Blooming and Forage Characteristics of Twelve Native Forbs Subjected to Repeated Defoliation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plot Establishment
2.2. Plant Heights and Plant Densities
2.3. Forage Mass
2.4. Forage Nutrient Composition
2.5. Flowering Characteristics
2.6. Statistical Analysis
3. Results
3.1. Temperature and Precipitation
3.2. Plant Heights and Plant Densities
3.3. Forage Mass
3.4. Forage Nutrient Composition
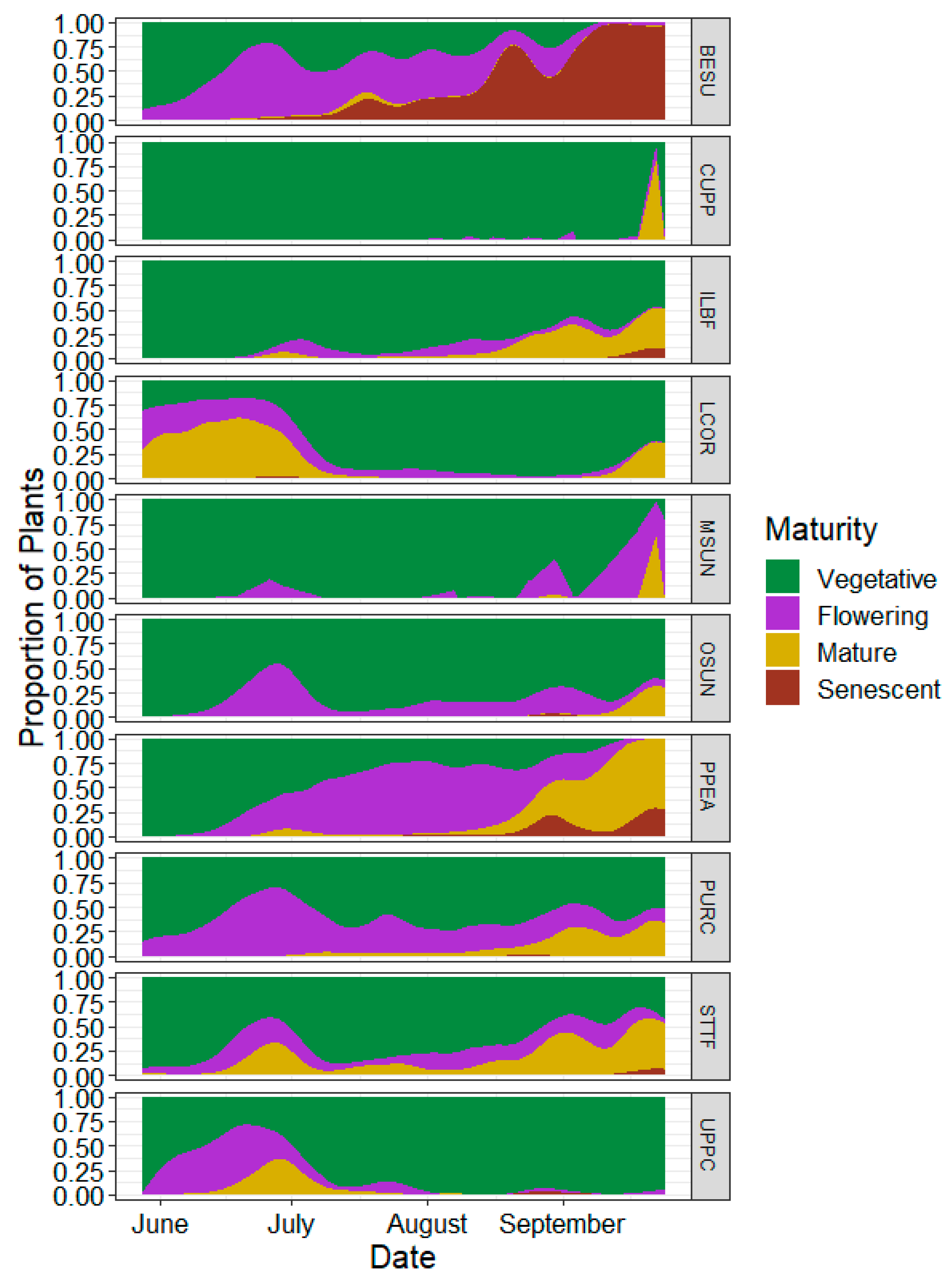
3.5. Flowering Characteristics
4. Discussion
4.1. Plant Persistence
4.2. Forage Characteristics
4.3. Flowering Patterns
4.4. Cost-Effective Forbs
4.5. Limitations and Further Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- The University of Texas at Austin Lady Bird Johnson Wildflower Center. Available online: https://www.wildflower.org/ (accessed on 23 February 2022).
