609/801/191/284/771 | Kaempferol 3-hydroxyferuloyl-diglucoside -7-diglucoside [32] | - | 0.02 ± 0.00 | 0.04 ± 0.00 |
| 4.08 | 993 | 284/609/785/429 | Kaempferol 3-caffeoyl-diglucoside-7-glucoside [31] | - | 0.06 ± 0.01 | 0.13 ± 0.01 |
| 4.14 | 1139 | 815/609/591/284 | Kaempferol 3-sinapoyl-diglucoside-7-diglucoside [32] | - | 0.04 ± 0.00 | 0.06 ± 0.01 |
| 4.18 | 1109 | 609/770/822/463 | Kaempferol 3-feruloyl-diglucoside-7-diglucoside [32] | - | 0.02 ± 0.00 | 0.02 ± 0.01 |
| 4.22 | 977 | 609/284/446/205 | Kaempferol 3-sinapoyl-diglucoside-7-glucoside [31] | 0.02 ± 0.00 | 0.04 ± 0.01 | |
| 5.18 | 831 | 300/271/255 | Quercetin 3-sinapoyl-diglucoside [32] | - | - | 0.02 ± 0.00 |
| 6.23 | 1361 | 993/1199/787/462/300 | Quercetin 3-disinapoyl-triglucoside-7-glucoside [33] | - | - | 0.05 ± 0.00 |
| Total flavonols | 0.06 ± 0.00 a | 0.34 ± 0.03 b | 0.70 ± 0.06 c | |||
| Sum of phenolic compounds | 1.43 ± 0.01 a | 1.69 ± 0.07 b | 1.52 ± 0.13 ab | |||
| tR (min) | [MS-H]− [MS+H]+ (m/z) | MS/MS (m/z) | Tentative Identification | Light Treatments | ||
|---|---|---|---|---|---|---|
| White | White + red | White + blue | ||||
| 3.60 | 315 | 108 | Dihydroxybenzoic acid-hexoside [34] | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.16 ± 0.00 |
| 3.79 | 353 | 134/191 | Chlorogenic acid | 0.01 ± 0.00 | 0.08 ± 0.00 | 0.20 ± 0.01 |
| 4.56 | 367 | 191/133/161 | Feruloylquinic acid [31] | - | - | 0.02 ± 0.00 |
| 4.70 | 385 | 175/119/190 | Sinapoyl-glucoside [33] | - | 0.01 ± 0.00 | 0.03 ± 0.01 |
| 4.79 | 353 | 128/144/119 | Caffeoylquinic acid [32] | 0.01 ± 0.00 | 0.03 ± 0.00 | - |
| 6.34 | 353 | 130/191 | Caffeoylquinic acid [32] | 0.13 ± 0.01 | - | 0.22 ± 0.02 |
| 7.78 | 753 | 205/190/164/223 | Disinapoyl-diglucoside [32] | - | 0,10 ± 0.01 | 0.19 ± 0.02 |
| 7.99 | 723 | 175/190/160/132 | Sinapoyl- feruloyl-diglucoside [32] | - | 0.12 ± 0.00 | 0.20 ± 0.02 |
| 8.93 | 959 | 205/223/190/164 | Trisinapoyl-diglucoside [32] | - | 0.08 ± 0.01 | 0.07 ± 0.01 |
| 8.95 | 929 | 175/205/223/160 | Disinapoyl-feruloyl-diglucoside [32] | - | 0.03 ± 0.00 | - |
| Total phenolic acids | 0.16 ± 0.01 a | 0.48 ± 0.03 b | 1.09 ± 0.05 c | |||
| 3.82 | 447 | 285/172/255 | Kaempferol 3-glucoside | - | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 3.97 | 1111 | 625/949/300/787 | Quercetin 3-triglucoside-7-diglucoside [32] | - | - | 0.04 ± 0.00 |
| 4.01 | 949 | 301/624/463 | Quercetin 3-diglucoside-7-diglucoside [32] | - | - | 0.02 ± 0.00 |
| 4.07 | 1125 | 609/801/284/191 | Kaempferol 3-hydroxyferuloyl-diglucoside-7-diglucoside [32] | - | 0.01 ± 0.00 | 0.03 ± 0.00 |
| 4.11 | 1095 | 625/607/949/300 | Kaempferol 3-triglucoside-7-diglucoside [31] | - | 0.01 ±0.00 | 0.06 ± 0.00 |
| 4.14 | 1301 | 933/771/1257/285 | Kaempferol 3-sinapoyltriglucoside-7-diglucoside [32] | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 |
| 4.16 | 933 | 284/446/609/339 | Kaempferol 3-diglucoside-7-diglucoside [32] | - | - | 0.05 ± 0.00 |
| 4.22 | 1125 | 755/609/285/591 | Kaempferol 3-hydroxyferuloyl-diglucoside-7-diglucoside [32] | - | - | 0.04 ± 0.00 |
| 4.26 | 993 | 284/446/609/339 | Kaempferol 3-caffeoyldiglucoside-7-glucoside [31] | - | - | 0.02 ± 0.00 |
| 4.34 | 1139 | 609/815/771/285 | Kaempferol 3-sinapoyldiglucoside-7-diglucoside [32] | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.03 ± 0.00 |
| 4.41 | 1109 | 609/447/284/815 | Kaempferol 3-feruloyldiglucoside-7-diglucoside [32] | - | - | 0.03 ± 0.01 |
| 4.45 | 977 | 285/609/446/591 | Kaempferol 3-sinapoyldiglucoside-7-glucoside [31] | - | - | 0.03 ± 0.01 |