- Harper, C.A. Strategies for Managing Early Succession Habitat for Wildlife. Weed Technol. 2007, 21, 932–937. [Google Scholar] [CrossRef]
- Jones, P.D.; Mixon, M.R.; Demarais, S. Habitat Quality Following Mid-Rotation Treatment in Conservation Reserve Program Pines. J. Wildl. Manag. 2009, 73, 1166–1173. [Google Scholar] [CrossRef]
- Ulappa, A.C.; Shipley, L.A.; Cook, R.C.; Cook, J.G.; Swanson, M.E. Silvicultural Herbicides and Forest Succession Influence Understory Vegetation and Nutritional Ecology of Black-Tailed Deer in Managed Forests. For. Ecol. Manag. 2020, 470–471, 118216. [Google Scholar] [CrossRef]
- Yeiser, J.M.; Baxley, D.L.; Robinson, B.A.; Morgan, J.J. Using Prescribed Fire and Herbicide to Manage Rank Native Warm Season Grass for Northern Bobwhite. J. Wildl. Manag. 2015, 79, 69–76. [Google Scholar] [CrossRef]
- Peters, D.C.; Brooke, J.M.; Tanner, E.P.; Unger, A.M.; Keyser, P.D.; Harper, C.A.; Clark, J.D.; Morgan, J.J. Impact of Experimental Habitat Manipulation on Northern Bobwhite Survival. J. Wildl. Manag. 2015, 79, 605–617. [Google Scholar] [CrossRef]
- Pollentier, C.D.; Lutz, R.S.; Drake, D. Female Wild Turkey Habitat Selection in Mixed Forest-Agricultural Landscapes. J. Wildl. Manag. 2017, 81, 487–497. [Google Scholar] [CrossRef]
- van Engelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony Collapse Disorder: A Descriptive Study. PLoS ONE 2009, 4, e6481. [Google Scholar] [CrossRef]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global Pollinator Declines: Trends, Impacts and Drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Shackelford, G.; Steward, P.R.; Benton, T.G.; Kunin, W.E.; Potts, S.G.; Biesmeijer, J.C.; Sait, S.M. Comparison of Pollinators and Natural Enemies: A Meta-Analysis of Landscape and Local Effects on Abundance and Richness in Crops. Biol. Rev. 2013, 88, 1002–1021. [Google Scholar] [CrossRef]
- Weiner, C.N.; Werner, M.; Linsenmair, K.E.; Blüthgen, N. Land-Use Impacts on Plant-Pollinator Networks: Interaction Strength and Specialization Predict Pollinator Declines. Ecology 2014, 95, 466–474. [Google Scholar] [CrossRef]
- Grab, H.; Branstetter, M.G.; Amon, N.; Urban-Mead, K.R.; Park, M.G.; Gibbs, J.; Blitzer, E.J.; Poveda, K.; Loeb, G.; Danforth, B.N. Agriculturally Dominated Landscapes Reduce Bee Phylogenetic Diversity and Pollination Services. Science (1979) 2019, 363, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Keyser, P.D.; Buehler, D.A.; Fike, J.H.; Finke, D.L.; Fuhlendorf, S.D.; Martin, J.A.; Naumann, H.D.; Smith, S.R. The Birds and the Bees: Producing Beef and Conservation Benefits on Working Grasslands. Agronomy 2022, 12, 1934. [Google Scholar] [CrossRef]
- Grass, I.; Albrecht, J.; Jauker, F.; Diekötter, T.; Warzecha, D.; Wolters, V.; Farwig, N. Much More than Bees-Wildflower Plantings Support Highly Diverse Flower-Visitor Communities from Complex to Structurally Simple Agricultural Landscapes. Agric. Ecosyst. Environ. 2016, 225, 45–53. [Google Scholar] [CrossRef]
- Bjørn, M.C.; Weiner, J.; Kollmann, J.; Ørgaard, M. Landscape and Urban Planning Increasing Local Biodiversity in Urban Environments: Community Development in Semi-Natural Species-Rich Forb Vegetation. Landsc. Urban Plan 2019, 184, 23–31. [Google Scholar] [CrossRef]
- Williams, D.W.; Jackson, L.L.; Smith, D.D. Effects of Frequent Mowing on Survival and Persistence of Forbs Seeded into a Species-Poor Grassland. Restor. Ecol. 2007, 15, 24–33. [Google Scholar] [CrossRef]
- Donkor, N.T.; Bork, E.W.; Hudson, R.J. Defoliation Regime Effects on Accumulated Season-Long Herbage Yield and Quality in Boreal Grassland. J. Agron. Crop. Sci. 2003, 189, 39–46. [Google Scholar] [CrossRef]
- Simanonok, S.C.; Otto, C.R.V.; Iovanna, R. Forbs Included in Conservation Seed Mixes Exhibit Variable Blooming Detection Rates and Cost-Effectiveness: Implications for Pollinator Habitat Design. Restor. Ecol. 2022, 30, e13657. [Google Scholar] [CrossRef]
- Keyser, P. Native Grass Forages for the Eastern U.S.; The University of Tennessee Institute of Agriculture: Knoxville, TN, USA, 2021. [Google Scholar]
- U.S. Drought Monitor. Available online: https://droughtmonitor.unl.edu/CurrentMap/StateDroughtMonitor.aspx?fips_47093 (accessed on 18 November 2023).
- McIntosh, D.; Aderson-Husmoen, B.J.; Kern-Lunbery, R.; Goldblatt, P.; Lemus, R.; Griggs, T.; Bauman, L.; Boone, S.; Shewmaker, G.; Teutsch, C. Guidelines for Optimal Use of NIRSC Forage and Feed Calibrations in Membership Laboratories, 2nd ed.; The University of Tennessee Press: Knoxville, TN, USA, 2022. [Google Scholar]