| 5.92 | 625 | 301/151/255 | Quercetin 3-glucoside-7-glucoside [32] | - | - | 0.01 ± 0.00 |
| 6.06 | 463 | 271/255/243/284 | Quercetin 3-glucoside | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 |
| Total flavonols | 0.03 ± 0.00 a | 0.06 ± 0.01 b | 0.40 ± 0.01 c | |||
| 6.99 | 1125+ | 287/449/177 | Cy 3-sinapoyl-p-coumaroyldiglucoside-5-glucoside [35] | - | - | 0.01 ± 0.00 |
| 7.17 | 1155+ | 287/449/207 | Cy 3-sinapoyl-feruloyldiglucoside-5-glucoside [35] | 0.01 ± 0.00 | 0.03 ± 0.00 | 0.07 ± 0.00 |
| 7.24 | 1185+ | 287/449/207 | Cy 3-sinapoyl-sinapoyldiglucoside-5-glucoside [35] | 0.03 ± 0.00 | 0.06 ± 0.00 | 0.08 ± 0.01 |
| 7.34 | 1125+ | 287/449/177 | Cy 3-sinapoyl-p-coumaroyldiglucoside-5-glucoside [35] | - | 0.01 ± 0.00 | 0.03 ± 0.00 |
| Total anthocyanins | 0.04 ± 0.00 a | 0.10 ± 0.00 b | 0.19 ± 0.01 c | |||
| Sum of phenolic compounds | 0.23 ± 0.01 a | 0.89 ± 0.04 b | 1.68 ± 0.08 c | |||
| Microgreens | Antioxidant Activity Assay | Light Treatments | ||
|---|---|---|---|---|
| White | White + Red | White + Blue | ||
| Green kale | ABTS | 30.16 ± 0.52 a | 39.29 ± 0.35 b | 50.35 ± 1.22 c |
| DPPH | 24.03 ± 1.74 a | 41.52 ± 1.82 b | 52.17 ± 1.64 c | |
| FRAP | 49.07 ± 1.07 a | 64.85 ± 1.12 b | 66.58 ± 2.21 b | |
| ORAC | 179 ± 7 a | 275 ± 8 b | 285 ± 12 b | |
| SARSA | 793 ± 26 b | 882 ± 11 c | 589 ± 7 a | |
| Red kale | ABTS | 18.57 ± 0.37 a | 55.35 ± 2.55 c | 50.97 ± 1.98 b |
| DPPH | 14.69 ± 0.48 a | 53.48 ± 0.82 b | 66.97 ± 2.12 c | |
| FRAP | 39.94 ± 1.32 a | 80.69 ± 1.34 b | 97.63 ± 0.84 c | |
| ORAC | 104 ± 8 a | 273 ± 10 b | 407 ± 11 c | |
| SARSA | 279 ± 18 a | 457 ± 7 b | 556 ± 19 c | |
| Green Kale Microgreens | Red Kale Microgreens | |||||
|---|---|---|---|---|---|---|
| White | White + Red | White + Blue | White | White + Red | White + Blue | |
| Total fiber | 31.53 ± 0.89 a | 44.90 ± 0.65 b | 45.30 ± 2.03 b | 32.56 ± 0.25 a | 47.04 ± 1.28 b | 45.86 ± 0.19 b |
| SDF total | 0.83 ± 0.00 a | 1.96 ± 0.08 b | 1.90 ± 0.03 b | 0.89 ± 0.04 a | 1.12 ± 0.06 b | 1.65 ± 0.10 c |
| IDF total | 30.70 ± 0.88 a | 43.27 ± 0.23 b | 43.40 ± 2.06 b | 31.67 ± 0.21 a | 45.92 ± 1.34 b | 44.21 ± 0.09 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podsędek, A.; Frąszczak, B.; Kajszczak, D.; Sosnowska, D. Evaluation of Bioactive Compounds and Antioxidant Activity of Green and Red Kale (Brassica oleracea L. var. acephala) Microgreens Grown Under White, Red, and Blue LED Combinations. Agronomy 2024, 14, 2454. https://doi.org/10.3390/agronomy14112454
Podsędek A, Frąszczak B, Kajszczak D, Sosnowska D. Evaluation of Bioactive Compounds and Antioxidant Activity of Green and Red Kale (Brassica oleracea L. var. acephala) Microgreens Grown Under White, Red, and Blue LED Combinations. Agronomy. 2024; 14(11):2454. https://doi.org/10.3390/agronomy14112454
Chicago/Turabian StylePodsędek, Anna, Barbara Frąszczak, Dominika Kajszczak, and Dorota Sosnowska. 2024. "Evaluation of Bioactive Compounds and Antioxidant Activity of Green and Red Kale (Brassica oleracea L. var. acephala) Microgreens Grown Under White, Red, and Blue LED Combinations" Agronomy 14, no. 11: 2454. https://doi.org/10.3390/agronomy14112454
APA StylePodsędek, A., Frąszczak, B., Kajszczak, D., & Sosnowska, D. (2024). Evaluation of Bioactive Compounds and Antioxidant Activity of Green and Red Kale (Brassica oleracea L. var. acephala) Microgreens Grown Under White, Red, and Blue LED Combinations. Agronomy, 14(11), 2454. https://doi.org/10.3390/agronomy14112454