- AOAC. Method 990.03: Protein (Crude) in Animal Feed: Combustion Method. In Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 2010; ISBN 0935584544. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Goering, H.K.; Van Soest, P.J. Forage Fiber Analyses (Apparatus, Reagents, Procedures, and Some Applications); U.S. Government Publishing Office: Washington, DC, USA, 1970. [Google Scholar]
- AOAC. AOAC Official Method 973.18 Fiber (Acid Detergent) and Lignin (H2SO4) in Animal Feed. In Official Methods of Analysis; AOAC International: Gaithersburg, MD, USA, 2005; ISBN 0935584544. [Google Scholar]
- Xu, S.; Chen, M.; Feng, T.; Zhan, L.; Zhou, L.; Yu, G. Use Ggbreak to Effectively Utilize Plotting Space to Deal with Large Datasets and Outliers. Front. Genet. 2021, 12, 2122. [Google Scholar] [CrossRef]
- Damhoureyeh, S. Effects of Simulated Grazing (Clipping) on Plant Population Responses and Resource Allocation Patterns in Semi-Arid Environment. Pak. J. Bot. 2017, 49, 981–986. [Google Scholar]
- Ditomaso, J.M. Invasive Weeds in Rangelands: Species, Impacts, and Management. Weed Sci. 2000, 48, 255–265. [Google Scholar] [CrossRef]
- Tracy, B.F.; Sanderson, M.A. Forage Productivity, Species Evenness and Weed Invasion in Pasture Communities. Agric. Ecosyst. Environ. 2004, 102, 175–183. [Google Scholar] [CrossRef]
- Tracy, B.F.; Renne, I.J.; Gerrish, J.; Sanderson, M.A. Effects of Plant Diversity on Invasion of Weed Species in Experimental Pasture Communities. Basic Appl. Ecol. 2004, 5, 543–550. [Google Scholar] [CrossRef]
- Deak, A.; Hall, M.H.; Sanderson, M.A. Grazing Schedule Effect on Forage Production and Nutritive Value of Diverse Forage Mixtures. Agron. J. 2009, 101, 408–414. [Google Scholar] [CrossRef]
- Richwine, J.D.; Keyser, P.D.; Hancock, D.W.; Ashworth, A.J. Using a Browntop Millet Companion Crop to Aid Native Grass Establishment. Agron. J. 2021, 113, 3210–3221. [Google Scholar] [CrossRef]
- Keyser, P.D.; Holcomb, E.D.; Lituma, C.M.; Bates, G.E.; Waller, J.C.; Boyer, C.N.; Travis Mulliniks, J. Forage Attributes and Animal Performance from Native Grass Inter-Seeded with Red Clover. Agron. J. 2016, 108, 373–383. [Google Scholar] [CrossRef]
- Tracy, B.F.; Davis, A.S. Weed Biomass and Species Composition as Affected by an Integrated Crop-Livestock System. Crop. Sci. 2009, 49, 1523–1530. [Google Scholar] [CrossRef]
- Liu, Y.; Miao, H.T.; Chang, X.; Wu, G.L. Higher Species Diversity Improves Soil Water Infiltration Capacity by Increasing Soil Organic Matter Content in Semiarid Grasslands. Land Degrad Dev. 2019, 30, 1599–1606. [Google Scholar] [CrossRef]
- Thompson, G.L.; Kao-Kniffin, J. Diversity Enhances NPP, N Retention, and Soil Microbial Diversity in Experimental Urban Grassland Assemblages. PLoS ONE 2016, 11, e0155986. [Google Scholar] [CrossRef]
- Brazil, K.A.; Keyser, P.D.; Bates, G.E.; Saxton, A.M.; Holcomb, E.D. Continuous Grazing of Mixed Native Warm-Season Grass in the Fescue Belt. Agron. J. 2020, 112, 5067–5080. [Google Scholar] [CrossRef]
- Rushing, J.B.; Maples, J.G.; Rivera, J.D.; Lyles, J.C. Early-Season Grazing of Native Grasses Offers Potential Profitable Benefit. Agron. J. 2020, 112, 1057–1067. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Beef Cattle, 8th ed.; National Academy Press: Washington, DC, USA, 2016; ISBN 9780309317023. [Google Scholar]
- Dado, R.G.; Allen, M.S. Intake Limitations, Feeding Behavior, and Rumen Function of Cows Challenged with Rumen Fill from Dietary Fiber or Inert Bulk. J. Dairy Sci. 1995, 78, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Prigge, J.L.; Sheaffer, C.C.; Jungers, J.M.; Jaqueth, A.L.; Lochner, H.L.; Martinson, K.L. Forage Characteristics and Grazing Preference of Cover Crops in Equine Pasture Systems. J. Equine Vet. Sci. 2021, 103, 103663. [Google Scholar] [CrossRef] [PubMed]
- Sousa, D.O.; Hansen, H.H.; Hallin, O.; Nussio, L.G.; Nadeau, E. A Two-Year Comparison on Nutritive Value and Yield of Eight Lucerne Cultivars and One Red Clover Cultivar. Grass Forage Sci. 2020, 75, 76–85. [Google Scholar] [CrossRef]
- Brunsvig, B.R.; Smart, A.J.; Bailey, E.A.; Wright, C.L.; Grings, E.E.; Brake, D.W. Effect of Stocking Density on Performance, Diet Selection, Total-Tract Digestion, and Nitrogen Balance among Heifers Grazing Cool-Season Annual Forages. J. Anim. Sci. 2017, 95, 3513–3522. [Google Scholar] [CrossRef]
- Vickery, J.A.; Feber, R.E.; Fuller, R.J. Arable Field Margins Managed for Biodiversity Conservation: A Review of Food Resource Provision for Farmland Birds. Agric. Ecosyst. Environ. 2009, 133, 1–13. [Google Scholar] [CrossRef]



| Common Name | Scientific Name | Abbreviation | Seeding Rate PLS kg ha−1 | 100,000 SEEDS ha−1 * |
|---|---|---|---|---|
| Canada goldenrod | Solidago canadensis L. | CAGO | 0.5 | 50.6 |
| Cup plant | Silphium perfoliatum L. | CUPP | 8.9 | 19.6 |
| Maximilian sunflower | Helianthus maximiliani Schrad. | MSUN | 4.1 | 17.6 |
| Oxeye sunflower | Helopsis helianthoides (L.) Sweet | OSUN | 8.9 | 19.6 |
| Eastern purple coneflower | Echinacea purpurea (L.) Moench | PURC | 7.7 | 19.3 |
| Lanceleaf coreopsis | Coreopsis lanceolata L. | LCOR | 4.0 | 19.6 |
| Upright prairie coneflower | Ratibida columnifera (Nutt.) Woot. & Standl. | UPPC | 1.8 | 29.2 |
| Black-eyed Susan | Rudbeckia hirta L. | BESU | 1.8 | 62.5 |
| Illinois bundleflower § | Desmanthus illinoensis (Michx.) MacMill. | ILBF | 7.4 | 14.1 |
| Partridge pea § | Chamaecrista fasciculata (Michx.) Greene | PPEA | 10.7 | 15.0 |
| Purple prairie clover § | Dalea purpurea (Vent.) Rydb. | PUPC | 2.9 | 19.1 |
| Showy tick-trefoil § | Desmodium canadensis (L.) DC. | STTF | 5.3 | 8.5 |
| Collection Schedule | |||
|---|---|---|---|
| Response Variable | 2020 | 2021 | 2022 |
| Plant heights | Weekly | Weekly | Weekly |
| Plant densities | June July September | June September | June September |
| Forage mass and forage nutrient composition | June July September | June September | September |
| Flowering characteristics | Weekly | Weekly | Weekly |
| Predictor | F | df | p-Value |
|---|---|---|---|
| Plant height | |||
| Species | 18.61 | 11 | <0.01 |
| Julian date | 20.11 | 1 | <0.01 |
| Year | 5.11 | 2 | 0.01 |
| Species × Julian date | 15.27 | 11 | <0.01 |
| Species × year | 3.71 | 20 | <0.01 |
| Julian date × year | 5.71 | 2 | 0.01 |
| Species × Julian date × year | 3.41 | 20 | <0.01 |
| Plant density | |||
| Species | 901.44 | 11 | <0.01 |
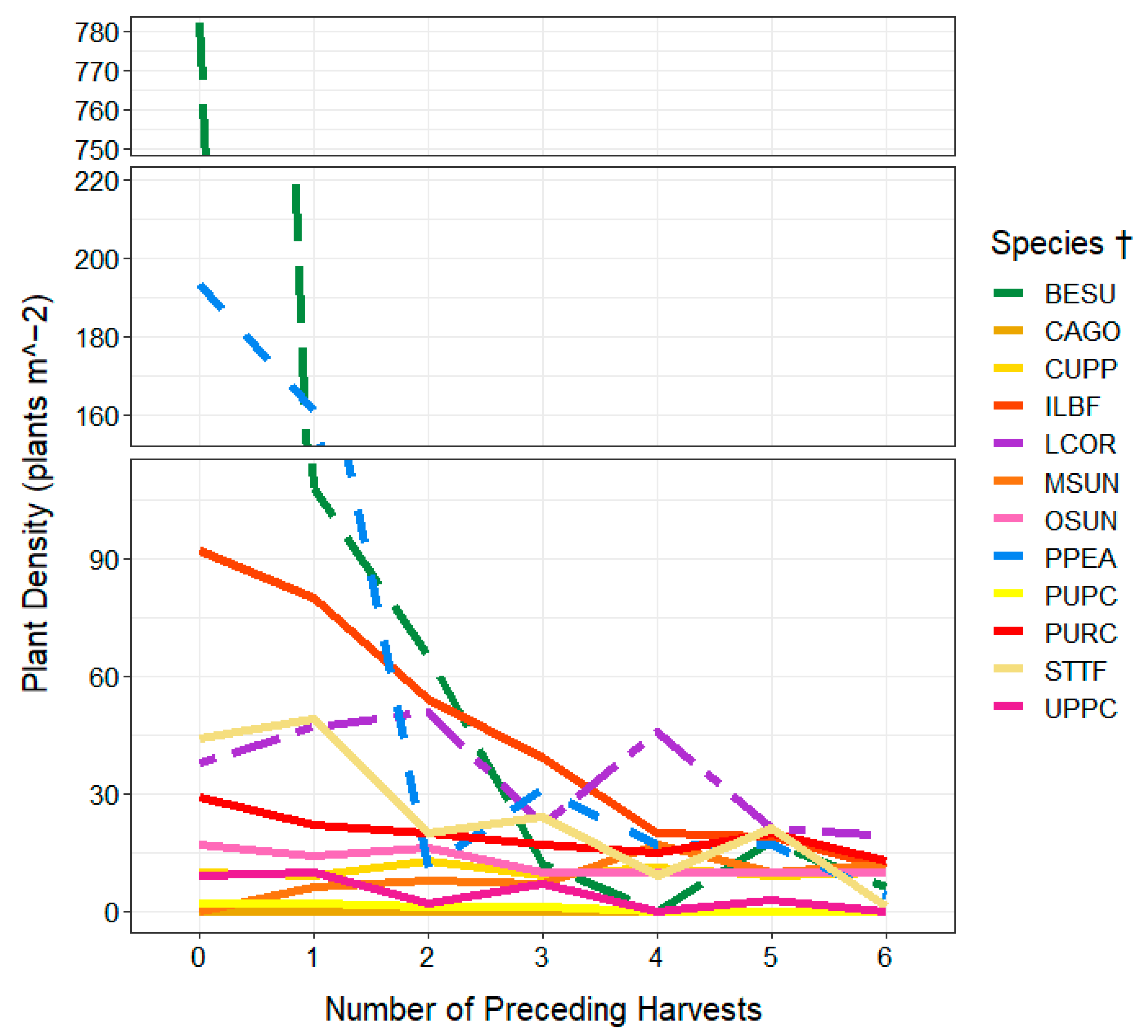
| Number of preceding harvests | 284.51 | 6 | <0.01 |
| Species × number of preceding harvests | 432.05 | 66 | <0.01 |
| Forage mass | |||
| Species | 29.95 | 11 | <0.01 |
| Year | 1.97 | 1 | 0.17 |
| Species × year | 6.42 | 11 | <0.01 |
| Crude protein | |||
| Species | 5.12 | 10 | <0.01 |
| Neutral detergent fiber | |||
| Species | 12.27 | 9 | <0.01 |
| Acid detergent fiber | |||
| Species | 12.35 | 9 | <0.01 |
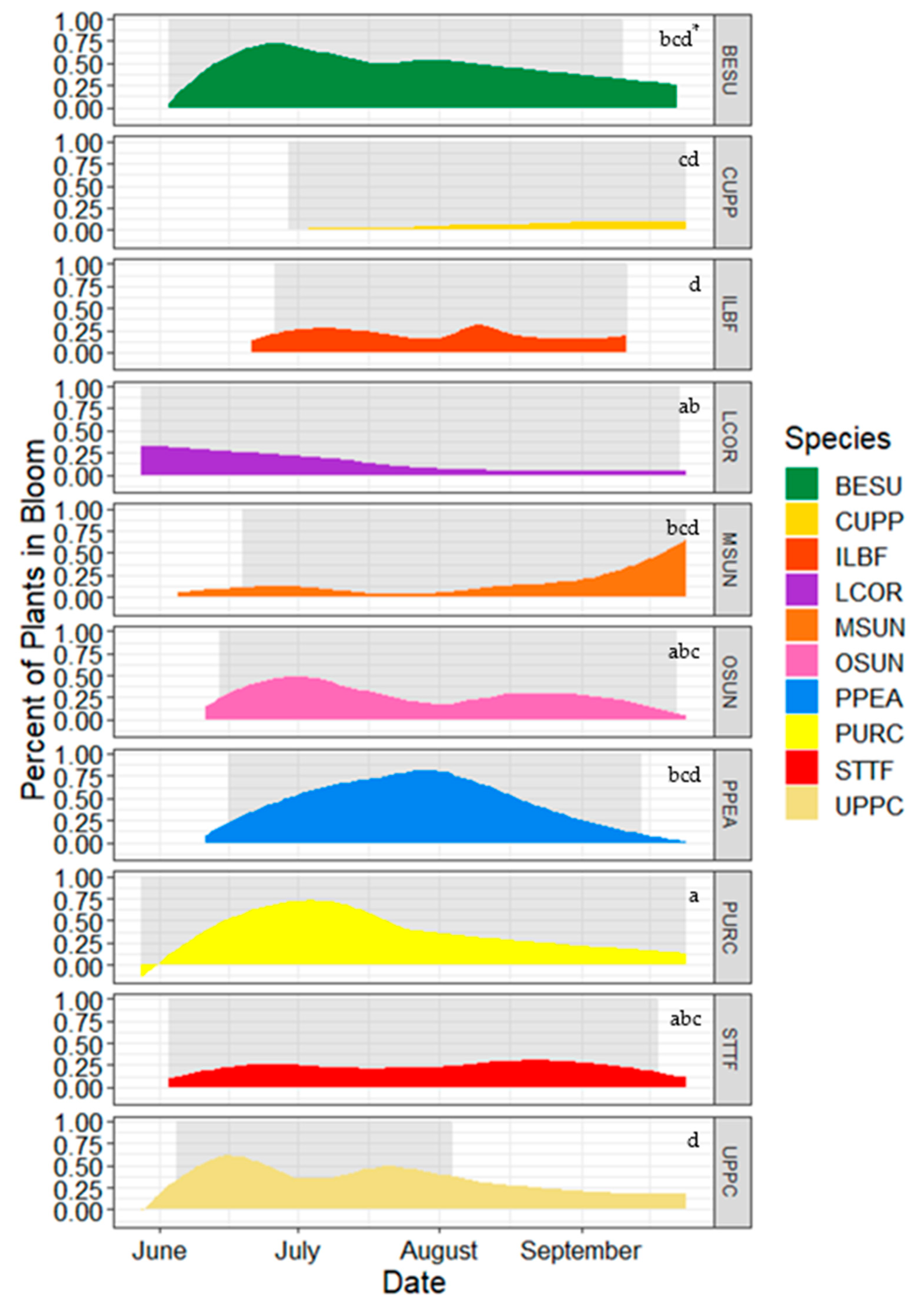
| Flowering length | |||
| Species | 9.96 | 9 | <0.01 |
| Median flowering date | |||
| Species | 55.41 | 9 | <0.01 |
| Flowering length | 40.25 | 1 | <0.01 |
| Species × flowering length | 22.84 | 9 | <0.01 |
| Year | ||
|---|---|---|
| Species | 2020 | 2021 |
| ––––––––––––––––––––––kg ha−1 DM–––––––––––––––––––– | ||
| BESU | 3374 | 1926 |
| CAGO | - | - |
| CUPP | 7248 b * | 14,803 a |
| ILBF | 3663 | 2016 |
| LCOR | 3177 | 4564 |
| MSUN | 773 b | 5242 a |
| OSUN | 3524 | 3623 |
| PPEA | 1250 a | 9 b |
| PUPC | 6 | 2 |
| PURC | 4284 | 4719 |
| STTF | 627 | 957 |
| UPPC | 823 | 1747 |
| Forage Nutrient Measures | |||
|---|---|---|---|
| Species † | CP | NDF | ADF |
| –––––––––––––––––––––––––––g kg−1 DM–––––––––––––––––––––––––– | |||
| BESU | 107 c * | 410 a | 368 a |
| CAGO § | - | - | - |
| CUPP | 135 bc | 221 d | 217 d |
| ILBF | 143 abc | 254 bcd | 225 bcd |
| LCOR | 105 c | 320 abc | 282 abc |
| MSUN | 142 abc | 306 abc | 294 ab |
| OSUN | 142 abc | 277 bcd | 234 bcd |
| PPEA | 189 a | 235 cd | 208 cd |
| PUPC | 168 abc | - | - |
| PURC | 126 bc | 326 abc | 278 a–d |
| STTF | 154 ab | 348 ab | 316 a |
| UPPC | 142 abc | 404 a | 353 a |
| Mean | 141 | 310 | 278 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prigge, J.L.; Bisangwa, E.; Richwine, J.D.; Swilling, K.J.; Keyser, P.D. Blooming and Forage Characteristics of Twelve Native Forbs Subjected to Repeated Defoliation. Agronomy 2024, 14, 28. https://doi.org/10.3390/agronomy14010028
Prigge JL, Bisangwa E, Richwine JD, Swilling KJ, Keyser PD. Blooming and Forage Characteristics of Twelve Native Forbs Subjected to Repeated Defoliation. Agronomy. 2024; 14(1):28. https://doi.org/10.3390/agronomy14010028
Chicago/Turabian StylePrigge, Jessica L., Eric Bisangwa, Jonathan D. Richwine, Keagan J. Swilling, and Patrick D. Keyser. 2024. "Blooming and Forage Characteristics of Twelve Native Forbs Subjected to Repeated Defoliation" Agronomy 14, no. 1: 28. https://doi.org/10.3390/agronomy14010028
APA StylePrigge, J. L., Bisangwa, E., Richwine, J. D., Swilling, K. J., & Keyser, P. D. (2024). Blooming and Forage Characteristics of Twelve Native Forbs Subjected to Repeated Defoliation. Agronomy, 14(1), 28. https://doi.org/10.3390/agronomy14010028







